Note: Descriptions are shown in the official language in which they were submitted.
CA 02277622 1999-07-14
AUTOCIrAVB BlIVI~Tt3 HIGB OBYG$N
TRADTSFBB BlITB TO 1LBTlIL-CO~tTAI~TI~TG BOLOTIONB
FIELD OF THE INVENTION
The present invention ie generally directed to autoclaves
and specifically to autoclaves having high rates of oxygen
transfer to metal-containing solutions.
BACKGROUND OF THE INVENTION
To oxidize sulfide sulfur and thereby permit
solubilization and/or liberation of metals compounded with the
sulfide sulfur, base metal ores and concentrates, and
refractory gold ores and Concentrates are commonly treated by
pressure oxidation. Pressure oxidation is typically performed
by passing a feed slurry of a metal-containing material
through a sealed autoclave (operating at superatmospheric
pressure) having multiple compartments. To provide for
oxidation of the sulfide sulfur in the slurry, oxygen is
typically fed continuously to the autoclave by means of a
sparge tube located below the impeller. Commonly a large
portion of the oxygen reacts with the sulfide sulfur, but
there is a smaller significant portion that is vented from the
autoclave and may be considered not effectively utilized.
In designing an autoclave, there are a number of
considerations. By way of example, the autoclave should
permit reaction of as much of the oxygen as possible with
sulfide sulfur. If the oxygen is inefficiently reacted with
CA 02277622 1999-07-14
the sulfide sulfur, the autoclave can have higher oxygen plant
capital and operating costs. The autoclave should provide as
short a residence time as possible for a given volume of
slurry while realizing a high rate of recovery for the metal.
Finally, the autoclave should vent inert gases that build up
in the autoclave above the slurry to prevent rupturing of the
autoclave from high pressure gas. Some oxygen gas is
inevitably vented along with these inert gases. Other
processes, which rely on efficient and effective gas/liquid
transfer of oxygen and which are commonly carried out in
autoclaves, include catalytic chemistry reactions, such as the
conversion of ferrous to ferric ions, reoxidation of NO by
oxygen, and cuprous amine conversion to cupric amine.
SU1~IARY OF THE INVENTION
These and other design objectives are satisfied by the
autoclave of the present invention. The autoclave includes a
vessel for containing a feed slurry material, such as a metal
sulfide-containing slurry, or a liquid comprising dissolved
chemical compounds and an impeller attached to a rotatable
shaft for agitating the feed slurry material. The shaft has
a passage for an oxygen-containing gas and an outlet in
communication with the passage for dispersing the oxygen-
containing gas in the slurry. In one configuration, the
passage passes along the length of the rotatable shaft, and
the outlet is located at or close to the tip of the impeller.
-2-
CA 02277622 1999-07-14
The autoclave can realize relatively high oxygen transfer
rates to the feed slurry material relative to conventional
autoclaves through better oxygen gas dispersion in the feed
slurry material. Commonly, the autoclave can yield an oxygen
transfer rate of at least about 2 kg moles oxygen/cubic meter
of slurry/hour. At such high oxygen transfer rates, a high
rate of metal recovery can be realized in a relatively short
residence time, and therefore lower capital and operating
costs for the autoclave equipment can be realized relative to
conventional pressure oxidation processes.
The autoclave is able to accomplish such high oxygen
transfer rates without the use of a sparge tube. The sparge
tube has proven to be an ongoing source of maintenance
problems in existing pressure oxidation processes.
To consume as much oxygen as possible, the rotatable
shaft can have an inlet for the oxygen containing gas located
at an upper end of the shaft that is above the slurry surface
yet is contained within the vessel. The inlet will provide a
suction, drawing the atmosphere in the autoclave into the
2o passage. After passing through the passage, the gas is
dispersed into the feed slurry material. In this manner, the
oxygen is continuously recycled during pressure oxidation to
provide a high rate of oxygen utilization. By efficiently
reacting the oxygen, the autoclave can have lower oxygen plant
capital and operating costs than conventional autoclaves.
-3-
CA 02277622 1999-07-14
New oxygen can be supplied to the autoclave either
directly through the rotatable shaft or through a separate
conduit such as one having an outlet in close proximity to the
impeller shaft gas inlet or above the feed slurry material.
In the latter case, the shaft must include the inlet at the
upper end of the shaft to permit oxygen escaping from the
agitated feed slurry material into the autoclave atmosphere
and/or supplied to the atmosphere to be drawn into the shaft
and thereby entrained in the agitated feed slurry material.
The rotatable shaft of the present invention can provide
improved reaction rates in the upstream compartments of the
autoclave. In conventional autoclaves, the initial
compartments frequently operate at a temperature below the
desired operating range (which is from about 180oC to about
220oC) because the exothermic conversion of sulfides to
sulfates in the initial compartments is insufficiently
complete to raise the temperature to the desired operating
range. To raise the temperature to within this range, it is
common to add steam (from a source external to the autoclave)
to the initial compartments to raise the temperature of the
slurry in the compartment and thereby increase the rate of
conversion of sulfides to sulfates. Steam can be costly to
add to the system. In contrast, in the autoclave of the
present invention the rotatable shaft draws steam in the
autoclave atmosphere through the shaft and into the slurry in
the initial compartments, thereby providing a higher
-4-
CA 02277622 1999-07-14
temperature in the slurry in these compartments and a
concomitant higher reaction rate. In other words, the
rotatable shaft increases the heat transfer from the discharge
end of the autoclave (i.e., the downstream compartments) to
the input end of the autoclave (i.e., the upstream
compartments). Accordingly, the autoclave of the present
invention can be less expensive to operate than conventional
autoclaves that inject steam into the initial compartments.
Autoclaves can include a discharge control means for
controllably removing the gas atmosphere from the sealed
autoclave to prevent rupture of the autoclave from high
pressure gases. The system includes:
(a) analyzing means (e.g., a gas analyzer) for analyzing
a selected component (e. g., carbon dioxide and/or molecular
oxygen) in the gas atmosphere inside the autoclave;
(b) an outlet for removing gas in the gas atmosphere
from the autoclave interior:
(c) a controller (e.g., a computer) for receiving a
signal from the gas analyzer and generating a control signal
in response thereto: and
(c) a control means (e.g., a valve) for controlling the
amount of gas removed in response to the control signal
received from the controller. The control means vents the gas
atmosphere when the amount of the component exceeds or falls
below a threshold amount. In this manner, the autoclave can
vent oxygen gas and other gases that build up in the autoclave
-5-
CA 02277622 1999-07-14
above the slurry while maintaining the oxygen gas in the
autoclave as long as possible for consumption in the oxidation
of sulfide sulfur.
In operation, pressure oxidation using the autoclave
follows the following steps:
(a) agitating a feed slurry material in the autoclave
using the impeller, and
(b) during the agitating step (a), passing an oxygen-
containing gas through the rotatable shaft and dispersing the
gas radially outward from the shaft into the feed slurry
material. In one autoclave configuration, the gas is passed
through a blade of the impeller outwardly into the slurry.
In another embodiment of the invention, the impeller is
used in conjunction with a sparge tube to provide a further
increase in the oxygen content of the feed slurry material.
The sparge tube is preferably located in the vicinity of the
impeller and more preferably is located beneath the impeller
such that bubbles of the oxygen-containing gas released by the
sparge tube are dispersed in the vessel by the impeller.
BRIEF DESCRIPTION OF THE DRAWINGS
Fig. 1 depicts a side view of the interconnected impeller
and the rotatable shaft of the agitation assembly, with
certain parts of the agitation assembly being shown in cross-
section;
-6-
CA 02277622 1999-07-14
Fig. 2 is a cross-sectional view taken along line 2-2 of
Fig. 1~
Fig. 3 depicts the agitation assembly operating in an
autoclave
Fig. 4 is a flow schematic depicting the discharge
control systems
Fig. 5 is a cross-sectional view of an autoclave of the
present invention showing the various compartments: and
Fig. 6 is a latitudinal cross-sectional view of an
autoclave containing an EIiATO agitator used in the
experiments.
DETAILED DESCRIPTION
The present invention is directed to a sealed autoclave
particularly useful for pressure oxidation of slurried ores
and concentrates. Although the autoclave is discussed with
reference to leaching processes, the autoclave is useful in
numerous other applications including catalytic chemistry
reactions. The autoclave includes an agitation assembly for
2o discharging oxygen directly into the slurry. In this manner,
the autoclave is able to realize relatively high rates of
oxygen transfer into the slurry and, therefore, high oxidation
rates and low residence times. The autoclave is particularly
effective in the pressure oxidation of slurried metal sulfide-
containing materials. The metal sulfides that can be
effectively utilized include without limitation gold sulfides,
CA 02277622 1999-07-14
iron sulfides, copper sulfides, zinc sulfides, nickel
sulfides, and arsenic sulfides.
Referring to Figs. 1 and 2, the agitation assembly 10 is
depicted. The agitation assembly 10 includes a rotatable
shaft 14, a gas injecting impeller 18 and a mixing impeller 22
connected to the lower end of the shaft 14, and a motor (not
shown) connected to the upper end of the shaft 14 for rotating
the shaft 14 during pressure oxidation.
The rotatable shaft 14 includes a gas inlet 26 in
communication with a conduit 30 extending longitudinally along
the shaft 14. The conduit 30 is in communication with a
number of conduits 34a-d in the gas injecting impeller 18 for
dispersing the gas substantially uniformly throughout the
slurry. A fresh oxygen-containing gas 37 from an oxygen
supply plant or the ambient atmosphere can be introduced to
the slurry via an inner conduit 25, the conduit 30, and
finally radially outward through the conduits 34a-d. An
oxygen-containing gas 38 is recycled from the autoclave
atmosphere via inlet 26 (which is open to the autoclave
interior) because rotation of the impeller 18 creates a
negative pressure at the tips 78 a-d of the blades which draws
the gas through the inlet 26. The fresh oxygen-containing gas
37 mixes with the recycled oxygen-containing gas 38 downstream
(or below) the outlet 39 of the inner conduit 25 and the mixed
gas is outputted by the conduits 34 a-d.
-g-
CA 02277622 1999-07-14
The relative orientations and dimensions of the inlet 26
and shaft conduit 30 are important. The longitudinal axis 42
of the conduit 30 is substantially normal (i.e., transverse)
to the longitudinal axis 46 of the inlet 26. The conduit 30
and shaft 14 are coaxial and therefore have the same
longitudinal axis 42. The relationship between the cross-
sectional area of the inlet 26 normal to the direction of flow
(i.e., normal to the inlet longitudinal axis 46) depends upon
a number of factors including the desired oxygen transfer
rate, the compartment size of the autoclave, the operating
oxygen partial pressure, the slurry viscosity, and the like.
The bottom 62 of the conduit 30 is may be comically
shaped in a convex orientation to effectuate redirection of
the gas into the conduits 34a-d of the impeller 18. In this
manner, eddies and other disturbances in the gas flow in
response to the sudden change of direction are substantially
minimized.
To facilitate dispersion of the gas in the slurry, the
gas injecting impeller 18 has the outlet face 74a-d of each
impeller blade 70a-d angled away from the direction of
rotation of the gas injecting impeller 18 such that a shear
zone exists at the tip 78a-d of each blade 70a-d to provide
superior atomization and dispersion of the oxygen-containing
gas (and therefore finer bubble formation). The outlet face
74a-d of each conduit 34a-d faces away from the direction of
rotation while the longest side of the blade 70a-d faces in
.g-
CA 02277622 1999-07-14
the direction of flow. The angle between the outlet face 74a-
d and the tangent 82 of a circle defined by rotation of the
tips 78a-d of the blades 70a-d is preferably about forty-five
degrees.
The gas injecting impeller 18 is located at a depth in
the autoclave slurry that maximizes effective gas transfer and
dispersion. Locating the impeller below this optimum depth
increases the hydraulic head that the impeller has to overcome
to draw down the gas phase into the agitated slurry. This can
significantly and unnecessarily increase the power required to
maintain a given oxygen transfer rate.
The mixing impeller 22 is located below the gas injecting
impeller 18 at a suitable depth to maintain in suspension the
solid particles in the autoclave in the slurry and to assist
in distribution of the entrained gas bubbles in the slurry.
Typically, the concentration of gas bubbles in the upper
portion of the slurry (which contains the gas-injecting
impeller 18) is greater than the gas bubble concentration in
the lower portion of the slurry (which contains the mixing
impeller 22).
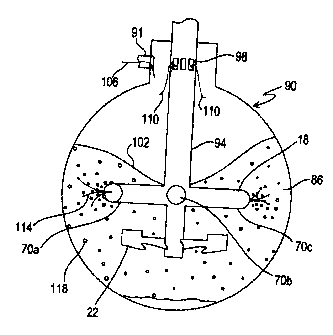
Referring to Fig. 3, the operation of the agitation
assembly will be described. During introduction of the
sulfide-containing slurry 86 into the autoclave 90, the
rotatable shaft 94 is rotated in a clockwise direction to
induce turbulence in the slurry. Unlike the rotatable shaft
14 of Figs. 1 and 2, the rotatable shaft 94 of Fig. 3 has a
-10-
CA 02277622 1999-07-14
plurality of open inlets 98 that are open to the atmosphere in
the autoclave 90 and an inner conduit extending the length of
the shaft 94 to transport fresh oxygen gas from a source
exterior to the autoclave. Rather, fresh oxygen 106 is
introduced directly into the autoclave atmosphere via inlet 91
and drawn into the open inlets 98 and through a conduit (not
shown) extending longitudinally along the shaft and finally
through the blades and dispersed into the slurry. A vortex
102 forms where the shaft 94 is immersed in the slurry 86. An
oxygen gas 106 is introduced into the autoclave and mixed with
recirculated gas 110 drawn into the shaft via the inlets 98.
The mixed gas 114 is dispersed radially outwardly, during
rotation of the blades 70a-d, in the slurry 86. The mixing
impeller 22, which rotates at the same rate and in the same
direction as the gas injecting impeller 18, further assists in
dispersing the gas bubbles 118 throughout the slurry 86,
maintains in suspension the solid particles in the slurry, and
provides a turnover of slurry from the bottom to the top of
the vessel on a continuous basis.
The autoclave 90 is able to realize high oxygen transfer
rates into the slurry 86. Typically, the oxygen transfer rate
is at least about 2kg moles and more typically at least about
4 kg moles and most typically ranges from about 2 kg moles to
about 12 kg moles of molecular oxygen/cubic meter of
slurry/hour. At such high transfer rates, the conversion of
the metal sulfides into soluble metal salts or oxidized metal
-11-
CA 02277622 1999-07-14
precipitates can be substantially completed (i.e., 90% or
more) in residence times as short as about 60 minutes and more
typically in as short as about 30 minutes.
Fig. 4 depicts a discharge control system for
controllably removing the gas atmosphere from the autoclave 90
to prevent rupture of the autoclave 90 from high pressure
gases. The system 130 includes a gas analyzer 134 for
analyzing, either continuously or at suitable intervals of
time, a selected component in the gas atmosphere in the
autoclave 90, a vent 138 for venting the gas in the
atmosphere, a controller 142 to monitor the signal 144 from
the gas analyzer 134 and generate a control signal 146 in
response thereto, and a control device 150 for controlling the
amount of gas discharged into the exterior atmosphere in
response to the control signal 146.
The selected component monitored by the gas analyzer 134
can be molecular oxygen, carbon dioxide, argon, and nitrogen,
with molecular oxygen being most preferred.
When a threshold concentration, or partial pressure, of
the selected component is reached, the controller 142 forwards
a control signal to the control device 150 to open and release
gas in the autoclave atmosphere. Preferably, the threshold is
set such that the ratio of the partial pressure of oxygen to
the partial pressure of nonoxygen compounds (e. g., carbon
dioxide) ranges from about 1:4 to about 4:1 and more
preferably from about 1:2 to about 2:1. Accordingly, when the
-12-
CA 02277622 1999-07-14
partial pressure of oxygen drops below a certain level, i.e.,
when the ratio falls below the threshold, the control device
150 opens and the autoclave gas phase is vented to the
atmosphere. Fresh "pure" oxygen is introduced at this time to
maintain the autoclave operating pressure setpoint. The
control device 150 closes either after the valve has been
opened for a specified predetermined time or alternately, may
be closed when the partial pressure of oxygen is restored to
a specified setpoint.
Referring to Figure 5, an autoclave 200 utilizing a
plurality of rotatable shafts 94a-j according to the present
invention is depicted. In the initial compartments 204a and
204b, the operating temperature of the slurry is within the
desired operating range because steam in the autoclave
atmosphere 208 above the slurry 202 is drawn through the shaft
and injected into the slurry at or near the impeller (i.e.,
mixing blades). As a result, additional steam is not
introduced into the initial compartments. The autoclave
further includes an optional sparge tube 212a-j in each
compartment 204a-j for additional oxygen enrichment of the
slurry 202. As will be appreciated, superheated steam 220
from the downstream compartments is drawn to the upstream
compartments where it is introduced into the slurry 202.
A vent 230 is located at the input end of the autoclave
to release inert gases, such as carbon dioxide, nitrogen and
argon. As will be appreciated, the atmosphere of the
-13-
CA 02277622 1999-07-14
autoclave typically contains about 80% steam, 8% molecular
oxygen, and 12% inert gases. The carbon dioxide is evolved by
the destruction of carbonate minerals in the autoclave feed by
the acid present in the autoclave oxidized slurry. Nitrogen
and argon can be present as impurities in the oxygen supply.
The vent is preferably located to the input end of the
autoclave because most of the carbon dioxide is evolved in the
initial compartments. This location of the vent allows
generally a higher carbon dioxide-to-oxygen ratio in the vent
gas which substantially minimizes the overall oxygen
consumption in the sulfur oxidation reaction.
EXPERIMENTAL
An experiment was performed using a type HWL2060~
standard agitator manufactured by EKATO~ of the type shown in
Figure 6. The agitator included a plurality of open inlets
300 that were open to the atmosphere in the autoclave. The
agitator did not have an inner conduit extending the length of
the shaft to transport fresh oxygen gas from a source exterior
to the autoclave into the slurry. The impeller blades 304
were made of round pipe. Alternatively, the blades could be
made of square pipe, rectangular pipe, or any other shaped
pipe. The blade tips 308 had an angle between the outlet face
and the tangent of a circle defined by rotation of the tips of
the blades 310 was about 450, though the angle could range
from about 30 to about 600. The oxygen re-entrainment ports
300 of the agitator were located on the upper (hollow) shaft
-14-
CA 02277622 1999-07-14
312. The motor 316 and a gear unit 320 were located at the
upper end of the agitator. An interprop 324 was located below
the blades. Modifications can be made to allow pure oxygen
being injected near or into these ports instead of sparging
oxygen into the slurry. A RUSHTOIJ~ interprop agitator was
also used by way of comparison to the EKATO~ agitator.
In two of the trials, a sparge tube was used with the
agitator to determine if the two oxygen introduction devices
would synergistically provide even higher oxygen transfer
rates compared to either device when used alone.
Data were measured in a solution containing about 0.5 M
sodium sulfite and 6 ppm cobalt. Oxygen partial pressure was
about 50 psi and the starting total pressure was about 386
psig at ambient temperature. The diameter of the agitator was
about 980 mm and the pressure vessel volume was around 5 cubic
meters.
-15-
CA 02277622 1999-07-14
TABLE OF TEST RESULTS
0.5 M NaZS03, 6 ppm Co2+, 50 psi O2, Total Pressure = 386 psig, ambient
temperature, 980 mm ERATO gassing impeller, 5 m' pressure vessel
Top Type Type of Oxygaa 7~gitator oxygen Oxygen
of
ImpellerTop Bottom SpargingBower DrswTransfer Transfer
Depth ImpellerImpeller Rats Energy
Requirement
(kW) (kg/m'.h) (kW.h/t-02)
700 RUSHTONeInterpropNO 14.4 39 87.6
700 NO 5.5 50 26.7
~
E1CAT0 Interprop -
Gassing 13.6 100 32.7
20.8 209 23.9
500 5.3 72 17.8
e
ERATO Iaterprop
Gassing 13.7 92 35.9
21.1 145 34.8
500 ERATO~ Pitch YES 6.3 87 17.3
Gassin Dower
g
Turbine 15.6 270 13.8
500 ERATO~ RU8HTOIJ~YE8 6.4 114 13.5
Gassing Turbine
16.5 273 14.5
As can be seen from the table, the oxygen transfer rate
was high in a number of the experiments, particularly when a
sparge tube was used with the impeller. To realize the same
benefits of sparging, the agitator design-of Figure 2 could
also be employed.
While carious embodiments of the present invention have
been described in detail, it is apparent that modifications
and adaptations of those embodiments will occur to those
-16-
CA 02277622 1999-07-14
skilled in the art. However, it is to be expressly understood
that such modifications and adaptations are within the scope
of the present invention, as set forth in the following
claims.
-17-