Note: Descriptions are shown in the official language in which they were submitted.
CA 02277815 1999-07-14
~....~ .,.
' "' ,' ~ ;: ~ ,
o o ~ v . ~ n a a a
0 o n a v
~, n n O a a ' a 7 ~ n , o s v s s ~
CELL-CULTURE SYSTEM
The present invention relates to substrates and vessels used
for cell-culture techniques. In particular it relates to substrates on
which cells may be grown for subsequent transfer to a pati~rt
requiring a layer of cells. ;-
W091 /13638 describes a wound dressing which comprises a
layer of polymeric material having a layer of cultured mammalian
cells anchored to one surface. Such a dressing is suitable for use in
the treatment of partial-thickness wounds such as burns and skin
donor sites. The cells are preferably epithelial cells which may be
cultured from autologous skin cells taken by biopsy or may be from
other sources. The cells are cultured and grown in the presence of
the polymer substrate to which they attach. When the substrate is
applied to a prepared site on the patient the cells transfer from the
substrate to the patient and continue to multiply to form a layer of
epidermal cells. The dressings described are made by culturing the
cells in contact with the surface of the polymeric dressing substrate,
removing the substrate from the culture medium, and then placing
the dressing on the patient such that the cells contact the wound-
bed on the patient.
An object of the present invention is to provide an improved
form of cell culture substrate device which is convenient to use in
the culture and anchorage of cells to the substrate and subsequent
transfer of cells to the patient. It is a further object to provide a cell
culture system which also achieves the above-mentioned objective.
According to the invention, a cell-culture substrate device
comprises a frame formed from a material which has no or low
toxicity to cells and cell culture substrate which is not toxic to cells
and having at least one surface to which cells may attach,
characterised wherein said frame is adapted to releasably secure
said culture substrate in a suitable configuration for attachment of
cells to said at least one surface and wherein the frame further
comprises upstanding walls extending around the said surface to
AMENDED SHEET
CA 02277815 1999-07-14
, ."
la ~ , ~ . ' . , ..:
-, .., ..
which cells may attach wherein the walls further comprises means
for retaining the frame within a container.
5 v~..
A~;'~l;v'~ ~HcET
CA 02277815 1999-07-14
' WO 98/31783 PCT/GB98100116 -
2
The frame is preferably of a suitable shape and size to fit into a
cell-culture vessel. Preferred forms are generally square,
rectangular or round although other shapes may be equally suitable.
The frame is adapted to secure a cell culture substrate in a
suitable configuration for the attachment of cells to a surface
thereof. The cell culture substrate preferably comprises a polymeric
film which has a surface suitable for the attachment of cells. The
film may be either hydrophilic or hydrophobic. The substrate may
comprise more than one layer of material.
Suitable cell culture substrate materials include those
mentioned in W091/13638, i.e. polyesters) polypropylene, blends or
copolymers of vinyl acetate, e.g. ethylene - vinyl acetate copolymer,
polyvinylidene chloride, polystyrene, polybutadienes, polyethylene,
ionomers, e.g. SURLYN (trade mark), or copolymers and blends
containing these compounds.
Alternatively hydrophilic polymers such as hydrophilic
polyurethanes may form the culture substrate. The culture substrate
has a first surface to which cultured mammalian cells may attach.
This surface may be treated to modify its properties such as by
coating with another material such as a collagen-based material, or
by other means) e.g. corona-discharge or plasma treatment. The
substrate material is preferably a film of polymeric material.
Preferably the substrate material is relatively conformable to body
contours. The substrate may be continuous or may be apertured.
The cell culture substrate material is preferably suitable for
application to a wound to serve as a wound dressing. Therefore the
cell culture substrate is preferably confom~able and elastomeric so
as to conform to the contours of the wound to which it is applied.
The material may be breathable. When the cell culture substrate
has epithelial cells attached to a surface thereof) it may be applied
to a wound such that the cells are in contact with the wound to effect
transfer of cells from the substrate to the wound to promote re-
~"i~~M''~~f e~i~#~eliau~s~tion of the wound.
CA 02277815 1999-07-14
' WO 98/31783 PCT/GB98/OOlI6 - -
3
When the substrate comprises a planar material such as a
polymeric film) the frame is preferably adapted to secure the
substrate in a substantially flat configuration. In this way pooling of
cells on the substrate may be minimised so that cells can be
relatively evenly distributed upon the surface to which they attach.
Therefore the frame preferably comprises a planar portion which
engages at least a part of said cell culture substrate to releasably
secure the substrate in a flat configuration in a plane which is
parallel to said planar portion. The planar portion may comprise an
open framework or a continuous layer of material. In a preferred
form the planar portion comprises an open frame comprising a
peripheral i.e. enclosed band, frame to which the cell culture
substrate may be attached.
The cell culture substrate may be releasably secured to the
frame e.g. by adhesive bonding, lamination) heat or radio-frequency
welding or mechanical means such as clips. The bond between the
frame and the cell culture substrate is preferably liquid-tight.
Alternatively the frame may serve to press against part or all of the
substrate to maintain it in a flat configuration against another
surface which may be the inner surface of a container or cell culture
vessel.
The frame may further comprise upstanding walls which may
or may not extend continuously around said planar portion. The
walls may comprise means for retaining said frame within a
container or vessel. For example, the walls may include a lip or
recess which may engage the edge of a container such as a petri
dish or cell culture vessel. Preferably the walls are integral with the
other parts of the frame. When the walls are continuous and
surround the planar portion of the frame, and the cell culture
substrate is bonded to the frame, the frame and substrate may,
together) form a container in which cells may be cultured.
The frame is made form materials which are non-toxic or not
significantly toxic to cells. Suitable materials include polyesters (e.g.
polyethylene terephthalate, polyethylene naphthalate), including
blends and copolymers thereof, polyolefins, polystyrene,
CA 02277815 1999-07-14
' WO 98/31783 PCT/GB98/00116 - -
4
polycarbonate, acrylic materials, polyetherimides, ethylene-vinyl
acetate copolymers and blends and copolymers containing any of
these or other suitable materials. The frame may be formed by
moulding e.g. blow moulding, thermoforming, vacuum or pressure
forming or injection moulding. The frame may be formed from a film
of polymer by vacuum forming and heating to form the desired
shape.
The device is preferably adapted to fit inside a vessel or
container, e.g. a petri dish. Therefore according to the invention a
cell culture system comprises a device comprising a frame formed
from a material which has no or low toxicity to cells and a cell culture
substrate which is not toxic to cells and having at least one surface
to which cells may attach, said frame being adapted to releasably
secure said cell culture substrate in a suitable configuration for the
attachment of cells to said surface and a container wherein said
frame is of a shape and size to fit into said container such that said
cell culture substrate is held substantially parallel to one internal
surface of said container.
The culture substrate may further comprise a support layer.
The culture substrate may be releasably attached to the support
layer over a part or all of one of its surfaces. Preferably the surface
to which a support layer is attached is opposed to the surface to
which cells attach. In this form, the culture substrate and support
layer are secured in a flat configuration by the frame. The support
layer preferably comprises a layer of a polymeric film material which
is stiffer than the culture substrate and thus it helps to maintain the
culture substrate flat and free of wrinkles. The support layer may
comprise a film of a material similar to those which are suitable to
form the frame. The support layer is preferably releasably attached
over the entire surface of the rest of the culture substrate which is
opposed to the surface to which cells attach. The support is
preferably attached in such a way that it may be removed from the
rest of the culture substrate by peeling when required.
In a preferred form of the invention, the cell culture substrate
material comprises a first surface to which cultured mammalian cells
CA 02277815 1999-07-14
' WO 98/31783 PCTlGB98/00116
may attach and a second opposed surface which is releasably
attached over at least the greater part of its second surface to the
first surface of a continuous polymeric film which forms the support
layer of the culture substrate.
5
In one embodiment the second surface of the cell culture is
releasably attached to a portion) preferably central portion, of the
support layer.
The remaining part of the support layer not in contact with the
second opposed surface of the substrate material may be releasably
or fixedly attached to the frame.
In accordance with a further aspect of the invention there is
provided a cell culture substrate device comprising a frame formed
from a material which has no or low toxicity to cells and a cell culture
substrate which is not toxic to cells and having at least one surface
to which cells may attach, wherein a portion of the substrate is
releasably attached to the other portion of the substrate, said other
portion being fixedly attached to said frame.
The invention will now be further described , by way of
example only with reference to the accompanying drawings which
are:-
Fig 1, a perspective view of a device according to the
invention;
Fig 2) a cross sectional view of the device shown in Fig 1;
placed within a vessel
Fig 3, a cross section through a second embodiment.
Fig. 4, a perspective view of a device according to a third
embodiment placed within a vessel;
Fig. 5, a cross-section of view of the device according to Fig. 4.
CA 02277815 1999-07-14
' WO 98/31783 PCT/GB98/00116 - -
6
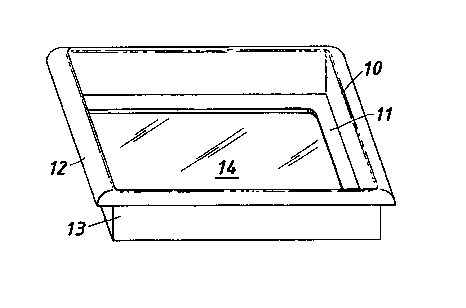
Fig 1 shows a cell culture device comprising a frame 10 and a
cell culture substrate 14. The frame 10 comprises an open
peripheral frame 11,which forms a planar portion of the frame, and
upstanding walls 13. Walls 13 and peripheral frame 11 are formed
integrally by vacuum forming a sheet of 200p,m thick polyethylene
terephthalate film. The walls 13 have a lip 12 extending around their
upper edges. The cell culture substrate film 14 is releasably
secured to the lower surface of peripheral frame 11 by lamination.
The cell culture substrate comprises a conformable sheet of
ethylene-vinyl acetate film which has been treated on its upper
surface by a corona discharge technique to modify its surface
properties for optimal cell attachment. The central portion of this
upper surface is accessible through the open part of peripheral
frame 11. The frame and culture substrate which form the device
may be placed within a petri-dish 15 as shown in Fig 2. The frame
and culture substrate are preferably sterile and supplied in a
bacteria-proof package.
To use the cell culture device, it is placed in a petri-dish or
other container and then a cell culture medium containing e.g.
human epithelial keratinocytes is added. The cells are cultured at
37°C for a number of days during which the cells multiply and attach
to the substrate until the required cell density and degree of
confluence of the cell layer is achieved. The medium is then
removed and the device is removed from the container. The cell
culture substrate may then be peeled from the frame and placed)
cell side downwards, on a wound to be treated.
In Fig 3, the culture substrate is attached over its lower surface
to a support layer 16 which is a 150 pm thick piece of PET film. The
support provides additional rigidity to the film 14 to help maintain it in
a flat and wrinkle-free condition. When the cells have been grown
and attached to the culture substrate, the support and substrate are
removed from the frame together and placed on a patient,
whereupon the support layer may be removed by peeling.
In Fig 4 and 5, the cell culture substrate material 14 is peelably
attached on its lower surface to support layer 16 over that part of
CA 02277815 1999-07-14
' WO 98/31783 PCT/GB98/00116 - ..-
7
support layer 16 defined by the peripheral frame 11. To aid
peelability, substrate material 14 is provided with a tab portion 17
towards a corner of substrate material 14. The remaining part of
support layer 16 is fixedly attached to frame 11.
10
20
,..~ $ 4~