Note: Descriptions are shown in the official language in which they were submitted.
CA 02278807 1999-07-19
I
A me~tl~od for element-selective detection, a micro plasma mass
spectrometer for use in the method and a micro plasma ion source,
together with applications thereof
The invention concerns a method for element-selective detection of
chromatographically or electrophoretically separated compounds, wherein for
the detection there is employed a micro plasma mass spectrometer with a
radio-frequency generator. a mass analyser and an ion detector. The
invention also concerns a micro plasma mass spectrometer, especially for
element-selective detection of chromatographically or electrophoretically ----
separated compounds, wherein the micro plasma mass spectrometer
comprises a radio-frequency generator, a mass analyser and an ion detector.
The invention further concerns a plasma ion source for use in a mass
spectrometer, especially a micro plasma mass spectrometer for element-
selective detection of chromatographically or electrophoreticallv separated
1 ~ compounds, the mass spectrometer comprising a radio-frequency generator, a
mass analyser and an ion detector, wherein the micro plasma ion source has
an inlet at one end and an outlet at the opposite end, and wherein the micro
plasma ion source is arranged in the mass spectrometer in such a manner that
the inlet and the outlet are located in the mass spectrometer's high vacuum
chamber. Finally, the invention concerns applications of the method, the
micro plasma mass spectrometer and the micro plasma ion source.
Element-selective detection of chromatographically and electrophoretically
separated compounds can be performed with a variety of detectors, but the
common feature of most of these is that they only respond to a few elements.
The so-called plasma atomic emission detector (AED) can be employed for
simultaneous detection of many elements (J.J. Sullivan and B.D. Quimby,
Anal. Chem. .62, 1990, pages 1034-1043: B.D. Quimby and J.J. Sullivan,
Anal. Chem. 62, 1990, pages 1027-1034), but due to high costs and limited
sensitivity its propagation has been limited. An alternative to AED is to
employ plasma mass spectrometry (E.H. Evans, J.J. Giglio, T.M. Castelliano
and J.A. Caruso, "Inductively coupled and microwave induced plasma
sources for mass spectrometry", The Royal Society of Chemistry, Cambridge,
UK, 1990. In detectors based on plasma mass spectrometry the compounds
are atomized and ionized in a plasma and the ions are detected by means of
3~ mass spectrometry. Inductively coupled plasma mass spectrometry (ICP-MS)
permits detection of several elements and has high sensitivity, but the
~EEZ
AM~N~EO
CA 02278807 1999-07-19
7
equipment is expensive to purchase and also to operate since it consumes
large amounts of noble gases. for example 1 ~ 1/min argon or helium. ICP-MS
has high detection limits for non-metals and is therefore used preferably for
determining metals. Another detector which is based on radio-frequency
glow discharge mass spectrometry (RF-GD-MS), can be employed at a gas
tZow rate of 0.6 1/min., but this detector is not commercially available at
present.
In order to reduce the operating costs in the use of microwave-induced
plasma (MIP) attempts have been made to employ discharge chambers with --~--
smaller dimensions. The introduction of the so-called "Beenaker" cavity has
made it possible to generate plasma by microwave induction down to flow
rates of ~0 ml/min. Mass spectrometers with microwave-induced plasma
(MIP-MS) are not commercially available at present.
Detection methods based on ICP-MS, RF-GD-MS and MIP-MS respectively
1 ~ employ the same method for transferring the ions from the plasma to the
mass analyser which works in a high vacuum. The ions are transferred via a
so-called "sampler" and a "skimmer". In this process the ions are transferred
from atmospheric pressure or low pressure to high vacuum. but the drawback
is that as much as 99% of the ions are lost.
?0 In the light of the disadvantages of the above-mentioned detection methods.
an object of the invention is therefore to provide a new element-selective
detector based on micro plasma ionization and mass spectrometric detection
of the ions.
A further object of the invention is that the detector should be able to be
used
2~ for detection of all elements in the periodic table. Yet another object of
the
invention is that it should be possible to directly transfer ions from the
plasma to the mass analyser under vacuum conditions in the mass
spectrometer. Yet a further object of the invention is to be able to use low
gas flow rates, typically less than 2~ ml/min, preferably less than 10 ml/min
30 and most preferably from 1-~ ml/min, and is able to employ all gases
suitable
as plasma forming gases, such as helium, neon, argon, hydrogen, nitrogen
etc. Finally. it is also an object of~the invention to provide a simple micro
plasma probe which can be used in existing commercial mass spectrometers.
e.g. as an option in addition to commonly used ion sources.
E~~E~ cr,~t~Z
P~
CA 02278807 1999-07-19
J
T'h~ above-mentioned objects are achieved according to the invention with a
method which is characterized by providing a micro plasma ion source in the
mass spectrometer's high vacuum chamber, connecting a radio-frequency
electrode in the micro plasma ion source with the radio-frequency generator,
introducing into the micro plasma ion source plasma gas which carries one or
more separated compounds which are to be detected in the mass
spectrometer, and creating a radio-frequency potential on the radio-frequency
electrode, thus creating micro plasma in the micro plasma ion source by
discharges between the radio-frequency electrode and an earth connection
provided at the micro plasma ion source, whereby the separated compounds)
which are to be analysed are atomized with the creation of atomic ions which
are subsequently expelled from the micro plasma ion source into the mass
spectrometer's high vacuum chamber for separation in the mass analyser and
detection in an ion detector provided near the mass analyser: a micro plasma
1 ~ mass spectrometer which is characterized in that it comprises a micro
plasma
ion source provided in the mass spectrometer's high vacuum chamber, the
micro plasma ion source being connected to the radio- frequency generator
for the creation of atomic ions in a gas introduced into the micro plasma ion
source: and a plasma ion source which is characterized in that between the
inlet and the outlet it is equipped with a capillary channel. that around the
channel there is provided a radio-frequency electrode which is arranged to be
connected to the radio-frequency generator for the creation of a radio-
frequency potential on the radio-frequency electrode, and that around or
adjacent to the capillary channel there are provided one or more earth
2~ electrodes, with the result that when plasma gas which carries one or more
of
the compounds which have to be detected is introduced at the inlet of the
channel, and when the radio-frequency potential is impressed on the radio-
frequency electrode, a plasma is capacitively created in the channel by
discharges between the radio-frequency electrode and the earth electrode or
electrodes. whereby the compound or compounds are atomized into atomic
ions.
The method. the micro plasma mass spectrometer and the micro plasma ion
source are employed according to the invention for selective detection of
halogens and carbon.
3~ The micro plasma ion source is employed according to the invention for
installation in existing, commercial mass spectrometers.
~ cJ,'~1~
P,~~,~OC~V
CA 02278807 1999-07-19
The-i~-dention will now be explained in more detail in connection with the
accompanying drawing, in which
Fig. 1 illustrates a micro plasma mass spectrometer according to the
invention.
Fig. 2 illustrates a first embodiment of a micro plasma ion source according
to the invention,
Fia. 3 illustrates a second embodiment of the micro plasma ion source
according to the invention,
Fig. 4 illustrates a third embodiment of the micro plasma ion.source
according to the invention,
Fig. ~ illustrates a fourth embodiment of the micro plasma ion source
according to the invention.
Fig. 6 illustrates a first plasma probe which realizes a practical embodiment
of the micro plasma ion source according to the invention for use in a mass
1 ~ spectrometer,
Fig. 7 illustrates a second plasma probe which realizes a practical
embodiment of the micro plasma ion source according to the invention for
use in a mass spectrometer, and
Fig. 8 illustrates an apparatus set-up for element-selective detection and use
of the micro plasma mass spectrometer according to the invention combined
with gas chromatographic separation.
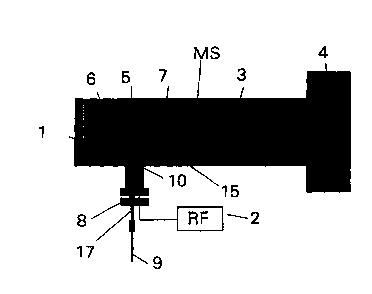
Fig. 1 illustrates a micro plasma mass~spectrometer according to the
invention. The mass spectrometer comprises a high vacuum chamber l, a
mass analyser 3 and an ion detector 4. All of this is well known to those
skilled in the art. In the high vacuum chamber 1 there is provided a cavity ~
in which there is inserted a micro plasma ion source 10 according to the
invention. The cavity ~ may be formed in a separate block in the high
vacuum chamber 1 of the mass spectrometer, and the block can include an
electrostatic repeller 6 together with electrostatic lenses 7 for focusing the
ion beam. as known from the use of standard ion sources for electron
ionization or chemical ionization. The supply of plasma gas and the sample,
i.e. the compound or compounds which are to be analysed is performed via a
pMEN~E~ ~EE~
CA 02278807 1999-07-19
J
supply--line 17. A transition piece 8 leads from the atmospheric pressure
outside the mass spectrometer into the mass spectrometer's high vacuum
chamber 1. The micro plasma ion source 10 is attached to the supply line 17
inside the high- vacuum chamber 1 ( with the result that the micro plasma ion
source projects into the high vacuum chamber. The supply line 17 passes
through the transition piece 8. A radio-frequency generator ? which Generates
a radio-frequency potential is connected to the micro plasma ion source 10
via the transition piece 8. A not shown lead 21 from the plasma ion source 10
to earth is also passed through the transition piece 8. __
The micro plasma ion source 10 is located as mentioned inside the mass
spectrometer's high vacuum chamber. In the micro plasma ion source 10 the
atomic ions which are created flow out of the micro plasma ion source's
outlet 1 ~, are deflected electrostatically by the repeller 6 and focused by
the
electrostatic lenses 7. whereupon the ions are separated in the mass analyser
1 ~ 3 and detected in the ion detector 4. Via the supply line 17 a suitable
plasma
Gas, for example helium or argon, mixed with the sample which is to be
analysed, is introduced into the micro plasma ion source 10. The supply line
17 is passed into the micro plasma ion source 10 via the transition piece 8
which forms a seal between the atmospheric pressure and the vacuum in the
mass spectrometer's high vacuum chamber 1. The micro plasma ion source
10 comprises a radio- frequency electrode 11 and one or more earth
electrodes 12, as will be discussed later. The radio-frequency Generator 2 is
connected to the radio- frequency electrode 1 1, and plasma is generated by
the radio-frequency generator impressing on the radio-frequency electrode a
2~ radio-frequency electrical potential. The frequency may, for example, be
between 100 kHz and 100 MHz. In a preferred embodiment 3~0 kHz is used.
By means of discharges between the radio-frequency electrode 1 1 and one or
more earth electrodes 12 the plasma gas is now mainly capacitively
converted to a plasma, and in this plasma the inserted sample is atomized,
with creation of atomic ions, which as mentioned flow out of the micro
plasma ion source's 10 outlet 1 ~ and are analysed in the mass spectrometer
for determination of the elements.
A~tirst embodiment of the micro plasma ion source 10 according to the
invention is illustrated in more detail in Fig. 2. The micro plasma ion source
3~ 10 has an inlet 14 which is connected to the supply line 17 in Fig. 1 and
an
outlet I ~ which projects into the mass spectrometer's high vacuum chamber
NO~O ~~~~Z
CA 02278807 1999-07-19
6
1. $etween the inlet l~ and the outlet 15 respectively the micro plasma ion
source 10 is designed as a capillary column or channel 13, for example in the
- form of a capillary tube whose internal diameter is preferably at most only
2
mm. Furthermore, the tube 13 may preferably be a quartz capillary tube. The
3 inlet 1 ~ of the micro plasma ion source 10 is connected to an earthed metal
tube 16 which contains the supply line 17 for the plasma gas and the sample
inserted in the plasma gas. The supply line 17 can also be designed as a
quartz capillary tube and may be formed in one piece with the micro plasma
ion source 10, with the result that the capillary channel 13 also acts
simultaneously as a supply pipe. Around the capillary channel 13 or the
capillary tube there is provided a radio-frequency electrode 11 which is
connected to the radio-frequency generator. In addition, around or adjacent to
the channel or the tube 13 there are provided one or more earth electrodes 12.
In Fig. 2 only one earth electrode 12 is shown, but there is no reason why
more earth electrodes cannot be provided. -The earthed metal tube 16 can thus
itself constitute a second earth electrode. When the radio-frequency generator
2 impresses a radio-frequency potential on the radio-frequency electrode 1 1,
discharges are obtained between the radio-frequency electrode and the earth
electrode or earth electrodes 12, the plasma gas introduced into the channel
13 is converted into a plasma and the accompanying sample is atomized with
subsequent creation of atomic ions, as discussed above in connection with the
description of the micro plasma mass spectrometer in Fig. 1.
The capillary column or channel 13 preferably has an internal cross section
which does not exceed 2 mm. The distance between the electrodes 11, 12
may be up to a few cm. IF a capillary tube is employed with an internal
diameter of, for example, 320 um, the volume of the discharge space
becomes very small and the gas consumption is correspondingly reduced. for
example to less than 2~ ml/min.
Fig. 3 illustrates a second preferred embodiment of the micro plasma ion
source 10 according to the invention. It differs from the embodiment in Fig. 2
in that the radio-frequency electrode 1 1 is mounted around the micro plasma
ion source's outlet 1 ~, i.e. the outlet of the capillary channel or the
capillary
tube 13. In this case the earthed metal tube 16 constitutes the earth
electrode
12. Otherwise the mode of operation of the micro plasma ion source 10 is as
mentioned above in connection with Fig. 2.
~~.~'~'~f0 ~~~'(
Ptj
CA 02278807 1999-07-19
Fia.-=1-t-llustrates a third preferred embodiment of the micro plasma ion
source
according to the invention. ft corresponds mainly to the embodiment in
Fig. 2, but in this case the capillary channel or capillary tube 1 ~ at the
outlet
1 ~ is provided with a narrowing 18. The narrowing 18 at the outlet 13 causes
the pressure in the plasma gas or the plasma in the channel or the tube 13 to
become higher, thus influencing the properties of the micro plasma ion
source, for example by increasing the energy density in the plasma.
Otherwise the mode of operation of the micro plasma ion source 10 is as
mentioned above in connection with the embodiment in Fig. 2. _ _»
10 Fig. 5 illustrates a fourth embodiment of a micro plasma ion source 10
according to the invention. In this case too the outlet 1 ~ of the capillary
channel or the capillary tube 13 is provided with a narrowing 18. The radio-
frequency electrode 11 is arranged around the narrowing 18 and in this case
too the earthed. metal tube 16 acts as an earth electrode 12, with the result
1 ~ that apart from the narrowing the embodiment corresponds to the
embodiment illustrated in Fig. 3. Otherwise the mode of operation of the
embodiment in Fig. ~ is as mentioned above in connection with the
embodiment in Fig. 2, apart from the fact that in this case the narrowing 18
at
the outlet 1 ~ also influences the properties of the micro plasma ion source,
as
mentioned in connection with Fig. 4.
The micro plasma ion source 10 according to the invention can be
advantageously implemented as a micro plasma probe, in which form it can
be installed in existing, commercial mass spectrometers. A plasma probe of
this kind is illustrated in Fig. 6. The micro plasma ion source 10. whose
mode of operation is as mentioned above in connection with one of the
figures 2-~, can be implemented by one of the embodiments illustrated in
these figures. In Fig. 6 the micro plasma probe is provided with a radio-
frequency electrode 11 arranged in the middle of the capillary channel or
capillary tube 13 and has an earth electrode 12 at the outlet 1 ~. The radio-
frequency electrode 1 1 is connected via a radio- frequency conductor 19 to
the radio-frequency Generator 2 provided outside the mass spectrometer.
while the earth electrode 12 is connected to earth via an earth conductor 21.
As mentioned above the micro plasma ion source 10 is connected to an
earthed metal tube 16 which contains the supply line 17 for the plasma Gas
and the sample included therein. At the end of the supply line 17 there is a
device 24 which enables the length of the supply line to be adjusted in
a.~L~S
;..i : ~. C-:J
CA 02278807 1999-07-19
8
relation to the electrode system 1 1. 12 in the micro plasma ion source 10.
~fhe supply line 17 is naturally connected as illustrated in Fig. 1 to a feed
line
9 for the plasma gas and the sample which has to be analysed. Around the
capillary channel 13 there are provided insulating pipes 20 for attaching the
electrodes I l, 12, while the electrode leads, i.e. the radio-frequency
conductor 19 and the earth conductor 21. may be flexibly connected by
means of screw devices 22. The transition piece 8 between the external
atmospheric pressure and the mass spectrometer's high vacuum chamber 1 is
formed as a cover and the micro plasma probe is mounted in this cover. The
cover 8 is made of an insulating material, such as transparent acrylic
plastic. ~y
The supply line 17, the radio- frequency conductor 19 and the earth
conductor 21 are passed through the cover 8. The metal tube 16 or the supply
1 ine 17 to gether with the radio-frequency conductor 19 are attached to the
cover 8 with plugs 23 which seal and insulate them, the plugs possibly being
l~ made of TetZon. Since the cover 8 is transparent and electrically
insulating,
the plasma in the micro plasma ion source 10 can be observed. The
electrically insulating material prevents short-circuiting between the radio-
frequency conductor 19 and the earth conductor 21. The micro plasma probe
as illustrated in Fig. 6 is easily attached to the mass spectrometer and is
secured by means of vacuum. Between the micro plasma probe and the
opening iri the mass spectrometer there is provided a not shown rubber O-
ring to ensure a good seal. The illustrated micro plasma probe is very simple
to install and dismantle, thus making it easy to equip existing commercial
mass spectrometers, so that they can be employed as a micro plasma mass
2~ spectrometer according to the invention. In a preferred embodiment the
supply line 17 for plasma gas and the sample included therein is a capillary
quartz tube with an internal diameter of 0.32 mm and external diameter of
0.45 mm. The channel or tube 13 in the micro plasma ion source 10 may also
be a capillary quartz tube, formed in one piece with the supply line 17. The
capillary tube of quartz is then passed through the radio-frequency electrode
l 1 and the earth electrode I 2 at the outlet. These electrodes 1 1, 12 may,
for
example, be in the form of metal tubes with an internal diameter of 0.~ mm
and external diameter of 1.6 mm.
Fig. 7 illustrates a second embodiment of the micro plasma ion source 10
3~ implemented as a micro plasma probe. This embodiment is similar to the one
amen tn Fig. 6, except that the part of the capillary tube 13 which protrudes
".. , .,,.
~~thC'~'.~~ ~'~
CA 02278807 1999-07-19
c)
out a-f xhe earthed metal tube l 6, is encapsuled by an outer fused silica
tube
'_'~. The fused silica tube ?~ is provided with a strong narrowing 18 at the
outlet 1 ~ and is tightly attached to tl:e earthed metal tube 16 by a
TetlonT""
tubing 26. Thus the encapsulation of the capillary tube 13 is almost tight and
a satisfactory pressure of the plasma gas can be maintained for very small gas
consumptions. The gas consumption may be as low as 1 ml/min, but it is
preferred to employ a consumption of 2.25 ml/min, which is the output of the
gas chromatograph. The radio-frequency electrode 1 1 and the outer earth
eletrode 12 are made of steel wire which is twisted around the fused silica
tube 2~.
Fig. 8 illustrates an apparatus set-up for element-selective detection in
micro
plasma mass spectrometry and the use of gas chromatographic separation.
The actual micro plasma mass spectrometer is designed as illustrated in Fig.
l , and reference is therefore made to the above discussion of this figure. A
1~ gas chromatograph has an open tubular column 37 which ends in a T-
connection 38 in order to mix the sample with oxygen-doped helium which is
used as plasma gas and supplied from a helium gas supply 30 with pressure
regulator and Gauge, while the oxygen. which in this case is used as
scavenger gas, comes from the oxygen gas supply 31 which is similarly
?0 equipped with pressure regulator and gauge. A T-connection 32 divides the
helium gas glow into a carrier gas tlow and an external helium flow. An
external helium flow gauge 33 and an external helium flow regulator 34 are
provided between the T-connection 32 and the T-connection 3~, the T-
connection 3 ~ being used to introduce oxygen to the external helium flow
25 through, for example, a 20 p.m microcapillary column of fused silica. The
helium carrier gas line is conveyed to a "split-splitless" injector 36. The
plasma gas doped with oxygen is transported from the T-connection 38 at the
end of the tubular column 37 and the separated sample is added through a
heated feed line 9 with a temperature control unit 39 to the supply line 17
30 and the inlet 14 of the plasma ion source 10. It should not be necessary to
provide a detailed description of this apparatus set-up. since the technique
will be well known to those skilled in the art.
For the implementation of the method according to the invention. helium was
employed as plasma gas in the micro plasma ion source. Helium has a high
33 ionization potential, providing a plasma with high energy. thus enabling
the
method and the micro plasma mass spectrometer according to the invention
~NO~~ f~aty
P~
CA 02278807 1999-07-19
tope-successfully employed for the detection of elements with a high
ionization potential. It is assumed that the flow rate of helium influences
the
plasma energy, the plasma pressure and the extension of plasma from the
foremost electrode in the micro plasma ion source's channel. Since collisions
in the plasma create the atomic ions, the amount of colliding species and
their energy will, for example, be important factors.
In the implementation of the method according to the invention. a scavenger
gas was added to the plasma gas in order to remove carbon deposits which
were formed on the quartz wall in the capillary tube 13. As mentioned in
~°"
connection with Fig. 8. oxygen was employed as scavenger gas. since oxygen
is considered to be effective with respect to chlorine-selective detection.
With the use of the method and the micro plasma mass spectrometer
according to the invention for detection of chlorine, a detection limit of 3.3
as-1 was achieved. With the use of hydrogen instead of oxygen as scavenger
l~ gas a somewhat higher detection limit for chlorine was achieved. Gas flow
rates of less than 2~ ml/s were employed, but higher flow rates were also
possible. A radio-frequency potential of 3~0 kHz was used. hut the frequency
can be higher or lower, for example in the range I00 kHz to 100 MHz. An
internal diameter of the tube or channel of only 320 ~m gave a narrow ion
beam from the outlet of the micro plasma ion source. The small volume of
the channel resulted in a power output of only 2.0 watt being employed for
the discharge.
Thus it will be seen that the micro plasma ion source as specified above and
employed in a mass spectrometer effectively realizes a micro plasma mass
2~ spectrometer according to the invention.
Experiments, however, also showed that the method and the micro plasma
mass spectrometer according to the invention, together with the micro plasma
ion source employed could probably be further improved. Ions could be
detected both in positive and negative mode, and with the invention the
opportunity is offered of detecting all elements of the periodic table.
0;
P~ENO~