Note: Descriptions are shown in the official language in which they were submitted.
CA 02278917 1999-07-26
-2-
Title: ELECTROLYSIS CELL AND INTERNAL COMBUSTION ENGINE
KIT COMPRISING THE SAME
FIELD OF THE INVENTION
This invention relates to the general field of combustion engines, and
more particularly, to combustion engines of the type that use conventional
hydrocarbon fuels such as gasoline or diesel fuel, natural gas and propane
in combination with fuel additives such as hydrogen and oxygen. Most
particularly, this invention relates to an electrolysis cell capable of
producing
hydrogen and oxygen for use in an internal combustion engine as a fuel
additive for improving performance and reducing pollution from the internal
combustion engine.
BACKGROUND OF THE INVENTION
Modern gasoline and diesel engines are much more efficient and less
polluting than similar engines of even a few years ago. However, due to the
increased number of vehicles in use, levels of air pollution continue to rise
even in light of more efficient and clean running vehicles. Therefore, there
has been increasing pressure to develop vehicles which have lower
emissions, and thus are less polluting than conventional automotive
technology permits.
For example, under certain government "Clean Air" legislation, a certain
number of vehicles are required to be emission free. This legislation has put
pressure on OEM's to develop alternate fuel technologies including electric
cars and vans, natural gas and propane fuelled vehicles, hydrogen cell
vehicles and the like. While a number of these technologies are promising,
some are still a long way from commercial implementation, and others
appear to have reached the limit of present design capabilities without
yielding a consumer acceptable product. Therefore, attention has refocused
CA 02278917 1999-07-26
-3-
on conventional gas and diesel burning engines, to try to develop a more
pollution free and efficient combustion system.
In the past, it has been discovered that the use of hydrogen and oxygen
as a fuel additive increases the efficiency of an internal combustion engine
and reduces pollution considerably. Both advantages appear to be the
byproduct of faster flame speed that is as much as 9 times that of gasoline,
resulting in more complete combustion of the fuel in the combustion
chamber, with the resultant reduction in soot (semi-burnt hydrocarbons) and
other pollutants such as nitrous oxide, carbon monoxide, and an increase
in output energy for a greater fuel efficiency and horsepower.
U.S. Patent No. 5,231,954, which issued on August 3, 1993, teaches an
electrolysis cell for use in connection with a combustion engine for
generating hydrogen and oxygen gases which are added to the fuel delivery
system as a supplement to the gasoline or other hydrocarbons burned
therein. While this patent proposes an electrolysis unit which is relatively
simple and easy to install, this prior device has a number of problems which
require considerable maintenance and lead to higher costs associated with
installing and using the device.
This patent teaches a low concentration of electrolyte, by weight, which
is created by pre-mixing and pre-charging a concentrate for 24 hours. This
results in a higher resistance electrolysis cell which can be connected
directly to a conventional vehicle battery. In the context of consumer
applications, it is too awkward to do this pre-charging and mixing. In
addition, since the resistance of the solution is relatively high, a high
amount
of heat is created in the cell during use, which can be problematic.
The preferred form of the electrolysis chamber taught by this patent is a
plastic walled chamber, into which the terminals are sealed. The terminals
project from the lower side of the plastic shell of the electrolysis cell
itself.
The unit comes on when the motor is turned on and the cell, and in
particular the terminals, tend to heat up considerably. Under the continual
heating and cooling cycling the seals around terminals can crack leading to
CA 02278917 1999-07-26
-4-
a loss of seal integrity and leaks. This requires more frequent replenishment
of the electrolyte, and a loss of function.
In addition, the electrodes of this prior device extend about two thirds to
three quarters of the way to the top of the device. Thus, there is not much
free board of solution above the top of the electrode which is
disadvantageous. After only a short period of operation, parts of the
electrodes become exposed, creating a need for addition of distilled water.
This requires frequent replenishment of the fluid, which is awkward and time
consuming. Further there is always a risk, when the electrodes are
exposed, of a spark causing an explosion of the highly combustible gases
in the unit.
In addition, the patent teaches that the gases produced in the
electrolysis chamber be introduced directly into the PVC vacuum line used
for circulating crank case gases to the intake manifold so that oxygen and
hydrogen generated in the electrolysis cell are withdrawn by the vacuum
effect in the vacuum line.
This has been found to be problematic. The introduction of the gases
into the PVC vacuum line creates considerable problems for modern
engines. Such engines typically include sensors for monitoring input air
quality (the so called "MAP" or mass air pressure sensors) which provide
output to a microprocessor which can for example adjust the fuel input to the
engine accordingly. Additional sensors monitor the combustion outputs.
Introducing these additional gases into the PVC means that they are put in
downstream of the MAP sensors which creates an imbalance, fooling the
microprocessor and causing the engine to misfire and behave poorly. Thus,
in some cases, introduction of the gases creates a worse polluting engine.
Considerable adjustment of the microprocessor controller is required to
make resolve this issue, which increases installation and servicing costs.
CA 02278917 1999-07-26
-5-
SUMMARY OF THE INVENTION
What is required is a simple and inexpensive system which overcomes
the problems associated with the prior art devices. Most particularly, this
system should include a sealed chamber, to prevent the electrolytic solution
from being lost to effects other than electrolysis. In addition, the device
should include electrodes which are located well beneath the surface of the
electrolytic solution, to allow the electrolytic solution to be used up
without
exposing the electrodes. Further the system should include an automatic
shut off switch to cause the unit to stop in the event the liquid level gets
low
enough to expose the electrodes. In addition, most preferably the device
will conduct electrolysis in a low resistance electrolysis fluid, permitting
it to
operate at relatively low temperatures to prevent damaging heating and
cooling cycles which can impair seal integrity. As well the device should
have any joints or openings in the sealed chamber formed above the highest
liquid level in the chamber. In this manner, even if a leak develops, the leak
will simply allow additional air into the electrolysis chamber rather than
leaking out electrolytic solution. Lastly, the system should preferably
compensate for loss of liquid water to decomposition to prevent over
concentration of the solution, which can lead to a higher resistance cell and
excessive heat generation.
Accordingly, there is provided an electrolysis cell according to the
present invention comprising:
a sealed plastic body;
an outlet vent on the body;
an inlet vent on the body;
a first terminal located at a top of said body;
a second terminal located adjacent to said first terminal;
an insulated conductor associated with each terminal extending through
said body and towards a bottom end thereof;
an anode operatively connected to one of said terminals; and
CA 02278917 1999-07-26
-6-
a cathode associated with the other of said terminals, said anode and
said cathode being spaced apart from one another within said body.
BRIEF DESCRIPTION OF THE DRAWINGS
Reference will now be made, by way of example only, to preferred
embodiments of the invention as illustrated in the attached figures.
Figure 1 is a perspective view of a kit of components, including an
electrical section and a fluid section, assembled according to the present
invention;
Figure 2 is a perspective view of the electrical section of Figure 1;
Figure 3a is a perspective view of the fluid section of Figure 1;
Figure 3b is the same view as Figure 3a, shown at a different angle;
Figure 4 shows the operator panel;
Figure 5 is an overview of the elements of the electrolysis cell;
Figure 6 is a schematic view of the electrode according to the present
invention;
Figure 7 is a plan view of the anode and cathode fingers, enclosed by
tube-shaped isolators; and
Figure 8 is an overview of the elements of the moisture trap.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
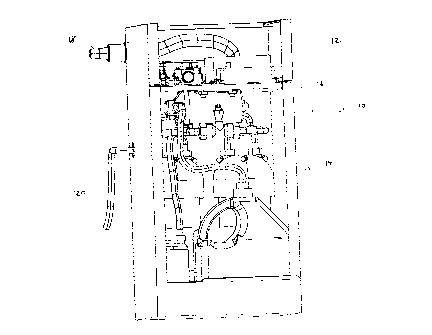
Figure 1 shows a perspective view of a kit of components for providing
combustion products to an internal combustion engine. The assembled kit
is generally indicated with reference numeral 10, and comprises an electrical
section 12, shown on top, for mostly electrical components, and a fluid
section 14, shown on bottom, for mostly electrolysis and fluid handling
components, all housed in a box enclosure 11. An internal shelf 16 divides
the two sections and supports the electrical section 12. It can be
appreciated that other configurations of the assembled kit of components
are possible, in addition to the rectangular box shown, as long as they
fulfill
the function of housing the kit of components in a convenient enclosure.
CA 02278917 1999-07-26
-7-
Figure 1 illustrates that the present invention may be viewed as a "black
box" with two outer connections. One connection is a wiring harness 18,
which carries a bundle of electrical wire and cable. A second connection is
output hose 20, which carries generated hydrogen and oxygen gas to a
vehicle internal combustion engine, not shown. For greater clarity, it may be
noted that the preferred embodiment of the present invention also includes
an operator panel and oil pressure switch that are located external to the
box enclosure 11. These elements are discussed further below.
The dimensions of the preferred embodiment of the box enclosure 11 are
about 12 inches by 12 inches by 24 inches. This size is small enough to
permit the assembled kit 10 to be conveniently attached, as an add-on, to
one or more locations in or on a typical commercial vehicle. In a large
tractor-trailer type vehicle, with an engine size of about 7-15 litres, the
box
enclosure 11 may be conveniently attached to the bracket that holds the fuel
tanks, outside the engine compartment. In that case the output hose 20 will
be run to the engine air intake. In some cases, this distance will be about
15 feet.
Figure 2 is a detailed view of the elements of the electrical section 12,
resting on the base or shelf 16. The wiring harness 18 carries electrical
power and communication signals into and out of the assembled kit 10.
Included among the electrical components mounted on the shelf 16 are a
C.P.U. 22, cell power supply 24, auto-refill electronics circuit 26, and relay
28.
The C.P.U. 22 may be any common programmable control device, such
as a microprocessor or programmable logic controller (PLC). In the
preferred embodiment of the invention, a PLC is preferred because of its low
cost and relatively simple and embedded programming scheme. The PLC
is typically a single chip device with a plurality of pins, most of which
connect
with one of the various switches, sensors, or display units that comprise
some of the components of the assembled kit 10, as further discussed
below.
CA 02278917 1999-07-26
_8_
Among the electrical signals carried in the wiring harness 18 is an input
power line from the vehicle battery. This line supplies power to the C.P.U.
and other digital electronic devices, and also to the cell power supply 24.
The cell power supply 24 is a passive device that contains a power
conditioning means in the form of a DC-DC converter to power the
electrolysis process. This direct current (DC) output is at a reduced voltage
and stepped-up current from the input vehicle battery power, and typically
maintains a current on the order of 23-26 amps. The relay 28 opens or
closes the connection between the vehicle battery and the cell power supply
24, under the control of the C.P.U. 22. When the connection is open the cell
power supply 24 is disabled and the electrolysis process is shut down. The
auto-refill electronics circuit 26 is a separate circuit maintained in its own
housing in the preferred embodiment. It can be appreciated however that
this circuitry could be incorporated into the C.P.U. 22 if desired.
The electrical section 12 also includes a number of switches and
sensors, including a temperature sensor 30, cover switch 32, inertia switch
34, and manual refill switch 36. The temperature sensor may be a simple
thermistor, and in the preferred embodiment is set to activate upon the
ambient temperature in the electrical section 12 rising above about 80
degrees Celsius. The box enclosure 11 also includes a removable cover,
not shown, to permit access to the components of the assembled kit 10.
The door switch 32 opens if the cover is not attached or improperly closed.
The inertia switch 34 responds with an open signal if the spatial orientation
of the assembled kit 10 is disturbed. This could occur, for example, if the
vehicle experiences a collision or other accident. The manual refill switch
36 acts to bypass the auto-refill electronics circuit 26, so that the refill
function performed by the circuit may be provided, upon manual
intervention, in the event of a failure by the circuit. The temperature sensor
30, cover switch 32, and inertia switch 34 connect with the C.P.U. so that,
upon activation by any one of them, the C.P.U. is notified and can take
appropriate action, generally consisting of shutting down the device.
CA 02278917 1999-07-26
_g_
The components of the fluid section 14 are shown in Figures 3a and 3b.
These figures both show the same elements from different perspectives.
Generally, the fluid section 14 of figures 3a and 3b describes two paths: a
water flow path and a gas flow path.
A starting point for the water flow path is water reservoir 38, which
contains a reservoir of distilled water 40. The reservoir 38 is held in place
by a water jug bracket 42. A water hose 44 connects the water reservoir to
a fill pump 46 through a first section, designated 44a. The fill pump 46
mounts just below the shelf 16 through an attached mounting bracket or
plate 48. The water hose 44 continues beyond the fill pump through a
second section 44b to a T-section, not visible in the drawings, that splits
the
water hose 44 into two further sections, designated as 44c and 44d. Water
hose section 44c connects with a first electrolysis cell 50 and, as seen in
Figure 3b, water hose section 44d connects with a second electrolysis cell
52. These connections are made through a water inlet 51 on each cell. A
pair of solenoids, designated as solenoid 54 and solenoid 56 respectively,
are located on either side of the T-section. Each solenoid attaches to a
separate gate and acts independent of the other so that, when the solenoid
is in a closed position, the path is blocked through water hose section 44c
and/or 44d, as the case may be. The entry of water hose sections 44c and
44d into cells 50 and 52, respectively, marks the other end point for the
water flow path in the fluid section 14.
Cells 50 and 52 are sealed chambers preferably constructed out of a
lightweight, heat resistant material. In the preferred embodiment a PVC
based plastic is used. Also in the preferred embodiment two cells are used
to provide better flexibility and capacity in meeting the combustion needs of
large commercial vehicles. However it can be appreciated that a smaller
apparatus using only one cell may also be constructed, and may be
advantageously used with smaller vehicles such as automobiles. The cells
in the preferred embodiment are generally cylindrical and are approximately
13 inches high, with a diameter of approximately 4 inches. The output
CA 02278917 1999-07-26
-10-
capacity of a cell is directly related to its volume, so in general a larger
cell
may be preferred. However, since it is also desired to minimize the size of
the overall assembled kit 10 to ease installation, there is an opposing
preference to keep the cell size small. It is a particular advantage of the
present invention that, while keeping the cell size relatively small, it is
nonetheless able to produce a useful amount of combustible gas. The
discussion further below concerning the design of the internal components
of the cells provides further information on how this beneficial effect is
achieved. There are also 4 electrical terminals on each cell representing a
cathode terminal 84, anode terminal 86, low level indicator terminal 88 and
high level indicator terminal 90. Not all of these are visible in the
drawings,
which are marked as appropriate.
The flow path of gas 57 through fluid section 14 may be described as
follows. In one embodiment of the present invention, each of cells 50 and
52 have a gas hose 58, designated 58a and 58b respectively, emanating
from a gas outlet 53 located on each cell. Gas hoses 58a and 58b then
attach to opposing ends of a T-section 60. A pressure sensor 62 is located
at the central junction of the T-section 60. The pressure sensor 62 is of a
conventional type, and is set to activate upon a pressure setting of
approximately 6 psi. The gas may be optionally routed from the boost side
of the vehicle turbocharger, in which case the pressure sensor would
preferably be set to activate in a range of approximately 20-30 psi. The gas
hose continues out of the T-section 60 as a single hose designated as gas
hose 58c, and connects at its other end to a moisture trap 64. Finally, the
gas flow path continues out of the moisture trap through output gas hose 20.
There is a second, preferred embodiment of the gas flow path that is not
directly illustrated in the drawings but that may be readily described. In
this
embodiment there is no gas hose 58a, T-Section 60, or gas hose 58c.
Rather, the moisture trap 64 sits directly on top of the gas output of cell
50,
at gas outlet 53. Gas hose 58b, emanating from the other cell, attaches
near the top and side of moisture trap 64. As there is no T-Section in this
CA 02278917 1999-07-26
-11-
embodiment, the pressure sensor 62 would be attached to the side wall of
the moisture trap. Output gas hose 20 extends from the moisture trap 64 to
the engine as before. The advantages of this embodiment relate to
improved efficiency in moisture capture and disposal, and are discussed
below in the section relating to the design of the moisture trap. Finally, it
may be noted that where only one cell is used, the first embodiment is
preferred, with the pressure sensor attached to the moisture trap as in the
second embodiment.
As noted above, there is an oil pressure switch 66 that is not shown as
part of the assembled kit 10 or in any of the drawings. This switch is an off-
the-shelf switch that is easily installed on the vehicle engine by inserting
it
in place of a non-functional oil gallery plug. The oil pressure switch 66 will
generally activate upon the presence of any oil pressure, such as occurs
upon engine start-up.
Also as noted, there is an operator panel that installs in the driver cabin.
This is shown generally as 68 in Figure 4. The operator panel has an on/off
switch 70 and a corresponding power I.e.d. (light emitting diode) 72. There
are also five I.e.d.'s which light up when their corresponding sensors are
active. These are: low engine oil pressure I.e.d. 74, which corresponds with
oil pressure switch 66, cover open I.e.d. 76, which corresponds with cover
switch 32, low water I.e.d. 78, which corresponds with a sensor to be
discussed below, high pressure Le.d. 80, which corresponds with pressure
sensor 62, and high temperature Le.d. 80, which corresponds with
temperature sensor 30.
The overall system operation of the assembled kit 10 may now be
described, referring to figures 2, 3a, 3b, and 4.
The system is enabled by the operator, who is generally the driver of the
vehicle, pressing the on/off switch 70 on the control panel. In the ordinary
course this informs the C.P.U. 22 to close the relay connecting the vehicle
battery input to the cell power supply 24, and alerts the operator through the
power I.e.d. 72, which lights up. The system however will not start if any of
CA 02278917 1999-07-26
-12-
the switches or sensors are activated. In particular, the engine must be
running, so that the oil pressure switch detects any level of oil pressure.
The
cover to the box enclosure 11 must be properly closed, or there will be a
disabling signal from the cover switch 32. The other sensors must also be
satisfied, but are less likely to be a problem on start-up.
Electrolysis is the well known technique whereby current is passed
through liquid, causing it to decompose. Where the liquid is water it can be
caused to decompose to hydrogen and oxygen. When the present invention
is in operation, the cathode terminal 84 and anode terminal 86 of each cell
receives the high current DC input from the cell power supply 24. The
electrolysis process commences in each cell, and generated hydrogen and
oxygen gas exits the gas hoses 58a and 58b, or, as described, exits from
cell 50 directly into the moisture trap 64.
Gas pressure is monitored by the pressure sensor 62, which alerts the
C.P.U. if the pressure rises above a pre-set level, generally about 6 psi, or
20-30 psi if routed from a turbocharger. The presence of the pressure
sensor guards against a condition such as a kink or obstruction in the gas
hose, which could raise the pressure to a dangerously high level. If the
C.P.U. receives a high pressure signal, it will light up the high pressure
I.e.d.
80 on the operator panel and also trigger the relay 28 to turn off power to
the
cells, stopping the electrolysis.
The generated gas generally retains some moisture as a residue of the
electrolysis process. The presence of moisture is undesirable as it acts to
undermine combustion efficiency. Accordingly, the generated gas is run
through the moisture trap 64 so that moisture is removed from the gas
before it enters the output gas hose 20. It is preferred that most moisture
is removed, and the present unit provides adequate results. Other
configurations are also possible which may remove more or less moisture,
but removing less moisture might negatively affect performance of the
assembled kit. In the preferred embodiment, the moisture trap 64 is made
from a PVC based plastic, and is in the shape of a cylinder, about 3 inches
CA 02278917 1999-07-26
-13-
high and 1 inch inside diameter. The internal operation of the moisture trap
is discussed in greater detail below.
As will be discussed, it is possible to configure the cells so that the
hydrogen and oxygen gases are separated at production. The hydrogen
gas may be directed to the vehicle engine, and the oxygen either allowed to
escape or re-directed as desired. One reason to pursue this course would
be to avoid confusing the MAP sensors present in many vehicles,
particularly passenger cars. These sensors monitor and adjust the fuel/air
combination to optimize combustion efficiency. Adding oxygen through
electrolysis without properly accounting for it can lead to increased
pollution
and reduced combustion efficiency.
As a response, the output gas from the output gas hose 20 may be
preferably introduced into the engine ahead of the MAP sensors, for
example at the air intake filter. In this way, the MAP sensors will be able to
detect and compensate for the extra input oxygen, so that an efficient
combustion can be maintained. This avoids the problems of unbalanced
readings, and removes the need to substantially revise the microprocessor
combustion controls on most vehicles. Thus, rather than removing the
oxygen, this embodiment relies on introducing the gases upstream of the
sensors to avoid the problems of the prior art.
As will be shown, the electrolysis process depends on there being an
electrolytic solution inside the cells. The electrolytic solution has a
substantial water component which is used up by the electrolytic process.
As the water is used up, the liquid level of solution declines and needs to be
replenished. In other systems, it would be necessary for the operator to stop
the vehicle and manually refill the cells. This is inconvenient and
inefficient,
and also raises the risk that the operator may inadvertently cause damage
to the device. The present invention avoids these problems by including a
refill process that operates automatically and in the background. It is
therefore an advantage of the present invention that it extends the length of
CA 02278917 1999-07-26
-14-
time during which the electrolysis cells can operate without service by the
operator.
As will be shown in greater detail below, when the level of liquid reaches
a pre-determined low level, a signal is sent from the cell to the C.P.U.
Similar to the high pressure situation described above, the C.P.U.
generates a signal that activates the low water I.e.d. 78 on the operator
panel, and also sends a signal to the relay that disconnects the cell power
supply, shutting off the electrolytic process. Then, through the auto-refill
electronics 26, the C.P.U. signals the fill pump 46 to begin pumping water
out of the water reservoir 38. The C.P.U. also signals either one or both of
the solenoids 54 and 56 to open their respective gates, so that the pumped
water can flow into either or both of cells 50 and 52, as the case may be.
The refilling continues until the level in the cell reaches a predetermined
high level, at which point a signal is sent from the cell to the C.P.U. The
C.P.U. then signals the appropriate solenoid to shut its gate so that no
further water is permitted to enter the cell. The C.P.U. will also signal the
fill
pump to stop, unless it is still waiting to receive a high signal from the
other
cell, in which case it will stop the other solenoid and the fill pump when the
second high signal is received. At that point, the C.P.U. will turn off the
low
water I.e.d. on the operator panel, and the system will resume as before. If
the cell cannot be refilled, as for example, if the water reservoir 38 is low,
a
high level signal will not be received and the system will remain shut down.
In the preferred embodiment with cell sizes as noted, the entire refill
process takes approximately one minute. During this time the operator may
possibly experience a slight loss in power, but in most cases will likely not
notice any performance related effect at all.
In this way, it can be seen that the electrolysis cell and assembled kit of
the present invention maintain a continuous flow of combustible gas to the
vehicle engine, enhancing its performance. The presence of various
sensors and switches keep the system operating safely, so that combustible
gas is not being produced when the engine is off, the vehicle is jarred in an
CA 02278917 1999-07-26
-15-
accident, the cover is not properly closed, pressure builds up in the gas
hose, or when the temperature has risen to a high level.
The configuration and operation of the electrolysis cells and moisture
trap may now be described in greater detail.
The broad elements of the electrolysis cell are shown in Figure 5. The
cell 50 comprises a body 92 and lid 93, joined at a sealed rim 96 where the
body meets the lid. There are six input/output ports or terminals on the lid.
The water inlet 51 receives water from the water hose 44c. The combustible
gas exits the cell through the gas outlet 53, either to gas hose 58a or
directly to the moisture trap 64. The cathode terminal 84 and anode terminal
86 receive high current DC power from the cell power supply 24, to drive the
electrolysis process. A low level terminal indicator 88 and high level
terminal
indicator 90 are electrical terminals that connect to pins on the C.P.U., to
alert the C.P.U. to a condition of low fluid level and high fluid level
respectively.
The interior of the cell 50 may now be viewed. There is an electrolyte
solution 15 that fills the interior up to a particular level. This solution is
composed of an electrolyte in solution with water. Although many different
electrolytes may be used, good results have been achieved with distilled
water and potassium hydroxide. The potassium hydroxide acts as an
electrolyte, in a known manner, to lower the resistance of the water and to
improve the performance of the electrolytic cell. An operating range of
potassium hydroxide to water is about 15% to 45% by weight, with a
preferred range being 25-35%, and 30% being the most preferred ratio. As
is known in the art, at this level the resistance of the solution is the
lowest,
meaning that a minimum amount of heat is generated during electrolysis.
Other electrolytes may also be used such as HN03, HZ S04, Cr03 and the
like. Notwithstanding the low resistance however the production of
hydrogen and oxygen is sufficient for the beneficial effects in combustion.
In addition, the concentration of potassium hydroxide noted will prevent the
solution from freezing up to a temperature of about minus 70 degrees C.
CA 02278917 1999-07-26
-16-
The low and high level indicator terminals connect to a low level indicator
rod 89 and high level indicator rod 91, respectively, inside the cell. The
rods
are made from 316L stainless steel, and descend vertically from the lid. The
low level indicator rod 89 is longer, and therefore descends farther, than the
high level indicator rod 91. The fluid level will always be somewhere
between the tip of the low level indicator rod 89 on the low end, and the tip
of the high level indicator rod 91 on the high end.
The cathode terminal 84 connects to an outer electrode conductor rod
95 which is a vertical rod that descends substantially into the cell.
Similarly
the anode terminal 86 connects to an inner electrode conductor rod 97 that
descends somewhat less substantially into the cell. The conductor rods are
made most preferably from stainless steel. Grade 316L stainless steel has
found to yield suitable results, but other conductor material may also be
suitable. Not shown on the drawing is that both conductor rods are
surrounded end to end by insulation, so there is no short circuiting or
electrical or electrolysis interaction between the rods and the surrounding
solution. It has been found that conventional shrink wrap plastic is suitable
for forming an insulation layer around the conductor rods.
Each conductor rod terminates in an electrode, preferably formed in a
ring. The outer electrode conductor rod 95 terminates in an outer electrode
96, and the inner electrode conductor rod 97 terminates in an inner
electrode 98. The electrodes, being extensions of the conductor rods, are
constructed of the same stainless steel and are exposed to the surrounding
solution. Each electrode also contains a plurality of projecting tab
extensions or fingers 99 that project in a perpendicular plane. The inner
electrode 98 is of smaller diameter than the outer electrode 96. The
electrodes are arranged so that a finger from the inner electrode 98 is
aligned with and inside of a corresponding finger on the outer electrode 96.
Each such electrode pair is itself surrounded by an isolator 100. The
isolators 100 act as an electrical insulator. In the preferred embodiment the
isolators are tube shaped and open at each end. The isolators are generally
CA 02278917 1999-07-26
-17-
made of standard PVC plastic tubing, about 3 inches high and 1 inch inside
diameter. The preferred embodiment also uses six fingers on each
electrode, enclosed as six pairs within six isolators. Figure 5 shows only
three fingers on each electrode, and three finger pairs, for clarity in
visualizing the relationship between the elements.
In the preferred embodiment of Fig. 5 the cathode terminal is associated
with the larger diameter outer electrode 96 and the anode terminal is
associated with the smaller diameter inner electrode 98. This assignment
is arbitrary and may be reversed without reservation. The preferred
embodiment also shows that the fingers 99 descend downward from the
inner electrode 98, but are reversed and project upwards from the outer
electrode 96. Again, this assignment is arbitrary in that the outer electrode
96 could have fingers 99 that descend and the inner electrode 98 could
have fingers that rise. Similarly, both electrodes could have fingers that are
oriented in the same direction. It can be appreciated as well that other
arrangements may be used instead of ring shaped electrodes. The key
requirement is that each of the anode and cathode maintain some sort of
broad surface area, with edges, in close proximity to each other so that
electrolysis can proceed efficiently.
Notwithstanding the above comments, there is some benefit to retaining
the ring shaped electrode and finger arrangement shown in the preferred
embodiment of Figure 5. By arranging for the two rings to have fingers
projecting in opposite directions, and by using a tube shaped isolator which
is open at both ends, the electrodes and isolators can be kept in position
easier. The isolators will be unable to slip out since they will be blocked at
either end by an electrode. Not shown in the drawing are tie strings that can
be looped around the electrodes and fingers to hold the elements in place.
In the preferred embodiment, one tie string is tied horizontally, making
contact with the isolators, and two tie strings are tied in a vertical loop,
making contact with the two electrodes. Maintaining these elements in a
CA 02278917 1999-07-26
-18-
tight connection is advantageous because once the lid is sealed it is not
possible to open the cell to repair or reinforce a loose electrode.
Figure 6 shows an electrode 96 or 98 stretched out flat. It will be
appreciated that the ends 103, 104 are joined, by any conventional means
such as a spot weld 101, to form a circular loop. This loop is then secured
by solder, welding, or the like to the ends of the conductor rods 95, 97.
Satisfactory results have been achieved with fingers of about 3'/2 inches
high, 30/1000 inches thick, and 3/4 inches wide. Other dimensions will also
provide reasonable results, provided that a sufficient surface area of the
electrodes 96, 98 is provided. It is reasonably important for smooth
operation of the device to ensure that the electrodes are evenly spaced
apart. Therefore, it is preferred to use at least a few spacers, not shown, in
between the electrodes 96 and 98 to make sure that there is an even
annular gap.
Figure 6 also show that the fingers each have three grooves 102. The
grooves are cut completely through the fingers 99 so that two new edges
are formed on each side of the finger, for each groove. Because of the
nature of the electrochemical reaction, gas bubbles tend to form on the
edges of the plates. Therefore, forming a plate with a plurality of edges,
such as the grooves 102 as shown, is believed to enhance the production
of hydrogen and oxygen gas in the cell.
For further clarity, Figure 7 provides a plan view of the isolators and the
cathode/anode finger pairs, viewed on edge.
An advantage of the above configuration of the cell of the present
invention is that all of the terminals are placed on the top lid, while the
electrolyte solution cannot rise above a predetermined maximum set below
the top lid. Unlike the prior art, where the terminals project from the side
of
the cell, there is never any contact between the fluid and the terminals. This
avoids the problem of loss of seal integrity and leaks that can occur when,
as a result of continual heating and cooling of fluid the seals are caused to
crack.
CA 02278917 1999-07-26
-19-
The operation of the electrolysis cell can now be described. With
reference to Fig. 5, the electrolyte solution 15 will be at a level somewhere
between the lower tip of low level indicator rod 89 and the lower tip of high
level indicator rod 91. The high current DC power is applied across the
cathode and anode terminals 84 and 86. The electrical charges are applied
to the two electrodes, causing electrical current to flow across the gap
between each cathode finger and its adjacent anode finger. The electrical
current flows through the electrolyte in a conventional manner, causing the
electrolysis reaction to take place, i.e. the decomposition of water into
hydrogen and oxygen. The hydrogen and oxygen gas form bubbles which
rise to the surface of the fluid, continue rising in the unfilled space above,
and exit the gas outlet 53. The electrolysis process is enhanced by
concentrating the effect in finger pairs, with extensive edge producing
grooves, and by enclosing each such pair with an isolator. The isolator has
the effect of minimizing inefficient electrical contact between non-adjacent
finger pairs. As a consequence, this configuration and design of the present
invention has the advantageous effect of reducing heat, so that more of the
energy input is directed to the desired electrolysis.
As the electrolysis proceeds the fluid level will decline as water is
decomposed. The potassium hydroxide electrolyte does not get used up in
the reaction, and therefore does not need to be added in the usual case.
However, the result is that as the water decomposes and is lost from the
cell, the concentration of potassium hydroxide will increase. Once the
concentration passes about 30% by weight, the resistance of the cell
increases with increasing concentration of electrolyte. This in turn increases
the amount of heat generated, which reduces the efficiency of the
electrolysis. Another factor is that the electrodes should always be covered
by the solution and should not be exposed. Exposure of the electrodes
creates a risk of a spark which, in the environment containing combustible
gas, could cause an explosion.
CA 02278917 1999-07-26
-20-
In the present invention, as noted the fluid level will always be
somewhere between the tip of the low level indicator rod 89 on the low end,
and the tip of the high level indicator rod 91 on the high end. It can be
predetermined that for fluid levels within this range the concentration of the
electrolyte, and corresponding electrolysis efficiency, will be acceptable.
Further, as indicated in Fig. 5, the lowest level that the fluid might reach,
that
of the low level indicator rod 89, can be set well above the electrodes to
avoid the risk of electrode exposure. In the preferred embodiment, this
distance is about 1 '/z to 2 inches.
The manner in which the fluid levels are maintained within these bounds
is as follows. The low and high level indicator terminals 88 and 90 are
driven by a square or alternating wave from the C.P.U. The low level
indicator rod 89 will generally have contact with the electrolyte solution at
its
tip, and thereby will maintain some current flow, which will be sensed by the
C.P.U. Conversely, the high level indicator rod 90 will generally not have
contact with the electrolyte solution, and thereby will tend to appear as an
open circuit to the C.P.U. When the fluid level descends below the tip of the
low level indicator rod, that terminal will suddenly appear open to the
C.P.U.,
which will inform the C.P.U. that the fluid is at a low level. Similarly, on
refill,
the high level terminal will suddenly close when the fluid reaches the tip of
the high level indicator rod, informing the C.P.U. that the high point has
been
reached. Once the C.P.U. receives the information, it is straightforward for
it to take the steps described earlier, i.e. turn display I.e.d's on or off,
or turn
the system on or off. A square or alternating wave is used to drive the
indicator rods to prevent the current through the rods from becoming a part
of the electrolytic process.
In another form of the present invention, the electrolysis process can be
configured to separate the hydrogen and oxygen gases. This could be
achieved by providing a liquid permeable but gas bubble proof barrier
between the inner and outer electrodes. This barrier may be made from
woven polypropylene for example. Above the barrier would be located an
CA 02278917 1999-07-26
-21-
impermeable cowl, which could be vented by a further nozzle or vent
provided on the cell lid 93. It can now be appreciated that this embodiment
ofthe invention comprehends separating produced hydrogen from produced
oxygen.
The broad elements of the moisture trap are shown in Figure 8. In the
preferred embodiment described above, the moisture trap sits directly above
one of the cells, for example, cell 50, and connects with the gas outlet 53
through a first gas entry 112. In practice, this connection may also include
a short section of hose 58a. The moisture trap also connects with the gas
output of the second cell, cell 52, through gas hose 58b that connects to
second gas entry 114, located on a side wall near the top of the moisture
trap 64. Output gas hose 20 emanates from the top of the moisture trap and
connects with the vehicle engine air intake.
The moisture trap 64 itself comprises three cylinders. There is a large,
outer cylinder 106 that contains the first and second gas entries to receive
the gas outputs of the two cells, and that connects with the output gas hose
20. There is a smaller middle cylinder 108 positioned inside outer cylinder
106, and that attaches to the inside top of the outer cylinder. The bottom of
middle cylinder 108 is open. Then there is an even smaller inner cylinder
110 positioned inside the middle cylinder 108, that also attaches to the
inside top. The inner cylinder 110 is open at the top, coinciding with the top
of the moisture trap, and maintains an open connection with the output gas
hose 20. The bottom of inner cylinder 110 is a small hole, inner cylinder
entry 118. There are additionally a series of small holes, inner cylinder
holes 120, located on the side of inner cylinder 110. In the preferred
embodiment there are 8 inner cylinder holes 120, formed by drilling four 2-
hole pairs through the inner cylinder 110. However it can be appreciated
that a different number of inner cylinder holes 120 could also be used.
In the preferred embodiment, the three cylinders are constructed from
standard PVC plastic tubing. The outer, middle, and inner cylinder are
respectively approximately 3 3/4, 3 '/2, and 2 3/4 inches in height, and
CA 02278917 1999-07-26
-22-
approximately 2 '/, 1 1/16, and 3/4 inches in diameter. It can be
appreciated that other materials and dimensions may also be used with
satisfactory results.
In operation, gas enters the moisture trap at the bottom from cell 50 and
at the side near the top from cell 52. The entry of gas at opposing ends,
coupled with the internal configuration ofthe moisture trap, being the various
inner and outer walls and holes and openings of the cylinders, combine to
produce an air turbulence effect, as represented by air flow 122. The flow
of air can be likened to a cyclone, in which air flow follows a circular spin
and
rises along a central column. The spinning air deposits moisture embedded
in the gas on the internal walls of the moisture trap, where it eventually
drips
to the bottom and exits through first gas entry 112 to cell 50. This is
represented by the arrow showing water 40 in Fig. 8. Simultaneously, the
rising column of gas proceeds through inner cylinder 110 and exits through
output gas hose 20. Due to the function performed by the moisture trap, the
exiting gas has a reduced moisture content, which produces an improved
combustion in the vehicle engine.
The main benefit of the above embodiment, in which the moisture trap
is positioned directly above one of the cells, is that it automatically drains
trapped moisture out of the moisture trap and into the cell. In the
embodiment shown in Fig. 3 the moisture trap would have to be drained
periodically to get rid of the accumulated water. This would also be the case
in a single cell system. Another benefit of the above embodiment is that the
entry of gas at opposing ends enhances the cyclone effect. Additionally, the
return of water to the cell helps to replenish water lost to electrolysis,
which
reduces some of the demand on the automatic refill system and the water
reservoir 38.
When the present invention is operated under cold weather conditions,
as noted the presence of electrolyte acts as an antifreeze to prevent the
fluid
inside the cells from freezing. However, the water 40 in the water reservoir
38 may be vulnerable to freezing, particularly when the vehicle is stopped.
CA 02278917 1999-07-26
-23-
One simple solution would be for the driver to remove the reservoir, which
is often just a commonly available distilled water jug, when the vehicle is at
rest. This solution is facilitated by the mounting of the reservoir or jug in
the
water jug bracket 42. Another possibility is to put a small amount of
electrolyte in the water reservoir. This may require careful monitoring of the
level of electrolyte in the cell. To avoid over-concentration, the system
could
also be designed to constantly cycle fluid from the cell to the reservoir. In
that case the fill pump would always be in operation. Yet another possibility
would be to cover the water reservoir with a thermal blanket, powered by the
vehicle battery. The power requirement for this however is quite large.
Finally, another solution would be to mount the cell power supply in close
proximity to the water reservoir. The heat of the power supply could be used
advantageously to thaw the water in the reservoir, if frozen.
Results from installation of the present invention in actual vehicles are
provided below.
Example I
A kit according to the present invention (but without reservoir) was
installed on a 1996 Ford Escort having about 130,000 kilometres on the
odometer.
Emissions
The following emission results were noted:
A. At Idle
GAS Reading (Unit Off) Reading (Unit On) % Change
C02 15.18 14.71 -3%
CO 0.07 0.03 -57%
02 0.07 0.10 +42%
HC 98 33 -66%
B. At 2500 RPM
Gas Reading (Unit Off) Reading (Unit On) % Change
Co2 15.25 14.78 -3%
CO 0.12 0.01 -92%
CA 02278917 1999-07-26
-24-
02 -0.02 -0.05 ____
HC 7 3 -57%
Example 2
This same vehicle was tested for gas mileage. Typically, without the
unit, highway mileage was 10.0 kilometres per litre. Results of two trials
with
the unit in place, and activated, yielded 15.29 kilometres per litre and 17.07
kilometres per litre respectively, and average increase of about 61.8%. This
was all highway driving, and with air conditioning on.
Example 3
In this example a 1994 Volvo with a 460 Detroit engine was subjected to
a series of opacity tests to measure the cleanliness of the exhaust. The
tests were conducted using a CaITestT"" 1000 Smokemeter, and followed the
Society of Automotive Engineers SAE J1667 procedures and standards,
which is also the EPA standard for opacity testing.
The results of four tests conducted without the kit according to the
present invention installed were:
Average Opacity Range
1. 20.8% 4.9%
2. 18.1 % 0.3%
3. 21.3% 3.4%
4. 23.0% 3.3%
A kit according to the present invention was then installed in the subject
vehicle, and the following seven results were obtained upon repeating the
opacity test:
Average Opacity Ran a
1. 10.3% 1.8%
CA 02278917 1999-07-26
-25-
2. 13.3% 3.2%
3. 14.0% 0.9%
4. 12.4% 2.4%
5. 12.7% 3.2%
6. 11.8% 3.9%
7. 11.9% 2.1
The above results indicate that the average opacity was substantially
reduced after the unit was installed.
The most preferred form of the present invention is in the form of an after
market add-on kit to an existing automobile. However, it will be appreciated
by those skilled in the art that the unit can also be installed by OEM's as a
factory installation and achieve the same results. The sealed electrolysis
chamber merely needs to be installed under the hood, in the trunk or on the
frame of an H.G.V. unit and appropriately connected.
It will be appreciated by those skilled in the art that the foregoing
description was in respect of preferred embodiments and that various
alterations and modifications are possible within the broad scope of the
appended claims without departing from the spirit of the invention such as
operating underfull vacuum with the necessary modifications. For example,
while reference is made to an electrolyte solution made with potassium
hydroxide, other forms of electrolyte solutions will also yield reasonable
results. Also, while reference was made to the electrodes being made with
stainless steel, other materials such as titanium plated with platinum may
also be used. Further, a third electrolysis cell may be added, which is
activated upon an increase in load to the vehicle engine. Various other
modifications will be apparent to those skilled in the art but are not
described
in any further detail herein.