Note: Descriptions are shown in the official language in which they were submitted.
CA 02279361 1999-07-30
WO 98/33450 PCT/US98/01922
_1_
PROGNOSIS OF CANCER PATIENTS BY DETERMINING EXPRESSION
OF CELL ('.YCLE REGULATORS P27 AND CYCLIN E
This invention was made in part with support under grants ROICA59736,
R21CA66186, and NO'L-CN-05:?30, awarded by the National Institutes of Health.
The United States government hays certain rights in the invention.
Field of the Invention
This invention provides methods for prognosis and staging in cancer patients
involving the measurement in biological samples from cancer patients of the
levels of
expression of both the call cycle regulators cyclin E and p27.
Bacl;~round of the Invention
Mutations in genes that regulate the cell cycle are the most common genetic
changes found in tumor cells (C'.lurman and Roberts, (1995)). Several studies
have
focused on the efforts to detect mutations associated with the cell cycle
genes that
encode cyclin E, a G 1 cyclin, a.nd p27-kip 1 (hereinafter, "p27"), a CDK
inhibitor.
(Pietenpol, J., et al. ( 1 ~~95); Bhatia , K., et al. ( 1995); Ponce-
Casteneda, M., et al.
(1995); Konstantin, S., et al. (1996); U.S. Patent No. 5,543,291 (1996)).
Various
studies have suggested that the '.proteins encoded by these genes contribute
to tumor
progression (Leach, F., et al. {1993); Keyomarsi, K., et al. (1994); Keyomarsi
and
Pardee (1993); Said and Medina (1995); Fero, M.L., et al. (1996); Kiyokawa,
H.,
et al. (1996); Nakayam~~, K., et al. (I996)).
Cyclin E, a regulator of the G 1 to S-phase transition in mammalian cells, has
been implicated in numerous types of human cancer (Koff et al. ( 1991 ); Lew
et al.
(1991)). Two isoforms of cyclin E, having sizes of 50 and 55 kilodaltons, have
been
CA 02279361 1999-07-30
WO 98/33450 PCT/US98/019Z2
-2
identified in cycling cells (Ohtsubo and Roberts (1993)). Cyclin E is a
nuclear
protein that attains its maximal level at the entrance to S phase, and then is
degraded
as S phase progresses (Gong et al. (1994); Ohtsubo et al. (1995)). Thus, the
amount
of cyclin E present at this critical stage in the cell cycle would appear to
be a factor in
a cell's ability to traverse S phase and to subsequently divide. Not
unexpectedly,
constitutive expression of cyclin E shortens the GI phase of the cell cycle
and
promotes an increased rate of cell division, although the cells constitutively
expressing cyclin E lack many of the characteristics of tumorigenic cells
(Ohtsubo
and Roberts ( 1993)). This latter finding suggests that overexpression of
cyclin E
alone is not sufficient to cause cancer.
Cyclin E, and other cyclins as well, manifests its control of the cell cycle
by
associating in the cell nucleus with other proteins called "cell division
kinases"
(CDKs) (U.S. Patent No. 5,549,755 (1995)). Association of CDKs with cyclins to
form cyclin/CDK complexes results in the activation of the previously dormant
kinase activity. Thus, the cyclins often are described as being the regulatory
subunits
of the CDKs. During the late G1 and early S phases of the cell cycle, cyclin E
binds
and activates at least two different kinases that belong to the "CDK2" family.
Targets for phosphorylation of cyclin E-activated kinase include, for example,
cyclin E itself, and histone H 1. Levels of the cyclin E/CDK2 polypeptide
complexes
normally are cell cycle-regulated, and peak in abundance in late G1 phase of
the cell
cycle in accordance with the peak levels of cyclin E itself.
In recent years it has emerged that cyclin E is aberrantly expressed in a
variety of human tumors (Leach et al. (1993) (cyclin E genes amplified in 2 of
47
colorectal carcinomas); Keyomarsi et al. (1994) (quantitative and qualitative
alterations in cyclin E protein production in human breast cancer and
leukemia;
Garcia-Foncillas, J., et al., (1996) (overexpression of cyclin E associated
with
decreased survival in nonmetastatic esophageal tumors); Gong et al. (1994)
(cyclin E
expressed in the wrong phase of the cell cycle in ductal breast carcinoma and
colon
carcinoma cells); Dutta et al. ( 1995) (cyclin E expression is deregulated in
breast
tumor samples); Said and Medina (1995) (elevated cyclin E expression is
correlated
with mouse mammary tumor progression); Nielsen, N.H., et al. ( 1996) (high
levels
of cyclin E in human breast cancer samples correlated with increased risk of
death
and relapse).
Like the cyclins that stimulate cell division, inhibitors of the cell cycle
also
play an important role in regulating progression through the cell cycle. The
p27
CA 02279361 1999-07-30
WO 98/33450 PCT/US98t01922
-3-
protein belongs to this c:lass of c;ell cycle regulators that, in contrast to
the cyclins,
inhibit cell division. The class of inhibitors to which p27 belongs acts by
inhibiting
the activity of the cycli:n-dependent kinases, thus are known as the "kip"
proteins.
Normally, p27 is present in high levels in quiescent cells, and declines in
proliferating cells in reaponse to mitogenic signals such as growth factors
and
cytokines (Firpo, E. et ail. ( 1994); Nourse, J., et al. ( 1994); Coats, S.,
et al. ( 1996)).
p27-kip 1 specifically inlhibits the kinase activity of the cyclin E/CDK2
complex by binding v~~ith cyclin E (Ponce-Castenada et al. (1995); Coats et
al.
( I 996)). Coats et al. demonstrated that enforced expression of p27 arrested
the cell
cycle in G 1, and that conver:;ely, decreasing the level of p27 with antisense
oligonucleotide inhibition resulted in a shortened G I phase, and an increased
rate of
cell division. The down regulation of p27 in response to mitogenic signals has
been
proposed to be a critical step in :mediating the response of normal cells to
mitogenic
stimuli (Coats et al. (19~~6)).
Because p27 slows cell proliferation by inhibiting CDKs, several groups have
raised the possibility that p27 alterations could be involved in
tumorigenesis. Not
only is p27 involved in regulating the cell cycle, but also it has been mapped
to the
human chromosome ~~rm 12p, which is a site of frequent deletions and
rearrangements in a number of human cancers, including germ cell tumors,
ovarian
teratoma, leukemia, peritoneal mesothelioma, and malignant ovarian neoplasms
(Pietenpol et al., (1995); Ponce-Castenada et al. (1995)). This circumstance
suggested the hypothesis that the non-deleted copy of p27 remaining in those
tumor
cells may have undergone further mutations such that the cells were left
without a
normal copy of p27. In this model, tumor progression would involve a process
wherein one copy of p27 is first deleted, and the remaining copy is
subsequently
mutated.
Because the p2'7 gene thus seemed to be a likely "target" for mutation in
tumor cells, Pietenpol Eat al. (1995) analyzed the p27 gene in bone marrow
samples
from 45 leukemia patients. ThE;y found a high proportion of hemizygosity for
p27,
but the remaining copies of the gene appeared normal. To explain their
results, they
proposed that the observed hemizygosity for p27 might result in reduced levels
of
p27 and a corresponding reduction in the level of CDK-inhibitory activity,
thus
resulting in uninhibited cell growth. Similarly, Ponce-Castenada et al. (
1995) found
no detectable cancer-specific deletions or point mutations in the p27 gene in
a study
of I47 human primary solid tumors, including bladder carcinomas, prostatic
CA 02279361 1999-07-30
WO 98/33450 PCTIUS98/01922
-4
carcinomas, pancreatic adenocarcinomas, breast carcinomas, lung carcinomas,
germ
cell tumors, melanomas, and soft tissue sarcomas. This group proposed that
although
abnormal p27 genes were uncommon in tumor cells, it remained possible that p27
expression was subject to posttranslational modifications or altered patterns
that
S could upset the stoichiometric balance between p27 and cyclin-CDC complexes,
thus
resulting in unregulated cell growth.
Prognostic indicators for cancer are desirable because they provide physicians
with a basis for determining the best treatment for individual patients. For
most
types of cancer, the prognostic indicators generally relied upon include tumor
size,
histopathological classification, and the results of lymph node biopsies.
Based on
these and other cancer-specific criteria, cancers typically are classified
into various
"stages," generally designated by the roman numerals I, II, III, or IV. In
many cases,
other indicators have been established whose prognostic value is associated
with only
one or a limited number of tumor types (McGuire and Clark ( 1992)).
Nonetheless,
prognostication of cancer remains imperfect, and many patients continue to be
either
undertreated or overtreated. Improved prognostic methods can assist physicians
in
better determining which patients require aggressive treatment, and which ones
will
thrive with only the minimal degree of therapy, thus improving the average
survival
of all cancer patients.
Summar~of the Invention
It has now been discovered that high levels of cyclin E and low levels of p27
are strongly predictive of increased mortality in cancer patients, both before
and after
adjustment for other clinical and pathological characteristics. Most
dramatically, it
has now been demonstrated that when cyclin E and p27 indices are combined, the
pattern of low p27 and high cyclin E expression is associated with a multi-
fold
increased relative risk of mortality.
Accordingly, this invention provides methods for determining the prognostic
outcome of cancer, and for assigning tumors to various stages of tumor
progression.
These methods involve obtaining from the patient a biological sample that
either
contains cancerous tissue, such as a tumor biopsy, or a sample such as blood
or urine
that contains materials or molecules derived from tumor tissues that have
become
necrotic or that have otherwise released their contents. The levels of
expression of
cyclin E and p27 are then determined for these tumor samples. By comparison
with
a set of standards, the observed levels of expression of cyclin E and p27 in
the patient
sample are classified as "high," "intermediate," or "low". When high levels of
CA 02279361 1999-07-30
1 92 2
~?C_T,~!~;; Q~~
IPE~,~~..'.'' ~ ~ '. °"" ,~o~
-S-
cyclin E and low levels of p27 are observed, this is indicative of advanced
stages in
tumor progression, anti the prognosis for such patients is poor with respect
to relapse
or death from cancer. A low level of cyclin E and a high level of p27
expression
indicates a good prognosis, and indicates a low grade of tumor corresponding
to the
lower stages in tumor progression. Intermediate levels of expression of the
two
markers will correspond to intermediate stages in progression of the disease.
Brief Description of the Drawings
FIGURES lA-1F illustrate the associations of cyclin E and p27 expression in
breast tumor samples and survival of the patients from whom the samples were
obtained (Kaplan-Maie:r plots). The FIGURES show survival in either the total
group
of women (FIGURES lA-1C), or in the subset consisting of node-negative women
'~ ~ ~ (FIGURES 1D-1F). :Each plot shows either the correlation between
survival and
levels of cyclin E alone (FIGURES lA and 1D), levels of p27 alone (FIGURES 1B
and lE), or cyclin E and p27 combined (FIGURES 1C and 1F).
Detailed Description of the Preferred Embodiment
This invention provides. methods for determining the prognostic outcome of
cancer and for staging tumors. It has been demonstrated that measuring the
levels of
expression of cyclin E and p2T in biological samples from cancer patients
provide a
prognostic index having greater- predictive value than measurements of either
cyclin E
or p27 alone. Thus, me:asure:ment of these two indicators in the same
biological
sample provides prognostic infiormation valuable for determining the best
therapeutic
protocol for treating cancer patients, a group that includes patients
suffering from
°"'=1 various types of cancer, inch.rding sarcoma, melanoma, leukemia,
myeloma, and
°- carcinoma, including breast carcinoma, prostate carcinoma,
colorectal carcinoma,
stomach carcinoma, esophagerrl carcinoma, bladder carcinoma, cervical
carcinoma,
lung carcinoma, as well as other cancers.
The subject inl~ention thus provides a method for determining the prognostic
outcome of cancer that involves measuring the levels of expression of cyclin E
and
p27 in a tumor sample and comparing the levels observed in the sample with the
levels of expression is a set of standards. For purposes of these
descriptions, it
should be understood that a "tumor sample" includes samples derived from any
patient suffering from cancer, including those forms of cancer, e.g.,
leukemia, that
are not typically associated with the formation of solid tumor masses. If the
patient
from whom the tumor sample is taken has, as compared with the standards, a
high
level of cyclin E expression, arid a low or undetectable level of p27
expression, this
AMENDED SHEET
CA 02279361 1999-07-30
WO 98/33450 PCTlUS98/01922
-6
circumstance indicates that a poor prognostic outcome can be expected for that
patient. Cancer patients having a "poor prognostic outcome" are those who are
significantly more likely than other patients having the same type of cancer
to have a
relapse or to die from the cancer at the end of any designated test period.
"Significantly more likely" in this context refers to statistical
significance, where the
relative risk of death (RR) is calculated according to conventional
statistical methods.
Moreover, the relative levels of cyclin E and p27 can be used to stage cancers
as an
adjunct or as an alternative to conventional methods for cancer staging.
The values for "low," "intermediate," or "high" levels of expression are
determined by comparison to reproducible standards in which low or high levels
of
expression have been demonstrated to be present. The tissues used to establish
these
standards can be derived from the normal tissue found adjacent to tumor tissue
in
biopsy samples, from normal tissue taken from the same tissue type in non-
cancer
patients, from normal tissues of other types, or from cultured cells that are
determined empirically to express low, intermediate, or high levels of these
two
prognostic markers. The levels measured in these control tissues thus
establish a
range from "low" to "high," and once these are established, the levels of
these
markers expressed in tumor tissues can be compared with levels present in the
standards, and thus be assigned a value of "low," "intermediate," or "high."
The term "tumor sample" may include any tissue or body fluid from a cancer
patient and includes either malignant cells or materials or molecules derived
from
malignant cells. Hence, a "tumor sample" includes a body fluid into which the
contents of tumor cells have been released (e.g., blood, or could be urine),
and which
may contain metabolic degradation products derived from the tumor. Thus, as
used
herein, "tumor sample" refers not only to tumor biopsy samples, but also to
samples
of blood, saliva, urine, skin scrapings, or any other tissue derived from the
patient's
body. Tumor samples can be analyzed by measuring intact mRNA or protein
expressed by the cyclin E or p27 genes, including aberrant forms of these
proteins.
and also can be analyzed by measuring various breakdown products of these
molecules that may be present, for example, in blood or urine.
By applying the subject method, cancer patients can now be subdivided into
groups having either significantly elevated or significantly reduced risk of
mortality
based on their levels of expression of cyclin E and p27. Thus, these indices
are
useful in determining which patients suffering from cancer will benefit from
more
aggressive therapy.
CA 02279361 1999-07-30
WO 98/33450 PCT/US98/01922
_'7_
Assays for determining levels of cyclin E and p27 in tumor samples include
methods for quantifying mRNA specific for these proteins (such as, e.g.,
Northern
blots), methods for direct detection of the proteins (e.g., western blots or
immunocytohistological methods), or assays based on detecting functional
activities
of free cyclin E (e.g., phosphorylase kinase activation) or complex-associated
cyclin E (ability to phosphoryiate histone HI). Similarly, functional assays
for p27
are based on this protei;n's ability to inhibit cyclin E/CDK kinase activity.
Functional
assays for both cyclin E and p27 are known in the art (e.g., Koff et al. (
1991 );
Polyak, K., et al. (1994 a); Polyak, K., et al. (1994b)).
The subject invention includes assays for: a) detecting the relative or
absolute levels and activities of cyclin E and p27 in tumor samples including
nonsynchronized cell populations (e.g., in tumor biopsy specimens or body
fluids of
cancer patients); and b) assays for determining the levels and activities of
cyclin E
and p27 in various ty~~es of biological samples (i.e., solid tissue samples,
primary
cultures of tumor cells. blood, urine, saliva, serum, plasma, mucus
secretions, CNS
fluid, cell extracts, an~3 the lik:e). The levels and activities of cyclin E
and p27
expressed in these tumor samples, taken together, provide an improved method
of
determining the stage .and predicting the outcome of cancer in individual
patients.
Analysis of this pair of markers can be used either as an alternative to
present
methods of diagnosis, prognosis, and staging, or as an adjunct to extant risk
factor
analysis.
Simultaneous analysis oif p27 and cyclin E levels can be used for determining
prognosis, i.e., predicting patient survivability and time to recurrence of
tumor, for
forming a basis for determining if aggressive anti-cancer therapies are
appropriate
(e.g., chemotherapy or irradiation therapy), for monitoring the effectiveness
of
ongoing therapy (e.g., by analyzing biopsies taken at various times during
treatment),
or for staging tumors.
Malignant condition often are classified into stages ranging from Stage I to
IV. Staging is useful to facilitate planning the most appropriate course of
therapy for
each patient and for predicting the likely outcome of the disease. Each
cancerous
condition has its own staging classification, as each cancer is different, but
the stages
are broadly defined as i:ollows. Stage I is defined usually as cases wherein
the cancer
is still confined to the organ in which it originated. Such tumors are
considered to be
operable, and the prognosis is l~ypically favorable. Stage II cancers usually
involve
some surrounding tissue, while 'Stage III cancers have invaded the local lymph
nodes.
CA 02279361 1999-07-30
WO 98/33450 PCT/US98/019Z2
_g_
In Stage IV patients, the cancer has metastasized into areas of the body
distant from
the original site, and organs besides the one in which the cancer originated
have
become involved. In general, the higher the staging number, the more serious
the
cancer, and the poorer the prognosis. However, prognostication based on
conventional histological staging is imperfect, and physicians usually take
other
factors into account as well in making decisions regarding treatment.
The subject invention offers methods for staging tumors that can improve the
physician's ability to predict the course of cancer in individual patients. To
establish
a correlation between tumor stages and expression of p27 and cyclin E,
conventionally staged tumors from various types of cancer are analyzed for
cyclin E
and p27 levels, and thereafter, measurement of these levels can serve as a
surrogate,
or as an adjunct, to the conventional staging analyses.
For staging, a tumor sample is obtained from a cancer patient, and the levels
of expression of cyclin E and p27 in the tumor sample are assayed. Thereafter,
the
levels measured in the tumor sample are compared with the levels present in a
set of
standards whose content of cyclin E and p27 have been established to
correspond to
the levels found in Stages I, II, III, or IV for the same type of cancer from
which the
tumor sample is derived. Thus, the cancer from which the tumor sample was
derived
can be assigned a classification based on the correspondence of the levels
measured
in the sample with the levels present in the standards.
In an exemplary application of the subject invention, the expression of
cyclin E and p27 was characterized in breast tumors from a group of 278 young
women. Also assayed in these samples were a number of other indicators that
had
been implicated previously as independent prognosticators of breast cancer.
The
results, described below in Example 1, indicated that when the cyclin E and
p27
indices were combined, this combination provided a prognostic indicator far
better
than any one of the indicators taken alone. The superiority of this combined
index is
apparent from the plots shown in Figures 1 A-1 F.
EXAMPLES
Example 1: Distribution of Cyclin E and X27 in Normal and Carcinoma Tissues
Because breast cancer is now recognized to disseminate early during its
course, prognostic indicators are of particular importance in this disease. To
date,
efforts to predict the course of the disease have relied largely on the
presence of
metastases in axillary lymph nodes, tumor size, and grade, and tumor
proliferation
markers. Adjuvant therapy after surgical removal of the tumor has become the
CA 02279361 1999-07-30
WO 98133450 PCT/US98101922
-9
standard of care for the majority of women with axillary node involvement,
however,
the efficacy of adjuvant therapy in node negative patients is much less
certain
{McGuire and Clark (1992); Conference, N.C. (1991)). Tumor size has been used
to
stratify patients into prognostic groups, however, almost 50% of node negative
women fall into a category of intermediate tumor size with no clear indication
for
treatment choice (McGuire and Clark (1992)).
Prognosis of bre;~st cancer is important, especially in node-negative
patients,
because while following; excision two-thirds of these women will do well
without
further adjuvant therapy, the remaining third will experience relapse (Dutta
et al.
( 1995)). Because of th~~ deleterious side effects, aggressive treatment of
all node-
negative women is not considered worth the risks and side-effects associated
with
such treatment. However, node-negative women having a poor prognosis could be
identified at the time of initial diagnosis, these women could receive the
aggressive
therapy that would incre;~se their survival.
The measurement of cyclin E levels has recently emerged as being a good
prognosticator for brea~~t cancer, and the overexpression of cyclin E protein
was
shown to be associated with a two-fold greater risk of death (Keyomarsi et al.
( 1994)
(altered expression of c;yclin E correlates with increased breast tumor stage
and
grade); Said and Medina (1995) (increased cyclin E expression in tumorigenic
cell
lines); Nielsen et al. (19'6) (increased cyclin E expression associated with
decreased
survival of breast cancer patient:.); Dutta et al. ( 1995) (elevated cyclin E
expression
associated with breast cancers having a high proliferative index). Moreover,
abnormal isoforms of cyclin E have been observed in breast tumors (Keyomarsi,
K.,
et al. (1994); Said and Medina (1995)).
To provide prognostic assays for breast cancer and other forms of cancer,
experiments were conducted as described below to determine the relative
distribution
of cyclin E, p27, and c-e;rbB-2, in human tonsil tissue, benign breast
epithelium, and
invasive ductal carcinorr~a.
Patient population
Available for this study vvere paraffin-embedded primary breast tumor tissue
samples, obtained prior to any adjuvant treatment, from a cohort of 1292
women,
aged 20 to 44, who were: identified through the Cancer Surveillance System
(CSS) of
western Washington and who were interviewed as part of a ongoing study at the
Fred
Hutchinson Cancer Research Center. These women were diagnosed between 1983
3 5 and 1992. A total of 278 ductal carcinoma samples from this cohort were
analyzed.
CA 02279361 1999-07-30
WO 98133450 PCT/US98/01922
-10
Forty-eight percent of the women from whom these samples were derived were
node
positive. Information concerning diagnosis date, tumor size, clinical stage,
and
lymph node status, and deaths was obtained from the CSS. Subjects were
followed
until the earliest of: their date of death, the date last known to be alive,
or the end of
the follow-up period. Observations were censored at either the date of last
known
follow-up or the end date of the follow-up period if death had not occurred.
Compared with women in the entire cohort, the subject of women tested for
this study were more likely to have been diagnosed in the early years of the
study
(40% vs. 57%) and were more likely to have died (53% vs. 87%). A weighted Cox
regression analysis accounted for differences in the probability of testing
and allowed
for valid relative risk evaluation (see statistical methods section). Other
clinical
characteristics (age at diagnosis, stage at diagnosis, lymph node status, and
tumor
size), varied no more than 7% between the entire cohort and the women tested
for
this study.
Tissue evaluation and characterization of antibodies
Each of the tumors analyzed for this study was assigned a histologic grade
from I (low} to III (high) according to the Bloom and Richardson grading
scheme for
invasive ductal carcinoma.
Antibodies used for this study included previously characterized affinity
purified polyclonal anti-cyclin E (Ohtsubo, M., et al. ( 1995)), anti-cyclin A
(Roberts'
lab), and anti-p27 (Nourse, J., et al. ( 1994)); rabbit polyclonal anti-c-erbB-
2 (Dako,
Denmark); anti-p53 clone 1801 (Oncogene Science, Uniondale NY); anti-Ki-67
clone MIB-1 (Immunotech, Westbrook ME). Experimental validation of
immunostaining with anti-cyclin E antibody was done by constructing cell lines
that
overexpress cyclin E from a transfected gene. To provide standards for
assessing the
levels of cyclin E in test samples, cyclin E immunostaining was compared with
western blotting and a direct correspondence between the amount of cyclin E
present
and the intensity of cyclin E immunostaining was established (Ohtsubo, M., and
Roberts, J. ( 1993)). Tissues from p27 null mice (Fero, M.L., et al. ( 1996))
provided
a negative control for the p27 antibody; in the absence of p27, no detectable
immunostaining was observed with the anti-p27 antibody. Standards for "low,"
"intermediate," and "high" levels of p27 immunostaining were established
using,
respectively, 1 ) p27 cells derived from p27 null mice (negative), 2)
proliferating rat
fibroblasts (intermediate) and 3) serum starved quiescent cells (high). By
performing
western blots on these three types of cells using anti-p27, it was established
that a
CA 02279361 1999-07-30
WO 98133450 PCT/US98/01922
-11
direct correspondence existed between the amount of p27 in the cells and the
intensity of p27 immunostaining.
Immunohistochemical Studies
All scoring and interpretations of immunocytochemical results were made by
a single pathologist (PL,P), who had no knowledge of the clinical outcome,
other
clinical variables, or pre:;ence or absence of tumor markers in each example.
Paraffin
blocks for testing were selected for presence of representative tumor and,
when
available, presence of adjacent benign epithelium in the same block.
Immunostaining was done using a modification of the standard immunoperoxidase
technique, which included microwave treatment of the tissue sections in the
presence
of citrate buffer (Gerdes, J., et al. ( 1992)).
Four high poweo fields (400X magnification) from a single representative
tissue section, chosen tc> reflect the area of highest cyclin E or p27
intensity, were
scored and each tumor section was assigned a single composite value from 0
(negative) to 6 (highest intensity) that reflected both staining intensity and
the
percentage of tumor cells positive. Categories of "high" and "low" cyclin E
and p27
immunostaining were determined by comparison of assays in benign breast
epithelium. For cyclin E, "low" intensity included all values of 0-3 (98% of
benign
epithelial samples) and "high" included values from U4-6 (2% of benign
epithelial
samples). Levels of staff ping for p27 in benign epithelium ranged widely from
values
of 2-6, and staining above levels 0-1 was almost always present (77%). "Low,"
"intermediate," and "hig;h" categories of p27 expression were assigned as 0-1,
2-3,
and 4-6 respectively. For regression analysis, intermediate and high
categories were
combined and compared to low. Five percent of the cyclin E and p27 assays were
re-
scored for a reliability assessment, blinded to the first reading. In these re-
assessments, the categories of straining intensity never varied by more than 1
integer
and there were no instances where the difference in the first and second
review
resulted in changing th.e case fiom one summary category ("high" vs. "low") to
another.
Statistical Methods
Associations between the: cell cycle proteins and covariates were calculated
using contingency table; methods and tested for significance using Pearson's
chi-
square test Survival curves were calculated using the Kaplan-Meier method.
These
curves are used to gra~~hically display subset comparisons and are not
intended to
characterize absolute mortality rates within the cohort particularly since the
sampled
CA 02279361 1999-07-30
WO 98133450 PCT/IJS98I01922
-12
subset has an over representation of deceased women. Univariate and
multivariate
relative risks were computed using Cox proportional hazards regression where
observations for this subset of women have been weighted proportional to the
inverse
of their sampling probability with respect to the larger study. Such weighting
allows
valid estimation of relative risks in non-standard sampling schemes such as
survey
data or where covariates are missing (Lin and Ying (1993); Binder, D. (1992)).
Sampling weights were 'used that depended on vital status and year of
diagnosis as
discussed in the patient population section above. All calculations were
performed
using S+ version 3.3 (StatSci, WA, USA).
Results
Sections of human tonsil tissue, benign breast epithelium, and invasive ductal
carcinoma were subjected to immunohistochemical analyses as described above.
When human tonsil was immunostained with anti-cyclin E, it was revealed that
cyclin E was present in the nuclei of scattered cells of the proliferative
germinal
center, and was absent from the quiescent mantle zone cells. p27 exhibited the
inverse pattern in human tonsil, in that it was absent from the germinal
center, and
instead was observed predominantly in the quiescent cells of the mantle zone.
Different staining patterns for cyclin E and p27 also were observed in benign
breast
sections, in which cyclin E was absent, and p27 was present in the nuclei of
epithelial
cells. Breast cancer cells exhibited high levels of cyclin E in a poorly
differentiated
tumor and, conversely, exhibited high levels of p27 in a well differentiated
tumor.
The levels of expression of cyclin E and p27 were compared in this same
group of samples to other tumor characteristics and risk factors (Table 1 )
and to
mortality after a median follow-up of 5.2 years. The relative risks (RR) (both
univariate and multivariate) of dying and 95% confidence intervals (CI) were
estimated using a weighted Cox proportional hazard model, among 237 women for
whom information concerning stage, age at diagnosis, tumor size, lymph node
status,
histologic grade, and assays of cyclin E, p27, Ki-67 proliferation index, p53,
and
c-erbB-2 were available. In univariate models, positive lymph nodes, large
tumor
size, intermediate, and high histologic grade, presence of c-erbB-2, high
levels of
cyclin E, and low or absent p27, were associated with increased risk of death
(Table
1). However, after adjusting for all other factors, only lymph node status,
presence of
c-erbB-2, high cyclin E levels, and low p27 levels, were associated with
decreased
survival (Table 1 ).
CA 02279361 1999-07-30
WO 98/33450 PCT/US98/01922
-13
Table 1: Univariate and multivariate analyses comparing overall survival to
prognostic factors in 246 breast cancer patients.
Overall Survival
Prognostic factorUnivariate RR (Univariate)Multivariate RR (Multi-
(P) (P) variate)
positive lymph <0.001 4.5 (2.8-7.0)<0.001 4.5 (2.3-8.8)
nodes
tumor size (cm)
2-4+ <0.001 2.8 ( 1.6-4.9)0.09 1.9 (0.9-4.0)
or > <0.001 7.0 (3.1-15.9)0.08 2.9 (0.9-9.7)
histological
grade 0.007 3.1 ( l .4-6.9)0.37 1.6 (0.6-4.8)
intermediate <0.0() 1 3.9 ( 1.7-8.6)0.18 2.1 (0.7-6.4)
high
c-erbB-2 <0.001 2.4 ( 1.5-3.9)0.04 2.0 (
1.1-3.9)
p53 0.1(1 1.5 (0.9-2.5)0.90 1.0 (0.4-2.2)
Ki-67 high 0.4(1 1.2 (0.8-2.0)0.49 0.7 (0.3-1.7)
high cyclin 0.001 2.1 ( 1.3-3.3)0.03 2.4 (
E level 1.1-5.2)
low p27 level <0.001 2.9 ( 1.7-4.9)0.01 2.7 (
1.3-6.0)
The factors analyzed for this group of samples are listed in Table 1. The Cox
5 regression model was used to evaluate the univariate and the multivariate
predictive
value of prognostic factors; results are shown as P (regression coefficient +/-
SE).
Relative risk was determined by the Cox regression model. Ninety-five percent
confidence intervals are shown in parentheses. For Ki-67 analysis, positive
tumor
nuclei were scored in 4 fields (4 OOX), which were selected to reflect areas
of highest
proliferation. For this analysis, "low" Ki-67 meant that 0-50% of the cells
were
positive for this marker, and "high" Ki-b7 meant that 51-100% of cells in the
observed fields were positive. rdo significant differences in the results were
observed
when the cut-off point between high and low values was set at either 25% or
50%.
For p53 analysis, positive tumor nuclei in 4 fields were assessed, and the
sample was
considered positive if > 10% of the tumor nuclei were positive for this
marker.
Membranous staining pattern of any intensity was considered a positive result
for the
c-erbB-2 marker.
When tumor and clinical characteristics were analyzed according to lymph
node status in univaria~;e regression models, large tumor size (RR 10.9, CI
1.4-83.1;
CA 02279361 1999-07-30
WO 98/33450 PCT/US98/01922
-14
p=0.02), presence of c-erbB-2 (RR 3.4, CI 1.4-7.9; p=0.005), presence of p53
protein
(RR 2.7, CI 1.2-6.5; p=0.02), high levels of cyclin E (RR 3.3, CI 1.5-7.4;
p=0.003),
and low or absent p27 (RR 4.1, CI 1.4-12.1; p=0.01), were associated with
increased
risk of dying in node negative women. In women with lymph node involvement,
only the presence of c-erbB-2 (RR 2.4, CI 1.2-4.9; p=0.01 ), and low or absent
p27
(RR 2.6, CI 1.3-5.4; p=0.007) were associated with decreased survival.
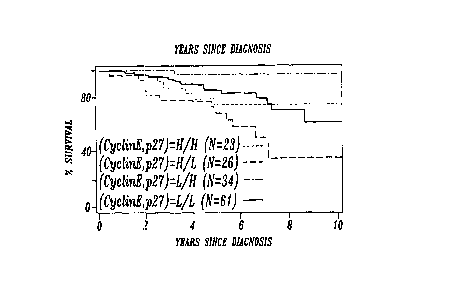
The Kaplan-Meier plots illustrated in FIGURES 1 A-1 F show the association
of survival and expression of cyclin E and p27 in all women tested
(FIGURES 1 A-1 C) and in node negative women (FIGURES 1 D-1 F). These plots
show an increased mortality risk associated with high levels of cyclin E (RR
2.1, CI
1.3-3.3, p=0.001 ) (FIGURES 1 A and 1 D), and low levels of p27 expression (RR
3.9;
CI 2.0-7.5; p=<0.001 ) (FIGURES 1 B and 1 E). Among FIGURES 1 A-1 D, the
greatest difference in survival was found between women having virtually no
p27
and women having the highest levels.
Stratification of survival based on 3 levels of p27 immunostaining was
possible in the entire group of tested patients and reflected the increasing
mortality
associated with low to absent p27 (p<0.001) (FIGURE 1B; thus, these data
provide a
basis for tumor staging based on p27 levels). The association was also present
in
node negative women with low p27 (p=0.01) (FIGURE lE) (the high and
intermediate levels were included in the "high" category for analysis).
When cyclin E and p27 results were combined, stratification based on
combinations of expression of the two proteins was possible in both groups of
women (FIGURES 1 C and 1 F). Women with high cyclin E and low p27 expression
experienced the highest mortality in the both groups (p<0.001 ). A striking
stratification of mortality risk was identified when four different
combinations of p27
and cyclin E proteins levels (i.e., both markers at high levels, both markers
at low
levels, cyclin E high when p27 is low and cyclin E low when p27 is high), were
evaluated for their relative effect on survival {FIGURES 1 C-1 F). The
combination
of expression that corresponded most closely to normal breast tissue (high p27
and
low cyclin E), was associated with the most optimal survival; the opposite
pattern
(low p27 and high cyclin E), was associated with the highest mortality (RR
8.6; CI
3.6-20.4; p<p.001 ).
Of the other tumor markers assessed in node negative women, increased
S-phase fraction measured by flow cytometry is one of the more reliable
indictors of
tumor behavior. Although a strong overall correlation of cyclin E expression
with
CA 02279361 1999-07-30
WO 98/33450 PCT/US98/01922
-15
proliferation (p<0.001 ) was found, in 27% of the tumors analyzed the Ki-67
index
and cyclin E expression levels were not concordant (in contrast to expression
of
cyclin A which invariably correlated with the proliferative status of the
tumor).
Compared with women whose tumors exhibited low tumor proliferation and low
levels of cyclin E, women with discordant cyclin E expression and
proliferation, i.e.,
low Ki-67 index and high cyclin E levels, showed a strong association with
mortality
(RR 3.3; CI 1.7-6.3; p<I).001 ).
These findings thus indicate that p27 and cyclin E are independent prognostic
markers for cancer, and that measuring the levels of both indicators provides
valuable
prognostic information.
While the preferred emhodiment of the invention has been illustrated and
described, it will be appreciated that various changes can be made therein
without
departing from the spirit and scope of the invention.
Citations
1. Clurman, B. & Roberts, J. Cell cycle and cancer. J. Natl. Cancer
Inst. 87, 1499-1501 (19'95).
2. Pietenpol, J., et al. Assignment of the human p27kip1 gene to 12p13
and its analysis in leukemias. Cancer Res. 55, 1206-1210 (1995).
3. Bhatia, lfC., et al. A mutant p21 cyclin-dependent kinase inhibitor
isolated from a Burkitt':; lymphoma Cancer Res. 55, 1431-1435 (1995).
4. Ponce-Castaneda, M., et al. p27kip1: Chromosomal mapping to
12p I 2-12p 13 . i and absence of :mutations in human tumors. Cancer Res. 55,
121 I -
1214 (1995).
5. Konstamrin, S., .et al. p27/kipl mutation found in breast cancer.
Cancer Res. 56, 2400-2404 ( 1996).
6. Leach, F., et al'. Amplification of cyclin genes in colorectal
carcinomas. Cancer Res. 53, 1986-1989 {1993).
7. Keyomarsi, K., et al. Cyclin E, a potential prognostic marker for
breast cancer. Cancer Res. 54, 380-385 (1994).
8. Keyomarsi, K., a.nd Pardee, A. Redundant cyclin overexpression and
gene amplification in breast cancer cells. Proc. Natl. Acad. Sci., USA 90,
1112-1 I 16
( I 993).
9. Said, T. & Medina, D. Cell cyclins and cyclin-dependent kinase
activities in mouse mammary tumor development. Carcinogenesis 16, 823-830
(1995).
CA 02279361 1999-07-30
WO 98/33450 PCT/US98/01922
-16
I0. Fero, M.L., et al. A syndrome of multi-organ hyperplasia with
features of gigantism, tumorigenesis and female sterility in p27kip 1-
deficient mice.
Cell ( 1996).
11. Kiyokawa, H., et al. Enhanced growth of mice lacking the cyclin-
dependent kinase inhibitor function of p27kip 1. Cell 85, 721-732 ( 1996).
12. Nakayama, K., et al. Mice lacking p27kip1 display increased body
size, multiple organ hyperplasia, retinal dysplasia and pituitary tumors. Cell
85, 707-
720 (1996).
13. Clurman, B., et al. Turnover of cyclin E by the ubiquitin-proteosome
pathway is regulated by CDK2 binding and cyclin phosphorylation. Genes & Dev.
in press( 1996).
14. Pagano, M., et al. Role of the ubiquitin-proteosome pathway in
regulating abundance of the cyclin-dependent kinase inhibitor p27. Science
269,
682-685 ( 1995).
15. Dulic, V., et al. Association of human cyclin E with a periodic
G1-S phase protein kinase. Science 257, 1958-1961 (1992).
16. Koff, A., et al. Human cyclin E, a new cyclin that interacts with two
members of the CDC2 gene family. Cell 66, 1217-1228 ( 1991 ).
17. Firpo, E., et al. Inactivation of a Cdk2 inhibitor during interleukin
2-induced proliferation of human lymphocytes. Mol. Cell. Biol. 14, 4889-4901
( 1994).
18. Nourse, J., et al. Interleukin-2-mediated elimination of p27kip1
cyclin-dependent kinase inhibitor prevented by rapamycin. Nature (London) 372,
570-573 (1994).
19. Coats, S., et al. Requirement of p27kip1 for restriction point control
of the fibroblast cell cycle. Science 272:877-880 (1996).
20. McGuire, W. and Clark, G. Prognostic factors and treatment decisions
in axillary-node negative breast cancer. N.E.J.M. 326, 1756-1761 (1992).
21. Conference, N.C. Treatment of early-stage breast cancer. J.A.M.A.
265, 391-398 (1991).
22. Gong, J., et al. Unscheduled expression of cyclin B 1 and cyclin E in
several leukemic and solid tumor cell lines. Cancer Res. 54, 4285-4288 (1994).
23. Dutta, A., et al. Cyclins as markers of tumor proliferation:
Immunocytochemical studies in breast cancer. Proc. Natl. Acad. Sci. USA 92,
5386
5390 (1995).
CA 02279361 1999-07-30
WO 98/33450 PCT/US98/01922
-17
24. Ohtsubo, M., et al. Human cyclin E, a nuclear protein essential for the
Gl-to S phase transition. Mol. (.ell. Biol. 15, 2612-2624 (1995).
25. Ohtsubo, M., and Roberts, J. Cyclin-dependent regulation of G1 in
mammalian fibroblasts. Science 259, 1908-1912 (1993).
26. Gerdes, J., et al. Immunohistological detection of tumour growth
fraction (Ki-67 antigen) in formalin-fixed and routinely processed tissues. J.
Pathol.
168, 85-86 ( 1992).
27. Lin, D., and Ying, Z. Cox regression with incomplete covariate
measurements. J. Am. ~Stat. Assoc. 88, 1341-1349 (1993).
28. Binder, D. Fitting Cox's proportional hazards models from survey
data. Biometrika 79, 1:i9-147 (1992).
29. Garcia-I~ oncillas, J., et al. Overexpression of Cyclin-D 1 and Cyclin-E
is Associated with Poorer Prognosis in Nonmetastatic Esophageal Cancer,
Gasteroenterology 110:A516 (1996).
30. Polyak, K., et al. p27K~P~, a cyclin-CDK inhibitor, links transforming
growth factor-(3 and r.ontact inhibition to cell cycle arrest. Genes Dev. , 8,
9-22
( 1994b).
31. Polyak, K., et al. Cloning of p27kip1, a cyclin-dependent kinase
inhibitor and a potential mediator of extra-cellular antimitogenic signals.
Cell 78, 59
66 ( 1994a).
32. U.S. Patent No. _'>,543,291
33. U.S. Patent No. t>,549,755