Note: Descriptions are shown in the official language in which they were submitted.
CA 02279608 1999-08-26
~!O 96/04189 ' PC'T/EP95/03130
CONTAINER FOR MOISTURE SENSITIVE MATERIAL
This invention relates to containers ) particularly to containers for moisture
sensitive materials, particularly pharmaceutical substances.
It is frequently necessary to store moisture sensitive materials for
relatively
long periods in containers. In a particular example) certain pharmaceutical
substances are supplied and/or stored in small vials containing one or more
unit
doses of the dry substance. Such vials are normally sealed with an elastomeric
closure including a closure wall across the mouth) and having a puncturable
region
such as a thin part of the closure wall through which a hypodermic needle may
be
inserted. By means of such a needle water or other suitable aqueous medium
tray
be injected into the vial, the substance dissolved in situ, and the solution
then
withdrawn via the needle into a syringe for use in the short term before
significant
hydrolysis of the moisture sensitive material occurs. Such an elastomeric
closure is
often retained on the mouth opening of the vial by a thin metal circlip. Such
puncturable seals enable this operation to Ix sterile. During storage the
presence of
atmospheric moisture within the container) or the ingress of atmospheric
moisture,
can cause decomposition of such materials
Often moisture sensitive pharmaceutical substances are provided in
containers together with an internal desiccant in the container) for example a
small
sachet of molecular sieve or silica gel. Clearly this is not practical when
the
substance has to be made up in situ within the container as described above,
as
contamination by desiccant on dissolution of the substance is likely.
It is known to compound polymeric materials with desiccants for various
applications, but mostly as moisture absorbing spacers for multiple glazing
panels.
Far example US 4485204 and US 4547536 disclose blends of polyester or
polyester
plus a butadiene polymer, plus a desiccant such as calcium oxide. EP 0599690
discloses a blend of a polymer such as styrene butadiene rubber) plus
molecular
sieve) plus also a fibrous material. EP 0599690 suggests the general
possibility of
use of such a polymer for drying of moisture sensitive pharmaceuticals, giving
results for moisture absorption at 80 °b RH.
An example of a moisture sensitive pharmaceutical substance is clawlanic
acid and its salts) such as potassium clawlanate. Potassium clawlanatc is both
hygroscopic and readily hydrolysed by water) so for handling and long term
storage
of potassium clawlanate it is necessary for the immediate envirotlment to be
kept
extremely dry ) e. g. 30 °h Relative Hunudity ("RH") or less,
preferably 10 °.& RH or
less ) ideally as low as possible. To obtain and maitain such conditions in a
container
such as a vial of the type mentioned above trquires quite a powerful desiccant
ability.
-1-
CA 02279608 1999-08-26
WO 96/04189 PCTfEP95/03130
Potassium clavulanate is a beta-lactamase inhibitor, and is often provided in
a formulation in combination with a partner beta-lactam antibiotic. A partner
which
is often used in such formulations is amoxycillin. For injectable formulations
amoxycillin is used in the form of sodium amoxycillin. In some forms sodium'
amoxycillin is a powerful desiccant, and when contained together with
potassium
clavulanate in a sealed vial such forms of sodium amoxycillin can exert a
dehydrating effect which helps to preserve the potassium clavulanate. Other
forms
of sodium amoxycillin, such as the anhydrous crystalline form disclosed in EP
0131147 are less desiccating, and although it would be desirable to use such
forms
in formulations together with potassium clavulanate, the problem arises that
these
forms can be insu~ciently desiccating to protect the potassium clavulanate
from
hydrolysis resulting from traces of moisture in the vial.
It is an object of this invention to provide a container having an internal
desiccant which inter alia is suitable for use with moisture sensitive
pharmaceutical
substances ) particularly potassium clavulanate and formulations containing
potassium clavulanate, and allows sterile dissolution without the problem of
contamination by desiccant. Other objects and advantages of the invention will
be
apparent from the following description.
The present invention provides a container for a moisture sensitive material)
having a container body of a substantially atmospheric moisture-impermeable
material) and incorporating a solid element which is made at least in part of
a
desiccant polymer and which is in contact with the atmosphere inside the
container.
The term "inwardly" used herein refers to directions toward the interior of
the vessel unless otherwise defined.
The term "desiccant polymer" means a polymer which absorbs water from
the surrounding atmosphere to the extent that it can exercise a desiccating
effect
upon the interior of a space within which it is contained or to the atmosphere
within
which it is exposed.
The desiccating polymer is suitably a polymer from which no or minimal
material can be extracted by liquid water, at least during the time period the
desiccant polymer is expected to be in contact with liquid water during the
making
up and subsequent storage of a solution in the container) e.g. during
injection of
water into a vial and make-up of a medicament for administration by injection.
Suitably the desiccant polymer is a biocompatible desiccant polymer.
The desiccant polymer may be an inherently desiccant polymeric material)
such as a hydrophilic polymer.
Suitable biocompatible inherently desiccant polymers are the lmown water-
absorbent hydrophilic polymers used for the manufacture of contact lenses)
artificial
cartilages and other bodily implants etc. Suitable such materials include
hydrogel
-2-
CA 02279608 1999-08-26
WO 96/04189 pCT/EP95/03130
polymers, such as polymers which comprise hydroxy alkyl methacrylates) for
example 2-hydroxyethyl methacrylate. Other suitable desiccant polymer include
the
homologous esters of the glycol monomethacrylate series such as diethylene
glycol
monomethacrylate and tetraethylene glycol monomethacrylate; slightly cross-
linked'
for example with a dimethacrylate of a glycol) copolymers of the higher glycol
monornethacrylates and 2-hydroxyethyl methacrylate) acrylamide hydrogels and 2-
hydroxyethyl methacrylate-vinylpyrrolidinone copolymers. Such polymers may be
cross linked for example with ethylene dimethacrylate and/or 1,1,1- triinethyl-
propane trimethacrylate. Other suitable polymers include water-insoluble
methacrylates copolymerised with 2-hydroxyethyt methacrylate. Poly (2-
hydroxyethyl methacrylate) polymers can for example absorb up to 40% w:w of
water. Copolymers of 2-hydroxyethyl methacrylate with a small amount of a
dimethacrylate) some methyl or other alkyl methacrylate and some methacrylic
acid)
which can be converted to their alkali salts) can absorb at least 45 % w:w of
water.
Copolymers of 2-hydroxyethyl methacrylate may for example also be
copolymerised
with n-pentyl met6acrylate, vinyl propionate) vinyl acetate, isobutyl and
cyclohexyl
methacrylate) to produce a suitable desiccant polymer. Copolymers of 2-
hydroxyethyl methacrylate with vinylpyrrolidinones, such as 1-vinyl-2-
pyrrolidinone, and which may be cross linked with ethylene glycol
dimethacrylate)
can produce hydrogels with a higher degree of hydration) suitable as desiccant
polymers. Other suitable hydrogel polymers include hydroxyethyl methacrylate -
N,N-dimethylacrylamide copolymers, hydroxyethyl methacrylate - N-vinyl
pyrrolidone copolymers) hydroxyethyl methacrylate - acryloyl morpholine
copolymers, N-vinyl pyrrolidone - methyl methacrylate copolymers) methyl
methaerylate - acryloyl morpholine copolymers, hydrozyethyl methacrylate -
acryloyl morpholine copolymers) methoxyethyl methacrylate - ethoxyethyl
methacrylate copolymers, and methoxy methacrylate - acryloyl morpholine
copolymers.
Alternatively the desiccant polymer may be a polymer material that includes
a desiccant filler) for example as particles thereof dispersed in its bulk.
An example of such a desiccant polymer is an elastomeric material, such as
a rubber) compounded with a desiccant material.
The compounding of the elastomeric material with a desiccant material
causes the compounded material to exercise a desiccant effect upon the
interior of
the container. The quantity of the said elastomeric material compounded with a
desiccant material should be sufficient to ensure absorption of sufficient of
the water
vapour in the container) or water in the moisture sensitive material contents
to
prevent or reduce to an acceptable degree any degradation of the material by
the
said water or water vapour.
-3-
CA 02279608 1999-08-26
WO 96/Od189 PCZ7EP95103130
The elastomeric material may be a rubber. Such a rubber may be a natural
rubber ) or a synthetic rubber such as a butadiene-based rubber, a . g . based
on
styrene-butadiene or cis-1,4-polybutadiene) butyl rubber) haloburyl rubber,
ethylene-propylene rubber, neoprene) nitrite rubber, polyisoprene, silicone
rubber,
chlorosulphonated polyethylene or epichlorhydrin elastomer) or a mixture,
blend or
copolymer thereof. Haloburyl, e.g. chlorobutyl, rubbers and silicone rubbers
are
pharmaceutically acceptable rubbers known for use as materials for stoppers
etc. to
be maintained in contact with pharmaceutical products. Such elastomeric
materials
are sufficiently permeable to atmospheric water vapour that the desiccant
material
compounded with the rubber can exert its desiccant effect through a thin layer
of the
material.
Such rubbers may be compounded in the manner with which they are
conventionally compounded for manufacture of a stopper as known in the art of
manufacture of rubber stoppers. For example they may be compounded with
reinforcing fillers, colouring agents) preservatives) antioxidants, additives
to modify
their stiffness ) chemical resistance etc. such as cuting/wlcanising agents.
Conventional reinforcing fillers include inorganic reinforcing fillers such as
zinc
oxide and silicas such as china clay and other clays. Suitable compounding
processes and compositions will be apparent to those skilled in the art of
compounding of rubbers.
The reinforcing filler) such as china clay, normally used in the rubber may
be totally or preferably partly replaced with a powdered solid desiccating
material.
Total replacement may lead to a loss of mechanical strength as compared to a
rubber using entirely china clay as its filler) although desiccants may be
found
which can be used as the entire filler without loss of strength. Such a
powdered
desiccating material may have a particle size the same as or similar to that
of the
conventional inorganic fillers referred to above, so that the desiccant can
serve as
the filler as well. The quantity of the powdered desiccating material used may
be up
to the quantity in which conventional inorganic fillers are used, that is )
they may
completely replace the usual inorganic filler. For example the powdered
desiccant
may replace up to 50 °6 of the weight of the normal weight of filler
used in the
rubber, a . g . 10-50 ~ , such as 20-40 ~ . The quantities of filler normally
used in a
rubber for a particular application such as a vial closure will be known to
those
skilled in the art.
The compounded rubber may also additionally include a conventional filler
as mentioned above) for example in a quantity which together with the powdered
desiccant comprises up to the weight °., of filler normally included in
such a rubber.
The quantity of desiccant necessary for a particular product contained in the
container will depend upon the application but can easily be determined by
-4-
CA 02279608 1999-08-26
W.lD 96/04189 pCT1EP95/03130
experiment.
The desiccating material should be one which is inert relative to the
elastomeric material) and vice versa. In the case of containers such as vials
in which
a solution is trade up in situ by introduction of water or aqueous medium the
desiccating material is suitably an inorganic desiccating material which is
wholly or
substantially insoluble in water so that none or only a pharmaceutically
insignificant
amount of the desiccant material or its hydration product, or undesirable
ions, is
likely to enter solution during the period when the desiccating polymer is in
contact
with water or aqueous medium. Preferred desiccants are those which can
chemically
or pysicochemically absorb or fix absorbed water; e.g. by formation of a
hydration
product, so that there is a reduced possibility of subsequent reversable
release of the
absorbed water, which might for example occur if the temperature of the
polymer
should rise) e.g to around 40°C subsequent after earlier desiccation at
a lower
temperature.
1 S Suitable inorganic desiccants are the known materials sold in the UK under
the names Grace A3~') Siliporite'~ and Ferben 200t". Particularly preferred
desiccant
materials are dried molecular sieves and calcium oxide, or mixtures thereof.
Calcium oxide chemically fixes water by formation of calcium hydroxide) from
which water can only be released at extreme temperatures, and absorbed water
can
generally only be released from molecular sieves at several hundred °C)
that is,
well above the temperatures containers of pharmaceutical substances would be
expected to experience under normal storage.
A preferred desiccating polymer is therefore a halobutyl) e.g. chlorobutyl)
rubber compounded with an inorganic desiccant such as a molecular sieve or
calcium oxide
The compounded elastomeric material may be made and formed into a solid
element by processes analogous to those by which solid products are made from
conventional compounded elastomeric materials which include the above-
mentioned
inorganic fillers are made.
In one embodiment of this invention the solid element comprises a closure
for the container) made wholly or partly of the said desiccating polymer.
Parts of .
such a closure other than the parts made of desiccant polymer which are to
come
into contact with the atmosphere within the container may be made of generally
conventional materials ) preferably pharmaceutically acceptable materials,
such as
plastics materials, elastomeric materials etc.) or composite materials such as
metal
and plastics or elastomeric materials. Preferably such parts are made of
plastics or
elastomeric materials which are of low moisture content) of low moisture
permeability and low moisture affinity.
Preferably parts of the closure which engage the mouth opening are at least
-5-
CA 02279608 1999-08-26
WO 96/04189 '
PC'T/EP93/03130
partly, more preferably wholly made of an elastomeric material comprising a
natural or synthetic rubber (which may be the above-described desiccating
rubber),
thereby allowing a tight compression fit with the mouth of the vessel. The
selling
engagement of the closure with the mouth opening may be by a generally
conventional construction e.g. similar to a conventional stopper. For example
the
closure may be engaged with the rim of the neck of a vial by a screw thread, a
friction/compression fitting, and/or a circlip-type clamp around the neck of
the vial.
Such constructions are known in the art. The closure may seal the mouth in a
generally conventional manner, e.g. by a compression fitting of the closure
wall
against the rim of the mouth, or by a sealing ring compressed between the
closure
face and the rim of the mouth etc.
In one embodiment the present invention provides a container for a moisture
sensitive material, having a container body of a substantially atmospheric
moisture-
impermeable material and having an opening sealed by a closure) characterised
in
that at least part of the closure which is exposed to the interior of the
container body
is made of a desiccant polymer, which is suitably an elastomeric material
compounded with a desiccant material or a hydrophilic polymer.
In another embodiment the present invention provides a container for a
moisture sensitive material, having a container body of a substantially
atmospheric
moisture-impermeable material and having an op~~g ~~ by a closure,
characterized in that at least part of the closure which is exposed to the
interior of
the container body is made of a desiccant polymer) which is suitably an
elastomeric
material compounded with a desiccant material or a hydrophilic polymer) the
closure comprising a closure wall having a puncturable region therein in
direct
communication with the interior of the vessel.
Such a last-mentioned container may be a vial as mentioned above suitable
for a moisture-sensitive pharmaceutical material) of generally conventional
construction) the mouth opening being defined by the rim of the neck of the
vial.
Such a vial may be made of conventional materials such as glass ) rigid
plastics
materials etc., but particularly glass.
By means of the invention, moisture-sensitive substances within the vessel
may be protected by the desiccant material, and in this last-mentioned
embodiment
water may be introduced into the vessel by means of a hypodermic needle
puncturing the closure face through the puncturable region) so as to dissolve
the
substance) and the so-formed solution of the substance may be withdrawn via
the
needle.
The puncturable region of the closure wall may suitably comprise a thinned
region of the closure wall) and is preferably provided in a region of
elastomeric
material (which may comprise the desiccating polymer) which can resiliently
seal
-6-
CA 02279608 1999-08-26
WO 96/04189 PCT/EP95/03130
around a hypodermic needle which is inserted therethrough, so as to facilitate
sterile
insertion and withdrawal.
Conveniently all the polymeric parts of the closure, e.g. of a vial closure
and
including the puncturable region) may be made of the desiccant polymer)
particularly an elastomeric material compounded with a desiccant material.
Such a
vial closure may correspond in shape and size to conventional vial closures
made of
elastomeric material) and may be retained on the mouth of the vial by a
conventional metal circlip. Elastomeric materials compounded with a desiccant
material may be moulded into such shapes and sizes by a moulding process
entirely
analogous to that used to mould closures out of conventional elastomeric
materials
such as rubbers.
Alternatively the closure may be of mufti-part construction having only
parts) including those parts which are exposed to the interior of the
container body,
made of the said desiccant polymer.
The distribution of the desiccant polymer may be such that the desiccant
polymer is located on only part of the closure wall, so that for example the
puncturable region may be situated between areas of the closure wall on which
is
the desiccant polymer, or to one side of such an area) thereby facilitating
the
construction of the puncturable region as a thinned region of the closure
face.
Such a mufti-part construction includes the possibility that the closure may
be integrally made of a co-moulded) or fused together) desiccating polymer and
an
elastomeric or plastics material making up parts of the structure of the
closure.
Alternatively the desiccating polymer may be provided as a separate part,
retained
by the closure on a suitable inward surface, e.g in an inwardly facing holder
or
cavity.
In one embodiment a mufti-part construction of closure of the invention) the
desiccant polymer may be in the form of a ring shape on the closure wall of a
closure ) with the puncturable region within) e. g. near or at the centre of,
the ring.
Such a ring shape may for example be circular, polygonal, or oval etc.
Such a ring-shape of desiccant polymer may be located in a corresponding
ring-shaped or cylindrical holder in the closure wall. Such a holder may
suitably be
in the form of two generally concentric walls extending inwardly from the
closure
wall, the space between the walls defining the ring-shaped cavity, and the
central
space within the inner wall defining a central passage in direct communication
with
the puneturable region) down which a hypodermic needle may be inserted. Such a
holder may be formed integrally with the closure wall, or may be separate part
of
the closure. Suitably both the walls may be integral with the closure wall) so
that
the closure wall forms the base of the cavity and of the central passage.
Suitably in
such a construction the base wall of the central passage includes the
puncturable
CA 02279608 1999-08-26
W O 96/04189 PCT/EP95/03130
region.
Alternatively such a ring-shape of desiccant polymer may be located in a
ring-shaped or cylindrical cavity in the closure wall, suitably in its inward
face) the
cavity opening into the interior of the container when the closure is in place
on the
vessel, and the central opening in the ring shape of desiccating polymer may
define
a central passage in direct communication with the puncturable region, down
which
a hypodermic needle may be inserted.
Alternatively the ring shape of desiccant polymer may be located adjacent to
the inner face of the closure wall.
The desiccant polymer may be simply physically attached to the closure) e.g
by cooperating parts such as projections and sockets) or simply be held in
place by
the inherent resilience of other parts of the closure) particularly when this
is made
of an elastomeric or other resilient material such as a plastics material)
alternatively
the desiccant polymer may be bonded to the closure c.g by adhesives or fusion
together etc.
Alternatively a closure for the container, e.g. a bottle or jar of glass or
plastics material, or a metal canister or keg, may be in the form of a
conventional
screw cap (optionally provided with tamper evident or child resistant
features) or
other form of closure (e.g. cam action closure, snap-fit closure) which relies
on a
compression fit on the lip of the mouth of the container) and having an insert
made
of the said desiccant polymer) e.g an elastomeric material compounded with a
desiccant material, in the form of a disc or ring washer or inward facing
coating
layer which forms a compression seal between the lip of the mouth of the
container
and the closure as the container closure is tightened down, e.g. by a screw
action.
Alternatively a closure for the container) e.g. a bottle or jar of glass or
plastics material) or a metal canister or keg) may be a screw / interference
friction / compression fit insertable bung or other insertable stopper, having
a part
of its surface exposed to the interior of the container made of the said
desiccant
polymer) c.g an elastomeric material compounded with a desiccant material.
Alternatively the container slay comprise a syringe barrel ) with a plunger
having at least part of its surface exposed to the interior of the container
madt of
the said desiccant polymer, e.g an elastomeric material compounded with a
desiccant material. Suitably the entire plunger may be made of the said
desiccant
polymer, e.g an elastomeric material compounded with a desiccant material.
Alternatively the said desiccant polymer) c.g an elastomeric material
compounded with a desiccant material may be included in other forms into the
container of the invention) for example as a removable resilient element such
as a
pad, wad) leaf, helix, coil or spiral spring which may be included in the
headspace
above the contents of a container and which exerts a restraining action on the
_g_
CA 02279608 1999-08-26
1N0 96/04189 PC'TlEP95103130
contents, such a tablets) pills, capsules ete. to prevent the contents
rattling about in
the container. Such an element may be made as part of the container closure.
Alternatively the said desiccant polymer) e.g an elastomeric material
compounded-with a desiccant material may be made in the form of a pad, e.g. a
flat
disc to be retained at the bottom of a container, e.g. beneath tablet, pill or
capsule
contents.
The nature and quantity of desiccant polymer used in the container of the
invention will vary with the nature of the moisture sensitive contents) and
can easily
be determined by straightforward experimentation or calculation) e.g. from the
moisture content of the contents of the vessel. Suitably in the case of the
moisture
sensitive material potassium clawlanate, at the usual quantities in which it
is
supplied mixed with sodium amoxycillin in vials) typically of a capacity 10-20
ml,
for reconstitution for an injectable formulation) e. g . 100 - 200 mg
potassium
clawlanate mixed respectively with 500 -.1000 mg sodium amoxycillin (expressed
as the parent free acid equivalent weight) the desiccant polymer should
scavenge 5-
8 milligrams of water with a residual RH of less than 10 % throughout a two
year
storage period.
Preferred desiccating polymers for use with formulations containing
potassium clavulanate, e.g. its coformulation with sodium amoxycillin, are
able to
take up atmospheric moisture at 30 % RH or less, preferably at 10 % RH or less
.
Preferred desiccating polymers excercise such a desiccant function for a long
period, ideally throughout the shelf life, typically two years ) of such a
formulation.
Preferred desiccant polymers should also be capable of being sterilised
without loss of their desiccant ability at these low RH values. For example
desiccant
polymer vial closures are ideally sterilised by washing prior to use) without
loss of
their desiccant ability. It is found that desiccant rubbers such as halobutyl)
c.g.
chlorobutyl, rubber compounded with calcium oxide or molecular sieves are
capable
of being washed without deleterious effect on their desiccant ability.
The container of the invention is particularly suitable for the containment of
moisture-sensitive pharmaceutical substances such as a formulation of
potassium
clavulanate and sodium amoxycillin) particularly crystalline sodium
amoxyciIlin e.g.
as disclosed in EP 0131147. The invention therefore further provides a
container as
described above ) containing a mixture which comprises potassium clawlanate
and
sodium amoxycillin.
Other pharmaceutical substances which may sefully be contained in the
container of the invention include lyophilised substances) for example those
often
employed in diagnostic assy kits.
The closure of the invention) independent of the vessel, is also believed to
be novel, and therefore the invention further provides a closure capable of
sealing
-9
CA 02279608 1999-08-26
WO 96/04189 PCTIEP95/03130
engagement with the mouth opening of a container) the closure comprising a
closure
wall) the inwardly facing region of the closure wall comprising or having
thereon a
desiccant polymer.
For example such a closure may be a closure capable of sealing engagement
with the mouth opening of a container, the closure comprising a closure wall
having
a puncturable region therein in direct communication with the interior of the
vessel,
and having on an inwardly facing region of the closure wall a desiccant
polymer.
Suitable and preferred forms of the closure are as described above.
The present invention also provides a method of desiccating a moisture
sensitive material, which comprises enclosing the said material in a container
and
maintaining a desiccant polymer in contact with the atmosphere inside the
container. This method may be a method of long-term storage and/or protection
against hydrolysis during storage. The moisture sensitive material may be
potassium
clavulanate or it$ coformulations with sodium amoxycillin. This method is
suitable
for use with lyophilised, freeze dried) materials. Normally lyophilised
materials are
desiccated by an intense drying process before vials containing them are
sealed) and
this method of the invention provides the advantage that less intense drying
processes may be used) and the desiccant polymer can thereafter complete the
dehydration process whilst in the sealed vial.
Suitable and preferred forms of the process are as described above.
The invention will now be described by way of example only with reference
to the accompanying drawings) which show:
Figs. 1, 2 and 3: longitudinal sections through alternative mufti-part
construction vials and closures of the invention.
Fig. 4: a sectional view through the closure of Fig.l about the line A-A of
Fig 1 looking in the direction of the arrows.
Figs. 5-7: graphs showing moisture uptake for rubbers compounded with
various listed desiccants.
Fig. 8: a graph of normalised moisture uptake for dried hydrogels (a) to (f)
tested in example 4.
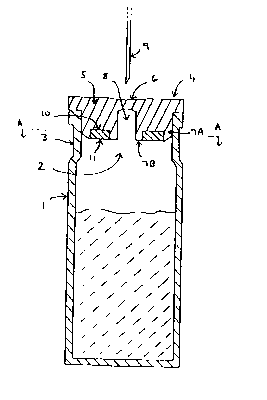
Referring to Figs.l to 4) a glass vial (1) has a mouth opening (2) defined by
the rim of an inwardly extending neck (3). In the neck (3) of the vial (1) is
a
closure (4 generally) integrally made of a synthetic rubber material, and
which
comprises a closure wall (5) which sealingly engages the rim of the mouth
opening
(2). Centrally located in the closure wall (5) is a thinned puncturable region
(6).
Referring specifically to Fig 1, extending inwardly into the vial ( 1 ) from
the
closure wall (5) is an integral holder (7) in the form of two concentric walls
(7A)
7B) the outer of which (7A) forms a aleck plug which sealingly engages the
neck (3)
with a compression fit. The inner wall (7B) defines a central space (8) with
the
- 10-
CA 02279608 1999-08-26
Vi~O 96/04189 PCTIEP95/03130
puncturable region (6) at its outer end. A hypodermic needle (9) may be
inserted
through the puncturable region (6) and passed along the passage into the vial
defined by the space (8).
Between the inner and outer walls (7A, 7B) is a ring-shaped cavity (10)
which contains a desiccant polymer (11 ) in the form of a ring with a central
opening. The ring (11) is retained in place in the cavity (10) by the inherent
resilience of the closure material.
Referring specifically to Fig. 2 an alternative construction of vial is shown.
Parts having a common identity with Fig. 1 are correspondingly numbered. In
the
vial of Fig. 2 the desiccant polymer is in the form of a ring (12) which is
bonded to
the inner face (13) of the closure wall (5) where this extends inwardly into
the
interior of the vial ( 1 ) in the form of a neck plug ( 14), with its central
opening in
communication with the central space (8) of the closure. The neck plug (14)
sealingly engages the neck (3) with a compression fit
Referring to Fig. 3 an alternative construction of vial is shown. Parts having
a common identity with Fig. 1 are correspondingly numbered. In the vial of
Fig. Z
the desiccant polymer is in the form of a ring (15) with a central opening
(16}. The
ring (15) fits into a central cavity (17) in the closure wall (5) where this
extends
inwardly into the interior of the vial (1) to form a neck plug (18) and is
held there
in place by the resilience of the material of the closure (4). The central
opening (16)
in the ring (15) defines a passage having the puncturable region (6) at its
outer end.
The neck plug (18) sealingly engages the neck (3) with a compression fit.
The closure wall (5) may be fastened tightly against the rim of the neck (3)
by means of a circlip (not shown). In another embodiment (not shown) a holder
for
the desiccant polymer (11) may be made as a separate part in the form of two
walls
analogous in shape to walls (7A) 7B) with a cavity ( 10) and desiccant polymer
( 11 )
between them) and with a base wall.
It should be noted that if the desiccant polymer is a hydrogel polymer
shrinkage may occur on drying which may affect the retention of the polymer on
a
rubber closure ) and steps ) e. g a suitable construction of holder, which
will be
apparent to those skilled in the art, might be necesary to overcome this.
In use, the hypodermic needle {9) is inserted through the puneturable region
(6), and along the passage (8)) into the vicinity of the contents (13) of the
vial (1),
a dry mixture of potassium clavulanate and anhydrous crystalline sodium
amoxycillin. Sterile water is injected down the needle (9) to dissolve the
contents
(13)) and the vial may be shaken to encourage dissolution. The solution may
then
be withdrawn through the needle (9) into a syringe (not shown) for subsequent
use.
Example 1: Rubbers compounded with desiccants.
A closure for a glass vial of the type conventionally used for the containment
-11-
CA 02279608 1999-08-26
WO 96/04189 PCT/EP95/0313a
made, using a standard known compounded haloburyl rubber formulation) but in
which 50% by weight of the conventional china clay filler was replaced with
calcium oxide ground to a particle size distribution similar to that of the
filler. The
shape and size of the closure corresponded to those of a conventional vial
closure.
The volume of the vial was ca. 10 ml. The molecular sieve was dried using a
standard process for drying the molecular sieve.
A moisture sensitive pharmaceutical formulation) being 500 mg crystalline
sodium amoxycillin prepared as described in EP 0131147 coformulated with 100
mg of potassium clavulanate was filled into the vial under conditions of less
than
30 % 1tH and the vial was sealed with the stopper as conventional ) with the
stopper
being retained on the vial using a conventional thin metal cover.
The vial containing the formulation was stored under ambient and
accelerated storage conditions. Colour measurements (a known sensitive method
of
assessing the degree of decomposition of potassium clavulanate) showed a
degree of
protection of the potassium clavulanate effectively equivalent to that shown
using
spray-dried sodium amoxycillin having desiccant properties, in a
conventionally
stoppered vial.
A similar result was achieved when calcium oxide instead of molecular sieve
was compounded with the rubber, and when all of the filler was replaced by
these
desiccants.
Example 2: Rubbers compounded with desiccants.
In a further experiment potassium clavulanate was enclosed within an
airtight glass vessel, and a piece of halobutyl rubber compounded with calcium
oxide as mentioned above in Example 1 was suspended inside the vial on a piece
of
wire. A control experiment was set up consisting of an identical vessel
enclosing the
same weight of potassium clavulanate but without the compounded rubber. The
decomposition of the potassium clawlanate under the action of traces of
moisture in
the atmosphere of the vial and in the potassium clavulanate itself) or
adsorbed on
the inner surface of the vial was monitored. Colour measurements showed that
decomposition of the potassium clavulanate was significantly retarded in the
vessel
containing the rubber compounded with the desiccant.
Example 3: Rubbers compounded with desiccants.
Fig 5 shows the moisture uptake (normalised data) in terms of weight % at
ca. 10 °h RH by desiccant polymers which are haloburyl rubbers of
standard
formulation except that 20-44 gfo of the china clay filler normally used has
been
replaced by the desiccant indicated. Grace A3~') Siliporite~' and Ferben 200'
are
commercially available powdered desiccants, sold under these trade names, and
were pre-dried according to the standard procedures for these desiccants.
Grace
-12-
CA 02279608 1999-08-26
WO 96/04189 PCT/EP95103130
A3~" and Siliporite"' are types of molecular sieve powder obtainable from W R
Grace Ltd. Northdale House) North Circular Road, London NW 10 7UH, GB. The
graph relates to the desiccant fillers:
(a) Siliporite~'
(b) molecular sieve
(c) Grace A3~'
(d) Ferben 200'
Fig 6 shows the moisture uptake (normalised data) in terms of weight % at
ca. 10% RH by desiccant polymers which are halobutyl rubbers of standard
formulation except that 20-40% of the china clay filler normally used has been
replaced by the desiccant, after the rubber has been tote washed. The graph
relates
to the desiccant fillers:
(a) calcium oxide
(b) molecular sieve
(c) Grace A3"'
(d) Siliporite ~'
Fig 7 shows the moisture uptake (normalised data) in terms of weight % at
ca. 10 % RH by desiccant polymers which are halobutyl rubbers of standard
formulation that 20-40 % of the china clay filler normally used has been
replaced by
the desiccant indicated) before and after the rubber has been tote washed. The
graph
relates to the desiccant fillers:
(a) molecular sieve - washed
(b) molecular sieve - unwashed
(c) Grace A3'" - washed
(d) Grace A3'" - unwashed
The data presented in these graphs show that rubber compounded with these
desiccants has a desiccant ability even at RH as low as 10 % RH, and this
desiccant
ability is relatively unaffected by washing.
Example 4: Hydrophilic Hydrogels.
Samples (a) - (f) of known hydrogels as tabulated below were obtained in a
hydrated state and were activated by heating to ca. 120 ° C under
vacuum for a
minimum of 3 hours.
(a) 90:10 hydroxyethyl methacrylate : N,N-dimethylacrylamide copolymer
(b) 90:10 hydroxyethyl methacrylate : N-vinyl pyrrolidone copolymer
(c) 90:10 hydroxyethyl methacrylate : acryloyl morpholine copolymer
(d) 70:30 N-vinyl pyrrolidone : methyl methacrylate copolymer
(e) 30:70 methyl methacrylate : acryloyl morpholine copolymer
(fj 50:50 hydroxy methacrylate : acryloyl morpholine copolymer
The moisture uptake of all six samples was evaluated in a standardised 24
-13-
CA 02279608 1999-08-26
WO 96/04189 PCT/EP95/03130
hour cycle on the Dynamic Vapour Sorption apparatus. The samples were prepared
and placed at a nominal 0 % RH (actual 2 % ) for 4 hours to complete
activation. The
RH was then raised to a nominal 10 % ( actual 12 °k ) for 1000 minutes
and then
returned to 0 % for a further 200 minutes completing the 24 hour cycle. Data
was
normalised to allow for any weight loss during the 4 hour activation stage,
and is
illustrated in Fig. 8.
In order to evaluate whether the samples had reached a stable equilibrium at
the end of the holding time at 10 °~ RH two samples (c) and (d) with
different
profiles in the screening test above were selected and held for 24 hours at 0
% RH
followed by ca. 45 hours at 10% RH. This confirmed that maximum moisture
uptake was achieved within 1000 minutes.
It was clear from these results that all hydrogels tested had highly
significant
water uptake at low RH) i. e. 10 k . The majority of the water uptake occurred
extremely rapidly and final equilibrium was attained within 17 hours or less.
The
maximum uptake using hydrogel polymers was for sample (d) which was able to
absorb approximately 1.7 °k of its own weight of water at 10 % RH when
fully
dried.
The hydrogel samples showed the physical changes listed below during the
test:
(a) very brittle when dried
(b) least brittle when dried
(c) very brittle when dried
(d) considerable shrinkage
on drying
(e) opaque when dried.
- 14-