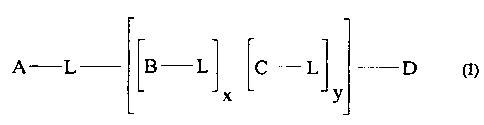Note: Descriptions are shown in the official language in which they were submitted.
CA 02279882 1999-07-29
WO 98/35003 PCT/US98/02366
DeterEent Compound
Technical field
The present invention relates to a polyamine cationic surfactant, containing
at least
one quaternary amine group and at least one primary, secondary or tertiary
amine
group and methods for making them. The surfactants may be used in any
application where surfactancy is required. In particular they may be used in
cleaning or detergent compositions or components thereof.
Background to the invention
The satisfactory removal of greasy soils/stains, that is soils/stains having a
high
proportion of triglycerides or fatty acids, is a challenge faced by the
formulator of
detergent compositions for use in laundry and dish washing methods. Surfactant
components have traditionally been employed in detergent products to
facilitate the
removal of such greasy soilslstains. In particular, surfactant systems
comprising
cationic surfactants have been described for use in greasy soil/stain removal.
A wide selection of surfactants for use in detergents can be found in the
literature,
but the reality is that many such surfactants are chemicals which are not
always
suitable in detergents, because of certain properties they have (such as
instability
in acidic or basic environment, incompatibility with bleach, malodour
problems,
biodegradability problems). Thus, the challenge to the detergent manufacturer
seeking improved performance has been increased by these various factors. For
example, some non-biodegradable ingredients have fallen into disfavour. As a
result, the manufacturer is somewhat more limited than the literature would
suggest in the selection of effective) yet affordable, ingredients.
A variety of cationic surfactants are suggested to be usable in detergents.
One
group of cationic surfactants which is widely studied is the group consisting
of
quaternary ammonium or imidazolinium compounds, which are often designed for
speciality use. For example, various quaternary ammonium surfactants have been
CA 02279882 1999-07-29
WO 98/35003 PCT/US98/02366
2
suggested for use in shampoo compositions and are said to provide cosmetic
benefits to hair.
US-A-4,228,042 discloses biodegradable cationic surfactants, including
cationic
ester surfactants for use in detergent compositions to provide greasy/oily
soil
removal. For example, US 3,567,729 discloses diquaternary ammonium
compounds for use in detergents. For example, US 5,068,431 describes
quaternary
ammonium compounds, containing amphoteric amine oxide groups.
The Applicants have now found that certain polyamine cationic compounds,
containing at least one cationically charged quaternary amine group and at
least
one primary, secondary or tertiary amine group are very good surfactants,
suitable
for use in cleaning or detergent compositions. These compounds are found to be
very surface active under alkaline washing conditions, and they are found to
give
excellent cleaning performance benefits. This is believed to be due to the
compounds containing both a positively charged group and a neutral, more
hydrophobic group.
Furthermore, several examples of these surfactants are found to be more
biodegradable and to have a very low aquatic toxicity, relative to most
quaternary
amine compounds.
It has been found that the stability of the polyamine cationic surfactants is
not
affected by changes of the pH. Furthermore, it has been found that, depending
on
their structure, most of the polyamine cationic compounds of the present
invention
and detergent compositions containing these polyamine cationic surfactants,
are
stable under standard storage and washing conditions.
Furthermore, it has been found that the polyamine cationic surfactants can be
compatible with bleach, especially oxygen-based bleaches, and with certain
bleach
activators.
The polyamine cationic surfactants can be obtained via various efficient
synthesis
routes, and the production of these compounds can be very cost-effective.
All documents cited in the present description are, in relevant part,
incorporated
herein by reference.
..r
CA 02279882 1999-07-29
WO 98135003 PCT/US98102366
3
Summarx of the Invention
The present invention relates to polyamine cationic surfactants, containing at
least
one quaternary amine group and at least one primary, secondary or tertiary
amine
group. The invention also relates to methods of making the polyamine cationic
surfactants. The polyamine cationic compounds of the invention can be used as
a
surfactant in any composition where surfactancy is required, for example in
cleaning or detergent compositions or components thereof.
Detailed description of the invention
Cationic surfactant
A cationic surfactant according to the present invention comprises at least
one
quaternized ammonium group and at least one primary, secondary or tertiary
amine group, whereby not more than one linear or branched polyoxyalkylene
group is present as substituent group.
Preferred cationic surfactant of the present invention are polyamine cationic
surfactants of the general formula (I):
LB- L L
A L J C -LJ -D ~I~
Y
wherein L is a linking unit, and each L is independently selected from the
group
consisting of C2-C3p linear or branched alkylene) alkenylene, alkarylene,
aralkylene, arylene, (poly) hydroxyalkylene) (poly) alkylenoxy, (poly) hydroxy
alkenylene; L can be substituted by one or more A, B, C or D units; x is a
number
CA 02279882 1999-07-29
WO 98/35003 PCTIUS98/02366
4
from 0 to 10, y is a number from 0 to I0; and wherein the units A- and D- are
each independently selected from
14
RZ-Nand -N
I
R3 RS
R
-B- - N ; and
M-
R~
-C- - N-
I
Rg
wherein R1, R2, R3, R4, R5, R6, R~ and Rg are independently selected from the
group consisting of Cl-C30 linear or branched alkyl, alkenyl, alkaryl,
aralkyl,
aryl, (poly) hydroxyalkyl, (poly) hydroxy alkenyl, alkoxy group and hydrogen,
one of R 1, R2, R3, R4, R5, R6, R~ or Rg can be a linear or branched
polyoxyalkylene group with from 2 to 2b oxyalkylene units or R1 and R2, Rland
RZ and R3, R4 and RS or R6 and R~ form together with the nitrogen atom part of
a ring structure; or R3 is not present and R 1 or R2 is double bonded to the
nitrogen; or R~ is not present and R6 is double bonded to the nitrogen; or RS
is
not present and R~ is double bonded to the nitrogen; or, when x and y are 0,
R, or
R2 or R3 and R4 or RS form together with the nitrogen atoms of A and D part of
a
ring structure; M- is one or more counterions, and at least one A or D
comprises a
quaternized ammonium group in which none of R1, R2 or R3 is hydrogen, or at
least one B is present in which neither R6 nor R~ is hydrogen, and at least
one A
or D comprises a primary, secondary or tertiary amine group, or at least one C
is
present.
........ T..... . . ..
CA 02279882 1999-07-29
WO 98135003 PCT/US98102366
The units B-L and C-L are linked when both are present (i.e. when x and y do
not
equal 0), and they can be randomly present along the chain between the end
units
A-L and D.
Preferably, the value of x+y is from 1 to 4. Preferably, when x+y is greater
than 1, at Ieast one of present groups A, B, C or D is a secondary or primary
ammonium group.
More preferably, x=0 and y is a number from i to 4. Even more preferably, both
xandyare0.
If x+y does not equal 0, it is preferred that the surfactant comprises only
one
quaternary group A or D.
Preferably R6, R~ and/or Rg are each independently selected from a C 1-C6,
more
preferably C I-C3 alkyl, alkoxyalkyl or (poly) hydroxyalkyl group or, most
preferably hydrogen.
Preferably, RI is a C6-C14 alkyl, (poly) hydroxyalkyl or alkoxy group or an
aralkyl group, most preferably a 2-ethylhexyl group, R2 and R3 are each
independently C 1-C6, more preferably C 1-C3 alkyl or hydroxyalkyl groups and
preferably R4 and RS (and R6, R~ and Rg when present) are each independently
C 1-C6, more preferably C 1-C3 alkyl, alkoxyalkyl or (poly) hydroxyalkyl
groups
or, most preferably, hydrogen atoms.
In a further preferred alternative, R4 is preferably a C6-C 14 alkyl, (poly)
hydroxyalkyl, alkoxy group or an aralkyl group, most preferably a 2-ethylhexyl
group RS is preferably a C I-C6, more preferably a C 1-C3 alkyl, (poly)
hydroxyalkyl group or hydrogen and R l , R2 and R3 (and R6, R~ and Rg when
present) are each independently preferably Cl-C6, more preferably Cl-C3 alkyl,
alkoxyalkyl or (poly) hydroxyalkyl groups or aralkyl groups.
When R1 and R2, Rl and R2 and R3, R,I and Rg or R6 and R~ form together with
the nitrogen atom part of a ring structure, the ring structure is preferably a
benzene ring structure, morpholino ring structure or a piperazino ring
structure, or
a subtituted benzene or substituted morpholino or substituted piperazino ring
structure.
CA 02279882 1999-07-29
WO 98/35003 PCT/ITS98102366
6
When x+y is 0 and R, or RZ or R3 and R4 or RS form together with the nitrogen
atoms of group A and D part of a ring structure, the ring structure is
preferably a
benzene ring structure, morpholino ring structure or a piperazino ring
structure, or
a substituted benzene or substituted morpholino or substituted piperazino ring
structure.
L groups are independently preferably a C2-Cg, more preferably a C2-C4 linear
or branched alkyl, hydroxy alkyl, alkoxy or hydroxy alkoxy group. If x+y is 0,
the 1 group is preferably a Cz alkyl group. If group L comprises more than 2
carbon atoms, the surfactant preferably comprises at least one primary or
secondary A, B, C or D group.
Examples of preferred polyamine cationic surfactants of the present invention
are:
R2
R 1 N~ L NH2 III)
M-
R3
R2
R 1 ~ L N CH3 III)
M-
R3 R4
i 2 CH3
RI -N~ L N~ L NH2 (IV)
R M_ ~ M_
3 R6
.. ...,... . . T ". ..
CA 02279882 1999-07-29
WO 98135003 PCTIUS98/02366
7
R2
R10 i ~~ ~2 (V)
R3
I2 R4
R10-N-L N (vi)
~M_
R3 RS
R2 R4
R~~-N-L--N-L-N ~,It)
R3 R8 RS
wherein Rl, R4, R6 and Rg are as described above; R2, R3 and RS are
independently selected from the group consisting of methyl, ethyl,
hydroxyethyl,
hydroxypropyl, polyhydroxy propyl, ethoxy, propoxy or 2,3,4,5,6-penta hydroxy
hexyl, and are most preferably methyl or hydroxyethyl groups; Rlp is a methyl
or
hydroxyethyl group; L is as described above; R 1 and/or R2 and/or R4 are most
preferably a 2-ethylhexyl group.
A highly preferred cationic polyamine surfactant is of formula VI, as defined
above, wherein R2 is a hydroxypropyl or hydroxyethyl group, R3 and Rip are
methyl groups, L is C2-C3 alkyl group.
' Highly preferred polyamine cationic surfactant are those of the formulas:
CA 02279882 1999-07-29
WO 98135003 PCT/US98/02366
8
M 3 M" ~ 3 ~2
Rl -N--CI~-CH2-NHZ or CH3 ~N-CHZ CI~-CH
E \
~3 ~3 Rl 1
or
M- I H3
Rl--N~ CHZ-CHZ-CHZ-NH2
CH3
wherein R 1 is as described above, preferably a C2-C 14, preferably C6-C 14
linear
or branched alkyl, (poly) hydroxy alkyl) alkoxy or araIkyl group; particularly
preferred Rl groups are hydroxyalkyl groups, where the alkyl groups have 2 to
5
carbon atoms, especially hydroxyethyl and hydroxypropyl are preferred;
particularly preferred alkyl R 1 groups have up to 9 carbon atoms, most
preferably
R 1 is a 2-ethylhexyl group; and R 11 is a C2-C 14 alkyl, (poly) hydroxy
alkyl,
alkoxy or aralkyl group or a A or D unit as described above .
The anion M' is a counterion for the canonically charged polyamine surfactant.
Therefore, the number of M' anions present will depend on the cationic charge
of
the polyamine surfactant, which depends on the groups A, B, C and D. The
number of M' anions will be at least 1. A preferred counterion is a halide
anion,
more preferably a sulphate anion.
Synthesis of the volyamine cationic surfac ant
A variety of synthesis routes may be used for making the polyamine cationic
surfactant of the present invention. Depending on which polyamine cationic
surfactant reaction product is to be prcparod and which starting material is
available therefor, one synthesis route will be preferred over other possible
routes
and this choice will be well within the ambit of the skilled person given the
examples below. The starting materials can be selected from a variety of
(readily),
commercially available compounds, depending on which polyamine cationic
. T.
CA 02279882 1999-07-29
WO 98135003 PCTIUS98/02366
9
surfactant needs to be prepared, and again, this selection is well within the
ambit
of the skilled person. Preferably, the starting material is an amine or
diamine
compound.
Preferred synthesis routes (A-D)
A. Reaction of two amine containing Compounds
A preferred synthesis route is as follows. In a preferably organic solvent one
or
more primary or secondary amine compound and a quaternary halide or sulphate
containing amine compound are mixed, preferably in a mole ratio of 1.5:1 to
3:1,
to make them react together. When the reaction is complete, a basic compound
is
added, preferably sodium hydroxide, and the polyamine cationic surfactant
reaction product can be removed from the reaction mixture by conventional
methods.
Examples of this synthesis route are the reactions of any of hexylamine,
octylamine, decylamine, dodecylamine or 2-ethylhexyfamine with (3-bromo,
chloro or sulphate propyl) trimethyl ammonium bromide, chloride or sulphate,
in
a ratio of about 2:1, in an organic solvent (ethanol), whereafter sodium
hydroxide
is added. Once the reaction is substantially completed (after reacting for up
to 24
hours), the organic solvent and the possibly unreacted amine compound are
removed from the reaction mixture via evaporation, and washed with (for
example) diethyl ether, to yield a white solid diamine cationic surfactant,
i.e. a (1-
hexyl, octyl, decyl, dodecyl or 2-ethylhexyl amine) (3-trimethylamine)
propane.
B. Alkylation of a diamine
Another preferred synthesis route is as follows.
A diamine compound and an alkylating agent are mixed in an organic solvent,
preferably the diamine to alkylating agent are in a mole ratio of from 3.5:1
to
1.1:1. When the reaction is completed the polyamine cationic surfactant
reaction
product can be removed from the reaction mixture by conventional methods.
Examples of this synthesis are the reactions of any of 1-bromo or chloro
hexane,
octane, decane, dodecane or tetradecane or 2- ethylhexylbromide with
tetramethylpropanediamine in an organic solvent (ethanol). The mole ratio of
the
CA 02279882 1999-07-29
WO 98135003 PCT/US98/02366
reactants is preferably about l: 1. Once the reaction is substantially
completed, the
organic solvent and any unreacted amine compound are removed from the reaction
mixture via evaporation. The remaining product is then washed with (for
example)
diethyl ether, to yield the diamine cationic surfactant, i.e. hexyl, octyl,
decyl
dodecyl, tetradecyl 2-ethylhexyl, tetramethyI propanediamine.
C. Prevaration of an imine reduction and alk, lation
A further preferred synthesis route is as follows.
In an organic solvent an aldehyde compound and a diamine compound are mixed
in a mole ratio of from 2:1 to 1:2, whereby an imine is formed. The imine
reaction product is reduced to a secondary diamine with a reducing agent. The
secondary diamine product is then selectively alkylated on the secondary amine
with an alkylating agent such as formaldehyde/. formic acid. The diamine is
then
quaternised/ alkylated using an alkylating agent to form a diamine cationic
surfactant.
Examples of this synthesis route are the reactions of any of 2-ethylhexanal,
hexanal, octanal, decanal or dodecanal with N, N, dimethylene diamine in an
organic solvent (toluene), in a mole ratio of about 1:1. Once the reaction is
substantially completed, the solvent is removed and borohydride is added to
effect
the reduction step. The reduced reaction product is then neutralised with
formic
acid and formaldehyde. Then, methyl bromide/ chloride is added to the reaction
product to yield a white solid diamine cationic surfactant, i.e. N'-hexyl,
octyl,
decyl, dodecyl or ethylhexyl N,N, dimethylethylene diamine.
D, Selective alkylation of diamine
Yet another preferred synthesis route is as follows:
In an organic solvent a tertiary/primary alkyl diamine and an anhydride, acid,
methyl ester or acid chloride are reacted, whereby the primary amine group is
acylated to produce an amide compound. The reaction product is then
selectively
alkylated with an alkylating agent, and the amide product is hydrolysed using
a
mineral acid, to produce a diamine cationic surfactant.
An example of this synthesis route is the reaction of the N, N
dimethylethylene
diamine with acetic anhydride in an organic solvent (toluene), followed by
~.
CA 02279882 1999-07-29
WO 98135003 PCTIUS98102366
11
alkylation in an organic solvent (ethanol) with bromo- or chloro- hexane,
octane,
decane or dodecane to yield a quaternary amine amide. This reaction product is
hydrolysed using hydrobromic acid in water, to produce a diamine cationic
surfactant, i.e. a N,N,N, hexyl, octyl, decyl or dodecyl-dimethyl ethylene
diamine.
Examples of small scale svnthesis of preferred polvamine cationic surfactants
Example I
1.1
Into a 250m1 round bottom flask fitted with reflux condenser and drying tube
was
placed 1-bromohexane ( 1 O.Og, 0.061 mol) and tetramethylpropanediamine
(8.68g,
0.067 mol) in 100m1 ethanol. The mixture was refluxed for 72 hours. Ethanol
was removed by rotary evaporation, adding additional ethanol to remove
unreacted
amine. The resultant sticky solid was triturated with diethyl ether, but
remained a
syrup. Analysis by 270 M Hzl H NMR in CDCl3 gave the following peaks: 8
0.9 (t, 4H), 1.3-1.5 (bs), 1.7 (m, 2H), 1.95 (m, 2H), 2.25 (s, 6H), 2.4 (t,
2H),
3.4-3.7 (2s, m, 14H inc. ethanol).
This synthesis was also carried out using the following materials:
1.2 replacing bromohexane with other alkyl bromides:
Bromooctane Yield 24 %
Bromodecane Yield 14 %
i.3 replacing tetramethylpropanediamine with tetramethylethanediamine and
using the following alkyl bromides:
Alkyl Bromide Y_ field
Bromohexane 85 %
Bromoctane 91 %
Bromodecane 85 %
Bromotetradecane75 %
2-Ethylexylbromide88 %
CA 02279882 1999-07-29
WO 98/35003 PCT/L1S98/02366
12
Example 2
Into a 250 ml round bottom flask fitted with reflux condenser and drying tube
was
placed octyIamine (7.SOg, 0.58 mol) and (3-
bromopropyl)trimethylammoniumbromide (10.15g, 0.0387 mol) in 100 ml
ethanol. The mixture was refluxed for 1 day. Further octylamine (2.SOg, 0.0193
mol) was added and the mixture refluxed for 1 day. Sodium hydroxide (1.56g)
was added and the mixture filtered to remove the resultant precipitate.
Ethanol
was removed by rotary evaporation, adding additional ethanol to remove
unreacted
amine. The resultant sticky solid was triturated with diethyl ether, to yield
a white
solid, ( lOg, 87.60 % yield). Analysis by 270 M Hz I H NMR in CD30D gave the
following peaks: $ 0.9(t), 1.2-1.4(bs), 1.6(m), 2.0(m), 2.5-2.7(2m), 3.2(2s),
3.4(m).
This synthesis was also carried out with the following materials:
2.2 replacing octylamine with the following alkylamines.
Butylamine
Hexylamine
Decylamine
Dodecylamine
2-Ethylhexylamine
Detereent comt~ositions and components thereof
The polyamine cationic surfactant of the present invention may be used in any
application where surfactancy is required. For example, the polyamine cationic
surfactant of the present invention can be used in detergent compositions or
components thereof.
Depending on the type of detergent composition or component the polyamine
cationic surfactant of the presen! invention can be present at a level of from
0.05 %'o
to 95 % by weight of the comp~~:cion or component.
CA 02279882 1999-07-29
WO 98135003 PCT/US98/02366
13
The detergent compositions or components thereof can contain any of the
traditionally known and used detergent ingredients or components. The precise
nature of these components, and levels of incorporation thereof will depend on
the
physical form of the composition, and the precise nature of the washing
operation
for which it is to be used.
The detergent compositions or components thereof preferably contain one or
more
detergent components selected from additional surfactants, bleaches, bleach
catalysts, bleach precursors, water-soluble and insoluble builders, chelants,
organic polymeric compounds, enzymes, suds suppressors, lime soap dispersants,
soil suspension and anti-redeposition agents, perfumes, brighteners and
corrosion
inhibitors.
The additional.surfactant can be selected from anionic, nonionic, additional
cationic, ampholytic, amphoteric and zwitterionic surfactants and mixtures
thereof.
Where present, ampholytic, amphoteric and zwitterionic surfactants are
generally
used in combination with one or more anionic and/or nonionic surfactants.
pH of the detergent compositions
The detergent compositions preferably have a pH measured as a 1 % solution in
distilled water of at least 8.5, preferabl y from 9.0 to 12.5, most preferably
from
9.5 to 11Ø
dorm of the compositions
The detergent or cleaning compositions, comprising the polyamine cationic
surfactant of the present invention, can take a variety of physical forms
including
granular, tablet, bar and liquid forms. The compositions are particularly the
so-
called concentrated granular detergent compositions adapted to be added to a
washing machine by means of a dispensing device placed in the machine drum
with the soiled fabric toad.
CA 02279882 1999-07-29
WO 98135003 PCT/US98/02366
14
In general, granular detergent compositions in accordance with the present
invention can be made via a variety of methods including dry mixing, spray
drying, agglomeration and granulation.
rt