Note: Descriptions are shown in the official language in which they were submitted.
CA 02280535 1999-08-20
1
PROCESS FOR SEPARATING POLYFUNCTIONAL ALCOHOLS FROM WATER
SOLUBLE SALTS IN AQUEOUS SYSTEMS
The present invention relates to a process for
separating polyfunctional alcohols from water-soluble salts in
aqueous systems.
Polyfunctional alcohols or polyols are produced in
large amounts for a wide variety of applications and are used,
for example, as heat transfer media, as viscosity modifiers, as
fragrance components, as intermediates for surfactants, as bases
for ointments, as antifreezes, as additives for the coatings
industry, as mold release agents, as adhesives, as plasticizers,
as starting materials in the production of synthetic resins
(e. g. polyesters or polyurethanes) and as lubricants. Polymeric
polyfunctional alcohols such as polyvinyl alcohol are used, for
example, as protective colloids, as suspension stabilizers, as
constituents of adhesives, as pigmented binders and as packaging
materials (Ullmann's Encyclopedia of Industrial chemistry, 5th.
Ed. (1985/1992), Vol. A1, Vol. A21).
Polyfunctional alcohols can be prepared in various
ways, for example by epoxidation reactions with subsequent
hydrolysis (e.g. in the preparation of ethanediol), by
Cannizzaro reactions (e. g. in the preparation of neopentyl
glycol) or by saponification of polymeric precursors with
alkalis or by transesterification (e.g. in the preparation of
polyvinyl alcohol). In some cases during the preparation, in
other cases during the subsequent work-up and use, one obtains a
mixture of salts and the polyfunctional alcohol which needs to
be separated.
Various methods of extracting the polyfunctional
alcohols and also processes for crystallizing out the salts have
been proposed in order to separate the water-soluble
polyfunctional alcohols from the likewise water-soluble salts.
CA 02280535 1999-08-20
2
Extraction using solvents for the polyfunctional
alcohols, for example using amyl alcohol, cyclohexanol or
various ester, requires large amounts of extractant which
subsequently have to be removed again from the desired product,
e.g. by distillation.
US-A 2468718 describes a process for separating
methylolalkanes from water-soluble salts by extraction of the
methylolalkanes using a low-boiling, water-soluble ketone.
Ullmann, Volume 7, 4th edition, p.231 (1974) describes
the work-up of the mixture of polyols and salts by extraction
with solvents such as amyl alcohol, cyclohexanol or ethyl
acetate and subsequent distillation of the polyols. However,
these extraction methods require the use of large amounts of
extractants which have to be removed afterwards. This gives
intensely colored products which contain many by-products and,
mainly because of their disadvantageous color, have to be
subjected to a further distillation.
The removal of the by-products is important because
these would lead to insuperable problems in the subsequent
applications. Thus, in the coatings application, colored by-
products have an adverse effect on the reproducibility of the
coatings. In addition, salt residues adversely affect possible
further reactions of the polyfunctional alcohols, e.g. in the
preparation of the corresponding esters.
It is likewise possible to concentrate the mixture of
polyols and salts and to crystallize the salts (CN-A-1076185).
However, this process also gives colored products. Furthermore,
considerable amounts of polyols remain in the crystallized
material and can be washed out only with significant losses in
yield.
It is an object of the present invention to provide an
improved process for separating polyfunctional alcohols from
water-soluble salts in aqueous systems which can be carried out
CA 02280535 1999-08-20
3
inexpensively, i.e. with a low consumption of energy, which
makes do without the use of solvents and can thus be carried out
in an environmentally friendly manner and which gives colorless
polyols which can be further processed directly without further
purification steps.
Attempting to achieve this object, the invention
provides a process for separating polyols from water-soluble
salts in aqueous systems by subjecting aqueous systems to
electro dialysis, with the proviso that the polyols do not
include trimethylolpropane.
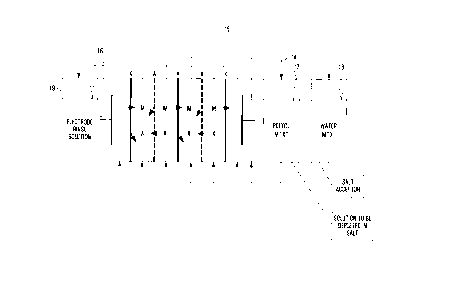
Figure 1 is a diagrammatic view of an electrodialysis
view of an electrodialysis apparatus that may be used for
carrying out a particularly preferred embodiment of the process
according to the present invention; and
Figures 2 to 5 are graphs showing the current and
voltage curves and conductivity changes in Salt-depletion and
Salt-uptake solutions employed in the working examples.
The polyols are water-soluble and may also be called
as polyfunctional alcohols hereinunder. However, "poly-
functional alcohols" may not be completely accurate since
polyphenols are also included.
Those polyols that can be used according to the
invention include:
a) diols of the formula HO-R1-OH, where R1 is:
1. a linear saturated aliphatic unit, preferably
containing 2 to 10 carbon atoms, such as -C2H4-, -C3H6-, -C4Hg-
or -C6H12- (e. g. ethylene glycol, 1,2- or 1,3-propylene glycol
and 1,2-, 1,3- or 1,4-butanediol),
2. a linear unsaturated aliphatic unit, preferably
containing 4 to 10 carbon atoms, such as -C4H6-, -C4H4- or
-C6H10- (e. g. 2,3-butene-1,4-diol, 2,3-butyne-1,4-diol and 2,3-
or 3,4-hexene-1,6-diol),
CA 02280535 1999-08-20
4
3. a branched saturated aliphatic unit, preferably
containing 3 to 10 carbon atoms, such as -C4Hg-, -C5H10- or
-C6H12- (e. g. 2-methyl-propane-1,2-diol, 2-methylpropane-1,3-
diol, 2,2-dimethylpropane-1,3-diol, 2-methylbutane-1,2-diol, 2-
methylbutane-1,3-diol, 2-methylbutane-1,4-diol, 2,3-
dimethylbutane-1,2-diol, 2,3-dimethylbutane-1,4-diol, 2,2-
dimethylbutane-1,3-diol and 2,2-dimethylbutane-1,4-diol),
4. a branched unsaturated aliphatic unit, preferably
containing 5 to 10 carbon atoms, such as -CSHg- or -C6H10- (e. g.
2-methyl-2,3-butene-1,4-diol and 2,2-dimethyl-3,4-pentene-1,5-
diol),
5. a cylic saturated aliphatic or alicyclic unit,
preferably containing 3 to 10 carbon atoms such as -C5H10-,
-C6H12- or -C~H14- (e. g. 1,2-cyclopentane-diol, 1,3-
cyclopentanediol, 1,2-cyclohexanediol, 1,3-cyclohexanediol and
1-methyl-1,2-cyclopent-anediol),
6. a cyclic unsaturated aliphatic or alicyclic unit,
preferably having 5 to 10 carbon atoms, such as -CSHg-, -C6H10-
or -C~H12- (e. g. 1,2-cyclopentene-3,4-diol, 1,2-cyclopentene-
3,5-diol, 1,2-cyclo-hexene-3,4-diol, 1,2-cyclohexene-3,5-diol,
1,2-cyclohexene-4,5-diol, 1,2-cyclohexene-3,6-diol, 1,2,3,4-
cyclohexadiene-5,6-diol and 1-methyl-1,2-cyclopentenediol),
7. an aromatic carbocyclic unit, preferably a phenylene
unit optionally substituted by a lower alkyl group, such as
-C6H4- or -C6H3(CH3)- (e. g. 1,2-dihydroxybenzene, 1,3-
dihydroxybenzene, 1,2-dihydroxy-3-methylbenzene and 1,3-
dihydroxy-2-methylbenzene), where further substituents may also
be present on the aromatic ring system or on alicyclic side
chains,
8. an aromatic heterocyclic unit, preferably a pyridine
unit, such as -C5H3N- (e.g. 2,4-dihydroxypyridine), or
9. a unit comprising a combination of the above-mentioned
units (a) 1-8;
CA 02280535 1999-08-20
b) monoglycerides of the formula: CH20Rr-CHORs-CH20Rt, where
two of the groups Rr, Rs and Rt are each hydrogen and the other
group is a saturated or unsaturated monocarboxylic acid residue
having from 1 to 22 carbon atoms, e.g. CH20H-CHOH-CH20-CO-
5 C17H35%
c) oligomeric and polymeric ether diols of the formula:
HO-[(CR2R3)n-0]m-H, where n >_ 2 and m >_ 2, preferably n is 2, 3
or 4 and m is 2-100, where R2 and R3 are each, independently of
one another, hydrogen or an aliphatic group such as -CH3 or
-C2H5 (preferably, (CR2R3)n is -CH2CH2- or -CH2CH(CH3)-);
examples include HO-CH2-CH2-O-CH2-CH2-OH (diethylene glycol),
HO[CH2CH20]3pH (polyethylene glycol having a mean molecular
weight of 1338) and HO [CH2CH (CH3) 0] 3pH (polypropylene glycol
having a mean molecular weight of 1758);
d) triols and higher-functional polyols of the formula:
R4(OH)x where x >_ 3, preferably x is 3-6, and R4 is:
1. a linear saturated aliphatic unit, preferably having
3-10 carbon atoms, such as -C3H5-, -C4H7-, -C5H9- or -CSHg-
(e.g. glycerol, 1,2,3-butanetriol, 1,2,4-butanetriol and 1,2,3-
pentanetriol),
2. a linear unsaturated aliphatic unit, preferably having
5-10 carbon atoms, such as -C5H7-, -C6H9- or -C6Hg (e.g. 1-
pentene-3,4,5-triol, 1-hexene-3,4,5-triol, 2-hexene-1,4,5-triol
and 3-hexene-1,2,5,6-tetrol),
3. a branched saturated aliphatic unit, preferably having
4 to 10 carbon atoms, such as -C4H7- or -CSHg-(e. g. 2-methyl-
1,2,3-propanetriol and pentaerythritol),
4. a branched unsaturated aliphatic unit, preferably
having 5 to 10 carbon atoms, such as -C5H7- or -C6H9-(e.g. 2-
methylol-2-butene-1,4-diol and 2-methylol-2-pentene-1,5-diol),
5. a saturated alicyclic or cyclic aliphatic unit,
preferably having 5 to 10 carbon atoms, such as -C5H7-, -C6Hg-,
-C6H8- or -C7H11- (e. g. 1,2,3-cyclopentanetriol, 1,2,3-
CA 02280535 1999-08-20
6
cyclohexanetriol, 1,2,3,4-cyclohexanetetrol, 6-methyl-1,2,3-
cyclohexanetriol and 6-methylol-1,3-cyclohexanediol),
6. an unsaturated alicyclic or cyclic aliphatic unit,
preferably having 5 to 10 carbon atoms, such as -C5H5- or -C6H7-
(e. g. cyclopentene-3,4,5-triol and cyclohexene-3,4,5-triol),
7. an aromatic carbocyclic unit, preferably a benzene
unit optionally substituted by a lower alkyl group, such as
-C6H3- or -C6H2(CH3)- (e. g. 1,2,3-trihydroxybenzene and 2,3,4-
trihydroxytoluene),
8. an aromatic heterocyclic unit, preferably a pyridine
unit, such as -C5H2N-(e.g. 2,3,4- trihydroxypyridine), or
9. a unit comprising a combination of the above-mentioned
units (d) 1-8; and
e) polymeric polyfunctional alcohols such as polyvinyl
alcohol, polysaccharides and branched or dendrimer-like
polyether polyols having, for example, the following structure:
HO-[CHz-CH2-O]d CH2\ / CHZ [O-CH2-CH2] a OH
C
2 0 HO-[CHz-CH2-O] f CH2 ~ CH2 [O-CH2-CH2]g OH
where d, e, f and g are, independently of one another, an
integer >_ 1, preferably 1-10.
The use of electrodialysis for desalination of sea
water, for brine recovery, for obtaining drinking water, for
regulating the hardness of water, for desalination of whey in
the food industry, for preventing cream of tartar deposits in
wine production or for recovering valuable materials from waste
solutions from electrolytic processes is known (Rompp Chemie
Lexikon, Volume 2, 10th edition, pp. 1113-1114 (1997)).
However, these applications, always involve aqueous systems
having a relatively low salt content (< 5% by weight).
CA 02280535 1999-08-20
7
Electrodialysis is a separation process in which the
migration of ions through a permeation-selective membrane is
accelerated by application of a direct current (DC) voltage. In
electrodialysis, ions are transported through a membrane under
the action of an electric field. If ion exchange membranes are
installed in the dialysis apparatus in such a way that anion
exchange membranes and cation exchange membranes are arranged
alternately between a cathode and an anode and divide a cell
into narrow chambers, appropriate connection of the chambers
gives streams which are depleted in salt and enriched in salt,
since when current is passed through the apparatus, the cations
can only pass through the cation exchange membranes and the
anions can only pass through the anion exchange membranes. The
enrichment occurs against the concentration gradient. This
means that connection of a plurality of anion- and cation-
selective ion exchange membranes in series makes it possible to
deionize the liquid to be dialysed while simultaneously
increasing the concentration of the ions in the concentrate
chambers.
In contrast to the other known membrane processes such
as membrane filtration, reverse osmosis or gas permeation, in
which the fluids are separated as a result of pressure or
concentration differences, electrodialysis enables a separating
force acting in a targeted way to be applied by means of the
electric current. In this way, charged constituents can be
moved selectively in the solution. The ion exchange membranes
used for the separation are organic polymer membranes having
functional, charge-bearing groups and act similarly to an
electrical rectifier. Ion exchange membranes allow passage of
only one type of ion, while the oppositely charged ions are
prevented from passing through. The construction of an
electrodialysis module corresponds approximately to that of a
chamber filter press, i.e. two or three solutions are fed
CA 02280535 1999-08-20
8
separately into the module frame via internal feed channels and
then flow, separated from one another by the ion exchange
membranes, through the respective hollow spaces in the frame.
Under the action of the applied electric potential, ions are
transported from the individual chambers through the ion
exchange membranes. Appropriate connection in series of anion
exchange membranes (A) and ration exchange membranes (K), i.e.
membranes which allow either only anions or only rations to pass
through, makes it possible for solutions to be reduced in salt
content or concentrated or for undesired ions to be replaced by
others.
An example of an apparatus which can be used according
to the invention for separating polyfunctional alcohols from
water-soluble salts is shown in Figure 1. The apparatus
consists of an electrodialysis module having three separate
circuits (a salt-depletion or salt-releasing circuit 14, a salt-
uptake or salt-accepting circuit 15 and an electrode-flushing or
electrode-rinse circuit 16) and the associated reservoirs 17, 18
and 19. In order to achieve a large membrane surface, five
successive cell units were installed.
In Figure 1:
M or M+ = metal ions, X or X - anions, Polyol - poly-
functional alcohol, K = ration exchange membrane and A = anion
exchange membrane.
To remove the reaction gases formed at the electrodes,
an Electrode-flushing solution may preferably be passed through
the end chambers. This electrode-flushing solution, if used,
should be inert toward the electrodes, have a low electrical
resistance and release no extraneous ions into the salt-uptake
and salt-depletion circuits.
It has now surprisingly been found that
electrodialysis can likewise be used for separating a mixture of
water-soluble polyols and water-soluble salts dissolved therein,
CA 02280535 1999-08-20
9
even if the salts are present in high concentration (> 50°s by
weight). High selectivities (S > 250) can be achieved in such a
process.
The selectivity of the separation between the
originally salt-containing and then salt-depleted solution and
the concentrated solution in respect of the neutral component
polyfunctional alcohol is defined as for other membrane
processes:
S = ( [polyol] conc [salt] conc) ~ ( [polyol] dil [salt] dil)
where: dil: salt-depletion solution
conc: salt-uptake solution
[polyol]: proportion by weight of polyol in the
solution
[salt]: proportion by weight of the salts in the
solution.
The selectivity of the salt transport is described by
the current yield, i.e. the actual amount of salt transported (N
measured in mole) divided by the maximum possible amount
(Ntheoretical) which can be transported by means of the electric
charge transport (electric current).
Nmeasured~Ntheoretical
In the salt removal from the alcoholic solution, a
current yield of over 95% can be achieved despite the high salt
content.
The electrodialysis apparatus used in the process of
the invention may employ commercially available electrodialysis
modules fitted with likewise commercially available anion and
cation exchange membranes. Here, the anion exchange membrane
may have to be selected according to the criteria: a) low
resistance, b) high selectivity in respect of the anion and c)
low solvent flux.
CA 02280535 1999-08-20
The electrodialysis apparatus preferably has a Salt-
depletion circuit 14 and a salt-uptake circuit 15, as shown in
Figure 1.
The mixture (which is typically an aqueous solution)
5 of polyfunctional alcohols and salts, as is formed, for example,
as crude product from the base-catalyzed aldol addition of a
relatively long chain aliphatic aldehyde and a shorter-chain
aliphatic aldehyde and subsequent Cannizzaro reaction in the
presence of stoichiometric amounts of base (e. g. alkali metal
10 hydroxide), can be fed directly to the salt-depletion circuit of
an electrodialysis apparatus as shown in Figure 1. The
concentration of the individual components in the mixture is
immaterial, as long as the mixture is pumpable. It is
preferable, however, that the concentration of the
polyfunctional alcohols is from about 5 to 80, more preferably
30 to 60% by weight and the concentration of the salt is from
about 2 to 40, more preferably 5 to 20% by weight. An overall
concentration is preferably 10-90% by weight.
The pH of the solution to be depleted in salt may be
adjusted to an approximately neutral value by means of an acid
or a base (preferably the same base which has been used for the
preparation of the polyols). Acidic or alkaline pH values are
possible as long as this does not affect the stability of the
membranes. Preference is given to pH values in the range from 4
to 10.
The upper temperature limit for the solution to the
depleted in salt is determined by the stability of the ion
exchange membranes; the lower temperature limit is determined by
the viscosity or the pumpability of the medium. However, the
temperature is preferably set to a value in the range from 10 to
50°C. Account needs to be taken of the fact that the salt-
depletion solution heats up during the separation process.
CA 02280535 1999-08-20
11
The solution to be depleted in salt is introduced into an
electrodialysis cell. When the apparatus of Figure 1 is used,
the solution is introduced into the salt-depletion circuit.
The salt-uptake solution in the salt-uptake circuit of
the electrodialysis apparatus shown in Figure 1 is preferably
water or an aqueous salt solution. The pH of the salt-uptake
solution is preferably set to a value in the range from 4 to 10
and the temperature of the solution is preferably in the range
from 10 to 50°C. In this case too, the upper concentration limit
for the medium is determined by the pumpability of the solution.
It is also important that the solubility product of the anions
and cations which permeate through the membranes is not exceeded
in the salt-uptake solution. Salt deposits in the salt-uptake
circuit can lead to irreversible damage to the entire apparatus,
in particular to the membranes.
The current density during electrodialysis is
preferably in the range from 50 to 750 A/m2, particularly
preferably in the range from 150 to 250 A/m2. The current yield
can be over 95% under optimum process conditions. The limiting
current density must be matched to the salt concentration in the
mixture from which salts are to be removed and can easily be
determined by a person skilled in the art. The limiting current
density as a function of concentration thus determines the
regulation of the current density during the separation process.
Electrode-flushing solutions are preferably used in
the electrodialysis apparatus used according to the invention,
as shown in Figure 1. The electrode-flushing solutions ensure
that no reaction of the electrodes with the materials in the
solution takes place, that gases formed in the electrode
reaction can be removed and that the electrical resistance is
reduced by a high conductivity and the energy consumption of the
electrode reaction is thus minimized. The electrode-flushing
solution should, if possible, comprise the same cations as the
CA 02280535 1999-08-20
12
other salt solutions in order to avoid introduction of further
rations into the process. Aqueous solutions of inorganic salts
can be used as electrode-flushing solutions.
The course of the separation process can be followed
via the conductivity of the salt-depletion and salt-uptake
solutions. The conductivity has to be correlated with the
analytically determined contents of polyol or salt, i.e. for a
given conductivity, the actual concentration of polyol. By-
products and salt may be determined by means of other analytical
methods (e.g. high performance liquid chromatography (HPLC), gas
chromatography (GC)). In general, it is sufficient to carry out
electrodialysis until the conductivity of the salt-depletion
solution has dropped to about 2 uS/cm. The result is an aqueous
solution of the polyfunctional alcohols substantially free of
the salts. The cleaning of the overall electrodialysis module
depends on the separation task. For example, flushing the
module with warm deionized water for about 2 hours every two
weeks may be sufficient for cleaning the system.
In the base-catalyzed reaction (e. g. using potassium
hydroxide or sodium hydroxide) of relatively long-chain
aliphatic aldehydes (e. g. butyraldehyde) with shorter-chain
aliphatic aldehydes (e.g. formaldehyde), the polyols are not
obtained as pure substances, but, depending on the process
conditions, as a mixture of the respective target product with
the dimers of the target product and further OH-functional
compounds as well as the corresponding salt of the acid from the
Cannizzaro reaction. if this crude product is desalted by means
of electrodialysis, there remains an aqueous, colorless solution
of the polyol mixture which can be subjected as such or in
concentrated form, without further purification processes, to
further reactions, for example condensation reactions such as
esterifications with, for example, oleic acid for producing
lubricants or with acrylic acid for producing coating additives.
CA 02280535 1999-08-20
13
If the polyfunctional alcohols are nevertheless to be
isolated from the mixture as pure substances, distillative
separation methods can be used.
The following examples illustrate the invention.
Examples
Various highly concentrated, aqueous solutions of
polyfunctional alcohols having a correspondingly high salt
content were worked up. A mixture of alcohol, salt and water
was placed in the salt-depletion circuit. The uptake phase
initially contained a small amount of the salt to be separated
off in order to produce a certain minimum conductivity. The
electrode-flushing solutions were selected for the respective
application according to the above-described criteria. In
general, they were intermediate-concentration solutions of the
same salts which were to be removed from the aqueous alcohol
solution.
The alcohols used were
diols: (ethanediol (ethylene glycol), 1,4-butane-diol,
diethylene glycol),
. triols: (glycerol, neopentyl glycol, pentaerythritol) and
also two
polyalcohols (polyvinyl alcohol, polyethylene alcohol)
having a mean molecular weight of about 1500 g/mol.
Apart from these aliphatic alcohols, 1,3-
dihydroxybenzene (resorcinol) was additionally studied as an
example of a polyhydric aromatic alcohol.
In each case, a 40% strength solution was prepared,
provided that the solubility of the alcohol in water permitted
this. Only in the case of pentaerythritol was it only possible
to make a 65 strength solution. The salt content varied from 5
to 20%, the remainder was then water. The salts were
inorganic salts such as NaCl and Na2S04 or
CA 02280535 1999-08-20
14
sodium and potassium salts of short-chain and relatively
long-chain organic acids (potassium acetate, sodium formate and
also the sodium salt of 2-ethyl-caproic acid).
CA 02280535 1999-08-20
Table 1
Alcohols Salts
Name Abbreviation Name Abbreviation
Ethanediol A1 Sodium S1
chloride
1,4-Butanediol A2 Potassium S2
acetate
Diethylene A3 Sodium S3
glycol sulfate
Glycerol A4 Sodium S4
formate
Neopentyl A5 Sodium salt S5
glycol of 2-ethyl-
caproic acid
Pentaerythritol A6
1,3-Dihydroxy- A~
benzene
Polyethylene A$
glycol
Polyvinyl A9
alcohol
The apparatus shown in Figure l~comprised an
5 electrodialysis module from Stantech provided with three
separate circuits and the associated reservoirs. Five
successive cell units were installed in the module which had an
effective membrane area of 100 cm2. The membranes used, namely
C66-10F* and AHA-2*, came from tokuyama Soda.
10 The precise proportions by weight together with the
corresponding combinations of alcohol, salt and water are shown
in Table 2.
*Trade-mark
CA 02280535 1999-08-20
16
Table 2
Alcohol M(alcohol) w(alcohol) Salt M(salt) w(salt) Water
g/mol % g/mol 0 0
A1 62 40.0 S1 58.5 12 48.00
A2 90 40.0 S2 98.0 20 40.00
A3 106 38.6 S3 146.0 5 56.37
A8 1500 40.0 Sl 58.5 12 48.00
A5 104 40.0 S4 68.0 14 46.00
A4 92 40.0 S5 167.0 5 55.00
A6 136 6.0 S4 68.0 14 80.00
A7 110 36.6 S3 146.0 16.5 46.90
-A9 20,000 5.0 S1 58.5 5 90.00
Initial concentrations in percent by weight and molar
masses of the alcohol/salt solutions studied.
The concentrations of the electrode-flushing solutions
were loo for the Na2S04 solution and 20s for the K2C03 and
Na2C03 solutions. They are indicated in Table 3 together with
the corresponding alcohols and salts in the solution from which
salt is to be removed. In place of chlorides which would be
reduced to elemental chlorine in the electrode reaction,
carbonates were used in the flushing solutions.
CA 02280535 1999-08-20
17
Table 3
Depletion Anode Cathode
Circuit
Alcohol Salt Salt o Salt s
A1 Sl Na2C03 20 Na2C03 20
A2 S2 K2C03 20 K2C03 20
A3 S3 Na2COq 10 Na2SOq 10
A8 S1 Na2C03 20 Na2C03 20
A5 S4 Na2C03 20 Na2C03 20
A4 S5 Na2C03 20 Na2C03 20
A6 S4 Na2C03 20 Na2C03 20
A7 S3 Na2SOg 10 Na2SOq 10
A9 S1 Na2C03 20 Na2C03 20
Composition and concentration of the electrode-
flushing solutions together with the alcohols and salts in the
depletion circuit.
Figures 2 to 5 show, by way of example, the current
and voltage curves and the conductivity changes in the salt-
depletion and salt-uptake solutions for the removal of Sl from
A8.
The concentrated salt/alcohol solution is in the
depletion circuit and the dilute salt solution is in the uptake
circuit. As soon as a voltage is applied to the electrodes, an
electric current flows as a result of ion conduction.
At a constant voltage, the current steadily decreases
as a result of the decrease in conductivity of the depletion
circuit. The increase in the conductivity of the uptake circuit
has no influence of the current, since when resistances are
CA 02280535 1999-08-20
18
connected in series it is the greatest resistance which
effectively determines the overall behavior.
Since the mass transport is proportional to the
electric transport, the decrease in the conductivity in the
depletion circuit also becomes smaller as a result of the
decreasing current. The experiment is continued until virtually
no change occurs in the conductivity, i.e. all salts have been
transported from the depletion circuit to the uptake circuit.
The jump in the conductivity in the uptake circuit results from
the temperature fluctuations when the experiment was interrupted
overnight.
The changes in the salt concentrations in the two
circuits are shown in Table 4 for all experiments. Apart from
the desalting of A7, all solutions tested could be desalted very
well. The lowest concentration achievable at the end is thus
only a question of time, i.e. how long the current has to flow.
Table 4
Alcohol Concentrate Depletion
Solution
Initial Final Initial Final
Salt o o Salt o $
A1 S1 1.00 10.17 S1 12.0 0.0100
A2 S2 0.98 19.09 S2 20.0 0.9200
A3 S3 1.50 4.57 S3 5.0 0.0010
A8 Sl 1.00 9.9 S1 12.0 0.1270
A5 S4 0.68 13.3 S4 14.0 0.0650
A4 S5 2.00 6.82 S5 5.0 0.0720
A6 S4 0.68 8.57 S4 14.0 0.0206
A7 S3 1.50 4.25 S3 16.5 15.2300
A9 Sl 1.00 5.59 S1 5.0 0.0380
CA 02280535 1999-08-20
19
Initial and final concentrations in the salt-depletion
circuit and the salt-uptake circuit
The selectivity of the salt transport is described by
the current yield ~, i.e. the actual amount of salt transported
(Nmeasured in mol) divided by the maximum possible amount
(Ntheoretical) which can be transported by means of the electric
charge transport (electric current):
~ = Nmeasured~Ntheoretical
The current yield can be calculated from the measured
concentrations and the amounts employed and the charge which has
passed through (integral of current x time) by accurate
balancing. With the exception of A7 which has already been
mentioned, it is from 0.8 to almost 1Ø In the desalting of A3
and A5, values of only about 0.6 were achieved.
CA 02280535 1999-08-20
Chemicals used
Ethanediol (ethylene Technical 99.9% HUls
glycol) grade
1,4-Butanediol Technical Huls
Grade
Diethylene glycol >99.9% Riedel-de
Haen
PEO (MW about 1500 Pure Fluka
g/mol
Neopentyl glycol Pure >98.Oo Fluka
Glycerol AR 99.5% Anhydrous
Pentaerythritol Pure >97.Oo Fluka
1,3-Dihydroxybenzene Pure >98.Oo Fluka
(resorcinol)
Polyvinyl alcohol Wacker
Polyviol
604/140
NaCl AR Merck
Potassium acetate AR Fluka
Na2S04 Technical >99.9o Riedel-de
Haen
Sodium salt of 2-ethyl-
caproic acid (Fluka);
the sodium salt was
prepared in situ by
neutralization of the
acid with NaOH
Sodium formate AR Fluka
Fluka Chemie AG, Industriestr. 25, CH-9471 Buchs Riedel-de Haen
Laborchemikalien GmbH & Co. KG, D-30918 Seelze
CA 02280535 1999-08-20
21
Merck KGaA, Frankfurter Str. 250, D-64293 Darmstadt
blacker-Chemie GmbH, Hanns-Seidel-Platz 4, D-81737 Munich