Note: Descriptions are shown in the official language in which they were submitted.
CA 02280727 2005-11-04
26474-452
- 1 -
Bicyclic aromatic amino acids
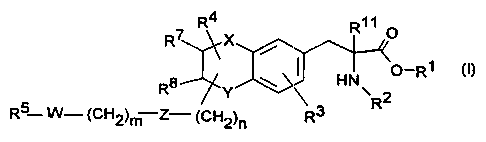
The invention relates to compounds of the
formula I
7 Rd R11 O
R X
HN O-Ri i
R$ Y \
2
R5 W- (CN2)m-Z- (CH2)n R3 R
in which
R1 is H, alkyl having 1-6 C atoms or benzyl,
2 10 10 6 10 6 10
R is R, C0-R , COOR , COOR , SOZR or S02R ,
R3 is H, Hal, OA, NHR10, N(R10) 2, -NH-acyl,
-0-acyl, CN, NO2, OR10, SR10, R2 or CONHRlo,
R4 is H, =0, =S, C1-C6-alkyl or acyl,
R5 is NH21 H2N-C(=NH) or H2N- (C=NH) -NH, where
the primary amino groups can also be provided
with conventional amino protective groups or
can be mono-, di- or trisubstituted by R''o,
CO-R10, COORlO or' S02R10,
or R6,
R', RB are each H or together form a bond,
X,.Y are each independently of one another =N-,
-N-, 0, S, -CH2- or =C-,
with the proviso that at least one of the two
definitions X, Y is =N-, -N-, 0 or S,
CA 02280727 1999-08-11
- 2 -
W, Z are each independently of one another absent,
0, S, NRl, C(=O), CONH, NHCO, C(=S) NH,
NHC ( =S ) , C ( =S ) , SO2NH, NHSO2 or CA=CA' ,
R6 is a mono- or binuclear heterocycle which has
1 to 4 N, 0 and/or S atoms and can be
unsubstituted or mono-, di- or trisubstituted
by Hal, A, -CO-A, OH, CN, COOH, COOA, CONH2,
NO2r =NH or =0,
R9 is H, Hal, OA, NHA, NAA', NHacyl, Oacyl, CN,
N02r SA, SOA, SO2A, S02Ar or S03H,
R10 is H, A, Ar or aralkyl having 7-14 C atoms,
R11 is H or alkyl having 1-6 C atoms,
A, A' are each independently of one another H or
unsubstituted or mono-, di- or tri-R9-
substituted alkyl or cycloalkyl, each of
which has 1-15 C atoms and in which one, two
or three methylene groups can be replaced by
N, 0 and/or S,
Ar is unsubstituted or mono-, di- or tri-A-
and/or R9-substitut:ed mono- or binuclear
aromatic ring system having 0, 1, 2, 3 or 4
N, 0 and/or S atoms,
Hal is F, Cl, Br or I ar.d
m, n are each independently of one another 0, 1,
2, 3 or 4,
and the physiologically accept:able salts thereof.
Similar compounds are disclosed, for example,
in WO 94/29273, WO 96/00730 arid WO 96/18602.
The invention was based on the object of
finding novel compounds witr. valuable properties, in
CA 02280727 1999-08-11
= - 3 -
particular those which can be used to produce
pharmaceuticals.
It has been found that the compounds of the
formula I and their salts have very valuable
pharmacological properties while being well tolerated.
In particular, they act as integrin inhibitors,
inhibiting in particular the interactions of the a,
integrin receptors with ligands. The compounds show
particular activity in the case of the integrins aõ(33
and av(35. The compounds are very particularly active as
adhesion receptor antagonists for the vitronectin
receptor aA. This effect can be demonstrated, for
example, by the method described by J.W. Smith et al.
in J. Biol. Chem. 265, 11008-11013 and 12267-12271
(1990). B. Felding-Habermann and D.A. Cheresh describe,
in Curr. Opin. Cell. Biol. 5, 864 (1993), the
significances of the integrins as adhesion receptors
for a wide variety of phenomena and pathological
states, specifically relating to the vitronectin
receptor av(33.
The dependence of the initiation of
angiogenesis on the interaction between vascular
integrins and extracellular matrix proteins is
described by P.C. Brooks, R.A. Clark and D.A. Cheresh
in Science 264, 569-71 (1994).
The possibility of inhibiting this interaction
and thus initiating apoptosis (programmed cell death)
of angiogenic vascular cells by a cyclic peptide is
described by P.C. Brooks,. A.M. Montgomery, M.
Rosenfeld, R.A. Reisfeld, T. Hu, G. Klier and D.A.
Cheresh in Cell 79, 1157-64 (1.994).
Experimental, demonstration that the compounds
according to the invention also prevent adhesion of
living cells to the appropriate matrix proteins and,
accordingly, also prevent the adhesion of tumour cells
to matrix proteins can be provided by a cell adhesion
assay carried out in analogy to the method of F.
Mitjans et al., J. Cell Science 108, 2825-2838 (1995).
CA 02280727 1999-08-11
- 4 -
P.C. Brooks et al. describe, in J. Clin.
Invest. 96, 1815-1822 (1995), a,R3 antagonists for
controlling cancer and for treating tumour-induced
angiogenic disorders. The contpounds of the formula I
according to the invention can therefore be employed as
pharmaceutical agents, in particular for treating
oncoses, osteoporoses and osteolytic disorders, and for
suppressing angiogenesis.
Compounds of the formula I which block the
interaction of integrin receptors and ligands such as,
for example, of fibrinogen ori the fibrinogen receptor
(glycoprotein IIb/IIIa) prevent, as GPIIb/IIIa
antagonists, the spread of tumour cells by metastasis.
This is proved by the following observations:
The spread of tumour cells from a local tumour into the
vascular system takes place by formation of
microaggregates (microthrombi) by the tumour cells
interacting with blood platelets. The tumour cells are
shielded by the protection in the microaggregate and
are not recognized by the ce_Lls of the immune system.
The microaggregates are able to become attached to
vessel walls, facilitating further penetration of
tumour cells into the tissue. Since the formation of
microthrombi is mediated by fibrinogen binding to the
fibrinogen receptors on activated blood platelets, the
GPIIa/IIIb antagonists can be regarded as effective
metastasis inhibitors.
Compounds of the formiila I inhibit not only the
binding of fibrinogen, fibronectin and Willebrand
factor to the fibrinogen receptor of the blood
platelets but also the binding of other adhesive
proteins, such as vitronectin, collagen and laminin, to
the corresponding receptors on the surface of various
types of cells. They prevent, in particular, the
development of blood platelet thrombi and can therefore
be employed to treat thromboses, stroke, myocardial
infarct, inflammations are arteriosclerosis.
The properties of the compounds can also be
demonstrated by methods described in EP-Al 0 462 960.
CA 02280727 1999-08-11
- 5 -
The inhibition of fibrinogen binding to the fibrinogen
receptor can be demonstrated by the method indicated in
EP-Al 0 381 033.
The platelet aggregation-inhibiting effect can
be demonstrated in vitro by the method of Born (Nature
4832, 927-929, 1962).
The invention accordingly relates to compounds
of the formula I according to Claim 1 and/or their
physiologically acceptable salts for producing a
pharmaceutical for use as integrin inhibitors. The
invention particularly relates to compounds of the
formula I according to Claim 1 and/or their acceptable
salts in which R 2 is camphor-l0-sulfonyl for producing
a pharmaceutical for controlling pathologically
angiogenic disorders, tumours, osteoporosis,
inflammations and infections.
The compounds of the formula I can be employed
as pharmaceutical agents in human and veterinary
medicine, for the prophylaxis and/or therapy of
thrombosis, myocardial infarct, arteriosclerosis,
inflammations, stroke, angina pectoris, oncoses,
osteolytic disorders such as osteoporosis,
pathologically angiogenic ciisorders such as, for
example, inflammations, ophthalmological disorders,
diabetic retinopathy, macular degeneration, myopia,
ocular histoplasmosis, rheumatoid arthritis,
osteoarthritis, rubeotic glaucoma, ulcerative colitis,
Crohn's disease, atherosclerosis, psoriasis, restenosis
after angioplasty, viral infection, bacterial
infection, fungal infection, in acute kidney failure
and in wound healing to assist the healing processes.
The compounds of the formula I can be employed
as substances with antimicrobial activity in operations
where biomaterials, implants, catheters or heart
pacemakers are used. They have an antiseptic effect in
such cases. The efficacy of the antimicrobial activity
can be demonstrated by the method described by P.
Valentin-Weigund et al. in Infection and Immunity,
2851-2855 (1988).
CA 02280727 1999-08-11
- 6 -
The invention furtherrnore relates to a process
for preparing compounds of the formula I according to
Claim 1 and salts thereof, characterized
a) in that a compound of the formula I is liberated
from one of its furictional derivatives by
treatment with a solvolysing or hydrogenolysing
agent,
or
b) in that a compound of the formula II
R7 R4 X R11 ,
R8 NH O-Rl 11
Y Z
RS W- (CH2)m Z- (CH2)n R3
in which R1, R3, R4, R5, R7, R8, R11, W, X, Y, Z, m
and n have the meanings stated in Claim 1,
is reacted with a compoun.d of the formula III
RZ-L III
in which
R 2 has the meaning stated in Claim 1,
and L is Cl, Br, I, OH or a reactively esterified
OH group,
or
c) in that an ester of the f:ormula I is hydrolysed,
or
d) in that a radical R1 and/or R5 is converted into
5
another radical R1 and/or R,
CA 02280727 1999-08-11
- 7 -
and/or
e) in that a basic or acidic; compound of the formula
I is converted by treatment with an acid or base
into one of the salts thereof.
The compounds of the formula I have at least
one chiral centre and may therefore occur in a
plurality of stereoisomeric forms. All these forms (for
example D and L forms) and mixtures thereof (for
example the DL forms) are included in formula I. The
compounds according to the irivention also include so-
called prodrug derivatives, that is to say compounds of
the formula I which have been modified with, for
example, alkyl or acyl groups, sugars or oligopeptides
and which are rapidly cleaved in the body to give the
active compounds according to the invention.
The abbreviations mentioned hereinbefore and
hereinafter represent:
Ac acetyl
BOC tert-butoxycarbonyl
CBZ or Z benzyloxycarbonyl
DCCI dicyclohexylcarbodii.mide
DMF dimethylformamide
DOPA (3,4-dihydroxyphenyl)alanine
DPFN 3,5-dimethylpyrazole:-1-formamidinium nitrate
EDCI N-ethyl-N'-(3-dimethylaminopropyl)carbo-
diimide
Et ethyl
Fmoc 9-fluorenylmethoxycarbonyl
HOBt 1-hydroxybenzotriazole
Me methyl
Mtr 4-methoxy-2,3,6-trimethylphenylsulfonyl
HONSu N-hydroxysuccinimide
OBn benzyl ester
OBut tert-butyl ester
Oct octanoyl
OMe methyl ester
CA 02280727 1999-08-11
- 8 -
OEt ethyl ester
Orn ornithine
POA phenoxyacetyl
TBTU 0-(benzotriazol-1-yl)-N,N,N',N'-tetramethyl-
uronium tetrafluoroborate
TFA trifluoroacetic acid
Trt trityl (triphenylmethyl)
Z or CBZ benzyloxycarbonyl.
All the radicals whic:h occur more than once,
such as, for example, A and A', can be identical or
different, that is to say are independent of one
another, and this applies to the entire invention.
In the formulae above, alkyl is preferably
methyl, ethyl, propyl, isopropyl, butyl, isobutyl, sec-
butyl or tert-butyl, also pentyl, 1-, 2- or
3-methylbutyl, 1,1-, 1,2- or 2,2-dimethylpropyl,
1-ethylpropyl, hexyl, 1-, 2-, 3- or 4-methylpentyl,
1,1-, 1,2-, 1,3-, 2,2-, 2,3- or 3,3-dimethylbutyl, 1-
or 2-ethylbutyl, 1-ethyl-l-methylpropyl, 1-ethyl-2-
methylpropyl, 1,1,2-, 1,2,2--trimethylpropyl, heptyl,
octyl, nonyl or decyl.
Cycloalkyl is preferably cyclopropyl,
cyclobutyl, cyclopentyl, cyclohexyl, cycloheptyl or
3-menthyl. Cycloalkyl is, in particular, the radical of
a bicyclic terpene, and the camphor-l0-yl radical is
very particularly preferred.
Alkylene is preferably methylene, ethylene,
propylene, butylene, pentylene, also hexylene,
heptylene, octylene, nonylene or decylene. Aralkyl is
preferably phenylalkyl and is, for example, preferably
benzyl or phenethyl..
Cycloalkylene is preferably cyclopropylene,
1,2- or 1,3-cyclobutylene, 1,2- or 1,3-cyclopentylene,
1,2-, 1,3- or 1,4-cyclohexylene, also 1,2-, 1,3- or
1,4-cycloheptylene.
CO-A is alkanoyl or cycloalkanoyl and is
preferably formyl, acetyl., propionyl, butyryl,
pentanoyl, hexanoyl, heptanoyl, octanoyl, nonanoyl,
CA 02280727 1999-08-11
- 9 -
decanoyl, undecanoyl, dodecanoyl, tridecanoyl,
tetradecanoyl, pentadecanoyl, hexadecanoyl,
heptadecanoyl or octadecanoyl.
Acyl is C1-C-7-acyl and :nas 1, 2, 3, 4, 5, 6 or 7
C atoms and is preferably, for example, formyl, acetyl,
propionyl, butyryl, trifluoroacetyl or benzoyl.
Preferred substituents for alkyl, alkylene,
cycloalkyl, cycloalkylene, al:kanoyl and cycloalkanoyl
are, for example, Hal, OA, NHA, NAA', CN, NOzr SA, SOA,
SO2A, SOzAr and/or S03H, in particular, for example, F,
Cl, hydroxyl, methoxy, ethoxy, amino, dimethylamino,
methylthio, methylsulfinyl, methylsulfonyl or
phenylsulfonyl.
Preferred substituents for Ar and arylene are,
for example, A and/or Hal, OA, NHA, NAA', CN, NO2, SA,
SOA, SOZA, SO2Ar and/or S0:3H, in particular, for
example, F, Cl, hydroxyl, methoxy, ethoxy, amino,
dimethylamino, methylthio, methylsulfinyl, methyl-
sulfonyl or phenylsulfonyl.
One, two or three methylene groups in each of
the radicals alkyl, alkylene, cycloalkyl,
cycloalkylene, alkanoyl and cycloalkanoyl can be
replaced by N, 0 and/or S.
Ar-CO is aroyl and is preferably benzoyl or
naphthoyl.
Ar is unsubstituted or preferably, as
indicated, monosubstituted phenyl, specifically
preferably phenyl, o-, m- or p-tolyl, o-, m- or
p-ethylphenyl, o-, m- or p-propylphenyl, o-, m- or
p-isopropylphenyl, o-, m- or p-tert-butylphenyl, o-, m-
or p-cyanophenyl, o-, m- or p-methoxyphenyl, o-, m- or
p-ethoxyphenyl, o-, :m- or p--fluorophenyl, o-, m- or
p-bromophenyl, o-, m- or p-chlorophenyl, o-, m- or
p-methylthiophenyl, o-, m- or p-methylsulfinylphenyl,
o-, m- or p-methylsulfon.ylphenyl, o-, m- or
p-aminophenyl, o-, m- or p-met.hylaminophenyl, o-, m- or
p-dimethylaminophenyl, o-, m- or p-nitrophenyl,
CA 02280727 1999-08-11
- 10 -
furthermore preferably 2,3-, 2,4-, 2,5-, 2,6-, 3,4- or
3,5-difluorophenyl, 2,3-, 2,4-, 2,5-, 2,6-, 3,4- or
3,5-dichlorophenyl, 2,3-, 2,4-,, 2,5-, 2,6-, 3,4- or
3,5-dibromophenyl, 2-chloro--3-methyl-, 2-chloro-4-
methyl-, 2-chloro-5-methyl--, 2-chloro-6-methyl-,
2-methyl-3-chloro-, 2-methyl-4-chloro-, 2-methyl-5-
chloro-, 2-methyl-6-chloro--, 3-chloro-4-methyl-,
3-chloro-5-methyl- or 3-methyl-4-chlorophenyl, 2-bromo-
3-methyl-, 2-bromo-4-methyl-, 2-bromo-5-methyl-, 2-
bromo-6-methyl-, 2-methyl-3-bi~omo-, 2-methyl-4-bromo-,
2-methyl-5-bromo-, 2-methyl-6-bromo-, 3-bromo-4-methyl,
3-bromo-5-methyl- or 3-methyl-4-bromophenyl, 2,4- or
2,S-dinitrophenyl, 2,5- or 3,4:-dimethoxyphenyl, 2,3,4-,
2,3,5-, 2,3,6-, 2,4,6- or 3,4,5-trichlorophenyl, 2,4,6-
tri-tert-butylphenyl, 2,5-dimethylphenyl, p-iodophenyl,
4-fluoro-3-chlorophenyl, 4-f_luoro-3,5-dimethylphenyl,
2-fluoro-4-bromophenyl, 2,5-difluoro-4-bromophenyl,
2,4-dichloro-5-methylphenyl, 3-bromo-6-methoxyphenyl,
3-chloro-6-methoxyphenyl, 2-methoxy-5-methylphenyl,
2,4,6-triisopropylphenyl, nap:rithyl, 1,3-benzodioxol-5-
yl, 1,4-benzodioxan-6-yl, benzothiadiazol-5-yl or
benzoxadiazol-5-yl.
Ar is furthermore preferably 2- or 3-furyl, 2- or 3-
thienyl, 1-, 2- or 3-pyrrolyl, 1-, 2-, 4- or 5-
imidazolyl, 1-, 3--, 4- or 5--pyrazolyl, 2-, 4- or 5-
oxazolyl, 3-, 4- or 5-isoxazolyl, 2-, 4- or 5-
thiazolyl, 3-, 4- or 5-isot:hiazolyl, 2-, 3- or 4-
pyridyl, 2-, 4-, 5- or 6--pyrimidinyl, furthermore
preferably 1,2,3-triazol-l-, -4- or -5-yl, 1,2,4-
triazol-l-, -3- or -5-yl, 1- or 5-tetrazolyl, 1,2,3-
oxadiazol-4- or -5-yl, 1,2,4-oxadiazol-3- or -5-yl,
1,3,4-thiadiazol-2- ;or -5-yl, 1,2,4-thiadiazol-3- or
-5-yl, 1,2,3-thiadiazol-4- or -5-yl, 2-, 3-, 4-, 5- or
6-2H-thiopyranyl, 2-, 3- or 4--4-H-thiopyranyl, 3- or 4-
pyridazinyl, pyrazinyl, 2-, 3-, 4-, 5-, 6- or
7-benzofuryl, 2-, 3-, 4-, 5-, 6- or 7-benzothienyl, 1-,
2-, 3-, 4-, 5-, 6- or 7-indolyl, 1-, 2-, 4- or
5-benzimidazolyl, 1-, 3-, 4-, 5-, 6- or 7-
benzopyrazolyl, 2-, 4-, 5-, 6- or 7-benzoxazolyl, 3-,
CA 02280727 1999-08-11
- 11 --
4-, 5-, 6- or 7-benzisoxazolyl, 2-, 4-, 5-, 6- or 7-
benzthiazolyl, 2-, 4-, 5-, 6- or 7-benzisothiazolyl,
4-, 5-, 6- or 7-benz-2,1,3-oxadiazolyl, 2-, 3-, 4-, 5-,
6-, 7- or 8-quinolyl, 1-, 3-, 4-, 5-, 6-, 7- or
8-isoquinolyl, 3-, 4-, 5-, 6-, 7- or 8-cinnolinyl, 2-,
4-, 5-, 6-, 7- or 8-quinazolinyl.
Arylene has the same meanings as indicated for
Ar with the proviso that a. further bond from the
aromatic system is linked to the nearest bonding
neighbour.
Heterocycloalkyl is preferably 1,2-, 2,3- or
1,3-pyrrolidinyl, 1,2-, 2,4-, 4,5- or 1,5-imidazol-
idinyl, 1,2-, 2,3- or 1,3-pyrazolidinyl, 2,3-, 3,4-,
4,5- or 2,5-oxazolidinyl, 1,,2-, 2,3-, 3,4- or 1,4-
isoxazolidinyl, 2,3-, 3,4-, 4,5- or 2,5-thiazolidinyl,
2,3-, 3,4-, 4,5- or 2,5-isothiazolidinyl, 1,2-, 2,3-,
3,4- or 1,4-piperidinyl, 1,4- or 1,2-piperazinyl,
furthermore preferably 1,2,3-tetrahydrotriazol-l,2- or
-1,4-yl, 1,2,4-tetrahydrotriazol-1,2- or 3,5-yl, 1,2-
or 2,5-tetrahydrotetrazolyl, 1,2,3-tetrahydrooxadiazol-
2,3-, -3,4-, -4,5- or -].,5-yl, 1,2,4-tetrahydro-
oxadiazol-2,3-, -3,4- or -4,5-yl, 1,3,4-tetrahydro-
thiadiazol-2,3-, -3,4-, -4,5- or -1,5-yl, 1,2,4-
tetrahydrothiadiazol-2,3-, -3,4-, -4,5- or -1,5-yl,
1,2,3-thiadiazol-2,3-, -3,4-, -4,5- or -1,5-yl, 2,3- or
3,4-morpholinyl, 2,3-, 3,4- or 2,4-thiomorpholinyl.
R6 is a mono- or binuclear heterocycle,
preferably 2- or 3-furyl, 2- or 3-thienyl, 1-, 2- or
3-pyrrolyl, 1-, 2-, 4- or 5-imidazolyl, 1-, 3-, 4- or
5-pyrazolyl, 2-, 4- or 5-oxazolyl, 3-, 4- or 5-
isoxazolyl, 2-, 4- or 5-thiazolyl, 3-, 4- or 5-
isothiazolyl, 2-, 3- or 4-pyridyl, 2-, 4-, 5- or 6-
pyrimidinyl, furthermore preferably 1,2,3-triazol-l-,
-4- or -5-yl, 1,2,4-triazol-l-, -3- or -5-yl, 1- or 5-
tetrazolyl, 1,2,3-oxadiazol-4- or -5-yl, 1,2,4-
oxadiazol-3- or -5-yl, 1,3,4-thiadiazol-2- or -5-yl,
1,2,4-thiadiazol-3- or -5-y:L, 1,2,3-thiadiazol-4- or
-5-yl, 2-, 3-, 4-, 5- or 6-2H:-thiopyranyl, 2-, 3- or 4-
4-H-thiopyranyl, 3- or 4-pyridazinyl, pyrazinyl, 2-,
CA 02280727 1999-08-11
- 12 -
3-, 4-, 5-, 6- or 7-benzofury:L, 2-, 3-, 4-, 5-, 6- or
7-benzothienyl, 1-, 2-, 3-, 9:-, 5-, 6- or 7-indolyl,
1-, 2-, 4- or 5-benzimidazolyl, 1-, 3-, 4-, 5-, 6- or
7-benzopyrazolyl, 2-, 4-, 5-, 6- or 7-benzoxazolyl, 3-,
4-, 5-, 6- or 7-benzisoxazolyl, 2-, 4-, 5-, 6- or 7-
benzthiazolyl, 2-, 4-, 5-, 6- or 7-benzisothiazolyl,
4-, 5-, 6- or 7-benz-2,1,3-oxadiazolyl, 2-, 3-, 4-, 5-,
6-, 7- or 8-quinolyl, 1-, 3-, 4-, 5-, 6-, 7- or 8-
isoquinolyl, 3-, 4-, 5-, 6-, 7- or 8-cinnolinyl, 2-,
4-, 5-, 6-, 7- or 8-quinazolinyl.
The heterocyclic radicals can also be partly or
completely hydrogenated.
R6 can thus also be, for example, 2,3-dihydro-2-, -3-,
-4- or -5-furyl, 2,5-dihydro-2-, -3-, -4- or -5-furyl,
tetrahydro-2- or -3-furyl, 1,3-dioxolan-4-yl,
tetrahydro-2- or -3-thienyl, 2,3-dihydro-l-, -2-, -3-,
-4- or -5-pyrrolyl, 2,5-dihydro-i-, -2-, -3-, -4- or
-5-pyrrolyl, 1-, 2- or 3-pyrrolidinyl, tetrahydro-l-,
-2- or -4-imidazolyl, 2,3-dihydro-l-, -2-, -3-, -4- or
-5-pyrazolyl, tetrahydro-l-, -3- or -4-pyrazolyl, 1,4-
dihydro-l-, -2-, -3- or -4-pyridyl, 1,2,3,4-tetrahydro-
1-, -2-, -3-, -4-, -5- or -6-pyridyl, 1-, 2-, 3- or 4-
piperidinyl, 2-, 3- or 4-morpholinyl, tetrahydro-2-,
-3- or -4-pyranyl, 1,4-dioxanyl, 1,3-dioxan-2-, -4- or
-5-yl, hexahydro-l-, -3- or -4-pyridazinyl, hexahydro-
1-, -2-, -4- or -5-pyrimidinyl, 1-, 2- or 3-
piperazinyl, 1,2,3,4-tetrahydro-1-, -2-, -3-, -4-, -5-,
-6-, -7- or -8-quinolyl, 1,2,3,4-tetrahydro-l-, -2-,
-3-, -4-, -5-, -6-, -7- or -8-isoquinolyl.
The said heterocyclic radicals may also be
substituted once, twice or three times by Hal, A, -CO-
A, OH, CN, COOH, COOA, CONH21 NOZ, =NH or =0.
R6 is very particularly 1H-imidazol-2-yl,
thiazol-2-yl, 1H-benzimidazol-2-yl, 2H-pyrazol-2-yl,
1H-tetrazol-5-yl, 2-iminoimidazolidin-4-on-5-yl,
i-alkyl-1,5-dihydroimidazol-4--on-2-yl, pyrimidin-2-yl
or 1,4,5,6-tetrahydropyrimidiii-2-yl.
R11 is H or alkyl with 1-6 C atoms, prefer-
ably H.
CA 02280727 1999-08-11
- 13 -
Accordingly the invention particularly relates
to those compounds of the formula I in which at least
one of the said radicals has one of the preferred
meanings stated above. Some preferred groups of
compounds can be represented by the following part-
formulae Ia to Ig which corres.oond to the formula I and
in which the undefined radicals have the meanings
stated for formula I, but in which
in Ia) R1 is H or alkyl with 1-6 C atoms,
R2 is R10, CO-R10, COOR10 or S02R10,
R3 is H,
R4 is H or =0,
R5 is HzN-C (=NH ) or H2N-C (=NH )-NH,
W, Z are each independently of one
another abserit, C(=0), NH, CONH or
NHCO,
X is -NH-, 0 or. -CH2-,
Y is NH or 0,
Rlo is H, A or benzyl,
R11 is H,
A is unsubstituted alkyl or cycloalkyl
with 1-15 C atoms and
m, n are each independently of one another
0, 1 or 2;
in Ib) R1 i.s H or alky:L with 1-6 C atoms,
RZ is R10, CO-R10, COOR10 or S02R10,
R3 is H,
R4 i.s H or =0,
RS is R6,
W, Z are each independently of one another
absent, C(=0), NH, CONH or NHCO,
X is -NH-, 0 or -CH2-,
Y is NH or 0,
R6 is a mono- or binuclear heterocycle
which has 1-4 N, 0 and/or S atoms and
which can be unsubstituted or mono-,
di- or trisubstituted by Hal, A,
CA 02280727 1999-08-11
- 14 -
-CO-A, OH, CN, COOH, COOA, CONH2, NO2,
=NH or =0,
R10 is H, A or benzyl,
R11 is H,
A is unsubstituted alkyl or cycloalkyl
with 1-15 C atoms and
m, n are each independently of one another
0, 1 or 2;
in Ic) R1 is H or a1ky:L with 1-6 C atoms,
RZ is R10, CO-R1 , COOR10 or S02R10,
R3 is H,
R4 is H or =0,
R5 is HZN-C (=NH) or H2N-C (=NH) -NH,
W, Z are each indi=_pendently of one another
absent, C(=O), NH, CONH or NHCO,
X is -NH-, 0 or -CH2-,
Y is NH or 0.
A is alkyl wit:z 1-6 C atoms,
Rlo is H, alkyl with 1-6 C atoms,
camphor-l0-yl or benzyl,
R11 is H,
m, n are each independently of one another
C), 1 or 2;
in Id) R' is H or alkyl with 1-6 C atoms,
RZ is R10, CO-R10, COOR10 or SO2R10,
R3 is H,
R4 is H or =0,
R5 is R6,
W, Z are each independently of one another
absent, C(=0), NH, CONH or NHCO,
X is =NH-, 0 or -CH2-,
Y is NH or 0,
R6 is a mono- cr binuclear heterocycle
which has 1-4 N, 0 and/or S atoms and
which can be unsubstituted or mono-,
di- or trisubstituted by Hal, A,
-CO-A, OH, C:N, COOH, COOA, CONH2,
CA 02280727 1999-08-11
- 15 -
NOZ, =NH or =0,
R10 is H, alkyl with 1-4 C atoms, camphor-
10-yl or benzyl,
R11 is H,
A is unsubstitiited alkyl with 1-6 C
atoms and
m, n are each independently of one another
0, 1 or 2;
in Ie) R1 is H or alky:L with 1-6 C atoms,
R2 i.s R10, CO-R10, COOR10 or SO2R10,
R3 is H,
R4 is H or =0,
RS i.s R6,
W, Z are each independently of one another
absent, C(=0), NH, CONH or NHCO,
X is -NH-, 0 or -CH2-,
Y is NH or 0,
R6 is 1H-imidazol-2-yl, thiazol-2-yl,
1H-benzimidazol-2-yl, 2H-pyrazol-
2-yl, 1H-tetrazol-5-yl, 2-imino-
imidazolidin-4-on-5-yl, 1-A-1,5-
dihydro-imidazol-4-on-2-yl, pyrim-
idin-2-yl or 1,4,5,6-tetrahydro-
pyrimidin-2-yl,
R1 is H, alkyl with 1-4 C atoms, camphor-
LO-yl or benzyl,
R11 is H,
A is unsubstituted alkyl with 1-6 C
atoms and
m, n are each inclependently of one another
0, 1 or 2;
in If) R1 is H or alkyl with 1-6 C atoms,
RZ is R10, CO-R~LO, COOR10 or S02R10,
R3 is H,
R4 is H or =0,
R5 is H2N-C (=NH ) or H2N-C (=NH )-NH,
W, Z are each independently of one another
CA 02280727 2005-11-04
26474-452
- 16 -
absent, C(=0), NH, CONH or NHCO,
X is -NH-, 0 or -CHZ-,
Y is NH or 0,
R10 is Ar,
Ril is H,
A is unsubstituted alkyl or cycloalkyl
with 1-15 C atoms and
m, n are each independently of one another
0, 1 or 2;
in Ig) R' is H or alkyl with 1-6 C atoms,
R2 is R10, CO-R10, COORlO or S02R10,
R3 is H,
R4 is H or =0,
R5 is R6,
W, Z are each independently of one another
absent, C(=O), NH, CONH or NHCO,
X is -NH-, 0 or -CH2-,
Y is NH or O,
R6 is a mono- or binuclear heterocycle
which has 1-4 N, 0 and/or S atoms
and which can be unsubstituted or
mono-, di- or trisubstituted by Hal,
A, -CO-A, OH, CN, COOH, COOA, CONH2,
N02, =NH or =0,
R10 is Ar,
R11 is H,
A is unsubstituted alkyl or cycloalkyl
with 1-15 C atoms and
m, n are each independently of one another
0, 1 or 2.
The compounds of the formula I and the starting
materials for preparing them are moreover prepared by
methods known per se, as described in the literature
(for example in the stardard works such as Houben-Weyl,
Methoden der organischen Chemie [Methods of Organic Chemistry],
Georg-Thieme-Verlag, Stuttgart, 4u' edition, 1952 to 1986),
specifically under reaction conditions known and suitable for
CA 02280727 1999-08-11
- 17 -
the said reactions. It is also possible for this
purpose to make use of variants which are known per se
but which are no mentioned in detail here.
The starting materials can, if required, also
be formed in situ so that they are not isolated from
the reaction mixture but immediately reacted further to
give the compounds of the formula I.
Compounds of the formula I can preferably be
obtained by liberating compounds of the formula I from
one of the functional derivatives thereof by treatment
with a solvolysing or hydrogenolysing agent.
Preferred starting materials for the solvolysis
or hydrogenolysis are those which otherwise correspond
to the formula I but comprise in place of one or more
free amino and/or hydroxy:L groups corresponding
protected amino and/or hydroxyl groups, preferably
those which have an amino protective group in place of
an H atom bonded to an N atom, especially those which
have an R'-N group in place of an HN group, in which R'
is an amino protective group, and/or those which have a
hydroxyl protective group in place of the H atom of a
hydroxyl group, for example those which correspond to
the formula I but have a group -COOR" in place of a
group -COOH, in which R" is a hydroxyl protective
group.
It is also possible for a plurality of
identical or different protected amino and/or hydroxyl
groups to be present in the molecule of the starting
material. If the protecti've groups present are
different from one another, they can in many cases be
eliminated selectively.
The term "amino protective group" is generally
known and refers to groups which are suitable for
protecting (blocking) an amino group from chemical
reactions but which can easily be removed after the
required chemical reaction elsewhere in the molecule
has been carried out. Typical groups of this type are,
in particular, unsubstituted or substituted acyl, aryl,
aralkoxymethyl or aralkyl groups. Since the amino
--- ----- ------
CA 02280727 1999-08-11
- 18 --
protective groups are removed after the required
reaction (or sequence of reac:tions), their nature and
size are not otherwise critical; however, those with 1-
20, in particular 1-8, C atoms are preferred. The term
"acyl group" is to be interpreted in the widest sense
in connection with the present process. It embraces
acyl groups derived from aliphatic, araliphatic,
aromatic or heterocyclic carboxylic acids or sulfonic
acids and, in particular, alkoxycarbonyl,
aryloxycarbonyl and, especially, aralkoxycarbonyl
groups. Examples of such acyl groups are alkanoyl such
as acetyl, propionyl, butyryl; aralkanoyl such as
phenylacetyl; aroyl such as benzoyl or toluyl;
aryloxyalkanoyl such as POA; alkoxycarbonyl such as
methoxycarbonyl, ethoxycarbonyl, 2,2,2-trichloroethoxy-
carbonyl, BOC, 2-iodoethoxycarbonyl; aralkyloxycarbonyl
such as CBZ ("carbobenzoxy"), 4-methoxybenzyloxy-
carbonyl, FMOC; arylsulfonyl such as Mtr. Preferred
amino protective groups are BOC and Mtr, also CBZ,
FMOC, benzyl and acetyl.
The amino protective group is eliminated,
depending on the protective group used, for example
with strong acids, preferably with TFA or perchloric
acid, but also with other strong inorganic acids such
as hydrochloric acid or sulfuric acid, strong organic
carboxylic acids such as trichloroacetic acid or
sulfonic acids such as benzene- or p-toluenesulfonic
acid. The presence of an additional inert solvent is
possible but not always necessary. Suitable and
preferred inert solvents are organic, for example
carboxylic acids such as acetic acid, ethers such as
tetrahydrofuran or; dioxane, amides such as DMF,
halogenated hydrocarbons such as dichloromethane, also
alcohols such as methanol, ethanol or isopropanol, and
water. Mixtures of the abovementioned solvents are also
suitable. TFA is preferably used in excess without
addition of another solvent, and perchloric acid is
used in the form of a mixture of acetic acid and 70%
perchloric acid in the ratio 9:1. The reaction
CA 02280727 1999-08-11
- 19 -temperatures for the cleavage are preferably between
about 0 and about 50 , preferably between 15 and 30
(room temperature).
The BOC, OBut and Mtr groups can be eliminated,
for example, preferably with '.CFA in dichloromethane or
with approximately 3 to 5N HC1 in dioxane at 15-30 ,
and the FMOC group can be eliminated with an
approximately 5 to 50% solution of dimethylamine,
diethylamine or piperidine in DMF at 15-30 .
Protective groups which can be removed by
hydrogenolysis (for example CBZ or benzyl) can be
eliminated, for example, by treatment with hydrogen in
the presence of a catalyst (for example of a noble
metal catalyst such as palladium, preferably on a
support such as carbon). Suitable solvents in this case
are those indicated above, in particular, for example,
alcohols such as methanol or ethanol or amides such as
DMF. The hydrogenolysis is, as a rule, carried out at
temperatures between about 0 and 100 under pressures
between about 1 and 200 bar, preferably at 20-30 under
1-10 bar. Hydrogeriolysis of the CBZ group takes place
satisfactorily, for example, on 5 to 10% Pd/C in
methanol or with ammonium formate (in place of
hydrogen) on Pd/C in methanol/DMF at 20-30 .
Compounds of the formula I can preferably be
obtained by reacting compounds of the formula II with
compounds of the formula III. The starting compounds of
the formula II and III are, as a rule, novel. However,
they can be prepared by methods known per se.
In the compounds of the formula III, L is
preferably Cl, Br, I or a reactively modified OH group
such as alkylsulfonyloxy with 1-6 C atoms (preferably
methylsulfonyloxy) or arylsulfonyloxy with 6-10 C atoms
(preferably phenyl- or p-toly:Lsulfonyloxy).
The compounds of the formula II are, as a rule, reacted
in an inert solvent in the presence of an acid-binding
agent, preferably of an organic base such as
triethylamine, dimethylaniline, pyridine or quinoline.
CA 02280727 1999-08-11
- 20 -
It may also be beneficial to add an alkali metal or
alkaline earth metal hyciroxide, carbonate or
bicarbonate or another salt of a weak acid of the
alkali metals or alkaline earth metals, preferably of
potassium, sodium, calcium or caesium.
The reaction time depends on the conditions applied and
is between a few minutes and :14 days, and the reaction
temperature is between about -30 and 140 , normally
between -10 and 90 , in particular between about 0
and about 700.
Examples of suitable inert solvents are
hydrocarbons such as hexane, petroleum ether, benzene,
toluene or xylene; chlorinated hydrocarbons such as
trichloroethylene, 1,2-dichloroethane, carbon tetra-
chloride, chloroform or dichloromethane; alcohols such
as methanol, ethanol, isopropanol, n-propanol,
n-butanol or tert-butanol; ethers such as diethyl
ether, diisopropyl ether, t:etrahydrofuran (THF) or
dioxane; glycol ethers such as ethylene glycol
monomethyl or monoethyl ether (methylglycol or
ethylglycol), ethylene glycol dimethyl ether (diglyme);
ketones such as acetone or butanone; amides such as
acetamide, dimethylacetamide or dimethylformamide
(DMF); nitriles such as acetonitrile; sulfoxides such
as dimethyl sulfoxide (DNISO); carbon disulfide;
carboxylic acids such as formic acid or acetic acid;
nitro compounds such as nitromethane or nitrobenzene;
esters such as ethyl acetate, water or mixtures of the
solvents mentioned.
It is furthermore possible to hydrolyse an
ester of the formula I. This is preferably carried out
by solvolysis or hydrogenolys:Ls as indicated above, for
example with NaOH or KOH in dioxane/water at
temperatures between 0 and 60 C, preferably between 10
and 40 C.
It is furthermore possible to convert a radical
R1 and/or RS into another radical R1 and/or R5. In
particular, it is possible to convert a carboxylic acid
into a carboxylic ester.
CA 02280727 1999-08-11
- 21 -
A cyano group is converted into an amidino group by
reaction with, for example, hydroxylamine and
subsequent reduction of the N-hydroxyamidine with
hydrogen in the presence of a catalyst such as, for
example, Pd/C.
It is furthermore possible to replace a conventional
amino protective group by hydrogen by eliminating the
protective group by solvolysis or hydrogenolysis as
described above, or by liberating an amino group
protected by a conventional protective group by
solvolysis or hydrogenolysis.
A base of the formula I can be converted with
an acid into the relevant acid addition salt, for
example by reacting equivaleni: amounts of the base and
of the acid in an inert solvent such as ethanol and
subsequently evaporating. Acids particularly suitable
for this reaction are those which provide
physiologically acceptable salts. Thus, it is possible
to use inorganic acids, for example sulfuric acid,
nitric acid, hydrohalic acids such as hydrochloric acid
or hydrobromic acid, phosphoric acids such as
orthophosphoric acid, sulfamic acid, also organic
acids, in particular aliphatic, alicyclic, araliphatic,
aromatic or heterocyclic mono-- or polybasic carboxylic,
sulfonic or sulfuric acids, for example formic acid,
acetic acid, propionic acid, pivalic acid,
diethylacetic acid, malonic: acid, succinic acid,
pimelic acid, fumaric acid, maleic acid, lactic acid,
tartaric acid, malic acid, citric acid, gluconic acid,
ascorbic acid, riicotinic acid, isonicotinic acid,
methane- or ethanesulfonic acid, ethanedisulfonic acid,
2-hydroxyethanesulfonic acid, benzenesulfonic acid, p-
toluenesulfonic ac:id, naphtha.lenemono- and -disulfonic
acids, lauryl sulfuric acid. Salts with physiologically
unacceptable acids, for example picrates, can be used
for isolating and/or purifying the compounds of the
formula I.
On the other hand, an acid of the formula I can
be converted by reaction with a base into one of its
CA 02280727 1999-08-11
- 22 -
physiologically acceptable metal or ammonium salts.
Suitable salts in this connection are, in particular,
the sodium, potassium, magnesium, calcium and ammonium
salts, also substituted ammonium salts, for example the
dimethyl-, diethyl- or diisopropylammonium salts,
monoethanol-, diethanol- cr diisopropanolammonium
salts, cyclohexyl, dicyclohexylammonium salts,
dibenzylethylenediammonium salts, furthermore, for
example, salts with arginine or lysine.
The compounds of the formula I contain one or
more chiral centres and may therefore exist in racemic
or in optically active form. Resulting racemates can be
resolved mechanically or chemically by methods known
per se into the enantiomers. Preferably, diastereomers
are formed from the racemic mixture by reaction with an
optically active resolving agent. Examples of suitable
resolving agents are optically active acids such as the
D and L forms of tartaric acid, diacetyltartaric acid,
dibenzoyltartaric acid, manclelic acid, malic acid,
lactic acid or the various optically active
camphorsulfonic acids such as P-camphorsulfonic acid.
An advantageous enantiomer resolution also makes use of
a column packed with an optically active resolving
agent (for example dinitrobenzoylphenylglycine); an
example of a suitable mobile phase is a
hexane/isopropanol/acetonitril.e mixture, for example in
the ratio 82:15:3 by volume.
It is, of course, also possible to obtain
optically active compounds of the formula I by the
methods described above by using starting materials
which are already optically active.
The invention furtherinore relates to the use of
the compounds of the formula I and/or the
physiologically acceptable salts thereof for producing
pharmaceutical compositions, in particular by non-
chemical means. For this purpose they can be converted
together with at least orie solid, liquid and/or
semiliquid excipient or ancillary substance and, where
CA 02280727 1999-08-11
- 23 -
appropriate, in combination with one or more other
active ingredients into a suitable dosage form.
The invention furthermore relates to
pharmaceutical compositions comprising at least one
compound of the formula I and/or one of the
physiologically acceptable salts thereof.
These compositions can be used as
pharmaceuticals in human or veterinary medicine.
Suitable excipients are organic or inorganic substances
which are suitable for enteral (for example oral),
parenteral, topical administration or for
administration in the form of an inhalation spray and
which do not react with the novel compounds, for
example water, vegetable oils, benzyl alcohols,
alkylene glycols, polyethyl-ene glycols, glycerol
triacetate, gelatin, carbohydrates such as lactose or
starch, magnesium stearate, talc, petrolatum. Used for
oral administration are, in particular, tablets, pills,
coated tablets, capsules, powders, granules, syrups,
solutions or drops, for rectal administration are
suppositories, for parenteral administration are
solutions, preferably oily or aqueous solutions, also
suspensions, emulsions or implants, and for topical
administration are ointments, creams or dusting
powders. The novel compounds can also be lyophilized,
and the resulting lyophilisates can be used, for
example, for producing products for injection. The
stated compositions can be sterilized and/or comprise
ancillary substances such as lubricants, preservatives,
stabilizers and/or wetting agents, emulsifiers, salts
to influence the osmotic pressure, buffer substances,
colourants, flavourings and/or several other active
ingredients, for example one or more vitamins.
The sprays which can be used for administration as
inhalation spray comprise the active ingredient either
dissolved or suspended in a propellant gas or mixture
of propellant gases (for example CO2 or chlorofluoro-
carbons). In this case, the active ingredient is
preferably used in micronized form, and it is possible
CA 02280727 1999-08-11
- 24 -
for one or more additional physiologically tolerated
solvents to be present, for example ethanol. Solutions
for inhalation can be administered using conventional
inhalers.
The compounds of the formula I and their
physiologically acceptable salts can be used as
integrin inhibitors for controlling diseases, in
particular pathologically angiogenic disorders,
thromboses, myocardial infarct, coronary heart disease,
arteriosclerosis, tumours, inflammations and
infections.
Compounds of the formula I according to Claim 1 and/or
their acceptable salts in which R 2 is camphor-l0-yl are
preferred for controlling pathologically angiogenic
disorders, tumours, osteoporosis, inflammations and
infections.
In this connection it is possible for the
substances according to the invention to be
administered, as a rule, in analogy to other known and
commercially available peptides, but especially in
analogy to the compounds described in US-A 4 472 305,
preferably in dosages between about 0.05 and 500 mg, in
particular between 0.5 and 100 mg per dosage unit. The
daily dose is preferably between about 0.01 and 2 mg/kg
of body weight. The specific dose for each patient
depends, however, on a wide variety of factors, for
example on the activity of the specific compound
employed, on the age, body weight, general state of
health, sex, on the diet, on the time and route of
administration, on the rate of excretion, combination
of medicinal substances and severity of the particular
disorder for which the therapy is applied. Parenteral
administration is preferred.
All temperatures hereinbefore and hereinafter
are stated in C. In the following examples, "usual
workup" means: i.f necessary, water is added, if
necessary, depending on the constitution of the final
product, the pH is adjusted to between 2 and 10,
extraction is carried out with ethyl acetate or
CA 02280727 1999-08-11
- 25 --
dichloromethane, and the organic phase is separated
off, dried over sodium sulfate, evaporated and purified
by chromatography on silica gel and/or by
crystallization.
Mass spectrometry (MS): EI (electron impact
ionization) M+
FAB (fast atom bombardment)
(M+H)+
Example 1
A solution of 12 g of BOC-3-nitro-L-tyrosine
benzyl ester ("1") in 200 ml of THF is hydrogenated in
the presence of 1 g of Raney riickel at room temperature
under atmospheric pressure for 6 hours. The catalyst is
removed, and the usual workup results in 11.7 g of BOC-
3-amino-L-tyrosine benzyl ester ("2"), FAB 387.
A solution of 9.3 g of "2", 2.36 g of maleic anhydride
and 3.3 ml of triethylamine in 150 ml of DMF is heated
to 80 and then stirred for 12 hours. The solvent is
removed and the residue is chromatographed on silica
gel with dichloromethane/meth,anol 20:1-10:1 as eluent.
5.1 g of benzyl (2S)-2-tert-b,.ityloxycarboxamido-3-(3,4-
dihydro-2-carboxymethyl-2H-1,4-benzoxazin-3-on-6-yl)-
propionate are obtained as diastereomer mixture ("3"),
FAB 485.
1 g of Z-guanidine and 1.75 ml of ethyldiiso-
propylamine are added to a solution of 1 g of "3" and
0.79 g of 2-chloro-1-methylpyridinium iodide in 20 ml
of DMF, and the mixture is stirred at room temperature
for 12 hours. The usual workup results after
chromatography on silica gel (toluene/methanol 10:1) in
0.2 g of benzyl (2S)-2-tert-butyloxycarboxamido-3-[3,4-
dihydro-2-(2-benzyloxycarbonyl.guanidino-2-oxoethyl)-2H-
1,4-benzoxazin-3-on-6-yl]propionate ("4"), FAB 660.
A solution of 200 mg of "4" and 3 ml of
water/3 ml of dioxane is hydrogenated in the presence
of 100 mg of palladium (10% on active carbon) at RT
under atmospheric pressure. '.Che pH is kept at 4-5 by
CA 02280727 1999-08-11
- 26 --
adding 1N HC1. The catalyst and the solvent are
removed. The residue is purified by preparative HPLC
(RP-18 with acetoni-trile/water + 0.3% TFA gradient from
1:80 to 99:1 in one hour) to result in 40 mg of (2S)-2-
tert-butyloxycarboxamido-3-[3,4-dihydro-2-(2-guanidino-
2-oxoethyl)-2H-1,4-benzoxazin-3-on-6-yl]pro-pionic acid
("5"), trifluoroacetate, FAB 660.
Example 2
2.3 g of potassium carbonate are added to a
solution of 6 g of Z-L-DOPA ethyl ester ("6") in 25 ml
of ethanol and 25 ml of water under protective gas. The
mixture is heated to 60 , 4.5 ml of epibromohydrin are
added, and the mixture is heated to 90 . After stirring
for 2 hours and the usual workup, the crude product is
purified on silica gel. 5.6 g of a mixture ("8") of the
diastereomeric pairs of positional isomers, which
cannot be separated, are obtained:
ethyl (2S)-2-benzyloxycarboxaniido-3-(3-(3R,3S)-hydroxy-
methyl-1,4-benzodioxan-6-yl)propionate ("7a") and
ethyl (2S)-2-benzyloxycarboxamido-3-(2-(2R,2S)-hydroxy-
methyl-1,4-benzodioxan-6-yl)propionate ("7b"), FAB 416.
0.413 ml of methanesulfonyl chloride is added
to a solution of 2 g of "8" in 30 ml of pyridine at 0
and, after stirring for 2 hours, the usual workup is
carried out. 2.2 g of ethy:L (2S)-2-benzyloxycarbox-
amido-3-(2/3-methyl.sulfonyloxymethyl-1,4-benzodioxan-6-
yl)propionate ("9") are obtained, FAB 494.
A solution of 1.6 g of "9", 1.6 g of sodium
azide and 30 ml of DMF is stirred at 75 for 12 hours.
The usual workup results in ethyl (2S)-2-benzyl-
oxycarboxamido-3-(2/3-azidomet:nyl-l,4-benzodioxan-6-yl)
propionate ("10"), FAB 441.
3.4 ml of 1N sodium hydroxide solution are
added to a solution of 1.25 g of "10" and 25 ml of
methanol and stirred at room temperature for 12 hours.
The usual workup results in 1.3 g of (2S)-2-benzyloxy-
carboxamido-3-(2/3-azidomethyl-1,4-benzodioxan-6-yl)-
propionic acid ("11"), FAB 413.
CA 02280727 1999-08-11
- 27 -
Hydrogen sulfide is passed into a solution of
1.3 g of "11" in 40 ml of pyridine and 20 ml of water
at room temperature for 30 mir.., and it is then left to
stand for 12 hours. Removal of the solvent results in
1.5 g of (2S)-2-benzyloxycarboxamido-3-(2/3-amino-
methyl-l,4-benzodioxan-6-yl)propionic acid ("12"), FAB
387.
A solution of 0.3 g of "12", 0.23 g of 3,5-
dimethylpyrazole-l-formamidiniiim nitrate (DPFN) and
0.22 ml of triethylamine in 10 ml of DMF is stirred at
60 for 12 hours. The usual workup with preparative
HPLC (conditions analogous to Example 1 for purifying
"5") results in separation of the 2-guanidinomethyl
compounds from the 3-guanidinornethyl compounds.
Yield: 80 mg of (2S)-2-benzyloxycarboxamido-3-(2-
(2R,S)-guanidinomethyl-1,4-benzodioxan-6-yl)propionic
acid ("13"), FAB 429.
Example 3
A solution of 0.95 g of BOC-glycine and 0.96 g
of carbonyldiimidazole in 20 ml of THF is stirred for
2 hours. Then 0.7 g of "12" is added and the mixture is
stirred for 12 hours. The usual workup results in
0.66 g of (2S)-2-benzyloxycarboxamido-3-(2/3-tert-but-
yloxycarboxamidoacetamidomethyl-1,4-benzodioxan-6-yl)-
propionic acid ("14"), FAB 544.
0.5 ml of trifluoroacetic acid is added to a
solution of 0.15 g of "14" in 5 ml of dichloromethane
and stirred for 8 hours. After removal of the solvent,
10 ml of DMF are added, followed by 80 mg of DPFN and
70 l of triethylamine. The r:tixture is heated to 80
and stirred for 12 hours. Purification and
fractionation of the 2/3 isomers take place by
preparative HPLC in analogy to Example 1.
42 mg of (2S)-2-benzyloxycarboxamido-3-(2-guanidino-
acetamidomethyl-l,4-benzodioxan-6-yl)propionic acid
("15") are obtained, FAB 486.
Example 4
Hydrogen is passed for 2 hours through a
solution of 0.45 g of "14" in 10 ml of dioxane and 5 ml
CA 02280727 1999-08-11
- 28 -
of water in the presence of 0.2 g of palladium (10% on
active carbon). Removal of the catalyst and the usual
workup result in 0.28 g of (2S)-2-amino-3-(2/3-tert-
butyloxycarboxamidoacetamidomet:hyl-1,4-benzodioxan-6-
yl)propionic acid ("16"), FAB 410.
430 l of N,O-bis(trimethylsilyl)trifluoro-
acetamide (BSTFA) are added to a solution of 0.28 g of
"16" in 5 ml of acetonitrile, and the mixture is then
boiled under reflux for 3 hours. Then 66 l of pyridine
and 0.188 g of R-camphor-10-sulfonyl chloride are
added, and the mixture is stirred at 70 for 3 hours.
The usual workup results iri 0.26 g of (2S)-2-(R)-
camphorsulfonamido-3-(2/3-tert--butyloxycarboxamido-
acetamidomethyl-1,4-benzodioxari-6-yl)propionic acid
("17"), FAB 624.
Elimination of the BOC group from 0.25 g of
"17" and guanylation in analogy to the preparation of
"15" result in 58 mg of (2S)-=2-(R)-camphorsulfonamido-
3-(2-guanidinoacetamidomethyl-L,4-benzodioxan-6-yl)pro-
pionic acid ("18"), FAB 566.
Analogous reaction of "16"
with butylsulfonyl chloride results in
(2S)-2-butylsulfonamido-3-(2/3-tert-butyloxy-
carboxamidoacetamidomethyl-1,4-benzodioxan-6-yl)prop-
ionic acid;
with 4-tolylsulfonyl chloride results in
(2S)-2-(4-tolylsulfonamido)-3-(2/3-tert-butyl-
oxycarboxamidoacetamidomethyl-l,4-benzodioxan-6-yl)-
propionic acid;
with benzylsulfonyl chloride results in
(2S)-2-benzylsulfonamido-3-(2/3-tert-butyloxy-
carboxamidoacetamidomethyl-1,4-benzodioxan-6-yl)prop-
ionic acid;
with phenylsulfonyl chloride results in
CA 02280727 1999-08-11
- 29 -
(2S)-2-phenylsulfonamido-3-(2/3-tert-butyloxy-
carboxamidoacetamidomethyl-l,4-benzodioxan-6-yl)prop-
ionic acid;
with 2-naphthylsulfonyl chloride results in
(2S)-2-(2-naphthylsulfona:mido)-3-(2/3-tert-butyl-
oxycarboxamidoacetamidomethyl-l,4-benzodioxan-6-yl)-
propionic acid
and with cyclohexylsulfonyl chloride results in
(2S)-2-cyclohexylsulfonamido-3-(2/3-tert-butyl-
oxycarboxamidoacetamidomethyl-l,4-benzodioxan-6-yl)-
propionic acid.
Elimination of the 130C group therefrom and
guanylation result in
(2S)-2-butylsulfonamido-3-(2-guanidinoacetamido-
methyl-l,4-benzodioxan-6-yl)propionic acid;
(2S)-2-(4-tolylsulfonamido-3-(2-guanidinoacet-
amidomethyl-l,4-benzodioxan-6-yl)propionic acid;
(2S)-2-benzylsulfonamido-3-(2-guanidinoacetamido-
methyl-1,4-benzodioxan-6-yl)propionic acid;
(2S)-2-phenylsulfonamido-3-(2-guanidinoacetamido-
methyl-l,4-benzodioxan-6-yl)propionic acid;
(2S) -2- (2-naphthylsulfona;:nido) -3- (2-guanidinoacet-
amidomethyl-l,4-berizodioxan-6-yl)propionic acid and
(2S)-2-cyclohexylsulfonamido-3-(2-guanidinoacet-
amidomethyl-l,4-benzodioxan-6-yl)propionic acid.
Example 5
0.26 g of TBTU, 26 mq of HOBT and 0.34 ml of
N-methylmorpholine are added t:o a solution of 0.3 g of
"3" and 0.248 g of 2-aminoben:zimidazole ("A") in 10 ml
CA 02280727 1999-08-11
- 30 -
of DMF and stirred at room temperature for 12 hours.
The usual workup results in 0.14 g of benzyl (2S)-2-
tert-butyloxycarboxamido-3-{3,4-dihydro-2-[N-(2-benzim-
idazolyl)carbamoylmethyl]-2H-1.,4-benzoxazin-3-on-6-yl)-
propionate ("19").
Hydrogenation of "19" in ana:Logy to Example 1 results
in 60 mg of (2S)-2-tert-butyloxycarboxamido-3-{3,4-di-
hydro-2-[N-(2-benzimidazolyl)c:arbamoylmethyl]-2H-1,4-
benzoxazin-3-on-6-yl}propionic: acid ("22"), FAB 510.
Analogous reaction of "3"
with 2-aminoimidazole ("B") results in
benzyl (2S)-2-tert-butyloxycarboxamido-3-{3,4-di-
hydro-2-[N-(2-imidazolyl)carbamoylmethyl]-2H-1,4-benz-
oxazin-3-on-6-yl}propionate ("20")
and subsequent cleavage of the benzyl ester results in
(2S)-2-tert-butyloxycarboxamido-3-{3,4-dihydro-2-
[N-(2-imidazolyl)carbamoylmethyl]-2H-1,4-benzoxazin-3-
on-6-yl}propionic acid ("23"),, FAB 460;
and with 2-aminomethylbenzimidazole ("C") results in
benzyl (2S)-2-tert-butyloxycarboxamido-3-{3,4-
dihydro-2-[N-(2-benzimidazoly:Lmethyl)carbamoylmethyl]-
2H-1,4-benzoxazin-3-on-6-yl}propionate ("21"),
and subsequent cleavage of the benzyl ester results in
(2S)-2-tert-butyloxycarboxamido-3-{3,4-dihydro-2-
[N-(2-benzimidazolylmethyl)ca:rbamoylmethyl]-2H-1,4-
benzoxazin-3-on-6-yl}propionic acid ("24"), FAB 524.
Example 6
5 ml of TFA are added to a solution of 2 g of
"3" in 50 ml of dichloromethane and stirred at room
temperature for 1 hour. Removal of the solvent results
in 2 g of benzyl (2S)-amino-3-(3,4-dihydro-2-carboxy-
methyl-2H-1,4-benzoxazin-3-on-6-yl)propionate,
trifluoroacetate ("25"), FAB 385.
CA 02280727 1999-08-11
- 31 -
1.2 ml of BSTFA are added to a solution of 1 g
of "25" and 0.3 ml of triethylamine in 25 ml of
acetonitrile and then boiled iinder reflux for 2 hours.
Then, at 401, 0.19 ml of pyridine and 0.55 g of (R)-
camphor-l0-sulfonyl chloride are added, and stirred at
700 for 12 hours. The usual workup results in 0.41 g of
benzyl (2S) -2- [ (R) -camphorsul.fonamido]-3- (3,4-dihydro-
2-carboxymethyl-2H-1,4-benzoxazin-3-on-6-yl)propionate
("26"), FAB 599.
Analogous reaction of "25"
with butylsulfonyl chloride results in
benzyl (2S)-2-butylsulfonamido-3-(3,4-dihydro-2-
carboxymethyl-2H-1,4-benzoxazi:n-3-on-6-yl)propionate;
with 4-tolylsulfonyl chloride :results in
benzyl (2S) -2- (4-to].ylsulfonamido) -3- (3,4-di-
hydro-2-carboxymethyl-2H-1,4-b:nzoxazin-3-on-6-yl)prop-
ionate;
with benzylsulfonyl chloride results in
benzyl (2S)-2-benzylsulf=onamido-3-(3,4-dihydro-2-
carboxymethyl-2H-1,4-benzoxazin-3-on-6-yl)propionate;
with phenylsulfonyl. chloride results in
benzyl (2S)-2-phenylsulfonamido-3-(3,4-dihydro-2-
carboxymethyl-2H-1,4-benzoxazin-3-on-6-yl)propionate;
with 2-naphthylsulfonyl chloride results in
benzyl (2S)-2-(2-naphthylsulfonamido)-3-(3,4-di-
hydro-2-carboxymethyl-2H-1,4-benzoxazin-3-on-6-yl)prop-
ionate
and with cyclohexylsulfonyl chloride results in
benzyl (2S)-2-cyclohexylsulfonamido-3-(3,4-di-
hydro-2-carboxymethyl-2H-1,4-benzoxazin-3-on-6-yl)-
propionate
Reaction of "26" in analogy to Example 5
CA 02280727 1999-08-11
- 32 -
with "A" results in
benzyl (2S)-2-[(R)-camphorsulfonamido]-3-{3,4-
dihydro-2-[N-(2-benzimidazolyl.)carbamoylmethyl]-2H-1,4-
benzoxazin-3-on-6-yl)propionat:e ("27"), FAB 714,
with "B" results in
benzyl (2S)-2-[(R)-camphorsulfonamido]-3-{3,4-
dihydro-2-[N-(2-imidazolyl)carbamoylmethyl]-2H-1,4-
benzoxazin-3-on-6-yl)propionate
and with "C" results in
benzyl (2S)-2-[(R)-camphorsulfonamido]-3-{3,4-
dihydro-2-[N-(2-benzimidazolyl.methyl)carbamoylmethyl]-
2H-1,4-benzoxazin-3-on-6-yl}propionate ("29").
Cleavage of the benzyl ester by hydrogenation
affords
from "27"
(2S)-2-[(R)-camphorsulforiamido]-3-{3,4-dihydro-2-
[N-(2-benzimidazolyl)carbamoyl.methyl]-2H-1,4-benzox-
azin-3-on-6-yl)propionic acid ("28"), FAB 624
and from "29"
(2S)-2-[(R)-camphorsulforiamido]-3-{3,4-dihydro-2-
[N-(2-benzimidazolylmethyl)cai:bamoylmethyl]-2H-1,4-
benzoxazin-3-on-6-yl}propionic: acid ("30"), FAB 638.
Analogous reaction
of benzyl (2S)-2-butylsulfonamido-3-(3,4-dihydro-2-
carboxy-methyl-2H-1,4-benzoxa2:in-3-on-6-yl)propionate
with "A" results in
benzyl (2S)-2-butylsulfonamido-3-{3,4-dihydro-2-
[N-(2-benzimidazolyl)carbamoylmethyl]-2H-1,4-benzox-
azin-3-on-6-yl}propionate,
with "B" results in
CA 02280727 1999-08-11
= - 33 -
benzyl (2S)-2-butylsulfonamido-3-{3,4-dihydro-2-
[N-(2-imidazolyl)carbamoylmethyl]-2H-1,4-benzoxazin-3-
on-6-yl}propionate,
with "C" results in
benzyl (2S)-2-butylsulfonamido-3-{3,4-dihydro-2-
[N-(2-benzimidazolylmethyl)carbamoylmethyl]-2H-1,4-
benzoxazin-3-on-6-yl}propionat.e;
of benzyl (2S)-2-1:4-tolylsulfonamido)-3-(3,4-dihydro-2-
carboxymethyl-2H-1,4-benzoxazi.n-3-on-6-yl)propionate
with "A" results in
benzyl (2S)-2-(4-tolylsulfonamido)-3-{3,4-dihydro-
2-[N-(2-benzimidazolyl)carbamoylmethyl]-2H-1,4-
benzoxazin-3-on-6-yl}propionat.e,
with "B" results in
benzyl (2S)-2-(4-tolylsulfonamido)-3-{3,4-dihydro-
2-[N-(2-imidazolyl)carbamoylmethyl]-2H-1,4-benzoxazin-
3-on-6-yl}propionate,
with "C" results in
benzyl (2S)-2-(4-tolylsulfonamido)-3-{3,4-dihydro-
2-[N-(2-benzimidazolylmethyl)c:arbamoylmethyl]-2H-1,4-
benzoxazin-3-on-6-yl)propionat.e;
of benzyl (2S)-2-benzylsulfonamido-3-(3,4-dihydro-2-
carboxymethyl-2H-1,4-benzoxazi.n-3-on-6-yl)propionate
with "A" results in
benzyl (2S)-2-benzylsulfonamido-3-{3,4-dihydro-2-
[N-(2-benzimidazolyl)carbamoyl.methyl]-2H-1,4-benzox-
azin-3-on-6-yl}propionate,
with "B" results in
benzyl (2S)--2-benzylsulfonamido-3-{3,4-dihydro-2-
[N-(2-imidazolyl)carbamoylmethyl]-2H-1,4-benzoxazin-3-
on-6-yl}propionate,
with "C" results in
benzyl (2S)-2-benzylsulfonamido-3-{3,4-dihydro-2-
[N-(2-benzimidazolylmethyl)carbamoylmethyl]-2H-1,4-
benzoxazin-3-on-6-yl}propionate,
CA 02280727 1999-08-11
- 34 -
of benzyl (2S)-2-phenylsulfonamido-3-(3,4-dihydro-2-
carboxymethyl-2H-1,4-benzoxazi.n-3-on-6-yl)propionate
with "A" results in
benzyl (2S)-2-phenylsulfonamido-3-{3,4-dihydro-2-
[N-(2-benzimidazolyl)carbamoyl.methyl]-2H-1,4-benzox-
azin-3-on-6-yl}propionate,
with "B" results in
benzyl (2S)-2-phenylsulfonamido-3-{3,4-dihydro-2-
[N-(2-imidazolyl)carbamoylmethyl]-2H-1,4-benzoxazin-3-
on-6-yl}propionate,
with "C" results in
benzyl (2S)-2-phenylsulfonamido-3-{3,4-dihydro-2-
[N-(2-benzimidazolylmethyl)carbamoylmethyl]-2H-1,4-
benzoxazin-3-on-6-yl}propionat:e,
of benzyl (2S)-2-(2-naphthylsulfonamido)-(3,4-dihydro-
2-carboxymethyl-2H-1,4-benzoxazin-3-on-6-yl)propionate
with "A" results in
benzyl (2S)-2-(2-naphthylsulfonamido)-3-{3,4-
dihydro-2-[N-(2-benzimidazolyl.)carbamoylmethyl]-2H-1,4-
benzoxazin-3-on-6-yl}propionate,
with "B" results in
benzyl (2S)-2-(2-naphthylsulfonamido)-3-{3,4-
dihydro-2-[N-(2-imidazolyl)carbamoylmethyl]-2H-1,4-
benzoxazin-3-on-6-yl}propionat:e,
with "C" results in
benzyl (2S)-2-(2-naphthylsulfonamido)-3-{3,4-
dihydro-2-[N-(2-be:nzimidazolyl.methyl)carbamoylmethyl]-
2H-1,4-benzoxazin-3-on-6-yl}pz=opionate;
of benzyl (2S)-2-cyclohexylsulfonamido-3-(3,4-dihydro-
2-carboxymethyl-2H-1;4-benzoxazin-3-on-6-yl)propionate
with "A" results in
benzyl (2S)-2-cyclohexylsulfonamido-3-{3,4-
dihydro-2-[N-(2-benzimidazolyl.)carbamoylmethyl]-2H-1,4-
benzoxazin-3-on-6-yl)propionat.e,
with "B" results i:n
CA 02280727 1999-08-11
- 35 -
benzyl (2S)-2-cyclohexylsulfonamido-3-{3,4-
dihydro-2-[N-(2-imidazolyl)carbamoylmethyl]-2H-1,4-
benzoxazin-3-on-6-yl)propionate,
with "C" results iri
benzyl (2S)-2-cyclohexylsulfonamido-3-{3,4-
dihydro-2-[N-(2-benzimidazolylmethyl)carbamoylmethyl]-
2H-1,4-benzoxazin-3-on-6-yl}propionate.
Analogous cleavage of the last-mentioned benzyl
esters by hydrogenation results in the following
compounds
(2S)-2-butylsulfonamido-3-{3,4-dihydro-2-[N-(2-
benzimidazolyl)carbamoylmethyl]-2H-1,4-benzoxazin-3-on-
6-yl}propionic acid;
(2S)-2-butylsulfonamido-3-{3,4-dihydro-2-[N-(2-
imidazolyl)carbamoylmethyl]-2H-1,4-benzoxazin-3-on-6-
y1}propionic acid;
(2S)-2-butylsulfonamido-3-{3,4-dihydro-2-[N-(2-
benzimidazolylmethyl)carbamoylmethyl]-2H-1,4-
benzoxazin-3-on-6-yl}propionic acid;
(2S)-2-(4-tolylsulfonamido)-3-{3,4-dihydro-2-[N-
(2-benzimidazolyl)carbamoylmethyl]-2H-1,4-benzoxazin-3-
on-6-yl}propionic acid;
(2S)-2-(4-tolylsulfonamid.o)-3-{3,4-dihydro-2-[N-
(2-imidazolyl)carbamoylmethyl]-2H-1,4-benzoxazin-3-on-
6-yl}propionic acid;
(2S)-2-(4-tolylsulfonamid.o)-3-{3,4-dihydro-2-[N-
(2-benzimidazolylmethyl)carbamoylmethyl]-2H-1,4-
benzoxazin-3-on-6-yl}propionic acid;
(2S)-2-benzylsulfonamido-3-{3,4-dihydro-2-[N-(2-
benzimidazolyl)carbamoylmethyl]-2H-1,4-benzoxazin-3-on-
6-yl}propionic acid;
CA 02280727 1999-08-11
- 36 -
(2S)-2-benzylsulfonamido-3-{3,4-dihydro-2-[N-(2-
imidazolyl)carbamoylmethyl]-2H-1,4-benzoxazin-3-on-6-
yl}propionic acid;
(2S)-2-benzylsulfonamido-3-{3,4-dihydro-2-[N-(2-
benzimidazolylmethyl)carbamoyl:methyl]-2H-1,4-
benzoxazin-3-on-6-yl}propionic acid;
(2S)-2-phenylsulfonamido-3-{3,4-dihydro-2-[N-(2-
benzimidazolyl)carbamoylmethyl]-2H-1,4-benzoxazin-3-on-
6-yl}propionic acid;
(2S)-2-phenylsulfonamido-3-{3,4-dihydro-2-[N-(2-
imidazolyl)carbamoylmethyl]-2H-1,4-benzoxazin-3-on-6-
yl}propionic acid;
(2S)-2-phenylsulfonamido-3-{3,4-dihydro-2-[N-(2-
benzimidazolylmethyl)carbamoylmethyl]-2H-1,4-
benzoxazin-3-on-6-yl}propionic acid;
(2S)-2-(2-naphthylsulfonamido)-3-{3,4-dihydro-2-
[N-(2-benzimidazolyl)carbamoylmethyl]-2H-1,4-
benzoxazin-3-on-6-yl}propionic acid;
(2S)-2-(2-naphthylsulfonamido)-3-{3,4-dihydro-2-
[N-(2-imidazolyl)carbamoylmethyl]-2H-1,4-benzoxazin-3-
on-6-yl}propionic acid;
(2S)-2-(2-naphthylsulfonamido)-3-{3,4-dihydro-2-
[N-(2-benzimidazolylmethyl)carbamoylmethyl]-2H-1,4-
benzoxazin-3-on-6-yl}propionic acid;
(2S)-2-cyclohexylsulfonam:ido-3-{3,4-dihydro-2-[N-
(2-benzimidazolyl)carbamoylmethyl]-2H-1,4-benzoxazin-3-
on-6-yl}propionic acid;
(2S)-2-cyclohexylsulfonam:ido-3-{3,4-dihydro-2-[N-
(2-imidazolyl)carbamoylmethy1]-2H-1,4-benzoxazin-3-on-
6-yl}propionic acid;
CA 02280727 1999-08-11
- 37 -
(2S)-2-cyclohexylsulfonamido-3-{3,4-dihydro-2-[N-
(2-benzimidazolylmethyl)carbamoylmethyl]-2H-1,4-
benzoxazin-3-on-6-yl}propionic acid;
Example 7
Elimination of the BOC group with TFA in
dichloromethane affords
from "19"
benzyl (2S)-2-amino-3-{3,4-dihydro-2-[N-(2-benz-
imidazolyl)carbamoylmethyl]-2I-[-1,4-benzoxazin-3-on-6-
yl}propionate ("31a"), FAB 500;
from "20"
benzyl (2S)-2-amino-3-{3,4-dihydro-2-[N-(2-
imidazolyl)carbamoylmethyl]-2H-1,4-benzoxazin-3-on-6-
yl}propionate ("31b")
and from "21"
benzyl (2S)-2-amino-3-{3,4-dihydro-2-[N-(2-benz-
imidazolylmethyl)carbamoylmeth.yl]-2H-1,4-benzoxazin-3-
on-6-yl}propionate ("31c").
22 l of butylsulfonyl chloride ("D") and 71 l
of triethylamine are added to a solution of 0.13 g of
"31a" in 15 ml of dichloromethane and stirred for 30
hours. The crude product after the usual workup is
hydrogenated in analogy to Example 1. Purification by
preparative HPLC results in 13 mg of (2S)-2-
butylsulfonamido-3-{3,4-dihydro-2-[N-(2-benzimidazol-
yl)carbamoylmethyl]-2H-1,4-benzoxazin-3-on-6-yl}prop-
ionic acid ("32a"), FAB 530;
analogous reaction of "D" and subsequent hydrogenation
with "31b" result in
(2S)-2-butylsulfonamido-3-{3,4-dihydro-2-[N-(2-
imidazolyl)carbamoylmethyl]-2H-1,4-benzoxazin-3-on-6-
yl}propionic acid ("32b")
CA 02280727 1999-08-11
- 38 -
and with "31c" result in
(2S)-2-butylsulfonamido-3-(3,4-dihydro-2-[N-(2-
benzimidazolylmethyl)carbamoyl.methyl]-2H-1,4-benzox-
azin-3-on-6-yl}propionic acid ("32c").
Example 8
A solution of 2.1 g of "1", 3.7 g of diethyl
2,4-dibromoadipate, 1.4 g of potassium carbonate and
0.137 g of 18-crown-6 in 100 ml of toluene is stirred
at 800 for 2 hours. The usual workup results in 1.8 g
of benzyl (2S)-2-tert-butyloxycarboxamido-3-[3-nitro-4-
(1,4-bis(ethoxycarbonyl)-4-bromobutyloxy)phenyl]propio-
nate ("34") as a colourless syrup, FAB 696.
A solution of 1.5 g o:= "34" and 0.7 g of sodium
azide in 60 ml of DMF is stirred at 60 for 12 hours.
The usual workup results in 1.3 g of benzyl (2S)-2-
tert-butyloxycarboxamido-3-[3--nitro-4-(1,4-bis(ethoxy-
carbonyl)-4-azidobutyloxy)pher..yl]propionate ("35"), FAB
658.
1.1 g of "35" are dissolved in 50 ml of
methanol and, after addition of 5.9 ml of 1N NaOH,
stirred for 5 hours. The usua:- workup results in 0.85 g
of (2S)-2-tert-butyloxycarboxamido-3-[3-nitro-4-(1,4-
biscarboxy-4-azidobutyloxy)phenyl]propionic acid
("36"), FAB 512.
A solution of 0.5 g of "36" in 10 ml of dioxane
and 5 ml of water is hydrogemated in the presence of
0.1 g of palladium (10% on active carbon) for 6 hours.
The pH is kept at between 4 and 6 with 1N HC1. Removal
of the catalyst and the solvents results in 0.21 g of
(2S)-2-tert-butyloxycarboxamic'.o-3-[3,4-dihydro-2-(3-
amino-3-carboxypropyl)-2H-1,4-benzoxazin-3-on-6-yl]pro-
pionic acid ("37"), FAB 456.
The crude product "37" (0.2 g) is dissolved in
10 ml of DMF, 2 ml of ethanol and 1 ml of water and
guanylated with 0.354 g of DPFN in the presence of
0.5 ml of triethylamin at 60'' for 24 hours. The usual
workup results in 0.1 g of (2S)-2-tert-
butyloxycarboxamido-3-[3,4-dih.ydro-2-(3-guanidino-3-
CA 02280727 1999-08-11
- 39 -
carboxypropyl)-2H-1,4-benzoxazin-3-on-6-yl]-propionic
acid ("38"), FAB 480.
32 mg of 2-chloro-i-methylpyridinium iodide and
60 l of ethyldiisopropylamir.ie are added to a solution
of 50 mg of "38" (trifluoroacetate) in 2 ml of DMF and
stirred for 12 hours. The usual workup results in 22 mg
of (2S)-2-tert-butyloxycarbo):amido-3-{3,4-dihydro-2-[3-
(2-imino-4-oxoimidazolidin-5-yl)propyl]-2H-1,4-benz-
oxazin-3-on-6-yl}propionic acid ("39").
Example 9
Equimolar amounts of tert-butyl bromoacetate
and NaH are added to a solution of benzyl (2S)-2-
butylsulfonamido-3-(3-hydroxynethyl-1,4-benzodioxan-6-
yl)propionate in DMF. The mixture is stirred for
2 hours, and the usual workup results in benzyl
2-butylsulfonamido-3-(2-tert-butoxycarbonylmethoxyme-
thyl-1,4-benzodioxan-6-yl)pro:oionate.
Analogously, subsequent elimination of the BOC group
with TFA, reaction with 2-aminobenzimidazole and
cleavage of the benzyl ester by hydrogenation result in
the compound (2S)-2-butyl:;ulfonamido-3-{2-[(1H-imid-
azol-2-ylcarbamoyl.)methoxymet:hyl]-1,4-benzodioxan-6-
yl}propionic acid.
2-(4-Tolylsulfonamido)-3-{2-[(1H-imidazol-2-
ylcarbamoyl)methoxymethyl]-1,,4-benzodioxan-6-yl}prop-
ionic acid is obtained analogously.
Example 10
Reaction of "25" with 2,2,2-trichloro-l,1-
dimethylethyl chloroformate and subsequent cleavage of
the benzyl ester by hydrogenation results in the
compound (2S) -2- {[(2, 2, 2-tric:hloro-l, 1-dimethyl) ethyl] -
carboxamido}-3-(3,4-dihydro-2-carboxymethyl-2H-1,4-benz
oxazin-3-on-6-yl)propionic acid ("40").
Reaction in analogy to Example 5 of "40" with "A"
affords the compound (2S)-2-{[(2,2,2-trichloro-l,l-
dimethyl)ethyloxy]carboxamido}-3-{3,4-dihydro-2-[N-(2-
CA 02280727 1999-08-11
- 40 -
benzimidazolyl)carbamoylmethyl]-2H-1,4-benzoxazin-3-on-
6-yl}propionic acid, trifluoroacetate, FAB 726
H O
N O O N ~ OH
Q ~ ~1_1 ~ ~ H
N
H y O ~O
O
--~'<x cl
CI CI
The compound (2S)-2-{[(neopentyloxy)ethyl]carboxamido}-
3-{3,4-dihydro-2-[N-(2-benzim.idazoyl)carbamoylmethyl]-
2H-1,4-benzoxazin-3-on-6-yl}p:ropionic acid, FAB 524, is
obtained analogously.
Example 11
Reaction of BOC-3-amino-L-tyrosine ethyl ester
with (2S) -bromopentanedioic acid 5-benzyl ester [obtain-
able by reacting L-glutamic acid y-benzyl ester with
NaNOZ and KBr in sulfuric acid], and EDC1 in dichloro-
methane at room temperature results, after stirring for
12 hours and the usual workup, in the compound benzyl
(4S)-4-bromo-4-[5-((2S)-2-tert-butyloxycarbonylamino-2-
ethoxycarbonylethyl)-2-hydroxyphenylcarbamoyl]butyrate,
FAB 608.
Heating with DBU (diazabicycloundec-7-ene) in toluene
at 100 for 12 hours results, after the usual workup,
in the compound ethyl (2S) -3- [(2R) -2- (2-benzyloxy-
carbonylethyl)-3-oxo-3,4-dihydro-2H-benzo[1,4]oxazin-6-
yl]-2-tert-butoxycarbonylaminopropionate, FAB 527.
Hydrogenation with Pd/C results in ethyl (2S)-2-tert-
butoxycarbonylamino-3-[(2R)-2-(2-carboxyethyl)-3-oxo-3,4-
dihydro-2H-benzo[1,4]oxazin-6--yl]propionate ("41"), FAB
437
CA 02280727 1999-08-11
- 41 -
0
N
HO 0 HN O
O ~
O
"(
Reaction in analogy to Example 5 of "41"
with "A" results in
ethyl (2S)-2-tert-butyloxycarboxamido-3-{3,4-
dihydro-2-[N-(2-benzimidazolyl.)carbamoylethyl]-(2R)-2H-
1,4-benzoxazin-3-on-6-yl}propi.onate ("42") and
with "B" results in
ethyl (2S)-2-tert-butyloxycarboxamido-3-{3,4-
dihydro-2-[N-(2-imidazolyl)carbamoylethyl]-(2R)-2H-1,4-
benzoxazin-3-on-6-yl}propionat.e ("43"), FAB 502.
Cleavage of the ethy:L ester in "42" and "43"
with aqueous NaOH results in the compounds
(2S)-2-tert-butyloxyca.rboxamido-3-{3,4-dihydro-
2-[N-(2-benzimidazolyl)carbamoylethyl]-(2R)-2H-1,4-benz-
oxazin-3-on-6-yl}p:ropionic acid, FAB 524 and
(2S)-2-tert-butyloxyca.rboxamido-3-{3,4-dihydro-
2-[N-(2-imidazolyl)carbamoylet.hyl]-(2R)-2H-1,4-benzoxa-
zin-3-on-6-yl}propionic acid, FAB 474.
The following compound is obtained analogously
(2S)-2-tert-butyloxyca.rboxamido-3-{3,4-dihydro-
2-[N-(2-imidazolyl)carbamoylet.hyl]-(2S)-2H-1,4-benzoxa-
zin-3-on-6-yl}propionic acid, FAB 474.
CA 02280727 1999-08-11
- 42 -
Example 12
Elimination of the BC>C group from "42" and "43"
with TFA in dichloromethane results in the following
compounds
ethyl (2S)-2-amino-3-{3,4-dihydro-2-[N-(2-benz-
imidazolyl)carbamoylethyl]-(2R)-2H-1,4-benzoxazin-3-on-
6-yl}propionate ("44") and
ethyl (2S) -2-amino-3--{3,4-dihydro-2- [N- (2-imida-
zolyl)carbamoylethyl]-(2R)-2H:-1,4-benzoxazin-3-on-6-yl~
propionate ("45"), FAB 402.
Reaction in analogy to Example 6 of "44"
with 2,3,5,6-tetramethylsulfcnyl chloride results in
ethyl (2S)-2-(2,3,5,6-tetramethylphenylsulfon-
amido)-3-{3,4-dihydro-2-[N-(2-benzimidazolyl)carbamoyl-
ethyl]-(2R)-2H-1,4-benzoxazin.-3-on-6-yl}propionate
and ester cleavage thereof results in
(2S)-2-(2,3,5,6-tetramethylphenylsulfonamido)-
3 - { 3 , 4 -dihydro- 2 - [N- ( 2 -benz iu~idazolyl ) carbamoylethyl ] -
(2R)-2H-1,4-benzoxazin-3-on-6-yl}propionic acid, FAB
620.
Analogous reaction of "45"
with 3-chloro-6-methoxyphenylsulfonyl chloride results
in
ethyl (2S)-2-(3-chloro-6-methoxyphenylsulfon-
amido)-3-{3,4-dihydro-2-[N-(2-imidazolyl)carbamoylethyl]-
(2R)-2H-1,4-benzoxazin-3-on-6-yl}propionate,
with 1-naphthylsulfonyl chloride results in
ethyl (2S)-2-(1-naphthylsulfonamido)-3-{3,4-
dihydro-2-[N-(2-imidazolyl)ca.rbamoylethyl]-(2R)-2H-1,4-
benzoxazin-3-on-6-yl}propiona.te,
CA 02280727 1999-08-11
- 43 -
with 2,3,5,6-tetramethylphenylsulfonyl chloride results
in
ethyl (2S)-2-(2,3,5,6-tetramethylphenylsulfon-
amido)-3-{3,4-dihydro-2-[N-(2-imidazolyl)carbamoylethyl]-
(2R)-2H-1,4-benzoxazin-3-on-6-yl}propionate,
with (R)-camphor-l0-sulfonyl chloride results in
ethyl (2S) -2- [ (R) -camphor-10-sulfonamido] -3-
{3,4-dihydro-2- [N- (2-imidazolyl) carbamoylethyl] - (2R) -2H-
1,4-benzoxazin-3-on-6-yl}propionate,
with butylsulfonyl chloride results in
ethyl (2S)-2-butylsu:_fonamido-3-{3,4-dihydro-2-
[N-(2-imidazolyl)carbamoylethyl]-(2R)-2H-1,4-benzoxazin-
3-on-6-yl}propionate,
with isopropyl chloroformate results in
ethyl (2S)-2-isopropoxycarboxamido-3-{3,4-dihydro-
2- [N- (2-imidazolyl) carbamoylethyl] - (2R) -2H-1, 4-benzoxa
zin-3-on-6-yl}propionate,
with isobutyl chloroformate results in
ethyl (2S)-2-isobutoxycarboxamido-3-{3,4-dihy-
dro-2- [N- (2-imidazolyl) carbamoylethyl] - (2R) -2H-1, 4-
benzoxazin-3-on-6-yl}propionate,
with neopentyl chloroformate results in
ethyl (2S)-2-neopentyloxycarboxamido-3-{3,4-di-
hydro-2- [N- (2-imidazolyl) carbamoylethyl] - (2R) -2H-1, 4-
benzoxazin-3-on-6-yl}propionate,
with benzyl chloroformate results in
ethyl (2S)-2-benzyloxycarboxamido-3-{3,4-dihydro-
2- [N- (2-imidazolyl.) carbamoylethyl] - (2R) -2H-1, 4-benzoxa-
zin-3-on-6-yl}propionate,
with benzylsulfonyl chloride results in
CA 02280727 1999-08-11
- 44 -
ethyl (2S)-2-benzylsulfonamido-3-{3,4-dihydro-
2-[N-(2-imidazolyl)carbamoylethyl]-(2R)-2H-1,4-benzoxa-
zin-3-on-6-yl}propionate,
and ester cleavage thereof results in the following
propionic acid derivatives
(2S)-2-(3-chloro-6-methoxyphenylsulfonamido)-3-
{3, 4-dihydro-2- [N- (2-imidazolyl) carbamoylethyl] - (2R) -2H-
1,4-benzoxazin-3-on-6-yl}propionic acid, FAB 578;
(2S)-2-(1-naphthylsulfonamido)-3-{3,4-dihydro-2-
[N- (2-imidazolyl) carbamoylethyl] - (2R) -2H-1, 4-benzoxain-
3-on-6-yl}propionic acid, FAB 564;
(2S)-2-(2,3,5,6-tetramethylphenylsulfonamido)-3-
{3,4-dihydro-2-[N--(2-imidazolyl)carbamoylethyl]-(2R)-2H-
1,4-benzoxazin-3-on-6-yl}propionic acid, FAB 570;
(2S)-2-[(R)-camphor-10-sulfonamido]-3-{3,4-di-
hydro-2-[N-(2-imidazolyl)carbamoylethyl]-(2R)-2H-1,4-
benzoxazin-3-on-6-yl}propionic acid, FAB 588;
(2S)-2-butylsulfonamido-3-{3,4-dihydro-2-[N-(2-
imidazolyl)carbamoylethyl]-(2R)-2H1,4-benzoxazin-3-on-
6-yl}propionic acid, FAB 494;
(2S)-2-isopropoxycar:Z)oxamido-3-{3,4-dihydro-2-
[N-(2-imidazolyl)carbamoylethyl]-(2R)-2H 3,4-benzoxazin-
3-on-6-yl}propionic acid, FAB 460;
(2S)-2-isobutoxycarbcxamido-3-{3,4-dihydro-2-[N-
(2-imidazolyl)carbamoylethyl]-(2R)-2H-1,4-benzoxazin-3-
on-6-yl}propionic acid, FAB 474;
(2S)-2-neopentyloxyca.rboxamido-3-{3,4-dihydro-
2- [N- (2-imidazolyl) carbamoylethyl] - (2R) -2H3, 4-benzoxa-
zin-3-on-6-yl}propionic acid, FAB 488;
CA 02280727 1999-08-11
- 45 -
(2S)-2-benzyloxycarbo:xamido-3-{3,4-dihydro-2-[N-
(2-imidazolyl)carbamoylethyl]-(2R)-2H-1,4-benzoxazin-3-
on-6-yl}propionic acid, FAB 508;
(2S)-2-benzylsulfonamido-3-{3,4-dihydro-2-[N-(2-
imidazolyl)carbamoylethyl]-(2R)-2H-1,4-benzoxazin-3-on-
6-yl}propionic acid, FAB 528;
(2S)-2-benzenesulfonamido-3-{3,4-dihydro-2-[N-
(2-imidazolyl)carbamoylethyl]-(2R)-2H-1,4-benzoxazin-3-
on-6-yl}propionic acid, FAB 514.
The compound (2S)-2-(1,1-dimethyl-2,2,2-trichloro-
ethyloxycarboxamido)-3-{3,4-dihydro-2-[N-(2-imidazolyl~
carbamoylethyl]-(2R)-2H-1,4-benzoxazin-3-on-6-yl}-
propionic acid, FAB 578, is obtained analogously.
Example 13
g of L-Orn(NS-Z) are dissolved together with
20 37 g of potassium bromide in 300 1 of 2.5 N sulfuric
acid and, at 01, 9.7 g of sociium nitrite are added. The
mixture is allowed to warm to room temperature and then
stirred for 12 hours. The usual workup results in 11 g
of (2S)-2-bromo-4-benzyloxycarbonylaminobutyric acid as
25 oil, EI 330.
Subsequent reaction with BCC-3-amino-L-tyrosine ethyl
ester and EDC1 in dichloromethane at room temperature
results, after stirring for 12 hours, the usual workup
and subsequent reaction of the product with DBU (diaza-
bicycloundec-7-ene) in toluerie at 1000, in the compound
ethyl (2S)-3-[(2R)-2-(3-benzyloxycarbonylaminopropyl)-
3-oxo-3,4-dihydro--2H-benzo[1,4]oxazin-6-yl]-2-tert-but-
oxycarbonylaminopropionate ("46"), FAB 556.
Ester hydrolysis with aqueous sodium hydroxide solution
and subsequent elimination of: the Z group by hydrogena-
tion (Pd/C) in dioxane/water results in the compound
(2S)-3-[(2R)-2-(3-aminopropyl)-3-oxo-3,4-dihydro-2H-
benzo[1,4]oxazin-6-yl]-2-tert-butoxycarbonylamino-
propionic acid, FAB 394.
CA 02280727 1999-08-11
- 46 -
Reaction in analogy to Exa;nple 3 thereof with DPFN
results in the compound (2S)-3-[(2R)-2-(3-guanidino-
propyl) -3-oxo-3, 4-dihydro- 2H--benzo [l, 4] oxazin-6-yl] -2-
tert-butoxycarbonylaminopropionic acid, FAB 436.
Elimination of the BOC group from "46" with TFA
in dichloromethane results in the compound ethyl (2S)-
2-amino-3-[(2R)-2-(3-benzyloxycarbonylaminopropyl)-3-oxo-
3,4-dihydro-2H-benzo[1,4]oxazin-6-yl]propionate ("47"),
trifluoroacetate, FAB 456
O
O N
H
0 ~N -, H2N
O =
Reaction in analogy to Example 6 of "47"
with 2,3,5,6-tetramethylphenylsulfonyl chloride results
in
ethyl (2S)-2-(2,3,5,6-tetramethylphenylsulfon-
amido)-3-{3,4-dihydro-2-(3-ben.zyloxycarbonylaminopropyl)-
(2R)-2H-1,4-benzoxazin-3-on-6-yl}propionate,
with 3-chloro-6-methoxyphenylsulfonyl chloride results
in
ethyl (2S)-2-(3-chloro-6-methoxyphenylsulfon-
amido)-3-{3,4-dihydro-2-(3-berizyloxycarbonylaminopropyl)-
(2R)-2H-1,4-benzoxazin-3-on-6-yl}propionate,
with 1-naphthylsulfonyl chloride results in
ethyl (2S)-2-(1-naphthylsulfonamido)-3-{3,4-di-
hydro-2-(3-benzyloxycarbonylaminopropyl)-(2R)-2H-1,4-
benzoxazin-3-on-6-yl}propiona.te,
with 2,3,5,6-tetramethylphenylsulfonyl chloride results
in
CA 02280727 1999-08-11
- 47 -
ethyl (2S)-2-(2,3,5,6-tetramethylphenylsulfon-
amido)-3-{3,4-dihydro-2-(3-benzyloxycarbonylaminopropyl)-
(2R)-2H-1,4-benzoxazin-3-on-6-yl}propionate,
with (R)-camphor-l0-sulfonyl chloride results in
ethyl (2S) -2- [ (R) -camphor-10-sulfonamido] -3-
{ 3 , 4 -dihydro- 2 - ( 3 -benzyloxyca:rbonylamincgDropyl ) - (2R) - 2H-
1,4-benzoxazin-3-on-6-yl}propionate,
with butylsulfonyl chloride results in
ethyl (2S)-2-butylsulfonamido-3-{3,4-dihydro-2-
(3-benzyloxycarbonylaminopropyl)-(2R)-2H-1,4-benzoxazin-
3-on-6-yl}propionate, FAB 576;
with isopropyl chloroformate results in
ethyl (2S)-2-isopropoxycarboxamido-3-{3,4-
dihydro-2-(3-benzyloxycarbonylaminopropyl)-(2R)-2H-1,4-
benzoxazin-3-on-6-yl}propionate,
with isobutyl chloroformate results in
ethyl (2S)-2-isobutoxycarboxamido-3-{3,4-dihydro-
2-(3-benzyloxycarbonylaminopropyl)-(2R)-2H-1,4-benzoxa-
zin-3-on-6-yl}propionate,
with neopentyl chloroformate results in
ethyl (2S)-2-neopentyloxycarboxamido-3-{3,4-
dihydro-2-(3-benzyloxycarbonylaminopropyl)-(2R)-2H-1,4-
benzoxazin-3-on-6-yl}propionate, FAB 570;
with benzyl chloroformate results in
ethyl (2S)-2-benzyloxycarboxamido-3-{3,4-dihydro-
2-(3-benzyloxycarbonylaminopropyl)-(2R)-2H-1,4-benzoxa-
zin-3-on-6-yl}propionate,
with benzylsulfonyl chloride results in
ethyl (2S)-2-benzylsulfonamido-3-{3,4-dihydro-2-
(3-benzyloxycarbonylaminopropyl)-(2R)-2H-1,4-benzoxazin-
3-on-6-yl}propionate,
- ---------- -
CA 02280727 1999-08-11
- 48 -
with benzenesulfonyl chloride results in
ethyl (2S)-2-benzenesulfonamido-3-{3,4-dihydro-
2-(3-benzyloxycarbonylaminopropyl)-(2R)-2H-1,4-benzoxa-
zin-3-on-6-yl}propionate.
Ester cleavage and hydrogenation of the above-
mentioned Z-protected propionates result in the follow-
ing compounds
(2S)-2-(2,3,5,6-tetramethylphenylsulfonamido)-3-
{3,4-dihydro-2-(3-aminopropyl)-(2R)-2H-1,4-benzoxazin-
3-on-6-yl}propionic acid,
(2S)-2-(3-chloro-6-methoxyphenylsulfonamido)-3-
{3,4-dihydro-2-(3-aminopropyl)-(2R)-2H-1,4-benzoxazin-
3-on-6-yl}propionic acid,
(2S)-2-(1-naphthylsulfonamido)-3-{3,4-dihydro-
2-(3-aminopropyl)-(2R)-2H-1,4-benzoxazin-3-on-6-yl}pro-
pionic acid,
(2S)-2-(2,3,5,6-tetramethylphenylsulfonamido)-3-
{3,4-dihydro-2-(3-aminopropyl)-(2R)-2H-1,4-benzoxazin-
3-on-6-yl}propionic acid,
(2S) -2- [ (F.) -camphor-l0-sulfonamido] -3- {3, 4-di-
hydro-2-(3-aminopropyl)-(2R)-2H-1,4-benzoxazin-3-on-6-
yl}propionic acid,
(2S)-2-butylsulfonamido-3-{3,4-dihydro-2-(3-
aminopropyl)-(2R)-.2H-1,4-benzoxazin-3-on-6-yl}propionic
acid,
(2S)-2-isopropoxycarboxamido-3-{3,4-dihydro-2-
(3-aminopropyl)-(2R)-2H-1,4-benzoxazin-3-on-6-yl}pro-
pionic acid,
CA 02280727 1999-08-11
- 49 -
(2S)-2-isobutoxycarboxamido-3-{3,4-dihydro-2-
(3-aminopropyl)-(2R)-2H-1,4-benzoxazin-3-on-6-yl}pro-
pionic acid,
(2S)-2-neopentyloxycarboxamido-3-{3,4-dihydro-
2-(3-aminopropyl)-(2R)-2H-1,4--benzoxazin-3-on-6-yl}pro-
pionic acid, FAB 408;
(2S)-2-benzyloxycarboxamido-3-{3,4-dihydro-2-
(3-aminopropyl)-(2R)-2H-1,4-benzoxazin-3-on-6-yl}pro-
pionic acid,
(2S)-2-benzylsulfonamido-3-{3,4-dihydro-2-(3-
aminopropyl)-(2R)-2H-1,4-benzc>xazin-3-on-6-yl}propionic
acid,
(2S)-2-benzenesulfonamido-3-{3,4-dihydro-2-(3-
aminopropyl)-(2R)-2H-1,4-benzc>xazin-3-on-6-yl}propionic
acid.
Reaction in analogy to Example 3 of the above-
mentioned propionic acids with DPFN results in the
following compounds:
(2S)-2-(2,3,5,6-tetramethylphenylsulfonamido)-3-
{3,4-dihydro-2-(3-guanidinopropyl)-(2R)-2H-1,4-benzoxa-
zin-3-on-6-yl}propionic acid,
(2S)-2-(3-chloro-6-methoxyphenylsulfonamido)-3-
{3,4-dihydro-2-(3-guanidinopropyl)-(2R)-2H-1,4-benzoxa-
zin-3-on-6-yl}propionic acid,
(2S)-2-(l-naphthylsulfonamido)-3-{3,4-dihydro-2-
(3-guanidino propyl)-(2R)-2H-1,4-benzoxazin-3-on-6-yl} -
propionic acid,
(2S)-2-(2,3,5,6-tetram.ethylphenylsulfonamido)-3-
{3,4-dihydro-2-(3-guanidinopropyl)-(2R)-2H-1,4-benzoxa-
zin-3-on-6-yl}propionic acid,
CA 02280727 1999-08-11
- 50 -
(2S)-2-[(R)-camphor-10-sulfonamido]-3-{3,4-di-
hydro-2-(3-guanidincpropyl)-(2:R)-2H-1,4-benzoxazin-3-on-
6-yl}propionic acid,
(2S)-2-butylsulfonamido-3-{3,4-dihydro-2-(3-
guanidinopropyl)-(2R)-2H-1,4-benzoxazin-3-on-6-yl} pro-
pionic acid, FAB 456;
(2S)-2-isopropoxycarboxamido-3-{3,4-dihydro-2-
(3-guanidino propyl)-(2R)-2H-1,4-benzoxazin-3-on-6-yl} -
propionic acid,
(2S)-2-isobutoxycarboxamido-3-{3,4-dihydro-2-
(3-guanidinopropyl)-(2R)-2H-1,4-benzoxazin-3-on-6-yl} -
propionic acid,
(2S)-2-neopentyloxycarboxamido-3-{3,4-dihydro-2-
(3-guanidinopropyl)-(2R)-2H-1,4-benzoxazin-3-on-6-yl} -
propionic acid, FAB 450;
(2S)-2-benz.yloxycarboxamido-3-{3,4-dihydro-2-
(3-guanidinopropyl)-(2R)-2H-1,4-benzoxazin-3-on-6-yl}-
propionic acid,
(2S)-2-benzylsulfonamido-3-{3,4-dihydro-2-(3-
guanidinopropyl)-(2R)-2H-1,4-benzoxazin-3-on-6-y1} pro-
pionic acid and
(2S)-2-benzenesulfonamido-3-{3,4-dihydro-2-(3-
guanidinopropyl)-(2R)-2H-1,4-benzoxazin-3-on-6-yl}pro-
pionic acid.
The following examples relate to pharmaceutical
compositions:
Example A: Vials
A solution of 100 g of an active substance of
the formula I and 5 g of disociium hydrogen phosphate in
3 1 of double-distilled water is adjusted to pH 6.5
CA 02280727 1999-08-11
- 51 -
with 2N hydrochloric acid, sterilized by filtration,
dispensed into vials, lyophilized under sterile
conditions and sealed sterile . Each vial contains 5 mg
of active substance.
Example B: Suppositories
A mixture of 20 g of an active substance of the
formula I with 100 g of soya lecithin and 1400 g of
cocoa butter is melted, poure d into moulds and left to
cool. Each suppository contains 20 mg of active
substance.
Example C: Solution
A solution is prepared from 1 g of an active
substance of the formula I, 9.38 g of NaH2PO4 = 2H2O,
28.48 g of Na2HPO4 = 12HzO and 0.1 g of benzalkonium
chloride in 940 ml. of double-distilled water. The pH is
adjusted to 6.8, the volume is made up to 1 1, and the
solution is radiation-sterilized. This solution can be
used in the form of eye drops.
Example D: Ointment
500 mg of an active Substance of the formula I
are mixed with 99.5 g of petrolatum under aseptic
conditions.
Example E: Tablets
A mixture of 1 kg of: active substance of the
formula I, 4 kg of lactose, 1.2 kg of potato starch,
0.2 kg of talc and 0.1 kg of magnesium stearate is
compressed to tablets in a conventional way so that
each tablet contains 10 mg of active substance.
Exaample F: Coated tablets
Tablets are compressed in analogy to Example E
and then provided with a coating of sucrose, potato
starch, talc, tragacanth and dye in a conventional way.
Example G: Capsules
2 kg of active substance of the formula I are
packed into hard gelatin capsules in a conventional way
so that each capsule contains 20 mg of the active
substance.
CA 02280727 1999-08-11
- 52 -
Example H: Ampoules
A solution of 1 kg o:~ active substance of the
formula I in 60 1 of double-distilled water is
sterilized by filtration, dispensed into ampoules,
lyophilized under sterile conditions and sealed
sterile. Each ampoule contains 10 mg of active
substance.
Example I: Inhalation spray
14 g of active substance of the formula I are
dissolved in 10 1 of isotonic NaCl solution, and the
solution is dispensed intc commercially available
spraying vessels with a pump mechanism. The solution
can be sprayed into the mouth or nose. One puff (about
0.1 ml) corresponds to a dose of about 0.14 mg.