Note: Descriptions are shown in the official language in which they were submitted.
CA 02281905 1999-08-20
WO 98/36683 PCT/US98/03464
-1-
NON-INVASIVE RADIOGRAPHIC METHOD FOR
ANALYZATION OF A BODY ELEMENT
Cross-Reference to Related Application
The present application is a continuation-in-part of co-pending
Application Serial No. 08/805,787, entitled NON-INVASIVE RADIOGRAPHIC
METHOD FOR ANALYZATION OF A BODY ELEMENT, filed February 25,
1997.
Field of the Invention
The present invention is directed to a system for imaging internal
body structures and, more particularly, to a system for non-invasively
analyzing and
diagnosing abnormalities in a body element.
Background of the Invention
Direct bronchoscopy of the tracheobronchial tree and endoscopy of
hollow visci, such as the gastrointestinal (GI) tract and pharynx, have been
performed for years. They are used to directly visualize masses, caliber
changes,
surface or mucosal abnormalities, and traumatic injuries. However, direct
endoscopy requires the invasive introduction of a scope into the lumen of the
structure under consideration to visualize its inside surface. Apart from the
surgical
requirements and complications involved in inserting the scope into the
patient, the
actual physical advancement of a scope within the patient may be hampered by
obstructions which prevent or limit viewing of distal abnormalities.
Furthermore,
the presence of a scope traversing the airways may in itself impose
abnormalities
in airway structure or function by altering the diameter or by inducing
mechanical
stimulation to airway components (i.e., mucous glands, smooth muscle, cillia).
Radiological and sonographic imaging has been used for decades to
non-invasively determine the internal status of the human body. Radiographic
SUBSTITUTE SHEET (RULE 26)
CA 02281905 1999-08-20
WO 98/36683 PCT/US98/03464
-2-
procedures, such as computed tomography (CT) and magnetic resonance imaging
(MRI), operate on the basis of distinct physical principles by detecting and
mapping
differences in the composition of a target object.
Conventional radiographic procedures utilize a beam of x-rays to
pass through a target object and expose an underlying photographic film. The
film
captures an image of the radiodensity pattern of the object. Areas of less
radiodensity (e.g., air pockets) produce a greater blackening of the film.
More
radiodense objects (e.g., bones) produce a light image. Contrast agents are
chosen
so as to provide either less or more radiodensity than body tissues of
interest.
Computed tomography is superior to conventional radiography in its
ability to image a sequence of thin sections of an object at specific planes
along the
X, Y or Z axis of the target object and to do so with extremely high
resolution.
Nuclear magnetic resonance imaging systems for body imaging
operate on a different physical principle. Some atomic nuclei, such as, for
example, hydrogen nuclei, have both nuclear spin and nuclear magnetic moment.
As such, these nuclei can be manipulated by applied magnetic fields. In the
convernion MRI system, a magnetic field is established across a body to align
the
spin axes of the nuclei of a particular chemical element, usually hydrogen,
with the
direction of the magnetic field. The aligned, spinning nuclei execute
precessional
motions around the aligning direction of the magnetic field. For the aligned,
spinning nuclei, the frequency at which they precess around the direction of
the
magnetic field is a function of the particular nucleus involved and the
magnetic field
strength. The selectivity of this precessional frequency with respect to the
strength
of the applied magnetic field is very sharp, and this precessional frequency
is
considered a resonant frequency.
After alignment of the selected nuclei, a burst of radio frequency
energy at the resonant frequency is radiated at the target body to produce
deflection
of the spin alignment of the selected nuclei. When the radio frequency energy
is
terminated, the deflected spin axes start to realign. The realignment of the
spin
axis emits a characteristic radio frequency signal which can be detected by an
external coil. The differences in the emitted radio frequency signal establish
contrast between the different tissues.
Since tissue cells of body parts are primarily composed of water,
radiog-raphy procedures typically do not sufficiently distinguish between
contiguous
body parts. In the diagnosis of disorders of the digestive tract, for example,
SUBSTITUTE SHEET (RULE 26)
CA 02281905 1999-08-20
WO 98/36683 PCT/US98/03464
-3-
blockage or abnormalities in the conformation of loops of intestine lying one
on the
other are difficult to identify. Currently, assessment of pulmonary function
requires
the use of supplemental equipment or procedures (i.e., pneumotachography,
spirometry, bronchoscope).
In order to assist in distinguishing between contiguous body parts,
contrast agents have recently been utilized in imaging processes. The use of
contrast agents in combination with radiological imaging make it possible to
determine the location, size and conformation of organs or other structures of
the
body in the context of their surrounding tissues.
Contrast agents may be introduced into the body space in various
ways depending on the imaging requirement. In the form of liquid suspensions
or
emulsions they may be placed into the area of interest by oral ingestion or
injection
into the bodily space (either directly or by channeling through selected
vessels).
A suitable contrast agent must be biocompatible, that is non-toxic, and
chemically
1 S stable, minimally absorbed or reactive with the tissue, and eliminated
from the body
within a short time.
Fluorinated hydrocarbons (FCs) have been demonstrated to be useful
in several clinical applications including vitreous fluid replacement,
emulsions for
blood substitutes, and have been used as contrast media in the lung and liver.
FC
liquid can be used as an alternative respiratory media to support gas
exchange. FC
liquids are characterized by high respiratory gas solubility, are bioinert,
nonbiotransformable, minimally absorbed, and have no deleterious histological,
cellular, or biochemical effects. These properties combined with FC's
radiopacity
suggest that they may be ideal contrast agents for the mucosal surface of the
tracheobronchial tree.
It is known to use fluorocarbons as a contrast enhancement medium,
see for example, Wolfson, et al., Utility of a Fluorochemical Liquid for
Pulmonary
Diagnostic Imaging", Artificial Cells Blood Substitutes Immobilization
Biotechnolo~v, Volume 23, Number 4, pp. 1409-1420 (1994). Fluorocarbon (FC)
liquids are derived from common organic compounds by the replacement of all
carbon-bound hydrogen atoms with fluorine atoms. These liquids are typically,
clear, colorless, odorless, nonflammable and essentially insoluble in water.
Perfluorinated compounds (e. g. , perfluorocarbons or PFCs) are generally the
preferred form of fluorinated hydrocarbons. FC liquids are denser than water
and
soft tissue, and have low surface tension. fluorocarbon liquids have a high
affinity
SUBSTITUTE SHEET (RULE 26)
CA 02281905 1999-08-20
WO 98/36683 PCT/US98/03464
-4-
for gases, dissolving more than 20 times as much O~ and over three times as
much
CO~ as water. FCs are also nontoxic and biocompatible.
The advent of helical computerized tomography (helical CT) has
allowed for non-invasive volumetric data acquisition. This technique provides
markedly improved multiplanar reconstruction as well as three dimensional
rendering. However, current pulmonary imaging techniques, including helical
CT,
are limited by the presence of air on both sides of the walls of the
progressively
narrowing airway inhibiting visualization of small airways.
Recently developed post-processing software based on virtual reality
techniques has permitted changing the viewer frame of reference and allowing
direct
visualization of the inside of hollow visci and tubes. This has been referred
to as
"endoscopic CT" or "virtual endoscopy. " The technique has been applied to air
distended bladders, colons, stomachs, tracheobronchial trees, as well as blood
vessels. Major requirements of the technique are 1 ) a marked contrast
difference
between the lumen and its wall; and 2) advanced novel software-design for edge
detection and branch point detection of the structures. Air within the lumen
of most
structures supplies the contrast; or in the case of blood vessels,
intravascular
contrast agents provide the contrast between the lumen and the wall. In the
case
of magnetic resonance, fluid or moving protons provide the contrast with the
wall.
See, for example, Rubin et al., "Perspective Volume Rendering of CT and MR
Images: Applications for Endoscopic Imaging", Radiology, pages 321-330, May
1996, incorporated herein by reference in its entirety.
Volume rendering is an alternative to conventional surface display
and projectional techniques and has significant advantages. Because volume
rendering uses information from all "voxels" within the volume, there is no
information loss. As a result, it is not subject to the limitations caused by
the
information loss that is inherent in maximum intensity projection or to
thresholding
that occurs in surface displays. The basic drawback to volume rendering is
that it
is computationally more time consuming and expensive than other methods.
An additional advantage of volume rendering is that the images can
be displayed as perspective views. That is, the images are rendered from a
point
source at a finite distance to approximate the human visual system. As a
result, a
close object appears larger than an object of identical size at a greater
distance from
the viewer.- Surface displays of convention CT and MR data are rendered
without
perspective. Hence" the distance between objects is not readily apparent.
Although
SUBSTITUTE SHEET (RULE 26)
CA 02281905 1999-08-20
WO 98/36683 PCT/US98/03464
-5-
a lack of perspective is not critical when visualizing CT and MR data, three
dimensional volumetric rendering allows striking visualization of nearly any
surface
or anatomic feature that has sufficient contrast (attenuation in CT or signal
intensity
difference in MR imaging) compared with neighboring structures.
At present, volumetric or three dimensional rendering of internal
structures has been used to provide either a static or dynamic depiction of
the
scanned objects for viewing by medical personnel. The data accumulated has
not,
to date, been utilized in combination with a computer software and/or hardware
system for analyzing and diagnosing abnormalities.
A need therefore exists for a system for scanning, analyzing and/or
diagnosing abnormalities or deviations in a scanned body structure from a
baseline
set of data with subsequent displaying of the results.
Summar~of the Invention
A non-invasive process is disclosed for analyzing an internal element
in a body of a human or animal. The process involves scanning the body to
acquire
data representing a portion of the body's internal structure. The data is
processed
into three dimensional volumetric data representing the scanned internal body
element. A portion of the volumetric data is selected from the processed
scanned
data. Baseline data representing three dimensional volumetric data for either
a
normal internal body element or the patient's actual internal body element as
determined from previous scanning processes is also used in the process. A
portion
of the baseline data is selected which corresponds to the selected portion of
the
scanned data. The selected portions of the scanned and baseline data are
preferably
compared to determine whether an abnormality exists in the patient. Output
data
is sent to a display for displaying information related to the selected
portions of the
scanned and normative data.
In one embodiment of the invention, the internal body element is a
tracheobronchial tree within the human or animal body. The scanned bronchiole
on a selected generation is compared against a baseline bronchiole on a
corresponding generation. Fluorochemicals can be administered to enhance the
scanned data and, thereby, facilitate the visualization and selection of the
portion
of the scanned data by determining branching along the tracheobronchial tree.
An apparatus according to the present invention is also disclosed.
The apparatus includes a scanner for scanning a portion of a body. A processor
is
SUBSTITUTE SHEET (RULE 26)
CA 02281905 1999-08-20
WO 98/36683 PCT/US98/03464
-b-
utilized to receive the scanned data. The processor converts the scanned data
into
three dimensional volumetric and functional data based on analytical models.
The
processor compares the converted data to baseline data. A monitor is utilized
to
display and compare data related to the scanned and baseline
volumetric/functional
data.
The present invention is useful for analyzing the pulmonary function
of a patient, such as the patient's airway capacity or resistance, pulmonary
volumes
and capacities, and airway reactivity of pharmaceutical agents. The present
invention is also useful for determining congenital anomalies, locating
obstructions
or masses, and/or reducing tissue damage during surgery. The present invention
is also useful for determining changes in a patient's internal structure as
caused by
disease processes, therapeutic or diagnostic intervention.
The foregoing and other features and advantages of the present
invention will become more apparent in light of the following detailed
description
of the preferred embodiments thereof, as illustrated in the accompanying
figures.
Brief Description of the Drawings
For the purpose of illustrating the invention, the drawings show a
form of the invention which is presently preferred. However, it should be
understood that this invention is not limited to the precise arrangements and
instrumentalities shown in the drawings.
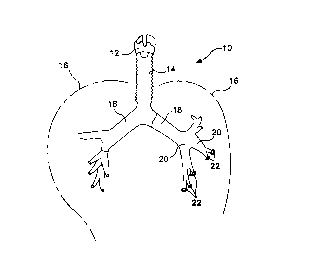
Figure 1 is a diagrammatical representation of a portion of a normal
respiratory system.
Figure 2 is a schematic representation of the Meyer model of the
tracheobronchial tree.
Figure 3 represents the process flow of the present invention.
Figures 4a through 4c are graphical representation of a comparison
between the frequency distribution in a baseiine structure and a scanned
structure.
Figure 5 illustrates a display of a lobar or segmental abnormality.
Figure 6 illustrates a display of a branch abnormality.
Detailed Description of the Preferred Embodiments
The present invention relates to a method for processing images into
structural mathematical models from which structure, function, and reactivity
of the
bodily system can be determined. For the sake of simplicity, the method is
SUBSTITUTE SHEET (RULE 26)
CA 02281905 1999-08-20
WO 98/36683 PCT/US98/03464
_7_
discussed herein as it is contemplated for use in analyzing the structure,
function
and reactivity of a pulmonary system. The invention, however, is equally
applicable to many other systems within the human body. Referring now to the
drawings wherein like reference numerals indicate corresponding or similar
elements throughout the several views, Figure 1 illustrates a diagrammatical
representation of a portion of a normal respiratory system 10. The respiratory
system 10 channels air from a larynx 12 through a trachea 14 into the lungs
16.
The lungs 16 include right and left bronchus 18 and segmental bronchi or
bronchioles 20. Air sacs or alveoli 22 are formed on the terminal ends of the
bronchioles 20. Air exchange occurs between the alveoli 22 and blood
capillaries
(not shown) which surround the alveoli.
There are a number of theoretical and conceptual models which are
useful for characterizing dichotomous, symmetrical, and asymmetrical nodes and
branch points throughout the tracheobronchiai tree. Some of the most well
known
are the Meyer, Horsfield, Strahler, and Weibel models. Figure 2 is a schematic
representation of the "Meyer model" for characterizing the tracheobronchial
tree.
This model identifies the various branches of the bronchus 18 and bronchioles
20.
The descending branches are identified as "generations" or "orders", the 1st
order
representing the right and left bronchus 18 and the subsequent orders
representing
the bronchioles 20. As shown in the figure, the numbering begins at the
trachea,
which is identified as generation 0. The numbering proceeds to the terminal
airways, increasing by one at each dichotomy. This approach can be applied to
both symmetric and asymmetric tree models. Other models, such as Horsfield or
Strahler, have a different counting and numbering system for identifying
branches
and, therefore, in certain cases can end up with fewer orders. The generation
system as described by the Meyer model is a useful method for locating a
branch
relative to the trachea (0) generation and, thus, is the most appropriate
relationship
for bronchoscopic or bronchographic investigations.
Referring to Figure 3, the process flow of the present invention is
illustrated as it is contemplated for use as a non-invasive medical imaging
and
analyzing procedure. The procedure involves the steps of imaging or scanning
the
desired area of a patient, analyzing the scanned data to establish a baseline
prior to
an airway challenge or therapeutic intervention to determine if abnormalities
or
changes exist, and displaying any abnormalities or changes. The imaging step
utilizes a standard imaging device, such as a computer tomography or nuclear
SUBSTITUTE SHEET (RULE 26)
CA 02281905 1999-08-20
WO 98/36683 PCT/US98/03464
_g_
resonance imaging machine. It is also contemplated that the present invention
can
be utilized with three dimensional data acquired by ultrasound. In one
preferred
embodiment, the imaging device is a Picker PQ 5000 helical CT device,
manufactured by Picker International, Cleveland, Ohio.
Although the following procedure is not dependent upon the
administration of a contrast agent, a contrast agent, such as a
fluorochemical, is
preferably administered into or near the area of interest prior to scanning
the
patient. For example, when imaging the internal bronchioles of the lung, it is
preferable to fill at least a portion of the lung with a suitable contrast
agent. The
contrast agent provides the high degree of differentiation between adjacent
structures for subsequent three dimensional rendering. The preferred type of
fluorochemical is a perfluorochemical (PFC). There are a large selection of
PFCs'
on the market and the one chosen for use in the present invention will depend
upon
the specific area of interest. For example, certain physicochemical
characteristics
including, but not limited to, vapor pressure, viscosity, and spreading
coefficients
will influence the rate of evaporation and pattern dispersion of the FC. A
fluid of
high vapor pressure and low viscosity is useful when it is desirable to
perform
imaging throughout the entire lung over a short period of time. A FC viscosity
above about 3 cS is generally considered to be a high viscosity. A FC
viscosity at
or below 3 cS is generally considered to be a low viscosity. A fluid of lower
vapor
pressure and high viscosity may be preferred for local imaging over a longer
time
period. Those skilled in the art would readily appreciate the FC that is
appropriate
for the chosen site and the imaging desired.
Another aspect of the FC is its radiopacity characteristic. A fluid
of marked radiopacity might be particularly useful to delineate larger regions
but
might in fact compromise detection of finer abnormalities. Thus, the physical
characteristics of the FC will determine the preferred FC for the imaging
desired.
Table 1 provides a list of preferred FCs which are contemplated for use in the
present invention. The table also provides the physical properties for each
FC. In
general, all the listed FCs are inert, odorless and colorless. The FCs have
low
surface tension (between approximately 10 and 19 dynes/cm) and high solubility
for
oxygen ( > 40 vol%}. These FCs are insoluble in water, sparingly soluble in
lipids
(as noted by the IogP values) and organic solvents, and completely soluble in
other
fluorinated-compounds. Other fluorochemicals may provide the required contrast
and, therefore, can be substituted for the preferred FC's listed in the table.
SUBSTITUTE SHEET (RULE 26)
CA 02281905 1999-08-20
WO 98/36683 PCT/US98/03464
-9-
TABLE 1
FC LIQUID COMPOUNDS AND PHYSICAL PROPERTIES
Va or PressureViscosi Densi Solubility
FC (mmHg) (cS) g/ml IogP
Com ounds
PP-2 141 0.873 1.78 6.05
PP-5 13.5 2.61 1.95 7.97
PP-9 2.9 3.25 1.97 8.84
PP-11 < 1. 0 14. 0 2 . 00 7 . 81
RM-101 51 0.85 1.78 6.42
PFOB 10.5 1.0 1.89 7.87
wnerem:
PP-2: perfluoromethylcyclohexane manufactured by BNFL
Fluorochemicals
Ltd.
PP-5: perfluorodecalin manufactured by BNFL Fluorochemicals
Ltd.
PP-9: perfluoromethyldecalin manufactured by BNFL Fluorochemicals
Ltd.
PP-11: perfluoroperhydrophenanthrene manufactured by
BNFL Fluorochemicals
Ltd.
RM-101: perfluoro-furan/pyranmixture manufactured by
Mercantile Development,
Inc.
PFOB: perfluorooctylbromide sold under the tradename
LiquiVentx and
manufactured by Alliance Pharmaceutical Corp.
When viewing other organs in the body, it may be more preferable
to provide a contrast agent external to the area of interest. Use of FCs as a
contrast
agent which enhances imaging is well known. See for example, U.S. Patent Nos.
4,993,415 and 5,350,359, which are both incorporated herein by reference in
their
entirety. The FC's can be provided to the patient in any suitable form, such
as neat
liquid, aerosol, vapor, or emulsion.
The preferred FC will have material properties which will allow for
the FC to remain in or around the structure of interest or coat the walls
until the
scanning is complete. For example, when scanning the small bronchioles in the
lungs, it is preferable to utilize a FC which will remain in the lung for a
sufficient
length of time to allow the FC to travel through the multiple branches of
bronchioles. The Houndsfield unit (HU) number of a suitable breathable FC
liquid
is in the range of 800-2700. As discussed above, the preferred form of
fluorocarbon is a perfluorocarbon.
SUBSTITUTE SHEET (RULE 26)
CA 02281905 1999-08-20
WO 98/36683 PCT/US98/03464
-10-
The amount of fluorocarbon necessary will vary depending on the
portion of the body being imaged. For example, when imaging the lung,
approximately 1 to 2 mils per kilo is needed if it is desired to coat only the
alveoli.
Approximately 20 mils per kilo is needed to coat everything, including the
branches. It is desirable to provide a sufficient amount of fluorocarbon to
leave the
airways free. Also, the amount of contrast desired will effect the amount of
fluorocarbon used.
The scanned or imaged data is transferred to a computer processor
or other processing unit. A variety of processing units exist which would be
capable of receiving and analyzing the scanned data. The processor may include
one or more forms of memory (e.g., EPROM, ROM, RAM, etc.) for storing
relevant data. The computations required to form volume rendered images
necessitates a relatively high speed computer. Those skilled in the art are
capable
of selecting a suitable processor for receiving and analyzing the scanned
data. The
imaged data is preferably in the form of numerical data. Any conversions
necessary to transform the scanned or imaged data to numerical data can be
performed either prior to or after transmission to the processor.
The processor preferably utilizes the scanned data to develop a
volumetric model of the scanned object. This is called volumetric rendering.
Conventional software is available to perform volumetric rendering of a
scanned
image (see for example, U.S Pats. Nos. 5,546,807, 5,315,512, and 5,594,842,
which are incorporated herein by reference in their entirety). Picker
International
also distributes Voyager software which is capable of performing volumetric
rendering. Therefore, no further discussion of the software is needed.
The processor also receives baseline data for comparing against the
current scanned or imaged data. The present invention contemplates various
types
of baseline data which can be utilized. In one embodiment, the baseline data
is data
representing a previously scanned portion of the patient's body. For example,
the
patient's lung may have been scanned at an earlier point in time. When the
previous scan was taken will depend on the intended analysis to be performed.
For
example, if it is desired to determine functional respiratory data, the first
scan
(previous scan) may be during inspiration and the subsequent scan may be
during
expiration. For this type of procedure, there may be only seconds or minutes
between scans. Other types of analysis may require scans that are, for
example,
minutes, hours, weeks, months or years old. The previously scanned data is
SUBSTITUTE SHEET (RULE 26)
CA 02281905 1999-08-20
WO 98/36683 PCT/US98/03464
-11-
utilized in the present invention for comparison against the current scanned
data.
The processor determines whether a deviation exists between the prior scanned
data
and the current scanned data as described in more detail below. Hence, in this
embodiment of the invention, the baseline data includes preexisting patient
data.
In an alternate embodiment of the invention, the baseline data is
scanned or stored data representing a "normal" structure. For example, data
representing healthy or normally developing lungs is utilized for comparison
against
the patient's current scanned data. The data is preferably generated from
scans of
a large segment of patients having a normal internal structure of interest
(e.g., a
normally developed lung structure). The data can be categorized based on
various
parameters, such as age, gender, etc. This data is referred to herein as
"normative
data" .
In this embodiment, the processor also preferably receives
background information associated with the scanned data, for example, the age
and
gender of patient and the location of the scanned area within patient. The
processor
preferably either receives this background information directly from the
scanner
(i.e., input into the scanner and transmitted along with the scanned image
data) or
the data can be entered directly into the processor (e. g. , by medical
personnel).
Utilizing this background information, the processor (or the
operator) selects an appropriate set of data representing a "normal" or
"average"
object under consideration (e.g., bronchioles) for use as the baseline data
for
comparison against the patient's scanned image. As described above, the
processor
preferably has available to it data representing a plurality of normal body
structures.
This normative data is either stored internally in the processor, or is
supplied
externally. The processor utilizes the background information to select the
appropriate data for comparing with the actual scanned image.
For example, if the patient is a 3 year old Caucasian male, and the
area of interest is the lungs, the processor will select predetermined data
representing a "normal" or "average" lung in a 3 year old Caucasian male. The
selected data is used as the baseline data for comparison against the imaged
data for
diagnostic analysis as described in more detail below. The criteria which is
used
to select the appropriate normative data can be, for example, age, gender,
race,
height, and/or weight, and is preferably based on a large segment of the
"normal"
population: Those skilled in the art would readily understand how to generate
the
normative high resolution CT data for airway dimensions, geometry, and
computer
SUBSTITUTE SHEET (RULE 26)
CA 02281905 1999-08-20
WO 98/36683 PCT/US98/03464
-12-
functional analysis (i.e., airway conductance) as a function of gender, race,
age,
lung pathophysiology, or differential diagnosis using airway challenge (e.g.,
cold
air, pharmacologic). These criteria should not be considered all encompassing
inasmuch as other criteria for separating the data can be substituted for any
of the
above criteria and are well within the purview of the claims.
Alternately, the normative data can be separated by suitable
background classifications or criteria and stored on individual data storage
media,
such as floppy disks. The appropriate storage media representing the
appropriate
"normal" object is selected by one of the medical personnel and input into the
processor. The normative data may be stored as raw numeric data similar to
scanned data or, more preferably, may be stored as volume-rendered data (i.e.,
data
converted into three-dimension volumes). Exemplary normative data for a
tracheobronchial tree for use in the present invention include, but are not
limited
to, the number of bronchi in a generation, and the diameter, length,
circumference,
cross-sectional area and volume of each bronchi in a given generation. This
type
of information can also be recorded from a prior scan of the patient according
to
the first embodiment of the invention described above.
The processor utilizes the two sets of data (scanned data and selected
baseline data} to determine if and where abnormalities or deviations exist in
the
current version of the patient's scanned data. As will be described in more
detail
below, the processor must first correlate or match the two sets of data in
order to
ultimately determine whether any differences exist. This can be achieved by
initializing or identifying portions of each data set. For example, if the
current
scanned data and the baseline data represent the structure of a lung, it is
desirable
to identify the data in both sets that corresponds to the trachea. The
software
identifies the branch point within the model. From that point, the processor
(or
more appropriately the software operating within the processor) can determine
the
structures that depend from the trachea and properly identify them according
to the
selected model.
An abnormality as determined by the processor is not necessarily
indicative of a unhealthy condition. Instead, an abnormality is, in its
broadest
sense, a difference (deviation) between the scanned data and the normative
data
which requires closer inspection by a physician. What is considered to be an
abnormality will vary depending on the organs that are being analyzed, the
procedures that are being performed, and/or the parameters that are being
SUBSTITUTE SHEET (RULE 26)
CA 02281905 1999-08-20
WO 98/36683 PCT/US98/03464
-13-
compared. A difference may exist between the current scanned data and the
prior
scanned data obtained in the same person, thus representing a return towards
"normal", new abnormalities, or worsening of abnormalities identified on
previous
scans.
If the processor determines that an abnormality exists, it then
displays the location of the abnormality and, preferably, the size of both the
baseline object and the scanned object. The processor can also visually
display the
abnormal structure (e.g., cross-section) if desired. It is also possible to
overlay the
scanned image and the baseline image on the display. The differences can be
highlighted (such as by coloring or shading). Conventional software exists
which
permits such manipulation of computer data. The following examples further
define
and illustrate some of the capabilities of the present invention.
Obstruction or Mass (Radiologic Applications)
Small endobronchial masses which have resolvable thickness or
i 5 distal obstructions can be identified using the present invention since
the small
bronchi are now navigable. The low surface tension of the FC liquid allows it
to
pass beyond any obstructions in the bronchi. The processor compares the cross-
sectional properties of the scanned bronchus and bronchioles (by order) to the
cross-section of the baseline bronchus and bronchioles. If the processor
determines
that the cross-section of the scanned bronchi is sufficiently different than
the
baseline cross-section as indicated by the baseline data, the processor
displays the
location of the abnormality and its size. The processor could also display the
size
of a normal (baseline) bronchi. It is contemplated that a range of values
around the
baseline would be considered "normal" (i.e., not a significant deviation from
the
baseline). A preferred range would be about ~ 2 standard deviations from the
mean. A reading outside of this range would represent an abnormality.
Congenital Anomalies
The caliber of the small bronchi could be determined which may be
important in stricture or hypoplasia. Anomalous bronchial origins and
congenital
or acquired fistulas from the tracheobronchial tree to other organs or spaces
could
be evaluated. The processor can determine this from the acquired data. A
branching -pattern typically is characterized by progressively smaller
diameter
SUBSTITUTE SHEET (RULE 26)
CA 02281905 1999-08-20
WO 98/36683 PCT/US98/03464
- 14-
airways. If the dimensions become larger, this would indicate an abnormality,
such
as a bronchi-bronchi fistula, bronchiectasis, or entry into another organ.
Non-invasive Radiologic Determination of Pulmonary Function
The ability to visualize the small airways non-invasively permits
non-pneumotach pulmonary function tests. The derivable information such as
diameter, length, and volume of the airways from the imaging study can be
broken
down and analyzed. The use of ultrafast electron beam CT significantly
decreases
motion artifact, enabling a greater variety of pulmonary parameters to be
determined at even high breathing frequencies.
a) Structural Analysis: Referring Figure 2 and Table 2, using the Meyer model
for
the tracheobronchial tree, a summarized structural profile table for each
patient can
be generated listing for each generation of bronchi, the number of that
generation,
mean diameter, mean length, cross-sectional area (CSA) and the volume.
SUBSTITUTE SHEET (RULE 26)
CA 02281905 1999-08-20
WO 98/36683 PCT/US98/03464
- 15 -
TABLE 2
Generation/t in DiameterLengthCircumferenceCross Volume
Generation(cm) (cm) Sectional
Area
0 1 2.5 8 7.9 calculatedcalculate
1 2 1.5 5 4.7 y y
2 4 1.0 3 3.2 y y
3 8 y y y y y
4 16 y y 1 y y
5 32 y y y y y
6 64 y y y y y
7 128 y y y y y
8 256 y y y y y
9 512 y y y y y
10 1024 y y y y y
11 2028 y y y y y
12 4056 y y y y y
13 8112 y 1 y y y
14 16,224 y y y y y
15 32,448 1 y l y y
16 64,896 y y y y y
17 129,792 0.08 0.2 0.25 y 1
As discussed above, this scanned data is then compared to baseline
data, which can be normative data based on age, sex/race, height and/or weight-
matched controls, or the patient's prior scanned data. In addition to the
summarized data for each generation (order), the scanned data would be
displayed
to demonstrate the frequency distribution of diameter, length, volume, and CSA
relative to normative values. This comparative analysis permits quick and
accurate
determination of abnormalities. Figures 4a-4c are graphical illustrations of
the
frequency distribution of the number of airways (Y-axis) of a certain diameter
(X-
axis). As shown in Figure 4a, a frequency distribution of the cross-sectional
diameter for the baseline 10th generation (order) bronchioles (solid Line) is
SUBSTITUTE SHEET (RULE 26)
CA 02281905 1999-08-20
WO 98/36683 PCT/US98/03464
- 16-
compared against the frequency distribution of the cross-sectional diameter
for the
current scanned data representing the 10th generation bronchioles for the
patient
(dashed line). From the visual display, the physician can readily determine
whether
or not any abnormalities exist in the scanned image of the patient. In the
scanned
data shown in Figure 4a, the physician can readily determine that the scanned
data
of the patient's 10th generation bronchiole shows abnormal development.
Specifically, all the bronchioles on the 10th generation have a cross-
sectional
diameter that is smaller than the baseline 10th generation bronchioles. In
order to
further assist the physician, it may be preferable to display only the
structures
which are determined to be "abnormal".
Figure 4b is a graphical representation of the frequency distribution
of the cross-sectional diameters for the baseline 10th generation (order)
bronchioles
(solid line) as compared against the frequency distribution of the cross-
sectional
diameters for the current scanned data representing the patient's 10th
generation
bronchioles (dashed line). In this figure, the processor displays (or
determines) that
most of the patient's 10th generation bronchioles have a normal cross-
sectional
diameter. However, a small population of bronchioles have cross-sectional
diameters which are smaller than the baseline and may be localized to a single
lung
segment.
Figure 4c is a graphical representation of a frequency distribution
of a patient's 10th generation bronchioles in which a diffuse abnormality is
seen
such that these airways are both larger and smaller than the baseline.
In addition, intrapulmonary variations can be assessed by the
processor and the anatomical location (i.e., lobar, segmental etc.) of the
abnormal
bronchi can be determined. That is, lobes or segments, as well as bronchial
generations within the lung, are compared against the baseline data and cross-
correlated. The comparison would identify the location of any abnormality
within
the lung (e.g, generations five through ten are abnormal but only in the lower
lobe).
This could be graphically displayed to illustrate the branching
tracheobronchial tree
and identify the location and generation of the abnormal airways. Figure 5
illustrates one such display which is contemplated by the present invention.
Furthermore, diagnosis and therapy of the pulmonary function of a
patient's lung may be determined by comparing ratios for various bronchi. The
present invention utilizes the processor to determine ratios of diameter,
length,
volume, CSA for different generations. These ratios can be displayed to
facilitate
SUBSTITUTE SHEET (RULE 26)
CA 02281905 1999-08-20
WO 98/36683 PCT/US98/03464
-17-
the rapid and accurate differential diagnoses. For example, it may be that in
lymphocytic interstitial pneumonia (LIP) the ratio of the diameters of the 7th
generation bronchioles to the 10th generation bronchioles is increased. This
profile
may occur only in the lower lobes. It is contemplated that the present
invention
would utilize the processor to compare the 7th and 10th generation bronchioles
of
the lower lobes. The ratio is displayed to allow the physician to diagnosis
LIP
earlier. A "normal" (or previous) ratio for the 7th and 10th bronchiole could
also
be displayed to facilitate comparison. This would also be important for
demonstrating the anatomic patterns of disease (i.e., specific lobes, segments
etc.).
Alternately, an index of obstruction can be assigned to a scanned component
(or
portion thereof) and compared to a baseline index (which can be the patient's
baseline or a normative baseline.)
b) Functional Anal, Referring generally to Figure 2, the airways of the lung
can be considered a large group of circuits in series and parallel. All of the
same
I S generation airways are in parallel and the airways from one generation to
the next
are in series. Utilizing the processor of the present invention, the overall
and site
specific pulmonary function can be calculated. That is, the processor can
compare
bronchi parameters for each generation against the baseline data. From this
comparison, the processor can display the specific generation of bronchi where
the
abnormality exists. Figures 5 and 6 illustrate this aspect of the invention.
Furthermore, if the processor determines that the diameter of the scanned
bronchi
is below a predetermined size (indicating substantially restricted flow), the
display
would also indicate the depending generations of bronchi as also being
functionally
abnormal.
Airway resistance: As part of the functional analysis of the lung,
the following equation can be used to determine the resistance in the
different
airway generations, individually, combined, or in the overall lung model
(figure 3).
R=8Lw/II r~
Where: R = resistance;
L = length of airway;
r = radius of the airway; and
w = viscosity of air.
The viscosity (~,) of air is known. The length (L) and radius (r) of the
airway can
be determined from the scanned data. Accordingly, the resistance along each
SUBSTITUTE SHEET (RULE 26)
CA 02281905 1999-08-20
WO 98/36683 PCT/US98/03464
- 18-
generation of airway can be derived. The processor can output the resistance
for
each airway or, alternately, can output airways which have a resistance below
a
normal resistance value as determined from the baseline data.
Airway Compliance (CAW): Functional analysis of the lung also
involves determining the airway compliance. The airway compliance of the
scanned structures are calculated using the following formula.
CAW = nV/nP
Where: o V = the calculated change in volume; and
o P = the calculated change in pressure.
In order to calculate the change in pressure ( o P), the imaging of the lung
structure
is performed twice under two different positive end distending pressure (PEEP)
conditions. In the first condition, there is zero PEEP (i.e., no pressure
applied to
airways). In the second condition, the airways are subjected to a PEEP of 5 cm
HZO (i.e., induced positive pressure). Also, from the two sets of imaged lung
structure the change in the airway volume ( o V) can be calculated. The airway
compliance CAW can then be determined for any visible generation of airway.
It is also 'contemplated that a comparison of the calculated scanned
airway impedance for the scanned data would be compared to the impedance for
the
baseline data. Airway impedance is a function of airway compliance and airway
resistance.
Pulmonary Volumes and Capacities: As discussed above with
respect to the airway compliance, volumes can be calculated from the scanned
data.
In order to determine the pulmonary capacity of the various airways, imaging
is
performed under different breathing conditions (i.e., static and dynamic).
First,
scanned or imaged data is acquired while the patient momentarily stops between
inspiring and expiring. This provides an index of tidal volume within the
airways.
Next, scanned data is acquired while the patient maximally inspires and
momentarily holds his/her breath. This provides data representing airway
volume
at total lung capacity (TLC). Lastly, scanned data is acquired while the
patient
maximally expires. This will provide data representing airway volume at
residual
volume (RV).
Lung volumes and capacities can then be derived by the processor
from this scanned data, such as vital capacity (VC), inspiratory and
expiratory
reserve volumes (IRV and ERV, respectively), and functional residual capacity
(FRC). Inspiratory and expiratory reserve volumes represent the volumes that
one
SUBSTITUTE SHEET {RULE 26)
CA 02281905 1999-08-20
WO 98/36683 PCT/US98/03464
-19-
could inspire/expire above and below a normal tidal volume breath and,
thereby,
increase the depth of breathing. Functional residual capacity represents the
volume
of gas that is in the lung at the end of a normal breath. It is determined by
a
balance of recoil forces across the lung (chest wall pulling outward; lung
pulling
inward) and provides a "buffer" volume of gas in the lung which prevents large
swings in arterial oxygen and carbon dioxide tension throughout a normal
breath.
With the use of a very rapid CT electron beam scanner, a forced
maximum expiratory maneuver, i.e., forced vital capacity (FVC) can be scanned
and analyzed with respect to time to provide standardized indices of airway
function, such as the forced expiratory volume per second (FEV,) of maximum
ventilation (VEmaxO From this data, the processor can analyze time dependent
data
relative to total effort (FEV,IFVC) or resting conditions (FVC/VC) to provide
indices of structural vs functional limitation of lung function.
Baseline datasets generated under any or all of the above conditions
can be utilized to further characterize and identify regional differences in
lung
function in a similar manner as discussed above with respect to the Structural
Analysis of the lung.
c) Airway Reactivity Analysis: It is also contemplated that the present
invention
can be used to determine airway reactivity to pharmacologic agents (e.g.,
vasodilator, bronchodilator, methacholine, etc.), physical agents (e.g., cold
air,
exercise, gases (O,, CO2, He, N20, NO2, etc.)), or various respiratory
maneuvers
(e.g., PIP, PEEP, inspiration, expiration, etc.). For example, after providing
the
FC contrast agent to the area of interest, the area is scanned before and
after
delivery of a pharmacologic agent. The scanned data would be compared to
baseline values representing, for example, standardized dose-response and
regional
airway site-specific nomograms.
In the past, only the overall pulmonary response could be evaluated.
However, by using the technique of the present invention, each generation of
airway could be evaluated to determine if the challenge affected all the
airways or
only specific generations of airways. Airway challenge refers to a stimulus
which
might induce bronchoconstriction, such as inspiration of cold air, inhalation
or
intravenous administration of an airway smooth muscle agonist. Similarly, the
present invention provides an important method for pharmacologic testing of
drugs
to dete-rmine which generations of airways are affected by which drugs. This
would
allow site specific ,pharmacologic intervention to ultimately be determined
for
SUBSTITUTE SHEET (RULE 26)
CA 02281905 1999-08-20
WO 98/36683 PCT/IJS98/03464
-20-
improving therapeutic management. The FC could also be combined with
pharmacologic agents and act as a carrier for delivery of the drug.
Improved Virtual Endosco~ic Technigue Anywhere in the Bodv
The present invention is not limited to performing analysis and
diagnosis of the lung structure but, instead, is applicable to any element or
component within the body. For example, the present invention can be used to
provide virtual endoscopy and diagnosis for the following body elements:
nasopharynx, nasal sinuses, peritoneum (i.e., virtual laparoscopy), GI tract,
urinary
tract, synovial spaces (i.e., virtual arthroscopy), pleural space, and
auditory canal
IO among others. Additionally, this technique is applicable to
intravasculature. When
using this technique on the intravasculature, it is contemplated that FC
liquid would
be used as a blood substitute to assist in imaging. In the In each case,
baseline data
(either based on prior scanned images from the patient or normative data)
would be
acquired for subsequent comparison with the current scanned data. A skilled
artisan would readily appreciate the diverse capabilities of the present
invention in
light of the above discussion.
Imaging the Tracheobronchial Tree and Small Airways External
Using a more viscous FC or by delivering the FC so that it remains
inside and fills the bronchial tree without entering the very tiny respiratory
bronchiolus and alveoli, the tracheobronchial tree could be viewed from the
perspective of its outer walls. In this embodiment, the contrast would be
between
the air filled lung and the very dense FC filled airways. Comparison against
baseline data would provide insight into the existence of any abnormalities in
or on
the walls.
Radioloeic Assisted Surgery
It is also contemplated that the present invention can be utilized to
assist during a surgical procedure. FC enhanced virtual endoscopy can be
applied
as an arthroscopy assistive modality in various procedures in which enhanced
edge-
detection would be advantageous and further minimize the need for invasive
procedures or production of iatrogenic trauma. In this embodiment tissue lined
lumen-may be protected from surgically related tissue trauma during diagnostic
or
therapeutic procedures, such as laparoscopy or "virtual laparoscopy". Scanned
and
SUBSTITUTE SHEET (RULE 26)
CA 02281905 1999-08-20
WO 98/36683 PCT/US98/03464
-21
processed data would provide the surgeon with actual dimensional data before
or
during surgery to facilitate an approximate surgical approach, choice of
instruments,
prosthetic devices, etc. One important advantage to using this information
during
surgery is the potential ability to minimize trauma and risk of infection by
reducing
tissue handling.
The present invention is extremely beneficial in the pediatric
population where the size of the pediatric bronchial tree prevents navigation
with
a conventional bronchoscope. Similarly, the present invention permits analysis
of
small airways which heretofore have not been viewable through non-invasive
procedures. For example, with the use of the present invention, it is possible
to
assess diseases down to approximately the 12th through 17th generation bronchi
(about 1 mm diameter in the adult). This is a much smaller size than is
reachable
by a bronchoscope.
As discussed above, with the use of fluorochemical contrast agents,
the present invention provides a non-invasive means for identifying and
analyzing
branching along the tracheobronchial tree to a degree previously unobtainable
through conventional techniques.
The present invention also provides a novel non-invasive method for
relating structure (e.g., normative data or prior patient scanned data,
obstruction
or mass identification, congenital abnormalities) to function (e. g. ,
pulmonary
function analysis section).
One key benefit of the present invention is the ability to provide
medical personnel with real-time, on-line analysis. When used with a high
speed
processor, the monitoring of the changes in the structure of the patient can
be
performed nearly instantaneously. When using the patient's previously scanned
data, it is possible to monitor changes in a body element due to therapy. For
example, the change in the size of a previously scanned mass may provide an
indication as to whether a change in therapy is warranted.
While the above discussion has described the use of a display for
providing a comparison of the actual scanned data against the baseline data,
or for
identifying an abnormality, it is also contemplated that the desired
information can
be "displayed" in printed format.
SUBSTITUTE SHEET (RULE 26)
CA 02281905 1999-08-20
WO 98/36683 PCT/US98/03464
-22-
Experimental Testing
Experimental tests were conducted utilizing several aspects of the
present invention. An eight week old 1400 gram New Zealand white rabbit was
locally anesthetized. A tracheotomy was performed with a Hi-Lo Jet
endotracheal
tube (Mallincrodt, Glen Falls, NY) inserted proximal to the caring. After
systemic
anesthesia, the rabbit was connected to a ventilator circuit and placed on a
CT table
in the supine position. The rabbit was gas ventilated at the same frequency
(30
bpm), tidal volume (9-5 ml/kg), temperature (37°C) and inspiratory time
(0.3 sec)
for the duration of the protocol with continuous cardiovascular monitoring for
data
comparison. The rabbit received an initial dose ( 17cc/kg) of
perfluorooctylbromide
(PFOB)(LiquiVent', Alliance Pharmaceutical. Corp. ) administered via the
endotracheal tube which equated to the measured gas functional residual
capacity
(FRC) as was determined by closed circuit helium dilution (PANDA, Medical
Associated Services, Hatfield, PA).
Imaging was performed on a Picker PQ 5000 helical CT scanner
before and after the administration of the PFOB during ventilated respiration.
Images were obtained wing a targeted 10 cm FOV, 3 mm slice thickness with a
pitch of 1.25, images reconstructed every 3 mm, 'smooth spatial reconstruction
algorithm, and a mA/kVp of 200/120. Each revolution of the helical tube
required
1 second. An additional set of images was obtained with the rabbit held at
peak
inspiratory pressure (PIP) with the same parameters except the images were
reconstructed every 1 mm. The images were then transmitted to an independent
work station (Voxel Q). Using software supplied by Picker International, three
dimensional rendering of the images was performed with identical windowing.
Using the three dimensional rendering, the operator was able to navigate
through
the lumen of the reconstructed tracheobronchial tree. The software permitted
viewing the inside wall of the tracheobronchial tree at any angle, navigating
from
distal to proximal, or entering distal to an obstruction. The entire process
required
only ten minutes of post-processing time. The maximum tracheal diameter of the
rabbit was approximately 5.2 mm.
The PFOB distributed evenly within the lungs with pooling in the
bronchi occurring only immediately after the PFOB was administered. Initially,
a
small amount of pooled PFOB was seen in the proximal right and left mainstem
bronchi on-the reconstructed bronchoscopic CT. The PFOB rapidly distributed
out
SUBSTITUTE SHEET (RULE 26)
CA 02281905 1999-08-20
WO 98/36683 PCT/US98/03464
- 23 -
of the larger airways into the very small airways and alveoli due to the
application
of PEEP (positive end expiratory pressure).
An endoscopic CT without PFOB was compared against the
endoscopic CT with PFOB. Without PFOB, the endoscopic CT only allowed
visualization of the trachea, carina, and the mainstem bronchi with very poor
visualization of the third order branches. By comparison the endoscopic CT
with
PFOB allowed improved visualization of the tracheobronchial tree down to
fourth
order branches with visualization of the orifices of fifth order branches in
some
locations .
The above results were obtained without suspension of ventilated
respiration. Respiratory motion results in the loss of resolution and contrast
between the air in the smaller airways and the PFOB and prevents navigation
into
these small bronchi. Endoscopic CT was performed with PFOB and peak
inspiratory breathhold to examine the effects of respiratory motion. The
endoscopic
CT which was performed with PIP and reconstructed every 1 mm, allowed
navigation into fifth order bronchi which were approximately 0.8 mm in
diameter.
The rabbit tolerated the PFOB well, maintaining physiologic gas
exchange and cardiopulmonary profile throughout the procedure.
The Houndsfield unit (HU) number of PFOB liquid is in the range
of 2700-2800. The Houndsfield unit number of PFOB in the rabbit lung is 1400
1700. PFOB inspired in the trachea creates a marked contrast difference
between
the air in the bronchi and the contrast in the lung. Since the bronchial wall
can be
assumed to have similar CT density to soft tissue and have a Houndsfield unit
number of approximately 40-60, the contrast difference achieved with FC is
much
greater than can be achieved with endogenous tissue densities in the lung. The
above described testing demonstrated that contrast improvement with PFOB
allows
markedly better visualization of significantly smaller bronchi with the
bronchoscopic
CT technique than the identical bronchoscopic CT technique without PFOB.
Since the airways of adult humans are much larger than those of the
1400 gram rabbit, the results of the testing in this small animal model
indicate that
the present invention would permit evaluation of small bronchi and be useful
in the
assessment of small airway disease down to approximately 12th order bronchi
(about 1 mm diameter in an adult human). In addition, the testing demonstrates
that the present invention might allow for the evaluation of changes in the
caliber
of small airways under different conditions such as inspiration, expiration,
PIP,
SUBSTITUTE SHEET (RULE 26)
CA 02281905 1999-08-20
WO 98!36683 PCT/US98/03464
-24-
PEEP (positive end expiratory pressure), bronchodilators or even slight
negative
pressure.
With respect to the pediatric population, the diameter of a steerable
pediatric bronchoscope is about 3mm. The size of the tracheobronchial tree in
premature infants is similar to the 1400 gram rabbit. As such, it is not easy
to
visualize the tracheobronchial tree in an infant utilizing conventional
bronchoscopy.
However, as demonstrated by the above tests, the present invention would be
useful
for non-invasively evaluating the bronchial tree in the pediatric population.
The present invention has been described thus far as it is intended
to be used for imaging and analyzing a body element on a macroscopic level.
However, it is contemplated that the present invention is also applicable for
imaging
and analyzing a body element on a cellular or molecular level. The
capabilities of
the invention are primarily dependent on the imaging resolution. Current and
developing technology is such that the visualization of the dynamics at the
level of
the cell membrane are readily foreseeable, even down to the molecular level.
The
present invention as described above would be readily applicable to such
images.
Hence, on both a cellular and molecular level, a comparison can be made
between
a body element of a patient and their own test profiles to evaluate a change
from
a prior state. Alternatively, cellular and molecular imaging of a body element
can
be compared to normative values based on selected criteria, such as age and
gender,
to evaluate the patient relative to a population standard.
The present invention can be applied to a cellular membrane to
calculate transmembrane flux of substrates by visualization of substrate
concentration on the two sides of the membrane. Also, imaging and analysis
according to the present invention permit direct observation of drug uptake
with the
resulting measurement of diffusion and partition coefficients. This permits an
assessment to be made of biovailability. The drug can be marked with a
biologically active or inert material, such as FC.
Using the present invention, physiologic responses on the cellular
level can be determined for any agent, such as coil contraction, secretion,
endocytosis, exocytosis etc. Also, direct observation can be performed of
functional cell actions at the tissue level (i. e. , Osteociast/osteoblast
interaction on
bone and changes/deviations from normal.
High resolution imaging permits the use of the present invention to
analyze and evaluate branching structure in blood vessels, nerves,
capillaries,
SUBSTITUTE SHEET (RULE 26)
CA 02281905 1999-08-20
WO 98/36683 PCT/US98/03464
- 25 -
lymphatics. As microscopic imaging techniques improve cellular and subcellular
structures may be analyzed to evaluate microtubular structure, actin
filaments,
membrane structure, and extraceilular "potential" space.
Contrast agents bound to antibodies for certain structures could
create boundaries (similar to cell membranes) which would allow evaluation of
extracellular space. In this regard, indium or gadolinium could be targeted to
the
epithelium/endothelium tissue of various organs which would enable the
epithelium/endothelium tissue to then be imaged. Epithelium or endothelium
could
be visualized in ureter, bile duct, pancreatic duct, lymphatic ducts, epidural
space,
meninges, inner ear/semi-circular canals, or other epithelium or endothelium
contammg structures.
It is also contemplated that ultra miniaturization will permit tiny
robot probes or sensors to be placed within the body at desired locations.
These
devices, operating in conjunction with an ultrasonic or magnetic resonance
imaging
system, would send back structural information from within the body that is
analyzed using the above-disclosed techniques.
Cellular and molecular imaging also permits evaluation of important
pulmonary structures and functions. For example, such imaging would permit
direct observation of ciliary function and clearance, single isolated airway
smooth
muscle cell assessment of airway contractility secondary to agonist
stimulation, and
assessment of other airway cell functions including secretory cells to assess
mechanisms of mucous secretion and surfactant production.
While the present invention has generally referred to radiographic
scanning as the preferred form of scanning, other non-invasive methods for
producing scanned images of a body element, such as sonographic imaging, are
also
contemplated for use in the present invention.
Although the invention has been described and illustrated with
respect to the exemplary embodiments thereof, it should be understood by those
skilled in the art that the foregoing and various other changes, omissions and
additions may be made therein and thereto, without parting from the spirit and
scope of the present invention.
SUBSTITUTE SHEET (RULE 26)