Note: Descriptions are shown in the official language in which they were submitted.
CA 02282622 1999-08-23
WO 98/38237 PCT/EP98/00717
POLYMERS COMPRISING A FLUORINATED CARBONATE MOIETY
The present invention is in the field of optical components, more
particularly, polymeric optical components. By optical components are
' S meant here, thermo-optical components, electro-optical components or
passive components.
Both thermo-optical and electro-optical components are known. The
working of thermo-optical components is based on the phenomenon of the
optical waveguide material employed exhibiting a temperature dependent
refractive index.
Polymeric thermo-optical components generally comprise a polymeric
three-layer structure on a substrate. The three-layer structure comprises a
low refractive index lower cladding layer, a high refractive index core layer,
and a low refractive index upper cladding layer. On top of the upper
cladding layer heating elements are provided (usually metal strips) to heat
the polymeric cladding and core material, in order to change the refractive
index for switching. The working of electro-optical devices is based on the
phenomenon of the non-linear optically active material employed exhibiting
an electric field dependent refractive index. Polymeric electro-optical
components in general also comprise a polymeric three-layer structure.
The three-layer structure comprises a low refractive index lower cladding
layer, a non-linear optically active, high refractive index core layer, and a
low refractive index upper cladding layer. On top of the upper cladding
layer electrodes are provided to apply an electric field to the non-linear
optically active material to change the refractive index for switching.
Optical components having an at least yenta-layered polymer structure on
' a substrate comprising:
a) a low refractive index lower cladding layer,
b) a core-matching refractive index lower cladding layer,
c) a core layer,
CA 02282622 1999-08-23
WO 98/38237 PCT/EP98/00717
2
d) a core-matching refractive index upper cladding layer, and
e) a low refractive index upper cladding layer, are also known in the art.
With this specific layer structure optimum transversal confinement can be
obtained, which results in less loss of light and an improved switching
efficiency.
For optical components preferably silicon substrates are used. These
substrates are readily available on the market and are of homogeneous
thickness. Furthermore, they are frequently used in integrated circuit
techniques and apparatus. One disadvantage of silicon is its high refractive
index. Due to this high refractive index the light of the propagating mode
might leak into the silicon substrate. The low refractive index lower cladding
layer a) is applied to prevent leaking of light from the propagating mode into
the silicon substrate. When other substrates are used, the low refractive
index lower cladding a) is also of advantage in controlling the confinement
of the propagating mode. Using a low refractive index lower cladding a) of
appropriate index and thickness gives ample freedom in designing the
core-matching refractive index cladding layers b) and d) and the core layer
c).
As described above, the optical components usually comprise metal
electrodes on top of the upper cladding layer, either for use as heating
elements or for applying an electric field. These electrodes are usually
made of gold and/or other metals such as chromium, nickel, copper,
platinum, or combinations or alloys thereof.
The low refractive index upper cladding e) is applied to prevent leaking of
the light from the propagating mode into the attenuating (gold) electrodes.
The refractive indices of the low refractive index lower and upper cladding
layers a) and e) are usually (approximately) the same.
CA 02282622 1999-08-23
WO 98/38237 PCTlEP98/00717
3
Employing a low refractive index upper cladding layer e) with a larger
thickness than that of the low refractive index lower cladding layer a)
makes it -possible to use a core-matching refractive index upper cladding
layer d) which is thinner than the core-matching refractive index lower
cladding b). In this case the resulting combined thickness of the low
refractive index upper cladding d) and the core-matching refractive index
upper cladding e) is smaller than the combined thickness of the low
refractive index lower cladding a) and the core-matching refractive index
lower cladding b). As a consequence, the structure is transversally
asymmetric, with the core layer being close to the electrodes and thus
experiencing stronger induced thermo-optical or electro-optical effects,
resulting in a more efficient component.
The core-matching refractive index lower cladding b) and the core
matching refractive index upper cladding d) are applied to obtain
transversal confinement of the propagating mode. The refractive index can
be chosen in a relatively wide range to achieve the required properties,
such as: monomode behavior, good overlap with a Standard Single Mode
Fiber (SMF).
Lateral confinement can be achieved by all known methods for defining
channels in planar waveguiding components. Suitable methods are:
1. shaping the core layer by etching techniques (for instance reactive ion
etching with oxygen plasma) to obtain a buried channel waveguide,
2. bleaching the core layer to obtain a buried channel waveguide,
3. shaping either of the core-matching refractive index upper and lower
cladding layers b) and d) to obtain a ridge (strip loaded) or an inverted
ridge waveguide,
4. bleaching either of the core-matching refractive index upper and lower
cladding layers b) and d) to obtain a ridge (strip loaded) or an inverted
ridge waveguide.
CA 02282622 1999-08-23
_ WO 98/38237 PCT/EP98/00717
4
All these techniques are known to the artisan and need no further
elucidation here. When using technique 1, the core layer is etched away,
leaving only the channel waveguide. Subsequently, core-matching
refractive index upper cladding material is applied both on top of the core
layer c) and onto the areas where the core material was etched away. This
technique and also technique 2 are preferred because they can result in
symmetrical channel waveguides. Symmetrical channel waveguides show
low polarization dependence of the modal properties. When the bleaching
technique is used, the refractive index of the core-matching refractive index
cladding layers b) and d) should be adapted to the refractive index of the
bleached parts of the core. When the shaping of the core technique is
used, the refractive index of the core-matching refractive index upper
cladding layer material is chosen such as to give the required properties,
such as: monomode behavior, good overlap with a Standard Single Mode
Fiber (SMF), low polarization dependence, low bend losses.
The polymers used for thermo-optical devices according to the invention
are so-called optical polymers. Many optical polymers are known in the art,
but there is still need for improvement. A particular problem of polymeric
waveguides is the difference between the refractive indices of the core
layer and the surrounding cladding layers. Typically, these index
differences are in the range of 0.003 to 0.008. In waveguide switches
switching is induced by index differences of 0.001 (digital switches) to
0.0001 (interferometer switches). These small index differences can be
induced by thermo- or electro-optical properties of polymeric materials. In a
thermo-optical switch the core index is lowered by focally heating the layer
stack by means of a heating element. The closer the heater is to the core,
the more efficiently this index lowering can be performed by a lower
switching power. To prevent unwanted light absorption by the heater
elements, it is advantageous to apply a low-index cladding layer between
the waveguide and the heating element. The lower the index of the
a
CA 02282622 1999-08-23
~;
. ,
, . ,,
-, ... .. ..'
AEM 2570 R
leakage to the substrate, this material must have a low absorption at the
operating wavelengths (1.3 and 1.5 ~~m). Low refractive index polymers
have been disclosed in WO 96128493, but their optical loss is
relatively high. It is therefore an object of the invention to provide
5 polymeric material with very low refractive index, and very low optical
loss,
preferably less than 0.15 dB/cm. However, the polymeric material must
also display high Tg because of chemical and optical stability, and be
cross-linkable to obtain cladding layers suitable for thermo-optical
waveguides. Moreover, when polymeric material is used as a waveguide
core, it is advantageous to use material with an index similar to that of the
optical fiber attached to said waveguide, which effective index for standard
single mode fiber is 1.467 at 1.3 ~m and 1.468 at 1.5 Vim. When doped
silica is used as a core, the polymers of the invention can be used
advantageously as cladding because their refractive indices can be lower
than that of the glass core, which has the advantage that these hybrid
waveguides can be rendered athermal.
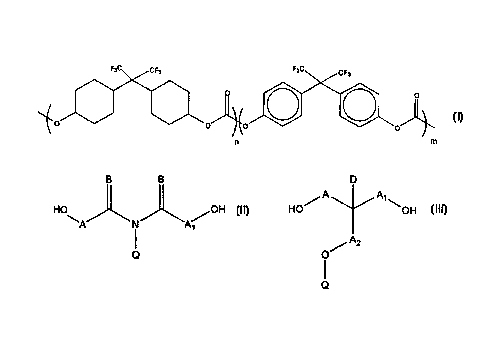
It has now been found that a cross-linkable fluorinated polymer comprising
the carbonate moiety having the formula:
F3C CFa FaC CF7
0 ~ O
~0 0 0 0
n m
wherein n = 1-10 and m = 0-9 meets these demands. Preferably n =1-3
andm=0-3.
The various layers can be applied by spin-coating. In order to be able to
spin-coat layer-on-layer, it is often necessary to cross-link one layer before
applying the next. Therefore, the optical polymers or NLO polymers are
AMENDED SHEET
CA 02282622 1999-08-23
WO 98/38237 PCT/EP9$/00717
6
rendered cross-linkable either by the incorporation of cross-linkable
monomers or by mixing cross-linkers such as polyisocyanates,
polyepoxides, etc. into the polymer.
Preferably, the polymer further comprises a cross-linkable moiety derived ,
from a diol selected from:
g B D
A A~
H \ /OH ~a HO~ OOH
A N A~
A2
i o
Q
wherein
A, A~, and A2 are independently a bond or C~_~2 alkyiene, or together with
the carbon atoms to which they are bonded form a 5- or 6-membered ring;
B is independently O or C~~ alkyl;
Q is -CO-C(=E)D, wherein
D is H or C~.~ alkyl; and
E is C~.~ alkylidene;
and each of the alkyl, alkylene, and alkylidene groups may be halogenated.
More preferably, the cross-linkable moiety is derived from the diol with the
formula HO-CH2-CH(OH)-CH2-O-CO-C(=CH2)CH~.
The term C» alkyl means an alkyl group with 1 to 4 carbon atoms, such as
methyl, ethyl, propyl, isopropyl, butyl, tent butyl, and the like. Methyl is
the
preferred alkyl group.
The term C~_~2 alkylene means an alkylene group with 1 to 12 carbon
atoms, such as methylene, ethylene, propylene, 2,2,-dimethylpropylene,
CA 02282622 1999-08-23
- WO 98/38237 PCT/EP98/00717
7
dodecylene, and the like. A, A~, and A2 are preferably a bond or an
alkylene group with 1-3 carbon atoms.
The term C~-s alkylidene means an alkylidene group with 1 to 6 carbon
atoms, such as methylene, ethylidene, propylidene, 2-methylpropylidene,
and the like. Methylene and ethylidene are the preferred alkylidene groups.
When the alkyl, alkylene, or alkylidene groups are halogenated, chlorine
and fluorine are the preferred halogens. Fluorine is the most preferred
halogen. The index of the polymer can be fine-tuned by selecting the
number, the type, and the combination of halogens.
The polymer of the invention can be prepared by standard methods known
in the art for the preparation of similar polymers. For instance, the
bischloroformate of hexafluorobisphenol A or hexafluoroisopropylidene-
dicyclohexanediol bischloroformate can be polymerized in suitable solvents
with the hexafluoro-perhydro-bisphenol A, the synthesis of which has been
disclosed in EP 0,279,462, optionally in the presence of suitable cross-
linkable moieties, such as the above-mentioned diols.
Non-linear electric polarization may give rise to several optically non-linear
phenomena, such as frequency doubling, Pockets effect, and Kerr effect. In
order to render polymeric non-linear optical material active (obtain the
desired NLO effect macroscopically), the groups present in the polymer,
usually hyperpolarizable side-groups, first have to be aligned (poled). Such
alignment is commonly effected by exposing the polymeric material to
electric (DC) voltage, the so-called poling held, with such heating as will
render the polymeric chains sufficiently mobile for orientation.
In order to enhance the stability of the thermo-optical component, oxygen
scavengers and radical scavengers and the like may be added to the
optical polymers.
CA 02282622 1999-08-23
WO 98138237 PCT/EP98/00717
8
The invention is further illustrated by the following examples.
EXAMPLE I
Hexafluoro-perhydro-bisphenol A (150.0 g), hexafluorobisphenol A
bischloroformate (398.7 g), and 2,3-dihydroxypropyl methacrylate (68.9 g)
were dissolved in a mixture of anhydrous dichloromethane (1.5 I) and
anhydrous tetrahydrofuran (1 I). After cooling to 0°C, anhydrous
pyridine
(133.4 g) was added dropwise. After the addition, the reaction mixture was
allowed to warm to room temperature and stirred overnight. The pyridine
hydrochloride was filtered off and the filtrate was precipitated in 50 I of
methanol. The precipitate was filtered off and washed with methanol. The
polymer was dried overnight in vacuo at 5 kPa at 50°C. Yield 460 g. Mw
26,000; Mn 13,000; Tg 119-126°C; TGA 190°C.
EXAMPLE II
The optical loss and the Tg of prior art polymers (A-C) with normal
refractive indices were compared with the optical loss of the very low
refractive index polymer of Example I:
Compound Refractive IndexOptical Loss Tg
(1565 nm) (dB/cm) (C)
A 1.5170 0.1 168/193
B 1.5111 0.1 168/190
C 1.4837 0.12 163/183
Example 1 1.4676 0.15 167/195
The optical loss and the Tg of prior art polymers (D-E) with very low
refractive indices were compared with the optical loss of the very low
refractive index polymer of Example I:
CA 02282622 1999-08-23
WO 98/38237 PCT/EP98/00717
9
Compound Refractive Optical Loss Tg
Index
{1565 nm) (dBlcm) (C)
D 1.4622 N.D. 88/97
E 1.4640 0.25 78/93
Example I 1.4676 0,15 167/195
N.D. = not determined
Key (monomer composition in mole%):
S T U V W
A 50 25 (R=H) - - 25
B 40 25 (R=H) - - 25
10 (R=COCI)
C - 25 (R=H) _ _ 25
50 (R=COCI)
D 50 (R=COCI) 25 - 25
E - 50 (R=COCI) - 25 25
legenda:
S=
Br
CIO( OCOC1
SUBSTITUTE SHEET (RULE 26)
CA 02282622 1999-08-23
- WO 98/38237 PCT/EP98/00717
9a
T=
F3C~ ~CF3
RO~ v v FOR
U=
CFg CFg
Ho-cl~-cl-~-o ~ ~ o-cl-~-ct-~-off
CF3 CFg
V=
HO-CH 2-(CF 2)4-CH 2-OH
w=_
OH
OOH
O O
X Y Z
Example I 50 25 25
SUBSTITUTE SHEET (RULE 26)
CA 02282622 1999-08-23
- WO 98/38237 PCT/EP98/00717
9b
legenda:
X=
C~oCO
Y=
Z=
OH
OOH
O O
It may be concluded that the polymer of the invention has a Tg and an
optical loss which are comparable with those of higher refractive index
materials, whereas the Tg of the polymer of the invention is
substantially higher than the Tg of other very low refractive index
materials.
1~
SUBSTITUTE SHEET (RULE 26)
CA 02282622 1999-08-23
- WO 98/38237 PCT/EP98/00717
Example III
A solution of 22.5 g of the polymer of Example I and 1.13 g of dicumyl
peroxide in 77.5 g of cyclohexyl acetate was filtered through a 0.2 p,m
filter.
5 The solution was spin-coated at 1000 rpm for 30 sec and then cured in a
vacuum oven which was heated from 20°C to 200°C in 1.5 h and
then kept
at 200°C for 30 min. The resulting product was cooled slowly to give a
cladding layer having a refractive index of 1.4676 and an optical loss of
0.15 dB/cm which was used in a penta-layered thermo-optical device
10 according to PCT application WO 97/01782.
Example IV
A reactor was charged with 122 g of hexafluoroisopropylidenedicyclo
hexanol, 100 g of triphosgene (bis(trichloromethyl) carbonate), and 1 I of
dry toluene, under a nitrogen atmosphere.
The temperature of the reactants was lowered to approx. -3°C with
stirring,
after which 86.3 g of N,N-dimethylaniline diluted in 100 ml of dry toluene
were added dropwise in 80 min.
The reaction mixture was diluted with 200 ml of dry toluene, the
temperature was raised to ca. 70°C, and the mixture was stirred for
approx.
50 h more under a stream of nitrogen to remove the excess phosgene and
other volatile products.
After working up (extraction with 10% hydrochloric acid, water, and brine,
drying on sodium sulfate, evaporation of toluene in vacuo, recrystallization
from toluene/n-hexane 1/1.35 v/v, 0.28 g/ml, and drying) pure hexafluoro
isopropylidenedicyclohexanediol bischloroformate was obtained in approx.
33% overall yield.
To a stirred solution of 11.45 g of hexafluoroisopropylidenedicyclohexane
diol bischloroformate, 4.17 g of hexafluoroisopropylidenedicyclohexanol,
and 1.95 g of dihydroxy-isopropylmethacrylate dissolved in 70 ml of tetra
CA 02282622 1999-08-23
WO 98/3$237 PCT/EP98/00717
11
hydrofuran/dichloromethane 2.4/1 v/v were added dropwise 3.83 g of
pyridine in 10 ml of tetrahydrofuran/dichloromethane 2.4/1 v/v at 20°C
in
approx. 60 min.
The reaction mixture was stirred overnight, the precipitate (pyridine.HCl
salt) was removed by filtration. The polymer was precipitated by vigorous
stirring in 1 I of methanol, collected, and stirred with fresh methanol. The
product was collected by filtration and left to dry in a vacuum oven at
65°C
to yield 11.2 g (approx. 70%) of a polymer the recurring unit of which has
the formula:
F3C CF3
O
O O O
3
HaC
Mw = 13,460 ; Mn = 6,470; polydispersity 2.08.
Refractive index at 1550 nm 1.4428 and at 1300 nm 1.4443.