Note: Descriptions are shown in the official language in which they were submitted.
CA 02282722 1999-09-O1
WO 98/39416 PCT/US98/04345
1
METHOD OF CLONING ANIMALS
S FIELD OF THE IN VENTION
The invention relates to the cloning of animals.
BACKGROUND OF THE INVENTION
The following discussion of the background of the invention is merely
provided to aid the reader in understanding the invention and is not admitted
to
describe or constitute prior art to the present invention.
Researchers have been developing methods for cloning mammalian animals
over the past two decades. These reported methods typically include the steps
of ( I )
isolating a cell, most often an embryonic cell; (2) inserting the cell or
nucleus isolated
from the cell into an enucleated oocyte (e.g., the oocyte's nucleus was
previously
extracted), and (3) allowing the embryo to mature in vivo.
The first successful nuclear transfer experiment using mammalian cells was
reported in 1983, where the pronuclei isolated from a marine (mouse) rygote
were
inserted into an enucleated oocyte and resulted in like offspring(s). McGrath
8
Solter, 1983, Science 110:1304-130. Subsequently, others described the
production
of chimeric marine embryos (~.R.. embryos that contain a subset of cells
having
significantly different nuclear DNA from other cells in the embryo) using
marine
primordial germ cells (PGC). These cells are and can give rise to pluripotent
cells
(e.g., cells that can differentiate into other types of cells but do not
differentiate into a
grown animal). Matsui et al., 1992, Cell 70:841-847 and Resnick et al., 1992,
Nature
359:550; Kato et al., 1994, Journal of Reproduction and Fertility Abstract
Series,
Society For the Study of Fertility, Annual Conference, Southampton, 13:38.
Some publications related to marine pluripotent cells stress the importance of
CA 02282722 1999-09-O1
WO 98/39416 PCT/US98/04345
2
steel factor for converting precursor cells into pluripotent cells. U.S.
Patents
5,453,357 and 5,670,372, entitled "Pluripotent Embryonic Stem Cells and
Methods of
Making Same," issued to Hogan. These same publications indicate that marine
pluripotent cells exhibit strong, uniform alkaline phosphatase staining.
Although marine animals were never clearly cloned from nuclear transfer
techniques using embryonic cells, some progress was reported in the field of
cloning
ovine (sheep) animals. One of the first successful nuclear transfer
experiments
utilizing ovine embryonic cells as nuclear donors was reported in 1986.
Willadsen,
1986, Nature 310:63-65. A decade later, others reported that additional lambs
were
cloned from ovine embryonic cells. Campbell et al., 1996, Nature 380:64-66 and
PCT Publication WO 95/20042. Recently, another lamb was reported to be cloned
from ovine somatic mammary tissue. Wilmut et al., 1997, Nature 385:810-813.
Some methods for cloning ovine animals focused upon utilizing serum deprived
somatic ovine cells and cells isolated from ovine embryonic discs as nuclear
donors.
PCT Publications WO 96/07732 and WO 97/07669. Other methods for cloning ovine
animals involved manipulating the activation state of an in at~o matured
oocyte after
nuclear transfer. PCT Publication WO 97/07668.
While few lambs were produced, publications that disclose cloned lambs
report a cloning efficiency that is, at best. approximatrlv 0.4°,0.
Cloning efficiency, as
calculated for the previous estimate, is a ratio equal to thr number of cloned
lambs
divided by the ntunber of nuclear transfers used to produce that numbs of
cloned
lambs.
Despite the slower progress endemic to the field of cloning bovine animals, a
bovine animal was cloned using embryonic cells derived from 2-64 cell embryos.
This bovine animal was cloned by utilizing the nuclear transfer techniques set
forth in
U.S. Patents 4,994,384 and 5,057,420. Others reported that cloned bovine
embryos
were formed by nuclear transfer techniques utilizing the inner cell mass cells
of a
blastocyst stage embryo. Sims & First, 1993, Theriogertology 39:313 and Keefer
et
al., 1994, Mol. Reprod. Dev. 38:264-268. In addition, another publication
reported
CA 02282722 1999-09-O1
WO 98/39416 PCT/US98I~4345
3
- that cloned bovine embryos were prepared by nuclear transfer techniques that
utilized
PGCs isolated from fetal tissue. Delhaise et al., 1995, Reprod. Fert. Develop.
7:1217
1219; Lavoir 1994, J. Reprod Dev. 37:413-424; and PCT application WO 95/10599
entitled "Embryonic Stem Cell-Like Cells." However, the reports demonstrated
that
cloned PGC-derived bovine embryos never clearly developed past the first
trimester
during gestation. Similarly, embryonic stem cell (e.g., cell line derived from
embryos
which are undifferentiated, pluripotent, and can establish a permanent cell
line which
exhibits a stable karyotype), ESC, derived bovine embryos never developed past
fifty-
five days, presumably due to incomplete placental development. Stice et al..
1996,
Biol. Reprod .54: 100-110.
Despite the progress of cloning ovine and bovine animals, there remains a
great need in the art for methods and materials that increase cloning
efficiency. In
addition there remains a great need in the art to expand the variety of cells
that can be
utilized as nuclear donors, especially expanding nuclear donors to non-
embryonic
cells. Furthermore, there remains a long felt need in the art for
karyotypically stable
permanent cell lines that can be used for genome manipulation and production
of
transgenic cloned animals.
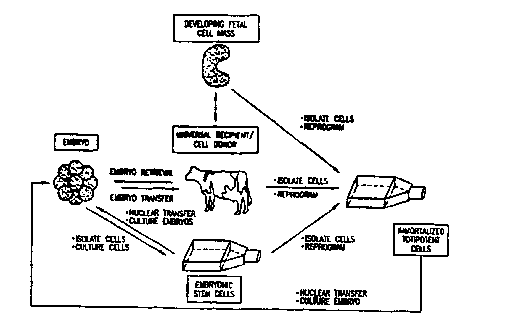
The present invention relates to cloning technologies. The invention relates
in
part to immortalized, totipotent cells useful for cloning animals, the embryos
produced
fmm these cells using nuclear transfer techniques, animals that arise from
these cells
and embryos, and the methods and processes for creating such cells, embryos,
and
animals.
The present invention provides multiple advantages over the tools and
methods currently utilized in the field of mammalian cloning. Such features
and
advantages include:
( 1 ) Production of cloned animals from virtually any type of cell. The
invention provides materials and methods for reprogramming non-totipotent
cells into
CA 02282722 1999-09-O1
WO 98/39416 PCT/US98/04345
4
totipotent cells. These non-totipotent cells may be of non-embryonic origin.
This
feature of the invention allows for the ability to assess the phenotype of an
existing
animal and then readily establish a permanent cell line for cloning that
animal.
(2) Creation of permanent cell lines from virtually any type of cell.
Permanent
cell lines provide a nearly unlimited source of genetic material for nuclear
uansfer
cloning techniques. In one aspect of the invention, non-totipotent precursor
cells can
be reprogrammed into totipotent and permanent cells. These non-totipotent
precursor
cells may be non-embryonic cells. Permanent cell lines provide the advantage
of
enhancing cloning efficiency due to the lower cellular heterogeneity within
the cell
lines (e.g., permanent cells that have lower rates of differentiation than
primary culture
cell lines currently used for cloning). In addition, the permanent cell lines
can be
manipulated in vitro to produce cells, embryos. and animals whose genomes have
been manipulated (e.g., transgenic). Furthermore, permanent cell lines can be
more
easily stored, transported. and re-established in culture than other types of
cell lines.
1 S (3) Enhancement of the efficiency for cloning embryos as a result of
utilizing
asynchronous, permanent, and l:aryotypically stable cell lines in a complete
in vitro
embryo production system.
Cloning efficiency can be expressed by the ratio between the number of
embryos resulting from nuclear transfer and the number of nuclear transfers
performed to give rise to the embryos. Alternatively, cloning efficiency can
be
expressed as the ratio between the number of live bom animals and the number
of
nuclear transfers performed to give rise to these animals.
In a first aspect, the invention features a totipotent mammalian cell.
Preferably, the totipotent mammalian cell is an immortalized cell. In
addition, the
mammalian cell is preferably an ungulate cell and more preferably a bovine
cell.
The term "mammalian" or "mammal" as used herein can refer to any warm-
blooded animal or cell from that animal. A mammalian animal of the invention
is
CA 02282722 1999-09-O1
WO 98139416 PCT/US98/04345
preferably an endangered animal, or, more preferably, a farm animal.
The term "ungulate" as used herein can refer to a four-legged animal having
hooves. In other preferred embodiments, the ungulate is selected from the
group
consisting of domestic or wild representatives of bovids, ovids, cervids,
suids, equids
5 and camelids. Examples of such representatives are cows or bulls, bison,
buffalo,
sheep, big-hom sheep, horses, ponies, donkies, mule, deer, elk, caribou, goat,
water
buffalo, camels, Llama, alpaca. and pigs. Especially preferred in the bovine
species are
Bos taurus, Bos indices, and Bos bu,~faloes cows or bulls.
The term "bovine" as used herein can refer to a family of ruminants belonging
to the genus Bos or any closely related genera of the family Bovidae. The
family
Bovidae includes we antelopes. oxen, sheep. and goats. for example. Preferred
bovine animals are the cow and ox. Especially preferred bovine species are Bos
taurus, Bos indices. and Bos bufjaloes. Other preferred bovine species are Bos
primixenius and Bos longifrons.
1 S The term "totipotent" as used herein can refer to a cell that gives rise
to all of
the cells in a developing body, such as an embn~o, fetus. and animal. The term
"totipotent" can also refer to a cell that gives rise to all of the cells in
an animal. A
totipotent cell can give rise to all of the cells of a developing cell mass
when it is
utilized in a procedure for creating an embwo from one or more nuclear
transfer steps.
An animal may be an animal that functions cx umro. An animal can exist, for
example, as a live born animal. Toapotent cclis may also be used to generate
incompietc animals such as those useful for organ harvesting, e.~., having
genetic
modifications to eliminate growth of a head such as by manipulation of a
homeotic
gene.
The terms "developing cell mass" as used herein can refer to a group of cells
in which all cells or a portion of the cells are undergoing cell division. The
developing cell mass may be an embryo, a fetus, andlor an animal, for example.
The
developing cell mass may be dividing in vitro (e.g., in culture) or in vivo
(e.g., in
utero). The developing cell mass may be a product of one or more nuclear
transfer
CA 02282722 1999-09-O1
WO 98/39416 PCT/US98/04345
6
processes or may be the product of oocyte activation (e.g., sperm mediated
fertilization).
The term "live born" as used herein preferably refers to an animal that exists
ex utero. A "live born" animal may be an animal that is alive for at least one
second
S from the time it exits the maternal host. A "live born" animal may not
require the
circulatory system of an in utero environment for survival. A "live bom"
animal may
be an ambulatory animal. Such animals can include pre- and post-pubital
animals. In
addition, a "live born animal" may also be deceased for a certain period of
time. As
discussed previously, a "live born" animal may lack a portion of what exists
in a
normal animal of its kind. For example, a "live born'' animal may lack a head
as a
result of the deletion or manipulation of one or more homeotic genes.
The term "totipotent" as used herein is to be distinguished from the tetin
"pluripotent." The tatter term can refer to a cell that differentiates into a
sub-
population of cells within a developing cell mass, but is a cell that cannot
give rise to
1 S all of the cells in that developing cell mass. The term "pluripotent" can
refer to a cell
that cannot give rise to all of the cells in a live born animal.
The term "toiipotent" as used herein is also to be distinguished from the term
"chimer" or "chimera." The latter term can refer to a developing cell mass
that
comprises a sub-group of cells harboring nuclear DIVA with a significantly
different
nucleotide base sequence than the nuclear DNA of other cells in that cell
mass. The
developing cell mass can, for example, exist as an embryo, fetus. and/or
animal.
The term "immortalized" or "permar>ent" as used herein in reference to cells
can refer to cells that have exceeded the Hayflick limit. The Hayflick limit
curt be
defined as the ntunber of cell divisions that occur before a cell line becomes
senescent.
Hayflick set this limit to approximately 60 divisions for most non-
immortalized cells.
See, e.g., Hayflick and Moorhead, 1961, Exp. Cell. Res. 25: 585-621; and
Hayflick,
1965, Exp. Cell Research 37: 614-636, incorporated herein by reference in
their
entireties including all figures, tables, and drawings. Therefore, an
immortalized cell
line can be distinguished from non-immortalized cell lines if the cells in the
cell line
CA 02282722 1999-09-O1
WO 98/39416 PCTlUS98104345
7
are able to undergo more than 60 divisions. If the cells of a cell line are
able to
undergo more than 60 cell divisions, the cell line is an immortalized or
permanent cell
line. The immortalized cells of the invention are preferably able to undergo
more than
70 divisions, are more preferably able to undergo more than 80 divisions, and
are
most preferably able to undergo more than 90 cell divisions.
Typically. immortalized or permanent cells can be distinguished from non-
immortalized and non-permanent cells on the basis that immortalized and
permanent
cells can be passaged at densities lower than those of non-immortalized cells.
Specifically. immortalized cells can be grown to confluence (e.g., when a cell
monolayer spreads across an entire plate) when plating conditions do not allow
physical contact between the cells. Hence, immortalized cells can be
distinguished
from non-immortalized cells when cells are plated at cell densities where the
cells do
not physically contact one another.
The term "plated" or ''plating" as used herein in reference to cells can refer
to
establishing cell cultures in vitro. For example, cells can be diluted in cell
culture
media and then added to a cell culture plate or cell culture dish. Cell
culture plates are
commonly known to a person of ordinary skill in the art. Cells may be plated
at a
variety of concentrations andlor cell densities.
The meaning of the term "cell plating" can also extend to the term ''cell
passaging." Immortalized cells of the invention can be passaecd using cell
culture
techniques well ktwwn to those skilled in the art. The term "cell passaging"
can refer
to such techniques which typically involve the steps of ( 1 ) ecleastng cells
from a solid
support and disassociation of these cells, and (2) diluting the cells in fresh
media
suitable for cell proliferation. Immortalized cells can be successfully grown
by plating
the cells in conditions where they lack cell to cell contact. Cell passaging
may also
refer to removing a portion of liquid medium bathing cultured cells and adding
liquid
medium from another source to the cell culture.
The term "proliferation" as used herein in reference to immortalized or
permanent cells can refer to a group of cells that can increase in size and/or
can
CA 02282722 1999-09-O1
WO 98/39416 PCT/US98/04345
increase in numbers over a period of time.
The term "confluence" as used herein can refer to a group of cells where a
large percentage of the cells are physically contacted with at least one other
cell in that
group. Confluence may also be defined as a group of cells that grow to a
maximum
cell density in the conditions provided. For example, if a group of cells can
proliferate
in a monolayer and they are placed in a culture vessel in a suitable growth
medium,
they are confluent when the monolayer has spread across a significant surface
area of
the culture vessel. The surface area covered by the cells preferably
represents about
50% of the total surface area, more preferably represents about 70% of the
total
surface area. and most preferably represents about 90% of the total surface
area.
The tetTn ''culture'' as used herein in reference to cells can refer to one or
more
cells that are static or undergoing cell division in a liquid medium. Nearly
any type of
cell can be placed in cell culture conditions. Cells may be cultured in
suspension
and/or in monolayers with one or more substantially similar cells. Cells may
be
cultured in suspension and/or in monolayers with a heterogeneous population
cells.
The term "heterogeneous" as utilized in the previous sentence can relate to
any cell
characteristics, such as cell type and cell cycle stage, for example. Cells
may be
cultured in suspension and/or in monolayers with feeder cells. The term
"feeder cells"
is defined hereafter. Cell cultures can be utilized to establish a cell line.
'The term "suspension" as used herein can refer to cell culture conditions in
which the cells are not attached to a solid support. Cells proliferating in
suspension
can be stirred while proliferating using apparatus well known to those skilled
in the
art.
The term "monolayer ' as used herein can refer to cells that are attached to a
solid support while proliferating in suitable culture conditions. A small
portion of the
cells proliferating in the monolayer under suitable growth conditions may be
attached
to cells in the monolayer but not to the solid support. Preferably less than
15% of
these cells are not attached to the solid support, more preferably less than
10% of
these cells are not attached to the solid support, and most preferably less
than 5% of
CA 02282722 1999-09-O1
WO 98/39416 PCT/US98/04345
9
these cells are not attached to the solid support.
The term ''substantially similar" as used herein in reference to immortalized
bovine cells can refer to cells from the same organism and the same tissue.
Substantially similar can also refer to cell populations that have not
significantly
differentiated. For example, preferably less than 15% of the cells in a
population of
cells have differentiated, more preferably less than 10% of the cell
population have
differentiated, and most preferably less than 5% of the cell population have
differentiated.
The term ''cell line" as used herein can refer to cultured cells that can be
passaced more than once. The invention relates to cell lines that can be
passaged
more than 2, 5, 10, 15, 20, 30, S0, 80, 100, and 200 times. The concept of
cell
passaging is defined previously.
In preferred embodiments, ( 1 ) the totipotent cells are not alkaline
phosphatase
positive; (2) the totipotent cells arise from at least one precursor cell; (3)
the precursor
cell is isolated from and/or arises from any region of an animal; (4) the
precursor cell
is isolated from and/or arises from any cell in culture; (5) the precursor
cell is selected
from the group consisting of a non-embryonic cell, a non-fetal cell, a
differentiated
cell, a somatic cell, an embryonic cell, a fetal cell, an embryonic stem cell,
a
primordial germ cell, a genital ridge cell. a cell isolated from an
asynchronous
population of cells. and a cell isolated from a synchronized population of
cells where
the synchronous population is not arrested in the G° stage of the cell
cycle; and (6) the
precursor cell is preferably isolated and~or arises from a mammalian animal,
mvrc
preferably an ungulate animal, and most preferably a bovine animal.
The term "alkaline phosphatase positive" as used herein can refer to a
detectable presence of cellular alkaline phosphatase. Cells that are not
alkaline
phosphatase positive do not stain appreciably using a procedure for
visualizing
cellular alkaline phosphatase. Procedures for detecting the presence of
cellular
alkaline phosphatase are well-known to a person of ordinary skill in the art.
See, e.g.,
Matsui et al., 1991, "Effect of Steel Factor and Leukemia Inhibitory Factor on
Murine
CA 02282722 1999-09-O1
WO 98139416 PCTlUS98/04345
Primordial Germ Cells in Culture," Nature 353: 750-752. Examples of cells that
stain
appreciably for alkaline phosphatase can be found in the art. See, e.g., U.S.
Patent
5,453,357, Entitled "Pluripotent Embryonic Stem Cells and Methods of Making
Same," issued to Hogan on September 26, 1995, which is incorporated by
reference
S herein in its entirety, including all figures, tables, and drawings.
The term "precursor cell" or "precursor cells" as used herein can refer to a
cell
or cells used to create a cell line of totipotent cells. The cell line is
preferably
permanent. Precursor cells can be isolated from any mammal, preferably from an
ungulate and more preferably from a bovine animal. The precursor cell or cells
may
10 be isolated from nearly any cellular entit)~. For example, a precursor cell
or cells may
be isolated from blastocysts, embryos, fetuses, and cell fines (e.~,~., cell
Iines
established from embryonic cells), preferably isolated from fetuses and/or
cell lines
established from fetal cells. and more preferably isolated from ex utero
animals andlor
cell cultures andlor cell lines established from such ex utero animals. An ex
utero
1 ~ animal may exist as a newborn animal, adolescent animal, yearling animal,
and adult
animal. The ex utero animals may be alive or post mortem. The precursor cell
or
cells may be immortalized or non-immortalized. These examples are not meant to
be
limiting and a further description of these exemplary precursor cells is
provided
hereafter.
The term "arises from" as used herein can refer to the conversion of one or
more cells into one or more other cells. For example, a non-totipotent
precursor ccll
can be converted into a totipotent cell by utilizing features of the invention
described
hereafter. This conversion process can be referred to as a rcpmgramming step.
In
another example, a precursor cell can give rise to a feeder layer of cells, as
defined
hereafter. In addition, the term "arises from" can refer to the creation of
totipotent
embryos from immortalized, totipotent cells of the invention, as described
hereafter.
The term "reprogramming" or "reprogrammed" as used herein can refer to
materials and methods that can convert a non-totipotent cell into an
totipotent cell.
Distinguishing features between totipotent and non-totipotent cells are
described
CA 02282722 1999-09-O1
WO 98/39416 PGT/US98/04345
11
previously. An example of materials and methods for converting non-totipotent
cells
into totipotent cells is to incubate precursor cells with a receptor ligand
cocktail.
Receptor ligand cocktails are described hereafter.
The term "isolated" as used herein can refer to a cell that is mechanically
separated from another group of cells. Examples of a group of cells are a
developing
cell mass, a cell culture, a cell line, and an animal. These examples are not
meant to
be limiting and the invention relates to any group of cells.
The term "non-embryonic cell" as used herein can refer to a cell that is not
isolated from an embryo. Non-embryonic cells can be differentiated or non-
differentiated. Non-embryonic cells can refer to nearly any somatic ezll, such
as cells
isolated from an cx mero animal. These examples are not meant to be limiting.
For the purposes of the present invention, the term "embryo" or ''embryonic"
as used herein can refer to a developing cell mass that has not implanted into
the
uterine membrane of a maternal host. Hence, the term ''embryo" as used herein
can
refer to a fertilized oocyte, a cybrid (defined herein). a pre-blastocyst
stage developing
cell mass, and/or any other developing cell mass that is at a stage of
development prior
to implantation into the uterine membrane of a maternal host. Embryos of the
invention may not display a genital ridge. flence, an "embryonic cell" is
isolated from
and~or has arisen from an embryo.
An embryo can represent multiple stages of cell development. For example, a
one cell embryo can be referred to as a rygote, a solid spherical mass of
cells resulting
from a cleaved embryo can be referred to as a morula, and an embn~o having a
blastocoel can be referred to as a blastocyst.
The term "fetus" as used herein can refer to a developing cell mass that has
implanted into the uterine membrane of a maternal host. A fetus can include
such
defining features as a genital ridge, for example. A genital ridge is a
feature easily
identified by a person of ordinary skill in the art, and is a recognizable
feature in
fetuses of most animal species. The term "fetal cell" as used herein can refer
to any
cell isolated from and/or has arisen from a fetus or derived from a fetus. The
term
CA 02282722 1999-09-O1
WO 98/39416
PCT/US98/04345
12
"non-fetal cell" is a cell that is not derived or isolated from a fetus.
The term "primordial germ cell" as used herein can refer to a diploid somatic
cell capable of becoming a germ cell. Primordial getirt cells can be isolated
from the
genital ridge of a developing cell mass. The genital ridge is a section of a
developing
cell mass that is well-known to a person of ordinary skill in the art. See,
e.g.,
Sirelchenko, 1996, Theriogenolo~ 45: 130-141 and Lavoir 1994, J. Reprod. Dev.
37:
413-424.
The term "embryonic stem cell" as used herein can refer to pluripotent cells
isolated from an embryo that are maintained in in vitro cell culture.
Embryonic stem
cells may be cultured with or without feeder cells. Embryonic stem cells can
be
established from embryonic cells isolated from embryos at any stage of
development,
including blastocyst stage embryos and pre-blastocyst stage embryos. Embryonic
stem cells are well known to a person of ordinary skill in the art. See. e.g.,
WO
97/37009, entitled "Cultured Inner Cell Mass Cell-Lines Derived from Uneulate
Embryos," Stice and Golueke, published October 9, 1997, and Yang R Anderson.
1992. Theriogenologv 38: 3l5-335, both of which are incorporated herein by
reference in their entireties, including all figures, tables, and drawings.
The term "differentiated cell" as used herein can refer to a precursor cell
that
has developed from an unspecialized phenotype to that of a specialized
phenotype.
For example, embryonic cells can differentiate into an epithelial cell lining
the
intestine. It is highly unlikely that differentiated cells revert into their
precursor cells
m vivo or in vrtro. However, materials and methods of the invention can
reprogram
differentiated cells into immortalized, totipotent cells. Differentiated cells
can be
isolated from a fetus or a live born animal, for example.
In contrast to the totipotent and/or immortalized cells of the invention that
arise from non-embryonic cells, an example of embryonic cells is discussed in
WO
96/07732, entitled "Totipotent Cells for Nuclear Transfer," hereby
incorporated herein
by reference in its entirety including all figures, drawings, and tables. The
WO
96/07732 publication relates primarily to ovine animals. A unique feature of
the
CA 02282722 1999-09-O1
WO 98139416 PCT/US98/04345
13
present invention is that immortalized, totipotent cells are reprogrammed from
non-
embryonic cells by utilizing the materials and methods described herein in
descriptions of the preferred embodiments and exemplary embodiments.
The tenor "asynchronous population" as used herein can refer to cells that are
not arrested at any one stage of the cell cycle. Many cells can progress
through the
cell cycle and do not arrest at any one stage, while some cells can become
arrested at
one stage of the cell cycle for a period of time. Some known stages of the
cell cycle
are G°, G,, S, GI, and M. An asynchronous population of cells is not
manipulated to
synchronize into any one or predominantly into any one of these phases. Cells
can be
arrested in the Go stage of the cell cycle, for example, by utilizing multiple
techniques
known in the art, such as by serum deprivation. Examples of methods for
arresting
non-immortalized cells in one part of the cell cycle are discussed in WO
97/07669.
entitled "Quiescent Cell Populations for Nuclear Transfer," hereby
incorporated
herein by reference in its entirety, including all figures, tables, and
drawings.
The terms "synchronous population' and "synchronizing" as used herein can
refer to a fraction of cells in a population that are arrested (i.c., the
cells are not
dividing) in a discreet stage of the cell cycle. Preferably, about 50% of the
cells in a
population of cells are arrested in one stage of the cell cycle, more
preferably about
70°0 of the cells in a population of cells are arrested in one stage of
the cell cycle, and
most preferably about 90°ro of the cells in a population of cells are
arrested in one
stage of the cell cycle. Cell cycle stage can be distinguished by relative
cell siu as
well as by a variety of cell markers well known to a person of ordinary skill
in the art.
For example, cells can be distinguished by such markers by using flow cvtometw
techniques well known to a person of ordinary skill in the art. Alternatively,
cells can
be distinguished by size utilizing techniques well known to a person of
ordinary skill
in the art, such as by the utilization of a light microscope and a micrometer,
for
example.
In preferred embodiments, ( 1 ) the totipotent cells of the invention comprise
modified nuclear DNA; (2) the modified nuclear DNA includes a DNA sequence
that
CA 02282722 1999-09-O1
WO 98/39416 PCT/US98/04345
14
encodes a recombinant product; (3) the recombinant product is a polypeptide;
(4) the
recombinant product is a ribozyme; (4) the recombinant product is expressed in
a
biological fluid or tissue; (5) the recombinant product confers or partially
confers
resistance to one or more diseases; (6) the recombinant product confers
resistance or
S partially confers resistance to one or more parasites; (7) the modified
nuclear DNA
comprises at least one other DNA sequence that can function as a regulatory
element;
(8) the regulatory element is selected from the group consisting of promotor,
enhancer, insulator, and repressor; and (9) the regulatory element is selected
from the
group consisting of milk protein promoter. urine protein promoter, blood
protein
promoter, tear duct protein promoter. synovial protein promoter, mandibular
gland
protein promoter, casein promoter, ~i-casein promoter, melanocortin promoter.
milk
serum protein promoter, a-lactalbumin promoter. whey acid protein promoter,
uroplakin promoter, a-actin promoter.
The term "modified nuclear DNA" as used herein can refer to the nuclear
1 ~ deoxyribonucleic acid sequence of a cell, embryo, fetus, or animal of the
invention
that has been manipulated by one or more recombinant DNA techniques. Examples
of
these recombinant DNA techniques are well known to a person of ordinary skill
in the
art. which can include ( 1 ) inserting a DNA sequence from another organism
(e.~., a
human organism) into target nuclear DNA. (2) deleting one or more DNA
sequences
from target nuclear DNA, and (3) introducing one or more base mutations (e.g.,
site-
dirceted mutations) into target nuclear DNA. Cells H7th modified nuclear DNA
can
be referred to as ''tr~ansgenic cells" for the purposes of the invention.
Transgenic cells
can be useful as materials for nuclear transfer cloning techniques provided
herein.
Methods and tools for insertion. deletion, and mutation of nuclear DNA of
mammalian cells are well-known to a person of ordinary skill in the art. See,
Molecular Cloning, a Laboratory Manual, 2nd Ed., 1989, Sambrook, Fritsch, and
Maniatis, Cold Spring Harbor Laboratory Press; U.S. Patent 5,633,067, "Method
of
Producing a Transgenic Bovine or Transgenic Bovine Embryo," DeBoer et al.,
issued
May 27, i 997; U.S. Patent 5,612,205, "Homologous Recombination in Mammalian
CA 02282722 1999-09-O1
WO 98/39416 PCT/(TS98/04345
Cells," Kay et al., issued March I 8, 1997; and PCT publication WO 93/22432,
"Method for Identifying Transgenic Pre-Implantation Embryos," all of which are
incorporated by reference herein in their entirety, including all figures,
drawings, and
tables. These methods include techniques for transfecting cells with foreign
DNA
fragments and the proper design of the foreign DNA fragments such that they
effect
insertion, deletion, and/or mutation of the target DNA genome.
Transgenic cells may be obtained in a variety of manners. For example,
transgenic cells can be isolated from a transgenic animal. Examples of
transgenic
animals are well known in the art, as described herein with regard to
transgenic bovine
10 and ovine animals. Cells isolated from a transgenic animal can be converted
into
totipotent and/or immortalized cells by using the materials and methods
provided
herein. In another example, transgenic cells can be created from totipotent
and/or
immortalized cells of the invention. Materials and methods for converting non-
transgenic cells into transgenic cells are well known in the art, as described
15 previously.
Any of the cell types defined herein can be altered to harbor modified nuclear
DNA. For example. embryonic stem cells, fetal cells, and any totipotent and
immortalized cell defined herein can be altered to harbor modified nuclear
DNA.
Examples of methods for modifying a target D1'A genome by insertion.
deletion, andlor mutation arc rrtroviral insertion, artificial chromosome
techniques.
Fcne inscrtion, random inscrtion with tissuc specific promotcrs, homologous
recombination, gone tarttcting. traruposablc clements. andior any other method
for
introducing foreign DNA. Other modification techniques well known to a person
of
ordinary skill in the art include deleting DNA sequences from a genome, andlor
altering nuclear DNA sequences. Examples of techniques for altering nuclear
DNA
sequences are site-directed mutagenesis and polymerise chain reaction
procedures.
Therefore, the invention provides for bovine cells that are simultaneously
totipotent,
immortalized, and transgenic. These transgenic, totipotent, immortalized cells
can
serve as nearly unlimited sources of donor cells for production of cloned
transgenic
CA 02282722 1999-09-O1
WO 98/3941b PCT/ITS98I04345
16
animals.
The term "recombinant product" as used herein can refer to the product
produced from a DNA sequence that comprises at least a portion of the modified
nuclear DNA. This product can be a peptide, a polypeptide, a protein, an
enzyme, an
antibody, an antibody fragment, a polypeptide that binds to a regulatory
element (a
term described hereafter), a structural protein, an RNA molecule. and/or a
ribozyme,
for example. These products are well defined in the art. This list of products
is for
illustrative purposes only and the invention relates to other types of
products.
The term "ribozyme" as used herein can refer to ribonucleic acid molecules
that can cleave other RNA molecules in specific regions. Ribozymes can bind to
discrete regions on a RNA molecule, and then specifically cleave a region
within that
binding region or adjacent to the binding region. Ribozyme techniques can
thereby
decrease the amount of polypeptide translated from formerly intact message RNA
molecules. For specific descriptions of ribozymes. see U.S. Patent 5.354.855,
entitled
I S "RNA Ribozyme which Cleaves Substrate RNA without Formation of a Covalent
Bond." Cech et al., issued on October 11, I 994, and U.S. Patent 5,591,610,
entitled
"RNA Ribozyme Polymerases, Dephosphorylases, Restriction Endoribonucleases and
Methods." Cech et al., issued on January 7, 1997, both of which are
incorporated by
reference in their entireties including all figures, tables, and drawings.
The term "biological fluid or tissue" as used herein can refer to any fluid or
tissue in a biological organism. The fluids may include, but are not limited
to. tears.
saliva, milk, urine. amniotic fluid. semen. plasma. oviductal fluid. and
synovial fluid.
The tissues may include. but are not limited to. lung, heart, blood, liver,
muscle, brain.
pancreas. skin, and others.
The term "confers resistance" as used herein can refer to the ability of a
recombinant product to completely abrogate or partially alleviate the symptoms
of a
disease or parasitic condition. Hence, if the disease is related to
inflammation, for
example, a recombinant product can confer resistance to that inflammation if
the
inflammation decreases upon expression of the recombinant product. A
recombinant
CA 02282722 1999-09-O1
WO 98f39416 PCT/US98I04345
17
product may confer resistance or partially confer resistance to a disease or
parasitic
condition, for example, if the recombinant product is an anti-sense RNA
molecule that
specifically binds to an mRNA molecule encoding a polypeptide responsible for
the
inflammation. Other examples of conferring resistance to diseases or parasites
are
described hereafter. In addition, examples of diseases are described
hereafter.
Examples of parasites and strategies for conferring resistance to these
parasites
are described hereafter. These examples include, but are not limited to,
worms,
insects, invertebrate, bacterial, viral, and eukaryotic parasites. These
parasites can
lead to diseased states that can be controlled by the materials and methods of
the
invention.
The term "regulatory element" as used herein can refer to a DNA sequence
that can increase or decrease the amount of product produced from another DNA
sequence. The regulatory element can cause the constitutive production of the
product
(e.g., the product can be expressed constantly). Alternatively, the regulatory
element
can enhance or diminish the production of a recombinant product in an
inducible
fashion (e.g., the product can be expressed in response to a specific signal).
The
regulatory element can be controlled, for example, by nutrition, by light, or
by adding
a substance to the trartsgenic organism's system. Examples of regulatory
elements
well-known to those of ordinary skill in the an are promoters. enhancers.
insulators,
and rcprrssors. See, e.~~.. TransgeW c Anrrrrals. Gtrreratron and Use, 1997,
Edited by
L. M. Houdebine, Hardwood Academic Publishers. Australia, hereby incorporated
herein by reference in its entirety including all figures, tables. and
drawings.
The term "promoters" or "promoter" as used herein can refer to a DNA
sequence that is located adjacent to a DNA sequence that encodes a recombinant
product. A promoter is preferably operatively linked to the adjacent DNA
sequence.
A promoter typically increases the amount of recombinant product expressed
from a
DNA sequence as compared to the amount of the expressed recombinant product
when no promoter exists. A promoter from one organism can be utilized to
enhance
recombinant product expression from a DNA sequence that originates from
another
CA 02282722 1999-09-O1
WO 98139416 PCT/US98/04345
18
organism. In addition, one promoter element can increase an amount of
recombinant
products expressed for multiple DNA sequences attached in tandem. Hence, one
promoter element can enhance the expression of one or more recombinant
products.
Multiple promoter elements are well-known to persons of ordinary skill in the
art.
Examples of promoter elements are described hereafrer.
The term "enhancers" or "enhancer" as used herein can refer to a DNA
sequence that is located adjacent to the DNA sequence that encodes a
recombinant
product. Enhancer elements are typically located upstream of a promoter
element or
can be located downstream of the coding DNA sequence (e.g., the DNA sequence
transcribed or translated into a recombinant product or products). Hence, an
enhancer
element can be located 100 base pairs. 200 base pairs, or 300 or more base
pairs
upstream of the DNA sequence that encodes the recombinant product. Enhancer
elements can increase the amount of recombinant product expressed from a DNA
sequence above the increased expression afforded by a promoter element.
Multiple
enhancer elements are readily available to persons of ordinary skill in the
art.
The term "insulators" or "insulator" as used herein can refer to DNA
sequences that flank the DNA sequence encoding the recombinant product.
Insulator
elements can direct the recombinant product expression to specific tissues in
an
organism. Multiple insulator elements are well known to persons of ordinary
skill in
the art. See, e.~., Geyer, 1997, Curr (~W . Genet. Dea. ?: 242-248, hereby
incorporated herein by reference in its entiret, , including all figures,
tables, and
drawings.
The term "repressor" or "rcprrssor element" as used herein can refer to a DNA
sequence located in proximity to the DNA sequence that encodes the recombinant
product, where the repressor sequence can decrease the amount of recombinant
product expressed from that DNA sequence. Repressor elements can be controlled
by
the binding of a specific molecule or specific molecules to the repressor
element DNA
sequence. These molecules can either activate or deactivate the repressor
element.
Multiple repressor elements are available to a person of ordinary skill in the
art.
CA 02282722 1999-09-O1
WO 98/39416 PCT/US98J04345
19
The terms "milk protein promoter," "urine protein promoter," "blood protein
promoter," "tear duct protein promoter," "synovial protein promoter," and
"mandibular gland protein promoter" refer to promoter elements that regulate
the
specific expression of proteins within the specified fluid or gland or cell
type in an
animal. For example, a milk protein promoter is a regulatory element that can
control
the expression of a protein that is expressed in the milk of an animal. Other
promoters, such as casein promoter, a-lactalbumin promoter, whey acid protein
promoter, uroplakin promoter, and a-actin promoter, for example, are well
known to a
person of ordinary skill in the ari.
In preferred embodiments, ( 1 ) the totipotent cell is subject to
manipulation; (2)
the manipulation comprises the step of utilizing a totipotent cell in a
nuclear transfer
procedure; (3) the manipulation comprises the step of cryopreserving the
totipotent
cells; (4) the manipulation comprises the step of thawing the totipotent
cells: (5) the
manipulation comprises the step of passaging totipotent cells; (6) the
manipulation
comprises the step of synchronizing totipotent cells; (7) the manipulation
comprises
the step of transfecting totipotent cells with foreign DNA; and (8) the
manipulation
comprises the step of dissociating a cell from another cell or group of cells.
The term "manipulation" as used herein can refer to the common usage of the
term, which is the management or handling directed towards some object.
Examples
of manipulations are described herein.
The term "nuclear transfer' as used herein can refer to introducing a full
complement of nuclear DNA from one ecll to an enucleated cell. Nuclear
transfer
methods arc well known to a person of ordinary skill in the art. Sec. U.S.
Patent No.
4.994,384. entitled "Multiplying Bovine Embryos." Prather er al., issued on
Februar~~
19, 1991 and U.S. Patent No. 5,057,420, entitled "Bovine Nuclear
Transplantation,"
Massey, issued on October 15, 1991, both of which are hereby incorporated by
reference in their entirety including all figures, tables and drawings.
Nuclear transfer
may be accomplished by using oocytes that are not surrounded by a zona
pellucida.
Although the basic principals of nuclear transfer have been described
CA 02282722 1999-09-O1
WO 98/39416 PCT/L1S98/04345
previously, the technique can be sensitive to the introduction of any new
parameters.
Therefore, significant modifications to the techniques described in the area
of nuclear
transfer may require some experimentation to determine the practical effect of
these
modifications upon the efficiency of nuclear transfer. An example of a
variable that
5 can affect nuclear transfer efficiency is the age of the oocyte utilized for
enucleation
and nuclear transfer.
The term "cryopreserving" as used herein can refer to freezing a cell. embryo,
or animal of the invention. The cells, embryos, or portions of animals of the
invention
are frozen at temperatures preferably lower than 0°C, more preferably
lower than -
10 80°C, and most preferably at temperatures lower than -196°C.
Cells and embryos in
the invention can be cryopreserved for an indefinite amount of time. It is
known that
biological materials can be cryopreserved for more than fifty years. For
example,
semen that is cryopreserved for more than fifty years can be utilized to
artificially
inseminate a female bovine animal. Methods and tools for cryopreservation are
well-
15 known to those skilled in the art. See, e.g., U.S. Patent No. 5.160.312,
entitled
"Cryopreservation Process for Direct Transfer of Embryos." issued to Voelkel
on
November 3, 1992.
The term "thawing" as used herein can refer to the process of increasing the
temperature of a cryopreserved cell, embryo, or portions of animals. Methods
of
r0 thawing cryopresrrved materials such that they are active after the thawing
process are
well-know to those of ordinary skill in the art.
The tams "transfected." "transformation," and "transfectiori ' as used herrin
refer to methods of inserting foreign DNA into a cellular organism. These
methods
involve a variety of techniques. such as treating the cells with high
concentrations of
salt, an electric field, liposomes, polycationic micelles, or detergent, to
render the host
cell outer membrane or wall permeable to nucleic acid molecules of interest.
These
specified methods are not limiting and the invention relates to any
transformation
technique well known to a person of ordinary skill in the art. See, e.g.,
Molecular
Cloning, a Laboratory Manual, 2nd Ed., 1989, Sambrook, Fritsch, and Maniatis,
Cold
CA 02282722 1999-09-O1
WO 98139416 PCT/US98104345
21
Spring Harbor Laboratory Press and Transgenic Animals, Ge»eration and Use,
1997,
Edited by L. M. Houdebine, Hardwood Academic Publishers, Australia, both of
which
were previously incorporated by reference.
The term "foreign DNA" as used herein can refer to DNA that can be
transfected into a target cell, where the foreign DNA harbors at least one
base pair
modification as compared to the nuclear DNA of the target organism. Foreign
DNA
and transfection can be further understood and defined in conjunction with the
term
"modified nuclear DNA," described previously.
The term "dissociating" as used herein can refer to the materials and methods
useful for pulling a cell away from another cell. For example, a blastomere
(i.e.. a
cellular member of a blastocyst stage embryo) can be pulled away from the rest
of the
developing cell mass by techniques and apparatus well known to a person of
ordinary
skill in the art. See. e.~.. U.S. Patent 4.994,384. entitled "Multiplying
Bovine
Embryos," issued on February 19. 1991. hereby incorporated herein by reference
in its
entirety, including all figures. tables, and drawings. Alternatively, cells
proliferating
in culture can be separated from one another to facilitate such processes as
cell
passaging, which is described previously. (n addition, dissociation of a
cultured cell
from a group of cultured cells can be useful as a first step in the process of
nuclear
transfer, as described hereafter. When a cell is dissociated from an embryo,
the
dissociation manipulation can be useful for such processes as re-cloning, a
process
described herein. as well as a step for multiplying the number of embryos.
In anottKr aspect. the invention features a totipotent mammalian cell. where
the cell is immortalized, prepared by a process comprising the steps of: Via)
isolating at
least one precursor cell: and (b) introducing a stimulus to the precursor cell
that
converts the precursor cell into the totipotent mammalian cell.
The term "converts" as used herein can refer to the phenomenon in which
precursor cells become immortalized and/or totipotent. The tenor "convert" is
synonymous with the term "reprogram" as used herein when the precursor cell is
non-
immortalized and/or non-totipotent. Precursor cells can be converted into
totipotent,
CA 02282722 1999-09-O1
WO 98/39416 PCT/US98104345
22
immortalized cells in varying proportions. For example, it is possible that
only a
small portion of precursor cells are converted into totipotent, immortalized
cells. In
the art, some researchers have discussed techniques for converting precursor
cells into
pluripotent cells. Matsui et al., 1992, Cell 70: 841-847.
The term "stimulus" as used herein can refer to materials and/or methods
useful for convening precursor cells into immortalized and/or totipotent
cells. The
stimulus can be electrical, mechanical, temperature-related, andJor chemical,
for
example. The stimulus may be a combination of one or more different types of
stimuli. A stimulus can be introduced to precursor cells for any period of
time that
accomplishes the conversion of precursor cells into immortalized and/or
totipotent
cells.
The term "introduce" as used herein in reference to a stimulus can refer to a
step or steps in which precursor cells are contacted with a stimulus. If the
stimulus is
chemical in nature. for example, the stimulus may be introduced to the
precursor cells
1 ~ by mixing the stimulus with cell culture medium.
In preferred embodiments ( 1 ) the precursor cells are co-cultured with feeder
cells; (2) the precursor cells are not co-cultured with feeder cells; (3) the
feeder cells
are established from fetal cells; (4) the fetal cells arise from a fetus where
no cell types
have been removed from the fetus; (5) the fetal cells arise from a fetus where
one or
more cell types have been removed from the fetus; (6) the stimulus is
introduced to
precursor cells by feeder cells; (7) the feeder cells are the only source of
the stimulus;
(8) the stimulus is introduced to the precursor cells in a mechanical fashion:
(9) the
only stimulus is introduced to the precursor cells in a mechanical fashion; (
10) the
stimulus is introduced to the precursor cells by feeder cells and in a
mechanical
2~ fashion; ( 11 ) the stimulus comprises the step of incubating the precursor
cells with a
receptor ligand cocktail; (12) the precursor cells are isolated from an
ungulate animal
and preferably a bovine animal; (13) the precursor cells are selected from the
group
consisting of non-embryonic cells, primordial germ cells, genital ridge cells,
differentiated cells, cells that originate from an animal, embryonic stem
cells, fetal
CA 02282722 1999-09-O1
WO 98/39416 PCTIUS98/Od345
23
cells, and embryonic cells; ( 14) the receptor ligand cocktail comprises at
least one
component selected from the group consisting of cytokine, growth factor,
trophic
factor, and neurotrophic factor, LIF, and FGF; (15) the LIF has an amino acid
sequence substantially similar to the amino acid sequence of human LIF; and (
16) the
FGF has an amino acid sequence substantially similar to the amino acid
sequence of
bovine bFGF.
The terms "mechanical fashion'' and "mechanical stimulus" as used herein can
refer to introducing a stimulus to cells where the stimulus is not introduced
by feeder
cells. For example, purified LIF and bFGF (defined hereafter) can be
introduced as a
stimulus to precursor cells by adding these purified products to a cell
culture medium
in which the precursor cells are growing.
The term "feeder cells" as used herein can refer to cells grown in co-culture
with tareet cells. Target cells can be precursor cells and totipotent cells,
for example.
Feeder cells can provide, for example, peptides, polypeptides, electrical
signals.
organic molecules (e.~., steroids), nucleic acid molecules, growth factors
(e.~., bFGF),
other factors (e.~., cytokines such as L1F and steel factor), and metabolic
nutrients to
target cells. Certain cells, such as immortalized, totipotent cells may not
require
feeder cells for healthy growth. Feeder cells preferably grow in a mono-layer.
Feeder cells can be established from multiple cell types. Examples of these
cell types arc fetal cells, mouse cells. Buffalo rat liver cells, and
oviductal cells. These
examples arc not meant to be limiting. Tissue samples can be broken down to
establish a feeder cell line by methods well known in the art le.~., by using
a blender).
feeder cells may originate from the same or different animal species as the
precursor
cells. In an example of feeder cells established from fetal cells, ungulate
fetuses and
preferably bovine fetuses may be utilized to establish a feeder cell line
where one or
more cell types have been removed fmm the fetus (e.g., primordial germs cells,
cells
in the head region, and cells in the body cavity region). When an entire fetus
is
utilized to establish a fetal feeder cell line, feeder cells (e.g., fibroblast
cells) and
precursor cells (e.g., primordial germ cells) can arise from the same source
(e.g., one
CA 02282722 1999-09-O1
WO 98/39416 PCT/US98/04345
24
fetus).
The term "receptor ligand cocktail" as used herein can refer to a mixture of
one or more receptor ligands. A receptor ligand can refer to any molecule that
binds
to a receptor protein located on the outside or the inside of a cell. Receptor
ligands
can be selected from molecules of the cytokine family of ligands, neurotrophin
family
of ligands, growth factor family of ligands, and mitogen family of ligands,
all of
which are well known to a person of ordinary skill in the art. Examples of
receptor/ligand pairs are: epidermal growth factor receptor/epidermal growth
factor,
insulin/insulin receptor, cAMP-dependent protein kinase/cAMP, growth hormone
receptor/growth hormone, and steroid receptoNsteroid. It has been shown that
certain
receptors exhibit cross-reactivity. For example. heterologous receptors, such
as
insulin-like growth factor receptor 1 (IGFR I ) and insulin-like growth factor
receptor 2
(1GFR2) can both bind IGF 1. When a receptor ligand cocktail comprises the
stimulus.
the receptor ligand cocktail can be introduced to the precursor cell in a
variety of
1 S manners known to a person of ordinary skill in the art.
The term "cytokine" as used herein can refer to a large family of receptor
ligands well-known to a person of ordinary skill in the art. The cytokine
family of
receptor ligands includes such members as leukemia inhibitor factor (L1F),
cardiotrophin 1 (CT-1 ), ciliary neurotrophic factor (CNTF), stem cell factor
(SCF),
.20 oncostatin M (OSM). and any member of the interleukin (IL) family,
including IL-6,
IL-I 1, and IL-1'_'. The teachings of the invention do not require the
mechanical
addition of steel factor (also known as stem cell factor in the art) for the
conversion of
precursor cells into totipotent cells.
The term "growth factor" as used herein can refer to any receptor ligand that
25 causes a cell growth andlor cell proliferation effect. Examples of growth
factors are
well known in the art. Fibroblast growth factor (FGF) is one example of a
growth
factor. The term "bFGF" can refer to basic FGF.
The term "substantially similar" as used herein in reference to amino acid
sequences can refer to two amino acid sequences having preferably 50% or more
CA 02282722 1999-09-O1
WO 98139416 PCTIU598/04345
amino acid identity, more preferably 70% or more amino acid identity or most
preferably 90% or more amino acid identity. Amino acid identity is a property
of
amino acid sequence that measures their similarity or relationship. Identity
is
measured by dividing the number of identical residues in the two sequences by
the
5 total number of residues and multiplying the product by 100. Thus, two
copies of
exactly the same sequence have 100% identity, while sequences that are less
highly
conserved and have deletions, additions, or replacements have a lower degree
of
identity. Those of ordinary skill in the art will recognize that several
computer
programs are available for performing sequence comparisons and determining
10 sequence identity,.
In another aspect, the invention features a method for preparing a totipotent
mammalian cell, where the cell is immortalized, comprising the following
steps: (a)
isolating one or more precursor cells: and (b) introducing the precursor cell
to a
stimulus that converts the precursor cell into the totipotent cell. Any of the
15 embodiments defined previously herein in reference to totipotent mammalian
cells
relate to the method for preparing a totipotent mammalian cell.
Cloned Totinotent Emhrv~s of the Invention
The invention rclates in part to cloned totipotent embryos. Hence, aspects of
20 the invention feature cloned mammalian embryos where ( 1 ) the embryo is
totipotent;
(21 the embryo arises from an immortalized and/or totipotent ccll: and (3) the
embno~
arises from a non-embryonic cell: and (4 ) any combination of the forc~toir~.
The term "totipotent" as used herein in reference to embryos can rcfcr to
embryos that can develop into a live born animal. The term "live born' is
defined
25 previously.
The term "cloned" as used herein can refer to a cell, embryonic cell, fetal
cell,
and/or animal cell having a nuclear DNA sequence that is substantially similar
or
identical to the nuclear DNA sequence of another cell, embryonic cell, fetal
cell,
and/or animal cell. The teens "substantially similar" and "identical" are
described
CA 02282722 1999-09-O1
WO 98/39416 FCT/US98/04345
26
herein. The cloned embryo can arise from one nuclear transfer, or
alternatively, the
cloned embryo can arise from a cloning process that includes at least one re-
cloning
step. If the cloned embryo arises from a cloning procedure that includes at
least one
re-cloning step, then the cloned embryo can indirectly arise from an
immortalized,
S totipotent cell since the re-cloning step can utilize embryonic cells
isolated from an
embryo that arose from an immortalized, totipotent cell.
In preferred embodiments, ( 1 ) the cloned mammalian embryo is preferably an
ungulate embryo and more preferably a bovine embryo; (2) the cloned bovine
embryo
can be one member of a plurality of embryos. where the plurality of embryos
share a
substantially similar nuclear DNA sequence; (3) the cloned mammalian embryo
can
be one member of a plurality of embryos, and the plurality of embryos can have
an
identical nuclear DNA sequence; (4) the cloned mammalian embryo has a nuclear
DNA sequence that is substantially similar to a nuclear DNA sequence of a live
bom
mammalian animal; (5) one or more cells of the cloned mammalian embryo have
1 S modified nuclear DNA; (6) the cloned mammalian embryo is subject to
manipulation;
(7) the manipulation comprises the step of culturing the embryo in a suitable
medium:
(8) the suitable medium for culturing the embryo is CR-2 medium; (9) the
medium
can comprise feeder cells; ( 10) the manipulation of an embryo comprises the
step of
implanting the embryo into the uterus of a female; ( 11 ) the female animal is
preferably
an ungulate animal and more preferably a bovine animal; ( 12 ) the estrus
cycle of the
female is synchronized with the development cycle of the embryo: and ( 13 )
the
manipulation comprises the step of incubating the embryo in an artificial
environment.
All preferred embodimenu related to modified nuclear DNA for totipotent
cells of the invention extend to cloned embryos of the invention. In addition,
any of
the manipulations described in conjunction with totipotent cells of the
invention apply
to cloned embryos of the invention.
The term "substantially similar" as used herein in reference to nuclear DNA
sequences refer to two nuclear DNA sequences that are nearly identical. The
two
sequences may differ by copy error differences that normally occur during the
CA 02282722 1999-09-O1
WO 98/39416 PCT/US98/04345
27
replication of a nuclear DNA. Substantially similar DNA sequences are
preferably
greater than 97% identical, more preferably greater than 98% identical, and
most
preferably greater than 99% identical. The term "identity" as used herein in
reference
to nuclear DNA sequences can refer to the same usage of the term in reference
to
amino acid sequences, which is described previously herein.
The term "plurality" as used herein in reference to embryos can refer to a set
comprising at least two embryos having a substantially similar nuclear DNA
sequence. In preferred embodiments, the plurality consists of five or more
embryos,
ten or more embryos, one-hundred or more embryos, or one-thousand or more
embryos. Because the occurrence of more than three embryos progressing to term
only occurs with a probability of approximately 1 /100.000, a piuraliy of at
least five
embryos or animals relates to cloned embryos or cloned animals rather than
naturally
occurrine embryos or animals.
The term ''culturing" as used herein with respect to embryos can refer to
I S laboratory procedures that involve placing an embryo in a culture medium.
The
embryo can be placed in the culture medium for an appropriate amount of time
to
allow the embryo to remain static but functional in the medium, or to allow
the
embryo to grow in the medium. Culture media suitable for culturing embryos are
well-known to those skilled in the art. Ser. e.~,~ , U.S. Patent No. Sw
13.979. entitle)
"!n v~rrro Culture of Bovine Embryos," First et ol., issued May 25. 1993. and
U.S.
Patent No. 5.096.8'', entitled "Bovine Embryo Medium." Rosenkrans. Jr. ct al..
sssucd March 17. 1992, incorporated herein by reference in their entireties
including
all figures. tables, and drawings.
The term "suitable medium" as used herein can refer to any medium that
allows cell proliferation. The suitable medium need not promote maximum
proliferation, only measurable cell proliferation. A suitable medium for
embryo
development is discussed previously.
The term "CR-2 medium" as used herein can refer to a medium suitable for
culturing embryos. CR-2 medium can comprise one or more of the following
CA 02282722 1999-09-O1
WO 98/39416 PCT/US98/04345
28
components: sodium chloride; potassium chloride; sodium bicarbonate;
hemicalcium
L-lactate; and fatty-acid free BSA. These components may exist in the medium
in
concentrations of about I 15 mM for sodium chloride; about 3 mM for potassium
chloride; about 25 mM for sodium bicarbonate; about 5 mM for hemicalcium L-
lactate; and about 3 mg/mL for fatty-acid free BSA. Alternatively, the
concentrations
of these components may exist in the medium in concentrations of 0-1 M sodium
chloride; 0-100 mM potassium chloride; 0-500 mM sodium bicarbonate; 0-100 mM
hemicalcium L-lactate; and 0-100 mg/mL fatty-acid free BSA.
The term "feeder cells" is defined previously herein. Embryos of the
invention can be cultured in media with or without feeder cells. In other
preferred
embodiments, the feeder cells can be cumulus cells.
The term "implanting" as used herein in reference to embryos can refer to
impregnating a female animal with an embryo described herein. This technique
is
well known to a person of ordinary skill in the ari. See, e.~., Seidel and
EIsJen. 1997,
Embryo Transfer in Dairy Cattle. W.D. Hoard & Sons, Co., Hoards Dairyman. The
embryo may be allowed to develop in utero, or alternatively. the fetus may be
removed from the uterine environment before parturition.
The term "synchronized" as used herein in reference to estrus cycle, can refer
to assisted reproductive techniques well known to a person of ordinary skill
in the art.
These techniques are fully described in the reference cited in the previous
paragraph.
Typically' estrogen and progesterone hotTrtones arc utilized to synchronize
the estrus
cycle of the female animal with the developmental cycle of the embryo. The
term
''developmental cycle" as used herein refers to embryos of the invention and
the time
period that exists between each cell division within the embryo. This time
period is
predictable for embryos from ungulates, and can be synchronized with the esws
cycle
of a recipient animal.
The term "artificial environment" refers to one that promotes the development
of an embryo or other developing cell mass. An artificial environment can be a
uterine environment or an oviductal environment of a species different from
that of
CA 02282722 1999-09-O1
WO 98139416 PCT/US98b4345
29
the developing cell mass. For example, a developing bovine embryo can be
placed
into the uterus or oviduct of an ovine animal. Stice & Keefer, 1993, "Multiple
generational bovine embryo cloning," Biology of Reproduction 48: 715-719.
Alternatively, an artificial development environment can be assembled in
vitro. This
type of artificial uterine environment can be synthesized using biological and
chemical
components known in the art.
In another aspect the invention features a cloned mammalian embryo, where
the embryo is totipotent, prepared by a process comprising the step of nuclear
transfer.
Preferably, nuclear transfer occurs between (a) a totipotent mammalian cell.
where the
cell is immortalized, and (b) an oocyte. where the oocyte is at a stage
allowing
formation of the embryo.
In preferred embodiments, ( 1 ) the oocyte is an enucleated oocvte; (2) the
totipotent mammalian cell and the oocyte preferably orieinate from an ungulate
animal and more preferably originate from a bovine animal; (3) the totipotent
I S mammalian cell can originate from one specie of ungulate and the oocyte
can
originate from another specie of ungulate; (4) the oocvte is a young oocyte;
(5) the
totipotent mammalian cell is placed in the perivitelline space of the oocyte;
{6) the
totipotent cell utilized for nuclear transfer can arise from any of the cells
described
previously (e.g~.. a non-embryonic cell, a primordial germ cell, a genital
ridge cell, a
differentiated cell, a fetal cell, a non-fetal cell, a non-primordial germ
cell, a cell
isolated from an asynchronous population of cells, a cell isolated from a
synchronous
population of cells. a cell isolated from an existing animal, and an embryonic
stem
cell); (7) the nuclear transfer comprises the step of translocation of the
totipotent
mammalian cell into the recipient oocyte; (8) the translocation can comprise
the step
of injection of the totipotent mammalian cell nuclear donor into the recipient
oocyte;
(9) the iranslocation can comprise the step of fusion of the totipotent
mammalian cell
and the oocyte; ( 10) the fusion can comprise the step of delivering one or
more
electrical pulses to the totipotent mammalian cell and the oocyte; ( 11 ) the
fusion can
comprise the step of delivering a suitable concentration of at least one
fusion agent to
CA 02282722 1999-09-O1
WO 98/39416 PtrT/US98I04345
the totipotent mammalian cell and the oocyte; ( 12) the nuclear transfer may
comprise
the step of activation of the totipotent mammalian cell and the oocyte; and
(13) the
activation is accomplished by introducing DMAP and/or ionomycin to an oocyte
and/or a cybrid.
S The term "enucleated oocyte" as used herein can refer to an oocyte which has
had part of its contents removed. Typically a needle can be placed into an
oocyte and
the nucleus can be aspirated into the inner space of the needle. The needle
can be
removed from the oocyte without rupturing the plasma membrane. This
enucleation
technique is well known to a person of ordinary skill in the art. See. U.S.
Patent
10 4,994.384; U.S. Patent 5.057.420; and Willadsen, 1986. Narure 320:63-6~. An
enucleated oocyte can be prepared from a young or an aged oocyte. Definitions
of
''young oocyte" and ''aged oocyte" are provided herein. Nuclear transfer may
be
accomplished by combining one nuclear donor and more than one enucleated
oocyte.
In addition, nuclear transfer may be accomplished by combining one nuclear
donor.
15 one or more enucleated oocytes, and the cytoplasm of one or more enucleated
oocvtes.
The term ''cybrid" as used herein can refer to a construction where an entire
nuclear donor is translocated into the cytoplasm of a recipient oocytc. See,
e.g.. In
vitro Cell. Dev. Biol. 26: 97-101 ( 1990).
The invention specifically relates to cloned mammalian embryos created by
20 nuclear transfer, where the nucleus of the oocyte is not physically
extracted from the
nucleus. It is possible to create a cloned embryo where the nuclear DNA from
the
donor cell is the material replicated during cellular divisions. See. ~.~..
Wagoner e~
al.. 1996. "Functional enucleation of bovine oocytes: effects of
centrifugation and
ultraviolet light," TherioRenology 46: 279-284.
25 The term "another ungulate" as used herein can refer to a situation where
the
nuclear donor originates from an ungulate of a different species, genera or
family than
the ungulate from which the recipient oocyte originates. For example, the
totipotent
mammalian cell used as a nuclear donor can arise from a water buffalo, while
the
oocyte recipient can arise from a domestic cow. This example is not meant to
be
CA 02282722 1999-09-O1
WO 98139416 PCT/US98/04345
31
limiting and any ungulate species/family combination of nuclear donors and
recipient
oocytes are foreseen by the invention.
The term "young oocyte" as used herein can refer to an oocyte that has been
matured in vitro and/or ovulated in vivo for less than 28 hours since the
onset of
maturation. Oocytes can be isolated from live animals using methods well known
to a
person of ordinary skill in the art. See, e.g., Pieterse et al., 1988.
"Aspiration of
bovine oocytes during transvaginal ultrasound scanning of the ovaries."
TherioRenology 30: 751-762. Oocytes can be isolated from ovaries or oviducts
or
deceased or live born animals. Suitable media for in vitro culture of oocvtes
are well
I O known to a person of ordinary skill in the art. See. e.~., U.S. Patent No.
5.057.420,
which is incorporated by reference herein.
The term "maturation" as used herein can refer to process in which an oocyte
is incubated in a medium in vitro. Oocvtes can be incubated with multiple
media well
known to a person of ordinary skill in the art. See. e.~., Saito et al., 1992.
Roux's
Arch. Der. Brol. 101: 134-141 for bovine organisms and Wells et al., 1997,
Biol.
Repr. 57: 385-393 for ovine organisms, both of which are incorporated herein
by
reference in their entireties including all figures, tables, and drawings.
Maturation
media can comprise multiple types of components, includins microtubulc
inhibitors
(e.~~., cytochalasin B). Other examples of components that can be incorporated
into
maturation media arc discussed in WO 97/07668, entitled "Unactivated Oocvtes
as
Cytoplast Recipients for Nuclear Transfer." Campbell & Wiimut, published on
March
6. 1997, hereby incorporated hcrcin by rcfercnce in its enttrcty, including
all fiFurcs,
tables, and drawings. The time of maturation can be determined from the time
that an
oocyte is placed in a maturation medium and the time that the oocyte is then
utilized in
a nuclear transfer procedure.
Young oocytes can be identified by the appeat~nce of their ooplasm. Because
certain cellular material (e.g., lipids) have not yet dispersed within the
ooplasm.
Young oocytes can have a pycnotic appearance. A pycnotic appearance can be
characterized as clumping of cytoplasmic material. A "pycnotic" appearance is
to be
CA 02282722 1999-09-O1
WO 98139416 PCT/LTS98104345
32
contrasted with the appearance of oocytes that are older than 28 hours, which
have a
more homogenous appearing ooplasm.
The term "translocation" as used herein in reference to nuclear transfer can
refer to the combining of a totipotent mammalian cell nuclear donor and a
recipient
oocyte. The translocation may be performed by such techniques as fusion and/or
direct injection, for example.
The term ''injection" as used herein in reference to embryos, can refer to the
perforation of the oocyte with a needle, and insertion of the nuclear donor in
the
needle into the oocyte. In preferred embodiments, the nuclear donor may be
injected
into the cytoplasm of the oocyte or in the perivitelline space of the oocyte.
This direct
injection approach is well known to a person of ordinary skill in the art. as
indicated
by the publications already incorporated herein in reference to nuclear
transfer. For
the direct injection approach to nuclear transfer. the whole totipotent
mammalian cell
may be injected into the oocyte, or alternatively. a nucleus isolated from the
totipotent
mammalian cell may be injected into the oocyte. Such an isolated nucleus may
be
surrounded by nuclear membrane only, or the isolated nucleus may be surrounded
by
nuclear membrane and plasma membrane in any proportion. The oocyte may be pre-
treated to enhance the strength of its plasma membrane, such as by incubating
the
oocyte in sucrose prior to injection of the nuclear donor.
Techniques for placing a nuclear donor (e.~., an immortalized and totipotent
cell of the invention) into the perivitelfine space of an enucleated oocvte is
well known
to a person of ordinary skill in the art. and is fully described in the
patents and
references cited previously herein in reference to nuclear tr~artsfer.
The term "fusion" as used herein can refer to the combination of portions of
lipid membranes corresponding to the totipotent mammalian cell nuclear donor
and
the recipient oocyte. Lipid membranes can correspond to the plasma membranes
of
cells or nuclear membranes, for example. The fusion can occur between the
nuclear
donor and recipient oocyte when they are placed adjacent to one another, or
when the
nuclear donor is placed in the perivitelline space of the recipient oocyte,
for example.
CA 02282722 1999-09-O1
WO 98/39416 PCTIUS98I0434S
33
Specific examples for translocation of the totipotent mammalian cell into the
oocyte
are described hereafter in other preferred embodiments. These techniques for
translocation are fully described in the references cited previously herein in
reference
to nuclear transfer.
The term "electrical pulses" as used herein can refer to subjecting the
nuclear
donor and recipient oocyte to electric current. For nuclear transfer, the
nuclear donor
and recipient oocyte can be aligned between electrodes and subjected to
electrical
current. The electrical current can be alternating current or direct current.
The
electrical current can be delivered to cells for a variety of different times
as one pulse
I 0 or as multiple pulses. The cells are typically cultured in a suitable
medium for the
delivery of eiectrical pulses. Examples of electrical pulse conditions
utilized for
nuclear transfer are described in the references and patents previously cited
herein in
reference to nuclear transfer.
The term "fusion agent" as used herein can refer to any compound or
biological organism that can increase the probability that portions of plasma
membranes from different cells will fuse when a totipotent mammalian cell
nuclear
donor is placed adjacent to the recipient oocyte. In preferred embodiments
fusion
agents are selected from the group consisting of polyethylene glycol (PEG),
trypsin.
dimethvlsulfoxide (DMSO), lectins. agglutinin, viruses. and Sendai virus.
These
examples arc not meant to be limiting and other fusion agents known in the art
are
applicable and included herein.
The term "suitable concentration" as used herein in reference to fusion agents
can refer to any concentration of a fusion agent that affords a measurable
amount of
fusion. Fusion can be measured by multiple techniques well known to a person
of
ordinary skill in the art, such as by utilizing a light microscope, dyes, and
fluorescent
lipids, for example.
The term "activation" can refer to any materials and methods useful for
stimulating a cell to divide before, during, and after a nuclear transfer
step. Cybrids
may require stimulation in order to divide after a nuclear transfer has
occurred. The
CA 02282722 1999-09-O1
WO 98/39416 PCT/US98/04345
34
invention pertains to any activation materials and methods known to a person
of
ordinary skill in the art. Although electrical pulses are sometimes sufficient
for
stimulating activation of cybrids, other means are sometimes useful or
necessary for
proper activation of the cybrid. Chemical materials and methods useful for
activating
embryos are described below in other preferred embodiments of the invention.
Examples of non-electrical means for activation include agents such as
ethanol; inositol trisphosphate (IP3); Ca'~ ionophores (e.g., ionomycin) and
protein
kinase inhibitors (e.g., 6-dimethylaminopurine (DMAP)) : temperature change;
protein synthesis inhibitors (e.g., cyclohexamide); phorbol esters such as
phorbol 12-
myristate 13-acetate (PMA); mechanical techniques: and thapsigargin. The
invention
includes any activation techniques known in the art. Sce, e.~,~.. U.S. Patent
No.
5.496,720, entitled "Parthenogenic Oocyte Activation," issued on March 5.
1996.
Susko-Parrish er al.. incorporated by reference herein in its entireh~,
including all
figures, tables. and drawings.
1 S In other preferred embodiments, ( 1 ) one or more cells of the cloned
embryo
comprise modified nuclear DNA; (2) the cloned embryo is subject to
manipulation;
(3) the manipulation comprises the step of disaggregating at least one
individual cell
from a cloned embryo; (4) the manipulation comprises the step of utilizing the
individual cell as a nuclear donor in a nuclear transfer procedure; (5) thr
individual
cell is disaggrcgated from the inner cell mass of a blastocyst stage embryo;
(6) the
individual cell is disagFregated from a prc-blastocyst stage embryo: (7) the
manipulation comprises the process of rc-cloning; (8) the rc-cloning process
comprises the steps of: (a) separating the embryo into one or more individual
cells.
and (b) performing at least one subsequent nuclear transfer between (i) an
individual
cell of (a), and (ii) an oocyte; (9) the oocyte utilized for the subsequent
nuclear
transfer is an aged oocyte; ( 10) the individual cell is placed in the
perivitelline space
of the enucleated oocyte for the subsequent nuclear transfer; ( I 1 ) the
subsequent
nuclear transfer comprises at least one of the steps of translocation,
injection, fusion,
and activation of the individual cell and/or the enucleated oocyte; ( 12) one
or more
CA 02282722 1999-09-O1
WO 98/39416 PCT/US98I04345
cells of the cloned mammalian embryo arising from the subsequent nuclear
transfer
comprises modified nuclear DNA; and (13) the cloned mammalian embryo arising
from the subsequent nuclear transfer may be subject to a subsequent
manipulation,
where the subsequent manipulation is any of the manipulation steps defined
5 previously herein in relation to immortalized cells and/or cloned embryos.
The tet~rn "individual cells" as used herein can refer to cells that have been
isolated from a cloned mammalian embryo of the invention. An individual single
cell
can be isolated from the rest of the embryonic mass by techniques well known
to
those skilled in the art. See, U.S. Patents 4,994,384 and 5,957.420,
previously
10 incorporated herein by reference in their entireties.
The term "subsequent nuclear transfer" as described herein is also referred to
as a "re-cloning" step. Preferably', a re-cloning step can be utilized to
enhance nuclear
reprogramming during nuclear transfer. such that the product of nuclear
transfer is a
live born animal. The re-cloning step is distinct, since previous efforts
towards re-
1 ~ cloning have been directed to multiplying embryo number and not for
enhancement of
nuclear reprogramming. The number of subsequent nuclear transfer steps is
discussed
in greater detail hereafter.
Any of the preferred embodiments related to the translocation, injection,
fusion. and activation steps dexribed previously herein relate to the
subsequent
20 nuclear transfer step.
The term "saner cell mass" as used herein can refer to the cells that gives
case
to the embryo proper. The cells that line the outside of a blastocyst can be
referred to
as the trophoblast of the embryo. The methods for isolating inner cell mass
cells from
an embryo arc well known to a person of ordinary skill in the art. See, Sims
and First,
25 1993, Theriogenology 39:313; and keefer et al., 1994, Mol. Reprod Dev.
38:264-268,
hereby incorporated by reference herein in their entireties, including all
figures, tables,
and drawings. The term "pre-blastocyst" is well known in the art and is
referred to
previously.
The term "aged oocyte" as used herein can refer to an oocyte that has been
CA 02282722 1999-09-O1
WO 98/39416 PCT/US98/04345
36
matured in vitro or ovulated in vivo for more than 28 hours since the onset of
maturation or ovulation. An aged oocyte can be identified by its
characteristically
homogenous ooplasm. This appearance is to be contrasted with the pycnotic
appearance of young oocytes as described previously herein. The age of the
oocyte
can be defined by the time that has elapsed between the time that the oocyte
is placed
in a suitable maturation medium and the time that the oocvte is activated. The
age of
the oocyte can dramatically enhance the efficiency of nuclear transfer.
The term "ovulated in vivo" as used herein can refer to an oocyte that is
isolated from an animal a certain number of hours after the animal exhibits
characteristics that it is in estrus. The characteristics of an animal in
estrus are well
known to a person of ordinary skill in the art. as described in references
disclosed
herein.
In another aspect the invention relates to a method for preparing a cloned
mammalian embryo. The method comprises the step of a nuclear transfer between:
(a)
I ~ a totipotent mammalian cell, where the cell is immortalized; and (b) an
oocyte, where
the oocyte is at a stage allowing formation of the embryo. !n preferred
embodiments,
any of the embodiments of the invention concerning cloned mammalian embryos
apply to methods for preparing cloned mammalian embryos.
Cloned Fetuses of the Invention
!n another aspect. the invention features cloned mammalian fetuses arising
from totipotent embryos of the invention. Preferably, the mammalian fetuses
are
ungulate fetuses. and more preferably, the ungulate fetuses arc bovine
fetuses. A fetus
may be isolated from the uterus of a pregnant female animal.
In preferred embodiments, ( 1 ) one or more cells of the fetuses harbor
modified
nuclear DNA (defined previously herein); and (2) the fetuses may be subject to
any of
the manipulations defined herein. For example, one manipulation may comprise
the
steps of isolating a fetus from the uterus of a pregnant female animal,
isolating a cell
from the fetus (e.g., a primordial germ cell), and utilizing the isolated cell
as a nuclear
CA 02282722 1999-09-O1
WO 98/39416 PGTIUS98104345
37
donor for nuclear transfer.
Other aspects of the invention feature ( 1 ) a cloned mammalian fetus prepared
by a process comprising the steps of (a) preparation of a cloned mammalian
embryo
defined previously, and (b) manipulation of the cloned mammalian embryo such
that it
develops into a fetus; (2) a method for preparing a cloned mammalian fetus
comprising the steps of (a) preparation of a cloned mammalian embryo defined
previously, and (b) manipulation of the cloned mammalian embryo such that it
develops into a fetus; (3) a method of using a cloned fetus of the invention
comprising
the step of isolating at least one cell type from a fetus (e.~., for creating
a feeder cell
1 U layer): and (4) a method of using a cloned fetus of the invention
comprising the step
of separating at least one part of a fetus into individual cells (e.R.. for
establishing a
feeder cell layer).
Cloned Animals of the Invention
In another aspect the invention features a cloned mammalian animal arising
from a cloned embryo of the invention. The embryo is totipotent and can arise
from
any of the processes or methods described previously herein.
In preferred embodiments, the cloned mammalian animal ( 1 ) is preferably a
cloned ungulate animal and more preferably a cloned bovine animal; and (2) is
equal
in age or older than an animal selected from the group consisting of pre- and
post-
pubcrtal animals..
In yet another aspect the invention relates to a cloned mammalian animal,
when the animal is one member of a pluraliy of animals, and where the
plurality of
animals have a substantially similar nuclear DNA sequence. The term
"substantially
similar" in relation to nuclear DNA sequences is defined previously herein.
In preferred embodiments, ( 1 ) the plurality consists of five or more
animals,
ten or more animals, one-hundred or more animals, and one-thousand or more
animals; and (2) the plurality of animals can have an identical nuclear DNA
sequence.
The term "identical" in reference to nuclear DNA sequences is described
previously
CA 02282722 1999-09-O1
WO 98/39416 PCT/US98I04345
38
herein.
In another aspect, the invention relates to a cloned mammalian animal having
one or more cells that comprise modified nuclear DNA. All of the preferred
embodiments relating to modified nuclear DNA described previously apply to
cloned
bovine animals of the invention.
In yet another aspect, the invention features a method of using a cloned
mammalian animal. comprising the step of isolating at least one component from
the
mammalian animal.
The term "component" as used herein can relate to any portion of an animal.
A component can be selected from the group consisting of fluid, biological
fluid, cell.
tissue. organ, gamete, embryo, and fetus. Precursor cells may arise from
fluids.
biological fluids. cells, tissues, organs, gametes, embryos, and fetuses
isolated from
cloned oreanisms of the invention.
The term "gamete" as used herein can refer to any cell participating. directly
1 ~ or indirectly, to the reproductive system of an animal. Examples of
gametes are
spenmatocytes, spermatogonia, oocytes, and oogonia. Gametes can be present in
fluids, tissues, and organs collected from animals (e.g., sperm is present in
semen).
For example, methods of collecting semen for the purposes of artificial
insemination
are well known to a person of ordinary skill in the art. See, e.~~.,
PhvsioJogy of
Reproduction a»d Artifrcial lrrserrri»atio» ojCattle (2nd edition), Salisbury
et al.,
coprvright 1961, 1978. WH Freeman 8 Co.. San Francisco. I~ovurver, the
invention
rciates to the collection of any type of gamete from an animal.
The term "tissue" is defined previousy. The term "organ" relates to any organ
isolated from an animal or any portion of an organ. Examples of organs and
tissues
are neuronal tissue, brain tissue, spleen, heart, lung, gallbladder, pancreas,
testis,
ovary and kidney. These examples are not limiting and the invention relates to
any
organ and any tissue isolated from a cloned animal of the invention.
In a preferred embodiments, the invention relates to ( 1 ) fluids, biological
fluids, cells, tissues, organs, gametes, embryos, and fetuses can be subject
to
CA 02282722 1999-09-O1
WO 98r39416 PCT/US98/04345
39
manipulation; (2) the manipulation can comprise the step of cryopreserving the
gametes, embryos, and/or fetal tissues; (3) the manipulation can comprise the
step of
thawing the cryopreserved items; (4) the manipulation can comprise the step of
separating the semen into X-chromosome bearing semen and Y-chromosome bearing
S semen; (5) the manipulation comprises methods of preparing the semen for
artificial
insemination; (6) the manipulation comprises the step of purification of a
desired
polypeptide(s) from the biological fluid or tissue; (7) the manipulation
comprises
concentration of the biological fluids or tissues; and (8) the manipulation
can comprise
the step of transferring one or more cloned cells, cloned tissues. cloned
organs. andlor
portions of cloned organs to a recipient organism (e.g.. the recipient
organism may be
of a different specie than the donor source).
The term "separating" as used herein in reference to separating semen can
refer to methods well known to a person skilled in the art for fractionating a
semen
sample into sex-specific fractions. This type of separation can be
accomplished by
using flow cytometers that are commercially available. Methods of utilizing
flow
cytometers from separating sperm by genetic content are well known in the art.
In
addition, semen can be separated by its sex-associated characteristics by
other
methods well known to a person of ordinary skill in the art. See, U.S. Patents
5.439.362. 5.346.990, and x.02 I ,24:1, entitled "Sex-Associated Membrane
Proteins
,20 and Methods for Increasing the Probability that Offspring Will Be of a
Desired Sex."
Spaulding, issued on August 8. ! 995. September 13. 1994, and Junc 4. ! 991
respectively, all of which are incorporated herein by reference in their
entireties
including all figures, tables, and drawings.
Semen preparation methods are well known to someone of ordinary skill in the
art. Examples of these preparative steps are described in Physiology
oJReproduction
and Artificial Insemination of Caule (2nd. edition), Salisbury et al.,
copyright 1961,
1978, W.H. Freeman & Co., San Francisco.
The term "purification" as used herein can refer to increasing the specific
activity of a particular polypeptide or polypepddes in a sample. Specific
activity can
CA 02282722 1999-09-O1
WO 98/39416 PCT/ITS9$l04345
be expressed as the ratio between the activity of the target polypeptide and
the
concentration of total polypeptide in the sample. Activity can be catalytic
activity
and/or binding activity, for example. Alternatively, specific activity can be
expressed
as the ratio between the concentration of the target polypeptide and the
concentration
5 of total polypeptide. Purification methods include dialysis, centrifugation,
and column
chromatography techniques, which are well-known procedures to a person of
ordinary
skill in the art. See, e.g., Young et al., 1997, "Production of
biopharmaceutical
proteins in the milk of transgenic dairy animals," BioPharm 10(6): 34-38.
The term "transferring" as used herein can relate to shifting a group of
cells,
10 tissues. organs, and/or portions of organs to an animal. The cells,
tissues, organs,
andlor portions of organs can be, for example. (a) developed in vitro and then
transferred to an animal, (b) removed from an animal and transferred to
another
animal of a different specie, (c) removed from an animal and transferred to
another
animal of the same specie, (d) removed from one portion of an animal (e.b~.,
the leg of
15 an animal) and then transferred to another portion of the same animal
(e.g.. the brain
of the animal), and/or (e) any combination of the foregoing. The term
"transferring"
can relate to adding cells, tissues, and/or organs to an animal and can also
relate to
removing cells, tissues. and/or organs from an animal and replacing them with
cells,
tissues, and/or organs from another source.
20 The term ''~ansferring" as used herein can refer to implanting one or more
cells. tissues, organs, andlor portions of organs from the cloned mammalian
animal
into another organism. For example, neuronal tissue from a clor~d mammalian
organism can be grafted into an appropriate arcs in the human nervous system
to treat
neurological diseases such as Alzheimer's disease. Alternatively, cloned
cells, tissues,
25 and/or organs originating from a porcine organism may be transferred to a
human
recipient. Surgical methods for accomplishing this preferred aspect of the
invention
are well known to a person of ordinary skill in the art. Transferring
procedures may
include the step of removing cells, tissues, or organs from a recipient
organism before
a transfer step.
CA 02282722 1999-09-O1
wo 9s~m6 rcrws~roa~s
41
In other aspects the invention features ( 1 ) a cloned mammalian animal
prepared by a process comprising the steps of (a) preparation of a cloned
mammalian
embryo by any one of the methods described herein for producing such a cloned
mammalian embryo, and (b) manipulation of the cloned mammalian embryo such
that
it develops into a live born animal; (2) a process comprising the steps of:
(a)
preparation of a cloned mammalian embryo by any one of the methods described
herein for preparing such a cloned mammalian embryo, and (b) manipulation of
the
cloned mammalian embryo such that it develops into a live born animal; and (3)
a
cloned mammalian animal, comprising the steps of: (a) preparation of a cloned
1 C mammalian embryo by any one of the methods for producing such an embryo
described herein, and (b) manipulation of the cloned mammalian embryo such
that it
develops into a live born animal.
In preferred embodiments, ( 1 ) the live born animal is preferably an ungulate
animal and more preferably a bovine animal; (2) the manipulation can comprise
the
1 S step of implanting the embryo into a uterus of an animal; (3) the estrus
cycle of the
animal can be synchronized to the developmental stage of the embryo; and (4)
the
manipulation can comprise the step of implanting the embryo into an artificial
environment.
The summary of the invention described above is not limiting and other
20 featttrrs and advantages of the invention will be apparent from tt>c
following detailed
description of the prcfemed embodiments. as wcll as from the claims.
~21EF DE~CRPTION OF THF Fl ~ I1ZS
FrRrrre 1 illustrates multiple embodiments of the invention relating to the
25 generation of immortalized, totipotent cells from precursor cells. The
figure indicates
that immortalized, totipotent cells can arise from embryonic stem cells,
primordial
germ cells, and cells isolated from an animal. The precursor cell sources
illustrated by
Figure 1 are not limiting and other precursor cell sources are described
herein.
Figure 2 illustrates an embodiment of the invention related to cloning. The
CA 02282722 1999-09-O1
WO 98/39416 PCT/US98/04345
42
figure illustrates a cloning procedure in which (a) a precursor cell is
reprogrammed
into an immortalized, totipotent cell; (b) the immortalized, totipotent cell
is utilized as
a donor for a first nuclear transfer, which utilizes a young oocyte; (c) the
embryo
arising from the first nuclear transfer is cultured; (d) a cell isolated from
the embryo
arising from the first nuclear transfer is utilized as a nuclear donor for a
second
nuclear transfer, which utilizes an aged oocyte; and (e) the embryo resulting
from the
second nuclear transfer may be cultured and then allowed to develop into a
live born
animal. The embryos resulting from the nuclear transfers may be cultured
and/or
cryopreserved and thawed.
Figure 3 illustrates multiple embodiments of the invention related to pathways
for establishing totipotent cell lines and cloned animals. Fibroblast cells
can be
isolated from any source described herein. This figure is described in further
detail in
the Examples section.
Figure 4 illustrates multiple embodiments of the invention for creating cloned
transgenic cell lines and cloned transgenic animals. This figure is described
in further
detail in the Examples section.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENT
The present invention relates to cloning technologies. The present invention
provides multiple advantages over the tools and methods currently utilized in
the field
of cloning technoloy. For example, the invention relates in part to
immortalized,
totipotent cells useful for cloning animals. These immortalized. totipotcnt
cells can
give rise to methods of producing cloned animals by utilizing virtually any
type of
cell. For example, cells isolated from a live born animal can be reprogrammed
into
immortalized, totipotent cells. This feattue of the invention provides the
ability to
assess the phenotype of an existing animal and then readily establish a
permanent cell
line for cloning that animal. As described previously herein, no methods in
the art
have allowed for such advantages.
In addition, the immortalized, totipotent cells of the invention allow for
CA 02282722 1999-09-O1
WO 98/39416 PCT1US98/04345
43
creating permanent cell lines from virtually any type of cell. This
reprogramming
method is described previously herein. These permanent cell lines offer a
nearly
unlimited source of donor cells for nuclear transfer cloning techniques.
Moreover,
this feature provides the advantage of enhancing cloning efficiency due to the
lower
differentiation rates of these cell lines than existing cell lines used for
cloning. For
example, embryonic stem cell lines can harbor multiple colonies of cells that
are not
totipotent. The totipotent, immortalized cells of the invention harbor a
higher
percentage of totipotent cells than cell lines previously reported.
Moreover, the methods and processes for creating the immortalized, totipotent
cells, totipotent cloned embryos, and cloned animals of the invention
demonstrate the
enhanced cloning efficiency over cloning tools and techniques previously
reported. In
particular, the totipotent, immortalized cell lines and the refined nuclear
transfer
techniques of the invention provide for this enhanced cloning efficienc~~.
This
enhanced efficiency satisfies a long felt need in the art.
A. Generation of lmmottalized and Totilaotent Cellc
immortalized, totipotent cells of the invention can be produced from virtually
any type of precursor cell. Preferred embodiments of the invention relate to
the
following types of precursor cells: ( 1 ) embryos arising from the union of
two gametes
err vitro or err vrv~n: (2) embryonic stem cells (ESC's) arising from embryos
(e.~., pre-
blastocyst cells and inner cell mass cells); (3 ) cultured and noo-cultured
cells der,ved
from the inner cell mass of embryos; (4) cultured and non-cultured cells
arising from a
fetus; (5) primordial germ cells arising from a developing cell mass (e.g.,
genital ridge
cells); (6) immortalized cultured cells arising from primordial germ cells,
where the
immortalized cells are referred to as embryonic germ cells (EGCs) in the art;
and (7)
cultured and non-cultured cells isolated from an animal.
ESCs and EGCs can be readily generated from methods known in the art. See,
e.g., Stice et al., 1996, Biology of Reproduction 54: 100-110, hereby
incorporated by
CA 02282722 1999-09-O1
WO 98/39416 PCT/US98/04345
44
reference herein in its entirety including all figures, tables, and drawings.
See also,
Strelchenko, 1996, Theriogenology 4~: 130-141. ESCs have been demonstrated to
give rise to fetuses, from which primordial germ cells and EGCs can be
derived.
Therefore, ESCs are a nearly unlimited source for primordial germ cells and
EGCs.
Cells derived from an animal can be isolated from nearly any type of tissue.
For example, an ear-punch can be taken from an animal, the cells from the
sample can
be separated, and the separated cells can be subsequently cultured in vitro by
using
cell culture techniques well known to a person of ordinary skill in the art.
Preferably,
cells of the invention are extracted from bovine animals. Examples of
materials and
methods for reprogramming primary culture cells into immortalized. totipotent
cells
are described in exemplary embodiments hereafter.
Although exemplary embodiments of the invention are directed to bovine
animals. materials and methods of the invention can be applied to the
generation of
immortalized, totipotent cells using precursor cells isolated from any mammal.
Preferably immortalized, totipotent cells are extracted from ungulates.
Examples of
preferred ungulates envisioned for the invention are described previously.
Immortalized, totipotent cells of the invention are preferably generated from
the examples of cells indicated in the preceding paragraph after treatment
with a
receptor ligand cocktail. Examples of receptor ligands are well known to a
person of
ordinary skill in the art. Cytokines and/or growth factors are preferred
receptor ligands
of the invention. See, e.g., R&D Systems Catalog. 614 McKinley Place N.E.,
Minneapolis, MN 55413. In exemplary embodiments, varying amounts of human
recombinant leukemia inhibitory factor (hrLIF) and basic bovine fibroblast
growth
factor (bFGF) can be added to the culture medium to reprogram the precursor
cells
into immortalized, totipotent cells. Varying concentrations of these two
cytokines can
be added to the culture medium, preferably in concentrations of I-1000 ng/mL,
more
preferably in concentrations between 10-500 ng/mL, and most preferably about
100
ng/mL. Exogenous soluble and membrane-associated forms of steel factor are not
required for converting precursor cells into totipotent, immortalized cells.
CA 02282722 1999-09-O1
WO 98/39416 PCT/US98/04345
These examples are not meant to be limiting and any cytokine or combination
of cytokines can be added or deleted from those described in exemplary
embodiments
described hereafter. Preferred cytokines for generating immortalized,
totipotent cells
can be selected from the group consisting of fibroblast growth factor (FGF),
leukemia
5 inhibitor factor (LIF), cardiotrophin 1 (CT-1 ), ciliary neurotrophic factor
(CNTF),
stem cell factor (SCF), oncostatin M (OSM), and any member of the interleukin
(IL)
family, including IL-6, IL-11, and IL-12.
Other cytokines and other molecules besides cytokines can be added or deleted
from the receptor ligand cocktail described in the exemplary embodiments
described
10 hereafter to create immortalized, totipotent cells from any of the cells
described in the
previous paragraph. Any of the conditions for generating immortalized.
totipotent
cells can be modified from those described herein. The ability of these
modified
conditions to generate immortalized, totipotent cells can be monitored by
methods
defined in the section "ldeniification of Immortalized and Totipotent Cells"
described
15 hereafter.
B. n ni E. Immortalized a_n_d Totinotent Cells
A variety of methods for culturing cells exist in the art. See, e.g., Culture
of
anmnal cells: a manual ojbasic technigue (2nd. edition), Freshney, copyright
1987,
20 Alan R. Liss. lnc., New York. Particularly the cells that are precursor
cells for
immortalized. totipotent cells, as well as the immortalized, totipotent cells
themselves,
can be grown on feodcr layers. Examples of foods layers are well known to a
person
of ordinary skill in the art. and can arise from a number of different cells
that are
cultured in vitro. See, e.g.. Strelchenko. 1996, TherioRenologv 45: 130-141,
as well as
25 exemplary embodiments described hereafter. However, precursor cells for
immortalized, totipotent cells as well as the immortalized, totipotent cells
themselves
need not be grown on feeder layers.
CA 02282722 1999-09-O1
WO 98/39416 PCT/I1S98/04345
46
C. Identification of Immortalized and Totinotent Cells
Identification of Immortalized Cells
Immortalized cells can be identified as those that are not confined to the
Hayflick limit. The Hayflick limit is defined by cells that divide for more
than 60 cell
divisions. Hence, cells that have divided for more than 60 cell divisions are
immortalized cells. In addition, immortalized cells typically can be passaged
at lower
cell densities than non-immortalized cells.
The materials and methods described above (e.g., culturing the cells with
cytokines) can convert non-immortalized cells into immortalized cells. Other
methods
exist in the art for generating immortalized cell lines from primary cells.
For example.
manipulating the activity of teiomerase within the cells can immortalize
cells. See.
e.g., U.S. Patent No. 5,645,986, entitled "Therapy and Diagnosis of Conditions
Related to Telomere Length andlor Telomerase Activity." West et al., issued
Julv 8,
1 S 1997, and hereby incorporated by reference herein in its entirety
including all figures,
drawings, and tables.
Moreover, cellular immortality can be detenmined by identifying both low
molecular weight and macromolecular markers that are specific for immortalized
cells. The existence or lack of existence of a marker can be a determination
of cell
immortalization. In addition, a phenomenon associated with a marker can be an
indication of immortality. For example, if the marker is an enzyme, an
indication of
the presence of the enzyme andlor a certain level of catalytic activity of
that enrymc
may be a suitable indication that a certain cell type is immortalized.
Low molecular weight markers include specific nucleosides, lipid associated
sialic acids, polyamines, and pseudouridine. These examples are not limiting
and the
invention relates to any other low molecular weight markers known in the art.
Macromolecular markers can be separated into several classes including
nucleic acid polymers, peptides, polypeptides, proteins, enzymes, growth
factors,
growth factor receptors, honmones, hormone receptors, oncogenes, oncogene
products, and specific glycoproteins. Macromolecular markers can be selected
from
CA 02282722 1999-09-O1
WO 98/39416 PCT/US98I04345
47
the group consisting of extracellular proteins, membrane associated proteins,
and/or
intracellular
proteins,
which
may
be
membrane
associated
or
soluble.
One
such
marker
for
immortalized
cells
is
telomerase
or
its
associated
activity,
for
example.
See,
U.S.
patent
5,645,986,
supra.
Other
examples
of
markers
specific
for
S immortalized
cells
can
be
selected
from
the
following
list:
1 ) Epidermal growth factor (EGF) and its receptor (EGF-R)
2) Transforming growth factor-alpha (TGF-alpha) and its
receptor
3) c-erbB2 receptor tyrosine kinase (HER2 product)
4) Hyaluronan receptor (probably CD44, an integral membrane
glycoprotein)
Carcinoembryonic antigen (CEAI family of tumor markers
(for example TI. a
glycosylated protein)
6) Telomerase. a ribonucleoprotein which maintains telomere
length in
immortalized cells
7) Phosphatases: placental alkaline phosphatase (PLAP),
germ cell alkaline
phosphatasc, prostate acid phosphatase (PAS) '
8) Cathepsin D (catalyzes degradation of laminin).
9) Ornithine decarboxylase (ODC) (catalyzes the rate-limiting
step in polyamine
synthesis)
10) Beta-glucuronidase
11 ) Alpha-6 integrin
l2 ) i~cratin K8
13) Oncogene products: ras oocogenes (k-ras. Ha-ras. p21
), v-src. c-myc
14) Cyclin Dl, cyclin A. and Retinoblastoma Gene Protein
(Rb)
I S) Changes in p53 expression or p53 mutations
l6) Heterogeneous ribonucleoprotein-A2 (hnRNP-A2) overexpression
17) L-plastin
18) Ganglioside fucosyl-GM 1
19) Mob-1 expression (mob-1) (homology to proinflammatory
cytokines)
CA 02282722 1999-09-O1
WO 98/39416 PCT/US98/04345
48
These examples are not limiting and the invention relates to any markers
specific for
immortalized cells that are known in the art.
In addition to markers for immortalization known in the art, markers for
immortalization can be identified using methods well known in the art. For
example,
immortalization markers can be identified by analyzing particular molecules
(e.g.,
nucleic acid molecules and polypeptide molecules) that are unique to specific
cell
types.
In examples pertaining to nucleic acid immortalization markers, immortalized
and non-immortalized cells may be subjected to analysis for nucleic acid
sequence
content (e.g., hybridization techniques with nucleic acid probes). Nucleic
acid
samples from particular immortalized cells and nucleic acid samples from
particular
non-immortalized cells can be screened for particular nucleic acid sequences.
If
samples from non-immortalized cells lack a nucleic acid sequence present in
immortalized cells, then this nucleic acid sequence could bc: a marker for
I S distinguishing immortalized cells from non-immortalized cells. Similarly,
if samples
from non-immortalized cells harbor a nucleic acid sequence that immortalized
cells
lack, this nucleic acid sequence could be a marker for distinguishing
immortalized
cells from non-immortalized cells. Similar methods can elucidate polypeptide
markers by utilizing polypeptide analytical techniques (e.~,~.. PAGE. SDS-
PAGE,
procedures comprising antibodies. and HPLC techniques known in the art).
Identi ~cation of Totinotent Cells
Totipotent cells can be identified by a number of tests. Examples of these
tests include:
( 1 ) identifying a marker specific for totipotent cells;
(2) performing one or more nuclear transfer cycles with a cell (as described
hereafter) and developing the resulting embryo into an animal.
CA 02282722 1999-09-O1
WO 98139416
PCT/US98/04345
49
Markers can be utilized to distinguish totipotent cells from non-totipotent
cells.
Markers can be selected from the group of low molecular weight markers,
macromolecular markers, cell surface markers, and intracellular markers.
Examples
of markers that may be suitable for identifying totipotent cells can be
selected from
S the group consisting of alkaline phosphatase, cytokeratin, vimentin,
iaminin, and c-kit.
These markers are well known to a person of ordinary skill in the art and
these
examples are not meant to be limiting.
Some of these markers have been tested for cultured bovine cells being
identified for totipotency. As noted previously, totipotent, immortalized
bovine cells
of the invention generally do not appreciably stain for alkaline phosphatase.
Therefore the cells of the invention are to be contrasted with pluripotent
cells
discussed in previously referenced publications. It should be noted that some
of the
exe:nplan~ markers lis:ed previously may not be specific for to:ipo:ent cells
as some of
these markers may exist in pluripotent cells as well as in totipotent cells.
For
example. although immortalized, totipotent bovine cells do not appreciably
stain for
alkaline phosphatase, immortalized, totipotent porcine cells may appreciably
stain for
alkaline phosphatase. The invention relates to any markers specific for
totipotent cells
that are known to a person of ordinary skill in the art.
Markers for totipotency that are not clearly defined in the art can be
elucidated
by processes defined in the previous section, which illustrates methods for
elucidating
immortalization cell markers.
A preferred test for determining totipotency of cells is determining whether
cells give rise to totipotent embryos and eventually cloned animals. This test
represents a definitive test for cellular totipotency. An example of such a
test includes
the following steps: ( 1 ) utilizing a potentially totipotent cell for nuclear
transfer with
an enucleated oocyte; (2) allowing the resulting cybrid to develop; (3)
separating an
embryo that developed from the cybrid into individual cells and subjecting one
or
more of the individual cells to a second round of nuclear transfer; (4)
allowing a
resulting cybrid from step (3) to develop into an embryo; (5) implanting the
embryo
CA 02282722 1999-09-O1
WO 98/39416 PCTII1S98/04345
from step (2) or (4) into a uterine environment; and (6) allowing the embryo
to
develop. If the ensuing fetus develops past the first trimester of pregnancy
then the
cells initially used for nuclear transfer are most likely totipotent cells. If
the cells
utilized for nuclear transfer develop into a live born cloned animal then the
cells are
5 definitively totipotent. Examples of the techniques utilized for this
exemplary test
(e.g., enucleation of oocytes and nuclear transfer) are described completely
in the art
and in exemplary embodiments defined hereafter.
Hence, the materials and methods provided herein are the first to feature
immortalized, totipotent cells for cloning a bovine animal. As described above
these
10 materials and methods can be applied to other ungulates due to the high
degree of
nuclear DNA sequence homology among ungulates. Using the tests for identifying
immortalized, totipotent cells, the methods and materials described herein can
be
modified by a person of ordinary skill in the art to produce immortalized.
~.o~.ipotent
cells from any type of precursor cell. Hence, the invention covers any of the
materials
15 and methods described herein as well as modifications to these methods for
generating
immortalized, totipotent cells, since a person of ordinary skill in the art
can readily
produce immortalized, totipotent cells by utilizing the materials and methods
described herein in conjunction with methods for identifying immortalized,
totipotent
cells.
ll. T~genic Immortai_ized end Totipotent Cells
Materials and methods readily available to a person of ordinary skill in the
an
can be utilized to convert immortalized. totipotent cells of the invention
into
transgenic immortalized. totipotent cells. Once the nuclear DNA is modified in
the
immortalized. totipotent cells of the invention, embryos and animals arising
from
these cells can also comprise the modified nuclear DNA. Hence, materials and
methods readily available to a person of ordinary skill in the art can be
applied to the
immortalized, totipotent cells of the invention to produce transgenic animals
and
chimeric animals. See, e.g., EPO 264 16b, entitled "Transgenic Animals
Secreting
CA 02282722 1999-09-O1
WO 98139416 PCT/LTS98/04345
51
Desired Proteins Into Milk"; WO 94/19935, entitled "Isolation of Components of
Interest From Milk"; WO 93/22432, entitled "Method for Identifying Transgenic
Pre-
implantation Embryos"; and WO 95/175085, entitled "Transgenic Production of
Antibodies in Milk," all of which are incorporated by reference herein in
their entirety
including all figures, drawings and tables.
Methods for generating transgenic cells typically include the steps of ( 1 )
assembling a suitable DNA construct useful for inserting a specific DNA
sequence
into the nuclear genome of a cell; (2) transfecting the DNA construct into the
cells; (3)
allowing random insertion and/or homologous recombination to occur. The
modification resulting from this process may be the insertion of a suitable
DNA
constructs) into the target genome: deletion of DNA from the target genome:
and/or
mutation of the target genome.
DNA constructs can comprise a gene of interest as well as a varien~ of
elements including regulatory promoters, insulators, enhancers. and repressors
as well
as elements for ribosomal binding to the RNA transcribed from the DPdA
conswct.
DNA constructs can also encode ribozymes and anti-sense DNA and/or RNA,
identified previously herein. These examples are well known to a person of
ordinary
skill in the art and are not meant to be limiting.
Due to the effective recombinant DNA techniques available in conjunction
with DNA sequences for regulatory elements and genes rradily available in data
bases
arid the commercial sector, a person of ordinary skill in the art can readily
generate a
DNA construct appropriate for establishing transgenic cells ruing the
materials and
methods described herrin.
Ttsnsfection techniques are well known to a person of ordinary skill in the
art
and materials and methods for carrying out transfection of DNA constructs into
cells
are commercially available. Materials typically used to transfect cells with
DNA
constructs are lipophilic compounds, such as LipofectinTM for example.
Particular
lipophilic compounds can be induced to form liposomes for mediating
transfection of
the DNA construct into the cells.
I
CA 02282722 1999-09-O1
WO 98139416 PCT/US98/04345
52
Target sequences from the DNA construct can be inserted into specific regions
of the nuclear genome by rational design of the DNA construct. These design
techniques and methods are well known to a person of ordinary skill in the
art. See,
U.S. Patent 5,633,067, "Method of Producing a Transgenic Bovine or Transgenic
Bovine Embryo," DeBoer et al., issued May 27, 1997; U.S. Patent 5,612,205,
"Homologous Recombination in Mammalian Cells," Kay e~ al., issued March 18,
1997; and PCT publication WO 93/22432, "Method for Identifying Transgenic Pre-
Implantation Embryos," both of which are incorporated by reference herein in
their
entirety, including all figures, drawings, and tables. Once the desired DNA
sequence
is inserted into the nuclear genome, the location of the insertion region as
well as the
frequency with which the desired DNA sequence has inserted into the nuclear
genome
can be identified by methods well known to those skilled in the art.
Once the transgene is inserted into the nuclear eenome of the immortalized.
totipotent cell, that cell can be used as a nuclear donor for cloning a
transgenic animal.
A description of the embodiments related to transgenic animals are described
in more
detail hereafter.
A. j?iseases and Parasites
Desired DNA sequences can be inserted into the puclear cellular) genome to
enhance the resistance of a cloned transgenic animal to particular parasites
and
diseases. Examples of parasites include worms. flies, ticks, and fleas.
Examples of
infectious agents include bacteria, fungi, and viruses. Examples of diseases
include
Johne's. BVD, tuberculosis, foot and mouth, BLV. BSE and brucellosis. These
examples are not limiting and the invention relates to any disease or parasite
or
infectious agent known in the art. See, e.g., Hagan & Bruisers Infectious
Diesases of
Domestic Animals (7th edition), Gillespie & Timoney, copyright 1981, Cornell
University Press, Ithaca NY.
A transgene can confer resistance to a particular parasite or disease by
completely abrogating or partially alleviating the symptoms of the disease or
parasitic
CA 02282722 1999-09-O1
WO 98/39416 PCT/US98/04345
53
condition, or by producing a protein which controls the parasite or disease.
B. Elements of DNA Constructs a_nd Production of DNA Concm»tt
A wide variety of transcriptional and translational regulatory sequences may
be employed, depending upon the nature of the host. The transcriptional and
translational regulatory signals may be derived from viral sources, such as
adenovirus,
bovine papilloma virus, cytomegalovirus, simian virus, or the like, whereas
the
regulatory signals are associated with a particular gene sequence possessing
potential
for high levels of expression. Alternatively, promoters from mammalian
expression
products. such as actin, casein, alpha-lactalbumin. uroplakin. collagen,
myosin, and
the like, may be employed. Transcriptional regulatory signals may be selected
which
allow for repression or activation, so that expression of the gene product can
be
modulateu. Of interest are regulatory signals which can be repressed or
initiated by
I S external factors such as chemicals or drugs. Other examples of regulatory
elements
are described herein.
C. Examples of Preferred Recombinant Products
A variety of proteins and polypeptides can be encoded by a gene harbored
within a DNA construct suitable for creating transgenic cells. Those proteins
or
polypeptides include hormones, growth factors. enzymes, clotting factors,
apolipoproteins, receptors. drugs, pharmaceuticals. bioceuticals.
nutraceuticals,
oncogencs, tumor antigens, tumor suppressors, cvtokincs, viral antigcru,
parasitic
antigens, bacterial antigens and chemically synthesized polymers and polymers
biosynthesized and/or modified by chemical, cellular and/or enzymatic
processes.
Specific examples of these compounds include proinsulin, insulin, gmwth
hormone,
androgen receptors, insulin-like growth factor I, insulin-like growth factor
II, insulin
growth factor binding proteins, epidermal growth factor, TGF-ac, TGF-(3,
dermal
growth factor (PDGF), angiogenesis factors (acidic fibroblast growth factor,
basic
fibroblast growth factor and angiogenin), matrix proteins (Type IV collagen,
Type VII
CA 02282722 1999-09-O1
WO 98/39416 PCT/L1S98/04345
54
collagen, laminin), oncogenes (ras, fos, myc, erb, src, sis, jun), E6 or E7
transforming
sequence, p53 protein, cytokine receptor, IL-I, IL-6, IL-8, IL-2, a, (3, or
~yIFN,
GMCSF, GCSF, viral capsid protein, and proteins from viral, bacterial and
parasitic
organisms. Other specific proteins or polypeptides which can be expressed
include:
S phenylalanine hydroxylase, a-I-antitrypsin, cholesterol-7a-hydroxylase,
truncated
apolipoprotein B, lipoprotein lipase, apolipoprotein E, apolipoprotein AI, LDL
receptor, scavenger receptor for oxidized lipoproteins, molecular variants of
each,
VEGF, and combinations thereof. Other examples are clotting factors,
apolipoproteins, drugs, tumor antigens, viral antigens, parasitic antigens,
monoclonal
antibodies, and bacterial antigens. One skilled in the art readily appreciates
that these
proteins belong to a wide variety of classes of proteins, and that other
proteins within
these classes can also be used. These are only examples and are not meant to
be
limiting in anv way.
It should also be noted that the genetic material which is incorporated into
thr
cells from DNA constructs includes ( 1 ) nucleic acid sequences not normally
found in
the cells; (2) nucleic acid molecules which are normally found in the cells
but not
expressed at physiological significant levels; (3) nucleic acid sequences
normally
found in the cells and normally expressed at physiological desired levels; (4)
other
nucleic acid sequences which can be modified for expression in cells: and (5)
anv
combination of the above.
In addition. DNA constructs may become incorporated into the nuclear DhA
of cells, where the incorporated DNA can be transcribed into ribonucleic acid
molecules that can cleave other RNA molecules at specific regions. Ribonucleic
acid
molecules which can cleave RNA molecules are referred to in the an as
ribozymes.
which are RNA molecules themselves. Ribozymes can bind to discrete regions on
a
RNA molecule, and then specifically cleave a region within that binding region
or
adjacent to the binding region. Ribozyme techniques can thereby decrease the
amount
of polypeptide translated from formerly intact message RNA molecules.
Furthermore, DNA constructs can be incorporated into the nuclear
CA 02282722 1999-09-O1
WO 98/39416 PGT/US98/04345
complement of cells and when transcribed produce RNA that can bind to both
specific
RNA or DNA sequences. The nucleic acid sequences are utilized in anti-sense
techniques, which bind to the message (mRNA) and block the translation of
these
messages. Anti-sense techniques can thereby block or partially block the
synthesis of
5 particular polypeptides in cells.
III. Nuclear Transfer
Nuclear transfer (NT) techniques using non-immortalized and non-totipotent
cells are well known to a person of ordinary skill in the art. See, U.S.
Patent
1 C 4.994.;84 (Prather et al.) and 5.057,420 (Massey et al.). All of the
advantages
inherent to using the immortalized, totipotent cells as described above are
also
advantages for NT techniques, specifically the fact that the immortalized,
totipotent
cells are a nearly unlimited source of nuclear donors and that these cells
increase the
efficiency of NT. Exemplary embodiments define a two-cycle NT technique that
1 ~ provides for efficient production of totipotent bovine embryos. This
technique can be
applied to any mammal, preferably ungulates.
A. Nuclear Donors
Immortalized. totipotent cells of the invention can be used as nuclear donors
in
20 a NT process for generating a cloned embryo. As described above, the
immortalized.
totipotem cells can be generated from nearly any type of cell. for NT
techniques, a
donor cell may be separated from a EtroHing call mass or isolatod from a cell
line. The
entire cell may be placed in the perivitelline space of a recipient oocw~te or
may be
directly injected into the recipient oocyte by aspirating the nuclear donor
into a needle,
2~ placing the needle into the recipient oocyte, releasing the nuclear donor
and removing
the needle without significantly disrupting the plasma membrane of the oocyte.
Alternatively, a nucleus (karyoplast) may be isolated from a nuclear donor and
placed
into the perivitelline space of or injected directly into the recipient
oocyte, for
example.
CA 02282722 1999-09-O1
WO 98139416 PCT/C1898I04345
56
B. Recinient Ooc_ es
A recipient oocyte is typically an oocyte with a portion of its ooplasm
removed, where the removed ooplasm comprises the oocyte nucleus. Enucleation
techniques are well known to a person of ordinary skill in the art. See e.g.,
U.S.
S Patents 4,994,384 and 5,057,420.
Oocytes can be isolated from either oviducts and/or ovaries of live animals by
oviductal recovery procedures or transvaginal oocyte recovery procedures well
known
in the art and described herein. Furthermore, oocytes can be isolated from
deceased
animals. For example, ovaries can be obtained from abattoirs and the oocvtes
aspirated from these ovaries. The oocytes can also be isolated from the
ovaries of a
recently sacrificed animal or when the ovary has been frozen and/or thawed.
Oocytes can be matured in a variety of media well known to a person of
ordinary skill in the art. One example of such a medium suitable for maturing
oocvtes
is depicted in an exemplary embodiment described hereafter. Oocytes can be
successfully matwed in this type of medium within an environment comprising 5%
CO, at 39°C. Oocytes may be cryopreserved and then thawed before
placing the
oocytes in maturation medium. Cryopreservation procedures for cells and
embryos
are well known in the art as discussed herein.
The nuclear donor may be incorporated into either a young or an aged oocyte.
The age of the oocyte can be determined by the time that has elapsed since the
oocvte
was placed in maturation medium and the timc it was activated. A young oocvte
can
be defined as an oocvte that is cultured m vuro less than 28 hours before
activation.
An aged oocvte is defined as an oocyte that is cultured rn vitro for more than
28 hours
before activation.
The age of the oocytes can be functionally identified by the appearance of
their
ooplasm. For example, because certain cellular materials have not yet
dispersed
within the ooplasm of a young oocyte, young oocytes have a pycnotic
appearance.
Aged oocytes, in comparison, are characterized by a more homogeneous
cytoplasm.
A publication discussing the use of aged oocytes for NT is WO 97/07662,
entitled
CA 02282722 1999-09-O1
WO 98139416 PCT/US98/04345
57
"Inactivated Oocytes as Cytoplast Recipients for Nuclear Transfer."
The nuclear donor cell and the recipient oocyte can arise from the same specie
or different species. For example, a bovine immortalized, totipotent cell can
be
inserted into a bovine enucleated oocyte. Alternatively, an immortalized,
totipotent
cell derived from a bison can be inserted into a bovine enucleated oocyte. Any
nuclear donor/recipient oocyte combinations are envisioned by the invention.
Preferably the nuclear donor and recipient oocyte arise from one specie or
different
species of ungulates. Cross-species NT techniques can be utilized to produce
cloned
animals that are endangered.
The ooeytes can be activated by electrical and/or non-electrical means before.
during. and/or after fusion of the nuclear donor and recipient oocyte. For
example. the
oocyte can be placed in a medium containing one or more components suitable
for
non-electrical activation prior to fusion. Alternatively, a fused cybrid can
be placed in
a medium containing one or more components suitable for non-electrical
activation.
The activation process is discussed in greater detail hereafter.
C. jyiection/Fusion
A nuclear donor can be translocated into an oocyte using a variety of
materials
and methods that are well known to a person of ordinary skill in the art. In
one
example, a nuclear donor may be directly injected into a recipient oocyte.
This dimt
injection cart be accomplished by gently pulling a ntxlear donor mto a needle,
piercing
a recipient oocyte with that noodle, releasing the nuclear donor into the
oocyte, and
removing the needle from the oocyte without significantly disrupting its
membrane.
Appropriate needles can be fashioned from glass capillary tubes, as defined in
the art
and specifically by publications incorporated herein by reference.
In another example, at least a portion of plasma membrane from a nuclear
donor and recipient oocyte can be fused together using techniques well known
to a
person of ordinary skill in the art. See, Willadsen, 1986, Nature 320:63-65,
hereby
incorporated herein by reference in its entirety including all figures,
tables, and
CA 02282722 1999-09-O1
WO 98139416 PCTlUS98/04345
58
drawings. Typically, lipid membranes can be fused together by electrical and
chemical means, as defined previously and in other references incorporated by
reference herein.
Other examples of non-electrical means of cell fusion involve incubating
S cybrids in solutions comprising polyethylene glycol (PEG), and/or Sendai
virus.
Various molecular weights of PEG can be utilized for cell fusion.
Although the efficiency of NT as a process is sensitive to minor
modifications,
other variables for fusion can be determined without undue experimentation.
For
example, modifications to cell fusion techniques can be monitored for their
efficiency
by viewinb the degree of cell fusion under a microscope. The resulting embryo
can
then be cloned and identified as a totipotent embryo by the same methods as
those
previously described herein for identifying immortalized, totipotent cells,
which can
include tests for selectable markers and/or tests for developing an animal.
1 S D. Activation
Methods of activating oocytes and cybrids are known to those of ordinary skill
in the art. See, U.S. Patent 5,496,720, "Parthenogenic Oocyte Activation,"
Susko-
Parrish et al., issued on March 5, 1996, hereby incorporated by reference
herein in its
entirety including all figures, tables. and drawings.
Both electrical and non-electrical means can be used for activating the
cybrids.
Although use of a non-electrical means for activation is not always necessary,
non-
electrical activation can enhance the developmental potential of cybrids,
panicularls
when young oocytes are utilized as recipirnts.
Examples of electrical techniques for activating cells are well known in the
art.
See, U.S. Patents 4,994,384 and 5,057,420. Non-electrical means for activating
cells
can include any method known in the art that increases the probability of cell
division.
Examples of non-electrical means for activating a nuclear donor and/or
recipient can
be accomplished by introducing cells to ethanol; inositol trisphosphate (IP3);
Ca**
ionophore and protein kinase inhibitors such as 6-dimethylaminopurine;
temperature
CA 02282722 1999-09-O1
WO 98139416 PCT/US98/04345
59
change; protein synthesis inhibitors (e.g., cyclohexamide); phorbol esters
such as
phorbol 12-myristate 13-acetate (PMA); mechanical techniques, thapsigargin,
and
sperm factors. Sperm factors can include any component of a sperm. Other non-
electrical methods for activation include subjecting the cell or cells to cold
shock
and/or mechanical stress.
Examples of preferred protein kinase inhibitors are protein kinase A, G, and C
inhibitors such as 6-dimethylaminopurine (DMAP), staurosporin, 2-aminopurine,
sphingosine. Potentially, tyrosine kinase inhibitors may also be utilized to
activate
cells.
Although the NT process is sensitive to minor modifications. other variables
for activation can be determined without undue experimentation. Other
activation
materials and methods can be identified h~~ modifying the specified conditions
defined
in the exemplary protocols described hereafter and in U.S. Patent 5.496.724.
The result of any modifications upon efficiency and totipotency of the
activated embryo can be identified by the methods described previously in the
section
entitled "Identification of Immortalized and Totipotent Cells." Methods for
identifying totipotent embryos can include one or more tests, such as (a)
identifying
specific markers for totipotent cells in embryos, and (b) by determining
whether the
embryos are totipotent by allowing them to drvelop into an animal. Therefore,
the
invention relates to any modifications to the activation procedures described
herein
even though these modifications may not be explicitly stated herein.
F. M~ninulation of Embryos Resulting from Nuclear Transfer
An embryo resulting from a NT can be manipulated in a variety of manners.
The invention relates to cloned embryos that arise from at least one NT.
Exemplary embodiments of the invention demonstrate that two or more NT
procedures may enhance the efficiency for the production of totipotent
embryos. The
exemplary embodiments indicate that incorporating two or more NT procedures
into
methods for producing cloned totipotent embryos may enhance placental
CA 02282722 1999-09-O1
WO 98/39416 PCT/US98/04345
development. In addition, increasing the number of NT cycles involved in a
process
for producing totipotent embryos may represent a necessary factor for
converting non-
totipotent cells into totipotent cells. The effect of incorporating two or
more NT
cycles on the totipotency of resulting embryos is a surprising result, which
was not
S previously identified or explored in the art.
Incorporating two or more NT cycles into methods for cloned totipotent
embryos can provide another advantage. Incorporation of multiple NT procedures
into methods for creating cloned totipotent embryos provides a method for
multiplying the number of cloned totipotent embryos.
10 When multiple NT procedures are utilized for the formation of a cloned
totipotent embryo, young or aged oocytes can be utilized as recipients i~ the
first,
seco.~.d or subsequent NT procedures. For example, if a first NT and then a
second
NT are performed, the first NT can utilize a young enucleated oocyte as a
recipient
a:n :::;; s;:cond NT may utilize an aaec'. enucleated oocyt~ ::.. .. ::=:ai:
~~. . ~1~~r
nati~~ 1~ ,
I 5 the first NT may utilize an aged enucleated oocyte as a recipient and the
second NT
may utilize a young enucleated oocyte as a recipient for the same two-cycle
model for
NT. In addition, both NT cycles may utilize young enucleated oocytes as
recipients or
both NT cycles may utilize aged enucleated oocytes as recipients in the two-
cycle NT
example.
20 For NT techniques that incorporate two or more NT cycles, one or more of
the
NT cycles may be preceded, followed, andlor carried out simultaneously W th an
activation step. As defined previously herein. an activation step may be
accomplished
by electrical and/or non-electrical means as defined herein. Exemplified
embodiments
described hereafter describe NT techniques that incorporate an activation step
after
25 one of the NT cycles. However, activation steps may also be carried out in
conjunction with NT cycles (e.g., simultaneously with the NT cycle) and/or
activation
steps may be carried out prior to a NT cycle.
A preferred embodiment of the invention, for example, relates to a first NT
utilizing a young enucleated oocyte as a recipient followed by activation.
This, in
CA 02282722 1999-09-O1
WO 98/39416 PCT/US98104345
61
turn, is followed by a second NT utilizing an aged enucleated oocyte as a
recipient.
This second NT procedure is not followed by activation. This example is not
meant to
be limiting and the invention relates to any number of NT cycles that are
optionally
preceded by, followed by, simultaneously can;ied out with an activation
procedure.
NT techniques may utilize virtually any cell as a nuclear donor. For example,
in a preferred embodiment, a first NT may utilize an immortalized, totipotent
cell of
the invention as a nuclear donor and a second NT may utilize an embryonic cell
as a
nuclear donor. The second NT cycle in this example may utilize a blastomere (a
cell
isolated from an embryo), a cell isolated from a fetus (e.g., a primordial
germ cell) as
a nuclear donor, or a synchronized cell (described herein). The invention
pertains in
part to utilizing nearly any type of cell as a nuclear donor in any NT. The
effect of
using different nuclear donors on the overall efficiency for producing cloned
totipotent embryos can be tested by practicing the tests for totipotency
described in the
preceding section entitled "Identification of Immortalized and Totipotent
Cells."
The cloned totipotent embryos resulting from NTs can be ( ~ ) disaggregated or
(2) allowed to develop further.
If the embryos are disaggregated, these embryonic derived cells can be
utilized
to establish cultured cells. Any type of embryonic cell can be utilized to
produce
cultured cells. These cultured cells are sometimes referred to as embryonic
stem cell
or embryonic stem-like cells in the scientific literature. The embryonic stem
cells can
be derived from early embryos, monrlac, and blastocyst stage embryos. Multiple
methods arc known to a person of ordinary skill in the art for producing
cultured
embryonic cells. These methods arc enumerated in specific references
previously
incorporated by rcfercnce herein.
If the embryos are allowed to develop in utero, cells isolated from the
developing fetus can be utilized to produce cultured cells. In preferred
embodiments,
primordial germ cells arc isolated from the genital ridge of 28 to 75 day old
developing cell masses for the establishment of cell lines. These cultured
cells are
sometimes referred to as embryonic germ cells (EG). These cultured cells can
be
CA 02282722 1999-09-O1
WO 98139416 PCT/US98/04345
62
generated using methods well known to a person of ordinary skill in the art.
The
methods are enumerated in references previously incorporated by reference
herein.
The cloned totipotent embryos resulting from NT can also be manipulated by
cryopreserving and/or thawing the embryos. See, U.S. Patent No. 5,160,312,
entitled
"Cryopreservation Process for Direct Transfer of Embryos," Voelkel, and issued
on
November 3, 1992; and U.S. Patent No. 4,227,381, entitled "Wind Tunnel
Freezer,"
Sullivan et al., issued on October 14, 1980, all of which are hereby
incorporated by
reference herein in their entireties including all tables, figures, and
drawings. Other
embryo manipulation methods include culturing, performing embryo transfer,
dissociating for NT, dissociating for establishing cell lines for use in NT,
splitting ,
aggregating, sexing, and biopsying the embryos resulting from NT; which are
described hereafter. The exemplary manipulation procedures are not meant to be
limiting and the invention relates to any embryo manipulation procedure known
to a
person of ordinary skill in the art.
IV. Development ofCloned Embryos
A. TotiQotencv
Totipotent embryos can be identified by the methods described in the section
"Identification of Immortalized and Totipotent Cclls." Individual cells can be
isolated
and subjected to these similar tests. The tests relate to similar markers for
identifying
totipotcnt cells, as well as a test for determining totipotency by allowing an
embryo to
develop until it passes the second trimester of gestation, or preferably,
gives rise to a
live bom animal. Methods for identifying other markers for totipotency are
also
described in that section.
B. Culture of Embryos in vitro
Methods for culturing embryos in vitro are well known to those skilled in the
art. See, U.S. Patent No. 5,213,979, entitled "In vitro Culture of Bovine
Embryos,"
First et al., issued on May 25, 1993, and U.S. Patent No. 5,096,822, entitled
"Bovine
CA 02282722 1999-09-O1
WO 98/39416 PCTIUS98I04345
63
Embryo Medium," Rosenkrans, Jr. et al., issued on March 17, 1992, both of
which are
incorporated by reference herein in its entirety, including all figures,
tables, and
drawings. In addition, exemplary embodiments for media suitable for culturing
cloned embryos in vitro are described hereafter. Feeder cell layers may or may
not be
utilized for culturing cloned embryos in vitro. Feeder cells are described
previously
and in exemplary embodiments hereafter.
The present invention is superior to existing materials and methods for
cloning
organisms, because embodiments of the invention allow for culturing all cells
and
embryos in vitro prior to implantation. For example, cloning methods described
for
cloning ovine organisms require an in vivv development step in the oviducts of
an
ovine host animal before the embryos are implanted in a suitable host. Because
embodiments of the present invention do not require in vrvo development steps
prior
to implantation into the uterus, the materials and methods of the present
invention
represent an inventive step over cloning methods previously described by
others.
C. Deyelopment of Embrync in ritprp
Cloned embryos can be cultured in an artificial or natural uterine environment
after NT procedures. Examples of artificial development environments are being
developed and some are know to those skilled in the art. Components of the
artificial
environment can be modified with tittle experimentation. for example. by
modifyinb
ore component and monitoring the FroHth rate of the embryo.
Methods for implarttlng embryos into the uterus of an animal arc also well
known in the art. Preferably, the developmental stage of the embryos) is
correlated
with the estrus cycle of the animal.
Embryos from one specie can be placed into the uterine environment of an
animal from another specie. For example it has been shown in the art that
bovine
embryos can develop in the oviducts of sheep. Stice & Keefer, 1993, "Multiple
generational bovine embryo cloning," Biology of Reproduction 48: 715-719. The
invention relates to any combination of ungulate embryo in any other ungulate
uterine
CA 02282722 1999-09-O1
WO 98/39416 PCT/US98/04345
64
environment. The cross-species relationship between embryo and uterus can
allow for
efficient production of cloned animals of an endangered species. For example,
a bison
embryo can develop in the uterus of a domestic bovine. In another example, a
big-
horn sheep embryo can develop in the uterus of a large domesticated sheep.
Once the embryo is placed in the uterus of an animal, the embryo can develop
to term. Alternatively, the embryo can be allowed to develop in the uterus and
then
can be removed at a chosen time. Surgical methods are well known in the art
for
removing fetuses from uteri before they are born.
V. Cloned Animals
A. Bovine Cloned Animals
As described previously herein, the invention provides the advantages of being
able to assess the phenotype of an animal before cloning. This is an advantage
of the
invention since previous reports have only allowed the cloning of bovine
animals from
blastomeres, a method that does not allow for phenotype assessment.
Multiple products can be isolated from a cloned animal. For example, semen
can be collected from an animal, such as a bovine bull. Semen can be
cryopreserved
as well as separated sperm into sex-specific fractions. See, U.S. Patent No.s
5.439.362. 5.346,990, and 5,021.244, entitled "Sex-associated Membrane
Proteins and
Methods for Increasing the Probability that Offspring Will be of a Desired
Sex,"
Spaulding. and issued on August 8, 1995. September 13. 1994, and June 4, 1991,
respectively, all of which are hereby incorporated by reference herein in
their
entireties including all figures, drawings, and tables. Methods of collecting
semen arc
well known to a person of ordinary skill in the art. Physiology oJReproduction
and
Art~cial Insemination oJCattle (2nd. edition), Salisbury et al., copyright
1961, 1978,
W.H. Freeman & Co., San Francisco.
The invention relates in part to any products collected from a cloned animal,
preferably a cloned bovine animal. The products can be any body fluids or
organs
isolated from the animal, or any products isolated from the fluids or organs.
In
CA 02282722 1999-09-O1
WO 98/39416 PCT/US98104345
preferred embodiments, products such as milk and meat may be collected from
cloned
animals, preferably cloned bovine animals. In another embodiment, the
invention
relates to determining the phenotype of a bovine steer, which is a neutered
animal, and
then cloning this animal such that the cloned animals are reproductively
functional and
5 can be used to produce semen. Other preferred embodiments of the invention
relate to
such products as xenograft materials, sperm, embryos, oocytes, any type of
cells, and
offspring harvested from cloned animals of the invention, preferably cloned
bovine
animals.
Xenograft materials, which are described previously herein, can relate to any
10 cellular material extracted from one organism and placed into another
organism.
Medical procedures for extracting the cellular material from one organism and
grafting it into another organism are well known to a person of ordinary skill
in the
art. Examples of preferable xenograft cellular materials can be selected from
the
group consisting of liver, lung, heart, nerve, gallbladder, and pancreas
cellular
I S material.
B. Non-Bovine Cloned Animals
Due to the high DNA sequence homology between bovine animals and other
ungulates. the materials and methods of the invention can be utilized to clone
other
20 ungulates. The materials and methods of the invention are the most
efficient means
for cloning a mammal as known in the state of the an.
In prefcrrcd embodiments the materials and methods of the invention can be
utilized to clone endangered species, such as bison. In addition, the
materials and
methods of the invention can be utilized to clone commercially relevant
ungulates,
25 such as pigs. Due to the methods for reprogramming primary cells isolated
from an
animal into immortalized, totipotent cells, the more closely related the
animal species
is to cattle, the higher probability that the cloning methods of the invention
will have
greater success. Exemplary embodiments are described hereafter for cloning non-
bovine animals.
CA 02282722 1999-09-O1
WO 98!39416 PGT1US98/04345
66
C. Cloned Animals with Modified Nuclear DNA
As discussed in a previous section, transgenic animals can be generated from
the methods of the invention by using transgenic techniques well known to
those of
ordinary skill in the art. Preferably, cloned transgenic bovine animals are
produced
from these methods. These cloned transgenic animals can be engineered such
that
they are resistant or partially resistant to diseases and parasites endemic to
such
animals. Examples of these diseases and parasites are outlined in a preceding
section.
Moreover, the cloned transgenic animals can be engineered such that they
produce a recombinant product. Examples of recombinant products are outlined
in a
preceding section. The expression of these products can be directed to
particular cells
or regions within the cloned trartsgenic animals by selectively engineering a
suitable
promoter element and other regulatory elements to achieve this end.
For example, human recombinant products can be expressed in the urine of
cattle by operably linking a uroplakin promoter to the DNA sequence encoding a
recombinant product. Alternatively, examples are well known to a person of
ordinary
skill in the art for selectively expressing human recombinant products in the
milk of a
bovine animal.
Once the recombinant product or products have been expressed in a particular
tissue or fluid of the cloned transgenic animal, the suitable tissue or fluid
can be
collected using methods well known in the art. Recombinant products can be
purified
from that fluid or tissue by using standard purification techniques well known
to a
person of ordinary skill in the art.
CA 02282722 1999-09-O1
WO 98/39416 PCT/US98I04345
67
The examples below are non-limiting and are merely representative of various
aspects and features of the present invention.
EXAMPLE 1: Feeder Laver Prep ration
A feeder cell layer was prepared from mouse fetuses that were from 10 to 20
days gestation. The head, liver, heart and alimentary tract were removed and
the
remaining tissue washed and incubated at 37°C in 0.025% trypsin-0.02%,
EDTA
(Difco, Cat # 0153-61-1 ). Loose cells were cultured in tissue culture dishes
containing MEM-alpha supplemented with penicillin, streptomycin. 10% fetal
calf
serum and 0.1 mM 2-mercaptoethanol. The feeder cell cultures were established
over
a two to three week period at 37.4°C, 3.5% CO; and humidified air.
Before being
used as feeder cells, mouse fibroblasts were pre-treated with mitomycin C
(Calbiochem, Cat # 47589) at a final concentration of 10 ~eg/ml for 3 hours
and
washed 5 times with PBS before pre-equilibrated Fmwth media was added.
Feeder cells can be established from bovine fetuses from 30 to 70 days using
the same procedure. Bovine fetal cells may be optionally treated with
mitomycin C.
?0 ~,QMPLE ~: Establishing Culttifed Cells From hion-Embp~onic Ti~cue
Unc advantage provided by the materials and methods defined herein is the
ability to create an immortalized and toupotent cell from virtually any type
of
precursor xll. These precursor cells can be tmbryonic cells, culturrd
embryonic
2~ cells, primordial germ cells, fetal cells, and cells isolated from the
tissues of adult
animals, for example. Cells isolated from the kidney and ear of an adult grown
bovine
have been utilized as prectusor cells for the generation of immortalized,
totipotent
cells.
After cells are isolated from their respective tissues, the cells can be
subjected
30 to the materials and methods defined in Example 3.
CA 02282722 1999-09-O1
WO 98139416 PCTII1S98/04345
68
A first step towards generating immortalized, totipotent cells from tissues of
grown animals includes a primary culture of isolated cells. A protocol for
culturing
cells isolated from the tissues of grown animals is provided hereafter.
Although the
illustrative protocol relates to ear punch samples, this protocol can apply to
cells
isolated from any type of tissue.
The following steps are preferably performed utilizing sterile procedures:
1 ) Wash each ear sample twice with 2 mL of trypsin/EDTA solution in two
separate 35 mm Petri dishes. Process each ear sample separately. Mince the ear
sample with sterile scissors and scalpel in a 35 mm Petri dish containing 2 mL
of
trypsin/EDTA solution. The minced pieces are preferably less than 1 mm in
diameter.
2) Incubate minced ear pieces in the trypsin/EDTA solution for 40-50 min. in a
37°C incubator with occasional swirling. The trypsin/EDTA solution is
described in
more detail hereafter. The dish may be wrapped with a stretchable material,
such as
Parafilm~', to reduce CO, accumulation.
3) Transfer digested ear pieces to a 1 ~ mL sterile tube. Wash the dish from
which the digested ear pieces were recovered with 2 mL of the trypsin/EDTA
solution
and transfer this wash solution to the sterile tube.
4) Vortex the tube at high speed for 2 min.
5) Add 5 mL of media (defined below) to inactivate the trypsin.
6) Centrifuge the 1 S mL tube at 280xg for 10 minutes.
7) Decant the supernatant and re-suspend the cell pellet in rrsidual solution
by
gently taping the side of the tube.
8) Add 2 mL of media to the tube and then centrifuge as described in step (6).
9) Decant the supernatant, re-suspend the pellet as described in step (7),
then add
2 mL of media.
10) Keep 2-3 pieces of the ear for DNA analysis and store at -20°C in a
15 mL
tube.
11 ) Transfer resuspended cells into a 35 mm Nunc culture dish and incubate at
37°C in a humidified 5% COZ/95% air atmosphere.
CA 02282722 1999-09-O1
WO 98/39416 PGTIUS98I04345
69
12) Change media every 2 days.
Trypsin/EDTA solution:
0.025% trypsin (w/v) (Bacto trypsin, Difco #cat 0153-61-I )
0.02% EDTA (Sigma) (w/v)
Add the trypsin and EDTA to Cap'-free and Mgz'-free Dulbecco's phosphate-
buffered
saline (PBS) (Gibco cat# 450-1600EA) and sterilize by filtration through a 0.2
pm
filter.
Media:
Combine Alpha minimum essential medium (MEM) (Biowhittaker) with 10% fetal
bovine serum (Hyclone), 4 mM L-glutamine. 100 U/mL penicillin, 100 pg/mL
streptomycin. 0.25 ug/mL amphotercin B (Fungizone).
This protocol has been also successfully utilized to establish cultures of
kidney
and liver cells isolated from grown bovine animals. As discussed above, the
protocol
can be utilized to create cell cultures from any type of cell isolated from a
grown
animal, for any species or family of animals.
~yMPLE 3: R~programmin~_~nd Establishment of Immortalized and Totiro, tent
.'.0 Cells from Precursor I~,gll,
The reprogramming procedures described hereafter can utilize any cell type of
cells as prxursor cells for ttu generatron of immortalized, totipotent cells.
As an
example, the cell cultures described previously can be utilized as precursor
cells for
the reprogramming procedures described below. As another example, the
following
procedure describes one embodiment of the invention, where primordial germ
cells
were utilized as precursor cells for the generation of immortalized,
totipotent cells.
An embodiment of the reprogramming process is illustrated in Figure 2.
A bovine fetus approximately 40 days old was obtained from a pregnant
animal. The genital ridges were located at the caudo-ventral part of the
abdominal
CA 02282722 1999-09-O1
WO 98/39416
PCT/US98/04345
cavity. Genital ridges were removed aseptically and washed in phosphate
buffered
saline (PBS) (Gibco, Cat # 14287-015) with 500 U/mL penicillin/500 ug/ml
streptomycin. The tissue was sliced into 1-1.5 mm pieces and placed into a
solution
containing pronase E (3mg/ml; Sigma Cat # P6911 ) in Tyrodes Lactate (TL)
HEPES
5 (Biowhittaker, Cat # 04-616F) for 30-45 minutes at 35-37°C. The
proteolytic action
of pronase E disaggregated the slices of genital ridges to a cell suspension.
Pronase E
was removed by dilution and centrifugation in TL HEPES solution. After this
step,
the cell suspension was frozen and stored at -196°C.
A thawed cell suspension (final concentration 100,000 cells/ml) was placed
10 into a 35 mm Petri dish containing a murine primary embryonic fibroblast
feeder
layer. The culture media used was MEM alpha (Biowhittaker, Cat # 12-169F )
supplemented with 0.1 mM 2-mercaptoethanol (Gibco, Cat # 21985-023), 4 mM
glutamine. 100 ng/ml human recombinant leukemia inhibitory factor (hrLIF; R&D
System, Cat # 250-L), 100 ng/ml bovine basic fibroblast growth factor (bFGF;
R&D
15 System, Cat # 133-FB) and 10% fetal calf serum (FCS, HyClone, Cat # A 1111
D) at
37.5°C and 3.5% CO=. Exogenous steel factor (e.g., membrane associated
steel factor
and soluble steel factor) was not added to the culture media. After 24 hours,
and again
at 48 hour intervals, supplemented culture media was replaced. After an
initial culture
of 6 days, concentrations of hrLIF and bFGF were lowered to 40 ng/ml,
respectively.
20 After nine days in culture. hrLIF and bFGF were removed from the medium
entirely.
At the beginning of in yore culture of genital ridge cells, simple embryonic
bodies were occasionally observed. These bodies eventually disappeared with
subsequent passages. The rate of establishing immortalized. totipotent cell
lines from
genital ridge cells was i 00% and did not appear to be sex dependent. Table 1
contains
25 data from establishment of seven immortalized, totipotent cell lines.
Established
immortalized, totipotent cell lines were maintained in MEM-alpha supplemented
with
10% FCS which was replaced every second or third day. High density population
cells were passaged every week at a dilution ratio of 1:4 to 1:8. Cells were
passaged
by incubating with 0.025% trypsin - 0.02% EDTA mixture and preparing new
cultures
CA 02282722 1999-09-O1
WO 98/39416 PCT/US98/04345
71
in fresh growth medium. The growth promoting capacity of MEM-alpha media for
immortalized, totipotent cells was enhanced by adding insulin-transferrin,
sodium
selenite supplement, diluted to 1:100 (Sigma Cat # 1 I 884). As a preventive
measure
against mycoplasma contamination, short term cultivation with tylosine
tartrate
(Sigma, Cat T31 S 1 ) was carried out. Before NT, cell lines were tested for
presence of
mycoplasma by PCR performed with DNA primers specific for mycoplasma
sequences (Stratagene, Cat 302007).
TABLE 1
Characterization of Established Bovine Immortalized and Totipotent Cell Lines
Cell Weight of fetus Days in cultureSex of Cell
line (gm) line
EG 14.? >400 male
EG-1 20.? >300 male
EG-'' 3.9 >30 female
EG- 4.8 >30 male
EG-4 39.6 > 100 female
EG-S 3.9 >250 male
EG-6 8.6 >30 male
j~XAMPLf= 4:
The following embodiment of the invention describes materials and methods
utilized to produce totipotent embryos of the invention. lmmottalizcd embryos
of the
invention can be produced by utilizing immortalized and totipotent cells of
the
invention as nuclear donors in NT procedures. As described previously.
multiple NT
procedures can be utilized to create a totipotent embryo. The following two
examples
describe a multiple NT procedure, which describes the use of two NTs.
Mycoplasma free immortalized, totipotent cells used in the NT procedure,
were prepared by cutting out a group of immortalized, totipotent cells from
the feeder
CA 02282722 1999-09-O1
WO 98/39416 PCT/US98/04345
72
layer using a glass needle. The isolated immortalized, totipotent cells were
then
incubated in a TL HEPES solution containing from 1 to 3 mg/ml pronase E at
approximately 32°C for 1 to 4 hours, the amount of time which was
needed in this
example to disaggregate the cells. Once the cells were in a single cell
suspension they
were used for NT within a 2-3 hour period.
Oocytes aspirated from ovaries were matured overnight (16 hours) in
maturation medium. Medium 199 (Biowhittaker, Cat #12-I 19F) supplemented with
luteinizing hormone lOIU/ml (LH; Sigma, Cat # L9773), I mg/ml estradiol
(Sigma,
Cat # E8875) and 10% FCS or estrus cow serum, was used. Within 16 hours of
maturation, the cumulus layer expanded and the first polar bodies were
extruded.
In the first NT procedure, young oocytes were stripped of their cumulus cell
layers and nuclear material stained with Hoechst 3334 ~mg/ml (Sigma, Cat #
2261 )
in TL HEPES solution supplemented with cytochalasin B (7~cglml, Sigma, Cat #
C6762) for 15 min. Oocytes were then enucleated in TL HEPES solution under
i S mineral oil. A single immortalized, totipotent cell of optimal size ( i 2
to I 5 ~cm) was
then inserted from a cell suspension and injected into the perivitelline space
of the
enucleated oocyte. The immortalized, totipotent cell and oocyte membranes were
then
induced to fuse by electrofusion in a 500 E.,em chamber by application of an
electrical
pulse of 90V for 15 acs.
Cybrid activation was induced by a 4 min exposure to 5 ~cM calcium
ionophorc A23187 (Sigma Cat. # C-75'_2) or ionomycin Ca-salt in HECM (hamster
embryo culture medium) containing I mglml BSA followed by a 1:1000 dilution in
HECM containing 30 mgiml BSA for 5 min. Eor HECM medium, see, e.g., Seshagiri
& Barister, 1989, "Phosphate is required for inhibition of glucose of
development of
hamster eight-cell embryos in vitro," Biol. Reprod 40: 599-606. This step is
followed
by incubation in CR2 medium containing 1.9 mM 6-dimethylaminopurine (DMAP;
Sigma product, Cat # D2629) for 4 hrs followed by a wash in HECM and then
cultured in CR2 media with BSA (3 mg/ml) under humidified air with 5% COz at
39°C. For CR2 medium, see, e.g., Rosenkrans & First, 1994, "Effect of
free amino
71
in fresh growth medium. The
CA 02282722 1999-09-O1
WO 98/39416 PCT/US98104345
73
acids and vitamins on cleavage and developmental rate of bovine zygotes in
vitro," J.
Anim. Sci. 72: 434-437. Mitotic divisions of the cybrid formed an embryo.
Three
days later the embryos were transferred to CR2 media containing 10% FCS for
the
remainder of their in vitro culture.
Table 2 shows the effect of oocyte age on blastocyst development. The data
was obtained utilizing blastomeres from in vitro produced embryos or
immortalized,
totipotent cells as donor nuclei in the NT procedure. Developmental potential
was
measured in young versus aged oocytes.
TABLE 2
Effect of Ooryte Timing for Different Cell Sources
ooc~~tc age lmmortalizcd and Blastomercs (n-192)
(hours)
Totipotent Cells
(n=174)
16-28 (n=175) 17.9% blastocyst no development (n=35)
(n=140)
28-48 (n=191 no development (n=34)17.3% blastocyst
) (n=157)
The data presented in Table 2 shows that oocytes maintained in culture for 16-
28h were more suitable recipients for immortalized, totipotent cells, while
aged
oocytes maintained in culture for 28-48 h were a more suitable recipient for
blastomeres derived from embryos. In addition. activation procedures differed
between young and aged oocytes. Young oocytes, when used in the I~"T
procedure,
appear to require chemical activation w7th ionomycin and nMAP from these
studies.
Aged oocvtes. on the other hand, appear to be easily activated by
electrofusion
according to these studies.
EXAMPLE 5: Second Nuclear TrancfPr rRP~i~r,;"o~
Embryos from the first generation NT at the morula stage were disaggregated
either by pronase E (1-3 mg/ml in TL HEPES) or mechanically after treatment
with
cytochalasin B. Single blastomeres were placed into the perivitelline space of
CA 02282722 1999-09-O1
WO 98139416
PCT/I1S98/04345
?4
enucleated aged oocytes (28-48 hours of incubation). Aged oocytes were
produced by
incubating matured "young" oocytes for an additional time in CR2 media with 3
mglml BSA in humidified air with 5% COz at 39°C.
A biastomere from an embryo produced from an immortalized, totipotent cell
S was fused into the enucleated oocyte via electrofusion in a 500 ~cm chamber
with an
electrical pulse of l OSV for 1 S yes in an isotonic sorbitol solution (0.25
M) at 30°C.
Aged oocytes were simultaneously activated with a fusion pulse, not by
chemical
activation as with young oocytes.
After blastomere-oocyte fusion, the cybrids from second generation NT were
cultured in CR2 media supplemented with BSA (3 mg/ml) under humidified air
with
5% CO, at 39°C. On the third day of culture, developing embryos were
evaluated and
cultured further until day seven in C1L? media containing 10% FCS. Morphology
ically
good to fair quality embryos were non-surgically transferred into recipient
females.
Table 3 shows the increased gestation length achieved by use of recloned
(double NTl
immortalized, totipotent cells.
TABLE 3
Development of Immortalized and Totipotent Cells
Derived Fetuses after Double NT
No. of No. of recipientsNo. of pregnantNo.
of
embryos transferred recipients calves
into after
transferred 140 davs
Exper 15 5 1 1
# I
Exper 18 6 1 (two fetuses)2
#2
EXAMPLE 6: Cloning Non-Bovine Ungulates
The specification provides for methods of cloning non-bovine ungulates.
Examples of such ungulates can be selected from the group consisting of
bovids,
ovids, cervids, suids, equids and camelids, such as bison, sheep, big-horn
sheep,
CA 02282722 1999-09-O1
WO 98/39416 PCTILIS98/04345
caribou, antelope, deer, goat, water buffalo, camel, and pig.
Immortalized, totipotent cell lines can be prepared from multiple types of
cells
isolated from the non-bovine ungulate by using the methods described in
previous
examples relating to bovine animals, or by using the screening procedures for
these
5 methods as described in the specification. Virtually any type of cell
isolated from the
non-bovine ungulate can be utilized to establish an immortalized, totipotent
cell line.
For example. an ear-punch sample taken from a bison can be cultured in vitro
using a
variety of cell culture media such as MEM-alpha medium.
Bison-derived primary cells can then be converted or reprogrammed into
1 C immortalized. totipotent bison cells by supplementing the cell culture
medium with
hrLIF and bFGF as described in previous examples and in the specification.
Alternatively, the bison-derived primary cells can be converted into
immortalized.
totipotent cells by supplementing the growth medium with other types of
molecules
identified by methods for identifying such reprogramming molecules as
described in
I 5 the specification. The reprogrammed bison-derived cells can then be tested
for
totipotency by analyzing selected markers, such as alkaline phosphatase,
laminin, and
c-kit. In addition, the bison-derived cells can be considered permanent if the
number
of cell divisions exceeds the Hayflick limit andlor if the cells can Brow to
confluence
after being replated under conditions where the cells arc not in physical
contact with
20 one another. for example.
Once totipotcnt, immortalized cells have been established as nuclezr donors.
proper enucleatod oocytes can be prepared for NT. Oocytcs from the same or
diflercnt specie as the nuclear donor can be used for NT. For example, a bison-
derived nuclear donor cell can be fused or directly injected into a bison-
derived
25 enucleated oocyte or an enucleated oocyte from another specie, such as a
bovine.
As described in the specification, the oocytes can be derived from any
ungulate in a variety of ways, such as sacrificing an animal and retrieving
oocytes
from its oviducts, or spaying the animals by ovarian hysterectomy and
isolating the
oocytes from the oviducts or ovaries. Oocytes can also be obtained from live
animals
CA 02282722 1999-09-O1
WO 98/39416 PCT/US98/04345
76
by utilizing such methods as transvaginal oocyte recovery. The oocytes can
then be
enucleated by using methods described herein as applied to sheep or cattle.
These
methods can be easily applied to oocytes derived from other ungulates.
Nuclear transfer techniques can be performed after enucleated oocytes and
nuclear donor cells are prepared. Young or aged oocytes can be utilized for
the NT
procedure, and the number of NTs can vary as described in the specification.
In
addition the parameters that define the fusion step for a NT may be varied as
described
herein. An activation step can be applied to one or more of the NT cycles. For
example, the NT cycles defined in a previous exemplary embodiment can be
applied
I C to the generation of cloned bison. The embryo resultin_ from the NT can be
tested for
totipotency by utilizing tests for one or more markers, such as alkaline
phosphatase,
cytokeratin, vimentin, laminin, and c-kit. In addition, the embryo can be
tested for
totipotency by implanting it into the uterus of an animal and allowing
development to
term.
Once a cloned totipotent embryo is produced from the methods described
above for a non-bovine ungulate, the embryo can be further manipulated. Such
manipulations include cryopreserving, thawing, culturing, disaggregating the
embryo
into single cells. and implanting the embryo. The embryo may be cultured in an
artificial development environment (as described previously) or may be placed
in
urero of a properly synchronized female animal. An embryo derived from one
specie
may be placed in a uterus of the same or different specie. For example. a
bison-
derived embryo can be placed in the uterus of a bovine. The embryo can be
allowed
to develop until term. or may be retrieved from the uterine environment before
birth.
EXAMPLE 7: Multiple Pathways for Cloning Animals
Figure 3 illustrates multiple embodiments of the invention. Animals can be
cloned from cells that are reprogrammed into totipotent and immortalized
cells.
Fibroblast cell cultures were prepared as defined above from ear punches
CA 02282722 1999-09-O1
WO 98/39416 PCT/US98I04345
77
extracted from an adult bovine animal. However, the cell cultures could be
established from any type of differentiated cell. lndividual cells isolated
from these
cultures were utilized as nuclear donors in a nuclear transfer process,
labeled as step 2
in Figure 3. Although one nuclear transfer cycle was utilized to obtain
embryos
(labeled as step 3 in Figure 3), multiple nuclear transfer cycles could be
applied to
obtain these embryos. Also optional is ( 1 ) the addition of a stimulus before
or after
nuclear transfer, and (2) an activation step before or after nuclear transfer.
The embryo of step 3 in Figure 3 was implanted into a recipient bovine female
as described herein and a fetus (step 7) was isolated from that female. Cells
isolated
1 C from embryos of step 3 may be utilized to establish embryonic stem cell
cultures (step
4). In addition. the embryos of step 3 may be implanted into a female host and
allowed to develop into a cloned animal (step 5).
The steps labelled 8, 9, 10, 11, and 12 in Frficnr 3 were performed to
establish
totipotent and immortalized cells. The fetus of step 7 was manipulated in
three
I S manners. The manipulation in step 8 involved the isolation of genital
ridge cells,
specifically primordial germ cells, from the fetus of step 7. In step 9, the
primordial
germ cells were placed in co-culture with feeder cells. The feeder cells were
either
established from mouse fibroblast cells or from the rest of the fetus from
which the
primordial germ cells were extracted. Example 1 defines a method for
establishing
20 feeder cells. The head region and body cavity contents were removed from
the fetus
before the fetus was digested into a consistency suitable for establishing
feeder cells.
However, the fetus may be digested before the head region and contents of the
body
caviy arc removed. in addition. feeder cells may be established from a fetus
other
than the fetus from which the primordial germ cells are isolated.
25 In step 10, a cell culture was established with a digested fetus from which
the
primordial germ cells, head region, and body cavity contents were removed.
Step 11
illustrates that cell cultures may be established utilizing fetuses from which
no cell
types have been removed.
In step 12, cell cultures were either ( 1 ) subjected to a mechanical
stimulus, or
CA 02282722 1999-09-O1
WO 98/39416 PCT/US98/04345
78
(2) not subjected to a mechanical stimulus. When applied, the mechanical
stimulus
was effected by supplementing the culture medium with a receptor ligand
cocktail
comprising 100 ng/ml human recombinant leukemia inhibitory factor (hrLIF; R&D
System, Cat # 250-L) and 100 ng/ml bovine basic fibroblast growth factor
(bFGF;
R&D System, Cat # 133-FB). After step 12, cells were isolated from the cell
cultures
and utilized as nuclear donors in nuclear transfer processes, which are
defined
previously. Although one nuclear transfer cycle was utilized for step 13, more
than
one nuclear transfer cycle could be utilized.
Embryos developed after the nuclear transfer process of step 13. The embryos
of step 14 may be implanted into a bovine recipient female and develop into a
cloned
bovine animal.
Cells isolated from any of the developing cell masses of steps 1, 3, 4, S, 7,
8,
9, 10. 1 1. 1'?. 13, and 16 in Figure 3 may be transfected with a DNA
construct to form
transgenic cells suitable for cloning transgenic animals. One embodiment for
cloning
transgenic animals is defined in the next example.
EhAMPLE 8: Cloning Transgenic Animals
Transeenic cells suitable for creating a cloned transgenic animal can be
prepared from cells isolated from an adult animal. Figure 4 illustrates
processes that
can be utilized to create such tn3nsgenic cells. Although transgenic cells can
be
created from nearly any cell type by using the teachings of the invention.
FrRure a
illustrates procedures for establishing transgenic embryonic stem cells and
transgenic
immortalized and totipotcnt cells.
Fibroblast cell cultures can be established from ear punches extracted from a
bovine animal as defined previously. Individual cells can be isolated from
this cell
culture and utilized as nuclear donors in a nuclear transfer process. A single
nuclear
transfer cycle or multiple nuclear transfer cycles can be applied. Other
optional steps
are defined in the previous example.
CA 02282722 1999-09-O1
WO 98/39416 PCT/US98104345
79
Pre-blastocyst stage embryos and/or blastocyst stage embryos developed from
the nuclear transfer process can be utilized to establish embryonic stem
cells.
Materials and methods for preparing embryonic stem cells are described by
Stice et
al., 1996, Biology ofReproduction 54: 100-110, hereby incorporated by
reference
herein in its entirety, including all figures, tables, and drawings.
Immortalized and
totipotent cells can be established according to the procedures defined in
previous
examples.
Cells can then be transfected with a DNA construct. Cells can be transfected
at multiple steps. as indicated in Figure -1. Materials and methods for
preparing
trans~enic cells are defined in publications referenced previously.
Immortalized and
totipotent cells of the invention were successfully transfected with a DNA
construct
comprising (a) a neomycin gene, which encodes a product that renders cells
resistant
to a compound designated 6418: (b) a gene encoding the enzyme a-elucosidase:
and
(c) a casein promoter element. The transfected cells were selected for
transgenic
modification by selecting for transgenic cells in cell culture conditions
harboring
0418. The transgemc cells are then screened for transgenic modification by
utilizing
one or more screening techniques. Examples of these techniques are: ( I )
polymerasc
chain reaction, (2) Southern blotting, and (3) FISH-filter procedures. These
techniques are well known to a person of ordinary skill in the art. The latter
twe>
techniques are utilized to determine the number of copies of an inserted gene
sequence
in embryonic stem cell nuclear DNA.
These screening procedures can be applied to transfxted cells at am~ of the
steps indicated in Frgure .1. Cloned ttsrtsgenic animals may be created from
transgenic fetuses.
While the invention has been described and exemplified in sufficient detail
for
those skilled in this art to make and use it, various alternatives,
modifications, and
improvements should be apparent without departing from the spirit and scope of
the
invention.
CA 02282722 1999-09-O1
WO 98/39416 PCTIUS98/04345
One skilled in the art readily appreciates that the present invention is well
adapted to carry out the objects and obtain the ends and advantages mentioned,
as well
as those inherent therein. The cell lines, embryos. animals, and processes and
methods for producing them are representative of preferred embodiments, are
S exemplary, and are not intended as limitations on the scope of the
invention.
Modifications therein and other uses will occur to those skilled in the art.
These
modifications are encompassed within the spirit of the invention and are
defined by
the scope of the claims.
It will be readily apparent to a person skilled in the art that varying
Z O substitutions and modifications may be made to the invention disclosed
herein without
departing from the scope and spirit of the invention.
all patents and publicatior.~ n~.cationed in the specification arc indicative
of
the levels of those of ordinary skill in the art to which the invention
pertains. All
patents and publications are herein incorporated by reference to the same
extent as if
15 each individual publication was specifically and individually indicated to
be
incorporated by reference.
The invention illustratively described herein suitably may be practiced in the
absence of any element or elements, limitation or limitations which is not
specifically
disclosed herein. Thus, for example, in each instance herein any of the terms
20 "comprising", "consisting essentially of and "consisting of may be replaced
with
either of the other two terms. The terms and expressions wfiich have been
employed
are used as terms of description and not of limitation, and there is no
intention that in
the use of such terms and expressions of excluding any equivalents of the
features
shown and described or portions thereof, but it is recognized that various
25 modifications are possible within the scope of the invention claimed. Thus,
it should
be understood that aithough the present invention has been specifically
disclosed by
preferred embodiments and optional features, modification and variation of the
concepts herein disclosed may be resorted to by those skilled in the art, and
that such
modifications and variations are considered to be within the scope of this
invention as
CA 02282722 1999-09-O1
WO 98139416 PCT/IJS98/04345
81
defined by the appended claims.
In addition, where features or aspects of the invention are described in terms
of
Markush groups, those skilled in the art will recognize that the invention is
also
thereby described in terms of any individual member or subgroup of members of
the
Markush group. For example, if X is described as selected from the group
consisting
of bromine, chlorine, and iodine, claims for X being bromine and claims for X
being
bromine and chlorine are fully described.
Other embodiments are set forth within the following claims.