Note: Descriptions are shown in the official language in which they were submitted.
CA 02283472 1999-09-09
WO 98/40093 PCT/US98/04699
METHOD OF ANTAGONIZING THE HUMAN SRC SH2 DOMAIN
BACKGROUND OF THE INVENTION
Mammalian bone is constantly undergoing bone remodeling, which is a
dynamic process of bone resorption and bone formation. These processes are
mediated by specialized cell types: bone formation is the result of the
deposition of
mineralized bone by osteoblast cells, and bone resorption is the result of the
dissolution of bone matrix by osteoclast cells. Many bone diseases are brought
about by an imbalance of bone formation relative to bone resorption. For
instance,
diseases such as osteoporosis and Paget's disease are characterized by a net
loss of
bone matrix. Thus, agents which inhibit bone resorption are useful for the
treatment
of such diseases.
An activated osteoclast resorbs bone by attaching to the bone matrix, and
secreting proteolytic enzymes, organic acids and protons into the sealed
compartment formed between its cell membrane and the bone matrix. The acidic
environment and proteolytic enzymes effect the dissolution of bone in the
sealed
compartment to crest pits, or lacuna, in the bone surface, which are apparent
when
the osteoclast detaches from the bone.
A number of polypeptide growth factors and hormones mediate their cellular
effects through a signal transduction pathway. Transduction of signals from
the cell
surface receptors for these ligands to intracellular effectors frequently
involves
phosphorylation or dephosphorylation of specific protein substrates by
regulatory
protein tyrosine kinases (PTK) and phosphatases. Tyrosine phosphorylation may
be
the primary, or possibly even the sole, indicator of signal transduction in
multicellular organisms. Receptor-bound and intracellular PTKs regulate cell
proliferation, cell differentiation and signaling processes in immune system
cells.
Aberrant protein tyrosine kinase activity has been implicated or is suspected
in a number of pathologies such as diabetes, atherosclerosis, psoriases,
septic shock,
bone loss, anemia, many cancers and other proliferative diseases. Accordingly,
tyrosine kinases and the signal transduction pathways which they are part of
are
potential targets for drug design. For a review, see Levitzki et al. in
Science 267,
1782-1788 ( 1995).
CA 02283472 1999-09-09
WO 98/40093 PCTNS98/04699
Many of the proteins comprising signal transduction pathways are present at
low levels and often have opposing activities. The properties of these
signaling
molecules allow the cell to control transduction by means of the subcellular
location
and juxtaposition of effectors as well as by balancing activation with
repression
such that a small change in one pathway can achieve a switching effect.
The formation of transducing complexes by juxtaposition of the signaling
molecules through protein-protein interactions are mediated by specific
docking
domain sequence motifs. Src homology 2 (SH2) domains, which are conserved
non-catalytic sequences of approximately 100 amino acids found in a variety of
signaling molecules such as non-receptor PTKs and kinase target effector
molecules
and in oncogenic proteins, play a critical role. The SH2 domains are highly
specific
for short phosphotyrosine-containing peptide sequences found in
autophosphorylated PTK receptors or intracellular tyrosine kinases.
Approximately 60 proteins having distinct catalytic or other functional
domains yet sharing conserved SH2 domains, conserved sequences of
approximately 100 amino acids, have been identified. It is not known precisely
which physiological responses in the body are controlled by each of these SH2
domains. Further, the SH2 domain-ligand/compound interactions are highly
specific such that minor modifications in the structure of the ligandlcompound
will
significantly alter the selectivity with which the ligand/compound binds to
the
various SH2 domains.
The consequences of non selective antagonism of SH2 domains can be quite
severe. For example, although the src SH2 domain, the lck SH2 domain and the
fyn
SH2 domain are structurally similar, possessing a high degree of conservation
between the domains, antagonism of the src SH2 domain is indicated herein as
effecting bone resorption while antagonism of the lck SH2 domain or the fyn
SH2
domain induces immunosuppression. The induction of immunosuppression would
be undesirable in long term therapy for bone resorption disease.
It would be desirable to provide methods and compounds which allow the
treatment of a bone resorption disease by antagonizing the src SH2 domain.
2
CA 02283472 1999-09-09
WO 98/40093 PCT/U598/04699
Disclosed herein is an improved method of antagonizing the human src SH2
domain.
SUMMARY OF THE INVENTION
The present invention provides a method of treating a bone resorption
~ 5 disease in a subject which comprises administering to the subject a
therapeutically
effective amount of a compound which forms a covalent bond or link to cys 185
of
the src SH2 domain.
The present invention also provides a method of treating osteoporosis in a
subject which comprises administering to the subject a therapeutically
effective
amount of a compound which forms a covalent bond or link to cys 185 of the src
SH2 domain.
The present invention also provides a method of impairing the function of
osteoclasts in a subject which comprises administering to the subject an
osteoclast
function-inhibiting amount of a compound which forms a covalent bond or link
to
cys 185 of the src SH2 domain.
The present invention also provides compounds and pharmaceutical
compositions of these compounds which are useful in antagonizing the human SRC
SH2 domain.
DETAILED DESCRIPTION OF THE INVENTION
As used herein, the term "a bone resorption disease" means any disorder
characterized by abnormal hone loss due to osteoclastic activity, preferably
osteoporosis.
As used herein, the term "treating" and derivatives thereof means
prophylactic or therapeutic therapy.
As used herein, the term "compound" means a peptide or chemical
compound.
As used herein, unless other wise defined, the term "peptidomimetic" is as
defined in J. Med. Chem. 1993, 36, 3039-3049.
3
CA 02283472 1999-09-09
WO 98/40093 PCTIUS98/04699
As used herein, unless other wise defined, the term " src SH2 domain
antagonists" means a compound which is capable of forming a covalent bond or
link
to cys 185 of the src SH2 domain.
As used herein the term "cys 185 of the src SH2 domain" refers to the
Cysteine at the 185 position of the src gene following conventional numbering
as
described in Nature 1997, 385, 595-602. All of the src gene numbering
references
used herein follow conventional numbering as described in Nature 1997, 385,
595-
b02.
The present invention provides a method of treating a bone resorption
disease in a subject which comprises administering to the subject a
therapeutically
effective amount of a compound which forms a covalent bond or link to cys 185
of
the src SH2 domain.
The invention also provides a method of treating osteoporosis in a subject
which comprises administering to the subject a therapeutically effective
amount of a
compound which forms a covalent bond or link to cys 185 of the src SH2 domain.
The invention also provides a method of impairing the function of
osteoclasts in a subject which comprises administering to the subject an
osteoclast
function-inhibiting amount of a compound which forms a covalent bond or link
to
cys 185 of the src SH2 domain.
The nonreceptor tyrosine kinase src is essential for resorption of bone by
osteoclasts. The src homology-2 (SH2} domain of src controls its association
with
other signaling molecules through binding to short peptide motifs containing
phosphotyrosine. Inhibition of these interactions blocks src-mediated signal
transduction by preventing recruitment of src into receptor-effector
complexes. In
the human src SH2 domain, cysteine 185 (cys 185) is located in the
phosphotyrosine
binding pocket, close to histidine 201, arg 155, arg 175 and 1ys203. Compounds
which form a covalent bond or Iink to cys 185 block the phosphotyrosine
binding
pocket of human src SH2 thereby irreversibly inhibiting human src SH2.
4
CA 02283472 1999-09-09
WO 98/40093 PCT/US98/04699
In a preferred aspect of the invention, the compound which forms a covalent
bond or link to cys 185 will also form a hydrogen bond with arg 175 and have a
hydrophobic interaction with the sidechain portion of 1ys203.
The human src SH2 domain construct used in the present invention is
~ 5 described in Seq. ID No. 5. Seq. ID No. 5 uses a portion of src gene. As a
reference, cys 185 corresponds to cys67 in Seq. ID No. 5, his201 corresponds
to
his83 in Seq. ID No. 5, arg 155 corresponds to arg37 in Seq. ID No. 5, arg 175
corresponds to arg 57 in Seq. ID No. 5 and Iys203 corresponds to 1ys85 in Seq.
ID.
No. 5.
Presently preferred compounds of this invention which form a covalent bond
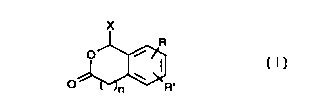
or link to cys185 of the src SH2 domain have the following Formula (I):
X
R
O
O ~ R~ (I)
X = OR", SR", NR" R"';
R" = H, methyl, alkyl;
R"' = CONH2, CONHMe, CO NHalkyl, SONH2, SONHMe, SONHalkyl,
S02NH2, SO~NHMe, SOZNHalkyl;
n = 0,1,2;
R = H, CH2CH(NHCOR"")CONHR""', organic moiety;
R"" = glu-glu-ileu-glu-NH2, peptide, peptidomimetic, alkyl, substituted alkyl,
aryl,
substituted aryl, heteroaryl, substituted heteroaryl;
R' =H, peptidomimetic;
or
- 25 R,R' = fused ring system substituted with H or peptidomimetic.
or a pharmaceutically acceptable salt, hydrate of solvate thereof.
. Compounds of Formula I are included in the pharmaceutical compounds of
the invention and used in the methods of the invention.
5
CA 02283472 1999-09-09
WO 98/40093 PCT/US98/04699
Scheme 1
CHO
HO
I ~ TFA, HO
AcOH, I ~ HS(CH2)3SH
., / _. _. ,
TiCl4
AcNH~C02Et ~N N CH CI
N AcNH C02Et z 2
~N
n
S S ~ Et3N,THF,
S S CO, S S
Pd(OAc)2, 2,4,6-trichloro-
HO ~ Tf2NPh, Tf0 dppt, HO C benzoyl chloride,
I Et3N, ~ _ _- ~ Z ~ t-BuOH, DMAP
__..-, I ~ KOAc, I
CH2CI2 ~ DMSO
AcNH~CO2Et AcNH CO Et
z AcNH CO"Et
n n
SS SS
t-BuOzC ~ NaOH, t-Bu02C
I MeOH, I ~ ~Bu-Glu-~Bu-Glu-Ile-~Bu-Glu-Rink resin
- .. -~ / _..
H20 HBTU, NMM, NMP
AcNH COzEt AcNH CO H
z
AgCl04,
S S S S
NCS,
acetone,
t-Bu02C ~ 5% H20, H02C ~ H20
I - _ , I __
i i
TFA
AcNH CO-t-Bu-Glu-t-Bu-Glu-Ile-t-Bu-Glu-Rink Resin AcNH CO-Glu-Glu-Ile-Glu-NHz
OH
O
O
I
AcNH CO-Glu-Glu-Ile-Glu-NHz
Scheme 1 depicts formation of (S)-alpha-(acetylamino)-1,3-dihydro-3-
hydroxy-1-oxo-5-isobenzofuranpropanamido-glutamate-glutamate-isoleucine-
glutamate-amine (Compound 1). N-acetyl tyrosine ethyl ester was formylated
with
hexamethylene tetramine (methenamine) in TFA, AcOH (J. Ind. Chem. 1987, 2bB,
6
CA 02283472 1999-09-09
WO 98/40093 PCT/US98/04699
7071 ). Then the aldehyde was protected as its 1,3-dithiane (Tet. Lett. 1983,
24,
1289), then the phenol was triflated using N-phenyl
trifluromethanesulfonimide.
Palladium catalyzed hydroxy-carbonylation followed by esterfication using
2,4,6-
trichloro benzoyl chloride gave the protected diester. Selective hydrolysis of
the
ethyl ester with sodium hydroxide in MeOH gave the desired amino acid analog,
which was coupled to an immobilized peptide using standard coupling chemistry
(HBTU (2-1H-benzotriazole-1-yl)-1,1,3,3-tetramethyluronium
hexafluorophophate),
N-methyl morpholine, DMF). Cleavage from the resin with concurrent
deprotection
of the t-butyl ester protective groups followed by a final deprotection of the
aldehyde gave the target compound for testing in vitro.
Compounds of Formula I are prepared by methods analogous to the methods
described in Scheme 1.
The inhibitory activity of compounds at the different human SH2 domains
was determined in vitro using SH2 domains expressed as fusion proteins in E.
coli
as further described in detail in Example 2 below.
The data shown in accompanying Table 1 indicates that src SH2 domain
antagonists will have significant efficacy in the fetal rat long bone (FRLB)
assay.
This in vitro activity is recognized in the art as correlating with efficacy
in treating a
bone resorption disease in vivo. This in vitro activity is also recognized in
the art as
correlating with efficacy in impairing the function of osteoclasts in vivo.
The present invention therefore provides a method of treating a bone
resorption disease, which comprises administering a quantity of a src SH2
domain
antagonists defined as herein in a quantity effective to inhibit bone
resorption. The
drug may be administered to a patient afflicted with a bone resorption disease
or in
danger of contracting a bone resorption disease by any conventional route of
administration, including, but not limited to, intravenous, intramuscular,
oral,
subcutaneous, intradermal, and parenteral. The quantity effective to inhibit
bone
resorption is from about 0.001 mg per kg to about 10.0 mg per kg of subject
body
weight. The selected dose will be an efficacious, nontoxic quantity selected
from
7
CA 02283472 1999-09-09
WO 98/40093 PCT/US98/04699
about 0.001 mg per kg to about 10.0 mg per kg of subject body weight. The
selected dose will be administered from about 1-6 times daily.
The method of treating a bone resorption disease disclosed in the present
invention may also be carried out using a pharmaceutical composition
comprising
an src SH2 domain antagonists defined herein and a pharmaceutically acceptable
carrier. The composition may contain between 0.05 mg and 500 mg of a src SHZ
domain antagonist, and may be constituted into any form suitable for the mode
of
administration selected. Compositions suitable for oral administration include
solid
forms, such as pills, capsules, granules, tablets, and powders, and liquid
forms, such
as solutions, syrups, elixers, and suspensions. Forms useful for parenteral
administration include sterile solutions, emulsions, and suspensions.
The present invention further provides a method of impairing the function of
osteoclasts, which comprises administering a quantity of a src SH2 domain
antagonists defined as herein in a quantity effective to inhibit bone
resorption. The
drug may be administered to a patient afflicted with a bone resorption disease
or in
danger of contracting a bone resorption disease by any conventional route of
administration, including, but not limited to, intravenous, intramuscular,
oral,
subcutaneous, intradermal, and parenteral. The quantity effective to impair
osteoclasts function is from about 0.001 mg per kg to about 10.0 mg per kg of
subject body weight. The selected dose will be an efficacious, nontoxic
quantity
selected from about 0.001 mg per kg to about 10.0 mg per kg of subject body
weight. The selected dose will be administered from about 1-6 times daily.
The method of impairing the function of osteoclasts disclosed in the present
invention may also be carried out using a pharmaceutical composition
comprising
an src SH2 domain antagonists defined herein and a pharmaceutically acceptable
carrier. The composition may contain between 0.05 mg and 500 mg of a src SH2
domain antagonist, and may be constituted into any form suitable for the mode
of
administration selected. Compositions suitable for oral administration include
solid
forms, such as pills, capsules, granules, tablets, and powders, and liquid
forms, such
8
CA 02283472 1999-09-09
WO 98/40093 PCT/US98/04699
as solutions, syrups, elixers, and suspensions. Forms useful for parenteral
administration include sterile solutions, emulsions, and suspensions.
The drug may otherwise be prepared as a sterile solid composition which
may be dissolved or suspended at the time of administration using sterile
water,
saline, or other appropriate sterile injectable medium. Carriers are intended
to
include necessary and inert binders, suspending agents, lubricants,
flavorants,
sweeteners, preservatives, dyes and coatings.
Optimal dosages to be administered may be readily determined by those
skilled in the art, and will vary with the particular src SH2 domain
antagonist in use,
the strength of the preparation, the mode of administration, and the
advancement of
the disease condition. Additional factors depending on the particular patient
being
treated will result in a need to adjust dosages, including patient age,
weight, diet,
and time of administration.
The invention also provides for the use of a src SH2 domain antagonists in
the manufacture of a medicament for use in the treatment of a bone resorption
disease.
The invention also provides for the use of a src SH2 domain antagonists in
the manufacture of a medicament for use in the treating osteoporosis.
The invention also provides for the use of a src SH2 domain antagonists in
the manufacture of a medicament for use in inhibiting osteoclast function.
The invention also provides for a pharmaceutical composition for use in the
treatment of a bone resorption disease which comprises a src SH2 domain
antagonists.
The invention also provides for a pharmaceutical composition for use in the
treatment of osteoporosis which comprises a src SH2 domain antagonists.
The invention also provides for a pharmaceutical composition for use in
inhibiting osteoclast function which comprises a src SH2 domain antagonists.
No unacceptable toxicological effects are expected when the methods of the
invention are utilized in accordance with the present invention.
9
CA 02283472 1999-09-09
WO 98/40093 PCTIUS98/04699
Without further elaboration, it is believed that one skilled in the art can,
using the preceding description, utilize the present invention to its fullest
extent.
The following Examples are, therefore, to be construed as merely illustrative
and
not a limitation of the scope of the present invention in any way.
Experimental Details
Example 1
Preparation of (S)-alpha-(acetylamino)-1,3-dihydro-3-h d~roxy-1-oxo-5-
isobenzofuranpropanamido-glutamate-glutamate-isoleucine-glutamate-amine
~Com~ound 1 )
a) N-Acetyl-3-formyl-tyrosine ethyl ester
Hexamethylene tetraamine (Aldrich, 25 g, 178 mmol) was added to a
solution of N-acetyl tyrosine ethyl ester mono hydrate (Aldrich, 10 g, 37.1
mmol) in
TFA (30 ml) and AcOH (30 ml) and the reaction was heated to 80 degrees C for
4.5
h. The reaction was cooled to RT, then H20 (200 ml) was added and the reaction
mixture was extracted with EtOAc {3 x 200 ml). The combined organics were
dried
with magnesium sulfate, filtered, concentrated in vacuo, and chromatographed
(silica gel, 5% MeOH/ CH2C12) to yield the title compound as a white solid
(4.5 g,
46% yield): MS ES M+H+ = 280.
b) N-Acetyl-3-( 1,3-dithiane)-tyrosine ethyl ester
A solution of TiCl4 (Aldrich, 1.0 M, 1.4 ml, 1.4 mmol) was added dropwise
to a solution of 1,3-propanedithiol ( 148 mg, 1.4 mmol) and N-Acetyl-3-formyl-
tyrosine ethyl ester (400 mg, 1.4 mmol) in CH2C12 (7.0 ml) at 0 degrees C. The
reaction was stirred for 2 h, then saturated aqueous NaHCO~ ( 10 ml) was
added, and
the reaction mixture was extracted with EtOAc (3 x 20 ml). The combined
organics
were dried with magnesium sulfate, filtered, concentrated in vacuo, and
CA 02283472 1999-09-09
WO 98!40093 PCT/US98/04b99
chromatographed (silica gel, 5% MeOH/ CH2C12) to yield the title compound as a
white solid (420 mg, 81 % yield): MS ES M+H+ = 370, M+Na+ = 392.
c) N-Acetyl-3-(I,3-dithiane)-4-triflyl-phenylalanine ethyl ester
N-phenyl trifluromethanesulfonimide (Aldrich, 1.0 g, 2.8 mmol) was added
to a solution of N-acetyl-3-( 1,3-dithiane)-tyrosine ethyl ester ( 1.0 g, 2.7
mmol) and
triethyl amine (0.41 ml, 3.0 mmol) in CHZCI~ (9.0 ml) at RT, and the reaction
was
stirred overnight. The reaction was diluted with H20 (20 ml), then the
reaction
mixture was extracted with EtOAc (3 x 20 ml). The combined organics were dried
with magnesium sulfate, filtered, concentrated in vacuo, and chromatographed
(silica gel, 5% MeOH/ CH~C12) to yield the title compound as a beige solid ( I
.14 g,
84% yield): MS ES M+H+ = 502, M+HCO~- = 546.
d) N-Acetyl-3-(1,3-dithiane}-4-carboxy-phenylalanine ethyl ester
Palladium (II) acetate (11 mg, 0.215 mmol) was added to a mixture of N-
acetyl-3-( 1,3-dithiane)-4-triflyl-phenylalanine ethyl ester (540 mg, 1.07
mmol),
1,1'=bis-(diphenylphosphino)ferrocene (Aldrich, 119 mg, 0.215 mmol), potassium
acetate (409 mg, 4.31 mmol) in DMSO {10.0 ml). The reaction mixture was heated
to 80 degrees C, then carbon monoxide was bubbled through the solution for 10
minutes. Then, the reaction was stirred overnight under a balloon of carbon
monoxide. The reaction was cooled to RT, diluted with HBO {30 ml), extracted
with
EtOAc (4 x 30 ml). Then, the combined organics were dried with magnesium
sulfate, filtered, concentrated in vacuo, and chrornatographed {silica gel, 5%
MeOH/
CH2Cl~) to yield the title compound as a white solid (320 mg, 76% yield): MS
ES
M+H+ = 398, M-H- = 396.
e) N-Acetyl-3-(1,3-dithiane)-4-(t-butyl-carboxylate)-phenylalanine ethyl ester
2,4,6-Trichlorobenzoyl chloride (Aldrich, 0.165 ml, 1.1 mmol) was added to
a solution of N-acetyl-3-( 1,3-dithiane)-4-carboxy-phenylalanine ethyl ester
(420 mg,
1.1 mmol}, triethyl amine, 0.295 ml, 2. I mmol) in THF (5.3 ml) and the
reaction
11
CA 02283472 1999-09-09
WO 98/40093 PCTIUS98/04699
was stirred for 0.25 h. Then, t-butanol (0.2 ml, 2.1 mmol) was added followed
by 4-
dimethyl amino pyridine (DMAP) (258 mg, 2.11 mmol) and the reaction was
stirred
overnight. The reaction mixture was loaded onto a chromatography column
(silica
gel, 5% MeOH/ CH~Cl2) to yield the title compound as a white solid (250 mg,
55%
yield): MS ES M+H+ = 454.
f) N-Acetyl-3-(1,3-dithiane)-4-(t-butyl-carboxylate)-phenylalanine
Aqueous sodium hydroxide (Aldrich, 1.0 N, 0.55 ml, 0.55 mmol) was added
to a solution of N-acetyl-3-(1,3-dithiane)-4-(t-butyl-carboxylate}-
phenylalanine
ethyl ester (250 mg, 0.55 mmol) in MeOH (1.5 ml) and the reaction was stirred
overnight. The reaction mixture was then diluted with AcOH (1.0 ml) and HBO
(10
ml) and the reaction mixture was extracted with EtOAc (4 x 30 ml). Then, the
combined organics were dried with magnesium sulfate, filtered, concentrated in
vacuo, and chromatographed {silica gel, 5% MeOH/ CH2C12) to yield the title
compound as a white solid ( 120 mg, 51 % yield): MS ES M+H+ = 426, M+NH4+ _
443, M-H- = 424.
g) y-t-butyl-glutamate- y-t-butyl-glutamate-isoleucine- y-t-butyl-glutamate-
Rink
resin
The title peptide was prepared by standard solid-phase chemistry on a
Symphony Multiple Peptide Synthesizer (Rainin) using standard FMOC protected
amino acids (2 x 1.5 h, 6 equivalents using HBTU (2-1H-benzotriazole-1-yl)-
1,1,3,3-tetramethyluronium hexafluorophophate)/ N-methyl morpholine in DMF
coupling conditions) and 20% piperidine/ DMF deprotection conditions ( 10 min)
starting with Rink Amide resin (Nova, 0.3 mmol/ g, PSl 1 % DVB, 100-200 mesh,
H. Rink Ter. Lett. 1987, 28, 3787).
12
. _ . _... .__ .:_
CA 02283472 1999-09-09
WO 98/40093 PCT/US98/04699
h) N-Acetyl-3-( 1,3-dithiane)-4-(t-butyl-carboxylate)-phenylalanine-y t-butyl-
glutamate- y-t-butyl-glutamate-isoleucine- y t-butyl-glutamate-Rink resin
HBTU {2-IH-benzotriazole-I-yl)-1,1,3,3-tetramethyluronium
hexafluorophophate) (61 mg, 0.16 mmol) was added to a slurry of N-Acetyl-3-
(1,3-
dithiane)-4-(t-butyl-carboxylate)-phenylalanine (68 mg, 0.16 mmol), y t-butyl-
glutamate- 'y-t-butyl-glutamate-isoleucine- 'y t-butyl-glutamate-Rink resin
(200 mg,
0.08 mmol), N-methyl morpholine (0.023 ml, 0.24 mmol) in DMF (5.0 ml) and was
shaken at RT for 48 h. The reaction mixture was filtered, washed with DMF (300
ml), then CH2C12 (300 mi), and was dried under vacuum overnight. The resin was
tested Kaiser ninhydrin negative consistent with quantitative coupling to give
the
title compound.
i) N-Acetyl-3-(1,3-dithiane)-4-(t-butyl-carboxylate)-phenylalanine-glutamate-
glutamate-isoleucine-glutamate-amine
N-Acetyl-3-( 1,3-dithiane)-4-(t-butyl-carboxylate)-phenylalanine-y-t-butyl-
glutamate-'y-t-butyl-glutamate-isoleucine- Y t-butyl-glutamate-Rink resin (100
mg,
0.04 mmol) was added to a solution of 95% TFA/ H20 and was shaken 4 h at RT.
The reaction mixture was filter;:d, diluted with 100 ml cold ether and the
precipitate
was collected, dissolved in AcOH (Sml), frozen to -78 degrees C, and
lyophilized to
give the title compound as a white, fluffy solid: MS ES M+H+ = 869, M-H- =
867.
j) (S)-alpha-(acetylamino)-1,3-dihydro-3-hydroxy-1-oxo-5-
isobenzofuranpropanamido-glutamate-glutamate-isoleucine-glutamate-amine
N-Acetyl-3-( I ,3-dithiane)-4-(t-butyl-carboxylate)-phenylalanine-glutamate-
glutamate-isoleucine-glutamate-amine {Smg, 0.006 mmol) was dissolved in 90 %
acetone/ H20 (0.3 ml), then N-chlorosuccinimide (5 mg, 0.037 mmol) and silver
perchlorate (10 mg, 0.04$ mmol) were added and the reaction was stirred 10
minutes at RT. The reaction mixture was chromatographed (C~g reverse phase
silica, MeCN, HZO), and the UV active fractions were combined, concentrated in
vacuo, and dissolved in MeOH (0.2 ml). Cold ether was added and the
precipitate
13
CA 02283472 1999-09-09
WO 98/40093 PCTIUS98/04699
was collected, washed with ether, dissolved in AcOH, frozen to -78 degrees C,
and
lyophilized to produce a white, fluffy solid (3 mg, 64% yield): MS ES M+H+ = M
+ Na+ = 801, M-H- = 777.
Example 2-Protocol for the Determination of the Potency of src SH2 Domain
Anta og nists
The inhibitory activity of compounds at the human src SH2 domain was
determined in vitro using the human src SH2 domain expressed as fusion
proteins in
E. coli.
The fusion protein containing the human SH2 domain was expressed as the
general sequence: DET1-DET2-spacer-ek-SH2, where DET1, DET2, spacer, ek and
SH2 are as described below. DETI ("defined epitope tag 1 ") (SEQ ID NO: 1) is
an
11 amino acid sequence found in the Human Immunodeficiency Virus Type 1 (HIV-
1 ) envelope protein gp 120 (or gp 160). Monoclonal antibodies to various
epitopes of
HIV-1 gp120 (or gp160) are known in the art, see, for example U.S. Patent
5,166,050. One preferred example is monoclonal antibody 178.1 (see, e.g.,
Thiriart
et al., J. Immunol., 143:1832-1836 (1989)), which was prepared by immunization
of
mice with a yeast-expressed HIV-1 gp160 molecule from strain BH10 (Ratner et
al.,
Nature, 313:277-284 ( 1985)). This tag was used for detection of expression
(by
Western blot), for purification of the protein (by affinity chromatography),
and for
configuring assays in which the fusion protein was captured or immobilized
using
the 178.1 antibody. DET2 is a hexa-histidine sequence tag (SEQ ID NO: 2) which
binds to nickel-containing resins and was used for purification purposes.
Spacer
(SEQ ID NO: 3) was utilized to design a BamHl restriction site at the
indicated
position of the construct. The term -ek- refers to a recognition sequence (SEQ
ID
NO: 4) for the enterokinase protease which provides for the optional removal
of the
tags from the SH2 domain, thus producing an SH2 domain that contains no
extraneous amino acids. SH2 domains which contain no extraneous amino acids
are
preferable to tagged protein for crystallography studies. SH2 refers to the
human
14
CA 02283472 1999-09-09
WO 98/40093 PCT/US98104699
src SH2 domain or, as described below, a construct used in the preparation of
the
human src SH2 domain.
The DNA sequence encoding each DET1-DET2-spacer-ek-SH2 was
designed such that the indicated restriction sites (BamH1 and Xbal) flank the
spacer-ek-SH2 region, thereby allowing different spacer-ek-SH2 contructs to be
readily substituted into any one of the vectors described in Procedures 2 or 3
below
to create a DET I -DET2-spacer-ek-SH2 tagged protein. The DNA sequence
encoding each DET1-DET2-spacer-ek-SH2 constructs was also designed such that
the entire tagged SH2 domain can be moved as an NdeI-XbaI fragment into any
expression vector containing an NdeI site at an appropriate distance
downstream of
E. coli transcription and translation regulatory sequences and a downstream
cloning
site compatible with XbaI. Although any suitable vector would yield similar
results(e.g., pET-1 la; Novagen, Inc.), the vector used in the instant
experiments was
E. coli expression vector pEA 1 KnRBS3. This vector is a derivative of the
series of
vectors described in Shatzman, A, Gross, M, and Rosenberg, M, 1990,
"Expression
using vectors with phage lambda regulatory sequences", In: Current Protocols
in
Molecular Biology (F.A. Ausubel et al , eds.), pp. 16.3.1-16.3.1 l, Greene
Publishing and Wiley-Interscience, N.Y. (hereinafter F.A. Ausubel et al.). The
specific vector pEAIKnRBS3 is described in Bergsma et al, 1991, J. Biol. Chem.
266:23204-23214.
The procedures below describe the expression of chicken src and human src
SH2 domains. First, the chicken src SH2 domain was expressed as DETI-DET2-
spacer-SH2. Then, the other was inserted into this vector in place of chicken
src to
express protein in the form DETI-DET2-spacer-ek-spacer-SH2.
Procedure 1: Cloning and Expression of chicken src SH2 domain containing tags
DET1 and DET2 (DET1-DET2-spacer-SH2).
A DNA sequence encoding the tagged protein DETI-DET2-spacer-SH2 was
PCR amplified from a cDNA clone containing the chicken src gene (pSH; Levy et
al
CA 02283472 1999-09-09
WO 98/40093 PCT/US98/04699
1986. Proc. Natl. Acad. Sci. USA 83:4228) by methods well known to those
skilled
in the art by using the following primers:
5'
TTCCATATGAAAAGTATTCGTATTCAGCGTGGCCCGGGCCGTCACCACC
ACCACCACCACGGGATCCCCGCTGAAGAGTGGTACTTT 3' (SEQ ID NO:
17)
The underlined sites are an NdeI recognition site (5') and a BamHI
recognition site (3').
5' GGAATTCTAGATTACTAGGACGTGGGGCAGACGTT 3' (SEQ ID NO: i8)
The underlined region is an XbaI recognition site.
The PCR product was digested with NdeI and Xbal, followed by isolation of
the digested fragment on an agarose gel. The fragment was ligated into NdeI-
Xbal-
digested pEAlKnRBS3 vector (Bergsma et aI, supra) that had been agarose gel
purified as a 6.5 kbp fragment. The ligation reaction was used to transform E.
coli
MM294cI+ (F.A. Ausubel et al., supra). A plasmid containing an inserticn of
the
correct fragment was identified and confirmed by DNA sequencing. The resultant
plasmid encodes DETI-DET2-spacer-SH2 under the control of the phage lamda P
promoter and regulatory system. Plasmid DNA was purified from MM294cI' and
used to transform E. coli strain AR120. In this host strain, expression of the
phage
promoter can be induced by addition of nalidixic acid to the growing culture
as
described in F.A. Ausubel et al, supra. Nalidixic acid induction of AR120
containing this plasmid, followed by analysis of the cellular proteins on an
SDS-
polyacrylamide gel stained with Coomassie Blue (F.A. Ausubel et al., supra),
resulted in appearance of a protein band with an apparent molecular weight of
15,000; this band was not seen in uninduced cells or in induced cells
containing
pEAIKnRBS3 lacking the PCR amplified fragment. Western blotting confirmed
16
CA 02283472 1999-09-09
WO 98/40093 PCTIUS98/04699
that the induced protein band reacted with the anti-DET1 monoclonal antibody
178.1.
Procedure 2: Cloning, expression and purification of human src SH2 domain
- 5 containing tags and an enterokinase proteolytic cleavage site (DET1-DET2-
spacer-
ek-src SH2).
A DNA sequence encoding protein ek-src SH2 was PCR amplified from a
cDNA clone containing the human src gene (c-src SH2 DNA sequence identical to
that described in Takeya,T. and Hanafusa, H, 1983 Cell 32:881-890) using the
following primers:
5' CGGGATCCTGGACGACGACGACAAAGCTGAGGAGTGGTATTTT 3'
(SEQ ID NO: 19)
The underlined site is a BamHI recognition site.
5' GGAATTCTAGACTATTAGGACGTGGGGCACACGGT 3' (SEQ ID NO: 20)
The underlined region is an XbaI recognition site.
The PCR product was digested with BamHI and XbaI, followed by isolation
of the digested fragment on an agarose gel. The fragment was ligated into
BamHI-
XbaI-digested expression vector containing the tagged chicken src gene DETI-
DET2-spacer-SH2 described in Procedure 1 above. In that vector, the BamHI site
is located between the coding regions for DET2 and SH2, and the XbaI site is
located after the 3' end of the SH2 coding region. The ligation reaction was
used to
transform E. coli MM294cI'. The construct DETI-DET2-spacer-ek-src SH2 was
confirmed by DNA sequencing (SEQ ID NO: 5) and induced in E. coli strain
AR120 as described in Procedure 1 above. A Coomassie-Blue-stained, Western-
17
CA 02283472 1999-09-09
WO 98140093 PCT/US98104699
blot-positive induced protein band with an apparent molecular weight of 16,000
was
observed after nalidixic acid induction.
Cells were lysed at neutral pH by sonication in the presance of lysozyme.
After centrifugation, the soluble extract was chromatographed on a Ni"NTA
column. After washing the column with equilibration buffer (Tris buffer pH 8
containing 0.5 M NaCI) and the same buffer containing I S mM imidazole, the
protein was eluted in highly purified form with 25 mM imidazole in
equilibration
buffer. The SH2 domain, purified in this fashion, was found to bind with high
affinity in a specific, saturable fashion to the appropriate pY peptide in the
"Binding
Assays" described below, demonstrating that the tag did not interfere with
function.
This expressed fusion protein, DET1-DET2-spacer-ek-src SH2, was utilized in
the
"Binding Assays" described below in order to determine the specificity of
compounds to selectively inhibit the human src SH2 domain.
Binding Assays: The potency of compounds at the human SH2 domain was
determined based on the ability of such compound to selectively inhibit the
SH2
domain from binding to its respective specific pY peptide.
The binding assay for the human SH2 domain and pY peptide was
performed in an ELISA-based 96 well plate assay. In Millipore 96 well filter
plates,
hydrophilic Durapore~ (pore size 0.65um Cat. No. MADVN6550), was added 2 ul
(50% suspension) of Protein-G Sepharose (available from Pharmacia of N.J. Cat.
No. 17-0618-O l ) and 2 ul of 2 mg/ml of MAB 178.1. 10 pmol of the subject SH2
domain fusion protein was added to one or more wells. The volume was brought
to
100 ul with TBS-T (tris buffered saline plus 0.05% tween-20), incubated and
shaken
at room trmperature for 1 hr. then washed lx with TBS-T (4°C). 90 ul of
TBS-T
was then added to each well. The specific pY biotinylated peptide was diluted
to a
concentration of 1.0 uM in TBS-T (this peptide can be obtained from Bachem
Bioscience of Pennsylvania, Genosys Biotechnologies of Texas and California
Peptide Research of California). 10 ul was aliquoted per well to yield a final
concentration of 0.1 uM (approx. the K~ for the SH2 domainlpeptide pair) and a
final volume of 100 ul. The assay plates were incubated until equilibrium
binding
18
r
CA 02283472 1999-09-09
WO 98/40093 PCT/US98/04699
was attained (3 hr at 4°C with shaking). The assay plates were washed 2
X per well
TBS-T (4°C), then 100 ul of SABC (Strepavidin biotinylated
horseradish
peroxidase complex, available from the Zymed corporation of California cat.
no. 93-
0043), 1 drop reagent A (streptavidin) and 1 drop of reagent B (AH-biotin
- 5 conjugated-horseradish peroxidase) per 10 ml of TBS-T, incubated at
37°C for 30
minutes, then cooled to 4°C) was added per well, then incubated at
4°C for 30-60
minutes. The plates were then washed 4 X with TBS-T (4°C) (250
ul/well)/wash).
100 ul of 1 mg/ml OPD (o-phenyldiamine, Sigma Chemical Corporation, St. Louis
Missouri) in Citrate Buffer was added per well. To stop development, 100 ul of
10% sulfuric acid was added per well. 150 ul from each well was then removed
from the assay plate and placed in an ELISA plate. The Aa"~ of each ELISA
plate
was then determined.
Determination of (ICS") for Table I
Each control or compound was assayed in duplicate. The duplicates were
averaged and the background subtracted and the maximal values with no
inhibition
were taken from the plate, then all other data points were expressed as a
percent of
the maximal value (or as % control). These % control data values were graphed
in
Kaleidagraph for Macintosh (Synergy Software}. The curves on these graphs were
nonlinear curve fitted with the following equation F(x)=Emax/(
1+(kd/conc)~slope),
wherein the k~ term represents the ICgfl for each of the curves.
Determination of (Ki) for Table II
The Ki for respective compounds is calculated via the following equation
(see reference). This expanded equation must be used under the conditions of
this
assay, due to the fact that the pY biotinylated peptide is not in vast excess
concentration ( 100X) over the SH2 domain fusion protein. The IC5« is an
extrapolated value from a nonlinear curve fit using Kaleidagraph. Rtot and *D
are
known values for reagents input into the assay. KD generally must be
experimentally determined for each combination of SH2 domain fusion protein
and
pY biotinylated peptide.
19
CA 02283472 1999-09-09
WO 98/40093 PCT/US98104699
~=(IC5«-
Rtot+Rtot/2((*D/(KD+*D))+(KD/(KD+*D+Rtot/2}))/( 1+*D/KD+Rtot/KD((KD+*
D/2)/(KD+*D)))
KI=(uM)KDof competitor
ICs, (uM) IC5" for inhibitor, derived via nonlinear curve fit of competition
selectivity assay data for the SH2 domain
Rtot=(uM)total SH2 domain concentration within 1 assay (microtitre plate) well
*D=(uM)concentration of specific pY and biotinylated peptide for the SH2
domain
KD=(uM)KD value for the specific pY and biotinylated peptide for the SH2
domain
ICS" is the concentration of inhibitor at which the response or signal is
inhibited by
50%
KD is the dissociation constant for a ligand in a receptor/ligand interaction,
normally equaling the concentration of ligand which is at 1/2 Vmax on a
saturation
binding curve>
The pY peptide ligand used in the above Binding Assays is as follows.
Biotinylated pY peptide ligand containing an aminocaproic acid (Aca) linker
used
for the human src SH2 domain.
Glu-Pro-Gln-pTyr-Glu-Glu-Ile-Pro-Ile-Tyr-Leu (SEQ ID NO: 13)
Results of Binding Assays:
Tables I and II illustrate the activity of SH2 antagonists at the human src
SH2
domain.
,,
CA 02283472 1999-09-09
WO 98/40093 PCT/fJS98/04699
Table I
ACTIVITY OF Src SH2 DOMAIN ANTAGONISTS AT CLONED HUMAN Src
- SH2 DOMAIN (IC-..)
Compound Src
1 0.25 uM
NI-No inhibition observed out to 300uM
X-not tested
Table II
ACTIVITY OF Src SH2 DOMAIN ANTAGONISTS AT CLONED HUMAN
Src SH2 DOMAIN {Ki)
Compound Src
I XX
NI-No inhibition observed below 1000 uM
X-not calculated
XX-not tested
Examt~le 3 Activity of src SH2 Domain Anta onists
The compounds of this invention which are antagonist of the human src SH2
domain are tested for their potency to inhibit osteoclast mediated bone
resorption in
the fetal rat long bone {FRLB) assay as described in Raisz LG (1965) J Clin
Invest
44: 103-116, Stern PH et al., ( 1979) Skeletal Research: Art experimental
Approach.
New York:Academic Press, 21-59 and Votta BJ et al., (1994) Bone 15:533-538).
To perform the experiment timed-pregnant Sprague Dawley rats (Taconic
Farms, Germantown, NY) are injected subcutaneously with 200 ~Ci of 45CaC12 on
day 18 of gestation, housed overnight, then anesthetized with Innovar-Vet
(Pittman
Moore, Mundelein, IL) and sacrificed by cervical dislocation. Fetuses are
removed
21
CA 02283472 1999-09-09
WO 98/48093 PCT/US98/04699
aseptically and radii and ulnae were dissected free of surrounding soft tissue
and
cartilaginous ends. The bones are cultured 18-24 hours in BGJb medium (Sigma)
containing 1 mg/ml bovine serum albumin, then transferred to fresh medium and
cultured for an additional 48 hours in the absence or presence of a compound
which
is an antagonist of the human src SH2 domain. 45Calcium released into the
medium
and total calcium in the bones are measured by liquid scintillation
spectrometry.
Data is expressed as the % 45calcium released from treated bones as compared
to
corresponding control bones. Statistical differences are assessed by employing
a
one-way analysis of variance for non-paired samples. Data are presented as
mean +
s.e.m., n=4. The experiment is generally repeated two times.
Data from the above experiment demonstrates the therapeutic effect of src
SH2 domain antagonists in treating a bone resorption disease.
While the preferred embodiments of the invention are illustrated by the
above, it is to be understood that the invention is not limited to the precise
instructions herein disclosed and that the right to all modifications coming
within
the scope of the following claims is reserved.
22
.y.........
CA 02283472 1999-09-09
WO 98/40093 PCT/US98/04699
SEQUENCE LISTING
(1) GENERAL INFORMATION
(i) APPLICANT: YAMASHITA, DENNIS
VEBER, DANIEL
HOLT, DENNIS
IO (ii) TITLE OF THE INVENTION: METHOD OF ANTAGONIZING THE
HUMAN SRC SH2 DOMAIN
(iii} NUMBER OF SEQUENCES: 10
IS (iv) CORRESPONDENCE ADDRESS:
(A) ADDRESSEE: SmithKline Beecham Corporation
(B) STREET: 709 Swedeland Road
(C) CITY: King of Prussia
(D) STATE: PA
2O (E) COUNTRY: USA
(F) ZIP: 19406
(v) COMPUTER READABLE FORM:
(A) MEDIUM TYPE: Diskette
25 (B} COMPUTER: IBM Compatible
(C) OPERATING SYSTEM: DOS
(D) SOFTWARE: FastSEQ Version 2.5
(vi) CURRENT APPLICATION DATA:
3O (A) APPLICATION NUMBER: Unknown
(B) FILING DATE: Concurrently
(C) CLASSIFICATION:
(vii) PRIOR APPLICATION DATA:
3S (A) APPLICATION NUMBER: 60/040,65-8
(B) FILING DATE: March 10, 1997
23
CA 02283472 1999-09-09
WO 98/40093 PCTlUS98/04699
(A) APPLICATION NUMBER:
(B) FILING DATE:
(A) APPLICATION NUMBER:
(B) FILING DATE:
(viii) ATTORNEY/AGENT INFORMATION:
(A} NAME: Dustman, Wayne J
IO (B) REGISTRATION NUMBER: 33,870
(C) REFERENCE/DOCKET NUMBER: P50630
(ix) TELECOMMUNICATION INFORMATION:
(A) TELEPHONE: 610-270-5023
IS (B) TELEFAX:
(C) TELEX:
(2) INFORMATION FOR SEQ ID N0:1:
2O (i) SEQUENCE
CHARACTERISTICS:
(A) LENGTH: 11 amino
acids
(B) Z'YPE: amino acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
25
(ii) MOLECULE TYPE: peptide
(iii) HYPOTHETICAL: NO
(iv) ANTISENSE: NO
(v) FRAGMENT
TYPE:
internal
3O (vi) ORIGINAL SOURCE:
(ix) FEATURE:
(xi) SEQUENCE DESCRIPTION: SEQ ID N0:1:
35 Lys Ser Ile Arg Ile Gln Arg Gly Pro Gly Arg
1 5 10
24
~......_~ ._......
r
CA 02283472 1999-09-09
WO 98/40093 PCT/US98/04699
(2) INFORMATION FOR SEQ ID N0:2:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 6 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: peptide
(iii) HYPOTHETICAL: NO
(iv) ANTISENSE: NO
(v) FRAGMENT TYPE: internal
(vi) ORIGINAL SOURCE:
IS
(xi) SEQUENCE DESCRIPTION: SEQ ID N0:2:
His His His His His His
1 5
(2) INFORMATION FOR SEQ ID N0:3:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 3 amino acids
2$ (B) TYPE: amino acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: peptide
3O (iii) HYPOTHETICAL: NO
(iv) ANTISENSE: NO
(v) FRAGMENT TYPE: internal
(vi) ORIGINAL SOURCE:
25
CA 02283472 1999-09-09
WO 98/40093 PCT/US98104699
(xi) SEQUENCE DESCRIPTION: SEQ ID N0:3:
Gly Ile Leu
1
(2) INFORMATION FOR SEQ ID N0:4:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 5 amino acids
(B} TYPE: amino acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: peptide
IS (iii) HYPOTHETICAL: NO
(iv) ANTISENSE: NO
(v) FRAGMENT TYPE: internal
(vi) ORIGINAL SOURCE:
2Q (xi) SEQUENCE DESCRIPTION: SEQ ID N0:4:
Asp Asp Asp Asp Lys
1 5
2S (2} INFORMATION FOR SEQ ID N0:5:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 130 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: peptide
(iii) HYPOTHETICAL: NO
3S (iv) ANTISENSE: NO
(v) FRAGMENT TYPE: internal
26
~ , , ~....
CA 02283472 1999-09-09
WO 98/40093 PCTlUS98/04699
(vi) ORIGINAL SOURCE:
(xi) SEQUENCE DESCRIPTION: SEQ ID N0:5:
Met LysSerIle ArgIle GlnArg GlyProGly ArgHisHis HisHis
S 1 5 10 15
His HisGlyIle LeuAsp AspAsp AspLysAla GluGluTrp TyrPhe
20 25 30
Gly LysIleThr ArgArg GluSer GluArgLeu LeuLeuAsn AlaGlu
35 40 45
Asn ProArgGly ThrPhe LeuVal ArgGluSer GluThrThr LysGly
50 55 60
Ala TyrCysLeu SerVal SerAsp PheAspAsn AlaLysGly LeuAsn
65 70 75 80
Val LysHisTyr LysIle ArgLys LeuAspSer GlyGlyPhe TyrIle
85 90 95
Thr SerArgThr GlnPhe AsnSer LeuGlnGln LeuValAla TyrTyr
100 105 110
Ser LysHisAla AspGly LeuCys HisArgLeu ThrThrVal CysPro
115 120 125
Thr Ser
130
(2) INFORMATION FOR SEQ ID N0:6:
2S (i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 19 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: peptide
(iii) HYPOTHETICAL: NO
(iv) ANTISENSE: NO
(v) FRAGMENT TYPE: internal
3S (vi) ORIGINAL SOURCE:
(ix) FEATURE:
27
CA 02283472 1999-09-09
WO 98/40093 PCT/US98104699
(A) NAME/KEY: Other
(B) LOCATION: 4...4
(D) OTHER INFORMATION: phosphorylated tyrosine residue
S
(xi) SEQUENCE DESCRIPTION: 5EQ ID N0:6:
Glu Pro Gln Tyr Glu Glu Ile Pro Ile Tyr Leu
10
(2) INFORMATION FOR SEQ ID N0:7:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 87 base pairs
1S (B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: cDNA
2Q (iii) HYPOTHETICAL: NO
{iv) ANTISENSE: NO
(v) FRAGMENT TYPE:
(vi) ORIGINAL SOURCE:
2S (xi) SEQUENCE DESCRIPTION: SEQ ID N0:7:
TTCCATATGA AAAGTATTCG TATTCAGCGT GGCCCGGGCC GTCACCACCA CCACCACCAC 60
GGGATCCCCG CTGAAGAGTG GTACTTT 87
3O (2) INFORMATION FOR SEQ ID N0:8:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 38 base pairs
{B) TYPE: nucleic acid
35 (C) STRANDEDNESS: single
(D) TOPOLOGY: linear
28
CA 02283472 1999-09-09
WO 98/40093 PCT/US98/04699
(ii) MOLECULE TYPE: cDNA
(iii) HYPOTHETICAL: NO
(iv) ANTISENSE: NO
S (v) FRAGMENT TYPE:
(vi) ORIGINAL SOURCE:
(xi) SEQUENCE DESCRIPTION: SEQ ID N0:8:
IO GGAATTCTAG ATTACTAGGA CGTGGGGCAG ACGTT
38
(2) INFORMATION FOR SEQ ID N0:9:
IS (i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 46 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(iij MOLECULE TYPE: cDNA
(iii) HYPOTHETICAL: NO
(iv) ANTISENSE: NO
(vj FRAGMENT TYPE:
2S (vi) ORIGINAL SOURCE:
(xi) SEQUENCE DESCRIPTION: SEQ ID N0:9:
CGGGATCCTG GACGACGACG ACAAAGCTGA GGAGTGGTAT TTT
46
(2) INFORMATION FOR SEQ ID NO:10:
(i) SEQUENCE CHARACTERISTICS:
3S (A} LENGTH: 38 base pairs
(B) TYPE: nucleic acid
29
CA 02283472 1999-09-09
WO 98140093 PCT/US98/04699
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: cDNA
(iii) HYPOTHETICAL: NO
(iv) ANTISENSE: NO
(v) FRAGMENT TYPE:
(vi) ORIGINAL SOURCE:
IO (xi) SEQUENCE DESCRIPTION: SEQ ID N0:10:
GGAATTCTAG ACTATTAGGA CGTGGGGCAC ACGGT
38