Note: Descriptions are shown in the official language in which they were submitted.
WO 98/40881 PCT/US98/04832
CORROSION PROTECTION OF ALUMINUM AND ALUMINUM ALLOYS
USING EMERALDINE BASE POLYANILINE
This application claims the benefit of U.S. Provisional Application Serial No.
60/036,685 filed on March 11, 1997, hereby incorporated herein by reference.
The present invention relates to the protection of aluminum and its alloys
from
corrosion.
Aluminum and its alloys are widely used in a variety of applications,
including aerospace and automotive construction, building construction, and
public
1 S utilities.
Many of these applications involve the use of aluminum and its alloys in
exposure to the environment or otherwise in corrosive atmospheres. Aluminum
typically undergoes relatively rapid oxidation which, over time, can detract
from its
functional and/or structural viability, and its appearance.
Accordingly, to preserve the functional and/or structural viability, as well
as
the appearance in some cases, of aluminum and its alloys, it is desirable to
reduce or
eliminate the corrosion of aluminum
CA 02283926 1999-09-10
SUBSTtTUTE SHEET (RULE 26)
WO 98140881 PCT/US98104832
Although the advantages and goals of the present invention are described with
reference to aluminum and its alloys, the present invention is not limited to
either
general or specific uses. Indeed, the potential uses of the present invention
are
numerous as may become apparent to one of ordinary skill in the fields of
endeavor to
which the present invention might be applied.
Accordingly, additional advantages or the solution to other problems may
become apparent to one of ordinary skill in these arts from the present
disclosure or
through practice of the present invention.
Summary of the Invention
Aluminum and aluminum alloys normally prone to corrosion when subjected
to potentially corrosive conditions (acid, alkaline or neutral) can be
protected by coats
of emeraldine base polyaniline (EB) (see Figure 1 ), and derivatives thereof.
The EB
containing film protects the aluminum surface it is coated on, but may also
protect the
uncoated surface areas of the metal object, including the opposite side of the
coated
surface, the sides and edges.
In broadest terms, the present invention includes a coating composition for
protecting aluminum and alloys thereof from corrosion. The composition
comprises
at least one substance selected from the group consisting of emeraldine base
polyanilines and oligomers thereof. Such oligomers may be trimers, tetramers,
octamers, hexadecamers, and/or mixtures thereof. The fraction of imine units
in the
emeraldine base may range from about .3 to about .75 units. The ring
structures of
emeraidine base or oligomers thereof may also be provided with additional ring
2
CA 02283926 1999-09-10
SUBSTITUTE SHEET (RULE 26)
WO 98/40881 PCT/US98/04832
substituents for purposes such as providing polymer cross linking, improved or
in
some cases even reduced solubility and/or better bonding with the carrier
resin. Such
substituents may be, for example, carbonate groups, alkyl groups, epoxy
groups, and
combinations thereof.
Nominally, it will be preferred that the coating composition also include a
resin which is used as a carrier for the active ingredient. Examples of such
resin
include acrylic and epoxy resins. Resins and blends containing one or more of
the
polymers/oligomers mentioned herein may also offer corrosion protection of
aluminum and aluminum alloys. In most applications, a top coat sealing the
corrosion
protecting polymers/oligomers from the environment is likely to be used,
enhancing
the durability of the corrosion protecting coat.
Adhesion-improving surface pretreatment prior to the coating with the
corrosion protecting polymer/oligomer also is likely to be utilized in order
to improve
the effectiveness of the protective coat.
The present invention also includes a coated metal piece which may be of
aluminum or any alloy thereof which has at least one surface adapted to
receive a
coating. The coated metal piece also has on at least one such surface a
coating
comprising at least one substance selected from the group consisting of
emeraldine
base polyaniline and oligomers thereof. The coating may also be provided with
a
coating sealant such as a lacquer or epoxy sealant.
The present invention also includes, in broadest terms, a coating composition
for protecting aluminum and alloys thereof from corrosion, the composition
comprising at least one substance selected from the group consisting of
sulfonated
CA 02283926 1999-o9-to gUBSTiTUTE SHEET (RULE 26)
WO 98/40881 PCT/US98/04832
polyaniline and salts and oligomers thereof. Such oligomers may include
trimers,
tetramers, octamers, hexadecamers, and/or mixtures thereof.
The sulfonated polyaniline coatings may also be born by resin such as acrylic
and epoxy resins.
Typically the sulfonated poiyaniline coatings will have a degree of
sulfonation
in the range of from about ~0% to about 100% (i.e. expressed in terms of the
percentage of ring structures bearing a sulfonate group). The ring structures
of the
sulfonated polyaniline may also be provided with additional ring substituents
for
purposes such as providing polymer cross linking and/or better sulfonation or
bonding with the carrier resin. Such substituents may be, for example,
carbonate
groups, alkyl groups, epoxy groups, and combinations thereof.
The present invention also includes a coated metal piece comprising a metal
selected from the group consisting of aluminum and alloys thereof, said metal
piece
having at least one surface adapted to receive a coating; and a coating on
said surface
which comprises at least one substance selected from the group consisting of
sulfonated polyaniline and oligomers thereof. Such oligomers may be trimers,
tetramers, octamers, hexadecamers, and/or mixtures thereof.
The coated metal piece may also have a coating sealant atop the coating, and
such sealants may be, for instance, lacquers and epoxy sealants.
4
CA 02283926 1999-09-10
SUBSTITUTE SHEET (RULE 26)
T ,.
WO 98/40881 PCT/US98/04832
Brief Description of the rawin~~
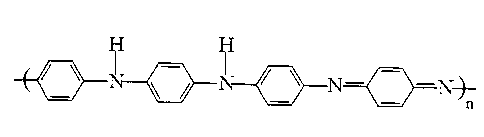
Figure 1 shows the schematic chemical structure of emeraldine base
polyaniline (EB).
Figure 2 shows the schematic chemical structure of sulfonated ( 100%)
S polyaniline (SPAN) in the emeraldine base form.
Figure 3 shows the XPS AI 2p core level depth profile of a polished A13003
sample that has not been exposed to a corrosive environment.
Figure 4 shows the XPS Al 2p core level spectra of (a) an A13003 sample
immersed in the 0.1 M HCl acid bath for 2 hours, (b) an EB/A13003 sample
immersed
in the 0. I M HCI acid bath for 2 hours (the metal side being depth profiled),
and (c) an
A13003 control sample not exposed to a corrosive environment. Data were
acquired
after argon ion sputtering in ultra-high vacuum to a depth of about 15 nm.
Figure 5 shows the XPS A12p core level spectra of (a) AI3003 exposed to
HCl and (b) the uncoated metal side of A13003/EB exposed to HCl where the EB
film
failed during the acid exposure. Data were acquired after argon ion sputtering
in
ultra-high vacuum to a depth of about 28 nm.
Figure 6 shows a graph of a potentiodynamic scan showing the results of a
potentiodynamic study on an uncoated aluminum 2024-T3 sample and an emeraIdine
base-coated aluminum 2024-T3 sample, in accordance with one embodiment of the
present invention. The surface was polished with 600 grit emery paper and
degreased
with ethanol before application of any coating. Reproducibility is evidenced
by scans
of multiple samples.
5
CA 02283926 1999-o9-io SUBSTITUTE SHEET (RULE 26)
WO 98/40881 PCT/U598/04832
Figure 7 shows a graph of a potentiodynamic scan showing the results of a
potentiodynamic study on an uncoated aluminum 2024-T3 sample and an emeraldine
base trimer-coated aluminum 2024-T3 sample, in accordance with one embodiment
of
the present invention. Reproducibility is evidenced by scans of multiple
samples.
Figure 8 shows a graph of a potentiodynamic scan showing the results of a
potentiodynamic study on a scribed emeraldine base-coated aluminum 2024-T3
sample in accordance with one embodiment of the present invention, as compared
to
an epoxy-coated aluminum 2024-T3 sample. The surface was polished with 600
grit
emery paper and degreased with ethanol before application of any coating.
Reproducibility is evidenced by scans of multiple samples.
Figure 9 shows a graph of a potentiodynamic scan showing the results of a
potentiodynamic study on a scribed emeraldine base trimer-coated aluminum 2024-
T3
sample in accordance with one embodiment of the present invention, as compared
to
an epoxy-coated aluminum 2024-T3 sample. The surface was polished with 600
grit
emery paper and degreased with ethanol before application of any coating.
Reproducibility is evidenced by scans of multiple samples.
Detailed Descri~ion of the Preferred Embodiments
In accordance with the foregoing summary of the invention, the following
presents a detailed description of the one embodiment of the invention which
is also
presently considered to be the best mode of the invention.
6
CA 02283926 1999-09-10
SUBSTITUTE SHEET (RULE 26)
.. _.. . . ~, . ~
WO 98/40881 PCT/US98/04832
EB films were coated onto A13003 alloys and were found to reduce the
corrosion damage on the uncoated backside as well as the coated side of the Al
samples when immersed in an 0.1 M HCl bath at 80 C for 2 hours, as compared to
corresponding uncoated A13003 control samples. Likewise, samples of pure
aluminum vapor deposited onto EB coated glass slides were also found to be
more
resistive to corrosion when exposed to hot acid vapor (0.1 M HCl) than the
control
samples consisting of Al vapor deposited onto glass.
Sample Prepara ion
Samples consisting of A13003 (alloy) coupons were polished on both sides
using emery paper and subsequently ultrasound washed in acetone and propanol.
Some of the coupons were then coated by emeraldine base polyaniline (EB) in an
N-
methylpyrrolidinone solution through drop-coating and dried over night under a
hood.
EB films also were drop-coated from N-methylpyrrolidinone solution onto glass
slides and dried over night under a hood. Thin {~ 1000 ~) Al films were then
vapor
deposited onto the EB coated glass slides and non-coated control glass slides.
The samples to be corroded where immersed in an acid bath kept at
80° C for
two hours. The acid used was 0.1 M HCI. After the acid exposure, the samples
were
quickly blow dried with N2 gas before being inserted into the UHV chamber.
Experimental Procedure
?0 X-ray photoelectron spectroscopy (XPS) was carried using a VG Scientific
ESCALAB MkII system and Mg Ka X-rays (1253.6 eV). Depth profiling was carried
out using an argon ion sputtering gun. The spectroscopy was earned out at a
background pressure of 109 mbar. XPS depth profiling experiments were carried
out
7
CA 02283926 1999-09-10
SUBSTITUTE SHEET (RULE 26)
WO 98140881 PCT/US98/04832
on the metal side for EB/A13003 and Al/EB/glass samples as well as for A13003
and
Al/glass control samples exposed to the corrosive environments mentioned in
the
sample preparation section. Depth profiling was carried out for polished AI
and
A13003 samples that had not been exposed to acid. In the case of the EB/A13003
samples, depth profiling was carried out both the A13003 side and the EB side.
In Figure 3 is depicted the Al 2p core level depth profile of a polished
AI3003
sample that has not been exposed to a corrosive environment. The peak situated
at
72.9 eV represents metallic Al and the peak at 76 eV results from A12O3. The
relative
amount of metallic A1 increases as the surface A1203 is sputtered away, with
the
metallic A1 being the dominating feature at depths of ~10 nm giving an
estimate of the
oxide layer's thickness. When aluminum and aluminum alloys are exposed to
corrosive environments, the protective A1203 oxide layer is damaged and Al3+
ions
from the underlying metal bulk are dissolved into the solution. Various new
chemical
species are formed at the surface layers, including chlorine containing
aluminum
hydroxides and aluminum oxides, with binding energies ranging from 76-79 eV.
This
new feature in the AI 2p core level spectrum will thus be a signature of
corrosion, and
the depth into the sample with which it extends will give an estimate of the
severity of
the corrosion damage.
In Figure 4 is shown the A1 2p core level spectra of (a) an A13003 sample
immersed in the 0.1 M HCl acid bath for 2 hours, {b) an EB/A13003 sample
immersed
in the 0.1 M HCI acid bath for 2 hours (the metal side being depth profiled),
and (c) an
CA 02283926 1999-09-10
SUBSTITUTE SHEET (RULE 26)
._
WO 98/40881 PCT/US98/04832
A13003 control sample not exposed to a corrosive environment. All spectra were
taken at a depth of ~15 nm. Whereas the intensities of the features at 75 eV
and
higher binding energies are practically the same for the control sample (c)
and the EB
protected one (b) with only a slight tail extending from 77 eV and out
signifying the
possible existence of corrosion products, the unprotected A13003 sample (a)
has a
much more intense peak in the non-metallic energy range giving evidence to a
corrosion attack. Qualitatively, the same results were obtained for the pure
Al
samples, where the features in the AI 2p spectrum signifying corrosion damage
extended much further down into the A1 films for the unprotected samples than
for the
EB protected ones.
A series of immersion tests were made and it was noted that the adhesion
between the polymer and oligomer films, EB included, and the aluminum alloy
substrates was quite poor leading to variations in the effectiveness of
corrosion
protection depending on the rate of ,film failure. In a practical application,
this
problem can be circumvented by crosslinking the polymers/oligomers, applying
them
in blends, pretreating the aluminum alloy surface to increase the adhesion or
applying
top coats typically used in the paint industry to protect the primer layer. An
example
of such a case where parts of the EB film failed during the acid exposure is
shown in
Figure 5. Here Al 2p spectra of (a) unprotected A13003 and (b) A13003/EB
samples
were taken at a depth of ~28 nm. Unlike the case depicted in Figure 4, the
peak
signifying corrosion damage (76-78 eV) is of higher intensity than the
metallic peak
(~73 eV), indicating that the corrosion protection was weaker in this case.
The EB
protected sample, however, still shows less corrosion damage as is evident
from the
9
CA 02283926 1999-o9-to SUBSTITUTE SHEET (RULE 26)
WO 98/40881 PCT/US98/04832
larger relative metallic/corrosion damage peak intensity as compared to the
unprotected sample. The unprotected sample has a distinct shoulder at ~78 eV,
significantly broadening the non-metallic peak giving further proof of
corrosion
damage since the number of different A1 containing oxides and hydroxides has
increased compared to the A13003/EB sample. It should be mentioned that in all
cases studied, there was always some corrosion damage visible. This is not
surprising, since, although not limited by theory, it is believed that the
corrosion
protection is of anodic origin (although based on our EB/steei studies we
expect that
there are other factors as well that contribute to the corrosion protection
when using
this class of polymers/oligomers) and anodic protection only reduces the
corrosion
current, generally not bringing it to zero.
For the electrochemical experiments, EB and trimer films were drop cast from
N-methylpyrrolidinone solutions on to 1.5 x 1.5 x 0.19 cm3 A12024-T3 coupons
that
had been polished with 600 grit emery paper and degreased with ethanol. The
thickness of the EB films were ~20 micrometers, and ~40 micrometers for the
trimer
films. The samples were stored for up to 24 hours in air prior to the
electrochemical
experiments. The EB-coated, trimer-coated and non-coated A12024-T3 samples
were
placed in a holder at the bottom of a vessel with the coated side exposed to a
deaerated
(argon) 0.1 M NaCI solution. The (coated) A12024-T3 coupons functioned as the
working electrode in a three electrode set up. A platinum foil was used as the
counter
electrode and a saturated calomel electrode (SCE) was used as the reference
electrode.
The potentiostat used for the potentiodynamic experiments was a Gamry
Instruments
CA 02283926 1999-09-10
SUBSTITUTE SHEET (RULE 26)
._.~_ ~.. r ,
WO 98/40881 PCT/US98/04832
model PC3 potentiostat/galvanostat equipped with the CMS 100 corrosion system
software. The scanning speed for the experiments was 5 mV/s.
The potentiodynamic experiments carried out in a deaerated O.1M NaCI
solution gave open circuit potentials of ~ -0.9 V vs SCE for the non-coated
A12024-
T3 coupons and ~ -0.5 V vs SCE for the EB-top-coated A12024-T3 samples, Fig.
6.
Trimer top coated A12024-T3 coupons had open circuit potentials of ~ -0.75 V
vs
SCE, see Fig. 7. The aluminum alloy is ennobled in the presence of an EB or a
trimer
top coat, i.e., some form of anodic protection is taking place. The pitting
potential
was ~-0.6 V for the uncoated A12024-T3 coupons. In contrast, no obvious
pitting
was found to occur for the EB-coated or trimer coated samples, even at
potentials as
high as 0.2 V.
Furthermore, the corrosion current was more than an order of magnitude lower
for the EB-coated coupons than the non-coated ones, clearly demonstrating the
corrosion protecting capability of EB coats in salt environments. The
corrosion
current was roughly five times less for the trimer coated coupons compared to
the
non-coated ones, also demonstrating an anti-corrosion effect.
Identical potentiodynamic studies were carried out for A12024-T3 samples
coated with EB, trimer and epoxy (Buehler Limited, epoxide resin #20-8130-032
and
epoxide hardener #20-8132-008) where a 0.2 cm2 scratch had been scribed into
the
films exposing the bare A12024-T3 metal to the electrolyte. Potentiodynamic
experiments carried out in a deaerated 0.1 M NaCI solution gave evidence that
the EB
and trimer films have throwing power, i.e., the ability to protect a metal
against
corrosion even for areas where the metal is exposed (cracks in the coating,
etc). In
CA 02283926 1999-o9-to SUBSTITUTE SHEET (RULE 26)
WO 98140881 PCT/US98/04832
Fig. 8 are shown the potentiodynamic scans for scribed EB and epoxy, and the
corrosion current was reduced by more than a factor of ten for the EB coated
samples
as compared to the epoxy coated coupons. The same anti-corrosion effect was
found
for the scribed trimer coatings, as depicted in Fig. 9, where the corrosion
current for
the trimer samples was reduced by an order of magnitude compared to the epoxy-
coated coupons.
There is evidence that EB and derivatives thereof spunlcast as filmslcoats
reduce the corrosion damage of aluminum and aluminum alloys when exposed to,
or
resident in, corrosive environments.
Sulfonated (50% - 100%) polyanilines (SPAN), depicted in Figure 2,
oligomers of EB and SPAN (trimers, tetramers, octamers, hexdecamers, etc.), as
well
as ring-modified derivatives of EB, SPAN and their oligomers are expected to
offer
similar corrosion protection given their electronic and chemical similarities
to EB.
The potentiodynamic studies also indicated that the coatings in accordance
with the present invention are capable of achieving throwing power levels such
that
the coatings are capable of providing an anti-corrosive effect even when there
are
exposed gaps in the coating.
In view of the foregoing disclosure, it will be within the ability of one
skilled in
the art to make alterations and variations to the present invention, such as
through the
substitution of equivalent materials and processing steps, without departing
from the
spirit of the invention as reflected in the following claims, the substance of
which is
included herein.
12
CA 02283926 1999-09-10
SUBSTITUTE SHEET (RULE 26)
n~