Note: Descriptions are shown in the official language in which they were submitted.
CA 02284195 1999-09-17
Apparatus and Method for Capillary Electrophoresis
The invention relates to an apparatus for capillary
electrophoresis with a large number of separation
capillaries and an optical detection system as well as a
method for using an apparatus of this kind.
Electrophoretic separation of substances and mixed
substances is an analytic procedure that is widespread
especially in biochemistry and molecular biology. The
substances to be separated are separated in a separation
medium subjected to an electric field and separately
detected. In capillary electrophoresis the separation medium
is in a capillary (typical inner diameter of < 150 um). The
separation procedure is performed in the capillary;
detection can be performed both inside and at the end of the
capillary. This is a special advantage in terms of speed,
resolving power and minimizing the amount of sample. For the
analysis of complex biochemical reactions or molecular
biological processes (eg for the analysis of complex genomes
or proteins) it is necessary to use an extremely large
number of different samples (eg 105 to 10') .
Consequently there is interest in apparatus for multiple
capillary electrophoresis with high sample throughput and
high-grade parallel analyses. For this purpose the
multichannel or multiplex arrangements, named as examples in
what follows, are known, which, although they allow highly
parallel processing, are usually so complicated in their
structure that routine use is restricted. US-A-5 498 324,
for instance, describes a multiplex fluorescence detector
CA 02284195 1999-09-17
system for capillary electrophoresis in which the
capillaries are connected to optical fibers through which
the excitation light is conducted separately to the
capillaries. Fluorescence detection is performed by a
microscope with a CCD camera. This structure is complicated
and subject to interference because of the coupling of
optical fibers to the capillaries. The amount of light that
can be coupled in is limited, so the sensitivity of
fluorescence detection is also restricted. The system is
unsuitable for routine use in particular because of the
substantial maintenance effort required by the capillary
array (difficult changing of capillaries) with the optical
fibers.
US-A-5 582 705 discloses of a multiplex capillary
electrophoresis system in which a CCD detector is optically
connected to the capillaries in such a way that the inside
of a capillary is imaged on a pixel of the CCD detector. The
disadvantage of this system is that the detector arrangement
is complicated and highly specialized, calls for the use of
specially matched optical components and is thus less
compatible with existing laboratory systems for fluorescence
detection. Furthermore, there is increased risk of crosstalk
from one capillary to another in the event that the
concentration differences of the analytes are very large.
US-A-5 584 982 and US-A-5 567 294 describe multiple
capillary systems with a socalled sheath flow cuvette that,
although it achieves an increase in detection sensitivity,
is disadvantageous because of the complicated structure
lacking the ruggedness required for routine laboratory
operation. The use of replaceable separation media is
2
CA 02284195 1999-09-17
especially difficult with such a cuvette. The cuvette can be
soiled when replacing the medium and there is the risk of
the separation medium slowly flowing out during separation.
Finally, US-A-5 675 155 discloses of a raster or scanning
system in which the fluorescence signals of a coplanar
capillary group are detected by a scanner detector. With
this detector the excitation or measurement light is
consecutively directed at the individual separation
capillaries by a moving mirror. The disadvantage here is the
susceptibility to disturbance because of the use of moving
parts and the restricted reading speed. In capillary
electrophoresis it is possible that the samples to be
detected separately will be so fast that reliable detection
is not possible during one raster scan. The capillaries at
the edge of the capillary array, in particular, are not
scanned at even intervals. What is more, the scanning
systems are not rugged enough for routine use.
In multiple capillary electrophoresis there is not only
interest in stability and parallelism of processing but also
in automation of the entire analytical procedure, starting
with the loading of a front-end reservoir through the actual
separation operations to cleaning of the separation
capillaries. Because of the disadvantages mentioned,
automation of capillary separation arrangements has not been
achieved to date with multiple capillary systems but only
with single capillary systems.
The object of the invention is to provide an improved
apparatus for capillary electrophoresis that is
characterized by a simplified and stable structure and
3
CA 02284195 1999-09-17
allows automation of the parallel separation of a large
= number of samples. It is also the object of the invention to
provide methods for the use of such an apparatus.
These objects are solved by an electrophoresis apparatus
with the features of patent claim 1 and a method with the
features of patent claim 17 respectively. Advantageous
embodiments are defined in the dependend claims.
The invention is based on the idea of arranging a large
number of separation capillaries, each having a detection
range, so that the samples in all detection ranges are
exposed to simultaneous and uniform illumination or
excitation and a detector device simultaneously detects the
images of all detection ranges. For this purpose the
following measures are implemented, singly or together, on a
generic multiple capillary separation apparatus with a
front-end reservoir with a large number of samples, a
correspondingly large number of separation capillaries (each
with a detection range), attached to a common holding
device, a collector device and a measurement system with an
illuminating device and a detector device.
The holding device is a support for the separation
capillaries on which the separation capillaries are arranged
so that the detection ranges form a straight row. The
detection ranges are, for example, detection windows on each
of the separation capillaries, which are also provided with
protective or shielding layers. The holder can also offer
"optical isolation" between the capillaries to prevent
crosstalk. In addition, the holder is modular (eg six
holders for 16 capillaries each), ie it allows replacement
4
CA 02284195 1999-09-17
of smaller capillary arrays without having to dismantle the
w entire arrangement. The illuminating device preferably forms
a line-type, uniform illumination field whose shape is
matched to the row of detection ranges. A special advantage
of the invention is that the illumination or excitation of
the samples in the capillaries is direct from the outside by
illumination of the capillary wall in the region of the
particular detection range. No additional devices are
necessary for input coupling, and adjustment is implemented
by the fixed but detachable location on the holding device.
The detector device is based on detection of the light
emitted in the detection ranges through the capillary wall.
All detection ranges are simultaneously imaged on a detector
camera by a suitable imaging device. Depending on the
analysis requirements, the detector device comprises imaging
on a single detector row or on a large number of detector
rows, forming a two-dimensional matrix of detector elements.
In the latter case at least one dispersion element can be
provided in the detector device allowing, in addition to
simultaneous detection of the detection ranges, analysis of
the spectral properties of the light emitted from the
detection ranges.
The separation capillaries exit into a common collector
device that fulfills a dual function. Firstly, the collector
device contains the carrier medium for loading the
separation capillaries. Secondly, the separated substances
are jointly collected on the collector device. For this
purpose the collector device will preferably contain a means
of collection for the molecules of the samples to be
separated. This means of collection, or molecule trap, is a
CA 02284195 1999-09-17
semi-permeable wall element that separates the ends of the
separation capillaries from the high-voltage power supply
for generating molecular movement in the separation
capillaries.
During electrophoretic separation the molecules are drawn
through the porous wall element to the electrode and thus
collected in the molecule trap. Passive back-diffusion
through the wall element is hindered to a large extent
because the pores are very small. After completion of
analysis a pressure of up to 5 bar is applied to the
collector device (reservoir), but in the region outside the
molecule trap. This prevents ready analyzed molecules from
being pressed back into the capillaries and disturbing
subsequent separation.
An important feature of the method according to the
invention is that both illumination or excitation of the
samples to be separated in the detection ranges and
detection of the light from the samples through the
capillary wall is from outside into the capillary or vice
versa. Thin-walled capillaries of approx. 35 to 50 um are
preferably used to reduce background signals. But larger
designs - with thicker walled capillaries - are also
possible. Furthermore, other forms of the detection range
are possible, eg by coupling the capillaries into a cuvette
or into a microstructure with channels. The design of the
detector unit with lenses and objectives allows simple
alteration of the imaging scale (for optimum imaging of the
detection range on the detector elements) and thus greater
flexibility in terms of the form of the detection range. The
separation device according to the invention is best
6
CA 02284195 1999-09-17
operated with a low-viscosity separation medium. In this way
- the loading pressure to be applied to the collector device
(or outlet vessel) is reduced and the loading speed
increased. Simple replacement of the separation medium
allows adequate flushing of the capillaries (either with the
separation medium itself or beforehand with a cleansing
agent) and thus extends the service life of the capillaries.
The invention possesses the following advantages. The
separation device is compact and without moving parts.
Illumination and detection are compatible with available
laboratory setups and with currently used dye markers. This
means advantages on the one hand in routine operation by
personnel without highly specialized training and on the
other hand in maintenance. The invention allows, for the
first time, an entirely automated analysis, details of which
are explained below. Some 15000 different samples can be
analyzed, for example, before an operator has to intervene
for the first time. The system possesses high multiplex
capability. Both the sample feed (preferably with common
formats, eg from microtiter plates) and the illumination and
detection are simultaneous in all channels formed by a
separation capillary. Special detection structures like a
sheath flow cuvette are unnecessary. The holding device for
the separation capillaries is of rugged design, prevents
stray light between the capillaries and allows bundled
attachment of the capillaries to simplify maintenance. The
loading pressure of the carrier medium can be reduced from
approx. 70 bar for conventional carrier media (eg 2~
hydroxyethylcellulose, viscosity approx. 1000 centiStokes)
to approx. 5 bar if carrier media with viscosity of 100
7
CA 02284195 1999-09-17
centiStokes (eg 10-15% dextran or 4-8$
- polydimethylacrylamide) are used.
Further advantages and details of the invention are
described in what follows with reference to the attached
drawings, which show:
Fig. 1: a schematic overview of the setup of an
electrophoresis apparatus according to the
invention,
Fig. 2: a partial view of a holding device that is part of
a
separation device according to Fig. l,
Fig. 3: an overview to illustrate spectrally resolved
detection according to the invention,
Fig. 4: a further overview to illustrate spectrally
resolved
detection according to the invention,
Fig. 5: an illustration of the detection of detector
signals,
Fig. 6: a schematic side view of a collector device that
is
part of a separation device according to Fig. 1,
Fig. 7: a special capillary form used for electrophoretic
separation according to the invention, whereby the
8
CA 02284195 1999-09-17
capillary is metallically coated at the end and
- serves simultaneously as an electrode,
Fig. 8: a curve illustrating uniform illumination by the
line
Generator,
Fig. 9: a curve illustrating detector signals of three
adjacent separation capillaries,
Fig. 10: curves illustrating the dependence on
concentration
of the separation medium viscosity, and
Fig. 11: curves illustrating experimental results with a
separation device according to the invention.
The invention is described in what follows with reference to
a preferred embodiment in which samples in microtiter plates
are electrophoretically separated by detecting the migration
of probe components through separation capillaries with a
carrier medium influenced by high voltage. But the invention
is not restricted to alignment of the capillary entrances
with reference to a microtiter plate or certain carrier
media or a certain separation effect. Instead it can be
implemented in all electrophoresis capillary systems with a
large number of separation capillaries.
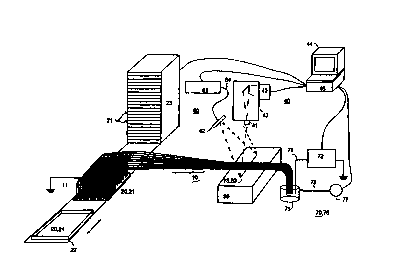
Fig. 1 shows an electrophoresis apparatus according to the
invention in which a large number of separation capillaries
are attached to a common holding device 50, the details
of which are explained below with reference to Fig. 2. The
9
CA 02284195 1999-09-17
separation capillaries 10 are aligned so that detection
- ranges, in the form of detection windows for example, form
a straight row 13. An illuminating device 60 with a light
source 61 and an imaging optical system 62 forms a line-type
illuminating field 63 that coincides with the row 13 of
detection windows of the capillaries 10. There is also a
detector device 40 containing an imaging device 41, a
detector camera 42 and a dispersion element 43. The
dispersion element 43 is an optional assembly that can be
dispensed with for certain applications, as explained below.
The detector camera 42 is connected to a control and data
storage device 44, for example in the form of a computer and
electronic controller 45.
The separation capillaries 10 lead from an inlet reservoir
20 (samples (21) or storage (24) reservoir) to a collector
device 70. The electrophoretic separation circuits are
formed by applying a high voltage between the inlet
reservoir 20 and the collector device 70 through electrodes
11 or 71. For this purpose the inlet reservoir 20 is
connected to ground potential, for example, and the
collector device 70 to a high-voltage power supply device
72. This high-voltage power supply device can produce DC
voltage or - for special purposes - a modulated voltage (eg
pulse or sinusoidal) and is controlled by the unit 44, 45.
The inlet reservoir 20 is a samples reservoir 21 (during
injection) or a storage reservoir 24 (during separation).
The samples reservoir 21 is preferably a flat substrate with
a large number of samples arranged in predetermined fashion,
which are to be subjected to electrophoretic separation in
parallel (or simultaneously). This substrate is preferably a
CA 02284195 1999-09-17
microtiter plate with a common format (eg 96-hole, 384-hole
- or 1536-hole plate). For complete automation of separation
starting with the feeding of samples, the samples reservoir
is arranged on a transport device 22 by means of which a
required samples reservoir 21 can be moved from a storage
device 23 to the operating position at the injection ends of
the separation capillaries by control signals from the unit
44, 45. For this purpose the transport device 22 and/or
storage device 23 are equipped with appropriate means of
actuation and positioners. The transport device 22 is also
intended, after loading the injection ends of the separation
capillaries, for replacing the samples reservoir 21 by a
storage reservoir 24 so that the circuit is again completed.
The injection ends 11 of the separation capillaries 10 are
aligned so that they match the positions of the samples to
be separated on the substrate or samples reservoir 21. The
separation capillaries 10 have an outer diameter of 100 to
400 um for example. Preferred forms are capillaries with an
outer diameter of 375 um and an inner diameter of 100 um, as
well as capillaries with an outer diameter of 200 um and an
inner diameter between 50 and 100 um, or capillaries with an
outer diameter of 150 um and an inner diameter of 75 um. The
capillary wall thickness may be of any other figure,
however, that allows reproducible detection with the
illuminating and detection devices described below. The
total length of the separation capillaries is approx. 40 to
50 cm in the shown implementation, although this length can
be modified as a function of application.
At the injection end the separation capillaries form what is
essentially a flat, two-dimensional "brush-like" fan, the
11
CA 02284195 1999-09-17
dimensions of which correspond more or less to those of the
- samples reservoir 21. Towards the holding device 50 the
separation capillaries are brought together in such a way
that they, at least for the length where the detection
ranges are arranged as a row 13, are closely adjacent (see
Fig. 2) .
In the region where the separation capillaries are brought
together before the holding device 50, it is possible to
provide a tempering device. This tempering device can be
designed to circulate a tempered medium (eg air) around the
separation capillaries and to regulate the heat. Preferably
a temperature between 10 and 60°C will be set.
The illuminating device 60 is intended to illuminate the row
of detection ranges of the separation capillaries on the
holding device 50 as uniformly as possible so that the
sample components, passing the detection ranges in the
separation process, are exposed to the same amount of light.
Seeing as the row 13 of detection ranges extends over the
approx. 5 cm width of the capillary group for example, a
laser light source 61 is preferably combined with an optical
system 62 forming a line-type illumination field (socalled
line generator) in order to produce sufficient illumination
or excitation intensity. The laser 61 is selected as a
function of the spectral properties of the fluorescence
marker that is used (eg Ar laser of approx. 50 to 200 mW)
and can also be driven through the control unit 44, 45.
The optical system 62 forms the line-type illumination
field. The optical system can take the form of a mirror
swinging or rotating at high speed for example, which may be
12
CA 02284195 1999-09-17
disadvantageous for the stability of the arrangement
- however. The use of a cylindrical lens is also possible,
preferably avoiding moving parts, but scaled so that,
despite the Gaussian distributed intensity of the
illumination field, sufficiently homogeneous illumination of
the capillary row is enabled. Consequently, in a preferred
implementation of the invention, the optical system 62 is
created by a socalled Powell lens (producer: OZ Optics,
Canada), allowing homogeneous illumination and optimum
utilization of laser power (see Fig. 8). The Powell lens can
also be connected to the laser 61 direct by an optical fiber
64, producing a rugged and transportable setup in which no
moving parts like rotating mirrors and the like are
contained, and ensuring that, apart from the line-type
illumination field, no coherent or highly focused light
escapes (user safety).
The optical fiber 64 also allows simple replacement of the
laser (eg for adaptation to other fluorescence markers) or -
combined with special couplers - conduction of the light
from two different lasers into one illumination field.
The light intensity of the illumination field of the Powell
lens is uniformly distributed in the direction of the line
and Gaussian distributed perpendicular to it (ie in a
direction parallel to the orientation of the separation
capillaries). The parameters of the Powell lens and the
configuration referred to the detection windows are selected
to focus into a line with a width of approx. 1 mm or less.
The narrower the illumination field, the higher is the
resolution achieved by the separation apparatus.
13
CA 02284195 1999-09-17
Details of the detection device 40 and the collector device
- 70 are explained below with reference to Fig. 3, 4 and 5.
Fig. 2 shows a section through a holding device 50 as a
schematic perspective to illustrate the planar, parallel
attachment of the separation capillaries on the top of the
holding device (Fig. 2 bottom) and a magnified perspective
with a capillary section as a phantom view (Fig. 2 top). The
holding device comprises at least the shown capillary holder
51, offering the separation capillaries 10 mechanical
support in the focus plane of the illuminating device and
ensuring optical isolation between the separation
capillaries. Several capillary holders of 16 capillaries
each, for example, can be provided, whereby the overall
holder 50 is then modular and single capillary holders can
be replaced as modules. Except for the detection ranges, the
separation capillaries are provided with protective layers
that may be impervious to light. In the detection ranges l0a
the separation capillaries are free of any coating. The
capillary holder 51 supports the separation capillaries at
least on a lengthwise section where the detection ranges are
located. In terms of the total length of the separation
capillaries, this is in the rear quarter or rear third of
the length of the separation capillaries looking from the
injection end. Thus the detection ranges or detection
windows of the separation capillaries, given a separation
capillary length of approx. 50 cm, form a straight row with
a perpendicular spacing of approx. 5 to 20 cm, preferably 10
cm, from the exit ends of the capillaries. Generally, to
improve resolving power, the detection windows should be as
far as possible downstream referred to the direction of
motion of the samples to be separated.
14
CA 02284195 1999-09-17
The capillary holder is a block with grooves 52 in which the
separation capillaries are inserted. The dividing walls 53
serve for optical isolation between the detection ranges of
the individual separation capillaries. The dividing walls
are formed of segments that are as thin as possible to allow
tight packing of the capillaries and to prevent shadowing of
the illuminating or excitation light from above.
Alternatively it is possible to create the capillary holder
51 without grooves and to lay the capillaries next to one
another flat on top of the capillary holder. But this
requires, instead of the dividing walls 53 for optical
isolation, the provision of coatings impervious to light in
the detection ranges on the sides of the separation
capillaries facing adjacent separation capillaries, or exact
optical imaging.
On the holding device there are also (not illustrated)
releasable means of attachment, eg clamps, for the
separation capillaries 10 in the grooves 52. It is possible
for the holding device to support the separation capillaries
in lengthwise sections outside the detection ranges. But as
a rule it is sufficient for the separation capillaries to be
conducted through the air from the inlet reservoir 20 to the
holding device 50 (see Fig. 1). But tempering devices may
also be provided in this region so that the separation
operation can be performed in predetermined temperature
conditions.
The principle of spectrally resolved multiplex detection is
explained in what follows with reference to Fig. 3 and 4. In
simple analysis, where only one fluorescence marker has to
CA 02284195 1999-09-17
be detected, it is sufficient to attach suitable filters to
the imaging device 41 of the detector device 40 (see Fig. 1)
to shield the excitation light in fluorescence measurement,
but more complicated analysis requires spectral separation
of the light from the detection ranges. This is the case in
DNA sequencing, for example, when nucleotides are
specifically provided with fluorescence tags and are to be
selectively detected as four separate fluorescence bands for
instance.
The invention simultaneously produces excellent spectral and
local resolution by mapping the detection window row on a
two-dimensional detector matrix with resolution
corresponding to the two matrix dimensions. This is
illustrated schematically in Fig. 3. The vertical array of
separation capillaries 10 with the detection window row 13,
differing from the operating position, is mapped by an
imaged device (not shown) and the dispersion element 43 on a
CCD matrix 42. The dispersion element 43 is symbolized by a
prism but can be formed of any wavelength-dispersive
structure with high local resolution. The imaging on the CCD
matrix produces the local resolution on the Y axis and the
spectral resolution on the X axis. Thus the matrix contains
illuminated pixel rows, the number of which corresponds to
the number of separation capillaries. Each pixel delivers a
detector signal as a function of the amount of incident
light, so that the detector signal response of each X row
corresponds to the spectral response of the light from a
separation capillary. It is possible, depending on the
fluorescence dye or tag that is used, for only a subrange of
one or more X rows to be read out, corresponding to the
expected wavelength emission band of the fluorescence dye or
16
CA 02284195 1999-09-17
tag. The spectral resolution is designed for measuring in at
- least three spectral bands.
Details of spectrally and locally resolved detection are
shown in Fig. 4. In a preferred embodiment the detection
window row 13 is imaged by a first objective 411 onto a slit
412. A second, inverted objective 413 sends the light from
the slit through the dispersion element 43. One or more
filters 414 for masking the excitation light may be located
either in front of the objective 411 (as shown) or between
the inverted objective 413 and the dispersion element 43.
The dispersion element 43 is either a classic spectral
device with prisms and/or gratings (drawback: alteration of
the imaging direction, reduced local resolution) or
preferably a direct-vision prism 431 (socalled Amici prism,
Fig. 4A) or a prism/grating/prism combination 432 (Fig. 4B)
or an L-shaped arrangement with a holographic transmission
grating 433 (Fig. 4C). In the last mentioned example of Fig.
4C the light is reflected at a certain angle by a mirror 434
onto the transmission grating 433. The direct-vision prism
431 or the combination 432 or the L-shaped arrangement with
transmission grating 433 offers the advantage of compactness
and extra ruggedness. Interference through aberration and
astigmatism is excluded for the most part. Furthermore, high
light throughput is guaranteed plus small focal length. In
the first two designs a straight optical axis from the
detection windows to the detector camera is maintained. The
entire structure of objectives, slit, filters and dispersion
element can be configured in a tube shielded against stray
light, even functioning as a portable spectrometer. Suitable
selection of the imaging parameters of the objectives and
the dispersion element makes the imaging scale variable over
17
CA 02284195 1999-09-17
a wide range, resulting in high flexibility in terms of the
number of separation capillaries and their diameter.
The light through the dispersion element 43 is imaged on the
camera 42 by an objective 415. The CCD chip 421 of the
camera 42 is 500 * 500 pixels, for example, from which pixel
groups are selected as a function of application (especially
the size and number of the separation capillaries to be
detected) for reading in the analysis operation. Given a
pixel size of 24 * 24 um for example, the slightest
misadjustment (eg shifting of the separation capillaries)
can cause the image on the CCD matrix to shift. To avoid
this effect, the invention uses a search algorithm that
determines the pixel groups which are to be read. This means
that the controller 44 automatically selects the required
pixels of the spectrally and locally resolved image of the
detection window row. The algorithm for reading is a
suitable one from image data processing, eg the socalled
watershed algorithm (see S. Wegner et al. in "Spektrum der
Wissenschaft", 1997, p 113) or what is called the chain code
algorithm, explained below with reference to Fig. 5.
Using 100 capillaries and n fluorescence emissions for
example, 100 * n pixel groups (regions of interest) are
imaged on the CCD chip. The pixels of the groups must be
binned and read out correlated. The pixel groups are defined
in a predetermined manner or automatically determined with
the data processing algorithm in a first trial phase. With
the chain code (Fig. 5) only a starting point of the matrix
coordinates is recorded and the remaining pixels of a group
are detected and read with predetermined direction codes.
Eight direction codes can be provided as shown, for example,
18
CA 02284195 1999-09-17
relating to the eight pixels that surround an observed
pixel. By entering a numeric sequence corresponding to the
numbered directions, all the pixels belonging to a pixel
group can be uniquely identified. This entry is insensitive
to slight image shifts (dislocation in the left part of the
image) and is advantageous in allowing reduction of the
memory requirement for characterizing a group.
Fig. 6 shows as a schematic side view details of the
collector device (or outlet vessel) 70 (see Fig. 1). The
collector device 70 consists of a pressure vessel 73 with a
pH buffered carrier medium 74. The pressure vessel 73 is
connected by a pressure line 75 to a pump device 77 that
produces compressed air for loading the separation
capillaries 10 with their exit ends in the carrier medium.
The pump device is controlled by the unit 44, 45. Also
projecting into the carrier medium is an electrode 71,
connected to a high-voltage power supply (not shown).
Between the electrode 71 and the exit ends of the separation
capillaries 10 is a molecule trap 76, indicated by dashed
lines, that is intended for collecting the separated
samples. Through the effect of an electric field, the
separated samples exit from the ends of the capillaries and
drift to the electrode 71. In doing this they come up
against the molecule trap in the form of a porous dividing
wall (eg a membrane or gel) with pores of a characteristic
diameter of approx. 10 to 100 nm. Collection of the
separated samples or molecules is implemented by one of the
following principles.
Seeing as the speed of motion under the influence of an
electric field is substantially greater than the speed of
19
CA 02284195 1999-09-17
diffusion motion, the probability of molecules passing
through the molecule trap from the electrode to the exit
ends during a turn-off time of the high voltage is very
slight. In this case it is not necessary to select the size
of the pores within very precisely defined limits. In an
alternative mechanism it is assumed that the separated
samples consist of field-dependent stretched molecules
(polyelectrolytes) that pass the pores relatively simply in
stretched form under the influence of the electric field,
but in globular form can only pass the pores with difficulty
when the field is turned off.
In a preferred embodiment of the invention the electrodes
are directly attached to the capillaries at the input, as
shown schematically in Fig. 7. For this purpose the inlet
ends of the separation capillaries have an outer metal
coating 14 (of silver or platinum for example),
simultaneously fulfilling the electrode function. This
allows substantial reduction of the minimum injection
volume. Electrical contact is produced by a contact device
15 (eg clip).
The procedure of a separation analysis using the separation
apparatus detailed above (see Fig. 1) is described in what
follows. Firstly, for loading the carrier media, an empty
plate or a vessel is moved under the injection ends of the
separation capillaries and pressure is applied to the
pressure vessel 73 of the collector device 70 so that the
carrier medium 74 enters the exit ends of the separation
capillaries and runs through them to the injection ends. The
pressure is reduced as soon as sufficient separation medium
has flowed through the capillaries. In the following step
CA 02284195 1999-09-17
the samples reservoir 21 is moved from below to the
- injection ends of the separation capillaries so that the
injection ends are immersed in the samples arranged on the
storage reservoir. Next a high voltage is applied to the
separation capillaries for a certain loading time to load
the samples, ie to inject very small amounts of sample into
the ends. The loading time and the high voltage are selected
so that the first millimeters of the separation capillaries
are filled with the samples to be separated. In the case of
the above mentioned capillaries with inner diameters between
50 and 100 um for example, and using common solvents, this
is produced in a loading time of 1 to 20 s and with high
voltage of approx. 100 to 400 V/cm (preferably approx. 10
kV). After sample loading the samples reservoir 21 is
replaced by the storage reservoir 24 with a buffer solution
(solution of electrolytes), with which the injection ends of
the separation capillaries are in contact during the
following separation process. For the actual separation
process the high voltage is applied again for a time
depending on the application, this being between 10 and 30
min or even amounting to hours.
During the entire separation process, automatically
controllable by the unit 44, the samples to be separated
(molecules, DNA fragments, proteins, etc) move to the exit
ends of the separation capillaries. As a result of the
separation medium there is "selection", ie the small
molecules reach the detection windows faster than the large
molecules, so that complete analysis of the sample
composition is possible because of the locally and
spectrally resolved detection on each window as a function
of time. Separation of the molecules is also possible by
21
CA 02284195 1999-09-17
other mechanisms, eg by socalled end-labeled free solution
electrophoresis.
Experimental results of the separation apparatus according
to the invention are presented in Fig. 8 through 11. Fig. 8
shows a comparison between illumination of the detection
range by a cylindrical lens and a line generator (here the
noisy signal is produced by the use of a multimode optical
fiber instead of a monomode one). The cylindrical lens
generates a socalled Gaussian profile, whereas the line
generator produces a plateau-shaped profile and thus more
homogeneous illumination. Fig. 9 shows a curve to illustrate
the practical elimination of crosstalk between different
separation channels (separation capillaries). The three
maxima correspond to the detector signals from pixel groups
assigned to adjacent separation capillaries. Crosstalk
between adjacent capillaries is less than 1~, so that unique
and reproducible assignment of the detector signals to the
separation channels is possible. In the example shown the
capillaries were arranged next to one another without a
dividing wall. Crosstalk is reduced even more by the optical
isolation of the capillary holder.
Fig. 10 illustrates the choice of a low-viscosity carrier
medium (separation matrix) according to the invention. The
curves show the dependence of carrier medium viscosity on
the particular carrier medium concentration. The
concentration is selected so that the viscosity amounts to
100 centiStokes (mm2/s) (corresponding to approx. < 100 cP).
Fig. 11 shows a control experiment to demonstrate the
reproducibility of the separation apparatus according to the
invention. 96 identical DNA samples were separated
22
CA 02284195 1999-09-17
simultaneously. By way of example, the detector signals of
eight capillaries are shown accumulated over a detection
interval of approx. 30 min, demonstrating excellent
correlation of the separation results in the different
capillaries.
The invention is explained above with reference to
fluorescence measurements. But, in analogous manner, optical
detection can also be implemented based on absorption,
reflection or transmission measurements.
23