Note: Descriptions are shown in the official language in which they were submitted.
CA 02284255 1999-10-12
1
PREVENTION OF Bt RESISTANCE DEVELOPMENT
This invention relates to plant cells and plants,
the genomes of which are transformed to contain at
least two genes, each coding for a different non-
competitively binding Bacillus thuringiensis
("B.thuringiensis" or "Bt") insecticidal crystal
protein ("ICP") for a specific target insect species,
preferably belonging to the order of Lepidoptera or
Coleoptera. Such transformed plants have advantages
over plants transformed with a single B. thuringiensis
ICP gene, especially with respect to the prevention of
resistance development in the target insect species
against the at least two B. thuringiensis ICPs,
expressed in such plants.
This invention also relates to a process for the
production of such transgenic plants, taking into
account the competitive and non-competitive binding
properties of the at least two B. thuringiensis ICPs in
the target insect species' midgut. Simultaneous
expression in plants of the at least two genes, each
coding for a different non-competitively binding B.
thurinctiensis ICP in plants, is particularly useful to
prevent or delay resistance development of insects
against the at least two B. thuringiensis ICPs
expressed in the plants.
This invention further relates to a process for
the construction of novel plant expression vectors and
to the novel plant expression vectors themselves, which
contain the at least two B. thuringiensis ICP genes
encoding the at least two non-competitively binding B.
thuringiensis ICPs. Such vectors allow integration and
coordinate expression of the at least two B.
thuringiensis ICP genes in plants.
CA 02284255 1999-10-12
2
BACKGROUND OF THE INVENTION
Since the development and the widespread use of
chemical insecticides, the occurrence of resistant
insect strains has been an important problem.
Development of insecticide resistance is a phenomenon
dependent on biochemical, physiological, genetic and
ecological mechanisms. Currently, insect resistance has
been reported against all major classes of chemical
insecticides including chlorinated _ hydrocarbons,
organophosphates, carbamates, and pyrethroid compounds
(Brattsten et al., 1986).
In contrast to the rapid development of insect
resistance to synthetic insecticides, development of
insect resistance to bacterial insecticides such as B.
thuringiensis sprays has evolved slowly despite many
years of use (Brattsten et al., 1986). The spore
forming gram-positive bacterium B. thuringiensis
produces a parasporal crystal which is composed of
crystal proteins (ICPs) having insecticidal activity.
Important factors decreasing the probability of
emergence of resistant insect strains in the field
against B. thuringiensis sprays are: firstly the short
half-life of B. thuringiensis sprays after foliar
application; secondly the fact that commercial B.
thuringiensis preparations often consist of a mixture
of several insecticidal factors including spores, ICPs
and eventually beta-exotoxins (Shields, 1987); and
thirdly the transitory nature of plant-pest
interactions. Many successful field trials have shown
that commercial preparations of a B. thuringiensis
containing its spore-crystal complex, effectively -
control lepidopterous pests in agriculture and forestry
(Krieg and Langenbruch, 1981). B. thuringiensis is at
present the most widely used pathogen for microbial
control of insect pests.
CA 02284255 1999-10-12
3
Various laboratory studies, in which selection
against B. thuringiensis was applied over several
generations of insects, have confirmed that resistance
against B. thuringiensis is seldom obtained. However,
it should be emphasized that the laboratory conditions
represented rather low selection pressure conditions.
For example, Goldman et al. (1986) have applied
selection with B. thuringiensis israelensis toxin over
14 generations of Aedes aegypti and found only a
marginal decrease in sensitivity. The lack of any
observable trend toward decreasing susceptibility in
the selected strains may be a reflection of the low
selection pressure (LC50) carried out over a limited
number of generations. However, it should be pointed
out that Georghiou et al. (In : Insecticide Resistance
in Mosquitoes : Research on new chemicals and
techniques for management. In "Mosquito Control
Research, Annual Report 1983, University of
California.") with Culex c;uinguefasciatus obtained an
11-fold increase in resistance to B. thuringiensis
israelensis after 32 generations at LC95 selection
presssure.
McGaughey (1985) reported that the grain storage
pest Plodia interpunctella developed resistance to the
spore-crystal complex of B. thuringiensis; after 15
generations of selection with the Indian meal moth,
Plodia interpunctella, using a commercial B.
thuringiensis HD-1 preparation ("Dipel"; Abbott
Laboratories, North Chicago, Illinois 60064, USA), a
100-fold decrease in B. thuringiensis sensitivity was
reported. Each of the colonies was cultured for several
generations on a diet treated with a constant B.
thuringiensis dosage which was expected to produce
70-90% larval mortality. Under these high selection
presssure conditions, insect resistance to B.
* Trademark
CA 02284255 1999-10-12
4
thuringiensis increased rapidly. More recently,
development of resistance against B. thuringiensis is
also reported for the almond moth, Cadra cautella
(McGaughey and Beeman, 1988). Resistance was stable
when selectionwas discontinued and was inherited as a
recessive trait (McGaughey and Beeman, 1988). The
mechanism of insect resistance to B. thuringiensis
toxins of Plodia interpunctella and Cadra cautella has
not been elucidated.
The main cause of B. thuringiensis resistance
development in both reported cases involving grain
storage was the environmental conditions prevailing
during the grain storage. Under the conditions in both
cases, the environment was relatively stable, so B.
thuringiensis degradation was slow and permitted
successive generations of the pest to breed in the
continuous presence of the microbial insecticide. The
speed at which Plodia developed resistance to B.
thuringiensis in one study suggests that it could do so
within one single storage season in the bins of treated
grain.
Although insect resistance development against B.
thuringiensis has mostly been observed in laboratory
and pilot scale studies, very recent indications of B.
thuringiensis resistance development in Plutella
xylostella populations in the (cabbage) field have been
reported (Kirsch and Schmutterer, 1988). A number of _
factors have led to a continuous exposure of P.
xylostella to B. thuringiensis in a relatively small
geographic area. This and the short generation cycle of
P. xylostella have seemingly led to --an enormous
selection pressure resulting in decreased
susceptibility and increased resistance to B.
thuringiensis.
CA 02284255 1999-10-12
11288-4
A procedure for c:xprc:;alnd a U. thurincliensis iCP
gene in plants_in order to render the plants insect-
resistant (European patent publication ("1:P") 0193259;
Vaeck et
al., 1987; Barton et al., 1987; Fischtioff et al., 1987)
provides an entirely new approach to insect control in
agriculture which is at the same tiine . safe,
environmentally attractive and cost-effective. An
important determinant for the success of this approach
will be whether insects will be able to develop
resistance to B. thuringiensis ICPs expressed in
transgenic plants (Vaeck et al., 1987; Barton et al.,
1987; Fischhoff et al., 1987). In contrast with a
foliar application, after which B. thurinqiensis ICPs
are rapidly degraded, the transgenic plants will exert
a continuous selection pressure. It is clear from
laboratory selection experiments that a continuous
selection pressure has led to adaptation to B.
thuringiensis and its coinponents in several insect
species. In this recjard, it should be pointed out that
ttie conditions in the laboratory which resulted in the
development of insect-resistance to B. thuringiensis
are very similar to the situation with transcgenic
plants which produce D. thuringiensis ICPs and pi=ovide
a continuous selection pressure ori insect populatioris
feeding on the plants. Mathematical rnodels of selection
pressure predict tliat, if etigineered insect-resistant
plants become a perinanent part of their environment,
resistance development in insects will emerge rapidly
(Gould, 1988). Thus, the cliances for the developnient of
insect resistance to B. ttiuringiensis in transgenic
plants may be considerably increased as conipared to the
field application of B. thuringiensis sprays. A
Heliothis virescens strain has been reported ttlat is 20
times more resistant to B. ttiurinctiensis IID-1 ICP
CA 02284255 1999-10-12
6
produced by transgenic Pseudomonas fluorescens and 6
times more resistant to the pure ICP (Stone et al.,
1989). Furthermore, the monetary and human costs of
resistance are difficult to assess, but loss of
pesticide effectiveness invariably entails increased
application frequencies and dosages and, finally, more
expensive replacement compounds as new pesticides
become more difficult to discover and develop.
Therefore, it would be desirable to develop means
for delaying or even preventing the evolution of
resistance to B. thuringiensis.
B. thuringiensis strains, active against
Lepidoptera (Dulmage et al., 1981), Diptera (Goldberg
and Margalit, 1977) and Coleoptera (Krieg et al.,.
1983), have been described. It has become clear that
there is a substantial heterogeneity among ICPs from
different strains active against Lepidoptera, as well
as among ICPs from strains active against Coleoptera
(Hofte and Whiteley, 1989). An overview of the
different B. thuringiensis ICP genes, that have been
characterized, is given in Table 2 (which follows the
Examples herein).
Most of the anti-Lepidopteran B. thuringiensis
(e.g., Bt3, Bt2, Bt73, Bt14, Bt15, Bt4, Bt18) ICP genes
encode 130 to 140 kDa protoxins which dissolve in the
alkaline environment of an insect's midgut and are
proteolytically activated into an active toxin of 60-65
kDa. These ICPs are related and can be recognized as
members of the same family based on sequence
homologies. The sequence divergence however is
substantial, and the insecticidal spectrum, among the
order Lepidoptera, may be substantially different
(Hofte et al., 1988).
The P2 toxin gene and the cry B2 gene are
different from the above-mentioned genes in that they
CA 02284255 1999-10-12
7 11288-4
do not encode high molecular weight protoxins but
rather toxins of around 70 kDa (Donovan et al., 1988
and Widner and Whiteley, 1989, respectively).
It has recently become clear that heterogeiieity
exists also in the anti-Coleopteran toxin gene fainily.
Whereas several previously reported toxin gene
sequences from different B. thuringiensis isolates with
anti-Coleopteran activity were identical (EP 0149162
and 0202739), the segueiices and struct:urP of bt2l and
bt22 are suhstartially divergent (Canadian patent
application No. 2,046,646).
While the insect-icidal spectra of B. thurincliensis
ICPs are different, the major pathway of their toxic
action is believed to be co-ninon. All B. thuringiensis
ICPs, for wliich the mechanism of action has been
studied in any detail, interact with the midgut
epithelium of sensitive species and cause lysis of the
epithelial cells (Knowles and Ellar, 1986) due to the
fact that the permeability cliaracteristics of the bt-ush
border menibrane and the osmotic balance over this
menibrane are perturbed. In the patliway of toxic action
of B. thurinqiensis ICPs, the binding of the toxin to
rec ptor sites on the brusli border membrane of these
cells is aii important feature (llofinann et al., 1988b).
'i'lie toxin bitiding sites in the inidgut can be re9arded
as an ICP-receptor since toxin is bound in a saturable
way and with high affinity (itofmann et al., 1988a).
Although this outline of ttie mode of action of B.
ttiuringiensis ICPs is generally accepted, it remaiiis a
matter of diacussion what the essential deterininaiit (s)
are for the differetices in their insecticidal spectra.
llaider at al. .(1986) emphasize the importance of
specific pi-oteases in the insect midgut. liofmann at al.
(1988b) indicate that receptor binding is a
prerequisite for toxic activity and describe lliat
CA 02284255 2000-11-30
27620-8D
- 8 -
Pieris brassicae has two distinct receptor populations for two
toxins. Other authors have suggested that differences in the
environment of the midgut (e.g., pH of the midgut) might be
crucial.
SUMMARY OF THE INVENTION
In accordance with this invention a plant is provided
having, stably integrated into its genome, at least two B.
thuringiensis ICP genes encoding at least two non-competitively
binding insecticidal B. thuringiensis ICPs, preferably the
active toxins thereof, against a specific target insect,
preferably against a Lepidoptera or Coleoptera. Such a plant
is characterized by the simultaneous expression of the at least
two non-competitively binding B. thuringiensis ICPs.
Also in accordance with this invention, at least two
ICP genes, particularly two genes or parts thereof coding for
two non-competitively binding anti-Lepidopteran or anti-
Coleopteran B. thuringiensis ICPs, are cloned into a plant
expression vector. Plant cells transformed with this vector
are characterized by the simultaneous expression of the at
least two B. thuringiensis ICP genes. The resulting
transformed plant cell can be used to produce a transformed
plant in which the plant cells: 1. contain the at least two B.
thuringiensis ICP genes or parts thereof encoding at least two
non-competitively binding anti-Lepidopteran or anti-Coleopteran
B. thuringiensis ICPs as a stable insert into their genome; and
2. express the genes simultaneously, thereby conferring on the
plant improved resistance to at least one target species of
insect, so as to prevent or delay development of resistance to
B. thuringiensis of the at least one target species of insect
feeding on the transformed plant.
CA 02284255 1999-10-12
- 9 -
Further in accordance with this invention, plant expression
vectors are provided which allow integration and simultaneous
expression of at least two B. thuringiensis ICP.genes in a
plant cell and which comprise one or more chimeric genes, each
containing in the same transcriptional unit: a promoter which
functions in the plant cell to direct the synthesis of mRNA
encoded by one of the ICP genes; one or more different ICP
genes, each encoding a non-competitively binding
thuringiensis ICP; preferably a marker gene; a 3' non-
translated DNA sequence which functions in the plant cell for
3' end formation and the addition of polyadenylate nucleotides
to the 3' end of the mRNA; and optionally a DNA sequence
encoding a protease-sensitive protein part between any two ICP
genes.
In one embodiment, this invention provides for cells
of a plant, characterized by: at least two DNA sequences
stably inserted into the genome of said plant; said DNA
sequences being under the control of the same or a distinct
promoter and each of said DNA sequences encoding a different
non-competitively binding Bacillus thuringiensis Insecticidal
Crystal Protein toxic for the same insect species wherein said
insect species is a Lepidopteran or a Coleopteran insect
species; and wherein at least two different Insecticidal
Crystal Proteins can be produced in said cells.
In a further embodiment, this invention provides for
a vector suitable for transforming cells of a plant, capable
of being transformed with Agrobacterium, comprising at least
two DNA sequences encoding a different non-competitively
binding Bacillus thuringiensis Insecticidal Crystal Protein
27620-8
CA 02284255 2004-11-30
29827-20D
- 9a -
for the same insect species wherein said insect species is a
Lepidopteran or Coleopteran insect species.
In another embodiment, this invention provides for
a process for producing a plant having improved insect
resistance and having DNA sequences encoding Insecticidal
Crystal Proteins selected from the group consisting of Bt2,
Bt73, Bt4, Bt14, Bt15, Bt18, Bt13, Bt21, and Bt22, stably
integrated into the nuclear genome of its cells,
characterized by the non-biological steps of transforming a
plant cell by introducing DNA sequences encoding the said
Insecticidal Crystal Proteins into the nuclear genome of
said cell and regenerating said plant and reproduction
material from said cell.
In a further embodiment, this invention provides
for a plant cell culture, consisting of the plant cells
described above.
In a further embodiment, this invention provides
for a method for rendering a plant resistant to an insect
species by transforming the plant with said DNA sequences
encoding said Insecticidal Crystal Proteins as described
above.
In another embodiment, this invention provides for
cells of a plant, characterized by: at least two DNA
sequences stably inserted into the genome of said plant;
said DNA sequences being under the control of the same or
distinct promoter and each of said DNA sequences encoding a
different Insecticidal Crystal Protein toxic to the same
insect species; wherein said Insecticidal Crystal Proteins
do not bind competitively to the midgut membranes of said
same insect species; and wherein said insect species is a
Lepidopteran or Coleopteran insect species.
CA 02284255 2004-11-30
29827-20D
- 9b -
Accordingly, one aspect of the invention relates
to a method for preventing or delaying development of
resistance of at least one target pest insect species to
Bacillus thuringiensis ICP proteins expressed in a plant,
comprising stably incorporating into the genome of a plant
two DNA sequences under the control of promoter sequences
which drive the expression of said DNA sequences in cells of
a plant, each of said DNA sequences encoding a different
Bacillus thuringiensis ICP protein, toxic to the same target
insect species, wherein the encoded Bacillus thuringiensis
ICP proteins bind non-competitively to the brush border
membrane of the midgut epithelial cells of said same insect
species; wherein said Bacillus thuringiensis ICP proteins
are produced in said plant; and wherein said Bacillus
thuringiensis ICP proteins are selected from the group of:
an intact protein or a part thereof which has insecticidal
activity and which can be produced in nature by Bacillus
thuringiensis, a Bacillus thuringiensis protoxin, an active
toxin of Bacillus thuringiensis, an insecticidal truncated
part of a protoxin which need not be crystalline and which
need not be a naturally occurring protein, and a chimeric
toxin encoded by the combination of two variable regions of
two different Bacillus thuringiensis genes.
Another aspect of the invention relates to a
method for producing a plant with increased resistance to a
target insect pest species, comprising expressing in cells
of a plant two DNA sequences encoding different Bacillus
thuringiensis ICP proteins, wherein said Bacillus
thuringiensis ICP proteins comprise a first Bacillus
thuringiensis ICP protein and a second Bacillus
thuringiensis ICP protein toxic to said target pest insect
species, and wherein said second Bacillus thuringiensis ICP
protein is selected using the following procedure:
CA 02284255 2006-10-03
75749-7D
- 9c -
a) obtaining a strain of said target insect pest
species that developed resistance to said first Bacillus
thuringiensis ICP protein,
b) carrying out insect bioassays and competitive
binding studies using said first Bacillus thuringiensis ICP
protein and a second Bacillus thuringiensis ICP protein, and
c) selecting a second Bacillus thuringiensis ICP
protein that remains fully insecticidal to said resistant
insect strain and binds to a different binding site in the
target insect gut membranes compared to the first Bacillus
thuringiensis ICP protein; wherein said Bacillus
thuringiensis ICP proteins are selected from the group of:
an intact protein or a part thereof which has insecticidal
activity and which can be produced in nature by Bacillus
thuringiensis, a Bacillus thuringiensis protoxin, an active
toxin of Bacillus thuringiensis, an insecticidal truncated
part of a protoxin which need not be crystalline and which
need not be a naturally occurring protein, and a chimeric
toxin encoded by the combination of two variable regions of
two different Bacillus thuringiensis genes.
Another aspect of the invention relates to an
isolated insecticidal protein comprising the amino acid
sequence of the Bt14 protein, or a fragment thereof which
includes the histidine residue at position 150 of the Bt14
protein.
Another aspect of the invention relates to an
insecticidal protein comprising the amino acid sequence of
the Bt15 protein, or an insecticidally effective fragment
thereof.
Another aspect of the invention relates to an
isolated DNA encoding an insecticidal protein comprising the
CA 02284255 2004-11-30
29827-20D
- 9d -
amino acid sequence of the Bt14 protein, or an
insecticidally effective fragment thereof.
Another aspect of the invention relates to an
isolated DNA sequence encoding an insecticidal protein
comprising the amino acid sequence of the Bt15 protein, or
an insecticidally effective fragment thereof.
Another aspect of the invention relates to a
chimeric gene, comprising a promoter which can direct
expression in plant cells of the DNA described herein.
Another aspect of the invention relates to a plant
cell, containing the chimeric gene described herein, stably
integrated into its genome.
Another aspect of the invention relates to a
method of controlling insects selected from the group of:
Pieris brassicae, Phtorimeae operculella, and Plutella
xylostella, which method comprises contacting said insects
with an insecticidal protein comprising the amino acid
sequence of the Bt14 protein, or an insecticidally effective
fragment thereof.
Another aspect of the invention relates to a
method of controlling insects selected from the group of:
Manduca sexta, Mamestra brassicae, Plodia interpunctella,
Spodoptera littoralis and Plutella xylostella, which method
comprises contacting said insects with an insecticidal
protein comprising the amino acid sequence of the Bt15
protein, or an insecticidally effective fragment thereof.
CA 02284255 2004-11-30
29827-20D
- 9e -
DETAILED DESCRIPTION OF THE INVENTION
Definitions
As used herein, "B. thuringiensis ICP" (or "ICP")
should be understood as an intact protein or a part thereof
which has insecticidal activity and which can be produced in
nature by B. thuringiensis. An ICP can be a protoxin, as
well as an active toxin or another insecticidal truncated
part of a protoxin which need not be crystalline and which
need not be a naturally occurring protein. In this regard,
an ICP can be a chimeric toxin encoded by the combination of
two variable regions of two different ICP genes as disclosed
in EP 0228838.
As used herein, "protoxin" should be understood as
the primary translation product of a full-length gene
encoding an ICP.
As used herein, "toxin", "toxic core" or "active
toxin" should all be understood as a part of a proto
CA 02284255 1999-10-12
which can be obtained by protease (e.g., by trypsin)
cleavage and has insecticitial activity.
As used herein, "gene" should be understood as a
full-length DNA sequence encoding a protein (e.g., such
as is found in nature), as well as a truncated fragment
thereof encoding at least the active part (i.e., toxin)
of the protein encoded by the full-length DNA sequence,
preferably encoding just the active part of the protein
encoded by the full-length DNA sequence. A gene can be
naturally occurring or synthetic.
As used herein, "truncated B. thuringiensis gene"
should be understood as a fragment of a full-length B.
thuringiensis gene which still encodes at least the
toxic part of the B. thuringiensis ICP, preferentially
the toxin.
As used herein, "marker gene" should be understood
as a gene encoding a selectable marker (e.g., encoding
antibiotic resistance) or a screenable marker (e.g.,
encoding a gene product which allows the quantitative
analysis of transgenic plants).
Two ICPs are said to be "competitively binding
ICPs" for a target insect species when one ICP competes
for all ICP receptors of the other ICP, which receptors
are present in the brush border membrane of the midgut
of the target insect species.
Two ICPs are said to be "non-competitively binding
ICPs" when, for at least one target insect species, the
first ICP has at least one receptor for which the
second ICP does not compete and the second ICP has at
least one receptor for which the first ICP does not
compete, which receptors are present in the brush
border membrane of the midgut of the target insect
species.
A "receptor" should be understood as a molecule,
to which a ligand (here a B. thuringiensis ICP,
CA 02284255 1999-10-12
11
preferably a toxin) can bind with high affinity
(typically a dissociation constant (Kd) between 10'11
and 10'6M) and saturability. A determination of whether
two ICPs are competitively or non-competitively binding
ICPs can be made by determining whether: 1. a first ICP
competes for all of the receptors of a second ICP when
all the binding sites of the second ICP with an
a f f inity in the range of about 10"11 to 10'6M can be
saturated with the first ICP in concentrations of the
first ICP of about 10"5M or less (e.g., down to about
10"11M) ; and 2. the second ICP competes for the all of
the receptors of the first ICP when all the binding
sites of the first ICP with an affinity in the range of
about 10'11 to 10'6M can be saturated with the second ICP
in concentrations of the second ICP of about 10'5M or
less.
General Procedures
This section describes in broad terms general
procedures for the evaluation and exploitation of at
least two B. thurinctiensis ICP genes for prevention of
the development, in a target insect, of a resistance to
the B. thuringiensis ICPs expressed in transgenic
plants of this invention. A non-exhaustive list of
consecutive steps in the general procedure follows,
after which are described particular Examples that are
based on this methodology and that illustrate this
invention.
In accordance with this invention, specific B.
thuringiensis ICPs can be isolated in a conventional
manner from the respective strains such as are listed
in Table 2 (which follows the Examples). The ICPs can
be used to prepare monoclonal or polyclonal antibodies
specific for these ICPs in a conventional manner (Hofte
et al., 1988).
CA 02284255 1999-10-12
12 11288-4
Tlie ICP genes can each be isolated froin thc--ir
respective stmins in a conventional maiu-ier.
Preferably, the ICP genes are each identified by;
digesting total DNA from their respective strains with
suitable restriction enzyme(s); size fractionating the
DNA fragments, so produced, into DNA fractions of 5 to
Kb; ligating such fractions to suitable cloning
vectors (e.g., pEcoR251, deposited at the Deutsche
Sainmlung von Mikroorganisnien und 2el lculturen ("DSM" ),
10 Braunschweig, Federal Republic of Germany, under
accession number no. 4711 on July 13, 1988);
transforining E.coli with tlie cloning vectors; and
screening the clones with a suitable DNA probe. The D14A
probe can be constructed from a highly conserved region
which is commonly present in different B. thuringiensis
genes which encode crystal protoxins against Coleoptera
or Lepidoptera, such as on the basis of an N-terminal
amino acid sequence determined by gas-phase seauencing
of the purified proteins (EP 0,305,275, filed August 16, 1988).
Alternatively, the desired fragnients, prepared
froin total DNA of the respective strains, can be
ligated in suitable expression vectors ( e.g., a pUC
vector (Yaniscli-Perron et al., 1985) with the iiisert
under the control of the lac proinoter) and transfoi-ined
in E. coll, and the clones can tlien be screeneci by
conventional colony immunoprobing methods (Frencli et
al., 1986) for expression of the toxins with monoclonal
or polyclonal antibodies raised against the toxins
produced by the strains.
The Isolated B. thurinc;iensis ICP genes can then
be sequenced in a conventional manner using well-known
procedures (e.g., Maxam and Gilbert, 1980).
At present, several ICP genes have been cloned
from different subspecies of B. thuringiensis (Table
2). The nucleotide sequences froin several of these U.
CA 02284255 1999-10-12
13
thuringiensis ICP genes have been reported. Whereas
several sequences are identical or nearly identical and
represent the same gene or slight variants of the same
gene, several sequences display substantial
heterogeneity and show the existence of different B.
thuringiensis ICP gene classes. Several lines of
evidence suggest that all these genes specify a family
of related insecticidal proteins. Analysis of the
distribution of B. thuringiensis ICPs in different B.
thuringiensis strains by determining the protein
composition of their crystals, by immunodetection using
polyclonal antisera or monoclonals against purified
crystals, or by using gene-specific probes, shows that
subspecies of B. thuringiensis might contain up to
three related B. thuringiensis ICP genes belonging to
different classes (Kronstad et al., 1983).
To express the isolated and characterized gene in
a heterologous host for purification and
characterization of the recombinant protein, the
preferred organism is Escherichia coli. A number of
expression vectors for enhanced expression of
heterologous genes in E. coli have been described
(e.g., Remaut et al., 1981). Usually the gene is
cloned under control of a strong regulatable promoter,
such as the lambda pL or pR promoters (e.g., Botterman
and Zabeau, 1987), the lac promoter (e.g., Fuller,
1982) or the tac promoter (e.g., De Boer et al., 1983),
and provided with suitable translation initiation sites
(e.g., Stanssens et al, 1985 and 1987). Gene cassettes
of the B. thuringiensis ICP genes can be generated by
site-directed mutagenesis, for example according to-the
procedure described by Stanssens et al. (1985 and
1987). This allows cassettes to be made comprising,
for example, a truncated ICP gene fragment encoding the
toxic core (i.e., toxin) of an ICP or a hybrid gene
CA 02284255 1999-10-12
14 11288-4
encoding the toxic core and a selectable marker
according to -the procedures described in EP
0,358,557 published March 14, 1990.
The cells of an E. coli culture, which has been
induced to produce a reconibinant ICP, are liarveste:d.
The method used to induce the cells to produce the
recombinant ICP depends on the choice of the promoter.
For example, the lac promoter (Fuller, 1982) is iilduc:ed
by isopropyl-B-D-thiogalacto-pyranoside ("IPTG"); the
1l) pL pronioter is induced by temperature shock (Bernard et
al., 1979). The recombinant ICP is usually deposited
in the cells as insoluble inclusions (lisuing and
Becker, 1988). The cells are lysed to liberate the
inclusions. Ttie bulk of E. coli proteins is removed in
subsequent washing steps. A semi-purified protoxin
pellet is obtained, from which the protoxin can be
dissolved in alkaline buffer (e.g. , NazCO3, pti 10) . The
procedure for the ICP Bt2, wliich is also applicable to
otlier recombinant toxins, has been described by liofte
111 et al. , 1986.
In accordance with this invention, the binding of
various ICPs to ICP receptors. on the brush border
membrane of ttie columnar midgut epithelial cells of
various insect species has been investigated. The brush
hordor inombrana iu the primary ti,rget of eaclj ICP, jtjjd
meinbrane vesicles, prefereiitially derived froin the
brush border meinbrane, can be obtained according to
tiJolfersberger et al., 1987.
The binding to ICP receptors of one or more ICPs
30 (e.g., ICP A, ICP B, etc.) can be characterized by the
following steps (ilofmann et al, 1988b):
1. ICP A is labelled with a suitable marker (usually
a radioisotope such as 125I) .
2. Brush border meinbranes are incubated with a small
amount (preferably less than l0"1 M) of labelled
------------
CA 02284255 1999-10-12
ICP A together with different concentrations of
non-labelled ICP A (preferably from less than 10'11
to 10"5 M) .
3. For all concentrations tested the amount of
labelled ICP A bound to the brush border membranes
is measured.
4. Mathematical analysis of these data allows one to
calculate various characteristics of the ICP
receptor such as the magnitude of the population
of binding sites (Scatchard, 1949).
5. Competition by other toxins (e.g. ICP B) is
preferably studied by incubating the same amount
of labelled ICP A with brush border membranes in
combination with different amounts of ICP B
(preferentially from 10'il to 10"6 M; and
subsequently, steps 3 and 4 are repeated.
By this procedure, it has been found, for example,
that Bt3 toxin, Bt2 toxin and Bt73 toxin are
competitively binding anti-Lepidopteran ICPs for
Manduca sexta and Heliothis virescens (See example 6
which follows). Various other combinations of toxins
have been found to be non-competitively binding anti-
Lepidopteran or anti-Coleopteran toxins (example 6).
Although the concept of competitivity versus non-
competitivity of ICP binding does not have any
practical importance by itself, the observation of the
non-competitivity of two B. thuringiensis ICPs, active
against the same target insect, can be put to very
significant practical use. This is because a
combination of two non-competitively binding B.
thuringiensis ICPs can be used to prevent development,
by a target insect, of resistance against such B.
thuringienis ICPs.
A selection experiment with M. sexta, using Bt2
toxin, Bt18 toxin, and a mixture of Bt2 and Bt18
CA 02284255 1999-10-12
16
toxins, has shown that Bt2 and Bt18 are two non-
competitively binding anti-Lepidopteran toxins. After
20 generations of selection, a very pronounced
reduction in ICP sensitivity was observed in the
selection experiments with Bt2 or Bt18 alone (>100
times). The reduction in sensitivity in the selection
experiment with a Bt2-BtlB mixture was only marginal (3
times). This demonstrates the unexpected practical
advantage of a simultaneous use of two non-
competitively binding ICPs in a situation which models
the high selection pressure which will exist with the
use of transgenic plants transformed with ICP genes. In
this regard, the two resistant strains showed a
specific loss in receptor sites for either the Bt2 or
Bt18 toxin. In each case, receptor sites for the toxin,
which was not used for selection, were not affected or
their concentration even increased. Thus, the Bt2
selected strain retained its Bt18 receptors, and the
Btl8 selected strain developed an increased number of
Bt2 receptors. Indeed, the Bt18 selected strain showed
an increased sensitivity for Bt2 along with its
increased Bt2 receptor concentration. No significant
changes in receptor sites were found in the strain
selected against the combined toxins. These findings
are described in detail in Example 7 which follows.
A similar mechanism of resistance to Bt has been
observed with respect to a strain of diamondback moth,
Plutella xylostella. This strain had developed
resistance in the field to Dipel which is a commercial
formulation of the Bt HD-1 strain. Crystals of Dipel*
comprise a mixture of several BtICPs, similar to the
Bt2, Bt3 and Bt73 proteins which are competitively-
binding ICPs. As shown by both insect bioassays and
competitive binding studies using Bt2 and Bt15, the
Dipel-resistant diamondback moth strain is resistant to
* Trademark
CA 02284255 1999-10-12
17
Bt2 protoxin and toxin but maintains full sensitivity
to Bt15 protoxin and toxin. This finding is relevant to
other combinations of non-competitively binding anti-
Lepidopteran or Coleopteran ICPs which are expected to
have the same beneficial effect against their common
target insects.
Hence, a combination of non-competitively binding
ICPs, when directly expressed in a transgenic plant,
offers the substantial advantage of reducing the
chances of development of insect resistance against the
ICPs expressed in the plant. There may be additional
benefits because the combined spectrum of two toxins
may be broader than the spectrum of a single ICP
expressed in a plant (See Examples 8, 9 and 10 which
follow).
If, among two competitively binding ICPs, one has
a larger binding site population than the other against
a given target insect, it will be most advantageous to
use the one with the larger population of binding sites
to control the target pest in combination with the most
suitable non-competitively binding B. thuringiensis
ICP. For example, as seen from Example 6, it is
preferred to use Bt73 against Heliothis virescens,
rather than Bt2 or Bt3, and it is preferred to use Bt3
against Manduca sexta rather than Bt2 or Bt73. The
selected gene can then be combined with the best
suitable non-competitively binding ICP.
Previously, plant transformations involved the
introduction of a marker gene together with a single
ICP gene, within the same plasmid, in the plant genome
(e.g., Vaeck et al., 1987; Fischoff et al., 1987). Such
chimeric ICP genes usually comprised either all or part
of an ICP gene, preferably a truncated ICP gene
fragment encoding the toxic core, fused to a selectable
marker gene, such as the neo gene coding for neomycin
CA 02284255 1999-10-12
18
phosphotransferase. The chimeric ICP gene was placed
between the T-DNA border repeats for Agrobacterium Ti-
plasmid mediated transformation (EP 0193259).
This invention involves the combined expression of
two or even more B. thuringiensis ICP genes in
transgenic plants. The insecticidally effective B.
thuringiensis ICP genes, encoding two non-competitively
binding ICPs for a target insect species, preferably
encoding the respective truncated ICP genes, are
inserted in a plant cell genome, preferably in its
nuclear genome, so that the inserted genes are
downstream of, and under the control of, a promoter
which can direct the expression of the genes in the
plant cell. This is preferably accomplished by
inserting, in the plant cell genome, one or more
chimaeric genes, each containing in the same
transcriptional unit: at least one ICP gene; preferably
a marker gene; and optionally a DNA sequence encoding a
protease (e.g., trypsin)-sensitive or -cleavable
protein part intercalated in frame between any two ICP
- genes in the chimaeric gene. Each chimaeric gene also
contains at least one promoter which can direct
expression of its ICP gene in the plant cell.
The selection of suitable promoters for the
chimaeric genes of this invention is not critical.
Preferred promoters for such chimaeric genes include:
the strong constitutive 35S promoter obtained from the
cauliflower mosaic virus, isolates CM 1841 (Gardner et
al., 1981), CabbB-S (Franck et al., 1980) and CabbB-JI
(Hull and Howell, 1987); the promoter of the nopaline
synthetase gene ("PNOS") of the Ti-plasmid (Herrera-
Estrella, 1983); the promoter of the octopine synthase
gene ("POCS" [De Greve et al., 1982]); and the wound-
inducible TR1' promoter and the TR2' promGrer which
drive the expression of the 1' and 2" genes,
CA 02284255 1999-10-12
19
respectively, of the T-DNA (Velten et al., 1984).
Alternatively, a promoter can be utilized which is
specific for one or more tissues or organs of the
plant, whereby the inserted genes are expressed only in
cells of the specific tissue(s) or organ(s). Examples
of such promoters are a stem-specific promoter such as
the AdoMet-synthetase promoter (Peleman et al., 1989),
a tuber-specific promoter (Rocha-Sosa et al., 1989),
and a seed-specific promoter such as the 2S promoter
(Krebbers et al., 1988). The ICP genes could also be
selectively expressed in the leaves of a plant (e.g.,
potato) by placing the genes under the control of a
light-inducible promoter such as the promoter of the
ribulose-1,5-bisphosphate carboxylase small subunit
gene of the plant itself or of another plant such as
pea as disclosed in EP 0193259. Another alternative is
to use a promoter whose expression is inducible (e.g.,
by temperature or chemical factors).
A 3' non-translated DNA sequence, which functions
in plant cells for 3' end formation and the
polyadenylation of the 3' end of the mRNA sequence
encoded by the at least one ICP gene in the plant cell,
also forms part of each such chimeric gene. The
selection of a suitable 3' non-translated DNA sequence
is not critical. Examples are the 3' untranslated end
of the octopine synthase gene, the nopaline synthase
gene or the T-DNA gene 7 (Velten and Schell, 1985).
The selection of marker genes for the chimaeric
genes of this invention also is not critical, and any
conventional DNA sequence can be used which encodes a
- protein or polypeptide which renders plant cells,
expressing the DNA sequence, readily distinguishable
from plant cells not expressing the DNA sequence (EP
0344029). The marker gene can be under the control of
its own promoter and have its own 3' non-translated DNA
- --- ---- ------
CA 02284255 1999-10-12
20 11288-4
sequence as disclosed above, provided the marker gene is in the
same genetic locus as the ICP gene(s) which it identifies. The
marker gene can be, for example: a herbicide resistance gene such
as the sfr or sfrv gettes (EP 0,242,246, published October 21,
1987); a gene encoding a modified target enzyme for a herbicide
having a lower affinity for the herbicide than the natural (non-
modified) target enzyme, such as a modified 5-EPSP as a target for
glyphosate (U.S. patent 4,535,060; EP 0218571) or a modified
glutamine synthetase as a target for a glutamine synthetase
inhibitor (EP 0240972); or an antibiotic resistance gene, such as
a neo gene (PCT publication WO 84/02913; EP 0193259).
Using A. tumefaciens Ti vector-mediated plant
transformation methodology, all chimeric genes of this invention
can be inserted into plant cell genomes after the chimeric genes
have been placed between the T-DNA border repeats of suitable
disarmed Ti-plasmid vectors (Deblaere et al., 1988). This
transformation can be carried out in a conventional manrier, for
example as described in EP 0116718, PCT publication WO 84/02913
and EP 0,242,246.published October 21, 1987. The chimeric genes
can also be in non-specific plasmid vectors which can be used for
direct gene transfer (e.g., as described by Pazkowski et al.,
1984; De La Pena et al., 1986). Different conventional procedures
can be followed to obtain a combined expression of two
B.thuringiensis ICP genes in transgenic plants as. summarized
below.
I Cliimc:ric gene constructs whereby two or more ICP genes and a
CA 02284255 1999-10-12
21 11288-4
marker gene are transferred to the plant genome as a single piece
of DNA and lead to the insertion in a single'locus in the genome
Ia The genes can be engineered in different transcriptional
units each under control of a distinct promoter.
To express two or more ICP genes and a marker gene as
separate transcriptional units, several promoter fragments
directing expression in plant cells can be used as described
above. All combinations of the promoters mentioned above in the
chimeric constructs for one ICP gene are possible. Examples of
such individual chimeric constructs are described for the bt2 gene
in EP 0193259,for the bt13 gene in EP 0,305,275 published March 1,
1990 and for the bt18 gene in EP 0,358,557 published March 14,
1990. The ICP gene in each chimeric gene of this invention can be
the intact ICP gene or preferably an insecticidally-effective par,t
of the intact ICP gene, especially a truncated gene fragment
encoding the toxic core of the ICP. The individual chinieric genes
are cloned in the same plasmid vector according to standard
procedures (e.g., EP 0193259).
Ib Two genes (e.g., either an ICP and a marker gene or two ICP
genes) or more can be combined in the same transcriptional unit.
To express two or more ICP genes in the same
transcriptional unit, the following cases can bedistinguished:
In a first case, hybrid genes in which the coding region
of one gene is in franie fused with the coding region of another
gene can be placed under the control of a single promoter.
CA 02284255 1999-10-12
21a 11288-4
Fusions can be made between either an ICP and a marker gene or
between two ICP genes. An example of an ICP gene-marker gene
fusion has been described in EP 0193~59 (i.e., a hybrid truncated
bt2-neo gene encoding a Bt2 toxin-NPTII fusion protein).
Another possibility is,the fusion of two ICP genes.
Between each gene encoding an ICP which still is insecticidally
active (i.e., a toxic part of the protoxin), a gene fragment
encoding a protease (e.g.,
CA 02284255 1999-10-12
22
trypsin) - sensitive protein part should be included,
such as a gene fragment encoding a part of the N-
terminal or C-terminal amino acid sequence of one of
the ICPs which is removed or cleaved upon activation by
the midgut enzymes of the target insect species.
In a second case, the coding regions of the two
respective ICP genes can be combined in dicistronic
units placed under the control of a promoter. The
coding regions of the two ICP genes are placed after
each other with an intergenic sequence of defined
length. A single messenger RNA molecule is generated,
leading to the translation into two separate gene
products. Based on a modified scanning model (Kozak,
1987), the concept of reinitiation of translation has
been accepted provided that a termination codon in
frame with the upstream ATG precedes the downstream
ATG. Experimental data also demonstrated that the plant
translational machinery is able to synthesize several
polypeptides from a polycistronic mRNA (Angenon et al.,
1989).
II Chimeric constructs with one or more ICP genes that
are transferred to the genome of a plant already
transformed with a one or more ICP genes
Several genes can be introduced into a plant cell
during sequential transformation steps (retrans-
formation), provided that an alternative system to
select transformants is available for the second round
of transformation. This retransformation leads to the
combined expression of ICP genes which.are introduced
at multiple loci in the genome. Preferably, two
different selectable marker genes are used in the two -
consecutive transformation steps. The first marker is
used for selection of transformed cells in the first
transformation, while the second marker is used for
selection of transformants in the second round of
_ . _ _ ..-. ._._ ;
-----
- - - -- - ------- .~ .
CA 02284255 1999-10-12
23
transformation. Sequential transformation steps using
kanamycin and hygromycin have been described, for
example by Sandler et al. (1988) and Delauney et al.
(1988).
III Chimeric constructs with one or more ICP genes,
that are separately transferred to the nuclear qenome
of separate plants in independent transformation events
and are subsequently combined in a single plant genome
through crosses.
The first plant should be a plant transformed with
a first ICP gene or an Fl plant derived herefrom
through selfing (preferably an Fl plant which is
homozygous for the ICP gene). The second plant should
be a plant transformed with a second ICP gene or an Fl
plant derived herefrom through selfing (preferably an
Fl plant which is homozygous for the second ICP gene).
Selection methods can be applied to the plants obtained
from this cross in order to select those plants having
the two ICP genes present in their genome (e.g.,
Southern blotting) and expressing the two ICPs (e.g.,
separate ELISA detection of the immunologically
different ICPs). This is a useful strategy to produce
hybrid varieties from two parental lines, each
transformed with a different ICP gene, as well as to
produce inbred lines containing two different ICP genes
through crossing of two independent transformants (or
their Fl selfed offspring) from the same inbred line.
IV Chimeric constructs with one or more ICP genes
separately transferred to the genome of a single plant
in the same transformation experiment leading to the
insertion of the respective chimeric genes at multiple
loci.
Cotransformation involves the simultaneous
transformation of a plant with two different expression
vectors, one containing a first ICP gene, the second
CA 02284255 1999-10-12
24
containing a second ICP gene. Along with each ICP
gene, a different marker gene can be used, and
selection can be made with the two markers
simultaneously. Alternatively, a single marker can be
used, and a sufficiently large number of selected
plants can be screened in order to find those plants
having the two ICP genes (e.g., by Southern blotting)
and expressing the two proteins (e.g., by means of
ELISA). Cotransformation with more than one T-DNA can
be accomplished by using simultaneously two different
strains of Agrobacterium, each with a different Ti-
plasmid (Depicker et al., 1985) or with one strain of
Agrobacterium containing two T-DNAs on separate
plasmids (de Framond et al., 1986). Direct gene
transfer, using a mixture of two plasmids, can also be
employed to cotransform plant cells with a selectable
and a non-selectable gene (Schocher et al., 1986).
The transgenic plant obtained can be used in
further plant breeding schemes. The transformed plant
can be selfed to obtain a plant which is homozygous for
the inserted genes. If the plant is an inbred line,
this homozygous plant, can be used to produce seeds
directly or as a parental line for a hybrid variety.
The gene can also be crossed into open pollinated
populations or, other inbred lines of the same plant
using conventional plant breeding approaches.
Of course other plant transformation methods can
be used and are within the scope of the invention as
long as they result is a plant which expresses two or
more non-competitively binding ICPs. In this regard,
this invention is not limited -to the use of
Agrobacterium Ti-plasmids for transforming plant cells
with genes encoding non-competitively binding ICPs.
Other known methods for plant cell transformations,
such as electroporation or by the use of a vector
CA 02284255 1999-10-12
25 11288-4
system based on plant viruses or pollen, can be used for
transforming monocotyledonous and dicotyledonous plants in order
to obtain plants which express two non-competitively binding ICPs.
Furthermore, DNA sequences encoding two non-competitively binding
ICPs other than tho-se disclosed herein can be used for
transforming plants. Also, each of the ICP genes, described
herein, can be encoded by equivalent DNA sequences, taking into
consideration the degeneracy of the genetic code. Also,
equivalent ICPs with only a few aniino acids changed, such as would
be obtained through mutations in the ICP gene, can also be used,
provided they encode a protein with essentially the same
characteristics (e.g., insecticidal activity and receptor
binding).
The invention will be further described with reference
to the accompanying drawings in which:
Figure 1 shows the percentage binding of the 125I
labelled Bt2 toxin (concentration 1.05 nM) to Manduca sexta brush
border membrane vesicles as a function of the concentration of
competitor (Bt2(*), Bt3(=), Bt73(A));
Figure 2 shows the percentage binding of the 125I
labelled Bt3 toxin (concentration 0.8 nM) to Manduca sexta brush
border membrane vesicles as a function of the concentration of
competitor (Bt2(*), Bt3(o), Bt73(A));
Figure 3 shows the percentage binding of the 125I
labelled Bt73 toxin (concentration 1.05 nM) to Manduca sexta brush
border membrane vesicles as a function of the concentration of
competitor (Bt2(*), Bt3(=), Bt73(A));
--- - -------- --
CA 02284255 1999-10-12
25a 11288-4
Figure 4 shows the percentage binding of the 125I
labelled Bt2 toxin (concentration 1.05 nM) to Heliothis virescens
brush border membrane vesicles as a function of the concentration
of competitor (Bt2(*), Bt3(e), Bt73(A));
Figure 5 shows the percentage binding of the 125I
labelled Bt3 toxin (concentration 0.8 nM) to Heliothis virescens
brush border membrane vesicles as a function of the concentration
of competitor (Bt2(*), Bt3(*), Bt73(A));
Figure 6 shows the percentage binding of the 125I
labelled Bt73 toxin (concentration 1.05 nM) to Heliothis virescens
brush border membrane vesicles as a function of the concentration
of competitor (Bt2(*), Bt3(*), Bt73(A));
Figure 7 shows the binding of 125I labelled Bt2 toxin
(1.05 nM) to Pieris brassicae brush border membrane vesicles in
the presence of increasing concentration of Bt2 toxin (o) or Bt14
toxin (e);
Figure 8 shows the binding of. 125I labelled Bt14 toxin
(1.4 nM) to Pieris brassicae brush border membrane vesicles in the
presence of increasing concentration of Bt2 toxin (o) or Bt14
toxin (o);
Figure 9 shows the binding of 125I-Bt2 toxin (1.05 nlt)
to Manduca sexta brush border membrane vesicles in the presence of
increasing concentrations of Bt2 toxin (o) or Bt15 toxin (o);
Figure 10 shows the binding of 125I-Bt15 toxin (0.7 nM)
to Manduca sexta brush border membrane vesicles in the presence of
increasing concentrations of Bt2 toxin (o) or Bt15 toxin (o);
CA 02284255 2006-10-03
75749-7D
25b
Figure 11 shows the binding of 125I-Bt2 toxin (1.05 nM)
to Manduca sexta brush border membrane vesicles in the presence of
increasing concentrations of Bt2 toxin (o) or Bt18 toxin (=);
Figure 12 shows the binding of 125I-Bt18 toxin (0.7 nt-f)
to Manduca sexta brush border membrane vesicles in the presence of
increasing concentrations of Bt2 toxin (o) or Bt18 toxin (o);
Figure 13 shows the nucleotide sequence and deduced
amino acid sequence of the open reading frame of the bt4 gene
extending from nucleotide 264 to nucleotide 3761;
Figure 14 shows the nucleotide sequence and deduced
amino acid sequence of the ORF of the bt15 gene, isolated from HD-
110, extending from nucleotide 234 to nucleotide 3803;
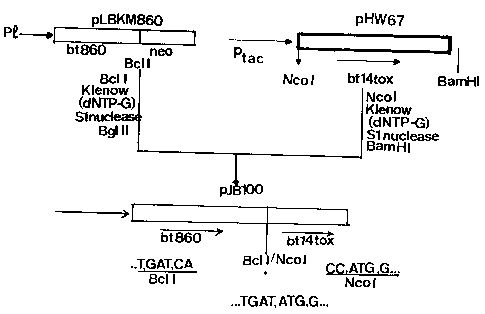
Figure 15 shows the construction of a chimeric bt15 gene
fragment under the control of the TR2' promoter in plasmid pTHW88;
Figure 16 shows the construction of a chimeric bt14 neo
hybrid gene under the control of the TR1' promoter in plasmid
pTHW94;
Figure 17 shows the construction of a hybrid bt2-btl4
gene in plasmid pJB100, consisting of a fusion of a C-terminally
truncated bt2 and bt14 gene.
Figure 18 shows the amino acid sequence of the Bt14
protein. .
The following Examples illustrate the invention. Those
skilled in the art will, however, recognize that other
combinations of two or more non-competitively binding B.
thuringiensis ICP genes can be used to transform plants in
accordance with this invention in order to prevent the
CA 02284255 1999-10-12
25c 11288-4
development, in a target insect, of resistance to B. thuringiensis
ICPs expressed in the transformed plants. Unless otherwise
indicated, all procedures for making and manipulating DNA were
carried out by the standardized procedures described in Maniatis
et al., Molecular Cloning - A Laboratory Manual, Cold Spring
Harbor Laboratory (1982).
Example 1: Collection of genes
The collection of anti-Lepidopteran and anti-Coleopteran
Bt genes encoding ICPs, which are the subject of the Examples, is
described in Table 2 (following the Examples). References for the
respective genes are indicated in Table 2. The origin, the
isolation and characterization of the Bt genes, which have not
been published, are described below. Bt
CA 02284255 1999-10-12
26
strains, such as strains HD-1, HD-68, HD-110, and
HD-73, are publicly available from the Agricultural
Research Culture Collection, Northern Regional Research
Laboratory, U.S. Dept. of Agriculture, Peoria, Illinois
61604, U.S.A.
bt3
gene: From B. thuringiensis var. kurstaki HD-i, the ICP
was cloned. Characterization of this gene revealed
an open reading frame of 3528 bp which encodes a
protoxin of 133 kDa. This gene was identical to
the one described by Schnepf et al. (1985).
bt73
gene: From B. thuringiensis var HD-73. The ICP gene was
cloned as described by Adang et al. (1985).
bt4
gene: A genomic library was prepared from total DNA of
strain B. thuringiensis aizawai HD-68. Using the
1.1 kb internal HindIII fragment of the bt2 gene
as a probe, a gene designated bt4 was isolated.
Characterization of this gene revealed an open
reading frame of 3495 bp which encodes a protoxin
of 132 kDa and a trypsin activated toxin fragment
of 60 kDa. This (insect controlling protein) gene
differs from previously identified genes and was
also found in several other strains of subspecies
aizawai and entomocidus including HD-110. Fig. 13
shows the nucleotide sequence and deduced amino
acid sequence of the open reading frame ("ORF") of
the bt4 gene extending from nucleotide 264 to
nucleotide 3761.
bt14 and bt15 -
genes: A genomic library was prepared from total DNA of
strain B. thuringiensis var. entomocidus HD-110 by
partial Sau3A digest of the total DNA and cloning
in the vector pEcoR251 (deposited at DSM under
_ ~.
CA 02284255 1999-10-12
27 11288-4
accession number 4711). Using monoclonal antibodies (Hofte et
al.,1988), at least three structurally distinct ICPs were
identified in crystals of B. thuringiensis entomocidus HD-110.
These monoclonal ant-ibodies were used to clone the three different
ICP genes from this B. thuringiensis strain. One of these genes
is the bt4 gene as described above.
The second gene was called "bt15". Figure 14 shows the
nucleotide sequence and deduced amino acid sequence of the ORF of
the bt15 gene, isolated from HD-110, extending from nucleotide 234
to nucleotide 3803. The Shine and Dalgarno sequence, preceding
the initiation codon is underlined. This gene has an open reading
frame of 3567 bp which encodes a protoxin of 135 kDa and a 63 kDa
toxin fragment. A similar gene has been described by Honee et al.
1988 (Honee, Guy; van der Salm, Theo and Visser, Bert: "Nucleotide
sequence of crystal protein gene isolated from B. thuringiensis
subspecies entomocidus 60.5 coding for a toxin highly active
against Spodoptera species"; Nucleic Acid Research 16, p.
6240(1988)), isolated from B. thuringiensis entomocidus 60.5. The
bt15 gene differs from the published sequence at three positions:
an Ala codon (GCA) is present instead of an Arg codon (CGA) at
position 925 and a consecution of a Thr-His codon (ACGCAT).is
present instead of a Thr-Asp codon (ACCGAT) at position 1400.
(The numbers of the positions are according to Honee et al.,
1988). Another similar gene has been described in EP 0295156,
isolated from B. thuringiensis aizawai 7-29 and entomocidus 6-01.
The bt15 gene is different from this published nucleotide sequence
CA 02284255 1999-10-12
27a 11288-4
at three different places 1) a Glu codon (GAA) instead of an Ala
codon (GCA) at position 700; 2) the sequence TGG, CCA, GCG, CCA
instead of TGC, CAG, CGC, CAC, CAT at position 1456 and 3) an Arg
codon (CGT) instead Qf an Ala codon (GCG) at
' :;
CA 02284255 1999-10-12
28 11288-4
position 2654. (The nuinbers of the positions are
according_to EP 0295156).
The third gene isolated was called "btl4". It has
an open reading frame of 3621 bp which encodes a
137 kDa protoxin and a 66 kDa activated toxin
fragment. A similar gene has been cloned froni
B.thuringiensis IID-2 (Brizzard and Whiteley,
1988). The bt14 gene differs from the published
nucleotide sequence by two nucleotide
subst.ltutions: a T instead of a C at position 126,
and a C instead of a T at position 448 (ttte
numbers of the positions are according to Brizzard
and Whiteley, 1988). In the first case, the Ile
codon (ATT or ATC) is conserved whereas in the
secorid case the '1'yr codon ('1'AT) is converted to a
tlis codon (CAC).
bt2
gene: The bt2 gene was cloned as described in
EP 0193259.
bt18
gene: Cloning of the bt18 gene was perforined as
described in EPO,358,557 published March 14, 1990.
bt13
gene: The btl3 qene was cloned as described in E!=
0,305,275 published March 1, 1990.
bt21 and bt22
genes: These genes, ericoding Colenoteran-active ICPs,
were cloned as described in CA 2,046,646.
EXAMPLE 2 Construction of gene cassettes and
expression of Bt genes in E.coli
1) bt2, bt18: the construction of bt2 and bt18 genP
cassett-as hae been areuiously desnribed in EP
0,193,259 published September 3, 1986 and EP 0,358,557
published March 14, 1990, respectively. Basically, the.y
eompri.se a Lruncated gene encoding Lhe Loxic core and a_:
hybrid gene comprising the
CA 02284255 1999-10-12
29 11288-4
truncated gene fused in frame to the N-terminus of the
neo gene. The gene cassettes are used to transform E.
coli to express the Bt2 and Bt18 ICP toxins.
2) bt14, bt15: as described in EP 0,358,557 published
March 14, 1990, gene cassettes for the bt14 and bt15
genes were constructed in order to express the genes in
E. coli and in plants.
First, a NcoI site was introduced at the N-terminus of
the genes by site-directed mutagenesis.
In the case of the bt15 gene, the conversion of the TT
nucleotides, immediately in front of the ATG codon, into CC
yielded a NcoI site overlapping with the ATG initiation codon.
This site was introduced using the pMa/c vectors for site-directed
mutagenesis (Stanssens et al., 1987) and a 28-mer oligonucleotide
with the following sequence:
5'-CGGAGGTATTCCATGGAGGAAAATAATC-3'.
This yielded the plasmid pVE29 carrying the N-terminal fragment of
the bt15 gene with a NcoI site at the ATG initiation codon.
According to Brizzard and Whiteley (1988), the
initiation codon of the bt14 gene is a TTG codon. Thus, a NcoI
site was created in a like manner at this codon for site directed
mutagenesis using a 34-mer oligonucleotide with the following
sequence:
5'-CCTATTTGAAGCCATGGTAACTCCTCCTTTTATG-3'.
In this case the sequence of the initiation codon was converted
from ATATTGA to ACCATGG. This yielded the plasmid pHW44 carrying
the N-terminal fragment of the bt14 gene with a NcoI site at the
CA 02284255 1999-10-12
29a 11288-4
initiation codon.
In a second step, the genes were reconstructed by
ligating the N-terminal gene fragments with a suitable C-terminal
gene fragment, yield-ing a bt15 gene and bt14 gene with a NcoI site
at the ATG initiation codon.
CA 02284255 1999-10-12
To express the bt14 and bt15 genes encoding the
protoxin in E. coli, the following constructs were
made: pOH50 containing the bt15 gene under the control
of the lac promoter; and pHW67 containing the bt14 gene
under the control of the tac promoter. Induction of a
culture of the E. coli strain WK6 carrying the
respective plasmids with IPTG yielded an overproduced
protein (Fuller, 1982).
The active toxic fragments of the Bt15 and Bt14
protoxins comprise 63 and 60 kDa trypsin digest
products respectively. Instead of expressing the whole
bt15 or bt14 gene, it is also possible to express a
toxin-encoding gene fragment or derivative thereof in
plants. To this end, truncated btl4 and btl5 gene
fragments were constructed. In order to be able to
select transgenic plants producing the ICP gene
products, hybrid genes of the truncated gene fragments
were also made with the neo gene encoding a selectable
marker as described in EP 0193259.
By comparison of the nucleotide sequence of the
bt4, bt14 and bt15 genes, respectively, with the bt2
and bt18 genes, respectively, the BclI site could be
identified as a suitable site localized downstream of
the coding sequence encoding the toxin gene fragment.
To construct a truncated gene fragment and a hybrid
gene of the truncated gene fragment with the neo gene,
the filled BclI site was ligated to the filled EcoRI
site of pLKM91 (Hofte et al., 1986) and the filled
HindIiI site of pLK94 respectively (Botterman and
Zabeau, 1987). pLIKM91 carries a 5' truncated neo gene
fragment which codes for an enzymatically active C-
terminal gene fragment of the neo gene, and pLK94
contains translation stop codons in three reading
frames. This yielded the following plasmids which are
then used to transform E. coli to express the ICP
CA 02284255 1999-10-12
31
genes: pHW71 carrying a truncated btl4-neo hybrid gene;
pHW72 carrying a truncated bt14 gene; pVE34 carrying a
truncated bt15-neo hybrid gene; and pVE35 carrying a
truncated bt15 gene.
In a similar way as described for the bt14 and
bt15 genes, gene cassettes are constructed for the bt3
and bt4 genes which are then expressed in E.coli.
EXAMPLE 3: Purification of recombinant ICPs
The ICPs expressed in E. coli in Example 2 are
purified by the method (described for recombinant Bt2
protoxin) by Hofte et al. (1986).
EXAMPLE 4: Purification of toxins
Solubilized protoxins of Bt2, Bt3, Bt73, Bt4,
Bt14, Bt15, Bt18, Bt13, Bt21 and Bt22 (in Na2CO3 50mM,
DTT 10 mM pH=10) are dialyzed against 0.5 $(NH4)2CO3 at
pH 8 and treated with trypsin (trypsin/protoxin=1/20
w/w) for 2h at 379C. The activated toxin is
chromatographically purified (Mono-Q column on FPLC) as
described by Hofmann et al.(1988b).
EXAMPLE 5: Determination of the insecticidal spectrum
The ICP protoxins and toxins of Examples 3 and 4
are evaluated for their insecticidal activity. Each
protoxin is dissolved in alkaline buffer containing a
reducing agent (Na2CO3 50 mM, DTT 10 mM pH=10), and
each toxin is used as soluble protein directly from
FPLC. Protein concentrations are determined.
Subsequently, dilutions of the resulting protoxin or
toxin solution are prepared in PBS buffer pH=7.4
containing 0.15 M NaCl and 0.1 % bovine serum albumin
("BSA") .
The artificial medium for- insect culture,
described by Bell and Joachim (1976) for Manduca sexta,
is poured in appropriate receptacles and allowed to
solidify. Subsequently a quantity of the (pro)toxin
dilutions is applied on this medium, and the water is
* Trademark
CA 02284255 1999-10-12
32
allowed to evaporate under a laminar flow. This results
in a medium with a certain quantity (in the range of
0.1 to 10000 ng/cm2) of toxin coated on its surface.
For example, for the Bt2 toxin, typical dilutions for a
toxicity test_on Manduca sexta are 1, 5, 25, 125 and
625 ng/cm2. First instar larvae of Manduca sexta are
then applied on the coated medium, and growth and
mortality are assessed after 6 days. Mortality
increases with dosage. Dose response data is analysed
in probit analysis (Finney, 1962), and the data are
best summarized by an LD50 value which is the amount of
toxin which kills 50 % of the insects. The LDSO for Bt2
toxin against Manduca sexta is around 20 ng/cm2.
Similar assays are carried out for other insect
species using a suitable diet or by applying the ICPs
on leaves for insects, for which no artificial diet is
used.
EXAMPLE 6: Binding studies
Toxins
All protoxins and their toxic fragments were
purified according to the methods described for the Bt2
protoxin and toxin in H6fte et al. (1986) and EP
0193259. The activated and purified toxins are further
referred to as the Bt2, Bt3, Bt73, Bt4, Bt14, Bt15,
Bt18, Bt13, Bt21 and Bt22 toxins.
By way of example for the Bt73 toxin, it has been
shown that B. thuringiensis var. kurstaki HD73 produces
a protein of 133 kDa encoded by a 6.6 kb type gene. A
culture of this strain was grown as described by
Mahillon and Delcour (1984). The autolysed culture was
spun down (20 minutes at 4500 rpm in a HB4 rotor) and
washed with a buffer containing 20 mM Tris, 100 mM NaCl
and 0.05 % Triton X-100; pH S. The final pellet was
resuspended in this buffer (4 ml buffer for 100 ml
culture). This solution was then layered onto a linear
* Trademark
- -- - - ----------
CA 02284255 1999-10-12
33
Urograff in*gradient (60-70%) which was centrifuged in a
SW 28 rotor for 90 minutes at 18000 rpm. Crystals were
collected and stored at -20' C until further use.
Activation was performed according to Hofte et al.
(1986). The _purified toxin is further referred to as
the Bt73 toxin.
Iodination of ICPs
Iodination of Bt2, Bt3, and Bt73 toxins was
performed using the Chloramin-T method (Hunter and
Greenwood, 1962). 1 mCi 125I-NaI and 20 to 37.5 ug
Chloramin-T in NaC1/P; were added to 50 ug of purified
toxin. After gentle shaking for 60 seconds, the
reaction was stopped by adding 53 ug of potassium
metabisulfite in H20. The whole mixture was loaded on
a PD 10 Sephadex*G-25M gelfiltration column to remove
free iodine. A subsequent run on a Biogel* P-60 column
was carried out in order to increase the purity.
Alternatively, toxins were labeled using the
Iodogen* method. Iodogen (Pierce) was dissolved in
chloroform at 0.1 mg/ml. 100 ul of this solution was
pipetted into a disposable glass vessel and dried under
a stream of nitrogen gas. The vessel was rinsed with
Tris buffer (20 mM Tris, pH 8.65,with 0.15 M NaCl). 50
ug of toxin (in Tris buffer) was incubated with 1 mCi
of 125I-NaI in the tube for 10 minutes. The reaction
was then stopped by the addition of 1 M NaI ( one
fourth of the sample volume) . The . sample was
immediately loaded onto a PD10 Sephadex* G-25M column
and later on a Biogel*P-60 column to remove free iodine
and possible degradation products.
Other toxins were iodinated using one of the above
mentioned procedures.
Determination of specific activity of iodinated toxin
Specific activity of iodinated Bt2, Bt3, and Bt73
toxin samples was determined using a "sandwich" ELISA
* Trademark
CA 02284255 1999-10-12
34
technique according to Voller, Bidwell and Barlett
(1976). Primary antibody was a polyclonal antiserum
raised against Bt2 toxin, and the secondary antibody
was a monoclonal antibody 4D6.
The conjugate used was alkaline phosphatase
coupled to anti-mouse IgG. The reaction intensity of a
standard dilution series of unlabeled toxin and
dilutions of the iodinated toxin sample (in NaCl/P; -
0.1 ~ BSA) was measured. Linear regression
calculations yielded the protein content of the
radioactive toxin sample. The samples with the highest
specific activities were used in the binding assays.
Specific activities were 59400, 33000 and 19800 Ci/mole
(on reference date) for Bt73 toxin (labeled according
to lodogen procedure), Bt2 toxin (Chloramin-T method)
and Bt3 toxin (Iodogen*method) respectively.
Specific activities of other toxins were
determined using a similar approach. Specific
monoclonal and polyclonal antibodies for each of these
toxins were raised and applied in ELISA.
Preparation of brush border membrane vesicles
Brush border membrane vesicles ("BBMV") from
Manduca sexta, Heliothis virescens, Plutella
xylostella, Phthorimaea operculella, Spodoptera exigua,
Spodoptera littoralis, Plodia interpunctella, Mamestra
brassicae, Pieris brassicae and Leptinotarsa
decemlineata were prepared according to the method of
Wolfersberger et al. (1987). This is a differential
centrifugation method that makes use of the higher
density of negative electrostatic charges on luminal
than -on basolateral membranes to separate these
fractions.
Binding assay
Duplicate samples of 12SI-labeled toxin, either
alone or in combination with varying amounts of
* Trademark
CA 02284255 1999-10-12
unlabeled toxin, were incubated at the appropriate
temperature with brush border membrane vesicles in a
total volume of 100 ul of Tris buffer (Tris 10 mM, 150
mM NaCl, pH 7.4). All buffers contained 0.1 t BSA. The
incubation temperature was 20 C. Ultrafiltration
through Whatmart GF/F glass fiber filters was used to
separate bound from free toxin. Each filter was rapidly
washed with 5 ml of ice-cold biuffer (NaCl/P;- 0.1 t
BSA). The radioactivity of the filter was measured in a
gammacounter (1275 Minigamma, LKB). Binding data were
analyzed using the LIGAND* computer program. This
program calculates the bound concentration of ligand as
a function of the total concentration of ligand, given
the affinity (Ka or its inverse Kd = 1/Ka, the
dissociation constant) and the total concentration of
receptors or binding site concentration (Rt).
Determination of protein concentration
Protein concentrations of purified Bt2, Bt3, Bt73
and Bt15 toxins were calculated from the OD at 280 nm
*
(measured with a Uvikon 810 P, Kontron Instruments
spectrofotometer). The protein content of solutions of
other toxins and of brush border membrane vesicles
(BBMV) as measured according to Bradford (1976).
Binding of Bt2, Bt3 and Bt73 toxins to BBMV of Manduca
sexta and Heliothis virescens: an example of 3
competitively binding Lepidopteran ICPs.
Bt2, Bt3 and Bt73 toxins are toxic to.both Manduca
sexta and Heliothis virescens: LC50 values for Manduca
sexta are respectively 17.70, 20.20 and 9.00 ng/cm2 ;
for Heliothis virescens the LC50's are 7.16, 90.00 and
- 1.60 ng/cm2.
Labelled toxin, either Bt3 (0.8 nM) or Bt2 (1.05
nM) or Bt73 (1.05 nM), was incubated with BBMV in a
volume of 0.1 ml. BBMV protein concentrations were 100
ug/ml for M. sexta and for Bt2-H. virescens, for Bt3-H.
* Trademark
CA 02284255 1999-10-12
36
virescens 150 and for Bt73-H. virescens 50 ug/mi. The
labelled toxin was combined with varying amounts of an
unlabeled toxin (competitor). After a 30 min.
incubation, bound and free toxins were separated
through filtration.
Figs. 1-3 show the percentages binding of
respectively labelled Bt2, Bt3 and Bt73 toxins as a
function of the concentration of competitor for Manduca
sexta. Figs. 4-6 show these data for Heliothis
virescens. The amount bound in the absence of
competitor is always taken as 100 % binding. Figs. 1-6
show the binding of 125I-labeled toxins to M. sexta (in
Figs. 1, 2 and 3) and H. virescens (in Figs. 4, 5 and
6) brush border membrane vesicles. Vesicles were
incubated with labeled toxin [in Figs. 1 and 4: 125I-
Bt2-toxin (1.05nM) ; in Figs. 2 and 5: 125I-Bt3-toxin
(0.8nM) ; in Figs. 3 and 6: 125I-Bt73-toxin (1.05nM) ] in
the presence of increasing concentrations of Bt2 toxin
(*), Bt3 toxin (9) or Bt73 toxin (A). Binding is
expressed as percentage of the amount bound upon
incubation with labeled toxin alone. On M. sexta
vesicles, these amounts were 1820, 601 and 2383 cpm,
and on H. virescens vesicles 1775, 472 and 6608 cpm for
125I-Bt2-, Bt3- and Bt73-toxin, respectively. Non-
specific binding was not substracted. Data were
analyzed with the LIGAND computer program. Each point
is the mean of a duplicate sample.
Figure 1: shows the binding of 1251 Bt2 toxin to
M. sexta BBMV
Figure 2: shows the binding of 1251 Bt3 toxin to
M. sexta BBMV
Figure 3: shows the binding of 125I Bt73 toxin to
M. sexta BBMV
Figure 4: shows the binding of 1251 Bt2 toxin to
H. virescens BBMV
CA 02284255 1999-10-12
37
Figure 5: shows the binding of 125I Bt3 toxin to
H.virescens BBMV
Figure 6: shows the binding of 125I Bt73 toxin to
H.virescens BBMV
The concl'iasions from Figures 1-6 are that Bt2 and
Bt3, Bt3 and Bt73, and Bt2 and Bt73 are competitively-
binding ICP's both for Manduca sexta and for Heliothis
virescens. Indeed Bt3 competes for the entire
population of receptor sites of Bt2 in Manduca sexta
(Fig.l): the % labelled Bt2 bound in the presence of
100 nM Bt3 is equal to the % Bt2 bound with 100 riM of
Bt2 itself. The opposite is not true: in the presence
of 100 nM Bt2 the % of labelled Bt3 is not reduced to
the same level as with 100 nM of Bt3 (Fig.2).
A similar reasoning is followed to observe
competitivity of other toxin combinations . Bt3
competes for the entire population of receptor sites of
Bt73 (Fig. 3) in M. sexta; the opposite is not true
(Fig. 2); Bt2 aA d Bt73 compete for the entire
population of each other's binding sites in M. sexta
(Figs. 1 and 3).
In Heliothis virescens . Bt2 competes for the
entire population of receptor sites of Bt3 (Fig. 5);
Bt73 competes for the entire population of receptor
sites of Bt3 (Fig. 5); Bt73 competes for the entire
population of receptor sites of Bt2 (Fig. 4); but the
opposite statements are not true (Figs. 4, 5 and 6).
The same data can be used in mathematical analysis
(e.g., Scatchard analysis according to Scatchard, 1949;
analysis with the LIGAND computer program according to
Munson and Rodbard, 1980) to calculate the dissociation
constant (Kd) of the toxin-receptor complex and the
concentration of binding sites (Rt); the results of
these calculations using the LIGAND computer program
were the following:
CA 02284255 1999-10-12
38
Bt2-M.sexta: Kd=0.4 nM Rt=3.4 pmol/mg vesicle
protein
Bt3-M. sexta: Kd=1.5 nM Rt=9.8 pmol/mg vesicle
protein
Bt73-M. sexta:_ Kd=0.6 nM Rt=4.0 pmol/mg vesicle
protein
Bt2-H. virescens: Kd=0.6 nM Rt=9.7 pmol/mg vesicle
protein
Bt3-H. virescens: Kd=1.2 nM Rt=3.7 pmol/mg vesicle
protein
Bt73-H. virescens: Kd=0.8 nM Rt=19.5 pmol/mg vesicle
protein
These data demonstrate the high affinity receptor
binding of the toxins (Kds in the range of 10-10 to 10"9
M.
Binding of Bt2 and Bt14 toxins to BBMV of P. brassicae,
Plutella xylostella and Phthorimaea opercullella: an
example two non-competitively binding Lepidopteran ICPs
Bt2 and Bt14 toxins are toxic to P. brassicae
(p.b.), P. xylostella (p.x.) and P. operculella (p.o.)
as seen from the table below.
LC50 of Toxins
Bt2 Bt14
P.b. 1.3 2.0
P.X. 6.7 5.4
P.O. 4.20 0.8-4.0
LC50 values of solubilized purified Bt2 and Bt14 toxins
for P.x. are expressed as ng protein spotted per cm2 of
artificial diet. LC50 values for P.b. are expressed as
ug2 toxin per ml solution into which leaf discs, fed to
first instar Pb larvae, were dipped. For P.o., LC50
values are expressed in ug/ml into which potato chips
were dipped prior to feeding.
Labelled Bt2 toxin (1.05 nM) or Bt14 toxin (1.4
nM) was incubated with BBMV from P. brassicae (100 ug
CA 02284255 1999-10-12
39
protein/ml) in a volume of 0.1 ml in combination with
varying amounts of unlabelled Bt2 or Bt14. After a 30
min. incubation period at 22 C, the bound and free
toxins were separated.
Figures 7 and 8 show the binding of 125I-labeled
toxins to P. brassicae brush border membrane vesicles.
Vesicles were incubated with labeled toxin [in Fig. 7:
"5I-Bt2-toxin (1.05nM); in Fig. 8: 12SI-Bt14-toxin
(1.4nM)J in the presence of increasing concentrations
of Bt2 toxin (o) or Bt14 toxin (o). Binding is
expressed as percentage of the amount bound upon
incubation with labeled toxin alone. Non-specific
binding was not substracted. Data were analyzed with
the LIGANV-computer program. Each point is the mean of
a duplicate sample. Figure 7 shows the binding of
labelled Bt2 toxin to P. brassicae BBMV, and Figure 8
shows the binding of labelled Bt14 toxin to P.
brassicae BBMV.
The competition data demonstrate the presence of
high affinity binding sites both for Bt2 and Bt14, as
well as the almost complete absence of competition of
Bt14 for the Bt2 binding sites and of Bt14 for the Bt2
binding sites. This demonstrates that Bt2 and Bt14 are
non-competitively binding toxins. Hence they are useful
to prevent the development of Pieris brassicae
resistance against B. thuringiensis ICP's expressed in
Brassica sp.
Calculated Kd and Rt values were from these experiments
were:
Bt2: Kd=2.8 nM, Rt=12.9 pmol/mg vesicle protein
Bt14: Kd=8.4 nM, Rt=21.4 pmol/mg vesicle protein.
Binding of Bt2 and Bt15 toxins to BBMV of M.sexta,
M.brassicae, P. xylostella and P.interpunctella an
example of two non-competitively binding Lepidopteran
ICPs
* Trademark
------------- - ---
CA 02284255 1999-10-12
Bt2 and Bt15 toxins are both toxic to M.sexta
(LC50's of 20 and 111 ng/cm2, respectively). They also
show activity against M. brassicae, P. xylostella and
P. interpunctella.
Labelled $t2 (1.05 nM) or Bt15 (0.7 nM) was
incubated with BBMV from M.sexta (100 ug protein/ ml)
in a volume of 0.1 ml in combination with varying
amounts of unlabelled Bt2 or Bt15. After a 30 min.
incubation period at 22 C, the bound and free toxins
were separated.
Figs. 9-10 show the binding of 125I-labeled toxins
to M. sexta brush border membrane vesicles. Vesicles
were incubated with labeled toxin [in Fig. 9: 125I-Bt2-
toxin (1.05nM); in Fig. 10: 125I-Bt15-toxin (0.7nM)) in
the presence of increasing concentrations of Bt2-toxin
(o) or Bt15-toxin (9). Binding is expressed as
percentage of the amount bound upon incubation with
labeled toxin alone. Non-specific binding was not
substracted. Data were analyzed with the LIGAND
computer program. Each point is the mean of a duplicate
sample. Figure 9 shows the data for binding of labelled
Bt2, and Figure 10 shows the binding of labelled Bt15.
The competition data demonstrate the presence of
high affinity binding sites for both Bt2 and Bt15, as
well as the complete absence of competition of Bt15 for
the Bt2 binding sites and of Bt2 for the Bt15 binding
_ sites. This demonstrates that Bt2 and Bt15 are non-
competitively binding toxins. Hence the combination of
Bt2 and Btl5 is useful to prevent the development of
resistance of M.sexta against B. thuringiensis ICP's
expressed in tobacco or other crops in which Manduca
sp. are a pest. Calculated Kd and Rt values are:
Bt2: Kd=0.4 nM, Rt=3.4 pmol/mg vesicle protein
CA 02284255 1999-10-12
41
Bt15: Kd = 0.3 nM Kd2=2.9 nM, Rtl= 5.9 and Rt2=6.7
pmol/mg vesicle protein (2 distinct high affinity
receptor sites are present).
Similar studies were performed for M. brassicae,
S. littoralis-and P. interpunctella. Although LD50, Kd
and Rt values differed substantially, the essential
observation that Bt2 and Bt15 are both toxic and are
non-competitively binding toxins was confirmed in these
three insect species. Thus, it is also a useful toxin
combination to prevent resistance of M. brassicae to
ICP's or to prevent resistance of Spodoptera species
against ICP's expressed in any of the crop plants in
which Spodoptera species are a pest.
Binding of Bt2 and Bt4 toxins to BBMV of M. sexta: an
example of two non-competitively binding Lepidopteran
ICPs
Both Bt2 and Bt4 toxins are toxic to Manduca
sexta. LD50 values are 20 and 5.4. ng/cm2,
respectively. No mutual competition of Bt2 for binding
of labelled Bt4 and of Bt4 for binding of labelled Bt2
was observed, demonstrating that Bt2 and Bt4 are non-
competitively binding toxins.
Binding of Bt15 and Bt18 toxins to BBMV of S.
littoralis: an example of two non-competitively binding
Lepidopteran ICPs
Both Bt15 and BtlB toxins are toxic to S.
littoralis. LD 50 values are 93 and 88 ng toxin/cm2,
respectively. Labelled Bt15 (0.7 nM) or Bt18 (0.9 nM)
was incubated with 100 ug of vesicle protein from S.
littoralis in combination with varying amounts of
unlabelled Bt15 or Bt18 toxin. After a 45 min. -
incubation period, bound and free toxins were
separated. Binding data demonstrate high affinity
binding for both Bt15 and Bt18 to S. littoralis BBMV.
As seen from Figures 11 and 12, the entire population
CA 02284255 1999-10-12
42
of receptor sites of Bt15 was not saturable with Bt18,
nor was the entire population of receptor sites of Bt18
saturable with Bt15.
Binding of Bt13 and Bt22 toxins to BBMV of L.
decemlineata : an example of two non-competitively
binding Coleopteran ICPs.
Both Bt13 and Bt22 toxins are toxic to L.
decemlineata. LD 50 values are 0.8 and 1.1 ug toxin/ml
respectively. Labelled Bt13 11 nM) or Bt22 (0.7 nM) was
incubated with 100 ug of vesicle protein/ml from S.
littoralis in combination with varying amounts of
unlabelled Bt13 or Bt22 toxin. After a 45 min.
incubation period, bound and free toxins were
separated. Binding data demonstrate high affinity
binding for both Bt13 and Bt22 to S. littoralis BBMV.
The entire population of receptor sites of Bt13 was not
saturable with Bt22. Nor was the entire population of
receptor sites of Bt22 saturable with Btl3.
Binding of Bt2 and Bt18 toxins to BBMV of M. sexta: an
example of two non-competitively binding Lepidopteran
ICPs.
Both Bt2 and Bt18 toxins are toxic to M. sexta,
and LD 50 values are 20 to 73 ng toxin/cm2
respectively. Labelled Bt2 (1.05nM) or Bt18 (0.7nM) was
incubated with 100 ug/ml of vesicle protein from M.
sexta in combination with varying amounts of unlabelled
Bt2 or Bt18 toxin. After a 45 min. incubation period,
bound and free toxins were separated. Binding data
(Figs. 11-12) demonstrate high affinity binding for
both Bt2 and Bt18 to M. sexta BBMV. The entire
population of receptor sites of Bt2 was not saturable
with Bt18. Nor was the entire population of receptor
sites of Bt18 saturable with Bt2. Calculated Kd and Rt
values are:
Bt2: Kd= 0.4 nM, Rt= 3.4 pmol/mg vesicle protein.
CA 02284255 1999-10-12
43
Bt18: Kdl= 0.04 nM, Rtl= 2.2 pmoles/mg vesicle protein
and Kd2= 168nM Rt2= 194 pmoles/mg vesicle protein (2
distinct receptor sites for Bt18 are present).
A list of non-competitively binding anti-
Lepidopteran ICP combinations and anti-Coleopteran ICP
combinations is given below, together with their common
target insect species in which non-competitivity has
been demonstrated:
Bt2-Bt15 (Manduca sexta, Plutella xylostella,
Pieris brassicae, Mamestra brassicae, Plodia
interpunctella)
Bt2-Bt18 (Manduca sexta, Spodoptera littoralis)
Bt2-Bt14 (Pieris brassicae, Plutella xylostella,
Phthorimaea operculella)
Bt2-Bt4 (Manduca sexta)
Bt15-Bt18 (Manduca sexta, Spodoptera littoralis)
Bt14-Bt15 (Pieris brassicae)
Bt15-Bt4 (Manduca sexta, Spodoptera exigua)
Bt18-Bt4 (Manduca sexta, Spodoptera littoralis)
Bt18-Bt14 (Pieris brassicae)
Bt18-Bt4 (Manduca sexta)
Bt13-Bt21 (Leptinotarsa decemlineata)
Bt13-Bt22 (Leptinotarsa decemlineata)
Bt21-Bt22 (Leptinotarsa decemlineata)
Of course, this list of specific non-competitively
binding ICP combinations for specific target insect
pests is not exhaustive, and it is believed that other
such ICP combinations, including combinations for yet-
to-be discovered ICPs, will be found using a similar
approach for any target insect species. Likewise, the
foregoing list of target insect pests- also is not
exhaustive, and it is believed that other target
insects pests (as well as the plants that are to be
transformed to prevent their attack by such pests),
against which the specific combinations of ICPs can be
CA 02284255 1999-10-12
44
used (e.g., the combination of the Bt2 and Bt14 ICPs in
Brassica to prevent resistance of Pieris brassicae
against the ICPs expressed in the plant), will be found
using a similar approach.
EXAMPLE 7: Selection for resistance of Manduca sexta
(tobacco hornworm)
A selection experiment involves exposing a large
number of larvae to a concentration of a toxin in a
diet killing (e.g., 50-90 %) of the larvae. The
surviving larvae are again exposed to toxin
concentrations killing a similar proportion of the
larvae, and this process is continued for several
generations. The sensitivity of the larvae to the toxin
is investigated after each four generations of
selection.
Selections for 20 generations of M. sexta were
performed with Bt2 toxin alone, with Btl8 toxin alone
and with a 1/4 (by weight) Bt2/Bt18 mixture. LC50
values of the reference strain for Bt2, Bt18 and the
1/4 Bt2/Btl8 mixture respectively were the following :
20 ng/cm2, 73 ng/cm2 and 62 ng/cm2 of diet.
Selection was initiated at concentrations killing
around 75 -t of the larvae. After 4 generations of
selection, survival increased in both the Bt2 and the
Bt18 selection to around 70 %, no such increase was
observed in the selection with the combination of Bt2
and Bt18. Dosages were again increased to calculated
LC75 values. This was repeated every 4 generations. The
selection process was thus continued to the 20th
generation. Final results were the following (LC50 of
the 20th generation): -
- Bt2 selection: LC50 was 6400 ug/g (320
times decreased sensitivity)
- Bt18 selection: LC50 was 15100 ug/g (207
times decreased sensitivity)
CA 02284255 1999-10-12
- Bt2/Btl8 selection: LC50 was 181 ug/g (3 times
decreased sensitivity).
Thus the decrease in sensitivity was about 100 times
slower in the combined selection experiment.
Receptor -binding in the three selected M. sexta
strains was investigated with Bt2 and Bt18 and compared
to those of the reference M. sexta strain (non-selected
strain). Binding characteristics of the reference
strain for the Bt2 and BT18 toxins were:
Bt2: Kd = 0.4 nM, Rt=3.4 pmol/mg vesicle protein
Bt18: Kdl=0.04 nM, Rtl=2.2 pmoles/mg vesicle protein
and Kd2=168nM, Rt2=194 pmoles/mg vesicle protein (2
distinct receptor sites for Bt18 are present).
Figures 11 and 12 show the binding of 'ZSI-labeled
toxins to M. sexta brush border membrane vesicle.
Vesicles were incubated with labeled toxin [in Fig. 11:
"SI-Bt2-toxin (1.05nM) ; in Fig. 12: 125I-Bt18-toxin
(0.7nM) ] in the presence of increasing concentrations
of Bt2-toxin (o) or Bt18-toxin (e). Binding is
expressed as percentage of the amount bound upon
incubation with labeled toxin alone. Non-specific
binding was not substracted. Data were analyzed with
the LIGAND computer program. Each point is the mean of
a duplicate sample.
The Bt2 selected strain showed no detectable high
affinity binding of Bt2 whereas its Bt18 binding
characteristics remained close to the reference strain.
(Btl8: Kdl=0.03 nM, Rtl=2.8 pmoles/mg vesicle protein
and Kd2=199nM, Rt2=109 pmoles/mg vesicle protein; 2
distinct receptor sites for Bt18 are still present).
The Bt18 selected strain lost the high affinity
receptor site for Bt18. The lower affinity site for
Bt18 was still present in lower concentration than in
the reference strain (Kd=189 nM, Rt=43 nM). Bt2 binding
site concentration increased markedly compared to the
CA 02284255 1999-10-12
46
reference strain (Kd=0.4 nM, Rt=20.8 pmoles/mg vesicle
protein). This strain had a Bt2 sensitivity of LC50=4
ng/cm2. Thus, its sensitivity fo-- Bt2 had increased as
compared to the reference strain (LC50=20 ng/cm2).
The Bt2/Bt18 selected strain showed a slight but
statistically non-significant decrease in Bt18 binding
site concentration. (Bt2 : Kd = 0.4 nM, Rt=3.4 pmol/mg
vesicle protein, Bt18 : Kdl=0.04 nM, Rtl=1.0 pmoles/mg
vesicle protein and Kd2=168nM, Rt2=194 pmoles/mg
vesicle protein; 2 distinct receptor sites for Bt18 are
present). These data demonstrate that, in the. two
selection lines where resistance occurred, the
mechanism was situated at the receptor level. Changes
in receptor site are shown to be the most likely
mechanism of resistance to B. thuringiensis ICPs.
EXAMPLE 8: Mechanism of resistance of the diamondback
moth to the microbial insecticide Bacillus
thuringiensis.
The mechanism of development of insect resistance
to ICPs has been investigated in a P. xylostella strain
("PxR"). This insect strain has developed a high level
of resistance in the field against Dipel': Crystals of
Dipel preparations contain a mixture of ICPs such as
Bt3, Bt2 and Bt73 ICPs; in Example 6, it has been shown
that these toxins are competitively binding ICPs.
Resistance to Dipel*was confirmed by the toxicity
data for the sensitive strain ("PxS") and for the
Dipel-resistant strain ("PxR"). High levels of
resistance are also observed for the Bt2 protoxin and
toxin as shown in the following table :
LC50 of Strains
PxS PxR
Bt2 6.7 > 1350.
Bt15 132.6 120.4
* Trademark =
CA 02284255 1999-10-12
47
LC50 data are expressed as ng protein spotted per cm2 of
artificial diet.
However, insect toxicity data show that there is
no resistance to the Bt15 protoxin and Bt15 toxin; this
ICP is not pr~sent in Dipel* crystals. To investigate
whether a change in toxin-membrane binding was
responsible for resistance, receptor binding studies
were performed with 125I-labeled Bt2 toxin and Bt15
toxin, with BBMV derived from larvae midguts of the PxR
and PxS strains. The results are summarized in Table
1, below.
Table 1. Binding characteristics of Bt2 and Bt15 toxins
to brush border membrane vesicles from sensitive and
resistant P. xylostella.
ICP strain Kd (nM) Rt (pmol/
mg protein)
Bt2 toxin PxS 8.1 1.6
PxR no binding detectable
Bt15 toxin PxS 1.9 4.2
PxR 3.7 5.8
Table 1 shows that there was high-affinity saturable
binding of the Bt2 toxin to midgut membranes of the PxS
strain, but the PxR strain showed no detectable level
of Bt2 toxin binding. With the Bt15 toxin, there was
significant binding to BBMW of both the PxR and PxS
strains, and values are not significantly different for
the two strains.
These data show that resistance in P. xylostella
is due to an alteration in toxin-membrane binding.
Resistance to the Bt2 toxin and the sensitivity toward
the Bt15 toxin of the PxR strain is reflected by the
binding characteristics shown in Table 1.
Hence, when different non-competitively binding
ICPs (i.e., Bt2 and Bt15) are available with activity
against the same insect species (e.g., P. xylostella)
,
* Trademark
CA 02284255 1999-10-12
48
resistance to one ICP(Bt2) does not imply resistance
against other ICPs (such as Bt15). Thus, ICPs with
different binding properties can be used in combination
to delay development of insect resistance to ICPs.
EXAMPLE 9: Separate transfer of two ICP genes within
individual transcriptional units to the genome of plant
cells
Two procedures are envisaged for obtaining the
combined expression of*two ICP genes, such as the bt2
and btl5 genes in transgenic plants, such as tomato
plants. These procedures are based on the transfer of
two chimeric ICP genes, not linked within the same DNA
fragment, to the genome of a plant of interest.
A first procedure is based on sequential
transformation steps in which a plant, already
transformed with a first chimeric ICP gene, is
retransformed in order to introduce a second ICP gene.
The sequential transformation makes use of two
different selectable marker genes, stch as the
resistance genes for kanamycin ("km") and
phosphinotricin acetyl transferase ("PPT"), which
confers resistance to phoshinotricin. The use of both
these selectable markers has been described in De Block
et al. (1987).
The second procedure is based on the
cotransformation of two chimeric ICP genes on different
plasmids in a single step. The integration of both ICP
genes can be selected by making use of the two
selectable markers conferring resistance to KYn and PPT,
linked with the respective ICP genes.
For either procedure, a Ti-plasmid vector is- used
for Agrobacterium-mediated transformation of each
chimeric ICP gene into plant cells.
Plasmid pGSH163, described in EP 0193259, contains
the following chimeric genes between the T-DNA border
CA 02284255 1999-10-12
49 11288-4
repeats: a gene fragment encoding the toxin part of the bt2 gene
under the control of the TR2' promoter and the neo gene under
control of the TR1' promoter. The 3' ends of the T-DNA gene 7 and
octopine synthase respectively provide information for the 3' end
formation of transcripts.
A chimeric bt15 gene containing a gene fragment encoding
the toxin of the Bt15 ICP under the control of the TR2' promoter,
was constructed in the following way (Figure 15). pOH50 consists
of pUC18 with the whole bt15 gene under the control of the lac
promoter. A HindIII-BglII fragment was cloned in pMa5-8 yielding
pJB3. By site-directed mutagenesis, a NcoI site was created at
the initiation codon to yield pVE29. A fragment containing the
truncated gene fragment of the bt15 gene, with a translational
stop codon, was obtained by isolation of BclI-ClaI from pOH50 and
cloning in pLK91, yielding pHW38. The whole toxin gene fragment
was reconstructed under the control of the tac promoter, yielding
pVE35, by ligation of a ClaI-PstI fragment from pHW38, a NcoI-
ClaI fragment from pVE29 and a NcoI-PstI fragment from pOH48. A
truncated bt15 gene fragment with a NcoI site at the initiation
codon was obtained from pVE35 as a 1980 NcoI-BamHI fragment and
cloned in pGSJ141, digested with ClaI and BamHI. pGSJ141 has been
described in EP 0,305,275 filed August 16, 1988. Ligation of the
filled C1aI site to the filled NcoI site yielded a chimeric TR2'
- truncated bt15 - 3'g7 construct (pTVE47). As a selectable
marker in this plasmid, the bar gene encoding phosphinothricin
acetyl transferase and conferring resistance to PPT was used. A
CA 02284255 1999-10-12
49a 11288-4
chimeric bar gene containing the bar gene under the control of the
35S promoter and followed by the 3' end of the octopine synthase
was introduced in pTVE47. From pDE110,a 35S-bar-3'ocs fragment
was obtained as a
CA 02284255 1999-10-12
StuI-HindIiI fragment and was cloned in pTVE47 digested
with PstI and HindIII. This yielded the plasmid pTHW88
(Figure 15) which contains the truncated btl5 gene
under the control of the TR2' promoter and the bar gene
under the control of the 35S promoter between the T-DNA
border repeats. Plasmid pGSH163 is cointegration type
Ti-plasmid vector, whereas pTHW88 is a binary type Ti-
plasmid vector as described in EPA 0193259.
Both plasmids were mobilized in the A. tumefaciens
strain C58C1Rif (pGV2260) according to Deblaere et al.
(1988). In the sequential transformation procedure,
tomato was transformed according to De Block et al.
(1987) with the A. tumefaciens strain C58C1Rif carrying
pGS1163 resulting from the cointegration of pGSH163 and
pGV2260. Individual transformants were selected for
kanamycin resistance, and regenerated plants were
characterized for expression of the truncated bt2 gene
according to Vaeck et al. (1987). One representative
transformant was subsequently retransformed with the A.
tumefaciens strain C58C1Rif (pGV2260 and pTHW88), and
transformants were selected for PPT resistance. Using
this cotransformation procedure, the respective
Agrobacteria strains, carrying the cointegrate vector
pGS1163 and the binary vector pTHW88, were used for
transformation of tomato. Individual plants were
selected for resistance to Km and PPT.
Schematically shown in Fig. 15 are:
a) construction of pVE29: bt15 N-terminal gene
fragment with NcoI site
introduced at ATG
initiation codon. .
b) construction of pVE35: bt15 C-terminal truncated
gene fragment under
control of the tac
promoter.
CA 02284255 1999-10-12
51
c) construction of pTHW88: binary T-DNA vector with a
chimeric bt15 gene and a
chimeric bar gene within
the T-DNA border repeats.
In both cases, co-expression of the two ICP
genes in the individual transformants was evaluated
by insect toxicity tests as described in EP 0193259
and by biochemical means. Specific RNA probes allowed
the quantitive analysis of the transcript levels;
monoclonal antibodies cross-reacting with the
respective gene products allowed the quantitative
analysis of the respective gene products in ELISA
tests (EP 0193259); and specific DNA probes allowed
the characterization of the genomic integrations of
the bt2 and bt15 genes in the transformants. It was
found that the transformed tomato plants
simultaneously expressed both the bt2 gene (8.1
ng/mg) and the bt15 gene (7.6 ng/mg) as measured by
ELISA, which would prevent or delay development of
resistance of M. sexta to the insecticidal effects of
the Bt2 and Btl5 toxins, being expressed.
These procedures also could be applied when one
or both ICP genes are part of a hybrid gene. For
example, the same strategy as described above could
be followed with the plasmid vectors pGSH152,
containing a chimeric truncated bt2-neo hybrid gene
under control of the TR2' promoter, and pTHW88 in
suitable Agrobacterium strains.
EXAMPLE 10: Separate transfer of two ICP genes to the
nuclear genome of separate plants in independent
transformation events and subsequent combination in a
single plant through crossing.
Tobacco plants have been transformed with either
the bt18 gene or the btl5 gene by applying the same
cloning strategies as described in EP 0358557 and EP
CA 02284255 1999-10-12
52
193259, respectively. For both genes, the plants were
transformed with plant expression vectors containing
either the truncated btl8 or bt15 gene, which just
encode the Bt18 or Bt15 toxin, respectively.
The mortality rate of Spodoptera littoralis
larvae feeding on the transformed plants is
significantly higher than the mortality rate of
larvae fed on untransformed plants.
The bt18-transformed plant, which is homozygous
for the bt18 gene, is then crossed with the bt15 -
transformed plant, which is homozygous for the bt15
gene. After selfing, a plant homozygous for both
genes is obtained.
The resulting tobacco plants, expressing both
the bt18 and bt15 genes, delay significantly
development of resistance by S. littoralis to either
the Bt18 or Bt15 toxin expressed by the plants.
EXAMPLE 11: Transfer of two chimeric ICP genes linked
within the same DNA to the genome of plant cells
The strategy used is based on the organization
of two independent chimeric ICP genes between the T-
DNA border repeats of a single vector. Binding
studies indicated that the Bt2 and Bt14 toxins are
two non-competitively binding ICPs with insecticidal
activity towards Pieris brassicae. For expression in
plants, both the bt2 and bt14 genes can be co-
expressed to prevent insect resistance development.
For the design of a plasmid vector with each ICP gene
under the control of a separate promoter, two
possibilities can be envisaged: 1) three chimeric
constructs carrying the truncated bt2 and bt14 genes
and a selectable marker, respectively; or 2) a hybrid
of a truncated gene fragment (bt2 or bt14) and the
neo gene can be used in combination with a truncated
btl4 or bt2 gene.
CA 02284255 1999-10-12
53
This Example describes the construction of the
vector pTHW94 for plant transformations carrying the
following chimeric ICP genes between the T-DNA border
repeats: a truncated bt2 gene fragment under the
control of the TR2' promoter and a hybrid truncated
btl4-neo gene under the control of the TR1' promoter.
The 3' end of the T-DNA gene 7 and octopine synthase,
respectively, provide information for proper 3' end
formation. pTHW94 has been deposited at the DSM under
accession no. 5514 on August 28, 1989.
Schematically shown in Fig. 16 are the:
a) construction of pHW44: bt14 N-terminal gene
fragment with NcoI site
introduced at ATG
initiation codon.
b) construction of pHW67: reconstruction of the
bt14 gene under the
control of the tac
promoter.
c) construction of pHW71: construction of a hybrid
truncated bt14-neo gene
under the control of the
tac promoter.
d) construction of pTHW94: binary T-DNA vector with
a chimeric bt14 gene and
a chimeric bt2 gene
within the T-DNA border
repeats.
The pTHW94 vector is mobilized into the
Agrobacterium strain C58C1Rif (pMP90) which is used
- to transform Brassica napus according to the
procedure described by De Block et al. (1989).
Transformants are selected on Km, and regenerated
plants are found to express both ICP gene products in
insect toxicity tests and biochemical tests.
CA 02284255 1999-10-12
54
EXAMPLE 12: Expression of two ICP genes in a hybrid
construct
In order to obtain a combined and simultaneous
expression of two ICP genes, truncated gene fragments
encoding the toxic parts of two different ICPs can be
fused in a proper reading frame and placed, as a
hybrid gene, under the control of the same promoter
in a chimaeric gene construct. Toxic cores from
certain ICPs can be liberated from their protoxins by
protease activation at the N- and/or C- terminal end.
Thus, hybrid genes can be designed with one or more
regions encoding protease cleavage site(s) at the
fusion point(s) of two or more ICP genes.
The simultaneous co-expression of the bt2 and
bt14 genes is obtained by constructing a hybrid gene
composed of a truncated bt14 gene fragment fused to a
truncated bt2 gene fragment. Schematically shown in
Figure 17 is the construction of such a hybrid
bt2-btl4 gene with a C-terminal bt2 gene fragment -
(bt860) encoding the toxic core of the Bt2 protoxin
in frame with a C-terminal truncated bt14 gene
fragment encoding the toxic core of the Bt14
protoxin. The BclI site in the bt2 gene, localized
downstream of the trypsin cleavage site, is fused in
frame with the NcoI site introduced at the N-terminal
end of the truncated bt14 gene fragment. To this end,
the plasmids pLBKm860 (EP 0193259) and pHW67 are
used. pLBKm860 contains a hybrid bt2-neo gene under
control of the lambda PL promoter. The bt2 gene
moiety in the hybrid gene is a C-terminal truncated
bt2 gene fragment, indicated as bt860 (in Fig. 17)
(see also Vaeck et al, 1987). The construction of
pHW67 is described in Fig. 16. pHW67 contains a C-
terminal truncated bt14 gene fragment (btl4tox) with
a NcoI site at the ATG initiation codon, a
CA 02284255 2006-10-03
75749-7D
translation stop codon located at the BclI site of
the intact bt14 gene and a BamHI site downstream of
the whole gene fragment. To fuse both gene fragments
in the proper reading frame, the BclI and NcoI ends
of the respective plasmids are treated with Kienow
DNA polymerase and S1 nuclease as indicated in Figure
16. The resulting plasmid pJB100 contains the hybrid
bt860-btl4tox gene under control of the lambda PL
promoter and directs the expression in E. coli of a
fusion protein with the expected mobility on SDS-
PAGE.
Crude extracts of the E. coli strain show the
toxicity of the fusion protein, expressed by the
strain, against P. brassicae. It has also been
confirmed by N-terminal amino acid sequence analyses
of the fusion protein produced by the E. coli strain
that the N-terminal amino acids from the Bt14
protoxin are processed upon activation. The bt2-bt14
hybrid gene product has thus two potential protease
cleavage sites.
Subsequently, this hybrid gene is inserted into
a vector for plant transformations and placed under
control of a suitable promoter and transferred to the
genome of brassica (EP 0193259) where both the bt2
and bt14 genes are expressed in insect toxicity
tests.
The sequence of the Bt14 protein is shown in
figure 18.
CA 02284255 1999-10-12
56 11288-4
Tab1e 2
Guiio I]L aL=rain llost amino predicted Dioc.looure
range acida rll=1(koa) oE
elicoded of ericoded nucleotide
antinoacicls seducnce
bL3 ND-1 kuraL=aki L 1176 133.2 Schnopf et
a1.,1985
bt2 b4irliner 1715 L 1155 131 Iiofte et
al.,1986
bt73 IID-73 L 1178 133.3 A3ajig eL
al,
1985
bt14 entomocldus L 1207 138 Brizzard
fiu-1 10 and
14hiteley,
1988
LL= 15 uritoinocidue
110-110 L 1189 134.8 Fig. 14
b14 110-68 L 1165 132.5 e19. 13
aizati+ai
btl8 darmstadlen9is L 1171 133 EP
tID-146 appin.
0,358,557
btt3 BL51,D5144288 C 644 73,1 EP
22/10/87 applit.
0,305,275
b121 OLPCS1208,
D5M 5131, C 651 74.2 CA
19/1/89 ap ln.
2,14f,6'{6
bC22 DtPGS1245,
D:;M 5132, C 1138 129 Cp
19/ 1 /89 ~p1~1n~
,U4b, ~
P2 11U-263 L/U 633 70.9 Donovn,l et
al, 1988
Cry II0-1 L 633 70.8 widner and
02 Hhiteley,
1)09
CA 02284255 1999-10-12
57
REFERENCES
- Adang M., Staver M., Rocheleau T., Leighton
J., Barker R. and Thompson D. (1985), Gene
36, 289-300.
- Angenon et al (1989), Molecular and Cellular
Biology 9, 5676-5684.
- Barton K., Whiteley H. and Yang N.-S.
(1987), Plant Physiol. 85, 1103-1109.
- Bernard H., Remaut E., Hersfield M., Das H.,
Helinski D., Yanofski C. and Franklin N.
(1979), Gene 5, 59-76.
- Bell R. and Joachim F. (1976), Ann. Entomol.
Soc. Am. 69, 365-373.
- Botterman J. and Zabeau M. (1987), DNA 6,
583-591.
- Bradford M. (1976), Anal. Biochem. 72,
248-254.
- Brattsten L., Holyoke C., Leeper J. and
Raffa K. (1986), Science 231, 1255-1260.
- Brizzard B. and Whiteley H. (1988), Nucleic
Acids Research 16, 4168-4169.
- Deblaere R., Reynaerts A., Hofte H.,
Hernalsteens J-P, Leemans J. and Van Montagu
M. (1988), Methods in Enzymol. 153, 277-292.
- De Block M., Botterman J., Vandewiele M.,
Dockx J., Thoen, Gossele V., Rao Movva,
Thompson C., Van Montagu M. and Leemans J.
(1987), EMBO J. 6, 2513-2518.
- De Block et al (1989), Plant Physiology 91,
694-701.
- De Boer H., Comstock L. and Vasser M. -
(1983), Proc. Nati. Acad. Sci. USA 80,
21-25.
CA 02284255 1999-10-12
58
- de Framond A., Back E., Chilton W., Kayes L.
and Chilton M-D (1986), Mol. Gen. Genet.
202, 125-131.
- De Greve et al (1982), J. Mo1. Appl. Genet.
1 (6), 499-511.
- De La Pena and Schell (1986), Nature 325,
274-276.
- Delauney A., Tabaeizadeh Z. and Verma D.
(1988), Proc. Natl. Acad. Sci. USA 85,
4300-4304.
- Depicker A., Herman L., Jacobs A.,Schell J.
and Van Montagu M. (1985), Mol. Gen. Genet.
201, 477-484.
- Donovan W., Dankoscik C. and Gilbert W.
(1988), J. Bacteriol. 170, 4732-4738.
- Dulmage H.T and cooperators (1981), In :
Microbial control of pests and plant
diseases 1970-1980 (Ed. H.D. Burges),
Academic Press, 193-222.
- Finney D. (1962), Probit Analysis
(University Press, Cambridge), pp. 50-80.
- Fischhoff D., Bowdish K., Perlak F., Marrone
P., McCormick S., Niedermeyer J., Dean D.,
Kuzano-Kretzmer K., Mayer E., Rochester D.,
Rogers S. and Fraley R. (1987),
Bio/Technology 5, 807-812.
- Franck, Guilley, Jonard, Richards and Hirth
(1980), Cell 21, 285-294.
- French B., Maul H. and Maul G. (1986), Anal.
Biochem. 156, 417-423.
- Fuller F. (1982), Gene 19, 43-54.
- Gardner, Howarth, Hahn, Brown-Luedi, Shepard
and Messing, Nucl. Acids Res. 9, 2871-2887.
- Goldberg L. and Margalit J. (1977), Mosq.
News 37, 355-358.
CA 02284255 1999-10-12
59 11288-4
- Goldman I., Arnold J. and Carlton B. (1986),
J. Invert. Pathol. 47, 317-324.
- Gould F. (1988), Bioscience 38, 26-33.
Haider M., Knowles B. and Ellar D. (1986),
Eur. J. Biochem. 156, 531-540.
- Herrera-Estrella (1983) Nature 303, 209-213.
- Hofmann, C., Ltlthy P., Hutter R. and Pliska
V. (1988a),Eur. J. Biochem. 173, 85-91.
- Hofmann C., Vanderbruggen H., Hdite H., Van
Rie J., Jansens S. and Van Mellaert H.
(1988b), Proc. Natl. Acad. Sci. USA 85,
7844-7848.
- Hofte H., Van Rie J., Jansens S., Van
Houtven A., Verbruggen H. and Vaeck M.
(1988), Appl. Environ. Microbiol. 54, 2010-2017.
- Hofte H., De Greve H., Seurinck J., Jansens
S., Mahillon J., Ampe C., Vanderkerkhove J.,
Vanderbruggen H., Van Montagu M., Zabeau M.
and Vaeck M. (1987), Eur. J. Biochem. 161, 273-280.
- Hofte H. and Whiteley H.R. (1989), Microbiological
Reviews 53, 242-255.
- Hofte et al. (1986), Structural and functional analysis
of a cloned delta endotoxin of Bacillus thuringiensis
berliner 1715; European Journal of Biochemistry 161,
273-280.
- Hsiung H. and Becker G. (1988), Biotech. and Genetic
Engin. Rev. 6, 43-65.
CA 02284255 1999-10-12
59a 11288-4
- Hull and Howell (1987),Virology 86, 482-493.
- Hunter W. and Greenwood F. (1962), Nature 194,
495-496.
- Kozak M. (1.987), Mol. Cell. Biol. 7, 3438-3445.
- Krebbers E., Herdies L., De Clercq A.,
Seurinck J., Leemans J., Van Damrne J.,
Segura M., Gheysen G., Van Montagu M. and
CA 02284255 1999-10-12
Vandekerckhove J. (1988), Plant Physiol. 87,
859-866.
- Knowles B. and Ellar D. (1986), J. Cell. Sci
83, 89-101.
- Krieg A:, Huger A., Langenbruch G. and
Schnetter W. (1983), Z. Ang. Ent. 96,
500-508.
- Krieg A. and Langenbruch G. (1981), In :
Microbial control of pests and plant
diseases 1970-1980 (Ed. H.D. Burges),
Academic Press, 837-986.
- Kirsch K. and Schmutterer H. (1988), J.
Appl. Ent. 105, 249-255.
- Kronstad J., Schnepf H. and Whiteley H.
(1983), J. Bacteriol. 154, 419-428.
- Mahillon J. and Delcour J. (1984), J.
Microbiol. Methods 3, 69-73.
- Maxam A. and Gilbert W. (1980), Methods in
Enzymol. 65, 499-560.
- McGaughey W. (1985), Science 229, 193-195.
- McGaughey W. and Beeman R. (1988), J. Econ.
Entomol. 81, 28-33.
- Munson P. and Rodbard D. (1980), Anal.
Biochem. 107, 220-239.
- Pazkowski and cooperators (1984), EMBO J 3,
2717-2722.
- Peleman J., Boerjan W., Engler G., Seurinck
J., Botterman J., Alliote T., Van Montagu M.
and Inze D. (1989), The Plant Cell 1, 81-93.
- Remaut E., Stanssen P. and Fiers W. (1981),
Gene 15, 81-93. -
- Rocha-Sosa et al (1989) EMBO J. 8, 23-29.
- Sandier S., Stayton M., Townsend J., Ralstan
M., Bedbrook J. and Dunsmuir P. (1988),
Plant Mol. Biol. 11, 301-310.
CA 02284255 1999-10-12
61
- Scatchard G. (1949), Ann. N.Y. Acad. Sci.
51, 660-672.
- Schocher R., Shillito R., Saul M.,Pazkowski
J. and Potrykus I. (1986) Bio/technology 4,
1093-1096.
- Shields (1987), Nature 328, 12-13.
- Schnepf H., Wong H. and Whiteley H. (1985),
J. Biol. Chem. 260, 6264-6272.
- Stanssens P., Remaut E. and Fiers W. (1985),
Gene 36, 211-223.
- Stanssens P., McKeown Y., Friedrich K. and
Fritz H. (1987) : "Oligo-nucleotide directed
construction of mutations by the gapped
duplex DNA method using the pMa/c plasmid
vectors", published in the Collection of
Experimental Procedures distributed at the
EMBO course entitled "Directed mutagenesis
and protein engineering" in July 1987 at the
Max Planck Institut fur Biochemie,
Martinsried, FRG.
- Stone T., Sims S. and Marrone P. (1989), J.
Invert. Pathol. 53, 228-234.
- Vaeck M., Reynaerts A., H6fte H., Jansens
S., De Beukeleer M., Dean C. Zabeau M., Van
Montagu M. and Leemans J. (1987), Nature
327, 33-37.
Voller, Bidwell and Barlett (1976), In:
Manual of Clinical Immunology (Eds. Rose and
Friedman), pp. 506-512, American Society of
Microbiology, Washington
- Velten J.; Velten L., Hain R. and Schell J.
(1984), EMBO J. 3, 2723-2730.
- Velten J. and Schell J. (1985), Nuci. Acids
Res. 13, 6981-6998.
CA 02284255 1999-10-12
62
- Widner W. and Whiteley H. (1989), J.
Bacteriol. 171, 965-974.
- Wolfersberger M., Luthy P., Maurer A.,
Parenti P., Sacchi V., Giordana B. and
Hanozet G. (1987), Comp. Biochem. Physiol.
86, 301-308.
- Yanish-Perron C., Veiera J. and Messing J.
(1985), Gene 33, 103-119.