Note: Descriptions are shown in the official language in which they were submitted.
CA 02284300 1999-09-29
REFERENCE TO CO-PENDING APPLICATIONS
This is a continuation-in-part of U.S. provisional application serial number
60/060,084 filed on September 26, 1997, the subject matter of which is also
incorporated
herein by reference.
BACKGROUND OF THE INVENTION
1. FIELD OF THE INVENTION
The present invention relates to novel polymers, to polymers for use as
pressure
sensors, more particularly phosphorescent oxygen sensors and more particularly
to
compositions for forming coatings therefor.
2. DESCRIPTION OF THE RELATED ART
The field of luminescent barometry has developed as a result of continuing
difficulties
encountered with other mechanical means to measure pressure distributions over
aerodynamic
surfaces. The theories of luminescent barometry are described in detail in
U.S. Patent
5,359,887 and U.S. Patent 5,151,603, the subject matter of each of which is
incorporated
herein by reference. Luminescent barometry is based on the phenomenon that
some
phosphorescent materials emit light at a unique wavelength and which is
'quenched' by the
presence of particular molecules such as oxygen. This quenching effect can be
quantified so
that the phosphorescent material, provided in an oxygen permeable matrix, can
be used to
measure, for example, the partial pressure of oxygen passing over aerodynamic
surfaces.
As a consequence of their considerable fabrication advantages, the use of
polymeric
materials for the construction of sensing devices using this quenching effect
is an area of
CA 02284300 1999-09-29
intense current interest. Luminescent sensors based on composites comprising
transition
metal phosphorescent dyes immobilized in polymer matrices have attracted
attention as
oxygen sensors for both biomedical and barometric applications. Conventional
phosphorescent dyes such as Pt (platinum) octaethylporphyrin (OEP) derivatives
or Ru"
(ruthenium) bipyridyl (bipy) or phananthroline (Phen) derivatives with oxygen
quenchable
excited states have been dispersed in a silicone (otherwise known as
polysiloxane) based
polymer matrices due to their high gas permeability.
However, these conventional silicone-based polymer systems require cross-
linking and
tend to be incompatible with the dyes and can lead to undesirable local dye
concentrations and
thus reduced sensitivity. Most PtOEP based systems in cross-linked silicone
polymer matrices
also have non-linear dependence on air pressure thereby making measurements
less accurate.
Furthermore, conventional polysiloxane coatings tend to continue cross-linking
as
I 5 their temperature rises which causes irreversible changes in their
phosphorescent properties
as a result, making their data subj ect to error and generally unsuitable for
measurements taken
in fluctuating temperature conditions.
It is therefore an object of the present invention to provide a novel
composition for
use as pressure sensors.
It is a further object of the present invention to provide a novel composition
for use
as phosphorescent sensors.
It is a further object of the present invention to provide a novel composition
for use
as phosphorescent oxygen sensors.
It is a further object of the present invention to provide a novel polymer
which
2
CA 02284300 1999-09-29
provides improved distribution of dye there through.
It is a further object of the present invention to provide a novel polymer
with a
backbone containing nitrogen and one or more of sulfur and phosphorous which
provides
improved distribution of dye there through.
SUMMARY OF THE INVENTION
Briefly stated, the invention involves a polymer material comprising a
backbone
containing nitrogen and one or more of sulfur and phosphorous, the polymer
material further
comprising at least one side chain, wherein either the at least one side chain
or the backbone
includes a phosphorescent dye agent.
Preferably, the backbone includes both sulfur and phosphorus having side
groups
selected from the group consisting of oxygen, a halogen, methyl, a substituted
or
unsubstituted CZ_ZO linear or branched alkyl group, a substituted or
unsubstituted CZ_ZO linear
or branched alkenyl group, a substituted or unsubstituted CZ_~o linear or
branched alkynyl
group, a substituted or unsubstituted C~_~o aryl group, a substituted or
unsubstituted C3_zo
cycloalkyl group.
Preferably, the polymer has a plurality of side chains at least one of the
side chains
including the phosphorescent dye agent. More particularly, the sulfur has a
first side group
including oxygen and a second side group, the phosphorous has first and second
side groups,
the first and second side groups on phosphorus and the second side group on
sulfur being
either NHBu" or a group including the dye agent.
In another aspect, the invention involves a pressure sensor comprising a
substrate
having a surface, a polymer material as defined herein above and applied to
the surface to
3
CA 02284300 1999-09-29
form a coating.
In still another of its aspects, the invention provides a polymer material
formed from
a phosphorescent dye agent contained in a polymer material of formula A,
wherein:
E 1, E2 and E3 are the same or are different and are selected from sulfur or
phosphorus;
RI to R6 are the same or different and are selected from the group comprising
, oxygen, a halogen, hydrogen, methyl a substituted or unsubstituted Cz_zo
linear or branched
alkyl group, a substituted or unsubstituted Cz_zo linear or branched alkenyl
group, a substituted
or unsubstituted Cz_zo linear or branched alkynyl group, a substituted or
unsubstituted C6_zo
aryl group, a substituted or unsubstituted C3_zo cYcloalkyl group, and wherein
at least one of
R1 to R6 is a group including a phosphorescent dye agent.
Preferably E 1 is in the form of sulfur VI, while E2 and E3 are each
phosphorus. Each
of R2 to R6 includes an oxygen or a nitrogen substituent. More particularly,
each of R3 to
R6 includes an aryloxy group or an alkamine group, and each is selected from
the group
consisting ofNHBu", OBu", OC6H~, OC6H,~CF3-m, OCHZCH=CHz and OC6H4CF3-p, and
the
group including the dye agent.
More preferably, R2 is a halogen, and more particularly R2 and R3 to RS are
the same
and R6 is the group including the dye agent. Still more particularly, R2 and
R3 to RS are
each NHBu" and the group including the dye agent includes a ruthenium
substituent. Still
more particularly, the group including the dye agent is Ru(4,7-diphenylphen)3.
In one embodiment, the group including the dye agent includes a heterocyclic
group
selected from the group comprising a substituted C3_zo cYcloalkyl group, a
substituted C6_zo
4
CA 02284300 1999-09-29
aryl group and a substituted or unsubstituted C6_zo aralkyl group.
In still another of its aspects, the invention provides a polymer material of
the formula
B wherein:
EI, E2 and E3 are the same or are different and are selected from sulfur or
phosphorus;
RI to R6 are the same or different and are selected from the group comprising
oxygen, a halogen, hydrogen, methyl a substituted or unsubstituted Cz_zo
linear or branched
alkyl group, a substituted or unsubstituted C,_zo linear or branched alkenyl
group, a substituted
or unsubstituted C,_zo linear or branched alkynyl group, a substituted or
unsubstituted C6_zo
aryl group, a substituted or unsubstituted C3_zo cycloalkyl group; and wherein
at least one of
RI to R6 is a group including a phosphorescent dye agent.
IS
R7 is selected from oxygen, nitrogen or from groups 15 and 16 of the periodic
table
of elements;
R8 is selected from the group comprising methylene, a substituted or
unsubstituted
Cz_,o linear or branched alkyl group, a substituted or unsubstituted Cz_zo
linear or branched
alkenyl group, a substituted or unsubstituted Cz_zo linear or branched alkynyl
group, a
substituted or unsubstituted C6_zo aryl group, a substituted or unsubstituted
C3_zo cYcloalkyl
group.
In still another of its aspects, the invention provides a method of forming a
copolymer
material of the formula B, comprising the steps of:
providing a first polymer block of the formula A, wherein:
5
CA 02284300 1999-09-29
E 1, E2 and E3 are the same or are different and are selected from sulfur or
phosphorus;
R 1 to R6 are the same or different and is selected from the group comprising
oxygen,
a halogen, hydrogen, methyl a substituted or unsubstituted Cz_zo linear or
branched alkyl
group, a substituted or unsubstituted Cz_,o linear or branched alkenyl group,
a substituted or
unsubstituted Cz_zo linear or branched alkynyl group, a substituted or
unsubstituted C6_zo ~'Yl
group, a substituted or unsubstituted C3_,o cycloalkyl group; and wherein at
least one of Rl
to R6 is a group including a phosphorescent dye agent.
carrying out a ring opening polymerization of an unsaturated heterocyclic
group
having at least one electron rich site therein to form a copolymer material of
formula B
wherein:
R7 is selected from oxygen, nitrogen or from groups 15 and 16 of the periodic
table
of elements;
R8 is selected from the group comprising methylene, a substituted or
unsubstituted
C,_,o linear or branched alkyl group, a substituted or unsubstituted Cz_zo
linear or branched
alkenyl group, a substituted or unsubstituted Cz_zo linear or branched alkynyl
group, a
substituted or unsubstituted C6_zo aryl group, a substituted or unsubstituted
C3_zo cycloalkyl
group.
In yet another of its aspects, the present invention provides a coating
composition
comprising a polymer material, the polymer having a backbone and at least one
side group
with a phosphorescent dye agent as a member of the backbone or the side group,
the polymer
being capable of being applied as a coating. Preferably, the polymer material
is in a solvent
mixture and the solvent mixture is homogeneous.
6
CA 02284300 1999-09-29
In yet another of its aspects the present invention provides a polymer
material formed
from a polymer material having a backbone containing nitrogen and one or more
of sulfur
and phosphorous, the polymer material including at least one side group
including a silicone
group.
Preferably, the backbone contains sulfur and phosphorous, the polymer material
is
stable and the sulfur is in the form of sulfur VI. More preferably, the
polymer material
includes a number of silicone side groups. Still more preferably, each of the
silicone side
groups has a trimethylsilyl constituent. Still more preferably, each
phosphorous in the
backbone has a side group including silicone. Still more preferably, each side
group on each
phosphorus in the backbone includes a trimethylsilyl group.
In yet another of its aspects, the present invention provides a polymer
material of
formula A as defined herein above wherein at least one of Rl to R6 includes
siloxane.
In yet another of its aspects, the present invention provides a method of
forming a
pressure sensor, comprising the steps of forming a stable polymer having a
backbone
containing nitrogen and one or more of sulfur and phosphorus, and with a
plurality of side
groups, and providing a silicone constituent on at least one of the side
groups. Preferably,
at least one silicone constituent is provided on a plurality of the side
groups. More preferably,
the backbone includes sulfur and phosphorous and each side group on the
phosphorus
includes a silicone constituent. Still more preferably, the sulfur has one
side group including
oxygen and a second side group including a silicone constituent.
In yet another aspect of the present invention, there is provided a polymer
material
having a backbone containing nitrogen and one or more of sulfur and
phosphorous, and at
least one silicone-bearing side group. Preferably, the polymer material has a
glass transition
temperature ranging from -20 ° C to 0 ° C.
7
CA 02284300 1999-09-29
In yet another aspect of the present invention, there is provided a pressure
sensor
comprising a stable polymer material as defined above and a phosphorescent dye
agent.
Preferably, the polymer and dye agent are in the form of a coating. More
preferably, the
pressure sensor is operatively characterized by a Stern V olmer plot having a
linearity ranging
from 0.980 to 1Ø More preferably, the sensor exhibits a Stern Volmer plot
having a linearity
ranging from 0.985 to 0.995, still more preferably 0.990 to 0.995.
Preferably, pressure sensor is operatively characterized by a Stern Volmerplot
having
the above ranges of linearity over a range of pressures, namely from about 0.1
to 75 psi,
more preferably 0.1 to 50 psi, still more preferably 0.2 to 40 psi.
BRIEF DESCRIPTION OF THE DRAWINGS
Several preferred embodiments of the present invention will be provided, by
way of
example only, with reference to the appended drawings, wherein:
Figures A and B are schematic diagrams of polymerizations;
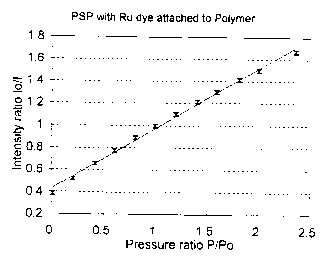
Figure 1 is a plot of a luminescence intensity ratio versus pressure ratio for
one
exemplified coating of the present invention;
Figure 2 is a PSP image of a wing model coated with the coating of figure l;
and
Figure 3 is a comparison of pressure distribution measurements.
DESCRIPTION OF THE PREFERRED EMBODIMENTS
Briefly stated, the invention involves a polymer material comprising a
backbone
8
CA 02284300 1999-09-29
containing nitrogen and one or more of sulfur and phosphorous, the polymer
material further
comprising at least one side chain, wherein either the at least one side chain
or the backbone
includes a phosphorescent dye agent.
Preferably, the backbone has sulfur and phosphorous, which have side groups
selected from the group consisting of oxygen, a halogen, methyl, a substituted
or
unsubstituted C~_ZO linear or branched alkyl group, a substituted or
unsubstituted CZ_ZO linear
or branched alkenyl group, a substituted or unsubstituted CZ_ZO linear or
branched alkynyl
group, a substituted or unsubstituted C~_zo aryl group, a substituted or
unsubstituted C3_zo
cycloalkyl group.
Preferably, the polymer has a plurality of side chains at least one of the
side chains
including the phosphorescent dye agent. More particularly, the sulfur has a
first side group
including oxygen and a second side group, the phosphorous has first and second
side groups,
the first and second side groups on phosphorus and the second side group on
sulfur being
either NHBu" or a group bearing the dye agent, as shown, for example, at 2a.
In another aspect, the invention involves a phosphorescent oxygen sensor
comprising
a substrate having a surface, a polymer material as defined above and applied
to the surface
to form a coating.
In still another of its aspects, the invention provides a polymer material
formed from
a phosphorescent dye agent contained in a polymer material of formula A,
wherein:
El, E2 and E3 are the same or are different and are selected from sulfur or
phosphorus;
R1 to R6 are the same or different and are selected from the group comprising
9
CA 02284300 1999-09-29
oxygen, a halogen, hydrogen, methyl a substituted or unsubstituted Cz_zo
linear or branched
alkyl group, a substituted or unsubstituted Cz_zo linear or branched alkenyl
group, a substituted
or unsubstituted Cz_zo linear or branched alkynyl group, a substituted or
unsubstituted C6_zo
aryl group, a substituted or unsubstituted C3_zo cYcloalkyl group, and wherein
at least one of
Rl to R6 is a group including a phosphorescent dye agent.
More particularly, the polymer material has a plurality of side chains, at
least one of
the side chains including the phosphorescent dye agent. Still more preferably,
the sulfur has
a first side group including oxygen and a second side group, the phosphorous
has first and
second side groups, the first and second side groups on phosphorus and the
second side group
on sulfur being either NHBu" or a group including the dye agent. In one
embodiment, at least
one of the side groups on the phosphorous bears the dye agent and the other
side groups of
the phosphorous are the same as the second side group on the sulfur.
1 S Preferably, the group including the dye agent includes a ruthenium
substituent, more
particularly a ruthenium phenanthroline complex.
The polymer material A is useful as an ingredient in phosphorescent oxygen
sensors
and coatings therefor. The polymer material A is polar, owing to the presence
of electron rich
sites in its backbone. The constituents of the polymer material A should be
selected having
regard to the oxygen environment in which the sensing is to take place and in
particular the
expected temperature ranges in which the sensor so formed is to be expected to
be operable
and this may be measured by the Glass Transition Temperature (T~), and which
may be
considered as the boundary of the temperatures substantially above which there
is sufficient
permeability for gases such as oxygen.
Preferably, the sulfur and phosphorus have side groups selected from the group
consisting of oxygen, a halogen, methyl, a substituted or unsubstituted Cz_zo
linear or branched
CA 02284300 1999-09-29
alkyl group, a substituted or unsubstituted Cz_zo linear or branched alkenyl
group, a substituted
or unsubstituted C,_,o linear or branched alkynyl group, a substituted or
unsubstituted C6_zo
aryl group, a substituted or unsubstituted C3_zo cycloalkyl group.
Preferably, the group including the dye agent includes a heterocyclic group
selected
from the group comprising a substituted C3_zo cycloalkyl group, a substituted
C6_zo aryl group
and a substituted or unsubstituted C6_zo aralkyl group.
More preferably, El is in the form of sulfur VI and R1 is oxygen, while each
of E2
and E3 are phosphorus, providing a repeating PN segment in the backbone along
with a
relatively small terminating S=O segment. The S=O segment contributes an
asymmetry to
the backbone to discourage crystallization, which is sensitive to the
regularity of the polymer
chain. Moreover, the S=O segment contributes to an amorphous structure which
is a
contributing factor to the gas permeability of resulting polymers.
More preferably, each of R2 to R6 includes an oxygen or a nitrogen substituent
and
each of R2 to R6 may be provided in the form of an aryloxy group, an alkoxy
group,
arylamine group or an alkamine group and may include therein a phenyl group.
In this case,
the aryloxy and the arylamine tend to increase T~ due to the fact that these
groups tend to be
relatively more rigid. In contrast, the alkoxy and alkamine groups tend to be
more flexible,
contributing to a lower T~ and higher permeability. In each of the oxy and
amine groups,
polarity is increased by the presence of oxygen and nitrogen substitutents.
These oxy and
amine groups can be selected with increased polarity by providing for
increasing numbers of
polar substituents such as oxygen and nitrogen.
If desired, the permeability, T~ and polarity can be tailored by the
selection, or for that
matter, a mixture of groups along the polymer depending on the contribution of
each group.
In some cases, the choice of the R groups may also influence the polarity of
the polymer and
11
CA 02284300 1999-09-29
thus the interactions between the polymer and the dye agent, leading in some
cases to
relatively uneven distribution and in other cases to relatively even
distribution. However, the
resulting composition, when used as a phosphorescent sensor, requires no cross-
linking and
therefore should be a desirable advance, in either case.
The polymers should also be formed so that they are stable for their intended
use. In
this case, the term stable polymer is intended to mean one which is stable in
its intended
environment, that is for a given period of time, and when subject to certain
conditions, such
as hydrolysis. For example, it is contemplated that some examples of such
polymers, although
stable in the short term may in fact be biodegradable, therefore with a
planned breakdown
beyond its intended useful life. Furthermore, the polymers should also have
photo stability,
that is be stable within reasonable tolerances to photo irradiation for the
purposes of exiting
the phosphorescent dye agent. In addition, the polymers should, to some degree
depending
on their intended life span, be resistant to attack by singlet oxygen, for
example, a byproduct
of the quenching process.
In a preferred embodiment, E 1 is in the form of sulfur and E2, E3 are each
phosphorus. In a still further preferred embodiment, each of R2 to RS are an
arylamine group
and R6 is a ruthenium phenanthroline complex, such as Ru(4,7-diphenylphen)3.
Synthesis of
an exemplified version of this further preferred version of the polymer
material A is shown
by the structures 1 to 3 and involves the thermal ring-opening polymerization
of the cyclic
monomer 1 followed by treatment of the halogenated polymer material 2 with an
excess of
n-butylamine and is further described below.
The polymer material 3 is a hydrolytically stable amorphous elastomeric
material and
possesses a Tg of-17 °C giving it both relatively high free volume and
gas permeability. The
polymer material may also be formed as a relatively high quality film coating
with dimensional
stability without the need for cross-linking.
12
CA 02284300 1999-09-29
The intensity characteristics of a phosphorescent material can be modeled by
the
Stern-Volmer equation which can be expressed as follows:
Ii.oo/I= A + B(P/P,.oo)~ where
I,.oo/I= Luminescence Intensity Ratio ( the 'LIR')
I - luminescence intensity,
I,,oo = luminescence intensity at 1.00 atmosphere. (used as a reference;
P - air pressure in atmospheres;
A - Coefficient for vacuum condition;
B - Coefficient corresponding to gradient of curve, or rate of change of
the Luminescence Intensity Ratio;
Compositions containing the polymer material 3 together with phosphorescent
dye
agents may show reasonably well-defined Stern-Volmer behaviour and
significantly improved
sensitivity.
Preferably, the dye agent includes a platinum or a ruthenium substituent. More
preferably, the dye agent is selected from the group consisting of Pt
octaethylpophyrin, Ru"
bipyridyl and Ru'~ phenanthroline derivatives, though other known organic and
inorganic dye
agents are contemplated.
Compositions made according to the present invention have been shown to be
usable
on substrates such as stainless steel and alumina, though other substrates
such as glass,
plastics and metals are also contemplated.
In another aspect of the present invention, there is provided a copolymer
material of
the formula B wherein:
13
CA 02284300 1999-09-29
E 1, E2 and E3 are the same or are different and are selected from sulfur or
phosphorus;
R1 to R6 are the same or different and are selected from the group comprising
oxygen, a halogen, hydrogen, methyl a substituted or unsubstituted Cz_zo
linear or branched
alkyl group, a substituted or unsubstituted Cz_zo linear or branched alkenyl
group, a substituted
or unsubstituted C,_,o linear or branched alkynyl group, a substituted or
unsubstituted C6_zo
aryl group, a substituted or unsubstituted C3_zo cYcloalkyl group; and wherein
at least one of
R1 to R6 is a group including a phosphorescent dye agent.
R7 is selected from oxygen, nitrogen or from groups 15 and 16 of the periodic
table
of elements;
R8 is selected from the group comprising methylene, a substituted or
unsubstituted
Cz_zo linear or branched alkyl group, a substituted or unsubstituted Cz_zo
linear or branched
alkenyl group, a substituted or unsubstituted C,_zo linear or branched alkynyl
group, a
substituted or unsubstituted C6_zo aryl group, a substituted or unsubstituted
C3_zo cYcloalkyl
group.
The copolymer material of the formula B may be formed by first providing a
first
polymer block of the formula A; and then carrying out a ring opening
polymerization of an
unsaturated heterocyclic group having at least one electron rich site therein.
Conveniently, the second polymer block can be formed by a ring opening
polymerization of a heterocyclic group in the presence of the first polymer
block, wherein the
heterocyclic group is selected from the group comprising a substituted C3_zo
cycloalkyl group,
a substituted C6_zo aryl group and a substituted or unsubstituted C6_zo
aralkyl group. More
preferably, the heterocyclic group is an unsaturated C3_5 cyclic group with
the oxygen or
14
CA 02284300 1999-09-29
nitrogen substituent therein. Still more preferably, the unsaturated
heterocyclic group is
tetrahydrofuran, ethylene oxide or propylene oxide.
More preferably, E1 is in the form of sulfur VI, while E2 and E3 are each
phosphorus
and R7 is an electron rich site such as sulfur, oxygen, nitrogen or any one of
groups 15 or 16
in the periodic table and provides the electron rich site by virtue of their
lone pair of unpaired
electrons. The electron rich site is thus able to form a stable electron bond
with the electron
deficient sulfur and thereby initiate the ring opening polymerization in the
presence of the first
polymer block.
Preferably, the sulfur in the first polymer block is in a stable form,
preferably a
hydrolytically stable form, more preferably in the form of sulfur VI in view
of the fact that
sulfur in other forms such as sulfur IV may be unstable in some cases, such as
for example
polythiophosphazene. Further examples of unstable sulfur IV polymers may be
found in I.
Manners (COORDINATION CHEMISTRY REVIEWS, 137, 1994, 109-129), the subject
matter of which is incorporated herein by reference.
The copolymer material made according to the present invention provides
improved
integrity and one example of the copolymer material is shown at 4. While the
polymer
material 3 may provide the coating with a generally tacky consistency, the
copolymer material
4 may be used to form a layer of material capable of withstanding its own
weight. In other
words, the copolymer material is envisaged in uses beyond mere coatings but
perhaps in the
formation or fabrication of devices with an inherent phosphorescent oxygen
sensing
capability.
It is also contemplated herein to form a polysiloxane polymer with a
phosphorescent
dye agent as described herein as a member of the polymer matrix.
CA 02284300 1999-09-29
A sample formed from polymer material 3 or copolymer material 4 with a T~ of -
17
°C will typically allow the sample to be used in environments whose
temperatures will
substantially exceed -17 °C, that is where the sample will allow for
the permeation of oxygen
and hence allow for the subsequent quenching of luminescence.
The present invention provides a coating which, in some cases, may provide
superior
characteristics over those currently available. Like their polysiloxane
counterparts, the
present coatings based on the polymer material A or the copolymer material B
present
phosphorescent properties that change with temperature. However, due to the
lack of cross-
linking in the matrix, the repeatability of the data presented by coatings
based on the polymer
material 3 or the copolymer material 4 can be substantially improved over
their polysiloxane
counterparts.
The present coatings form improved films with relatively faster drying times
and
without supplemental curing which is necessary for polysiloxane polymers. The
present
coatings have improved mechanical integrity and are believed to have
substantially no long
term flow (creep) on the surface.
Tables 1 to 4 are provided to illustrate a selection of possible groups for R1
to R6 in
the polymer material of the formula A . These tables are obtained from several
published
papers, namely I. Manners (COORDINATION CHEMISTRY REVIEWS, 137, 1994, 109-
129), the subject matter of which is incorporated herein by reference, and Y.
Ni et al
(MACROMOLECULES 1992, 27, 7119), the subject matter of which is also
incorporated
herein by reference. It is worth noting that a number of R groups have T~ in
the region of
-18°C to 25°C and these may be considered desirable R groups for
some applications.
Further details of the formation of copolymer material 4 can be seen in figure
A and
is believed to be representative of one method of forming in general the
copolymer materials
16
CA 02284300 1999-09-29
of formula B. In this case, the sulfur VI canon from poly(thionylphosphazene)
attacks an
oxygen site on a THF molecule to form an oxonium ion. Further reaction ofthis
oxonium ion
with more monomer generates a poly(THF) block.
In yet another of its aspects, the present invention provides a coating
composition
comprising a polymer material, the polymer having a backbone and at least one
side group
with a phosphorescent dye agent as a member of the backbone or the side group,
the polymer
being capable of being applied as a coating. Preferably, the polymer material
is in a solvent
mixture and the solvent mixture is homogeneous.
In yet another of its aspects, the present invention provides a phosphorescent
dye
agent, comprising a phenanthroline complex which is reactive to form a polymer
with the dye
agent as a constituent thereof. Preferably, the complex is a ruthenium
phenanthroline
complex.
In still another of its aspects, the present invention provides a polymer
material
comprising the phosphorescent dye agent as a constituent thereof or as a
substituent therein.
In still another of its aspects, the present invention provides a method of
forming a
phosphorescent dye agent, comprising the steps o~
providing a phenanthroline complex with a dye agent as a constituent thereof;
and
forming a reactive site on the complex which is reactive to form a polymer.
Preferably, the complex is a ruthenium phenanthroline complex.
In yet another of its aspects, the present invention provides a method of
forming a
17
CA 02284300 1999-09-29
phosphorescent polymer material, comprising the steps of:
providing a phenanthroline complex with a dye agent as a constituent thereof;
forming a reactive site on the complex; and
reacting the complex with a monomer to form a polymer.
Thus, the materials as described above provide for the use of
poly(thionylphosphazenes) as shown at 3 which contain the phosphorescent dye
agent as a
constituent part of the polymer structure. The materials described herein
provide increased
loading of the dye agent which allows higher intrinsic intensity of
phosphorescence making
observation of light emission from the coatings easier. The materials herein
also provide
improved compatibility between the dye agent and the polymer matrix which may,
in some
cases, lead to more predictable Stern-Volmer behaviour. It is believed that
the dye agent
loading may also raise the potential use of thinner films which would thus
allow more rapid
response, which in turn may permit measurements in such applications as
fluctuating pressure
systems.
The dye bound polymer may be formed, for example, by providing a dye agent
with
at least one functional group, such as an amino group, which is a candidate
for reaction with
one or more side groups on halogenated polymer 2 to substitute at least one
halogen with the
functionalized dye agent, followed by treatment of the halogenated polymer
with an excess
of an amino group such as, for example, n-butylamine, as is described below,
to form the
polymer 2a. The polymer 2a may then be present in a ring opening
polymerization of an
unsaturated heterocyclic group having at least one electron rich site therein,
such as
tetrahydrofuran or the others named above.
18
CA 02284300 1999-09-29
In yet another of its aspects the present invention provides a polymer
material formed
from a polymer material having a backbone containing nitrogen and one or more
of nitrogen
and phosphorous, the polymer material including at least one side group having
a silicone
constituent.
Preferably, the backbone has sulfur and phosphorous, the polymer is stable,
more
preferably hydrolitically stable, still more preferably with its sulfur in the
form of sulfur VI.
More preferably, the polymer material includes a number of silicone side
groups. Still more
preferably, each of the silicone side groups has a trimethylsilyl constituent.
Still more
preferably, each phosphorous in the backbone has a side group including
silicone. Still more
preferably, each side group on each phosphorus in the backbone includes a
trimethylsilyl
group.
In yet another of its aspects, the present invention provides a polymer
material of
formula A wherein E 1, E2 and E3 are the same or are different and are
selected from sulfur
or phosphorus and any one or more of R1 to R6 includes a siloxane group.
In still another of its aspects, the present invention involves a copolymer
material of
formula B wherein:
E1, E2 and E3 are the same or are different and are selected from sulfur or
phosphorus;
R1 to R6 are the same or different and is selected from the group comprising
oxygen,
a halogen, hydrogen, methyl a substituted or unsubstituted Cz_zo linear or
branched alkyl
group, a substituted or unsubstituted Cz_zo linear or branched alkenyl group,
a substituted or
unsubstituted Cz_zo linear or branched alkynyl group, a substituted or
unsubstituted C6_zo ~'Yl
group, a substituted or unsubstituted C3_zo cYcloalkyl group; and wherein at
least one of R1
19
CA 02284300 1999-09-29
to R6 is a group including a siloxane group;
R7 is selected from oxygen, nitrogen or from groups 15 and 16 of the periodic
table
of elements;
R8 is selected from the group comprising methylene, a substituted or
unsubstituted
C,_zo linear or branched alkyl group, a substituted or unsubstituted Cz_zo
linear or branched
alkenyl group, a substituted or unsubstituted CZ_zo linear or branched alkynyl
group, a
substituted or unsubstituted C6_zo aryl group, a substituted or unsubstituted
C3_zo cycloalkyl
group.
In yet another of its aspects, the present invention provides a method of
forming a
pressure sensor, comprising the steps of forming a stable polymer having a
backbone
containing nitrogen and one or more of sulfur and phosphorus, and with a
plurality of side
groups, and providing a silicone constituent on at least one of the side
groups. Preferably,
the backbone has sulfur and phosphorous, and at least one silicone constituent
is provided
on a plurality of the side groups. More preferably, each side group on the
phosphorus
includes a silicone constituent. Still more preferably, the sulfur has one
side group including
oxygen and a second side group including a silicone constituent.
In yet another aspect of the present invention, there is provided a polymer
material
having a backbone containing nitrogen and one or more of nitrogen and
phosphorous, and
at least one silicone including side group. Preferably, the polymer material
has a backbone
including sulfur and phosphorous and has a glass transition temperature
ranging from -20 °C
to 0°C.
In yet another aspect of the present invention, there is provided a pressure
sensor
comprising a stable polymer material as defined above and a phosphorescent dye
agent.
CA 02284300 1999-09-29
Preferably, the polymer and dye agent are in the form of a coating. More
preferably, the
pressure sensor is operatively characterized by a Stern Volmer plot having
ranging from 0.980
to 1Ø More preferably, the sensor exhibits a Stern Volmer plot having a
linearity ranging
from 0.985 to 0.995, still more preferably 0.990 to 0.995. Still more
preferably, the pressure
sensors herein can in some cases be operatively characterized by a Stern
Volmer plot having
the above ranges of linearity over a range of pressures, namely from about 0.1
to 75 psi,
more preferably 0.1 to 50 psi, still more preferably 0.2 to 40 psi.
Pressure sensors made according to the present invention can, in some cases,
also be
operatively characterized by a Stern Volmer plot having a slope ranging from
0.1 to 1.0, more
preferably 0.2 to 0.9, more preferably 0.4 to 0.7 still more preferably 0.49
to 0.6 (for example
ranging from 0.17 - 0.18, for one example of PTP(aminopropyltrisiloxane), and
ranging
from 0.49 to 0.60 for examples of the copolymer of Poly(THF) and
PTP(aminopropyltrisiloxane)).
A polymer with at least one silicone-bearing side group may be formed, for
example,
by reacting the halogenated polymer 2 with a silicone-bearing side group
having at least one
functional group, such as an amino group, the latter to react with at least
one of the halogen
side groups on the polymer 2 to yield a polymer such as that shown at 5.
The co-polymer 4 may also include at least one silicon-bearing side group and
this
copolymer may be formed by subjecting the halogenated polymer 2 to a ring-
opening
polymerization of an unsaturated heterocyclic group having at least one
electron rich site
therein, such as tetrahydrofuran or the others named above, and thereafter
subjecting the
resulting halogenated copolymer to an excess of a silicone group having at
least one
functional site, such as an amino group, leading to a co-polymer such as that
shown at 7.
Thus, the polymer of formula 5 incorporates silicones as side groups and thus
should,
21
CA 02284300 1999-09-29
in some cases, make such polymers more permeable to oxygen while the backbone
allows for
film formation without the need for cross-linking. The combination of the non-
cross-linking
capability provided in this case by the sulfur, nitrogen and/or phosphorous,
and the improved
permeability provided by one or more side groups should, in some cases,
provide improved
sensitivity as well as more predictable and linear Stern-Volmer behaviour.
Copolymers and
blends with organic monomers (such as THF as shown above) should in some cases
also
provide additional improvements and performance.
Thus, the polymer materials disclosed herein and the polymer materials formed
with
these polymer materials and a dye agent may be useful in a number of
environments, including
that ofpressure sensors, particularly phosphorescent sensors when used with
phosphorescent
dye agents, in a number of different oxygen environments, such as in the
atmosphere, in other
oxygen-containing fluid environments, such as in gases and liquids containing
oxygen, with
particular applications including that of measuring the efficiency of
aeronautic and aquatic
I 5 planforms (such as air craft fuselages and boat hulls), the measurement of
the presence of (and
possibly the content of) oxygen in ground water and the like.
While discussions herein are focused on polymers having nitrogen and both
sulfur and
phosphorous in the backbone, there are contemplated other polymers which do
not
necessarily have both sulfur and phosphorous. For example, other polymers may
just have
repeating S-N-S-N or P-N-P-N backbones, or of other irregularly or regularly
repeating
combinations of N, S and/or P.
Embodiments of the present invention will be described with reference to the
following examples which are presented for illustrative purposes only and are
not intended
to limit the scope of the invention.
EXAMPLE: SYNTHESIS OF A DYE-BOUND POLYMER
22
CA 02284300 1999-09-29
5-amino-1,10-phenanthroline:
To a solution of 5-nitro-1,10 phenanthroline (1.00g) in ethanol (40 ml) with
Pd/C
(0.40 g) were added sodium borohydride (2.00 g) in several portions over 2
hours. The
resulting solution was kept stirring for 12 hours under N,. The solution was
filtered and the
filtrate was concentrated. Then ether was added to the filtrate to crystallize
the product (0.33
g, yield: 38%).
4-aminomethyl-1,10-phenanthroline:
This synthesis was accomplished in 3 steps from 4-methyl-1,10-phenanthroline.
1,10-phenanthroline-4-carboaldehyde: Se02 ( 1.22 g, 99%) was dissolved in 20
ml refluxing
dioxane/water (v/v, 94:4). 4-methyl-1,10-phenanthroline (1.00 g) in 80 ml
dioxane/water
(v/v, 94:4) was added drop wise over 1 hour. Refluxing was continued for 2
hours under N2.
The resulting solution was filtered through celite when hot. The product (1.00
g, containing
selenium residues) crystallized as yellow solids and were used without further
purification.
1,10-phenanthroline-4-carboaldoxime: A solution of the 1,10-phenanthroline-4-
carboaldehyde product (1.00 g), hydroxyamine hydrochloride (2.00 g) and
pyridine (4 ml) in
ethanol (60 ml) was heated under reflux for 12 hours under N2. The resulting
grey solids
were separated from the ethanol solution. The grey solids were recrystallized
in hot ethanol.
The product was dried in vacuo (0.65 g, yield: 61 %).
4-aminomethyl-1,10-phenanthroline: A suspension solution of 1,10-
phenanthroline-4-
carboaldoxime (0.5 g) in 100 ml ethanol containing 2% perchloric acid was
hydrogenated at
atmospheric pressure, over 10% Pd/C ( 150 mg) for 12 hours and then was
refluxed for 2
hours. The solution was filtered and the filtrate was concentrated. Ether was
added to the
23
CA 02284300 1999-09-29
filtrate to crystallize the product (0.44 g, 97%).
Synthesis of Ru Dyes Functionalized with Amino Groups
Cis-Dichlorobis (4,7-diphenyl-1,10-phenanthroline)ruthenium: Ruthenium
trichloride (2.0
g), 4,7-diphenyl-1,10-phenanthroline (5.8 g), lithium chloride (2.2 g) were
heated at reflux
in 30 ml of DMF for 3 hours. The solution was then added with 80 ml of acetone
and stored
at 0°C overnight. Purple solids were collected by suction filtration
and washed by water. The
crude products were stirred in the cold acetone for 3 hours and filtered. The
product
suspended in water (100 ml) was heated under reflux for 2 hours and treated
with lithium
chloride (5.0 g). The purple solids precipitated from the cold solution and
were filtered and
dried in vacuo (2.5 g).
Ru[4,7-diphenyl-1,10-phenanthroline)2(L)][C104]z (L=5-amino-1,10-
phenanthroline or 4-
I 5 aminomethyl-1,10-phenanthroline):
Ru(4,7-diphenyl-1,10-phenanthroline),C1, (200 mg) and 60 mg of L were heated
under reflux
in 200 ml ethanol/water (v/v 70:30) for 18 hours under N,. The resulting
solution was treated
with LiCIOa (4.0 g). Red orange solids were collected by filtration and washed
by water,
hexane and ether (200 g, yield: 90%).
Synthesis of Dye Bound Polymer:
A CHZC12 solution of Ru[4,7-diphenyl-1,10-phenanthroline)2(L)]z (L=5-amino-
1,10-
phenanthroline or 4-aminomethyl-1,10-phenanthroline) (1.0 mg) was added to
poly(pentachlorothionylphosphazene) (200 mg) in CH,C12 solution while being
stirred at room
temperature. After 4 hours, the mixture was cooled to 0°C and excess n-
BuNHz (10 equiv.)
was added into the mixture and was stirred for overnight. After this reaction
finished, the
24
CA 02284300 1999-09-29
polymer was precipitated in water (three times) and then precipitated in water
methanol (three
times). Characterization was achieved by 1 H NMR and 31 P NMR. Films were
spray coated
from organic solvents such as CH,CI, or 1,2-dichloroethane.
ANALYSIS
Samples were placed in a pressure chamber which was equipped with a viewing
window to permit in-situ pressure measurements. The pressure chamber was
equipped with
an adjustable pressure supply of compressed air. All the measurements were
made at room
temperature (22 ~ 1 °C).
An imaging system was used to detect changes in luminescence intensity as a
function
of pressures. A 75 Watt tungsten Halogen lamp was used as a light source with
a 40 nm band
pass filter centred at 450 nm to obtain the blue light. A cut-off filter,
corresponding to OG
590 nm for the Ruthenium dye-bound polymer, was placed in front of a liquid
nitrogen cooled
CCD detector (MODEL LN/CCD, PRINCETON INSTRUMENTS, INC.) with 578 x 384
pixels in a cell size of 13.25 X 8.83 mm'-. Luminescent light from the sample
surface was
collected with a camera lens (NIKON, SSmm,1:1.2) and imaged onto the CCD
detector. The
pressure of the sample chamber was measured by a pressure gauge (MODEL FA233,
WALLACE AND TIERNAN) with an accuracy of ~ 0.1 psi. The results are shown in
figure
1, as a plot of a luminescence intensity ratio versus pressure ratio, showing
a substantially
linear relationship therebetween.
A scale wing model was also coated with the dye-bound polymer as discussed
herein
above and was positioned in a 5X5 foot wind tunnel and subjected to wind
speeds of Mach
0.8. Two images were taken in order to calculate the pressure distribution
over the model
surface. One image (hereinafter referred to as the 'wind-on' image) was taken
when the wind
speed was Mach 0.8 where the pressure distribution on the model is unknown.
The other
CA 02284300 1999-09-29
image (hereinafter referred to as the 'wind-off image) was taken when the wind
was off so
that the pressure distribution on the model was a constant of 1 atm. The wind-
off image was
divided by the wind-on image and the resulting image was shown in figure 2,
which presents
a complete static pressure mapping. Figure 2 also demonstrates, for example,
the transition
from relatively low pressure present at the leading edge of the wing (as shown
by the dark
colour) to a relatively high pressure at the trailing edge of the wing, as
would be expected.
Figure 3 is a comparison of pressure distribution measurements on a wing model
at Mach 0.8.
The solid circles are pressure tap measurements and the solid line is a
pressure sensitive paint
measurement.
EXAMPLE: SYNTHESIS OF POLYMER WITH SILOXANE SIDE GROUP
(3-aminopropyl)-heptamethyltrisiloxane.
In a 250 mL round bottom flask was placed freshly distilled (N-
trimethylsilyl)allylamine ( 10
g, 0.07 mol) (which was prepared via the literature procedure of Bachrach and
co-workers
in Bachrach, A., Zilkha, A. Eur. Polym. J. 1984, 20, 493. Also see Speir, J.
L. Adv.
Organomet. Chem. 1979, 17, 407.) was mixed with freshly distilled
1,1,1,3,3,5,5-
heptamethyltrisiloxane ( 17.2 g, 0.07 mol). To this was added THF 0100 mL) and
chloroplatinic acid hexahydrate (0.5 mol. %). The reaction mixture was
refluxed for 12
hours or until no Si-H stretching peak in the infrared spectrum at 2145 cm'
could be
detected. The reaction mixture was then cooled to room temperature, and a
large excess of
95% ethanol (~SOmL) was added. The reaction mixture was then refluxed for 12
hours to
remove the N-trimethylsilyl protecting group. The product was isolated by
vacuum
26
CA 02284300 1999-09-29
distillation and purified by vacuum fractional distillation from Ba0 [bp 45 ~C
(0.05
mmHg)]. (18 g, 83 %). 'H NMR (CDCl3) 8(ppm): 2.65 (1H, m, CHZCHZCHZ), 1.46
(2H,
m, CHZCHzCH2), 0.52 (2H, m, CHZCHZCHZ), 0.13 (9H, s, Si(CH3)3), 0.11 (6H, s,
Si(CH3)z),
0.05 (6H, s, Si(CH3)Z). '3C NMR (CDZCIZ) S(ppm): 45.85 (H2NCH2CHZCH2-), 28.01
(HZNCHZCHZCHZ-), 15.80 (HZNCHZCH2CHz-), 2.32, 1.78, 0.65 (Si(CH3)).
Poly(aminopropyl-heptamethyltrisiloxane)thionylphosphazene
The cyclic thionylphosphaaene (2.0 g) was heated in an evacuated sealed Pyrex
tube
at 165 ~C for 4 h. The tube contents were then dissolved in ca. (ie.
approximately) 40 ml
of CHZCIz and the solution was concentrated to ca 10 ml and was then added
dropwise to
200 ml of stirred hexanes via cannula. The colorless, moisture sensitive,
elastomeric
polymer was dissolved in 100 ml of CHZClzand (3-aminopropyl)-
heptamethyltrisiloxane was
added dropwise to the polymer solution which was cooled to 0 ~C. White
precipitation
formed immediately after the addition. The solution was concentrated to ca. 20
ml and
filtered through a filter frit. the precipitation was carried out by first
redissolving the dried
crude product in ca. 10 ml of THF then precipitating into water three times
followed by
precipitating from CHZCIz into hexanes three times. Final product was dried
from CHZC12
under high vacuum for 24 hours at ambient temperature (84 %). 3'P NMR (CHZCIz)
8(ppm):
1.45, 0.94. 'H NMR (CDC13) 8(ppm): 2.85 (m, br, propyl), 1.48 (m, br, propyl),
0.63 (m,
br, propyl), 0.12(s, br, Si(CH3)), 0.04 (s, br, Si(CH3)). '3C NMR (CDZCl2)
8(ppm): 44.76
(propyl), 26.17 (propyl), 16.15 (propyl), 2.02, 1.62, 0.67 (Si(CH3)). 29Si NMR
(CHZCIz)
27
CA 02284300 1999-09-29
8(ppm): 9.22, -0.80, -18.70. GPC measurement: Mw = 1.44 x 105, PDI = 1.8.
Poly[(aminopropyl-heptamethyltrisiloxane)thionylphosphazene]-b-
poly(tetrahydrofuran)
The cyclic thionylphosphazene (2.0 g) was heated in an evacuated sealed Pyrex
tube
at 165 ~C for 4 h. The tube contents were then dissolved in ca. 40 ml of
CHZC12 and the
solution was concentrated to ca 10 ml and was then added dropwise to 200 ml of
stirred
hexanes via cannula. The colorless, moisture sensitive, elastomeric polymer
was dissolved
in 100 ml of THF and the solution then stored in the refrigerator at -14 ~C
for 48 h. A
significant solution viscosity increase was noticed. (3-aminopropyl)-
heptamethyltrisiloxane
was added dropwise to the polymer solution which was cooled to 0 oC. White
precipitation
formed immediately after the addition. The solution was concentrated to ca. 20
ml and
filtered through a filter frit. The precipitation was carried out by first
redissolving the dried
crude product in ca. 10 ml of THF then precipitating into water three times
followed by
precipitating from CHzCl2 into hexanes three times. Final product was dried
from CHzCIz
under high vacuum for 24 hours at ambient temperature. Yield of 5, a colorless
film forming
material (79 %). 3'P NMR (CHZC12) 8(ppm): 1.39, 0.94. 'H NMR (CDCl3) 8(ppm):
3.45
(s, br, poly-THF), 3.01 (m, br, propyl), 1.65 (s, br, poly-THF), 1.37 (m, br,
propyl), 0.94 (m,
br, propyl), 0.15 (s, br, Si(CH3)), 0.05 (s, br, Si(CH3)). '3C NMR (CDZC12)
8(ppm): 71.33
(poly-THF), 45.16 (propyl), 27.43 (poly-THF), 26.51
(propyl), 16.44 (propyl), 2.40, 1.57,
1.07 (Si(CH3)). GPC measurment: Mw = 2.93 x 105, PDI = 1.8.
28
CA 02284300 1999-09-29
ANALYSIS
Samples utilizing the siloxane-bound polymers were placed in a pressure
chamber
which was equipped with a viewing window to permit in-situ pressure
measurements. The
pressure chamber was equipped with an adj ustable pressure supply of
compressed air. All the
measurements were made at room temperature (22 ~ 1 °C).
An imaging system was used to detect changes in luminescence intensity as a
function
of pressures. A 75 Watt tungsten Halogen lamp was used as a light source with
a 40 nm band
pass filter centred at 450 nm to obtain the blue light. A cut-off filter,
corresponding to OG
590 nm for the Ruthenium dye-bound polymer, was placed in front of a liquid
nitrogen cooled
CCD detector (MODEL LN/CCD, PRINCETON INSTRUMENTS, INC.) with 578 x 384
pixels in a cell size of 13.25 X 8.83 mm'. Luminescent light from the sample
surface was
collected with a camera lens (NIKON, SSmm, 1:1.2) and imaged onto the CCD
detector. The
pressure of the sample chamber was measured by a pressure gauge (MODEL FA233,
WALLACE AND TIERNAN) with an accuracy of ~ 0.1 psi.
Testing of several samples in this manner yielded standard Stern Volmer plots
with
slopes ranging from 0.17 - 0.18, and for the copolymer of Poly(THF) and
PTP(aminopropyltrisiloxane) ranging from 0.49 to 0.60, with a linearity
ranging from 0.990
to 1Ø
29