Note: Descriptions are shown in the official language in which they were submitted.
CA 02284618 1999-09-23
WO 98/42895 PCT/NZ98/00039
.. _1_
"COLOURING MAGNESIUM OR MAGNESIUM ALLOY ARTICLES"
Introduction
The present invention relates to a method of colouring magnesium ormagnesium
S alloy material having an anodized or other oxidised surface (as in the
chemical meaning,
ie; a transfer to a higher oxidation state, not limited to magnesium oxide) of
the
magnesium or magnesium alloy and to the product of such a process and related
apparatus and procedures. The present invention also consists in a related
procedure for
increasing corrosion resistance.
The colouring of magnesium alloy articles that have been anodized (or
otherwise
chemically oxidised) has defeated most attempts by experimenters attempting to
provide
a range such as is available for anodized aluminium. Whereas the colouring of
anodized
aluminium is available through several methods, there are no simple analogies
available
for magnesium even though much research has been directed towards this goal
over a
period of some years.
As most anodic films generated on aluminium have a regular hexagonal pore
structure, characterised by the spacing, size and even distribution of the
pores, several
options for colouring aluminium involve inducing colouring agents (dyes, metal
ions
or pigments) to enter the pores. Once the colouring step has concluded, the
article is
generally "sealed", a treatment that closes the pores and in effect, locks the
colouring
material into place. Transition metal ions achieve a strong colouring effect
when used
in this manner.
Magnesium anodic films feature a pore structure as well, but unlike aluminium
this is not well characterised and the pores are unevenly distributed. The
pores range in
diameter, but are generally larger than the aluminium pores. Also, the anodic
film is
translucent or opaque, not transparent, therefore any colouring effect from a
species
introduced into a pore may be more limited.
Nonetheless, early experimenters attempting to produce a range of colours on
anodized magnesium articles tried to use aluminium colouring systems. These
were
completely unsuccessful. One reason is that most aluminium colouring systems
operate
under conditions of acid pH at which aluminium is relatively impervious to
dissolution.
CA 02284618 1999-09-23
WO 98/42895 PCT/NZ98/00039
-2-
Magnesium does not exhibit the amphoteric behaviour of aluminium, which is
usually
anodized under acid conditions, and most magnesium anodising processes are
alkaline.
Acid processes applied to magnesium articles normally result in rapid
dissolution of the
metal, with the exception of a few acids which form insoluble magnesium
compounds
that cover and passivate the metal.
Acid colouring systems are therefore usually inapplicable to magnesium and
will
ordinarily result in attack of the substrate metal as well as the anodic film.
As one of
the principal reasons for anodising the metal is to increase corrosion
resistance, using
a colouring process that attacks the substrate or anodic film is contra-
indicated.
Even if a method of introducing transition metal ions into anodic film pores
was
developed it is unlikely to be satisfactory. This is because magnesium is a
highly
reactive metal, considerably more so than aluminium, and the presence of such
species
at the base of the film pores would introduce micro galvanic cells ready to
initiate
corrosion. As corrosion resistance may be a reason for anodising magnesium
substrates
an increase in the corrosion susceptibility of the substrate is not usually
commercially
acceptable.
US Patent 4,551,211 (Ube Industries) discloses a method of anodising a
magnesium article in a solution containing very high concentrations of
aluminium (as
aluminate ion) resulting in a combined magnesium/aluminium oxide matrix which
can
then be coloured using conventional aluminium dyeing technology. The process
is
notable for its difficulties in application and expensive chemicals being
required
(iodides). The colouring method there disclosed cannot be extended torother
anodic
films. US Patent 4,551,211 admits to the complexity of the process.
Other attempts at colouring have resulted in a loss in corrosion resistance.
An
example is the MAGOXIDT~' process described in US Patent 4,978,432
(Schmeling).
This teaches a method of anodising magnesium in which the formation of
phosphate
and aluminate salts of magnesium is preferred above the oxide. A licensee of
this
Magoxid process, Luke Engineering of Ohio report in their process literature
that:
"Some progress is being made in coloring this coating. Currently, however,
significant
reduction in corrosion resistance is an unwanted side effect of dyeing."
"Operations in Magnesium Finishing", of the Dow Chemical Company, ( 1990)
CA 02284618 1999-09-23
WO 98/42895 PCT/NZ98/00039
-3-
includes the statement:
"Anodizing of magnesium essentially follows the same procedure used on other
metals: soil
removal, deoxidation and anodisation. Both alkaline and acid anodizes are
available. Unlike
such coatings on aluminium, however, anodic coatings on magnesium do not lend
themselves
to dyeing. For colour effects on magnesium, clear anodizes or bright pickles
are used in
combination with tinted or dip-dyed clear lacquers."
Until the present invention there has been no elegant answer to a desire to
colour
magnesium. Thus far prior art processes that have achieved limited success
have done
so at the expense of some other desirable property. We believe there are
several reasons
why this should be so. Whilst we theorise in this respect hereinafter we do so
on a
without prejudice basis to the invention hereinafter defined.
The present invention is directed to a method of colouring magnesium or
magnesium alloys which provides a material having a coloured yet protection
providing
anodized or other oxidised surface. By the term "other oxidised surface" is
not meant
a modification of the surface with an oxidised metal of a different species as
in US
Patent 4,551,211.
It is also the object of the present invention to provide products modified by
a
method of the present invention and related methods and means.
In a first aspect the present invention consists in a method of colouring a
magnesium or magnesium alloy article, said method comprising or including the
steps
of ensuring the article has, or providing the article with an anodized or
other oxidised
surface of the magnesium or magnesium alloy, and
contacting said anodized or other oxidised surface with at least ane species
of
chromophoric moiety in a liquid carrier in conditions, or in a sequence of
conditions,
not substantially prejudicial to the integrity of said anodized or other
oxidised surface
yet which results in the chromophore(s) becoming associated (directly or via a
moiety
or species attached to the chromophore) with the surface by a reaction or
adsorption.
Preferably said conditions exclude the prospect of an aluminium build up on
said
surface even should the magnesium have been alloyed with aluminium.
Preferably the carrier is aqueous.
Preferably said surface is anodized.
In some forms of the invention said surface is chemically oxidised.
CA 02284618 1999-09-23
WO 98/42895 PCT/NZ98/00039
-4-
Preferably the conditions include elevated temperature(s).
Preferably the step of contacting is by immersion although spraying is another
option to find favour.
Preferably the method includes washing the anodized or other oxidised surface
prior to contact with said at least one species of chromophoric moiety in a
liquid carrier.
Preferably the method includes a washing step subsequent to the contact with
said at least one chromophoric moiety in a liquid carrier.
Preferably said conditions involves a near neutral, neutral or alkaline pH.
Preferably said pH is alkaline or at least as alkaline as the dye process will
allow.
Preferably said species is selected from the group consisting' of reactive
dyes,
direct dyes, VAT dyes, sulphur dyes and disperse dyes (as defined in the
Colour Index).
Most preferably the species is one of the group consisting of reactive dyes,
VAT
dyes and sulphur dyes.
Preferably said species is selected from the group consisting of vinyl
sulphones,
monochloro triazines, dichloro triazines, pyrimidines, phthalocyanines,
quinoxalines,
aniline derivatives, monofluoro triazines, anthraquinones, indigo and
halogenated
indigo derivatives, polysulphides, and diazo phenols.
Preferably said species is selected from the group consisting of vinyl
sulphones,
monochloro triazines, dichloro triazines, pyrimidines, phthalocyanines,
quinoxalines,
monofluoro triazines, anthraquinones, indigo and halogenated indigo
derivatives,
polysulphides, and diazo phenols.
In another aspect the invention comprises or includes
(i) providing a bath containing a sulphur textile dye together with alkali and
a
reducing agent such that the dye is present in its water-soluble reduced leuco
form;
(ii) immersing said article in said bath; and
{iii) subsequently immersing said article in an oxidising solution to form the
dye on
its surface.
Preferably said bath is at approximately 90 ° C for approximately 1/2
an hour.
Preferably said sulphur textile dye is provided at approximately double the
normal concentration employed for textile dyeing.
CA 02284618 1999-09-23
WO 98/42895 PCT/NZ98/00039
- -5-
In still another aspect the invention comprises or includes
(i) providing a bath containing a VAT textile dye together with an alkali and
reducing agent such that the dye is present in its water soluble leuco form;
(ii) immersing said article in said bath; and
(iii) immersing said article in a mildly acidic oxidising solution to form the
dye on
the surface of the article.
Preferably said reducing agent is sodium dithionite.
Preferably said article is immersed at approximately 70 ° C for
approximately 30
minutes.
Preferably said VAT textile dye is provided at double the normal concentration
employed for textile dyeing.
Preferably said article is immersed in said mildly acidic oxidising solution
for
approximately five minutes.
In yet another aspect the invention comprises or includes
(i) providing a bath containing a vinyl sulphone reactive textile dye;
(ii) immersing said article in said bath; and
(iii) washing said article.
Preferably said bath is provided at a pH of approximately 12 and said article
is
immersed for approximately 30 minutes at at least 60 ° C (preferably 80
° C),
Preferably said bath contains a reactive dye, and at least one of sodium
carbonate, sodium hydroxide or sodium silicate.
Preferably said bath contains a reactive dye in a concentratiorrof 5g/L and
sodium carbonate, also at a concentration of Sg/L and sodium hydroxide at a
sufficient
concentration to raise the pH to about 12.
Preferably said washing is in water at at least 60°C (preferable with a
wetting
agent, eg; ethylene glycol).
In yet another aspect the invention comprises or includes
(i) providing a bath containing a monochloro triazine reactive textile dye;
(ii) immersing said article in said bath; and
(iii) washing said article.
Preferably said bath and said article is immersed for approximately 30 minutes
CA 02284618 1999-09-23
WO 98/42895 PCT/NZ98/00039
-6-
at approximately 60 ° C.
Preferably said bath contains a reactive dye, and at least one of sodium
sulphate,
sodium chloride, carbonate and sodium hydroxide.
Preferably said bath comprises a reactive dye in a concentration of 0.5 - 2%
and
NazS04 concentration is equal to dye concentration, sodium carbonate 0.5 - 1%
and
NaOH 0.25%.
Preferably said washing is in water at 80°C.
In one embodiment of the invention said surface is anodized and said at least
one
species of chromophoric moiety in a liquid carrier is a reactive dye of a kind
having a
vinyl sulphone functional group and wherein the conditions are within a
temperature
range of from 30 to 100 ° C during an immersion or spray presentation
of the liquid dye
composition to the anodized surface for at least 10 minutes and thereafter a
rinsing step
with water at an elevated temperature.
Preferably said contact time is about 30 minutes.
Preferably said rinsing step is with hot water containing a wetting agent and
the
temperature of the aqueous rinsing composition is 80°C or greater.
Preferably said temperature of the immersion or spraying with the liquid dye
composition is at about 60 ° C.
Preferably the dye has a concentration of at least about 0.5% w/v in an
aqueous
system.
In another embodiment of the invention said surface is anodized and wherein
the
at least one species of chromophoric moiety in a liquid carrier is a
reactiv~dye of a kind
having a monochloro triazine functional group and the conditions during the
contact by
immersion or spraying is contact at an elevated temperature.
Preferably the contact time is for a period of from 30 to 60 minutes.
Preferably the elevated temperature of the contact is at about 70 ° C
or higher.
Preferably the dye concentration is from 0.5% to 2% w/v.
Preferably there is also present sodium sulphate at substantially equal
concentration to the dye, sodium carbonate at 0.5 to 1.0% w/v and sodium
hydroxide
to a concentration of about 0.25% w/v.
Preferably there is a rinsing step with hot water containing a wetting agent
and
CA 02284618 1999-09-23
WO 98/42895 PCT/NZ98100039
_ _ 'j _
at a temperature of 80°C or greater.
In another embodiment ofthe invention said at least one species of
chromophoric
moiety in a liquid carrier is that of a reactive dye having a dichloro
triazine functional
group or a monofluoro or difluoro triazine functional group.
Preferably the conditions are similar to those for monochloro triazine
functional
groups.
In still another embodiment the at least one species of chromophoric moiety in
a liquid carrier is a reactive dye of the pyrimidine type.
In a different embodiment the at least one species of chromophoric moiety in a
liquid carrier is that of a vat dye.
Preferably the dye is an anthraquinone or indigo derivative.
Preferably the vat dye is dispersed in water and reduced to water soluble
leuco
form using a combination of a reducing agent and alkali.
Preferably said leuco dye is used to colour anodized magnesium article by
immersion or spraying.
Preferably the temperature of leuco dye is elevated. Most preferable
60°C or
higher.
Preferably pH of leuco dye is 11.5 or greater.
Preferably leuco dye dyed article is rinsed in cold water (eg; 5 seconds) and
then
oxidised so that leuco dye is converted to insoluble pigment.
In another embodiment the at least one species of chromophoric moiety in a
liquid carrier is that of a sulphur dye. _
Preferably the moiety is attached to a polysulphide.
Preferably the sulphur dye is dispersed in water and reduced to water soluble
leuco form (thiol form) using a combination of a reducing agent and alkali.
Preferably said leuco dye is used to colour anodized magnesium article by
immersion or spraying.
Preferably the temperature of leuco dye is elevated. Most preferable 60
° C or
higher.
Preferably pH of leuco dye is 11.5 or greater.
Preferably Ieuco dye dyed article is rinsed in cold water (eg; 5 seconds) and
then
CA 02284618 1999-09-23
WO 98/42895 PCT/NZ98/00039
_8_
oxidised so that leuco dye is converted to insoluble pigment.
In still another embodiment the at least one species of chromophoric moiety in
a liquid carrier is a direct dye.
Preferably said direct dye comprises a chromophoric moiety attached to a
sodium
S sulphonate group.
Preferably direct dye is present in solution 0.5 to 2% w/v with sodium
sulphate
0.5 to 2% w/v and, optionally, sodium carbonate to a concentration of about
0.25 to 1
w/v.
Optionally an anodic potential of approximate 10 to 20 V DC may be applied to
the anodized article.
Preferably temperature of dye solution is at least 60°C.
Preferably dyed article is rinsed after dyeing.
In yet another embodiment the at least one species of chromophoric moiety in
a liquid Garner is a disperse dye.
Preferably dye is evenly dispersed in water to the extent of at least 0.25%
w/v.
Preferably a Garner being an ester is added to aid dyeing.
Preferably the article is introduced into the dye dispersion at 100°C
or greater
{eg; under pressure) and maintained in such contact for 20 to 90 minutes.
Preferably the article is subsequently rinsed.
In still a further aspect the present invention consists in a method of
increasing the
corrosion resistence of an anodized magnesium or magnesium alloy surface which
comprises the step of colouring such a surface by a process as previously def
ned
Preferably the at least one species of chromophoric moiety in liquid carrier
is that
of a reactive dye of a kind having a vinyl sulphone functional group.
In some forms of the present invention the at least one species of
chromophoric
moiety in a liquid carrier is an acid dye using an acid which forms magnesium
salts.
Preferably said acid is lactic acid.
Optionally the contact step with the liquid carried chromophoric moiety or
moieties is a multiple step process. Such multiple step process may result
from multiple
dipping, sequential spraying or a hybrid of dipping and spraying.
Optionally a plurality of compatible chromophoric moieties are used such as
_ _- i _ __._..._. - __ T _
CA 02284618 1999-09-23
WO 98/42895 PCT/NZ98/00039
- -9-
might result from mixed dyes.
In a further aspect the present invention consists in a magnesium or magnesium
alloy material having a coloured anodized or other oxidised surface, said
colouring
having been achieved using a method in accordance with the present invention.
Preferred forms of the present invention will now be described.
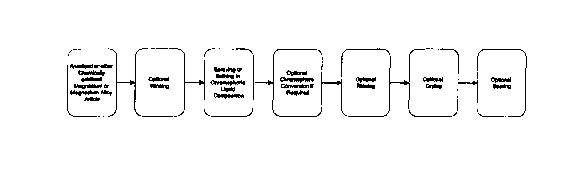
Figure 1 is a simple flow diagram showing the optional steps sometimes
preferred.
Preferred surfaces to be coloured include those anodized by a process such as
disclosed in PCT/NZ96/00016 (WO 96/28591 ) (Barton) or as disclosed in our PCT
International Application filed simultaneously herewith.
We believe (without wishing to be bound to such a theory) that historically
attempts to anodize magnesium have been made under the shadow of already known
aluminium techniques and it has been presumed that the technologies applicable
to
aluminium can be modified in some way to make them work on magnesium.
However, this approach we believe to be flawed. The metals aluminium and
magnesium are fundamentally different. Aluminium is amphoteric and is usually
anodized in acidic media. In solutions of strong alkalies, the metal dissolves
releasing
hydrogen:
Al + 20H~ ~ AIOz'_ + H,
Magnesium does not react with strong alkalis, and magnesium oxide is insoluble
in bases such as sodium hydroxide whereas aluminium oxide is readily soluble
in such
bases.
The oxidation of aluminium results in a well-defined surface oxide layer which
features regular hexagonal pores having a diameter of about O.l~m. These
result from
the dissolution of the anodic film in the electrolyte in which it is partially
and slowly
soluble. Magnesium forms pores as well, but not because of any solubility of
the anodic
film in its electrolytes. In alkaline electrolytes it appears that the film is
insoluble. In
such acidic electrolytes as are used it may be slightly soluble but it is only
with the
presence of added species that an impervious barrier film results. For example
anodisation of magnesium may be conducted in acid fluoride solutions in which
an
CA 02284618 1999-09-23
W O 98/42895 PCT/NZ98/00039
- 10-
impervious layer of magnesium fluoride results.
The anodic film formed on aluminium is transparent in thin films and colours
resulting from pigments or dyes deep within the pores therefore impart an
overall colour
to the film. Aluminium dye systems cannot be applied directly to magnesium
anodic
films.
Films formed on magnesium substrates are not transparent and have a natural
colour. This is sometimes slightly off white, but can be quite dark, depending
on the
electrolyte and conditions of anodisation. Colouring such a film would not be
a simple
matter of introducing a dye into the pores, which in any case are irregular
and typically
have diameters ranging from 2~cm to 5~m.
A colorant suitable for use on magnesium therefore has to mask whatever
natural
colour the surface has in addition to providing a strongly adherent layer
itself. It is
almost inevitable that any suitable method for colouring magnesium will add
another
layer to the surface owing to the translucent nature of the coating.
Some conversion coatings are available for use on magnesium substrates and
these produce, in some instances, strongly coloured substrates. For instance,
British
Patent 493,935 describes a method by which a chromate or permanganate
conversion
coating may be formed on a magnesium article. The coatings formed by this
method are
typically very dark and even black. Such is not considered "colouring" for the
present
purpose as the colour cannot be controlled and the coating formed is not a
true anodic
film. The process cannot be applied to an anodic film or an already existing
other
oxidised coating. As transition metal compounds such as permanganatc or
chromate
are present the corrosion resistance rendered by these processes may be
reduced owing
to micro-galvanic cell formation.
One class of aluminium dyes comprises inorganic compounds that result from
a two-step process in which an insoluble colour is precipitated in the second
stage of the
process. The f rst stage introduces a water-soluble species to the pores in
the aluminium
oxide anodic film. This species is adsorbed onto the surface of the anodic
film. The
diffusion of these species into the pores in the anodic f lm is governed by
the surface
charge on the film, known as the zeta potential. Ordinarily this is positive,
but in
suitable electrolytes it may be changed.
_... .._.... .T.. _..._.... _..__.... ~...._. _._....
CA 02284618 1999-09-23
WO 98/42895 PCT/NZ98/00039
-11-
Double dip processes only work in the correct sequence. Thus for instance, in
producing a bronze colour by this method using cobalt acetate and potassium
permanganate, it is necessary to dip the aluminium article into the cobalt
acetate
solution first. It is believed that if the permanganate is used first,
potassium cations are
S adsorbed preferentially leaving no permanganate ions available for reaction
with the
cobalt acetate.
Examples of binary inorganic salt pairs that may be used to colour an anodic
coating on aluminium include:
~ Cobalt acetate/potassium permanganate Bronze
~ Ferric ammonium oxalate/potassium ferrocyanide Blue
~ Ferric ammonium oxalate/tannic acid Black
~ Copper sulphate/ammonium sulphide Green
~ Lead nitrate/potassium chromate Yellow
~ Ferric sulphate/potassium ferrocyanide Prussian blue
This process is specific to aluminium as the adsorption of the soluble species
occurs inside the pores and relies on the surface chemistry and charge of the
aluminium
oxide anodic film. Consequently, a parallel process involving inorganic ions
does not
exist for magnesium anodic films.
Organic colouring may also be employed on aluminium, although in most cases
the dyes are adsorbed rather than undergoing a chemical reaction withthe
substrate.
Acid and substantive dyestuffs normally react with the substrate but on
aluminium
anodic films they do not, although a colour is produced. The colour is not
particularly
wash or light fast.
In cases where there is a true chemical reaction the result is a light fast
colour.
Dyes of the metal complexing acid colour type come into this category. These
appear
to form new complexes with aluminium atoms.
There are also single dip inorganic baths which colour aluminium. It is
believed
that the pigments are adsorbed directly into the pores. These processes tend
to be very
sensitive to pH.
CA 02284618 1999-09-23
WO 98/42895 PCT/NZ98/00039
-I2-
Two points are evident in connection with the use of these agents on
magnesium:
1. The dyes that work on aluminium are acid based and will usually have an
adverse effect on the anodic film produced on magnesium.
2. Precipitating inorganic compounds inside the pores will not be effective
on magnesium due to the translucent nature of the film.
Accordingly, the use of aluminium dyes to colour magnesium is not effective
except in the special case described above where the anodic film is modified
to have a
very high content of aluminium oxide (US Patent 4,551,21 I).
Dyes for alkaline conditions
We believe that since magnesium is best anodized under alkaline conditions a
suitable colouring technology should also operate under conditions of alkaline
pH.
Such a technology would have to provide effective bonding between the
magnesium
oxide substrate and good colour fastness.
1 S An extensive range of dyes and colouring chemicals is available world-wide
yet,
these have been designed for specific applications.
The range of existing colouring compounds includes simple pigments, such as
titanium dioxide, which is used very widely as a brilliant white colour in
paints, and
exotic chemically-formed dyes which are the basis of photography.
In this discussion, a "pigment" may be regarded as being a coloured molecule
which is insoluble and unreactive. A pigment may colour an article by virtue
of being
in close contact with it although usually a carrier is required to ensure that
it stays in
place. For example, titanium dioxide is chemically inert and insoluble in
aqueous
solution or solvents. It is dispersed in paints and forms an opaque,
brilliantly white layer
when applied to articles in suspension in suitable Garners. Insolubility alone
does not
result in a pigment, as some insoluble colouring compounds may still react
chemically
with the substrate.
A "dye" on the other hand is often soluble in its Garner and in many cases it
is
chemically reactive with the substrate to which it will bond. In some cases,
the
"reaction" may be the formation of a clathrate compound, hydrogen bond or Van
der
Waals bond, while in others it may be a true chemical reaction.
T
CA 02284618 1999-09-23
WO 98/42895 PCT/NZ98/00039
-13-
There is overlap between pigments and dyes, because in some cases, a colouring
agent is initially a dye, but is converted to a pigment once it has penetrated
the substrate
that is to be coloured. In some cases, a pigment is converted to a soluble,
reactive farm,
which is then a dye, after application of which, it is converted back to a
pigment.
We have determined that some colouring compounds designed for textile
applications have application in colouring magnesium anodic films. In most
cases
optimisation of the process has required alterations to conditions of
application and the
use. In some cases we have found an anodic or cathodic potential may be
applied to the
magnesium article. Such a potential is not applied to any textile material for
use with
the same dye systems. Also, there is no simple parallel to the fibre
penetration
processes that are usually part of the mechanism by which a textile fibre
adsorbs dye
molecules. In many cases where alkaline textile dyes are employed, sodium
hydroxide
present in the dye bath assists in penetration of the fibre because it
promotes softening
and swelling of the fibre. The most applicable dyes are those applied to
cellulosic fibres,
such as cotton. The cotton fibre consists of a central core of polyhydric
alcohols
surrounded by inert cellulose.
An anodic magnesium substrate has no such compounds on its surface and is
completely inorganic in composition.
The exact chemical composition of an anodic film produced on magnesium
depends on the process being employed, but there are some common general
features.
Typically, the electric fields employed in anodising magnesium are very high,
resulting in deprotonation of the forming film and therefore the formation of
magnesium
oxide, rather than magnesium hydroxide. Ordinarily the oxide ion, Oz', is
instantly
hydrolysed in aqueous solution:
OZ- + H,O '-' 20H-
In the case of anodisation of magnesium, the electric fields are often in the
region
of 1 x 109 volts per metre, sufficient to ensure the presence of oxide ions at
active
reaction sites.
The generic processes widely employed to "anodize" magnesium are based on
electrolytes containing sodium hydroxide and additives, or an acid fluoride
salt plus
CA 02284618 1999-09-23
WO 98/42895 PCT/NZ98/00039
- 14-
additives. Typically these processes produce a rough, porous, partially
sintered anadic
film comprising magnesium oxide together with included species. On the
surface,
owing to hydrolysis, some magnesium hydroxide is formed.
Specialised processes are often designed to produce other species, or to
include
S species that are present in the electrolyte. Such species may include
silicates or borates.
The process described in US Patent 4,978,432, mentioned above, is designed to
ensure
cationic species are available at reaction sites with the intention that
magnesium
phosphate and magnesium aluminate be formed. Even so, it is expected that
magnesium
oxide and hydroxide will feature as part of the film as well.
The process disclosed in PCT NZ96J00016 (WO 96/28591 ) also creates an
anodic film comprising principally magnesium oxide, with some surface
magnesium
hydroxide formation, but it does this in a manner which leads to less
sintering of the
coating and more uniform distribution of pores.
Clearly anodized metal substrates are unlike the surfaces normally coloured
using fibre-penetrating textile dyes.
Despite these differences, we have determined that certain classes of
compounds
described in the Colour Index and intended for textile application can be
applied to
anodized magnesium substrates resulting in satisfactory colours. Amongst these
are the
following classes of compounds, although there are others that work as well:
Vinyl sulphones, Monochloro triazines, Dichloro triazines, Pyrimidines,
Phthalocyanines, Quinoxalines, Aniline derivatives, Monofluoro triazines,
Anthraquinones, Indigo and halogenated indigo derivatives, Polysnlphides, and
Diazo phenols.
Commonly, these dyes comprise a chromophore which is a functional group
which is attached to a reactive group that may bond either chemically or
physically to
the substrate. Since the same chromophore may be used in dyes that react
differently
to form the final coloured article, there is considerable overlap in the
functional groups
and a consideration of each class of reactive group is required. A good
example of
chromophore overlap is in the area of turquoise dyes. Most turquoise dyes are
based on
phthalocyanine, which can form a range of blue and green hues when
appropriately
substituted, but will not readily form other colours. This chromophore is
widely used
__. _. _ T _ .. . __ ________-__
CA 02284618 1999-09-23
WO 98/42895 PCT/NZ98/00039
- 1S -
to produce turquoise shades in reactive dyes and direct dyes, as well as other
classes
where appropriate.
Within classes of compounds, dyes may be mixed to obtain colours different
from those of the individual compounds. When mixing of dyes is carried out it
must be
S borne in mind that the most effective mixing strategies are those which mix
similar
types of chromophores as well as reactive groups. Accordingly, while it is
possible to
mix a vinyl sulphone reactive dye with a monochloro triazine reactive dye, the
results
that are obtained may not be as expected owing to differences in reactivity.
Also, even
if, for instance, two vinyl sulphone reactive dyes are mixed, the result may
not be the
intermediate colour expected as the chromophores may not be compatible. Very
often,
for any given set of dyes, there is a trichromic set specified which are three
dyes, most
commonly a yellow, blue and red dye, from which it is possible to generate a
wide
range of intermediate colours as these dyes are able to be mixed in all
proportions
without ill effect.
1 S Also, in some circumstances, it may be desirable to double or multiple dip
a
magnesium article in a second dye after completing a process in a first dye.
Provided
there are no fundamental chemical incompatibilities between stages, this is
possible and
could commonly be employed to produce the corrosion resistant substrate that
results
from infusion with a reactive dye, but to use a more light fast dye to colour
the article.
The hydrogen bonding of direct and vat dyes is not usually affected by a
process
involving a reactive dye as a first step and in some cases when not all
reactive sites have
been chemically bonded to a reactive dye, it is possible to double dip an
article in a
second reactive dye.
Dyeing an article that has been anodised may also be conducted for the reason
2S of increasing the salt spray resistance of the article and not for any
specific colour
requirement in the final product. In tests it has been found that the salt
spray resistance
of anodised magnesium test plates has been considerably enhanced when those
test
plates have been coloured. Improvements of 100% in salt spray resistance may
be
obtained in some circumstances, thereby justifying the colouring of anodised
articles
for this reason alone.
CA 02284618 1999-09-23
WO 98/42895 PCT/NZ98/00039
- 16-
Reactive Dyes
These compounds are so named because normally there is a true chemical
reaction between the dye molecule and the substrate. A considerable range of
reactive
dyes may be found listed in the Colour Index, but new compounds are
continually being
synthesised. In a cellulose molecule, there are secondary hydroxyl molecules
attached
to the ring:
R-CH,OH
The reactive dye bridges the oxygen by the following reaction:
Cell-CHZOH + Dye-X ~ Cell-CH,-O-Dye + HX
The result is a strongly adherent, chemically bonded colour that exhibits a
high
degree of wash-fastness. However, an alternative and undesired side reaction
also takes
place:
Dye-X + HBO ~ Dye-OH + HX
This hydrolysis of the dye proceeds more rapidly under alkaline conditions,
which unfortunately, are also the very conditions which are required to
activate and
catalyse the dyeing reaction. Since hydrolysed dye is normally poorly
adherent, it must
be rinsed out thoroughly after dyeing. Most reactive dyes have low
substantivity so that
hydrolysed dye may be removed from dyed articles easily. The hydrolysis of the
dye
has the effect of lowering the pH of the dye bath.
Since an anodic film on a magnesium substrate comprises essentially magnesium
oxide, it would not ordinarily be expected that such a dye would work well on
magnesium. However, some surface hydrolysis of the magnesium oxide is favoured
thermodynamically:
Mg0 + HBO ~ Mg(OH)z
The dye may therefore react with the magnesium hydroxide bridging an oxygen
atom as happens in the cellulose molecule. Since the magnesium hydroxide is
formed
on the surface, this form of dyeing would meet the requirements of the
foregoing
discussion.
Application of a reactive dye to a magnesium anodic film is conducted in a
CA 02284618 1999-09-23
WO 98/42895 PCT/NZ98/00039
- 17-
solution containing reactive dye, and a base, at a bare minimum. Often
additional alkali,
and sometimes an ionic salt can improve the dye exhaustion. The dyeing
reaction is
more active under more alkaline conditions and the presence of salt may help
aggregate
the dye on the substrate surface owing to the common ion effect. The reaction
normally
S takes place at a temperature of around 60 ° C and sometimes
higher.
Under some circumstances, the dyeing reaction can be influenced favourably by
applying an electric charge to the surface of the magnesium oxide. This can be
done
easily by means of the electrolytic apparatus used to anodize the substrate.
Hydrolysed dye is unavoidably present in the dye bath and some of this would
diffuse into the pores of the magnesium article and adhere weakly to the
surface. It
seems that long after a reactive dye would be regarded by the textile industry
as
unsuitable for use owing to the extensively hydrolysed nature of the dye
molecules, it
is still possible to dye anodic films on magnesium. Often there is a slight
shift in the
shade obtained, presumably because the hydrolysed dye cannot bond chemically
to the
substrate and must be physically adsorbed.
Although it appears that hydrolysed dye may still provide satisfactory
colouring,
it is normal after dyeing to have to rinse well in hot aqueous solutions
containing a
wetting agent, to remove quantities of unreacted and poorly adherent dye as
otherwise
drying marks will affect the finish of the magnesium article.
The functional groups present in a reactive dye are normally vinyl sulphone or
derivatives of trichloro triazine (cyanuric trichloride), such as monochloro
triazine.
Some reactive dyes comprise two or more reactive groups. In cases wher~two
reactive
groups are present, a benefit may accrue from combining, for example, a vinyl
sulphone
with a monochloro triazine group. If one group is hydrolysed, the dye may
still bond
chemically to the substrate.
CA 02284618 1999-09-23
WO 98/42895 PCT/NZ98/00039
-18-
Reactive dyes have to be small molecules so as to penetrate the cellulose
fibre
thus enabling the reaction. This generally means one chromophore only, and
this should
most advantageously be a small group, for instance a monoazo or diazo
compound.
Often a reactive dye will have the following structure:
S
Chromophore-NH-(Reactive group)
Sometimes it is possible to produce intermediate colours by attaching a second
chromophore to a reactive dye, but in such cases the molecule is generally too
large to
function properly as a reactive dye. An example is CI Direct Green~26, in
which the
molecule is based on a reactive dye, but contains two chromophores, a yellow
and a
blue, attached to a molecule of cyanuric trichloride.
To colour an anodized magnesium article in a reactive dye we recommend the
following steps be carried out:
1. Article thoroughly rinsed following anodising process, especially if
process is
an acid-fluoride process.
2. Prepare reactive dye by heating deionised water bath to around 60°-
80°C.
3. Add salt (if required), base (such as sodium carbonate, if required) and
alkali
(such as sodium hydroxide, if required).
4. Add dye and mix well to ensure complete dissolution.
5. Introduce article to be coloured and maintain temperature. Most reactive
dyeing
processes will require 20-60 minutes.
6. Rinse article in hot water, preferably 85 ° C or higher, containing
a wetting agent
such as ethylene glycol, maintaining article in rinse bath for at least two
minutes.
7. Repeat step #6 until all residues of poorly adherent hydrolysed dye are
removed.
If the rinsing process is not carried out correctly, hydrolysed dye will leach
from
areas that are drying leaving drying marks on coloured articles. Ifnecessary,
such marks
may be removed by re-rinsing in very hot or even boiling water. Concentrations
of
reactive dyes used to colour magnesium are often greater than those employed
on textile
materials. There is no need to use auxiliaries which for textile application
are present
to aid in fibre penetration.
__ _ ~ . ___ ____ ___ _.__ _ ___. T
CA 02284618 1999-09-23
WO 98/42895 PCT1NZ98/00039
-19-
It is common for reactive dyes for textile application to be applied
progressively
in a dye bath to which the dye is added, then after a period of time, salt and
alkali. Such
a method does not appear to be beneficial when dyeing magnesium substrates.
Reactive Dyes (Most Preferred Conditions):
(a) Vinyl sulphone functional group dyes.
* Relatively thick anodic film ( 15 microns plus)
* Temperature at 60 ° C
* Dye concentration 0.5%
* Sodium carbonate concentration 0.5%
* Sodium hydroxide to adjust pH of 1% dye bath solution to 11.5 - typically
this
is around 0.25% concentration
* Dyeing time at least 10 minutes. Preferably about 30 minutes
* Rinse step, highly preferred - hot water containing a wetting agent such as
ethylene glycol 0.1 %, most preferably 80 ° C (or greater) for one
minute or
longer.
Preferably the article is a temperature where it will quickly dry and there
will be
no leaching of hydrolysed dye.
(b) Monochloro triazine functional groups:
* Anodic film 15 microns or greater most preferred
* Dye concentration 0.5% to 2%
* Sodium sulphate concentration 0.5% to 2%, equal to dye concentration
* Sodium carbonate 1
* Sodium hydroxide 0.25%
* Temperature -- most preferably 70 ° C (the process probably works
quite well at
any temperature above this, but fairly slowly at lower temperatures)
* Time - at least 10 minutes - 30-60 minutes is typical
(c) Other types of reactives - conditions most favourable to our applications
are
similar to those above e.g. a dichloro triazine dye requires similar
temperatures and
concentrations to a monochloro triazine dye. Pyrimidine based reactive can
also be
used.
CA 02284618 1999-09-23
WO 98/42895 PCT/NZ98/00039
-20-
Direct Dyes
Compounds described in the Colour Index as direct dyes bond to cellulosic
fibres
by way of secondary chemical bonds, such as hydrogen bonds or van der Waals
forces.
As these bonds are not as strong as true chemical bonds, the molecules are
designed to
be large, thereby strengthening the bond. Most direct dyes range in molecular
weight
from 400 to 1,200. Most direct dyes are azo dyes, and possess a linear
structure.
Direct dyes must be soluble in water. Generally in practice, a direct dye is
applied to a cellulosic fibre by means of an aqueous solution in which a
soluble ionic
salt, commonly sodium sulphate or sodium chloride is dissolved. The ionic salt
assists
in the dyeing process as it is believed that it aids in the charge transfer
between the dye
and the fibre, thus enhancing dye uptake by the fibre. Often direct dyes
contain sodium
sulphonate groups. These ensure high water solubility and enable the dye
molecule to
form an ionic aqueous solution under conditions of alkaline pH:
Dye-S03H + OH' '-' Dye-SO,-+ H20
As the dye chromophore is negatively charged, it is attracted to any ions that
are
positively charged and repelled from negatively charged ions or surfaces. The
addition
of an ionic salt is thought to assist in dyeing partly because of the "common
ion" theory
in which the dye is "salted out" or aggregates are formed. As there is a
balance between
water solubility and affinity for the substrate, a balance between exhaustion
of the dye
onto the substrate and that remaining in solution is established. The more
alkaline the
pH, the greater the water solubility of the dye. After a point, it also
increases the affinity
of the dye for the aqueous phase, rather than the insoluble fibre, hence it
reduces
exhaustion of the dye.
It is possible in some cases to improve the fastness of the dyed article by
after
processing the dyed article to render the dye in an insoluble form. This may
be done by
increasing the size of the molecule. Direct dyes which contain a primary amino
group
attached to an aromatic ring may be diazotised and coupled to a naphthol
component.
This process tends to alter the dye colour as an azo group is added:
Ar-NH, + NaNO, + 2HCI ''' Ar-NNCI + 2H,0 + NaCI
_ _..__ _~. _.-__.__ _ _ _.___.T
CA 02284618 1999-09-23
WO 98/42895 PCT/1~1Z98/00039
-21 -
Ar-NNCI + C,~H,OH* '-' Ar-NN-C,oH60H *(3-naphthol (as below)
i
Cationic agents, comprising a long chain hydrocarbon tertiary amine may be
added to improve wash-fastness:
CxHyN+(CH3), + Dye-SO,Na '-~ C~HyN(CH,),SO,-Dye + Na+
The resulting organic molecule is very large and not soluble in water. Such
agents added to an aqueous solution of a direct dye will cause the
precipitation of the
dye.
In the case of magnesium, the dye may have affinity for the layer of hydroxide
formed at the surface of the anodic film, the bonding that results being
hydrogen
bonding similar to that existing in water.
Application of a direct dye to a magnesium article may involve a solution
being
made comprising the direct dye, a salt (commonly sodium sulphate) and in some
cases,
sodium carbonate or other suitable base. The use of sodium sulphate is
preferred over
the use of sodium chloride as chloride ions are noted as initiators of pitting
corrosion
and are therefore an unwanted species inside the pores of the anodic film. The
dye is
normally heated to around 70°C, although in some cases higher
temperatures may be
desired.
The dyeing is normally complete after 60 minutes.
Since the soluble dye molecule tends to be negatively charged when the sodium
sulphonate group dissociates, an anodic voltage applied to the dye bath can
improve dye
deposition substantially. Such a voltage is ordinarily fairly low but depends
on the
thickness of the anodic film as this is an efficient insulator. Voltages less
than 50 volts
are normal.
CA 02284618 1999-09-23
WO 98/42895 PCT/NZ98/00039
-22-
Direct Dyes (Most Preferred Conditions)
* Prepare dye bath at a concentration ranging from 0.5% to 2% w/v
* Add sodium sulphate at similar concentration; viz 0.5% to 2% w/v
* Add sodium carbonate, 0.5% w/v, especially to direct black dyes
S * Heat to 60 ° C and immerse article for 60 minutes
* Optionally, apply an anodic voltage to the part in the order of IOV.
* Remove, rinse and dry.
VAT Dyes
Vat dyes are in fact insoluble pigments that are reduced to a water soluble
leuco
form in alkaline conditions for application to a fabric or anodized magnesium
article,
prior to being converted back to an insoluble pigment that is then highly
light and wash
fast on the finished article. According, the vat "dye" as such has a
transitory existence
in the leuco form. Vat dyes are described in the Colour Index under the
heading "Vat
Dyes". Most are based on 9,10 anthraquinone, which of itself is colourless.
However,
once azo or other chromophores are attached to the ring structure, a wide
range of
colours may be obtained.
0
Vat dyes include indigo, a substance originally of natural origin, which is
not
light fast, but which over time fades gradually in successive washes and is
favoured for
denim to create the characteristic "faded denim" look. Indigo and a few
halogenated
derivatives are still in use, although only indigo and tetrabromoindigo are of
commercial importance currently.
There are also fused ring polycyclic compounds that form good vat dyes.
Vat dyes are characterised by the presence of a keto group which can be
reduced
to an alcohol. This may then form a salt under conditions of alkaline pH:
CA 02284618 1999-09-23
WO 98/42895 PCT/NZ98/00039
- -23-
~C=O '-' ~CHOH (enol form) '-' ECHO- (salt of enol form)
The "salt" of the enol form is the leuco vat dye. This is water soluble and is
therefore a true dye, rather than a pigment whereas the keto form is insoluble
and is
S classed as a pigment.
It is believed that vat dyes form hydrogen bonds to magnesium hydroxide
molecules, thereby forming a dyed surface. To dye an anodized magnesium or
magnesium alloy article with a vat dye we recommend the following steps:
1. Dispersion of the dye in water. This must be done evenly, without the
formation
of lumps. Generally anthraquinone and other vat dyes are now available with a
particle size of less than l,um, enabling an even dispersion in water to be
made.
2. Reduction - while various agents may be used, the most common in dyeing
textiles is sodium dithionite, usually known as sodium hydrosulphite. This
produces the enol form of the dye. Sodium dithionite gives satisfactory
results
when the dye is to be used on magnesium substrates.
3. Adsorption of the dye - in alkaline solutions, the leuco vat dye is
adsorbed onto
the surface of the magnesium article, forming an evenly coloured surface.
Excessive quantities of undissolved pigment present in the solution may result
in "bronzing" of the surface so this should be avoided.
4. Oxidation of the reduced dye, once it has formed a layer on the substrate,
may
be conducted by an oxidising bath or simply by sufficient exposure to air.
Suitable oxidising baths comprise hydrogen peroxide or sodium perborate
solutions, but alternatives including dichromates may be used. The optimum
oxidising bath is acidified to a pH of 5-6 using acetic acid. It should be
noted
that the colour of the reduced leuco form of the dye may not be identical to
the
insoluble pigment form and thus to some extent the final shade obtained may
depend on the oxidant used. An example of this is CI Vat Green 9, which is a
very dark olive green as oxidised by air or hydrogen peroxide. However, it can
be oxidised in stronger oxidants to a neutral shade of black.
5. Rinsing to remove any excess dye follows oxidation and in most cases there
is
a rinse stage between dye adsorption and oxidation to remove excess reducing
CA 02284618 1999-09-23
WO 98/42895 PCT/NZ98/00039
-24-
agent and alkali, in addition to any unadsorbed dye.
The procedure must be carefully balanced for excessive reduction may result in
reduction of the enol form of the dye and therefore a loss of shade and
fastness. Some
vat dyes are affected by light in the leuco form, while others are
precipitated by calcium
ions in the water supply. Deionised water should be used for preparation of
vat dyes.
Anodized magnesium substrates must be very carefully rinsed prior to
introduction to
the bath containing the reduced leuco dye as free magnesium ions may also
precipitate
an insoluble compound from the dye bath.
Vat Dyes (Most Preferred Conditions):
All vat dyes have some common features regardless of whether the dye is a
indigoid or anthraquinone type.
For a few black vat dyes the oxidation procedure is slightly different because
of
the difficulties inherent in achieving black through dye systems. A
chromophore
molecule possesses a colour, as viewed under white light because of the
absorbance of
certain portions of the visible light spectrum by the molecule. True black
requires the
absorption of all of the visible light spectrum. Since this ideal case is not
in practice
readily obtainable, the most common procedure is to take a very dark colour
and apply
it in a manner intended to generate such a dark tone that it appears black.
This may be
done in a few instances by a more aggressive oxidation of the leuco dye. CI
Vat Green
9, as referred to above, may be made to appear black in this manner whereas it
is
normally a dusky olive green shade. It is more difficult to achieve this
result on a
magnesium anodic film substrate than on a cellulosic textile substrate and it
is thus not
part of the preferred procedure, nor are the oxidants (chrome VI) commonly
employed
for this purpose regarded as satisfactory for application generally.
Common features:
Preparing vat stock solution:
* Heat deionised water to 85 ° C
* Add 100g/litre of dye (as supplied, the dye is actually a pigment,
comprising the
water insoluble keto form of the dye)
CA 02284618 1999-09-23
WO 98/42895 PCT/NZ98/00039
-25-
* Add 250m1/litre of 10 molar sodium hydroxide solution (400g/litre)
* Mix thoroughly, then add:
* 100g/litre sodium dithionite
* Agitate at 85°C for fifteen minutes then cool and hold in dark,
stoppered flask
(this is necessary because the solution will absorb oxygen from the air and
denature otherwise; also some leuco vat dyes are light-sensitive).
To "vat" the dye, ready for use:
* Deionised water at 60°C -- 800m1/litre (ie: to make 10 litres of dye,
start with 8
litres of deionised water)
* Add lOml/litre of lOM sodium hydroxide
* Add Sg/litre sodium dithionite
* Add 100mI/litre stock dye solution
Make up to full volume with deionised water and dye parts to be coloured at
60 ° C or greater for 30 minutes.
* After dyeing, rinse in cold water for 5 seconds
* After rinsing, oxidise in a solution containing sodium perborate (15.4
grams/litre) or hydrogen peroxide (0.5%) or a mixture, adjusted most
preferably
with acetic acid to a pH of approx 5, for five minutes.
* Remove, rinse and dry.
Instead of oxidising in hydrogen peroxide/sodium perborate, the article may be
oxidised in air (but the full oxidation process takes several days) or other
oxidants.
Sodium or potassium dichromate, acidified with acetic acid is quite effective.
The vat dyes may be operated at a higher concentration than this - these
figures
were intended for textiles. We found that a better shade resulted from
doubling the
concentration (this meant using 200m1/litre stock solution instead of
100m1/litre).
Vat dyes do not last in air because of the absorption of atmospheric oxygen
and
the downward drift of pH which should remain above 12. The pH drift is due to
the
acids released by the sodium dithionite as it reduces the keto pigment to the
enol form.
If extra sodium dithionite has to be added, sodium hydroxide must be added
with it, in
a ratio of 1.2 parts sodium hydroxide to 1 part of sodium dithionite.
CA 02284618 1999-09-23
WO 98/42895 PCT/NZ98/00039
-26-
Sulphur Dyes
Sulphur dyes offer only a limited range of colours, none of them bright,
because
of the large size of the complex polysulphide rings formed, but they are fast
and
economical to apply to magnesium substrates. They are described in the Colour
Index.
Like vat dyes, sulphur dyes are in fact finely dispersed pigments which must
be reduced
to a soluble form in order for dyeing to proceed satisfactorily. After dyeing,
the dye is
oxidised back to the insoluble form, either by air or by an oxidising solution
containing
a suitable agent, often hydrogen peroxide or sodium perborate in aqueous
solution.
A sulphur dye is normally a disulphide, which may be reduced to a thiol. The
exact chemistry of a sulphur dye is still not fully understood, but the
reduction reaction
proceeds:
Ar-S-S-Ar + [2H] '-' 2Ar-SH
The leuco form of the sulphur dye is applied to the article in aqueous
solution at
elevated temperatures, often around 80 ° C, in the presence of an ionic
salt, often sodium
chloride or sodium sulphate, which assists the exhaustion of the dye.
The reducing agent for the dye may be sodium dithionite but there are also
proprietary agents available. Glyceraldehyde, hydroxyacetone, certain sugars,
and
various other agents are commonly found constituents of such reducing agents.
Some
proprietary reducing agents also contain alkalfies, as these are also required
to generate
the soluble leuco form of the dye. This limits the requirement to add alkali
separately
to the bath.
Alkali is also required to enhance the solubility of the thiol. in commercial
applications, sodium hydroxide is normally used although other alkalis serve
equally
well.
One problem in connection with sulphur dyes is that some dye decomposition
may take place on the finished article. This results in the liberation of acid
residues that
may damage a magnesium part. For this reason, a finish in a mildly alkaline
oxidising
bath, rather than an acid solution is advised.
To dye an anodized magnesium article using a sulphur dye we recommend the
following steps:
CA 02284618 1999-09-23
WO 98/42895 PCT/NZ98/00039
-27-
1. Anodized article rinsed thoroughly
2. Sulphur dye bath made up and heated to around 90 ° C. Dye bath
contains an
ionic salt, sodium sulphate being preferred, although sodium chloride may also
be used*. Reducing agent is added to form the leuco dye. If sodium dithionite
is
used, sodium hydroxide or another base should be added as the reduction
process
generates acid, lowering the pH.
3. Maintain the dye bath at 90 ° C for 30 minutes with the magnesium
article
present.
4. Wash off excess dye.
5. Oxidise the dye to the insoluble pigment form, either by atmospheric
oxidation
(which takes several days to go to completion) or by using agents such as
hydrogen peroxide or dichromate solutions. As is the case with vat dyes, the
nature of the oxidant used can affect the final shade of the dye and the leuco
dye
may not be the same colour as the insoluble pigment formed upon oxidation.
SULPHUR DYES (Preferred Conditions)
The sulphur dyes work much the same way as the vats, except the complex
polysulphide rings are actually split into two by the reducing action of the
reducing
agent which is then soluble in alkali. The most favoured procedure, at the
moment, is:
* Start with 800m1 deionised water at 40 ° C
* Add 80m1/litre of sulphur dye suspension (CI Leuco Sulphur Dye Mack 1 is
sold
as an aqueous suspension of the pigment).
* Add 80m1/litre of Reducer NSTM a proprietary alkaline reducing formulation.
* Add 1 g/litre sodium hydroxide
* Agitate for ten minutes then heat to 85 ° C
* At 85°C, add 60 g/litre sodium chloride or sodium sulphate over ten
minutes.
Raise temperature to 95°C.
* Introduce article to be coloured and hold at this temperature for 30
minutes.
* Rinse in cold water for five seconds
* Oxidise using hydrogen peroxide/sodium perborate solution as per vat dyes
for
CA 02284618 1999-09-23
WO 98/42895 PCT/NZ98/00039
- 28 -
twenty minutes at 50°C. As for vat dyes, various oxidants may be used.
One problem with sulphur dyes is that there is a tendency for the dyes to
release
acid upon decomposition, which is a negative feature for this will attack the
anodic film.
We therefore recommend not making the oxidising bath acidic in order to
maintain an
alkaline surface on he magnesium anodic film.
Disperse Dyes
The Colour Index lists a number of compounds where the chromophore is
bonded to an intentionally insoluble compound to create a dye, which is
applied as a
f nely dispersed suspension to dye certain types of fibres, such as
polyesters. These dyes
are thought to be solubilised either to a very slight extent either by a
carrier, which is
added to the bath, or at the surface of the fibre. The dissolved dye molecule
can then
migrate into the fibre. Monoazo and anthraquinone disperse dyes are common,
but a
range of varieties exist including diazo, nitrodiphenyl, methane, styryl,
benzodifuranone
and quinophthalone compounds. Carriers such as chlorobenzenes or aromatic
esters
increase the affinity of the substrate for the dye.
Since these compounds work by intermolecular penetration into textile fibres,
it is not obvious why they dye magnesium,anodic films although these films are
not
simple compounds such as magnesium oxide as a variety of species is present
and there
is some hydration at the surface. It is known that staining sometimes occurs
with
disperse dyes and this may result in quite a wash resistant colour, hence it
may be that
the bond between the anodic film and the dye is more of the nature of a stain.
This does
not of itself imply that the coloured article is unsuitable for general
application. It is
believed that these dyes enter and plug up pores in the anodic film thereby
imparting
a colouring effect.
Disperse Dyes (Most Preferred Conditions)
* Make up dye dispersion in water to required strength 0.5% to 2%
* Add carrier generally an aromatic ester), 2%, to dye bath
* Heat to boil and introduce article for 30-GO minutes.
* Remove, wash and dry
__T~..~___.._ _ _ ___._.. _ _....__ T
CA 02284618 1999-09-23
WO 98/42895 PCT/NZ98/00039
.. -29-
Other Dyes
In addition to the categories listed above it is possible to obtain
satisfactory
colours on magnesium anodic or oxidised film substrates using some other dyes.
Acid dyes normally attack both the substrate and anodic film, a clearly
' S unsatisfactory result. However, by substituting lactic acid or similar
organic acid which
forms an insoluble magnesium salt, for acetic acid in the general formulation
it is
possible to obtain a less aggressive formulation which can, for very short
immersions
or spray contact times, result in a stain that may be regarded as satisfactory
colouration.
To conduct this procedure would entail:
* Lactic acid -- 1-2%
* Acid dye -- 0.5-2%
* Optionally sodium sulphate 0.5-2%
* Heat dye bath to 60 C or greater
* Immerse magnesium article for short period, ideally shorter than 2 minutes
* Wash thoroughly
A uniform stain may result without any significant surface or substrate
degradation. However a longer immersion may result in a loss of the anodic or
oxide
film and attack of the substrate.
AZOIC DYES
These are really a special category of direct dyes in which the adsorbed
species
is colourless until diazotised by the procedure described on pages 19 and 20.
The
diazotisation creates the required colour. Generally these dyes are naphthol
or
phthalocyanine based. The intermediates involved in this process include
relatively
unstable diazonium compounds thus this procedure is quite sensitive to
environmental
fluctuations.
It is best to use these dyes at very low temperatures, around freezing point.
The
optimal procedure involves:
* Introduce magnesium article to colourless coupling component in solution in
CA 02284618 1999-09-23
WO 98/42895 PCT/NZ98/00039
-30
bath. The coupling component is often a derivative of 3-hydroxy-2-naphthoic
acid. Heat bath to elevated temperature, 70 C or greater.
* Optionally salt (sodium sulphate) may be added to improve uptake of coupler.
* In separate vessel, prepare diazonium salt. While this is best stabilised as
the
S hydrochloride salt, it is preferable to avoid mineral acids because of their
tendency to attack the substrate.
* Remove article from coupling agent and immerse it in diazonium salt bath.
The coloured dye will be formed inside the anodic f lm.
Application to anodized magnesium
Clearly, the ability to colour an anodized magnesium article is a considerable
advantage when such methods are not generally available, or are highly process-
specific
and awkward to apply. The dyes and methods of application outlined above are
generally applicable to magnesium anodising processes, including that outlined
in PCT
NZ96/00016 (WO 96/28591 ) and the prior art processes known as "TAGNITETM",
"MAGOXIDTM", "DOW 17TM" and "HAETM"
The MAGOXIDTM process is disclosed in US Patent 4,978,432. TAGNITETM
is described in the Society of Automotive Engineers (SAE) Aerospace
specification
AMS#2467 and US Patent 5,470,664.
TAGNITETM is a "spark" anodisation process characterised by the presence of
plasma discharges all over the surface of the part during the process.
"MAGOXIDTM" is intended to produce a layer comprising magnesium aluminate
and magnesium phosphate, using a pulsed or AC current to achieve cationic
presence
near the substrate surface. It is described in US Patent 4,978,432 and has
been referred
to herein above.
DOW 17TM is an acid fluoride based anodisation process that produces an
intrinsic green film whose colour intensity depends on the end point voltage
of the
process. In some cases it may be very dark. Clearly the reason why such a film
would
be coloured would not normally be to produce a range of colours as the
intrinsic
substrate colour limits this, but to benefit from the improved corrosion
resistance
CA 02284618 1999-09-23
WO 98/42895 PCT/NZ98/00039
-31
imparted by colouring. DOW 17TM colours well in these dyes although its
intrinsic
colour limits the range of shades obtainable.
HAETM is an alkaline anodising process using an alkali hydroxide, potassium
fluoride, trisodium phosphate and potassium manganate in solution. It produces
varying
S shades of brown coating which is not intrinsically amenable to taking a
colour, but
which may be coloured using the dyes described herein to take advantage of the
improvements in salt spray corrosion resistance obtained in doing this.
It is our understanding that these colouring processes require the presence of
magnesium hydroxide, at least in so far as reactive dyes are concerned, to
form a true
chemical bond with the substrate. While not wishing to be confined to that
theory, it is
our supposition that there would be surface deposits of magnesium hydroxide,
formed
by hydrolysis of the magnesium oxide anodic film, on the surfaces of parts
anodized
using either TAGNITETM or the process described within PCT NZ96/00016 (WO
96/28591 ). Furthermore, we believe that MAGOXIDTM also produces such a
surface,
even though the inventors have sought to form other species within the film.
The colouring processes described herein are applicable to all film
thicknesses
although optimum results are obtained on thicker films.
The reactive dyes will selectively split a secondary hydroxyl group in a
cellulose
molecule, as pictured below:
CHZOH CHz-O-Dye
+ Dye-C 1 <a HC I ~ I
It is our understanding (although we do not wish to be so bound) that the
reactive
groups within the dye molecules will cleave the magnesium hydroxide molecule
in a
similar way, bridging the remaining oxygen atom to form a true chemical
compound.
The other dyes, which do not necessarily form a true chemical compound, are
believed to bond to magnesium hydroxide in much the same manner as they do to
a
cellulose fibre inside a cellulosic fibre, such as cotton. Therefore, hydrogen
bonding and
van der W aals forces are presumed to exist between molecules of magnesium
hydroxide
CA 02284618 1999-09-23
WO 98/42895 PCT/NZ98/00039
-32-
and the dyes, just as they do within the fibre.
If a colour is applied incorrectly, or the wrong shade results, it is possible
to strip
the dye from the surface either progressively or completely to enable
recolouration.
Methods of stripping are mostly simple, for instance, by immersion in
solutions of lactic
acid, which does not adversely affect the substrate.
As used herein percentages where the context allows are expressed as weight to
volume.
EXAMPLES
- Example 1
A direct black dye was used to colour a 10 micron anodic film on magnesium
alloy AZ91 D: a 0.5% solution of Ciba-Geigy's Solophenyl~Direct Black GN dye
was
prepared and heated to 60 ° C. 0.5% sodium sulphate by weight was added
and an anodic
voltage of 10 volts was applied to the article. After 30 minutes the article
was found to
be a deep black in colour.
- Example 2
A second article was introduced to the dye bath outlined in example #1 above,
but no anodic voltage was applied to it. After 60 minutes it was found to be a
charcoal
grey in tone.
- Example 3
A strip of hot rolled anodized AZ31 magnesium alloy, having an anodic film of
1 S,um was introduced to a dye bath containing a monochloro triazine reactive
blue dye
which was freshly prepared. The bath contained:
(a} 1% dye (Ciba Geigy's Cibacron~ Blue HGN)
(b) 1 % sodium sulphate
{c) 0.5% sodium hydroxide
(d) 1 % sodium carbonate
The dye bath was heated to 70 ° C and the sample held for twenty
minutes. At the
end of the time, the article was found to be a deep and uniform blue in
colour.
CA 02284618 1999-09-23
WO 98/42895 PCT/NZ98/00039
- 33 -
Hydrolysed dye was rinsed from the article which was then allowed to dry
naturally in
air.
- Example 4
A die casting composed of AZ91 D magnesium alloy was coloured black using
CI Leuco Sulphur Dye Black 1, a liquid dispersion of sulphur dye. The sulphur
dye was
converted to the soluble leuco form by adding a proprietary reducing agent,
Reducer NS
and salt as follows:
(a) CI Leuco Sulphur Dye Black 1 - 8%
(b) Reducer NS - 8%
(c) Sodium chloride - 80 grams per litre
The solution was heated to 90 ° C and the magnesium article introduced
to the
bath for thirty minutes after which it had an even flat black appearance.
Finishing
comprised a rinse in hot water then an oxidising bath comprising hydrogen
peroxide,
0.5% and sodium perborate 10 grams per litre. This bath was heated to
40°C. After this
treatment, the article was dried and when dry, had a uniform flat black
appearance.
Example 5
A die cast component comprising AZ91 D magnesium alloy was coloured in a
vat black dye using sodium dithionite as a reducing agent and sodium hydroxide
as an
alkali. The leuco dye was prepared in the following manner:
A stock vat dye dispersion was prepared from 100 grams per litre vat dye and
250m1 per litre 10 molar sodium hydroxide solution. To this was added 100
grams per
litre of sodium dithionite to solubilise the dye, converting the dispersion to
the water
soluble enol salt form.
This stock dye was then added to deionised water at 60 ° C in the
following
proportions
Deionised water 900m1 per litre
Sodium hydroxide solution { 10 molar) 1 Oml per litre
Sodium dithionite 5 grams per litre
Stock solution (as described) 100m1 per litre
CA 02284618 1999-09-23
WO 98/42895 PCT/NZ98/00039
-34-
This solution of leuco dye was maintained at 60 ° C and the anodized
part was
introduced to it. It was coloured a charcoal grey shade which did not visibly
change as
the dyed part was rinsed and oxidised in a solution of 0.5% hydrogen peroxide
and 14
grams per litre sodium perborate.
- Example 6
A strip of hot rolled AZ31 magnesium alloy was introduced to a suspension of
a disperse dye, CI Disperse Red 1 to which had been added 0.1 % Pecar~ GL, a
proprietary carbonyl acid ester carrier compound. The dye bath was heated to
boiling
point and maintained for 30 minutes after which time the magnesium article was
found
to be a pastel orange-pink colour.
- Example 7
A number of magnesium alloy AZ91 D test plates were anodised to a nominal
film thickness of 20 microns using the process described in PCT/NZ96/00016. A
number of these were coloured using several different vinyl sulphone reactive
dyes,
whereas the remainder were not coloured.
These samples were then subjected to a neutral salt spray test as defined in
ASTM B 117. The samples were evaluated according to the two part method
described
in ASTM D1654. Under part B of this method, which deals with general
corrosion, the
samples which were not coloured failed after about 500 hours, whereas those
which
were coloured were in most cases still passing after 1,034 hours.
- Example 8
An acid dye, CI Acid Blue 62, was prepared as follows:
* Lactic acid 2%
* CI Acid Blue 62 (Everacid TM Blue RRL) - 1
* The dye bath was heated to 70°C and an anodised magnesium substrate
on
magnesium alloy AZ31 was introduced to it for two minutes after which time a
wash
resistant stain was formed without degradation of the surface film.
CA 02284618 1999-09-23 _
WO 98/42895 PCT/NZ98/00039
-35
- Example 9
A tube composed of Melram~ 072 TS alloy (magnesium containing 1-2% silicon
carbide in a mixed metal composite) was anodised by the procedure_ described
in
PCT/NZ96/00016 to a thickness of about S~cm. This was then coloured yellow
using
a vinyl sulphone reactive dye, Eversol~ Yellow, at 60 ° C for 3 0
minutes, at a
concentration of 0.5% with a concentration of 0.5% sodium carbonate and 0.25%
sodium hydroxide. The result was a bright yellow-gold colour.
- Example 10
A sample of hot rolled magnesium alloy AZ31 sheet was anodised to a thickness
of l5~cm and coloured using a vinyl sulphone reactive dye, Eversol~ Blue, at a
concentration of 0.5%, togetherwith 0.5% sodium carbonate and 1 °~o
sodium hydroxide,
yielding a dark blue colouration of the sample.