Note: Descriptions are shown in the official language in which they were submitted.
CA 02284967 1999-09-29
METHOD OF FORMULATING ALKALI EARTH SALTS
20
TECHNICAL FIELD
The present invention relates to a method of formulating alkali earth salts
and
more particularly, the present invention relates to a method of generating
food grade
sodium bicarbonate and fertilizer grade potassium sulfate.
BACKGROUND ART
A significant amount of prior art has been promulgated with respect to the
formulation of alkali earth salts. Sodium bicarbonate, as an example, has been
prepared in as many different ways as it has been known. Despite this fact,
previous
unit operations for bicarbonate synthesis have been hampered by inefficient
energy
use which results directly in increased synthesis costs. As a further
limitation, known
processes do not make efficient use of the unit operations involved in the
preparation of salts. Typically, a single high quality product is formulated
with
concomitant byproduct formation of a quality inadequate for commercial
purposes or
that would require too substantial an investment to render them commercially
viable.
Representative of the prior art is United States Patent No. 3,429,657,issued
February 25, 1969, to D'Arcy. The reference discusses a method for recovering
and
producing potassium salts. In the reference, a potassium bearing brine is
reacted
with sodium perchlorate to precipitate potassium perchlorate. The potassium is
removed by ion exchange with sodium and the free potassium is then combined
with
chloride, sulfate, nitrate inter alia.
INDUSTRIAL APPLICABILITY
30 The present invention has applicability in the fertilizer art.
CA 02284967 1999-09-29
2
h) contacting the liquor from step g) with sulfuric acid to precipitate
carbonates;
i) cooling the liquor from step h) to 0°C to form Glauber's salt
precipitate;
j) heating the liquor from step i) to between 30° to 40°C; and
k) precipitating potassium sulfate by contacting the liquor from step j)
with potassium chloride.
A further aspect of one embodiment of the present invention is to provide a
method of formulating food grade sodium bicarbonate and potassium sulfate,
comprising the steps of:
a) providing a source of liquid sodium sulfate;
b) providing a source of ammonium bicarbonate;
c) contacting the sodium sulfate and the ammonium
bicarbonate;
d) precipitating sodium bicarbonate and forming
a liquor;
e) precipitating sodium bicarbonate and forming
a liquor by contacting
the liquor
from
step
e) with
sodium
sulfate;
f) saturating the liquor from step e) with anhydrous
sodium sulfate;
g) filtering solids from the liquor of step f);
h) contacting the liquor from step g) with at least one of ammonium
bicarbonate, ammonia gas or carbon dioxide to precipitate sodium bicarbonate;
i) cooling the liquor from step h) to 0°C to a precipitate of sodium
bicarbonate and sodium sulfate; and
j) precipitating potassium sulfate by contacting the liquor from step i)
with potassium chloride.
It has been found that following the sodium bicarbonate formulation,
significant success in cooling the liquor to 0°C is realized for
removing sodium
sulfate as Glauber's salt and sodium bicarbonate. Glauber's salt solubility in
the
system is contemplated by the ammonium sulfate-sodium sulfate phase diagram.
By increasing the sodium sulfate in the bicarbonate circuit with increased
Glauber's
salt recycle, there is a tendency to decrease the bicarbonate solubility and
increase
the process efficiency.
CA 02284967 1999-09-29
3
Regarding the conversion of the starting reagents to potassium sulfate,
particular success has been encountered by maintaining a mole ratio of five
(5) or
greater for the potassium and ammonium ions. This ratio ensures high
conversion
efficiency in the second stage of the process.
Having thus described the invention, reference will now be made to the
accompanying drawings illustrating preferred embodiments, and in which:
Figure 1 is a process flow diagram illustrating a first part of one process
according to the present invention;
Figure 1a illustrates a second part of the process illustrated in Figure 1;
Figure 1 b illustrates a third part of the process illustrated in Figure 1;
Figure 2 is a is a process flow diagram illustrating a first part of a
variation of
the process according to the present invention;
Figure 2a illustrates a second part of the process illustrated in Figure 2;
and
Figure 2b illustrates a third part of the process illustrated in Figure 2.
Similar numerals in the figures denote similar elements.
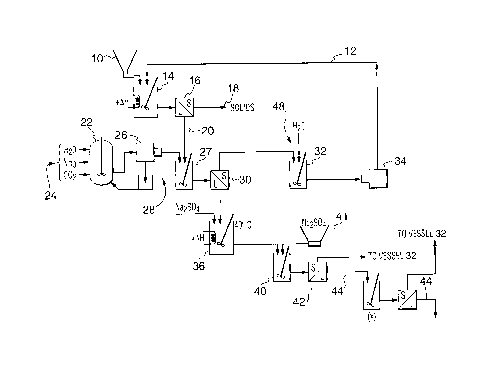
Referring now to the drawings, Figures 1 through 1 b illustrate the process
according to a first embodiment.
A source of liquid sodium sulfate 10 dissolved in fresh water and centrate
water 12 discussed herein after. The solution is mixed in vessel 14 at
40°C to a
specific gravity of 1.30. The solution is filtered in filter 16 which, as an
example, may
comprise a 5 micron filter. The solids 18 are disposed of while the filtrate
20 is
passed into a first sodium bicarbonate crystallization vessel 27.
CA 02284967 1999-09-29
4
Feeds of water, ammonia and carbon dioxide all denoted by numeral 24 are
reacted in vessel 22 in order to synthesize ammonium bicarbonate. Formulated
ammonium bicarbonate is centrifuged in centrifuge 26, with the solid product
being
passed into crystallization vessel 27. A recycle loop 28 recirculates ammonium
bicarbonate solids and liquor into reaction vessel 29. The result of the
combination
in vessel 29 is the formulation of sodium bicarbonate. The mixture is filtered
by filter
30 and centrifuged. The sodium bicarbonate is washed with water in vessel 32,
centrifuged in centrifuge 34 and the solid retained as food grade sodium
bicarbonate.
The wash water is returned to vessel 14.
The liquor from filter 30 has a specific gravity of 1.25 with the contents
including approximately 10.4% sodium sulfate, 17.1 % ammonium sulfate, 8%
sodium bicarbonate and excess ammonium bicarbonate for reaction with the
Glauber's salt (discussed herein after). The liquor is reacted in a vessel 36
at 40°C
with Glauber's salt formulated in the cooling phase of the process, which will
be
discussed later, to produce sodium bicarbonate from the excess of ammonium
bicarbonate from crystallization vessel 29. Alternatively, the ammonium
bicarbonate
may be added to the second stage (vessel 36) as solid, slurry or solution.
To the liquor from vessel 36 is added to solid sodium sulfate from source 41
in vessel 40 to formulate a saturated liquor of sodium sulfatelammonium
sulfate.
Sufficient ammonium bicarbonate may be present to complete the reaction is
solution or some may be added to result in the liquor having a specific
gravity of
1.285. The slurry from vessel 40 is filtered with filter 42. The sodium
bicarbonate
solids 48 are passed to vessel 32 and the liquor 44 is further processed with
additional separation of sodium bicarbonate, which is returned to vessel 32.
The
liquor 44, is then passed to vessel 46 (Figure 1A). Circuit volume from the
sodium
bicarbonate circuit can be controlled by evaporating the purified sodium
sulfate in the
feed to produce solid sodium sulfate to ensure circuit saturation.
Returning to Figure 1A, vessel 46 contains sulfuric acid to precipitate
carbonate compounds. The so treated liquor is cooled to 0°C in chiller
48 to recover
Glauber's salt and filtered in filter 50. The recovered Glauber's salt is
returned to the
sodium bicarbonate crystallization vessel 36.
CA 02284967 1999-09-29
The filtrate contains 25.25% by weight ammonium sulfate and up to 11 % by
weight sodium sulfate and is passed into a vessel 52 heated to between
30°C and
40°C and combined with solids 65 from filter 66. This solution is
passed into vessel
54 where solid potassium chloride is reacted therewith to formulate a 20% by
weight
solution of ammonium chloride also containing, by weight approximately, 20.2%
ammonium chloride, 6.7% potassium chloride, 4.9% sodium chloride, 2.3% as
(x)ZS04, where x = Na, K, and solid mixed crystals of potassium sulfate with
10% -
20% ammonium sulfate.
The solution is filtered in filter 56, with the solid fraction containing
approximately by weight, 5% potassium chloride, 80% - 85% potassium sulfate,
10%
- 15% ammonium sulfate. The solid fraction is combined in vessel 58 with water
and
potassium chloride brine from vessel 60. The potassium sulfate solid is
centrifuged
and filtered in filter 62 and recrystallized with a solution of potassium
chloride at
25°C. The remaining ammonium sulfate is converted to potassium sulfate.
Grades
of greater than 98% potassium sulfate are achievable.
In further unit operations, the liquor or filtrate from the potassium sulfate
operations and specifically from filter 56 is processed in accordance with the
unit
operations set forth in Figure 1 c. The liquor is evaporated in evaporator in
order to
concentrate the ammonium chloride liquor such that upon cooling the potassium
chloride and residual sulphates are minimized in solution. The solution is
filtered
with filter 66 with the solid material 67 recycled to vessel 54. The filtrate
containing
approximately 22% to 30% ammonium chloride is reacted with lime in reactor 68
with
liberated ammonia recycled. The calcium chloride formed may be passed to a
settler 70 or scrubber 72 depending on intended subsequent uses.
Having set forth the process according to this first embodiment, reference
will
now be made to an example of the process.
CA 02284967 1999-09-29
6
EXAMPLE 1
BICARBONATE KILL PRIOR TO
POTASSIUM SULFATE PROCESS
Feed - 1 litre @ 1.3 S.G.
360 gll Na2S04
1 st STAGE
Production of NaHC03
Brine Exit at reaction termination:
130g NazS04 10.4% Na2S04 40'C
213.8g (NH4)ZS04 17.1% (NH4)ZS04 1.250 S.G. @ 0.95 I
100g NaHC03 8.0% NaHC03 solution
907a HZO
1350.8
This makes 172g NaHC03 solids SECOND STAGE ESTIMATE
consumes 55g NH3 A) 25.07g NH3 + 64.9g CO2
142.5g CO2 B) 51.2g NH3 + 132.6g COZ
CA 02284967 1999-09-29
7
2nd STAGE 0.95 I of brine will dissolve the following:
A) 1 Moles ) Z Moles
NazS041 OH20 azS041 OH20
(3328) 6448)
2728 NazS04 16.2% NazS04 148 NazS04 20.7% NazS04
213.88 (NH4)zSO412.8% (NH4)zSO4 13.98 (NH4)zSO410.7% (NH4)zSO4
1008 NaHC03 5.9% NaHC03 1008 NaHC03 5.0% NaHC03
1087C! Hz0 65.1 % H20 1267 HZO 63.4% H20
1672.8 999
X1.275 S.G. and 1.313 I brine ~ X1.300 S.G. and 1.5 I brine
2nd STAGE Final Solution Composition
67.38 NazS04 10% NazS04 OOg NazS04 10% NazS04
118 (NH4)zSO4 18.9% (NH4)zSO4128 (NH4)zSO4 20.2% (NH4)zSO4
318 NaHC03 8% NaHC03 1608 NaHC03 8% NaHC03
1087 Hz0 63.1 % Hz0 1267 HZO 61.8% H20
644.58 Solution 0398 Solution
reduction of NaHC03 92.98 reduction of 193.28 NaHC03
.G, 1.265 and makes 1.31 .G. 1.285 and makes 1.6 I
I brine of Solution
BICARB KILL
4128 (NH4)zS04
2008 NaS04 + 160 X 98 =93.38 HZS04
1608 NaHC03 84(2)
12678 Hz0
20398 (1.6 1 )
1.285 S.G.
This becomes:
CA 02284967 1999-09-29
8
412g (NH4)ZS04
335g Na2S04
1267g H20
2014g @ 1.265 = (1.61 )
must add Na2S04 to Saturation of 1.30 S.G.
1.61 x 1.30 = 2080
Therefore:
412g (NH4)2S04
400g Na2S04
1267g Hz0
2079g total (1.61)
Cooling
4128 (NH4)ZS04 28.7%
116g Na2S04 8.0%
907 H20 63%
1435g Solution
Feed to Evaporator
NH4C1 330.8 g 21.9
KC1 130 g 8.6%
NaC1 94.7 g 6.3%
x-S04 50 3.3%
H20 907a 60.0
1512g
@ 33% NH4CI then: - 2.8% KCI
then: - 2.0% KZS04
Therefore: 330.8 = 1002 g
.33
Evaporation Load = 907 - 623 = 2844
CA 02284967 1999-09-29
9
0.79t1t NaZS04
add 0.5 t for washing
1.29 t HZO / t Na2S04
KZSO~ Reaction
a) K2S04 from (NH4)ZS04 = 412 x 174 = 543g
132
b) KZS04 from NazS04 = 116 x 174 = 142g
142
c) Losses of KZS04 - -43g
TOTAL KZS04 642g
KCI Recovery
a) KCI intermig reaction = 685 x 2 x 74 = 5828
174
b) KCI lost to tails = 50g
c) Therefore: KCI need = 632g
KZSO~ yield = 642 x 100 = 93.7%
685
KCI Conversion Efficiency = 582 x 100 = 92.1
632
CA 02284967 1999-09-29
BASIS: One Tonne of Na2S04 feed
INPUTS PRODUCT
First Stage 0.153t NH3 0.48t NaHC03
0.396t C03
2.52t H O
Second Stage 6448 Na2S0410H200.53t NaHC03
0.142t NH3
10 0.368t CO
Bicarb Kill + Na2S04 SaturationFilter to Produce clear brine
0.26t HzS04
0.18t Na SO
Cooler to 0 C -BTU's 1.8t Na SO 10H O
Cooler brine 1.14t (NH4)ZS04 28.7%
0.32t Na2S04 8.0%
2.52at Hz0 63%
3.99 t Tota I
KC1 = 1.76t 1.78t K SO
Evaporation to 33% NH4C1 0.92t NH4CI brine
1.29t1t Na2S04 0.08 t KCI SOLIDS
0.05t KZS04 0.28t KCI
1.73t H20 0.08t KZSO
2.78 Total 0.36t Rec cle
Lime Process @ 85% off
0.57t Ca0 0.29t NH3
Brine: 0.955 CaCl2
0.08t KCI
0.05t KZSOQ
1.73t H20
2.815t 75 to 90' C
CA 02284967 1999-09-29
11
Turning to Figures 2 through 2b, an alternative processing scheme is
schematically depicted. In this reaction scheme, prior to the production of
sodium
bicarbonate, the liquors are saturated with anhydrite.
In this embodiment, sodium bicarbonate is produced in crystallization unit 22
and undergoes generally similar steps as set forth for Figures 1 through 1 B.
The
brine or filtrate is saturated with anhydrous sodium sulfate in vessel 36 and
filtered
with filter 38 to remove insolubles which are discarded. The filtrate from
this
operation is reacted with ammonium bicarbonate in vessel 80. As an
alternative, the
filtrate could be reacted with ammonia or carbon dioxide to precipitate the
sodium
bicarbonate. The solution is filtered with filter 82 and the sodium
bicarbonate
remains. The latter is combined with the sodium bicarbonate from filter 30 and
then
washed, centrifuged and dried. These steps are not shown.
The filtrate remaining has a composition of approximately, on a by weight
basis, 10% sodium sulfate, 24% ammonium sulfate and 8% sodium bicarbonate.
The solution has a specific gravity of 1.285 at 40°C.
From this stage, the filtrate solution is cooled in a chiller 84 to
approximately
0°C in order to produce a filtrate containing approximately, on a by
weight basis 5%
sodium sulfate, 28% ammonium sulfate and 6% sodium bicarbonate. The solution
is
filtered with filter 86 and precipitated sodium bicarbonate and sodium sulfate
are
recycled back to the bicarbonate crystallization vessel 32, while the filtrate
is reacted
with potassium chloride in vessel 88 to synthesize first stage potassium
sulfate in a
purity range of about 75% to 90%. The solid potassium sulfate is repulped with
potassium chloride brine from vessel 92 in vessel 94. This results in high
quality,
high grade potassium sulfate. The product is washed with water in a
conventional
washing stage 96 with recycle to vessel 94.
The solution from filter 90 is evaporated in evaporator 98 (Figure 2A) to
concentrate ammonium chloride liquor whereby upon cooling the potassium
chloride
and sulfates are minimized. The solution is filtered using filter 100 with the
precipitated potassium chloride and (x)S04, where x = K, Na, recycled to
vessel 88.
CA 02284967 1999-09-29
12
The filtrate from filter 100 containing ammonium chloride, potassium chloride
and potassium sulfate is passed into evaporator 102. The sodium bicarbonate
backs
the reaction and as a result, ammonia and carbon dioxide are released. These
gases are then scrubbed/handled using suitable techniques. The calcium
chloride
generated is then discarded or sold.
EXAMPLE 2
NO BICARBONATE KILL
Feed -1 litre @ 1.3 S.G.
360 gll Na2S04
1 st STAG E
Production of NaHC03
Brine Exit at reaction termination:
130g Na2S04 10.4% Na2S04 40°C
213.88 (NH4)ZS04 17.1 % (NH4)ZS04 1.250 S.G. @ 0.95 I
100 g NaHC03 8.0%NaHC03 solution
9078 Hz0
1350.8
This makes 1728 NaHC03 solids
consumes 558 NH3
142.58 COZ
Resaturation with NaZS04: brine will hold 1508 Na2S04. This brine is then
filtered
and fed to the second stage NaHC03 crystallizer.
CA 02284967 1999-09-29
13
FEED REACTION EXIT BRINE PRODUCT
2808 Na2S04 35.98 NH3 1308 Na2S04 1778 NaHC03
213.88 (NHQ)ZS04 92.98 COZ 3538 (NHQ)zS04
1008 NaHC03 1008 NaHC03
9074 H20 9074 H20
1490.88 14908
1.151 @ 1.32 S.G. 1.285 S.G.
1.151
23.7% NH SO
The exit brine is then cooled to 0'C.
Brine composition is : 5.0% Na2S04 which mean 608 NazS04 precipitates as 1368
of
Na2S0410Hz0 precipitate and remove 768 of H20.
Therefore: 907 - 76 = 831 g H20.
Brine composition @ 0'C and 1.26 S.G.
708 Na2S04
3538 (NH4)zS04
1008 NaHC03
831 cLHzO
1354a TOTAL
About 1 litre brine
KzSO
a) 708 Na2S0~ x 174 = 85.8
142
CA 02284967 1999-09-29
14
b) 353 NH ~2SOA x 174 = 465.3a
132
EXIT BRINE:
283g NH4CI 21.9%
57g NaCI 4.8%
1198 (KNaHC03) 9.2%
8318 H20
1290
Boil up to 33.0% NH4C1.
Release of NH3 and COZ from evaporator but NH4CI salts out KCI and not the
NaCI.
KCI is recovered same as in Example 1.
BASIS: One Tonne Na2~ feed
INPUTS PRODUCT
First Stage 0.15t NH3 0.48t NaHC03
0.396t CO2
2.52t H O
Second Stage 0.10t NH3 0.49t NaHC03
0.26t C03
0.42t Na SO
Cooled to 0 C 0.4t of Na SO 10H O
Cooler Brine 0.19t Na2S04 5%
0.98t (NH4)ZS04 26%
0.28t NaHC03 7.4%
2.31 t H20 61.4%
3.76t Total
CA 02284967 1999-09-29
INPUT PRODUCT
KCI 1.62t ~~ 1.8t K SO
Evaporation to 33% NH4CI Brine Solids
Circuit Control = 0.7t H20 0.98t NH4C1 0.28t KCI
5t 0.08t KCI 0.08t KZSOa
Washing = 0-5 t
To evaporator 1.2t HzOlt NazS040.15t NaCI 0.36t
0.19t NaCI from C03
1.57t Hz0
2.97t
10 Lime Process @ 85% efficiency 1.01t CaCl2
0.61 t Ca0 0.08t KCI
0.34t NaCI
1.57t H20
3.Ot 75 - 90'C
EXAMPLE 3 - BICARBONATE KILL - NO EVAPORATION OF AMMONIUM
CHLORIDE
Feed Solution: from #1 412 g (NH4)ZS04
335 g NazS04
1267 4 H20
2014 g @ 1.265 = 1.60 I
Cooling to 0'C yields a filtered solution of:
412 g (NH4)zS04 28.7%
116 g Na2S04 8.0%
9. 07 ct H20
1435 g solution
CA 02284967 1999-09-29
16
This brine is then
heated to 25'C where
KCI solid is added
to produce KZS04.
The exit brine from circuit has the following composition:
the K2S04
NH4CI 330.8 g 21.9
KCI 130 g 8.6
NaCI 94.7 6.3
x-S04 50 g 3.3 % x = Na/K
H20 907 a 60
1512 g
This brine is than heated and reacted with lime to recover the ammonia and
bypass the evaporator. The KCI reports to the CaCl2 brine rather than being
recovered in the evaporator. This represents a 15 to 20 % loss of K to the
CaCl2
brine. The KCI in the CaClz brine can be reduced to as low as 1.0% by adding
solid
Na2S04 to CaCI2IKCl brine. The potassium is effectively collected as
apprecipitated
of syngenite (CaS04 ~ KzS04 ~ xHzO) at 0 to 100'C with preferred temperatures
of
to 30'C so that S04 solubility is kept to minimum and the reaction occurs at a
reasonable rate.
CaCl2 Brine composition
20 343.3 g CaCl2 22.5
130 g KCI 8.5
94.7 g NaCI 6.3
50 g x S04 32.% (NaIK)
907 g HZO 59.5%
1525 g 100
140 g Na2S04 addition: Exit BrineExit Cake
234.8 g CaCl2 17.8%
5.25 g KCI 1.1 % 310 g CaS04 ~ KzS04
209 g NaCI 15.9 % + 100 g Hzo
50gxS04 3.8%
807 61.3
CA 02284967 1999-09-29
17
The exit brine can be deep well disposed of and cake can be blended into the
KZS04 product as binder or further processed to remove the CaS04.
The cake can be reacted with (NH4)ZHC03 from the NaHC03 process feed
and the CaS04 reacts quickly to produce a brine of (NH4)2S04 and KZS04 and a
filter
CaCl3 precipitate which is disposed of. The (NHa)ZS041KZS04 brine is recycled
to
K2S04 first stage crystallizer.
Although embodiments of the invention have been described above, it is not
limited thereto and it will be apparent to those skilled in the art that
numerous
modifications form part of the present invention insofar as they do not depart
from
the spirit, nature and scope of the claimed and described invention.