Note: Descriptions are shown in the official language in which they were submitted.
CA 02285479 1999-09-22
WO 99/01417 , PCT/EP98/03825
- 1 -
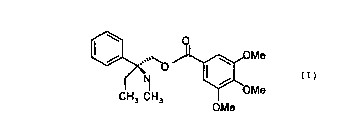
(S) 2-Methylamino-2-phenyl-n-butyl 3,4,5-trimethoxy
benzoate, its application to the treatment
of chronic pain
S Field of the invention
The subject of the present invention is optically pure
(S) 2-methy.lamino-2-phenyl-n-butyl 3,4,5-trimethoxy-
benzoate (I) of formula
,,,,~ OMe
O
N
CH3 CH3 OMe
its addition salts with pharmaceutically acceptable
acids, their preparation and their therapeutic applica-
tion in the form of medicaments to the treatment of
chronic pain of various etiologies.
Technological backcrround of the invention
Pain is a complex subjective phenomenon comprising a
sensation reflecting a real or potential tissue lesion
and the affective response which it generates. In the
first place, pain is described as acute or chronic
according to its intensity and its duration. Thus, pain
lasting for more than 6 months is described as chronic
pain. In addition to this first differentiation, pain is
classified in many ways, a summary of which can be found
on p. 1422 and subsequent pages of the Merck Manual
(French ed. 1988 - Merck and Co., INC.). A particularly
major problem is that of the treatment of chronic pain.
Conventiona'11y, it is treated with compounds with
so-called analgesic activity which eliminate pain while
preserving the patient's conscious state and sensitive
CA 02285479 1999-09-22
WO 99/01417 PCT/EP98/03825
- 2 -
perceptions. Essentially, these compounds belong to the
nonsteroidal anti-inflammatory class (NSAID) or to the
opiate analgesics and are chosen and administered to
patients according to the intensity of the pain felt and
the duration of the treatment envisaged. It is known that
these compounds cause undesirable side effects, in.
particular during prolonged treatments such as precisely
those necessary for chronic pain, which requires a
frequent therapeutic check up, which often places
excessive constrain on the patient. Thus, the NSAIDs
which, in the arachidonic cascade, decrease the synthesis
of prostaglandins and of algogenic thromboxanes by
inhibition of cyclooxygenases, are known to be able to
cause serio~:s, in particular gastrointestinal and
hematological, side effects (The Pharmacological Basis of
Therapeutics - Gooddman & Gilman - 9th ed. p. 617-655).
Furthermore, the opiate analgesics, in addition to
central effects (respiratory depression, nausea and
vomiting) or peripheral effects (gastrointestinal
transit) are known to cause drug dependence phenomena
(ibid. p. 521-555 and p. 557-577).
To this day, a major problem therefore exists, the
resolution of which has been quite unsatisfactory, for
the treatment of chronic painful conditions which, in
various forms, affect a considerable number of people
including for example, according to the World Health
Organization, 4 million cancer sufferers who, worldwide,
suffer as a result of a lack of suitable care. In
addition to cancer, in the area of pain or of chronic
painful syndrome with a somatic and psychological com-
ponent, there are a number of conditions such as
musculoskeletal or vertebral pain, neurological pain,
headaches or vascular pain.
._ _ __ ._.T _. _._._ r~._.__ __.._._._ .___ . ~ _.
CA 02285479 1999-09-22
WO 99/01417 PCT/EP98/03825
- 3 -
In the context of the compounds of the prior art related
to the product of the invention, British Patent
-GB 1,434,826 published on 5 May 1976 claims esters and
carbamates of racemic amino alcohols and among them a
family of esters of formula (II)
R 4
R
CHZOCOR~
Rz I
N'
R '3
R ~ R 6
in which, more particularly:
R'1, R'~, R'; are hydrogens
R'9 is a C1 to C~ alkyl
R' S and R' 6, which are similar or different, are hydrogen
or a C, to Cq alkyl
R'-, is aryl optionally substituted with 1 to 3 groups
chosen from a set comprising, inter alia, a C1 to C9
alkoxy radical.
These esters, in addition to motility- and
gastrointestinal transit-inhibiting properties and local
anesthetic properties, are said to comprise analgesic and
anti-inflammatory compounds which rnay be useful in the
treatment of pain in digestive disorders. Moreover, in
the Japanese Patent Application published under the
No. 16416/1980 on 1 May 1980, there is described and
claimed a process for the preparation of 2-dimethylamino-
2-phenyl-n-butyl 3,4,5-trimethoxybenzoate, whose racemate
is trimebutine (INN) with spasmolytic activity (Patent
FR 2,369M - 1962). The Japanese application also
exemplifies the synthesis of the (+) and (-) enantiomers
by the claimed process without mentioning any activity of
the compounds.
The study of the metabolism of trimebutine maleate leads
to the identification, in addition to other metabolites,
CA 02285479 1999-09-22
WO 99/01417 PCT/EP98/03825
- 4 -
of (R, S) 2-methylamino-2-phenyl-n-butyl
3,4,5-trimethoxybenzoate (Magaribuchi, T. et al. Oyo
~Yakuri (1982), 24 (5), 625-9). This compound, adminis-
tered by the i.v. route, is shown to inhibit gastric
motility in rats and to reduce blood pressure and to
lower the heart rate. _
Additional studies of this racemic metabolite, called
NDTMB for N-demethyltrimebutine, demonstrate:
- in vitro, an agonist affinity for the ~t type opiate
receptors, an affinity which is more moderate for the K
and 8 type opiate receptors, which affinities confirm the
activity of the studied products on gastrointestinal
motility via the peripheral opiate receptors (Roman F. et
al. J. Pharm. Pharmacol. (1987), 39 (5), 404-7). Without
any marked specificity for a subtype, this opiate
affinity is confirmed (Pascaud, X. et al., Gastroenterol.
Clin. Biol. (1987), 11 (3), 77B-81B),
- in vivo, in dogs, administered by the oral route or the
i.v. route, it is established that NDTMB participates in
the effects of trimebutine on the gastrointestinal and
colic motilities (Bueno, L. et al., Gastroenterol. Clin.
Biol. (1987), 11 (3), 90B-93B). By the oral route in
dogs, NDTMB promotes emptying of the stomach, an effect
which is inhibited by K antagonists, which suggests the
effect of the product by local stimulation of the x
receptors of the mucous membrane or submucous membrane at
the antroduodenal level (Gue, M. et al., J. Pharm.
Pharmacol. (1988), 40 (12), 873-5).
As reported, the prior art most closely related to the
subject of the invention is (R, S) 2-methylamino-2-phenyl-
n-butyl 3,9,5-trimethoxybenzoate, a known metabolite of
trimebutine. Up until now, the studies carried out on
this metabolite demonstrate activities similar to those
__._. _
CA 02285479 1999-09-22
WO 99/01417 PCT/EP98/03825
- 5 -
of its precursor, which leads to the metabolite being
credited with participation in the overall effect of
-trimebutine, reported to be a musculotropic antispasmodic
compound which modifies the digestive motility, involving
a peripheral encephalinergic agonist activity which
stimulates the intestinal motility and makes it possible
to inhibit it after stimulation (Vidal pharmaceutical
dictionary - 1997 ed. - p. 434). Thus, the racemate, a
parent compound of the (S) enantiomer which is the
subject of the invention, is potentially useful, like
trimebutine, in the treatment of functional disorders of
the gastrointestinal tract and of the bile ducts and,
consequently, in relieving patients of the painful
manifestations resulting from these disorders.
As regards the study of the compounds having affinity for
the opiate receptors, when the compound has an asymmetric
carbon atom, thereby generating two enantiomers with
opposite absolute configuration (R or S) and their
equimolecular mixture called (R, S) racemate, it is known
that the affinity is stereospecific, that is to say
preferential for one of the enantiomers. By convention,
the enantiomer with the highest affinity for the
receptors is called eutomer as opposed to the less active
or even inactive enantiomer called distomer.
Trimebutine and its metabolite NDTMB comprise the same
asymmetric carbon atom and are racemic mixtures. Poten-
tially, in relation to the opiate receptors, they both
have a eutomeric form having an identical absolute
configuration given the nature of the substituents of the
asymmetric carbon atom which does not allow inversion of
configuration and also due to the fact that the
N-demethylation does not modify this configuration
determined according to the Cahn Ingold and Prelog rules.
However, while the enantiomers of trimebutine were
CA 02285479 1999-09-22
WO 99/01417 PCT/EP98/03825
- 6 -
prepared as mentioned in Patent JP 16416/1980, those of
its metabolite, NDTMB, are new. Given the advantageous
properties of the racemates and of the likelihood of
eutomeric forms for these products, the Applicant has
undertaken the in vitro and in vivo study of these
compounds, enantiomers and racemates. _
The work reported in this report has shown particularly
surprisingly that the enantiomer of (S) configuration of
the metabolite NDTMB, established as a distomer in the
light of its affinity for the opiate receptors, exhibits,
in vivo, particularly advantageous analgesic properties
for application in the treatment of chronic pain. Indeed,
in spite of its low affinity for the opiate receptors
compared with its (R) antipode, this enantiomer proves,
in animals, to be a potent analgesic, lacking opiate side
effects, in pharmacological models which are
representative of chronic pain.
Summary of the invention
The invention relates to optically pure (S) 2-methyl-
amino-2-phenyl-n-butyl 3,4,5-trimethoxybenzoate, its
addition salts with pharmaceutically acceptable acids,
and the process for preparing them.
According to another aspect, the invention relates to
medicinal compositions comprising these compounds, their
preparation and their therapeutic application in the
treatment of chronic pain of various etiologies.
Detailed description of the invention
In a first aspect, the invention relates to the optically
pure (S) 2-methylamino-2-phenyl-n-butyl
r T
_.__.__.._.._ _ a.__.,___.__._.__~_ .
CA 02285479 1999-09-22
WO 99/01417 PCT/EP98/03825
_ 7 _
3,4,5-trimethoxybenzoate and its addition salts with
pharmaceutically acceptable acids.
By optically pure, there is understood that, in addition
to the chemical purity established according to pharma-
ceutical industry standards, the compound is substan-
tially purified from its (R) antipode, which means that
for a minimum optical purity, the product according to
the invention contains at least 90'~ by weight of (S)
enantiomer or, expressed differently, contains less than
100 of undesirable (R) enantiomer. In any case, the
preferred optical purity corresponds to that of a
compound according to the invention containing at least
99o by weight of (S) enantiomer or, at most, l~ by weight
of (R) enantiomer which is considered as an optical
impurity, these levels being determined by appropriate
analytical methods.
By addition salts with pharmaceutically acceptable acids,
an extensive list of these acids will be found in J.
Pharm. Sci., 1977, 66, 1-19. These inorganic or organic
acids are recognized as being nontoxic, such as for
example hydrobromic, hydrochloric, sulfuric, phosphoric,
nitric, acetic, succinic, tartaric, citric, malefic,
hydroxymaleic, benzoic, fumaric, toluenesulfonic and
isethionic acids and the like. However, the hydrochloric,
malefic and tartaric acids are preferred, especially the
natural L(+) isomer of tartaric acid.
The invention also relates to a process for the
preparation of the compound of the invention and of its
salts. The pr.~cess for the preparation of (S) 2-methyl-
amino-2-phenyl-n-butyl 3,4,5-trimethoxybenzoate consists
either in preparing the racemic mixture and then in
resolving it, or, according to the preferred process, in
CA 02285479 1999-09-22
WO 99/01417 PCT/EP98/03825
_ g _
carrying out its chemical synthesis from enantiomeric
precursors of (S) configuration.
The process involving resolution of the racemic compound
consists in using an optically active acid to obtain with
the racemic compound treated two diastereoisomeric
addition salts which are separated and from which there
is generated, by an appropriate treatment, the enantiomer
of (S) configuration, eutomer for the purposes of and
according to the subject of the invention, and also for a
comparative study of its distomeric (R) antipode. It is
also possible to use a method for direct resolution of
the racemic compound by liquid chromatography on a chiral
column.
However, the preferred method of preparation of (S)
2-methylamino-2-phenyl-n-butyl 3,9,5-trimethoxy-benzoate
is that by chemical synthesis from precursors of (S)
configuration which essentially consists, as shown in
20 diagram l:
- in reducing, with a complex hydride derived from boron
or aluminum, (S) 2-formylamino-2-phenyl-n-butyric acid
(III) in order to obtain (S) 2-methylamino-2-phenyl-n-
butanol (II),
25 - in esterifying the amino alcohol (II) with a reagent
chosen from 3,4,5-trimethoxybenzoyl chloride according to
a procedure A and methyl 3,4,5-trimethoxybenzoate
according to a procedure B in order to obtain (S)
2-methylamino-2-phenyl-n-butyl 3,4,5-trimethoxybenzoate
(I) .
i
___. ~ _ _.._.. ___ _ . __ . . _ __._
CA 02285479 1999-09-22
WO 99/01417 PCTlEP98/03825
_ g _
0
OCH3
CI
OCH3
~ ~ \ A +
OCH
/ COOH hYdfld2 ~ CHZOH
---~ ( I )
NH NH
I I _
CH3 CHO CH3 CH3 O
g +
(III) (~I) Me0 ~ OCH3
OCH3
OCH3
The subject of the invention is also the use, as
medicament, of the compound (I) of the invention and of
its salts, as well as the compositions in which these
products are used as active ingredients and which are
intended for the preparation of medicinal forms useful
for the treatment of chronic pain.
As previously stated, the Applicant has undertaken
studies to determine the stereospecificity of the
affinity for the opiate receptors of the (R) and (S)
enantiomers ci the metabolite NDTMB compared with the
latter which is a racemate and with the precursor, namely
trimebutine TMB. This in vitro study reveals a
preferential affinity for the (R) configuration, eutomer
in this tria'. with a eudismic ratio in relation to the
antipode of 0.3 to 0.4 on the various ~, 8 and K subtypes,
or in other words the affinity of the (R) eutomer is 2.5
to 3 times higher than the (S) antipode for these
receptors. Moreover, the respective affinities of the TMB
and NDTMB rac~~mates are not very different, the affinity
of NDTMB being, however, lower for the b and K subtypes.
Continuing the in vivo study, the Applicant used NDTMB
and the (R) and (S) enantiomers in tests which are
recognized as being representative of chronic pain, such
as:
CA 02285479 1999-09-22
WO 99/01417 PCT/EP98/03825
- 10 -
- the analgesic activity on the (tardive) tonic painful
phase caused by injecting a formaldehyde solution into
-the leg in mice,
- the analgesic activity on chronic hyperalgesia induced
by PGE2 in rats.
The results of the tests show surprisingly that the (S)
enantiomer, which has a lower affinity for the opiate
receptors, possesses, according to the tests, an
analgesic activity equal to or greater than the racemic
NDTMB and its (R) antipode, which activity should be
considered to be particularly advantageous since it is
only very partially of an opiate nature. Indeed, in
contrast to the other two compounds, the analgesic effect
of the (S) enantiomer is only partially inhibited by
naloxone, which is known as an antagonist of the
so-called opiate analgesics, especially of those with
affinity. Further study of the product on models
representative of specific chronic pain such as neuro-
pathic pain caused by ligation of the sciatic nerve or
pain caused by experimental diabetes induced by strep-
tozocin in rats, and also behavioral study in rats in
which an inflammatory pain has been triggered by
carrageenin confirm this advantage. Finally, the (S)
enantiomer shows no morphine-type withdrawal effect
unlike its (R) antipode, when used in a test reputed to
reveal dependence phenomena following repeated adminis-
tration of opiate drugs.
Considering these in vivo studies these analgesic
and
properties, the (S) which
enantiomer is the
subject
of
the invention is, in contrast to the
in vitro
receptor
study presented above, the eutomer as regards the
application to the treatment chronic pain such as for
of
example during osteoarthritis, polyarthritis,
spondylarthritis, postsurgical pain, trauma, painful
_...__. ~.~_ ... _. ~__.___.._ . _ _
CA 02285479 1999-09-22
WO 99/01417 PCT/EP98/03825
- 11 -
sequela of zona, polyneuropathy, amputation of a limb and
also vascular conditions such as arteritis or varicose
-ulcer .
The compound cf the invention and/or one of _its salts is
administered to the patient in a medicinal form adapted .
to the nature and the seriousness of the condition to be
treated. The daily dosage in humans is usually between
2 mg and 1.0 g of active ingredient which may be taken in
single or divided doses. Compositions prepared according
to techniques which are common for persons skilled in the
art comprise from 0.5 to 75~ by weight of active
ingredients and from 99.5 to 25'~ of appropriate
excipients which are chemically and physically compatible
with the active ingredient so as to obtain medicinal
forms adapted to the route of administration envisaged.
As nonlimiting examples, tablets, sugar-coated tablets,
capsules, suppositories, gels for local application or in
occlusive supports, solutions for injections or
administration by the oral route, are prepared.
More precisely, as regards the process for the prepara-
tion of the compound of the invention,
- by resolution of the racemate:
a) as regards the method of resolution via diastereo-
isomeric salts, it consists in reacting the racemate, in
an appropriate solvent, with an optically active acid so
as to form two diastereoisomers which are separated by
the difference in their solubility in the solvent. The
diastereoisomer with the lowest solubility precipitates
and it is isolated by filtration. The salts separated may
be recrystallized from selective solvents to give a
satisfactory optical purity or they may be treated in a
basic medium in order to regenerate the respective
enantiomers. These may then be purified by
recrystallization or used in a second purification step
CA 02285479 1999-09-22
WO 99/01417 PCT/EP98/03825
- 12 -
by forming new diastereoisomers, optionally with an
optically active acid different from the preceding one.
-The enantiomers of acids commonly used for the
preparation of diastereoisomeric salts are, by way of
nonlimiting examples, those of a-phenylglycine, a-
phenylalanine and their N-carboxylated derivatives, of
malic acid, of mandelic acid, of tartaric acid and their
esterification derivatives with acids, or alternatively
camphanic acid, 3-bromo-8-camphorsulfonic acid and its
position isomers, a-methoxy-a-trifluoromethylphenylacetic
acid. The enantiomers of these acids are commercially
available and their use has been documented; they are
therefore appropriate for the resolution of the racemate
or the purification of any mixture enriched with one of
the enantiomers.
The preparation of the diastereoisomeric salts consists
in reacting, for one mol of racemate or of mixture to be
separated or purified, from 0.25 to 2.00 mol of the acid
enantiomer in solution in a solvent or a mixture of
solvents which are preferably completely or partially
miscible with water, and which are usually chosen from
alcohols, ketones, low-molecular-weight ethers or
acetonitrile.
Preferably, the reaction is carried out in solution in
ethanol, methanol or acetone by reacting, for one mol of
product to be treated, from 0.5 to 1.25 mol of acid
enantiomer, at a temperature between 20°C and that of the
boiling temperature of the solvent used, which is
generally preferred. The salification is complete after a
period of between 5 minutes and 3 hours. After that, the
reaction mixture is left, first at room temperature, and
then optionally at around 0°C in order to crystallize the
least soluble diastereoisomer, which crystallization is
optionally facilitated by seeding with crystals of the
expected salt. Upon completion of the crystallization or
at a time which is judged to be appropriate in order to
I ........_._...._~.....__...v__. . _
CA 02285479 1999-09-22
WO 99/01417 , PCT/EP98/03825
- 13 -
obtain the ~3esired optical purity, the crystallized
diastereoisomer is separated by filtration. The two
phases, each containing a diastereoisomer purified to a
greater or lesser degree, are treated separately, either
for purification of the salts, or for release of the
enantiomer from its salt, which is carried out by an .
alkaline treatment in aqueous medium, followed by
filtration or extraction of the enantiomer released.
b) As regards the resolution of the racemate or the
purification of any mixture of enantiomers by column
liquid chromatography, various chiral or nonchiral
supports can be used for this separation.
Thus, an appropriate method is adapted from that
described in the publication JP 05215736 and consists in
using, as stationary phase, carboxylic esters of
polysaccharide such as the phase Chiralcel OJ (supplier
Diacel Chem.) and in carrying out the elution using a
mixture of a buffer solution (for example HClOa - NaClO~
0.1 M) with a water-miscible organic solvent such as
acetonitrile.
- by chemical synthesis from precursors of (S); the (S)
enantiomer and the (R, S) racemic mixture of 2-formyl-
amino-2-phenyl-n-butyric acid are known and their
preparation described (H. Sobotka et al., J of Am. Chem.
Soc., 54, 1932, p. 4697). To prepare these compounds, an
advantageous adaptation of the process consists in
reacting propiophenone with potassium cyanide and
ammonium carbonate, by the Bucherer-Berg reaction, in
order to obtain (+/-) 5-ethyl-5-phenylhydantoin which is
subjected, in an alkaline aqueous-acetone medium to a
stereospecific salification with (R)-a-methylbenzylamine
in order to obtain the addition salt with the (S)
enantiomer of hydantoin, an insoluble salt which is
filtered off and from which, by treatment with an acidic
solution, (S) 5-ethyl-5-phenylhydantoin is obtained in a
CA 02285479 1999-09-22
WO 99/01417 PCT/EP98/03825
- 14 -
state of optical purity greater than 98o according to the
process of patent EP 0,510,168.
-The (S) enantiomer of hydantoin is conventionally
hydrolyzed, in an alkaline aqueous medium at 190°C, in
order to obtain (S) 2-amino-2-phenyl-n-butyric acid on
which N-formylation is carried out by reacting with pure -
formic acid and acetic anhydride in order to obtain (S)
2-formylamino-2-phenyl-n-butyric acid (III).
(R) 2-Formylamino-2-phenyl-n-butyric acid and (R,S)-2
formylamino-2-phenyl-n-butyric acid are prepared
respectively from the (R) antipode or the (R, S) racemate
of 5-ethyl-5-phenylhydantoin.
As regards the simultaneous reduction of the carboxylic
and amide functional groups of the intermediate (III) in
order to obtain respectively the primary alcohol
functional group and the secondary amine functional group
of the amino alcohol (II), use is made of known methods
and reagents which are summarized in the summary table
presented in Advanced Organic Chemistry p. 1095 (J. March
3r'j ed. 1985 McGraw Hill). Reducing agents such as
derivatives of boron hydride and of aluminum hydride are
recommended as being effective for carrying out these
reactions, in particular lithium aluminum hydride and the
borane-dimethyl sulfide (BSM in the remainder of this
text) complex which is the preferred reagent.
The reductions are carried out in aprotic media which do
not dissociate in solvents such as diethyl ether,
diisopropyl ether, 1,2-dimethoxyethane or tetrahydrofuran
(THF) which is preferred.
The BMS complex is used in a stoichiometric excess
relative to the products to be reduced in order to obtain
a complete reaction. Thus, 2 to 8 mots of BMS are used
per mol of product to be treated, the preferred
quantities being, however, from 4 to 6 mols of BMS per
mol of compound (II).
T i
....~..._.____..~__
CA 02285479 1999-09-22
WO 99/01417 PCT/EP98/03825
- 15 -
The product is subjected to reduction at the rate of 1 to
parts by weight in 100 parts by volume of THF and more
-particularly from 3 to 7 parts in 100, protected from
moisture and under a nitrogen atmosphere. The quantity of
S BMS is introduced and then the mixture is heated under
reflux in order to have a complete reaction, a duration
which may be between 30 minutes and 10 hours; however, a
duration of 3 to 5 hours is more favorable. The amino
alcohol is then released from its complex with the
10 reducing agent by adding methanol and then sodium
hydroxide and finally isolated by conventional processes
described in the example presented.
As regards thE~ esterification of the (S) precursor (II),
the reaction, as presented above, may be carried out
according to ~ procedure A which consists in carrying out
the alcoholysis of 3,4,5-trimethoxybenzoyl chloride with
the amino alcohol (II) in order to obtain the eutomer
amino esters (I) according to various widely described
techniques (Sonntag, Chem. Rev. 52, 237-416 (1953)
pages 312 to 324) which consist, for example, in reacting
the reagents in an aprotic anhydrous solvent such as
benzene, toluene, diethyl ether, tetrahydrofuran,
dioxane, acetone, acetonitrile, pyridine,
dimethylformamide, methylene chloride or chloroform which
is the preferred solvent. Optionally, an acid acceptor is
added to the reaction medium. This agent may be inorganic
or organic. Sodium or potassium carbonates, the
corresponding bicarbonates or triethylamine or pyridine
are used to this effect. An alternative method, which is
preferred, consists in previously preparing in situ a
metal alcoholate of the alcohols (II) which is then
subjected to the reaction with the acid chloride. The
metal alcoholate is prepared by the action of alkali
metals such as sodium or its hydride or alternatively its
amide, or by reaction with sodium ethoxide or of
CA 02285479 1999-09-22
WO 99101417 PCT/EP98/03825
- 16 -
potassium tert-butoxide in the presence of an inert
compatible solvent. For example, the metallation reaction
-consists in reacting 1 mol of amino alcohol (II) in
solution in 5 to 20 parts of THF with 0.9 to 1.1 mol of
sodium hydride, the reaction of formation of the
alcoholate being generally complete between 1 to 3 hours .
and at a temperature between 0 and 67°C.
The eutomeric amino ester (I) is obtained by reacting one
mol of (II) or of its alcoholate in solution in 5 to
20 parts of solvent with 0.6 to 1.8 mol of 3,4,5
trimethoxybenzoyl chloride. However, the preferred ratios
for one mol of (II) or of its alcoholate are 8 to
parts of solvent and 0.8 to 1.3 mol of acid chloride.
The reaction is then continued at a temperature of
15 between 10 and 110°C for a period of between 1 and
24 hours. Preferably, the reaction, carried out between
and 67°C for 3 to 7 hours in THF, makes it possible to
obtain the ester (I) with a satisfactory yield. The
compound (I) is isolated from the reaction medium and
20 purified by conventional methods such as extractions,
crystallizations and column chromatographies.
According to procedure B which is particularly preferred,
the alcoholysis of methyl 3,4,5-trimethoxybenzoate with
the amino alcohols (II) leads to the product of the
invention (I) by transesterification which is carried out
in solution in anhydrous aprotic solvents in the presence
of catalysts of an acidic or basic nature. The latter
method is preferred and leads, on the one hand, by
extension, to the choice of 3,4,5-trimethoxybenzoic acid
esters in which the alkyl radical comprises from 1 to 3
carbon atoms in order to obtain, by transesterification,
low-boiling point alcohols such as methyl, ethyl, propyl
or isopropyl a_Lcohols and, on the other hand, the use, as
reaction sol~~ents, of benzene, toluene or xylenes which
form azeotropes with these alcohols. The removal, during
the reaction, of the alcohol formed is favorable, which
~ ____..
CA 02285479 1999-09-22
WO 99/01417 PCT/EP98/03825
- 17 -
is carried out by adding to the medium agents for
sequestering this alcohol, such as molecular sieves of
appropriate porosity or by removal by distillation as it
is formed. Particular preference is given to the use of
methyl 3,4,5-trimethoxybenzoate which, under these
transesterification conditions, generates methanol which,
itself, with toluene which is the preferred reaction
solvent, gives an advantageous azeotropic mixture by
virtue of its composition and its relatively high boiling
point which allows rapid progress of the reaction. The
acid or basic catalysts may be of an inorganic and/or
organic nature. The basic catalysts are however
preferred, such as alkali or nonalkali metals and their
derivatives such as, for example, potassium tert-
butoxide, aluminum triisopropylate, magnesium methoxide,
sodium, sodium hydride, sodium ethoxide and methoxide,
the latter alcoholate being particularly preferred.
In practice, the reaction consists in dissolving one mol
of amino alcohol ( I I ) in 10 to 50 parts by weight of the
appropriate solvent, and then in adding to the solution
from 1.1 to 2.0 mol of benzoic ester. After addition of
0.025 to 0.05 mol of catalyst, the mixture is heated at a
sufficient temperature to remove, by distillation, the
alcohol formed or its azeotrope with the solvent. This
temperature may be between 50 and 130°C and may be
maintained for between 1 to 6 hours in order to obtain a
satisfactory result. A particularly valuable technique
consists in the addition of the catalyst in fractions or
continuously during the reaction, and also in the case of
an azeotropic distillation, to add solvent during the
reaction in order to make up for the loss caused by the
distillation.
The preferred conditions consist in dissolving one mol of
amino alcohol (II), 1.25 to 1.75 mol of methyl 3,4,5
trimethoxybenzoate in 25 to 35 parts by weight of toluene
relative to the quantity of (II) used. The solution is
CA 02285479 1999-09-22
WO 99/01417 PCT/EP98/03825
- 18 -
heated to 65-75°C, 0.025 to 0.05 mol of sodium methox_ide
is added and the toluene-methanol azeotrope formed is
-slowly distil~.ed for 1 to 2 hours. 0.0225 to 0.025 mol of
sodium methoxide is again added and distillation is
carried out for 30 min to 1 h and this last operation is
again repeated once. The products of the reaction are
then isolated and purified according to the techniques
mentioned above.
The invention is illustrated in a nonlimiting manner by
the following examples which refer to procedures A and B
described above.
The purity, identity and physicochemical characteristics
of the products of the invention are determined, thus:
- the purity is checked by thin-layer chromato
graphies on silica gel. The Rf observed in the elution
solvent used is reported in the examples.
- the identity of the products obtained with the
proposed structures is checked by their proton nuclear
magnetic resonance spectrum at 400 MHz, the products
being dissolved in deuterochloroform with tetramethyl-
silane as internal standard. The nature of the signals,
their chemical shifts in ppm as well as the number of
protons which they represent are noted.
- for the products obtained in solid form, the
value of the melting point obtained by differential
thermal analysis is indicated.
The technical description of the following examples
illustrates the scope of the invention without, however,
limiting it.
Experimental section
a) - Chemistry
Example 1 (S) 2-Methylamino-2-phenyl-n-butyl 3,4,5-tri-
methoxybenzoate (I)
_ _
___ ___.~._._.___ _ _ _._- ..___ _ __ __ _ __..__.~ _______ _. _ _
CA 02285479 1999-09-22
WO 99/01417 PCT/EP98/03825
- 19 -
- Stage l: (S) 2-Methylamino-2-phenyl-n-butanol (II)
55.0 g (0.27 mol) of (S) 2-formylamino-2-phenyl-n-butyric
acid (a~° - + 123°, c = 1 NaOH N) in 1375 ml of anhydrous
tetrahydrofuran (THF) are introduced into a reactor
protected from moisture and under a nitrogen atomsphere.
125.9 ml, that is to say 100.8 g (1.33 mol) of borane-
dimethyl sulfide complex are added, with stirring, at
20°C, to the suspension over one hour. After introduc-
tion, the solution which is obtained is heated and
maintained under reflux for 4 h.
The suspension is cooled to 0-5°C and 200 ml of methanol
are added without exceeding 10°C, followed under the same
conditions with 220 ml of a 10° NaOH solution.
The mixture is stirred for one hour at 20-25°C and then
the solvents are removed by vacuum distillation and on a
water bath. The residue is taken up in 1250 ml of water
and, after cooling to 0°C, acidified, with stirring, to
pH 1 with about 40 ml of concentrated HC1.
The acidic phase is extracted with 5 times 220 ml of
methyl-t-butyl ether and then alkalinized at t < 10°C,
with stirring, by addition of 26.5 g of NaOH pellets.
The mixture is extracted 3 times with 170 ml of
dichloromethane. The combined organic phases are washed
with water, dehydrated over Na-.SO:,, and the solvent
removed by vacuum distillation. The crude product is
obtained in a satisfactory state of chromatographic
purity to be used as such in the next stage.
Weight = 35.3 g Y = 74.20
TLC: Rf 0.50-0.60 (butanol-acetic ac. - water 8-2-2 v/v/v)
~a~z - +13.6° (c = 2, HC1 N)
- 1H - NMR S (ppm): 0.70 (t, 3H); 1.55-1.65 (m, 1H); 1.75-
1.85 (m, 1H); 2.15 (s, 3H); 2.20-2.40 (m, 2H); 3.70-3.85
(m, 2H); 7.20-7.30 (m, 3H); 7.35-7.50 (m, 2H)
IR (film - NaCl pellets): 3300, 2900, 1600, 1490, 1440,
1380, 1330, 1130, 1040, 1010, 840, 760, 700 cm ' .
CA 02285479 1999-09-22
WO 99/01417 PCT/EP98/03825
- 20 -
- Stage 2: (S! 2-Methylamino-2-phenyl-n-butyl 3,4,5-tri-
methoxybenzoate ( I ) .
Procedure A
7.03 g of a 60° (w/w) suspension of sodium hydride in a
mineral oil (that is to say 4.22 g - 0.176 mol of pure
product) are introduced into 42 ml of anhydrous THF in a-
reactor protected from moisture and under a nitrogen
atmosphere. The suspension is heated under reflux, with
stirring, and a solution of 30.0 g (0.167 mol) of (S) 2-
methylamino-2-phenyl-n-butanol (II) in 21 ml or THF is
added dropwise over about 30 min.
The mixture is kept for 1 h under reflux and then a
solution of 93.3 g (0.184 mol) of 3,4,5-trimethoxybenzoyl
chloride at 98'~ in 89 ml of THF is added at 50-60°C over
about 1 h. After addition, the mixture is kept for 2 h
under reflux and then 110 ml of THF are distilled off at
ordinary pressure, 105 ml of toluene are added to the
concentrated medium and, after having cooled to 20°C,
60 ml of water are added. After stirring for 15 min, the
organic phase is acidified with a solution of 9.0 ml of
93° H~SO~ in 225 ml of water, the acidic phase is
separated, alkalinized in the cold state with 33 ml of
~ NaOH solution and then extracted with 3 times 100 ml
of dichloromethane. The combined organic phases are
25 washed with ;cater, dehydrated over NazS04 and then the
solvent is removed by vacuum distillation. The residue is
taken up in 250 ml of hexane at 50°C. Upon cooling, the
expected compound crystallizes. After stirring for 2 h at
10°C, the product is filtered off and then dried.
30 Weight = 37.2 g Y = 59.5'o m.p. - 46°C
TLC: Rf 0.10-0.20 (toluene-acetone 9-1 - v/v)
(a~o - not significant in a 10 cm polarimetric tube for a
4°s ethanolic solution
- optical purity determined by HPLC > 990
- 1H - NMR 8 (ppm) : 0. 80 (t, 3H) ; 1 .50-1 .70 (m, 1H) ; 1 . 80-
1.95 (m, 2H); 2.25 (s, 3H); 3.80 (s, 6H); 3.90 (s, 3H);
T _ __.. ..__ _ ____._~. _ _
CA 02285479 1999-09-22
WO 99/01417 PCT/EP98/03825
- 21 -
4.50-4.65 (m, 2H); 7.15 (s, 2H); 7.20-7.25 (m, 1H); 7.30-
7.40 (m, 2H); 7.50-7.55 (m, 2H)
- IR (KBr pellet): 2800, 1710, 1590, 1500, 1460, 1910,
1360, 1330, 1220, 1180, 1120, 1000, 760, 700 cm-l
Example 2 (S) 2-methylamino-2-phenyl-n-butyl 3,4,5-tri- -
methoxybenzoate (I)
Procedure B (preferred)
In a reactor protected from moisture and under a nitrogen
atmosphere, there are introduced in a mixture composed of
440 ml of anhydrous toluene and 22 ml of absolute
ethanol:
- 10.0 g (0.056 mol) of (S) 2-methylamino-2-phenyl-n-
butanol as prepared in stage 1 of Example 1,
- 19.0 g (0.084 mol) of methyl 3,4,5-trimethoxybenzoate.
The solution is heated with stirring and, at ordinary
pressure, 110 ml of a mixture of solvents are distilled
off. After cooling to 100°C, 180 ml of anhydrous toluene
and 2.7 ml of a methanolic solution of 4 N sodium
methoxide are added. The mixture is heated and 150 ml of
solvent are distilled off over one hour, following which
the mixture is cooled to 100°C, 150 ml of anhydrous
toluene and 0.9 ml of 4 N sodium methoxide solution are
added, the mixture heated so as to distill off over 1 h
140 ml of solvent, cooled to 100°C and the addition of
toluene and methoxide repeated so as to finally distill
off 120 ml of solvent over one hour.
The reaction mixture is concentrated under vacuum, the
residue is taken up in 120 ml of 1N HC1 and the mixture
extracted with 3 times 70 ml of dichloromethane. The
combined organic phases are washed and dried and then the
solvents are evaporated under vacuum. The oily residue
(17.6 g) is purified by rapid chromatography on a silica
column. Elution with a dichloromethane-acetone 95-5 (v/v)
mixture make: it possible to obtain the product in the
pure state.
CA 02285479 1999-09-22
WO 99/01417 PCT/EP98/03825
- 22 -
Weight = 15.6 g Y = 74.60
Analyses in accordance with and identical to that for the
product obtained in Example 1 - stage 2.
Example 3 (S) 2-methylamino-2-phenyl-n-butyl 3,4,5-tri
methoxybenzoate L(+)-tartrate (I)
15.0 g (0.040 mol) of (S) 2-methylamino-2-phenyl-n-butyl
3,4,5-trimethoxybenzoate and 6.03 g (0.040 mol) of L(+)
tartaric acid are added to 150 ml of absolute ethanol in
a reactor.
The mixture is heated and kept under reflux for 5 min,
with stirring. 50 ml of ethanol are then distilled off at
ordinary pressure, and then 1 ml of water is added
dropwise.
The salt crystallizes upon cooling. The mixture is left
for 4 h at 15-20°C and then the L(+)-tartrate monohydrate
crystals are filtered off and dried.
Weight = 16.0 g Y = 73.90
m.p. - 94 - 97°C
Analysis in conformity for C2,H->-,NO" CqH50:; ~ H_O.
According to the procedures of these examples, starting
with (R, S) or (R) 2-formylamino-2-phenyl-n-butyric acids,
there are respectively prepared:
a) (R, S) 2-methylamino-2-phenyl-n-butyl 3,4,5-trimethoxy-
benzoate and its hemimaleate (m.p. - 138°C)
b) (R) 2-methylamino-2-phenyl-n-butyl 3,4,5-trimethoxy-
benzoate and its anhydrous salt with D(-)-tartaric acid
(m.p. - 132°C)
b) - Receptor study in vitro
The study consisting in evaluating the respective
affinity of the (S), (R) enantiomers and of NDTMB which
is their racemic mixture in solution in their salified
forms on the ~, b and K opiate receptors was carried out
in comparison to their racemic precursor trimebutine TMB.
T ~
.~___ . ... __._______. . _ . _.._. ...._ _ .
CA 02285479 1999-09-22
WO 99/01417 PCT/EP98/03825
- 23 -
The tests consist, on preparations appropriate for each
receptor, in putting the affinities of the compounds in
competition with those of radioactive ligands specific
for each of the subtypes according to a technique adapted
from that described by F. Roman et al. in J. Pharm.
Pharmacol. (1987), 39, p. 404-907, which consists in-
incubating solutions of appropriate concentration of test
compounds with preparations loaded with the radioactive
ligand, and then, after competition and displacement of
the latter, in filtering and then in determining the
radioactivity.
Thus, for the determination of the affinity for the
receptors, a suspension of rat brain membranes previously
incubated with ['H]-DAGO is used, for the determination of
the affinity for the 8 receptors, a suspension of guinea
pig brain membranes previously incubated with ['H]-DADLE
and for the determination of the affinity for the k
receptors rKOR-CHO cells previously incubated with ['H]-
U69,593. For each experiment, the radioactivity filtered
is determined, and then, by computer processing of the
results, the inhibition constant for 50'-~ of the
radioactive ligand is calculated and expressed by a Ki
value in nM. The results of the study are reported in the
table below:
Compound Ki nM ~ Ki nM b Ki nM K
(S) enantiomer 221 2456 1053
(R) enantiomer 82 735 405
(R,S) NDTMB 152 943 491
(R, S) TMB ~ 123 ~ 302 ~ 145
The affinities are evidence of the stereospecificity for
the (R) enantiomer which, in these tests, is the eutomer
CA 02285479 1999-09-22
WO 99/01417 PCT/EP98/03825
- 24 -
regardless of the opiate subtype considered, the
reciprocal of the eudismic ratio (Ki (S)/Ki (R)) being
respectively 2.7 for the ~ subtype, 3.3 for the 8 subtype
and 2.6 for the K subtype. Moreover, a decrease in
affinity is observed overall in particular for the b and K
subtypes of NDTMB and its enantiomers compared with the
affinities of the racemic TMB which is their biological
precursor.
c) - Analgesic activity: studies in vivo
In these tests, the products were used in salified form,
namely in tartrate form for the (S) and (R) enantiomers
and in maleate form for the racemic NDTMB.
c.l) - Comparative activity of the (R), (S) enantiomers
and of their racemic mixture NDTMB
This activity was evaluated in tests recognized to be
representative of chronic pain namely:
- the analgesic activity on the (tardive) tonic painful
phase caused by an injection of a formaldehyde solution
into the leg in mice,
- the analgesic activity on chronic hyperalgesia induced
by PGE2 in rats,
c.l.l) - Formaldehyde test in mice
The technique is adapted from that described by
S. Hunskarr (J, of Neurosciences Methods, 1985, 14,
p. 69-76) and consists in causing, in one leg of the
animal, a painful inflammation by intraplantar injection
of 20 ~1 of a 5~> (v/v) sterile saline solution of
formaldehyde. The duration of the reactions of licking
the treated leg by the animal are compatible in the
period between the twentieth and twenty-fifth minute
after the injection of formalin considered as t0 of the
test.
_~_. ___ _ . ....___ ____ ._. _ _.
CA 02285479 1999-09-22
WO 99/01417 PCT/EP98/03825
- 25 -
For the evaluation of the activity of the products, they
are administered by the s.c. route in an amount of
1 ml/100 g as a pretreatment 10 min before the
intraplantar injection of formalin. In tests where the
opiate component of the test is sought, 1 mg/kg of
naloxone in solution is also administered by the s.c.
route 5 min after the injection of formalin, that is to
say 15 min after the s.c. administration of the test
product. Comparison of the times for the batches of
treated animals compared with the time for the batch
having received the vehicle makes it possible to
determine a result expressed in ~ analgesic activity.
(S) enantiomer (R, S) (R)
NDTMB enantiomer
s.c.
dose
pdt + pdt + Pdt +
mg/kg
alone nalox. alone nalox. alone nalox.
10 16 13 22 2 19 2
33 44 36 11 26 6
65 64 65 46 37 28
15 These results are evidence of the particularly advan-
tageous activity of the (S) enantiomer which is the
subject of the invention and this in two respects in the
light of the preceding in vitro study:
i) - the (S) enantiomer shows an activity which is
20 practically equal to that of the racemate but in any case
greater than that of the (R) antipode,
ii) - whereas the analgesic activity is notably inhibited
by naloxone both for the (R,S) racemate and for the (R)
antipode, it is unchanged as regards the (S) enantiomer,
which suggests that, under test conditions related to a
chronic pain, the compound acts by a non-opiate mechanism
different from the products to which it is compared.
CA 02285479 1999-09-22
WO 99/01417 PCT/EP98/03825
- 26 -
c.1.2) - Analgesic activity on chronic hyperalgesia
induced by PGE2 in rats
The test consists in determining the analgesic effect of
the test compounds by the Randall and Selitto test in
rats in which chronic hyperalgesia has been triggered by
intraplantar injection of PGE~ over four days into a leg--
according to a protocol adapted from that described by
M. Nakamura-Craig et al. (Pain, 63, (1995) 33-37).
Practically, the study is carried out on batches of 120-
140 g Sprague-Dawley rats to which 100 ng of PGE; is
administered in a volume of 100 ~tl by the intraplantar
route for four consecutive days at a rate of twice a day,
which causes chronic hyperalgesia in the leg from the
fifth day, and this for at least one week. On the day of
the test, in the morning, the threshold of reaction to
pain is checked by the Randall and Selitto test and
animals whose threshold is <_ 110 arbitrarily defined
units are selected and then, in the afternoon, the
measurement is repeated after prior administration by the
s.c. route of a solution of the test product. This
administration is carried out 30 min before the
determination of the pain threshold. The mean of the
thresholds determined before and after treatment is
calculated for each batch and makes it possible, compared
with the animals receiving only the vehicle for the test
product to determine a result in ° analgesic activity.
In a first test series, the administration of increasing
doses made it possible to determine the comparative
activity of the (R), (S) enantiomers and of their racemic
mixture NDTMB. A second test series, in which the
products are administered in a single dose with and
without naloxone, made it possible to determine the
opiate participation in the observed effect. The results
of these tests are reported in o analgesic activity in
the tables below.
T i
_. _ _ __ .__ _
CA 02285479 1999-09-22
WO 99/01417 PCT/EP98/03825
- 27 -
- Comparative activity of the products:
Comp . ( S ) ( R, S ) NDTMB( R )
mg/kg s.c. enantiomer enantiomer
6 80 18o NT -
8 42$ NT NT
480 350 llo
16 59$ NT NT
67 o NT NT
NT . not tested
5
- Evaluation of the opiate participation in the analgesic
effect for an s.c. administration of 10 mg/kg with and
without naloxone in an amount of 0.3 mg/kg
(S) enantiomer (R, S) (R) enantiomer
NDTMB
pdt + pdt + pdt +
alone naloxone alone naloxone alone naloxone
550 370 330 3s 200 3
These tests are evidence of the analgesic activity of the
(S) eutomer of the invention compared to its antipode and
the racemic mixture but, in particular, that the activity
is only slightly due to an opiate effect since, in the
presence of naloxone, 670 of the activity is maintained
whereas for the other two compounds, naloxone inhibits
practically the entire activity which is therefore
attributable to an opiate mechanism.
CA 02285479 1999-09-22
WO 99/01417 PCT/EP98/03825
- 28 -
c.2) - Activity of the (S) eutomer on various
representative models of chronic pain
- Activity on a hyperalgesia model in diabetic rats
The test is carried out in rats according to a technique
described by Burchiel et al. (Diabetes, 34 (1985) 1210-
1213) which consists in causing, in the animal, an-
experimental diabetes by injecting streptozotocin, and,
after verifying the diabetic condition and the
hyperalgesia, in carrying out the test for determining
the analgesic effect of the test compounds. This
determination is carried out by immersing the tail of the
animals in a water bath whose temperature is kept at
44°C, the nociceptive reaction being determined by the
time in seconds before the tail is removed from the hot
water bath. In this test, the (S) eutomer of the present
invention exr:ibits, administered by the s.c. route, an
analgesic activity at the dose of 30 mg/kg.
- Activity on neuropathic pain
The mononeuropathy model in rats is produced by loose
legations of the sciatic nerve according to the Bennett
and Xie technique, the effect of the test compounds is
determined according to a modification of the Randall and
Sellitto method with the aid of a U. Basile analgesimeter
according to the technique described by G. Catheline et
al. (European Journal of Pharmacology, 318 (1996) 273
281) . In this test, the (S) eutomer which is the subject
of the invention shows analgesic activity from the dose
of 1 mg/kg by the s.c. route, an effect which is not
modified by naloxone.
- Activity on behavioral pain
The test is carried out with rats in which a lasting pain
has been triggered by injecting carrageenin into the hind
legs and which are placed in an area whose exploration is
made painful. Different behavioral variables in the
__.._.._ _.___T _ __.____~~.~.__ _.. . .__.__.. 1 _
CA 02285479 1999-09-22
WO 99/01417 PCT/EP98/03825
- 29 -
animal are observed which make it possible to
qualitatively and quantitatively assess a possible
analgesic effect of a test product. In this test, the (S)
eutomer shows an analgesic effect from the dose of
3 mg/kg by the s.c. route.
c.3) - Evaluation of an opiate dependence effect:
Saelens test
The test according to J.K. Saelens et al. (Arch. Int.
Pharmacodyn. (1971), 213-218) consists in causing
addiction in mice by repeated administrations of a
putative opiate product, followed by administration of
naloxone, in bringing about a sudden withdrawal which
causes characteristic reactions in the animal, in
particular jumps of which the number, over a given lapse
of time, reflects the morphine-type state of dependence
in the animal under the action of the test product.
The (S) eutomer which is the subject of the invention,
its (Ft) antipode and, as reference, morphine were used in
the test. In male mice, repeated quantities, in
increasing doses, of the products are administered by the
s.c. route in an amount of 0.20 ml/20 g of weight of
animal over two days: at Z0, 11, 12, 14 and 16 hours on
the first day and at 10 and 12 hours on the second day
and then at I9 hours a solution of naloxone is injected
by the intraperitoneal route in an amount of 100 mg/kg
and, immediately, the number of jumps per mouse is
counted over 15 minutes. The mean number of jumps per
batch of mice and the percentage per batch of dependent
animals are then calculated. The results are presented in
the table below:
CA 02285479 1999-09-22
WO 99/01417 PCT/EP98/03825
- 30 -
Total (S) enantiomer (R) enantiomer Morphine
d.
mg/kg Jumps /batch Jumps /batch Jumps '-./batch
8.56 0 0 NT - 5.8 10
17.13 0 0 NT - 3.1 40
34.25 0 0 0.3 20 4.6 70
68.50 0 0 0.3 20 17.0 80
137.0 0 0 3.2 40 31.5 100
275.0 0 0 12.2 90 41.1 100
NT , not tested
The results clearly show that the (S) enantiomer causes
no addiction after administration by the s.c. route
whereas its (R) antipode causes this condition without,
however, reaching the degree observed with the morphine
used as positive reference in the test.
d) - Galenic section
By way of illustration of the pharmaceutical dosage forms
according to the invention, the composition and the
preparation of tablets comprising, as active ingredient,
(S) 2-methylamino-2-phenyl-n-butyl 3, 4, 5-trimethoxybenzoate
L(+) tartrate (product of Example 3) is described.
Product of Example 3 1 to 75 mg
Lactose 124 to 74 mg
Microcrystalline cellulose 36 to 60 mg
Polyvinylpyrrolidone 6 mg
Sodium carboxymethylstarch g mg
Magnesium stearate 1 mg
Mix the active ingredient, the lactose, the microcrystal-
line cellulose and the carboxymethylstarch. Wet with the
r. _ ___...._-....__....__ _ _..__..._.. _. __ ._ .._
CA 02285479 1999-09-22
WO 99/01417 PCT/EP98/03825
- 31 -
aid of an alcoholic solution of polyvinylpyrrolidone of
appropriate concentration and granulate. Dry and size the
granule. Mix homogeneously the magnesium stearate and
then tablet at. the rate of 200 mg per tablet.