Note: Descriptions are shown in the official language in which they were submitted.
CA 02286107 2005-07-28
IMPROVED CATHETER CALIBRATION
FIELD OF THE INVENTION
The present invention relates generally to systems for medical diagnosis and
treatment, and specifically to medical catheters whose location can be
detected.
BACKGROUND OF THE INVENTION
Various methods and devices have been described for determining the position
of a probe or catheter tip inside the body using electromagnetic fields, such
as in U.S.
Pat. No. 5,042,486 and PCT patent publication WO 94/04938. Other
electromagnetic
tracking systems, not necessarily for medical applications, are described in
U.S. Pat.
Nos. 3,644,825, 3,868,565, 4,017,858, 4,054,881 and 4,849,692.
U.S. patent. 5,391,199 describes a system that incorporates a catheter, which
includes a position measuring device that can determine the position of the
catheter in
three dimensions, but not its orientation.
PCT patent application PCT/W096/05768, which is assigned to the assignee of
the present patent application describes a catheter system including means for
determining the six-dimensions of position and orientation of the catheter's
distal tip.
This system uses a position sensor, formed of a plurality of non-concentric
coils,
adjacent to a locatable site in the catheter, for example near its distal tip.
Preferably
three orthogonal coils are used. These coils generate signals in response to
externally
applied magnetic fields, which allow for the computation of six position and
orientation
coordinates, so that the position and orientation of the catheter are known
without the
need for imaging the catheter.
U.S. patent 4,580,557 describes a surgical laser system for connection to
various peripheral surgical devices. The system identifies to which device it
1s
connected according to characteristics of a signature resistor embedded within
the
device. The resistor uniquely identifies the device in which it is embedded.
U.S. patent 5,383,874 describes a system for identifying and monitoring
catheters, including identification means carried within the handle of the
catheter body.
In one embodiment
1
CA 02286107 1999-10-08
WO 99/40856 PCT/IL98/00064
of the catheter in this patent, the handle includes a solid-state microchip
pre-programmed with a
digital value representing an identification code and other operational and
functional
characteristics of the catheter. The handle is connected by a cable to a
control console, which
receives data from the microchip. In one disclosed embodiment, the microchip
may record the
number of times the catheter has been used.
U.S. patent 5,617,857 describes an imaging system which determines the
location of a
medical instrument. A read-only storage device is positioned on or in the
medical instrument for
storing initialization information characteristics of the instrument. Thus,
the system may
determine the type of the instrument connected thereto, and receive
initialization information
associated with the instn.~ment type. This patent further suggests preventing
use of the
instrument unless the initialization information was transferred from the
storage device to the
ima;in~ system. A verification method is also described in which the
initialization information is
verified for correctness. Two alternatives are sng~ested for the location of
the storage device.
One alternative susgests embedding the device directly in the instrument. A
second alternative
sugjests embedding the storage device within an attachment, essentially an
instrument handle, to
which a certain type of instrument may be fit.
Thus, in some of the embodiments described in the above-referenced patents,
information
regarding a catheter (or other medical tool) is stored in an attachment to the
catheter and not in
the catheter itself. These embodiments are not suitable for storm; item-
specific information such
2 0 as calibration information.
In other embodiments of the above-referenced patents, information is stored in
the
catheter. These embodiments, however, suffe;~ from over-complexity, requiring
for example
multiple di_ital si;nal wires to run along the catheter. This complexity is
not feasible for mass
use in disposable catheters.
CA 02286107 1999-10-08
WO 99/40856 PCT/IL98/00064
SUMMARY OF 'fHE INVENTION
It is an object of some aspects of the present invention to provide means for
convenient
electronic storage and recall of calibration inforrnation regarding a
catheter.
It is another object of some aspects of the presem invention to provide means
for
convenient electronic storage and recall of calibration information regarding
a catheter, in which
the recall time of the information is minimal.
It is another object of some aspects of the present invention to provide means
for
providing improved communication between the catheter and a control console.
It is a further object of some aspects of the present invention to provide
catheters of
1 0 minimal cost and complexity, which are capable of storing and recallinV
calibration information.
In one aspect of the present invention, a catheter assembly for connection to
a control
console comprises two parts: a catheter of minimal complexity which is
inserted into a patient's
body, and a connection cable which connects between the proximal end of the
catheter and the
console. The catheter comprises a microcircuit which carries substantially
only information
specific to the catheter, which is not in common with other catheters of the
same model. Such
information includes, for example, item-specific: calibration data and a date
of first use of the
catheter. The cable comprises an access circuit which receives the information
from the catheter
and passes it in a suitable form to the console.
Preferably, the cable operates with all catheters of a specific model or type,
and therefore
2 0 when a catheter is replaced, there is no need to replace the cable.
Particularly, catheters which
are planned for one-time use do not require replacement of the cable, which
does not come in
contact with patients.
In a preferred embodiment of the present invention, the access circuit
verifies that the
catheter is of the model which is compatible wu:h the cable. Preferably, the
connection between
2 5 the catheter and the cable is unidue for each c,~theter model.
Alternatively or additionally, the
model identification is stored in the microcircuit and the access circuit
verifies that the model
identification is the same in the cable and the catheter.
In some preferred embodiments of the present invention, each cable is
associated with a
few catheter models, and the model identification stored in the microcircuit
is used by the access
3 0 circuitry to identify which catheter model is in u~;e.
In some preferred embodiments of the present invention, the catheter
microcircuit
contains data which is stored digitally. Preferably, the leads of the
microcircuit are coupled
3
CA 02286107 1999-10-08
WO 99/40856 PCT/IL98/00064
directly to sockets in a receptacle at the distal end of the cable. Thus, the
catheter does not
contain digital signal wires, and still allows quick access to the information
in the microcircuit.
Also, digital electronic signals transmitted from the microcircuit to the
console via the cable do
not interfere with low-level analog signals conveyed by wires from the distal
end of the catheter
to the cable. Preferably, the access circuit is located within the receptacle
at the distal end of the
cable and includes the socket which receives the leads of the microcircuit.
In preferred embodiments of the present invention, the microcircuit stores
minimal
calibration and/or initialization information re~a.rdin~ the catheter.
Alternatively or additionally,
the microcircuit stores usage information regarding the catheter, such as the
date of first use of
the catheter.
In preferred embodiments of the present invention, the catheter comprises at
its proximal
end a handle which contains controls which are used to manipulate the
catheter. It is noted that
in catheters in which the handle is not at the proximal end of the catheter,
the len;th beyond the
handle does not add to the functionality of the catheter but raises the cost
of the catheter and of
sterilization thereof. Preferably, the catheter microcircuit is contained in
the handle.
Alternatively, the handle is separate from the ca~:heter, and is rather fined
at the distal end of the
cable, and the microcircuit is contained within a connector at the proximal
end of the catheter,
which connects to the handle.
In some preferred embodiments of the present invention, the catheter includes
coils
which generate analo; signals indicative of t~~e position of the catheter, as
described, for
example, in the above-reference PCT/W096/C~~768 patent application. The cable
preferably
comprises amplifcers which are used to amplify the analog signals.
Alternatively or additionally,
the amplifiers may be used to amplify other si'nals, such as physiological
measurements.
Preferably, the amplifiers are within the receptacle so that they are as close
as possible to the
coils and/or the other sources of signals. It is nosed that placing the
amplifiers within the console
is not desirable due to interference from circ°,~itry of the console
which generates noise in
neighboring wires and due to noise pickup over the long distance between the
distal end of the
catheter and the console. The si~nais generated within the catheter are
relatively weak and must
be protected from attenuation and noise. Placing the amplifiers in the
catheter adds to the
3 0 complexity and cost of the catheter, which is also undesirable.
In some of these preferred embodiments, the access circuitry includes one or
more
analog-to-digital (A/D) converter circuits, which convert analog signals from
the catheter into a
CA 02286107 2005-07-28
digital form, which is conveyed to the console. Thus, the attenuation and
noise problems
mentioned above are substantially eliminated.
In other preferred embodiments of the present invemion, the catheter itself
includes the
one or more analog-to-digital (:a/D) converter circuits. In these embodiments,
the access circuit
couples onlv digital signals from the catheter to the console. In one such
preferred embodiment,
an A/D converter is adjacent to the distal tip of the catheter.
In some preferred embodiments of the present invention, the cable comprises an
additional microcircuit in which information characteristic of the one or more
models of
catheters associated with the cable, is stored. Such information may include,
for example, the
0 configuration of the catheters and usage codes. Preferably, the additional
microcircuit also
includes calibration information for the access circuit and the amplifiers
within the cable. The
calibration information of the ampliners may include, for example, their zero-
gain, DC oft'set and
linearity. Thus, information which does not have to be in the catheter is
stored in the cable, and
the catheter is less complex and costly. Preferably, the console substantially
does not require any
S other catheter-specific information beyond that supplied by the
microcircuits in the catheter and,
preferably, the cable, so that newer models of catheters may be used with the
console without
updating software or hardware of the console.
Preferably, the microcircuits comprise a read/write memor<~ component, such as
an
EEPROi\I. EPROM, PROM, Flash R01I or non-volatile R~'~~I, and the information
is stored in
.0 digital for~tn. :~lternativeiy or additionally, either of the microcircuits
may comprise a read-only
memory which is pre-programmed at the time of manufacture.
In preferred embodiments of the present invention, the calibration information
includes
data relating to the relative displacement of the distal tip of the catheter
from the coils. In some
other preferred embodiments of the present invention, the calibration
information also includes
2 S data relating to deviation of the coils from orthogonalitv, or data
relating to the respective gains
of the coils, or a combination of these data. The above calibration
information ;enerallv varies
from one catheter to the other and therefore is preferably stored in the
microcircuit within the
catheter. Preferably, the data is determined in a calibration method. Other
calibration information
may include the general configuration of the catheter and the gain and offset
of the access
3 ~ circuitry, and is preferably stored in the microcircuit in the cable.
In some preferred embodiments of the present invention, the catheter is
electrically
isolated from signal processing and computing apparatus in the console, and
the calibration
CA 02286107 1999-10-08
WO 99/40856 PCT/IL98/00064
information includes data relating to isolation circuitry in the catheter.
Preferably, the catheter is
isolated by at least one inductive element, such as an isolation transformer,
adjacent to the
proximal end of the catheter or in the catheter handle. Alternatively, the
catheter may be isolated
by one or more opto-isolators, or other types of isolation circuitry known in
the art. Such
S inductive elements and other isolation circuitry typically introduce non-
linearities in signals
conveyed thereby. Such non-linearities may lead to significant distortions
particularly in analog
signals conveyed by wires from the distal end of the catheter to the signal
processin<= circuits.
Therefore. the calibration information preferably includes data relating to
signal non-Iinearities
introduced by the inductive elements and/or othc;r isolation circuitry.
The calibration data may be recorded in the microcircuit in the catheter in
the form of
lookup tables, polynomial coefficients or any other suitable form known in the
art.
In preferred embodiments of the present invention, calibration data are
produced and
recorded at or close to the time of manufacture, and the microcircuits are
confiVured so as to
prevent subseduent recording of calibration data by a user. For example, when
the microcircuit
1 S comprises an EPRONI or PROM, a suitable programming device connects to the
catheter
connector and programs the EPROM or PROI~9f by inputting digital signals
thereto through the
connector from a computer used in calibration. Thereafter, the EPROM or PR01VI
may not be
re-programmed.
In other such preferred embodiments wherein the microcircuit comprises an
EEPROM or
non-volatile R.WI device, the EEPROM or non-volatile RAW device includes a
write-enable
input connection, of a type known in the art. which is connected to a write-
enable pin in the
connector at the proximal end of the catheter. ~3t the time of calibration,
the write-enable input
is enabled, and calibration data are recorded in the microcircuit. Thereafrer
the write-enable
input is disabled, for example by removing the write-enable pin or by
connecting it to electrical
2 S ground, so that further calibration data may not be recorded in the
microcircuit.
Alternatively, in preferred embodimems of the present invention wherein the
microcircuit
comprises an EEPROVI device, the write-enable input may be disabled by sending
a write-
protect command to the device. This command may be reversible or irrever
Bible.
In still other preferred embodiments of the present invention, the
microcircuit in the
catheter and/or the microcircuit in the cable comprise access control
circuitry, such as, for
example, the X76F041 Password Access Security Supervisor (PASSTM) SecureFlash
ROM
device, manufactured by Xicor, Inc. The microcircuit is preferably pro;rammed
with a
passworu, so that after calibration data are produced and recorded at the time
of manufacture,
CA 02286107 1999-10-08
WO 99/40856 PCT/IL98/00064
further calibration data may not be recorded in the microcircuit, with the
possible exception of
data recording by factory-authorized personnel to whom the password is known.
In some preferred embodiments of the present invention, data recorded in the
microcircuit include a calibration code, which is encrypted in accordance with
methods known in
S the art, so as to ensure that calibration data have not been altered or
corrupted. When a user
connects the catheter to a suitable console, which console comprises a
computer, the computer
reads the calibration code and compares the code with pre-pro;rammed values.
If the code does
not match the desired pre-programmed value, 'the computer causes a message to
be displayed
indicatin~~ that the catheter may not be approF>riately calibrated. The
computer may prevent
i 0 further operation until a catheter having a code matching the desired pre-
pro;rammed value is
connected thereto.
Preferably the calibration code is encrypted using a method that prevents
decryption by
unauthorized parties, for example the RSA encryption scheme, using a public
key and a private
key, or other methods known in the art. When a method such as RS:~ encryption
is used, the
15 private key is known only to authorized manufacturers of the catheter, so
as to prevent the
possible use of unauthorized substitutes of possibly inferior duality.
In further preferred embodiments of the present invention, data recorded in
the
microcircuit include an expiration date and time, after which the catheter may
not be used. When
a user connects the catheter to a suitable console, which console comprises a
computer, the
2 0 computer reads the expiration date and time and compares them to the
actual date and time,
generated, for example, by a real-time clock circuit. If the expiration date
and time have passed,
the computer causes a messa~~e to be displayed indicating that the catheter is
unsuitable for
further use. The computer may prevent further operation until a catheter
having a valid
expiration date and time is connected thereto.
S Preferably the expiration date and time are recorded by the console computer
by
programming the microcircuit in the catheter when the catheter is first used.
Thus, when the
catheter is connected to a console for the first tine, the computer detects
that no expiration date
and time have yet been recorded in the microcircuit, and programs the
microcircuit with the
appropriate expiration data and time, at a pre-set inten~al afrer the actual
date and time.
Preferably, the pre-set interval is stored within tl'~e cable and is
determined by the manufacturer,
based on the expected useful life of the catheter.
In a preferred embodiment in which the ,microcircuit comprises access control
circuitry,
the micrucircuit is programmed so that a memory location therein is operable
in a "read access
7
CA 02286107 1999-10-08
WO 99/40856 PCT/IL98/OOOb4
and program only" mode. The mode may be changed only by entry of an
appropriate password,
which is generally not available to users of the catheter. In the "read access
and program only"
mode, a number stored in the memory location may be decreased, by changing a
bit from "1" to
"0", but not increased, since the microcircuit as programmed will not permit a
"0" to be changed
to a "1 ". Preferably the memory location is set at the time of manufacture to
contain a maximum
value, i.e., all bits set to "1". Then, as described above, at the time of
first use, the computer
programs the microcircuit with the appropriate. expiration time and date by
changing one or
more bits in the memory location from "1" to "0". Thereafter, the expiration
date cannot be
changed to any later date (unless the correct password is first entered).
Alternatively or additionally, the microcircuit comprising access control
circuitry, as
described above, may be used to track the numb~:r of times the catheter has
been used and/or the
duration of use, in a manner that is protected from possible tampering or
error by a user thereof.
Preferably, a record corresponding to the number of times and/or the length of
time that the
catheter may be used is stored in a memory location in the device or in the
microcircuit within
the catheter, at the time of manufacture, and the microcircuit is programmed
so that this memory
location is operable in the "read access and program only" mode, as described
above. Each time
the catheter is used, and/or at regular time intervals during use, the
computer reads the record in
the memory location and reduces it by changing one or more bits therein from
"1" to "0". When
the record stored in the memory location reaches zero, or some other
predetermined minimum
2 0 value, the computer causes a message to be displayed to the user
indicating that the catheter is
unsuitable for further use and, preferably, prevents further operation until a
suitable catheter is
connected thereto.
There is therefore provided in accordance with a preferred embodiment of the
present
invention, a probe assembly for connection to a console including a probe for
insertion into the
2 ~ body of a subject, said probe having distal and proximal ends and a
microcircuit which stores
information relating to the probe and a cable for connecting,; the probe to
the console, said cable
including access circuitry for accessing the microcircuit in the probe.
Preferably, the cable is interchangeably connectable to two or more different
probes of a
common type, and wherein the microcircuit stores information relating uniquely
to the probe and
3 0 substantially not in common with other probes of the type.
Preferably, the access circuitry includes a cable-microcircuit, which stores
information
relating commonly to different probes of the common type.
a
CA 02286107 1999-10-08
WO 99140856 PCT/IL98/00064
Further preferably, the cable-microcircuit stores information identifying the
type of the
probe.
Preferably, the information relating to the probe includes usage-related
information of the
probe.
S Preferably, the usage related information includes a usage code, which
controls
availability of the probe to a user thereof.
Preferably, the access circuitry allows the usage code to be changed so as to
reduce the
availability of the probe, but not to increase the availability thereof.
Preferably, the microcircuit stores the usage code in a memory location
therein that is
controlled by the access circuitry so as to operate in a read access and
program only mode.
Preferably, the mode may be changed by entry of a password to the access
circuitry.
Alternatively or additionally, the usage code includes date information.
Preferably, the microcircuit is adjacent to the proximal end of the probe.
Preferably, the microcircuit includes leads which protrude from the proximal
end of the
1 S probe and wherein the access circuitry includes a socket which receives
the leads of the
mtcroctrcuit.
Preferably, the probe includes a functional portion which generates analog
signals and
wherein the access circuitry includes one or more amplifiers which amplify the
analog signals.
Preferably, the access circuitry includes one or more analog to digital
converters.
Preferably, the access circuitry includes a cable-microcircuit, which stores
information
relating to calibration of the one or more amplifiers.
Alternatively or additionally, the access circuitry includes a cable-
microcircuit which
stores information relating to the probe assembly.
Preferably, the cable-microcircuit stores information descriptive of a
configuration of the
2 5 probe.
Preferably, the cable-microcircuit stores an allowed usage period for the
probe.
Alternatively or additionally, the cable includes an internal clock for
measuring the time
from a first usa~,e of the catheter.
Preferably, at least a portion of the information on the microcircuit is
encrypted.
Preferably, the information relating to tlae probe includes calibration
information of the
probe.
Preferably, the probe includes a device that generates signals responsive to
the position
or orientation of the probe, and the calibration information of the probe
includes information
9
CA 02286107 1999-10-08
WO 99/40856 PCT/IL98/00064
relating to calibration of the signal generating device.
Preferably, the signal generating device is adjacent to the distal end of the
probe.
Further preferably, the signal generating device includes one or more coils.
Preferably, the calibration information includes information relating to a
gain of at least
one of the one or more coils.
Alternatively or additionally, the calibration information includes
information relating to
an angular orientation of at least one of the one or more coils.
Alternatively or additionally, the calibration information includes
information relating to a
positional displacement of the signal generating device, relative to the
distal end of the probe.
Preferably, the probe includes isolation circuitry, and wherein the
information relating to
the probe includes information relating to a non-liinearity of the isolation
circuitry.
Preferably, the microcircuit includes a programmable memory device, .an EEPROM
device, an EPROM or PROM device, a non-volatile R~Nf device or a Flash ROM
device.
Preferably, the cable includes means for disablinV at least one of the
connections for
programmin' the programmable memory device.
There is further provided in accordance with a preferred embodiment of the
present
invention, apparatus for determining the position of a probe in the body of a
subject, including a
probe for insertion into the body of a subject, said probe including a
microcircuit which stores
calibration information of the probe, a cable for connecting the probe to the
console. said cable
including access circuitry for accessing the microcircuit in the probe, and a
console, including a
computer, which receives said position- or orientation-responsive signals and
said information
relating, to calibration and determines therefrom the position of the probe.
Preferably, the probe includes a device tl~~at generates signals responsive to
the position
or orientation of the probe, and the calibration information of the probe
includes information
2 5 refatinQ to calibration of the signal generating device.
Preferably, the microcircuit includes a programmable memory device.
Preferably, the computer is adapted to program the pro~rammabie memory device.
There is further provided in accordance with a preferred embodiment of the
present
invention, a method of initializing a console for u;~e with a probe assembly
including a probe and
a connection cable, including connecting the probe to the console using the
cable, loading the
console with general model information from a microcircuit within the cable,
and loading the
console with specific catheter information from a microcircuit within the
catheter.
CA 02286107 2003-08-26
Preferably, the specific catheter information includes calibration
information, a usage code and/or a first usage date.
Preferably, the general model information includes a permitted usage
duration.
S Preferably, the method includes displaying a warning message if the
usage duration from the first usage date has expired.
Preferably, connecting the probe to the console includes connecting the
probe via access circuitry in the cable.
Preferably, the method includes loading the console with calibration
information regarding the access circuitry
According to a further broad aspect of the present invention there is
provided a probe assembly for connection to a console. The probe assembly
comprises:
a probe for insertion into the body of a subject, said probe having distal
and proximal ends and comprising a microcircuit which stores information
relating to
the probe, wherein the information relating to the probe comprises usage-
related
information of the probe and the usage-related information comprises a usage
code,
which controls availability of the probe to a user thereof; and
a cable for connecting the probe to the console, said cable comprising
access circuitry for accessing the microcircuit in the probe.
According to a still further broad aspect of the present invention there is
provided an apparatus for determining the position of a probe in the body of a
subject.
The apparatus comprises:
a probe for insertion into the body of a subject, said probe comprising a
microcircuit which stores calibration information of the probe, said probe
generating
position or orientation responsive signals;
a cable for connecting the probe to the console, said cable comprising
access circuitry for accessing the microcircuit in the probe; and
a console, comprising a computer, which receives said position or
orientation responsive signals and said information relating to calibration
and
determines therefrom the position of the probe.
According to a still further broad aspect of the present invention there is
provided a method of initialising a console for use with a probe assembly
including a
probe and a connection cable. The method comprises:
cormecting the probe to the console using the cable;
11
CA 02286107 2003-08-26
loading the console with general model information from a microcircuit
within the cable; and
loading the console with specific probe information from a microcircuit
within the probe wherein the specific probe information comprises calibration
information.
The present invention will be more fully understood from the following
detailed description of the preferred embodiments thereof, taken together with
the
drawings in which:
11B
CA 02286107 1999-10-08
WO 99/40856 PCT/IL98/00064
BRIEF DESCRIPTION OF THE DRAWINGS
Fig. 1 is a perspective view of a system including a catheter and a connection
cable, in
accordance with a preferred embodiment of the present invention;
Fig. 2 is a detailed sectional view of the distal end of the catheter of Fig.
1; and
Fig. 3 is a detailed schematic view of a connection site- between the catheter
and the
cable, in accordance with a preferred embodiment of the present invention.
12
CA 02286107 1999-10-08
WO 99/40856 PCT/IL98/00064
DETAILED DESCRIPTION OF' PREFERRED ENIBODI1'(ENTS
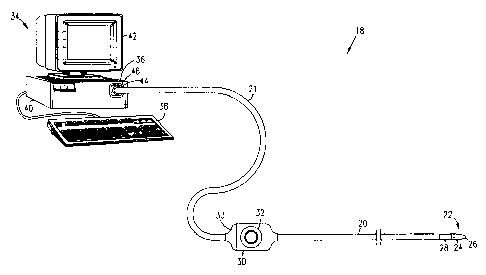
Fig. I shows a probe system 1$ in a~~cordance with a preferred embodiment of
the
present invention. System 18 comprises an elon;ate probe, preferably a
catheter 20, for insertion
into the human body. It will be understood that although the following
preferred embodiments
are described with reference to a catheter, the present invention is edually
applicable to other
types of probes.
A distal end 22 of catheter 20 includes <i functional portion 24 for
performing diagnostic
and/or therapeutic functions, adjacent to distal tip ?6. Functional portion 24
mav, for example,
comprise electrodes (not shown in the figure) for performing
electrophysiological measurements
or for electrosurgical ablation of areas of pathology in the heart.
Alternatively or additionally,
the functional portion may comprise other types of sensors, or optical or
ultrasound imaging
devices.
Distal end 22 of catheter ?0 further includes a device 28 that generates
signals used to
determine the position and orientation of the c~itheter within the body.
Device 3S is preferably
adjacent to functional portion 34. There is preferably a fixed positional and
orientational
relationship between device 23, tip 2G and portion 24.
Catheter 30 preferably includes a handle: 30, which includes controls 32 which
are used
by a surgeon to steer the distal end of the catheter in a desired direction,
or to position and/or
orient it as desired.
0 The system shown in Fi';. 1 further comprises a console 34, which enables
the user to
observe and regulate the functions of catheter :'0. Console 34 preferably
includes a computer
36, keyboard 3S, signal processing circuits 40, which are typically inside the
computer, and
display 43. Signal processing circuits 40 typically receive, amplify, filter
and digitize signals
from catheter 20, including signals ~=enerated by position signal generating
device 28, whereupon
2 5 these digitized signals are received and used by computer 36 to compute
the position and
orientation of the catheter. Alternatively, apI>ropriate circuitry may be
associated with the
catheter itself, as described below, so that circuits 40 receive signals that
are already amplified,
filtered and/or di;itized.
Catheter 20 is coupled to computer 36 via an extension cable ?1, which at its
proximal
30 end comprises a connector 44 adapted to fit in a mating receptacle 46 on
console 34. The distal
end of cable 2t comprises a receptacle 33 which connects to handle 30.
Receptacle 33 is
preferably configured to receive catheters of a. specific model, and
preferably includes user-
13
CA 02286107 1999-10-08
WO 99/40856 PCT/IL98/00064
tangible identification of the specific model. One of the advantages in using
cable 21 is the ability
to connect different models and types of catheters, possibly havin' dit~erent
handle
configurations, to the same console 34. Different cables 31 can be used to
connect a large
variety of catheters to console 34. Another advantage in having a separate
cable 21 is in the fact
that the cable does not come into contact with patients and therefore it is
possible to re-use the
cable without sterilization.
Preferably, cable 21 further contains one or more isolation transformers (not
shown in
the figures), which electrically isolate catheter 20 from console 34. The
isolation transformers
are preferably contained in receptacle 33.
i 0 Reference is now made to Fix. 2, whnich shows a detailed view of distal
end 22 of
catheter ?0 in accordance with a preferred embodiment of the present
invention. Device 28
comprises three non-concentric coils 60, 63 and 64, such as described in PCT
patent publication
number W096/0~768, whose disclosure is incorporated herein by reference. This
device enables
continuous generation of six dimensions of position and orientation
information with respect to
an externally-applied magnetic field. Coils 60. 62 and 64 have respective ayes
66, 68 and 70,
which preferably define orthovonal Cartesian aces Z, X and Y, respectively, as
shown in Fig. 2,
wherein the Z-axis is parallel to the long axis of catheter 20 and the X- and
Y-axes define a plane
perpendicular thereto. The coils each have a fi~;ed position and orientation
with respect to each
other.
Although preferred embodiments of the present invention are described herein
with
reference to position signal generating device ?8 shown in Fi~_. 2 and
described above, it will be
understood that the inventive concepts of the present invention are similarly
applicable to probes
including other position sensing' devices. For example, in other preferred
embodiments of the
present invention, the probe may comprise a single coil for generating
position signals, or two or
2 S more such coils, which may be concentric or non-concentric. Other
preferred embodiments of
the present invention may comprise other types of position sensing devices
known in the art,
such as Hall effect devices or ultrasonic or optical sensors.
As shown in Fig. 2, device 28 is located in catheter 20 at a distance L from
distal tip ?6,
where L is here defined for convenience as the distance alon; the Z-axis from
the central axis 68
of coil 6? to tip 26. Respective axes 66 and 70 of coils 60 and 6~ are
displaced from axis 68 by
respective distances dy and dz.
When a time-varyins: external magnetic field is applied to distal end 22 of
catheter 20,
coils 60, 62 and 64 'enerate analog signals, which are preferably conveyed
through the catheter
1 'i
*rB
CA 02286107 1999-10-08
WO 99/40856 PCT/IL98/00064
by coil wires 72. The amplitudes of these analog signals are typically small
relative to other
electrical signals in and around catheter 20, such as the electrophysiological
signals measured by
functional portion 24 and conveyed through the catheter by functional wires
76. Furthermore,
external magnetic fields may also cause undesired electrical currents, not
generated by coils 60,
62 and 6-1, to flow in coil wires 72. These other electrical signals and
undesired electrical
currents can cause noise or interfering signals to appear together with the
signals generated by
the coils. Therefore, in preferred embodiments of the present invention, wires
72 are configured
as twisted pairs and may also be shielded from electromagnetic interference by
shields 74, so as
to maintain a high signal-to-noise ratio in the position and orientation
signals received from the
coils.
As described in the above-mentioned 05763 PCT patent publication, si~,nal
processing
circuits 40 in console 3~t receive the signals carried by coil wires 72 and
convey them to
computer 36, which computes the three-dimensional translational position of
device 28 and the
rotational orientation of axes 66, 6S and 70, relative to a fixed, external
coordinate frame. The
l 5 actual position and orientation of distal tip 26 are then computed by
taking into account the
distance L of tip 26 from the center of device :'3, as defined by axis 63, and
the orientation of
axes 66, 63 and 70.
It has been found empirically that due to deviations in the process of
manufacturing
catheter 20, the distance L typically varies from one catheter to another,
leading to errors in
calculating the position of tip 26. Furthermore, axis 66 of coif 60 typically
deviates' from
absolute alignment with the long= axis of catheter 20, which passes through
tip 26, and axes 63
and 70 of coils 62 and 64 respectively are typically not precisely orthogonal
to axis 66 or to each
other, thereby inducing additional errors in determination of position and
orientation of the
catheter. Finally, variations in the respective gains of coils 60, 62 and 64
and in the distances dg
and dZ may cause additional errors in determination of position and
orientation of the catheter.
Therefore, in preferred embodiments of the present invention, device 28 that
is used to
determine the position and orientation of catheter 20 is calibrated before the
catheter is inserted
into a patient's body. This calibration may be pei-Formed using any suitable
method including the
methods described in PCT/IL97/00060. The determined calibration correction
function is
thereafter stored electronically in a memory device, which device is
preferably in catheter 20.
When the catheter is coupled to console 3=i, this memory device is accessible
to computer 36 in
the console.
CA 02286107 1999-10-08
WO 99/40856 PCT/IL98/00064
Fig. 3 shows details of receptacle 33 and handle 30, in accordance with a
preferred
embodiment of the present invention. Handle 30 includes a digital microcircuit
90 in which
calibration data for catheter 20 are electronically stored. RMicrocircuit 90
preferably includes an
EEPROM or Flash ROM, but may alternatively include EPROi\-t, PROM, non-
volatile RAM, or
other types of programmable memory device.; known in the art. When a catheter
20 is
calibrated, its specific calibration data are stored in microcircuit 90 and
thus the data is
conveniently accessible to computer 36, as will be described below.
Preferably, another microcircuit 88 is included in receptacle 33 of cable 21.
~~ticrocircuit
88 preferably includes a programmable memory similar to that of microcircuit
90. Information
regarding initialization of catheter 20 which is common to all catheters of a
certain mode! is
preferably stored in microcircuit SS rather than in microcircuit 90, which is
embedded within the
catheter itself. l~fost catheters are limited in the number of times they may
be used, because of
problems of cleaning, sterilization and wear. Commonly catheters may be used
only once.
Therefore, it is desirable to minimize the cost of the catheter itself by
incorporating into catheter
20 only the minimal circuitry necessary, including a minimal size microcircuit
90. All other
information which is characteristic commonly of all catheters of a given model
is stored within
receptacle 33, which is not inserted into the patient's body. Alternatively or
additionally,
information characteristic of a family of catheters is stored within console
36 while cable 21 only
holds minimal information identifying which catheter model is being used.
An advantage of havinv the model information in receptacle 33 rather than in
console 36
lies in allowing use of a lar<~e variety of catheters with console 36 without
loading large
databases into the console. Furthermore, microcircuit S8 preferably stores
calibration
information relating to circuitry in the receptacle, as described below. These
features allow
using a standard console with various catheter types, rather than havin~l a
single console 36
2 5 associated with each type of catheter. Furthermore, newer models of
catheters may be used with
console 36 simply by connecting them via their compatible cable 21 to the
console, thus reducing
the need to update software in the console or to acquire a new console.
In the preferred embodiment shown in Fi;:~. 3, handle 30 further includes pins
92, 94, 96
and 98, which mate with corresponding sockets 93 in receptacle 33. Functional
pins 94 couple
3 0 analog electrophysiological signals conveyed over functional wires 7G to
signal processing
circuits 40 Coil pins 92 couple analog position and orientation signals
conveyed by coil wires
72 from coils 60, 62 and 64 to signal processin; r.ircuits 40 and computer 36,
which compute the
position and orientation of catheter 20. The computer further reads the
di;ital calibration
to
CA 02286107 1999-10-08
WO 99/40856 PCT/IL98/00064
correction data stored in microcircuit 90 via memory pins 96, and uses these
data in computing
the correct catheter position and orientation.
Receptacle 33 preferably comprises one: or more amplifiers 80 which amplify
the position
and orientation signals conveyed by coil wires 72. These signals are generally
very weak, and
therefore it is important to locate amplifiers 80 as close as possible to
coils 60, 62, and 64 which
produce the signals. However, it is advantageous not to locate amplifiers 80
within catheter 20
since they increase the cost and complexity c>f the catheter unduly.
Preferably, receptacle 33
further comprises one or more analog-to-digital (AID) converters 82 which
convert the analog
signals from amplifiers 80 to digital form.
0 Preferably, the physiological si~,=nals conveyed over functional wires 76
are also
amplified by amplifiers 84 and are then converted to digital form via A.~D
converter 86.
Preferably, calibration information for amplifiers 80 and 84. such as gain and
ot~'set, is stored in
microcircuit 88.
One or more write-enable pins 104 are preferably coupled to microcircuit 90.
These pins
are used to enable pro~;rammin'~ of the microcircuit with the desired
calibration data. At the time
of calibration, the write-enable input is enabled, and calibration data are
recorded in the
microcircuit. Thereafter the write-enable input is disabled, for example by
removing the write-
enable pin or by connecting it to electrical ground 106, as shown in Fig. 3,
so that further
calibration data may not be recorded in the microcircuit, and the microcircuit
functions in a read-
2 0 only mode. l~-ficrocircuit 88 may be proVrammed in like fashion.
Alternatively, in preferred embodiments of the present invention wherein
microcircuit 90
comprises an EEPRO:~~I device, the write-enable input may be disabled by
sending a write-
protect command to the device This command may be reversible or irreversible.
In other preferred embodiments of the present invention, microcircuit 90
comprises a
25 device incorporating password-secured access control, and write-access to
the microcircuit
requires that an appropriate password first be entered. For example, in one
such preferred
embodiment, microcircuit 90 comprises a Password Access Security Supervisor
(PASST")
X76F041 SecureFlash ROi\I device, manufactured by Xicor, Inc. The microcircuit
is
programmed with calibration data at the time of manufacture, and thereafter
operates in a "read
30 access only" mode, with all write operations locked out, or in a "read
access and program only"
mode, in which certain data, but not calibration data, may be written to the
device, as will be
described below. Changing, the mode of operation of the microcircuit reduires
that an
appropriate password be entered, which password is generally unavailable to
users of the system.
17
CA 02286107 1999-10-08
WO 99/40856 PCT/IL98/00064
In another preferred embodiment of the present invention, microcircuit 90
comprises an
EPROI~~I or PROM. Calibration data are recorded in the EPROM or PROM at the
time of
manufacture using a suitable programming deviate, not shown in the figures,
which receives data
from the computer used in calibration. The programming device is connected to
handle 30 via a
S calibration socket, not shown in the figures, which like receptacle 33 is
adapted to receive handle
30. The programming device programs the EPROM or PROM by inputting digital
signals
thereto through the connector. Thereafter, the I~PROM or PROivI may not be re-
programmed.
In some preferred embodiments of the present invention, data recorded in
microcircuit 90
and/or microcircuit 88 include a calibration code:, which is encrypted in
accordance with methods
known in the art, so as to ensure that the calibration data have not been
altered or corrupted.
Preferably the calibration code includes a checksum. When the user connects
catheter 20 to
console 3~, computer 36 reads the calibration code and compares the code with
pre-
programmed values. If the code does not match the desired pre-programmed
value, the
computer causes a message to be displayed by display 42 indicating that the
catheter may not be
appropriately calibrated. The computer may further cause the system to cease
operation until a
catheter having a code matchin'; the desired pre-programmed value is connected
thereto.
Preferably the calibration code is encrypted using a method that prevents
decryption by
unauthorized parties, for example the RSA encryption scheme, using a public
key and a private
key, or other methods known in the art. When a method such as RSA encryption
is used, the
private key is known only to authorized manufacturers of the catheter, so as
to prevent the
possible use of unauthorized substitutes of possibly inferior quality.
In further preferred embodiments of the present invention, data recorded in
microcircuit
90 include an expiration date and time, after which the catheter may not be
used. Microcircuit 88
may similarly include data relating to the maxirr~al period over which the
catheter may be used.
When a user connects catheter 20 to a console 3-I, computer 36 reads the
expiration date and
time and compares them to the actual date and time, generated, for example, by
a real-time clock
circuit. If the expiration date and time have passed, the computer causes a
messa;e to be
displayed by display 42 indicating that the catheter is unsuitable for further
use. Alternatively or
additionally, the computer may prevent usage of catheter 20 after the
expiration date.
In a preferred embodiment of the present invention, cable 21 includes an
internal clock
which keeps track of the time and date. Alternatively or additionally, the
internal clock of cable
21 keeps track of the relative time from the fir~;t use of catheter 20. Thus,
it is not possible to
avoid the usage prevention by changing the date in the console.
16
CA 02286107 1999-10-08
WO 99/40856 PCT/IL98100064
Preferably the expiration date and time are recorded by computer 36 by
programming
microcircuit 90 when catheter 20 is f rst used. When catheter 20 is connected
to console 34 for
the first time, computer 36 detects that no expiration date and time have yet
been recorded in
microcircuit 90, and programs the microcircuit with the appropriate expiration
date and time, at
a pre-set interval after the current date and time. The pre-set interval is
preferably determined by
the manufacturer, based on the expected useful life of the catheter.
In preferred embodiments of the present invention in which microcircuit 90
comprises a
device including access control circuitry, such as the aforementioned X76F041
device, the
microcircuit is programmed so that a memory location therein is operable in a
"read access and
program only" mode. The mode may be chan;,ed only by entry of an appropriate
password,
which is generally not available to users of the :system. In the "read access
and program only"
mode, a number stored in the memory location may be decreased, by changing a
bit from "I" to
"0", but not increased, since the microcircuit as programmed will not permit a
"0" to be changed
to a "I". Preferably the memoy.~ location is set at the time of manufacture to
contain a maximum
value, i.e., all bits set to "1". Then, as described above, at the time
catheter 20 is first used,
computer 36 pro?rams the microcircuit with the appropriate expiration time and
date by
changing one or more bits in the memory location from "I" to "0". Thereafter,
the expiration
date cannot be changed to any later date (unless the correct password is first
entered).
Alternatively or additionally, microcircuit 90 comprising_ access control
circuitry, as
described above, may be used to track the nurnber of times catheter 30 has
been used, in a
manner that is protected from possible tampering or error by a user thereof.
Preferably, a record
corresponding to the number of times catheter 20 may be used is stored in a
memory location in
the device at the time of manufacture, and the microcircuit is programmed so
that this memory
location is operable in the "read access and program only" mode, as described
above. Each time
2 5 the catheter is used, computer 36 reads the record in the memory location
and reduces it by
changing one or more bits therein from "1" to "t)". When all the bits in the
record are equal to
zero, or the record reaches some other predetermined minimum value, the
computer causes a
message to be displayed to the user indicating that the catheter is unsuitable
for further use and,
preferably, prevents further operation until a suitable catheter is connected
thereto.
Similarly, either alternatively or additionally, microcircuit 90 may be used
to track the
duration of use of catheter 30. In this case. a record corresponding to the
duration of use of the
catheter is stored in a "read access and prod?ra.m only" memory location in
the microcircuit.
While the catheter is in use, at re;ular, predetermined intervals, computer 36
reads the record
19
CA 02286107 1999-10-08
WO 99!40856 PCT/IL98/00064
and reduces it by changing one or more bits therein from "1" to "0". When the
entire record
reaches zero, or some other minimum value, further operation is prevented, as
described above.
As noted earlier, the low-level analoj signals conveyed from coils 60, 62 and
64 over coil wires
72 must generally be protected from interference due to other analog si~,;nals
in functional wires
76 and digital signals conveyed to an from microcircuit 90. Therefore, in
preferred embodiments
of the present invention, as shown in Fig. 3, '.handle 30 includes
electromagnetic shields 74,
which are coupled to ground via pin 98 on the connector.
In another preferred embodiment of the present invention, shields 74 are
active shields,
which are driven by noise canceling circuitry (not shown).
Although most of the features and capabilities of system l S, particularly
features related
to access control, have been described above with reference to microcircuit 90
in catheter handle
30, it will be clear to those skilled in the art that many of these features
and capabilities could be
implemented using microcircuit SS in cable 21, as well.
. Furthermore, although the above preferred embodiments have been described
with
reference to calibration of position and orientation sensing apparatus, in
other preferred
embodiments of the present invention, calibration data stored in catheter 20,
and specifically in
microcircuits SS and 90, may relate to other aspects of the catheter. For
example, in some
preferred embodiments of the present invention, calibration data relating to a
physiological
sensor, actuator or therapeutic tool are stored in the catheter. In another
preferred embodiment
2 0 of the present invention, calibration data may be stored in the catheter
regarding the gain of a
piezoelectric motion control device used in steering the catheter's distal
end.
It will be appreciated that the preferred embodiments of the invention
described above
are cited by way of example. and the full scopf; of the invention is limited
only by the claims
which follow.