Note: Descriptions are shown in the official language in which they were submitted.
CA 02286362 1999-10-O1
WO 98/45378 PCT/US98/06292
CORRECTION FLL!ID
This invention is concerned with aqueous correction
fluids, with a method of .preparing them and with a composite
particulate material useful there:in_
correction fluids are fluids which are used by
typists and others to cover typing, writing, printing or
drawing errors or the like and which when dried sufficiently
can, for example, be typed, written or drawn over. The
fastest writeover times for correction fluids have been
achieved using volatile organic solvents, but the use of
such materials has become much less desirable for
environmental reasons and, in socne cases, because of the
risk of abuse. Correction fluids containing water-based
liquid media are not subject to these disadvantages, but
they usually have greater writeover times than the organic
solvent compositions. "Writeover time" for the purposes of
this discussion means the time re=quired for the surface of
the applied correction fluid to dimensionally stabilize
sufficiently enough so the subsequent mark applied to the
surface of the correction pigments with polymeric material.
U.S. Patent no. 4,665,107 describes the preparation
of sub-micron-sized pigment particles encapsulated by a
polymer matrix, for use, for example, in inks. The pigment
particles are dispersed in a solution of the polymer, and
this mixture is dispersed in water to form an emulsion'.
Solvent is then removed to form the polymer-encapsulated
pigment particles.
CA 02286362 1999-10-O1
WO 98/45378 PCT/US98/06292
U.S. Patent no. 3,884,871 describes coating pigment
particles with polymer to prevent or reduce agglomeration of
the pigment in paints. An anchoring agent is first adsorbed
on the pigment particle surfaces and then organic vinyl
monomer is added and polymerized to form a coating on the
particles. The pigment particles are individually coated
and remain in the sub-micron size range. U.S. Patent nos.
4,421,620, 4,665,107, 3,281,344, 3,897,586, 4,023,981, and
4,036,652 also describe similar processes in which pigment
particles are encapsulated with polymer.
U.S. Patent no. 4,194,920 describes an
intrachromoleucospheruloid pigment composition. Spheruloids
of organic polymer (from ethylenically unsaturated monomer)
of a size up to 4 microns have pigment particles embedded
therein. The spheruloids are produced by aqueous emulsion
polymerization. U.S. Patents 4,264,700 and 4,358,388
describe particulate composite materials which contain
magnetic particles dispersed in a polymer. The particulate
composite materials are made by polymerizing monomer in the
presence of the magnetic particles.
U.S. Patent no. 4,254,201 describes a pressure
sensitive toner which is a particulate composite material.
The toner particles are porous aggregates each consisting of
a cluster of a multiplicity of granules of
pressure-sensitive adhesive encapsulated by a relatively
thin, frangible coating of, for example, a film.forming
material. Particles of pigment or magnetic material are
contained within the aggregates in the interstices between
the granules. The granules adhere to each other owing to
adhesion that occurs between the encapsulating films during
spray fluid is neatly legible and not smeared or smudged
into the correction fluid surface.
- 2 -
CA 02286362 1999-10-O1
WO 98/45378 PCT/US98/06292
It should be well understood that the correction
fluid may still contain water, and therefore not yet be
completely dry; yet the surface will have dimensionally
stabilized sufficiently to pertr~it writeover. The water
remaining in the correction fluid at the time of writeover
will thereafter evaporate from the surface, and/or dissipate
into the supporting paper. "Writeover time" therefore
typically occurs before the correction fluid is completely
dry.
With a view to reducing the writeover time of
aqueous correction fluids, attempts have been made to
increase their solids loading (and thus reduce the amount of
aqueous liquid therein). This approach has given some
success, but can no longer be used because, in general,
current aqueous correction fluids already have the minimum
writeover time achievable by maximizing solids loading. If
attempts are made to include more solids, the dispersions
become unstable due for example to settling and
agglomeration of the solids, anal there is a loss of lay-down
facility. The addition of extra solids, e.g., fillers,
leads to a significant increase in viscosity without any
concomitant reduction in writeover time. That is to say,
the fluids become thicker but d.o not permit writeover any
more quickly. Indeed, the addition of extra conventional
fillers to an aqueous correction fluid can result in an
increase in writeover time. Pu.t shortly, we believe it is
not possible by simple changes in proportions of solids, to
reduce any further the writeove:r times of current aqueous
correction fluids (without adversely affecting their
performance).
We have now found that the writeover times of
. aqueous correction fluids can be reduced, and other
- 3 -
CA 02286362 1999-10-O1
WO 98/45378 PCT/US98/06292
improvements obtained, by including in the fluids novel
particles of composite materials.
Particles composed of two or more discrete
components held together to form an integral whole,
S (hereinafter generally referred to as "composite
particles"), are known. There are, for example, a large
number of patents describing particulate composite materials
formed by coating particles such as drying. A binder can
also be provided in the spaces between the granules, to_
improve the adhesion. The individual porous aggregates, as
a whole, are approximately spherical in shape with a rough
surface like an orange. In use, under the pressure applied
to fix the toner, the aggregates are broken apart. into
separate granules, and the encapsulating films break to
release the pressure sensitive adhesives therein.
U.S. Patent Nos. 4,206,094 and 4,157,323 disclose
polymeric functional microspheres containing metal or metal
compounds, the microspheres being formed by addition
polymerization of a covalently bondable olefinic monomer in
the presence of finely divided metal or metal oxide
particles, such as iron, gold, platinum or magnetite, which
are embedded in the resulting microspheres. The
microspheres are then used in labelling and separating
biological cells. The addition polymerization can be
effected by high energy gamma radiation. The process is
either an aqueous suspension polymerization process or an
aqueous emulsion polymerization.
We have now devised some particulate composite
materials which are especially useful in aqueous correction
fluids. The presence of these particles can reduce drying
time and/or provide an improved stability in the correction
- 4 -
CA 02286362 1999-10-O1
WO 98/45378 PCT/US98/06292
fluids, and can also provide much higher solids loadings, if
desired.
In accordance with a first aspect of the present
invention, there is provided a material for use in a fluid,
said material comprising: a plurality of film forming
binder particles; a polymerized polymeric material
containing a portion of said film forming binder particles;
said portion of said binder particles in combination with
said polymerized polymeric material forming composite
material particles; said composite material particles when
polymerized, fixing said binder particles contained within
said composite.material particles relative to each other,
said composite material particles having a substantially
spherical outer surface and a diameter of from about 0.1 to
about 100 micrometers, and a portion of said binder
particles being bound adjacent the outer surface of said
composite material particles.
In a second aspect, the invention provides a method
of making a material for use in an aqueous correction fluid,
said method comprising the steps of: providing film-forming
binder particles; providing a p~~lymerisable liquid; mixing
said polymerisable liquid and said film-forming binder
particles together; and polymerizing the polymerisable
liquid to form a suspension of ~~omposite material particles;
each composite material particle having a portion of said
film forming binder particles i~acluded therein; said
polymeric material of said composite material particles
contacting said binder particles to fix said binder
particles relative to each other, a portion of said film
forming binder particles bound adjacent the outer surface of
said composite material particles; and said composite
material particles have a diameter of from about 0.1 to
about 100 micrometers.
- 5 -
CA 02286362 1999-10-O1
WO 98/45378 PCT/US98/06292
In another aspect, the invention provides an aqueous
correction fluid which comprises:
(a) an aqueous latex comprising particles of at
least one film-forming binder suspended in an
S aqueous medium;
(b) opacifying particles; and
(c) material of the invention;
wherein said opacifying particles and said
material are in suspension in said aqueous
medium.
In a further aspect of the invention, there is
provided a method of making an improved correction fluid,
which comprises the steps of:
(a) dispersing a polymerisable liquid in an aqueous
correction fluid, said correction fluid
including particles of at least one
film-forming binder;
(b) polymerizing the polymerisable liquid to form
an aqueous suspension of composite material
particles; said polymeric material contacting a
portion of said film- forming binder particles
to fix them relative to one another to form
said composite material particles; and
(c) adding the aqueous suspension to further
correction fluid.
Preferably, in this method:
(d) said composite material particles have a
substantially spherical outer surface;
(e) said outer surface includes some of said
particles of film-forming binder; and
(f) said composite material particles are of a size
in the range of from about 0.1 to about 100
micrometers in diameter. The film-forming
- 6 -
CA 02286362 1999-10-O1
WO 98/45378 PCT/US98/06292
binder particles are. of course smaller than the
composite material particles.
In a further aspect, the invention includes a
correction fluid applicator which comprises a container for
correction fluid, an outlet for dispensing fluid from the
container and an edge shear member integral with the
applicator for spreading dispensed fluid under shear.
The invention also provides a method of coating a
surface with a correction fluid, said method comprising the
steps of: providing a correction fluid of the invention and
applying the correction fluid to said surface.
The particles of composite material of the invention
each comprise a plurality of (by which we mean at least two)
smaller particles of at least one film forming binder, which
smaller particles are bound together and so fixed relative
to each other by the polymer formed on polymerization of the
polymerisable liquid. This polymer is cross-linked. The
composite material particles can also include smaller
particles of other solids such as opacifying particles, e.g.
pigments. Generally, in each composite material particle,
the plurality of smaller particles will be very close to
each other or be touching, so that the polymer formed from
the irradiation-polymerisable liquid fills the gaps or
interstices between the plurality of smaller particles. The
smaller particles are thus bound together and thus fixed
relative to each other, by the hardened polymer. Some
binding can also occur by surface entanglement processes
between adjacent binder particles such that some binder
particles can become bound directly to each other. By
entanglement processes, we mean that polymer chains
extending from a binder particle surface tangle with similar
chains from an adjacent binder particle to bind the binder
particles together.
CA 02286362 1999-10-O1
WO 98/45378 PCT/US98/06292
The composite material particles will preferably
have film-forming binder particles on their outer surface as
well as binder particles partially and totally within the
composite particle.
The outer surface of each particle of composite
material is substantially spherical, i.e., the composite
particles will be generally rounded and substantially free
of edges and will promote easy close packing in films. It
is felt that a novel feature of the invention lies in the
compaction of the binder particles and composite particles
when the correction fluid is applied.
The particles of composite material in accordance
with the invention generally have a relatively low surface
area in relation to their volume. They are generally
non-porous and will generally be relatively non-absorbent
towards the aqueous suspending medium of the correction
fluid. In preference, they will generally have a relatively
smooth surface.
The size (diameter) of the composite particles will
normally be in the range of from 0.1 to 100 micrometers,
preferably 0.1 to 50 micrometers, the average composite
particle size being about 20 to 30 micrometers. By average
particle size we mean the size of particle at which 50% of
the sample is smaller and 50% is larger than this size:
this value is also known as the mass median diameter. For
use in correction fluids of the ir_vention, the cpmposite
material particles will generally be of larger size than the
opacifying particles and film-forming binder particles
normally used in aqueous correction fluids. Thus, for
example, with opacifying particles and film-forming binder
particles of a size of about 0.5 micrometers, a typical
composite material particle of about 20 micrometers in size
_ g _
CA 02286362 1999-10-O1
WO 98/45378 PCT/I1S98/06292
would contain about 50,000 of tine smaller binder particles
therein.
We have found that it i~~ advantageous (though not
essential) for the size distribution of the composite
material particles to be about one order of magnitude or
more (expressed in micrometers). Thus, for example, if the
average particle size of the composite material particles is
about 20 micrometers, the compo;aite particles will
preferably range in size from, i°or example, about 0.1 to
about 50 micrometers. This promotes good.packing of the
particles on application of the correction fluid and is also
believed to contribute to a pari:icularly preferred fast -
hardening effect to be described hereinafter. It is also
preferred that the composite material particles are at least
ten times, and preferably up to about one hundred times, the
size of the film-forming binder particles: the opacifying
particles are normally of a similar size to the film-forming
binder particles.
More specifically, the composite material particles
of the invention, for use in correction fluids, are
preferably made by dispersing an irradiation polymerisable
liquid, as a multiplicity of discrete volumes, in an aqueous
latex of at least one film-forming binder, and then
irradiating the liquid to polymerize it. This process is
not the same as a conventional ;suspension polymerization
process, in which globules of a polymerisable liquid are
suspended in a medium and polymerized, without interaction
with the medium, to produce polymerized "globules". In the
present invention, the discrete dispersed volumes of
polymerisable liquid are not present in the latex in the
form of spherical globules, but surprisingly are of a
flattened laminar shape. For example, the volumes can be of
a generally disc-like shape through oval to a ribbon-like
- g _
CA 02286362 1999-10-O1
WO 98/45378 PCT/US98/06292
shape. The size can vary but we generally prefer the
volumes to have a thickness of about 1 to 6 micrometers and
a "diameter" or length of about 5 to 20 micrometers.
Smaller or greater volumes can be used. The shapes and
sizes of these discrete dispersed volumes are quite
different from the shapes (generally spherical) and sizes
(larger) of the composite particles formed therefrom. In
the spaces between the discrete dispersed volumes of
polymerisable liquid is the aqueous latex of film-forming
binder particles. Figure 2 of the accompanying drawings
illustrates the dispersion before polymerization, and is
described in more detail hereinafter.
Upon irradiation, the dispersed volumes of
polymerisable liquid are polymerized and adjacent volumes of
the polymerisable liquid combine to form the composite
material particles incorporating the film-forming binder
particles. The resulting composite material particles are
far larger than the discrete dispersed laminar volumes of
polymerisable liquid. For example, whilst the size of a
discrete dispersed laminar-shaped volume of polymerisable
liquid could be from 5 to 20 micrometers in length and from
1 to 6 micrometers in thickness (smaller volumes may also be
present), the size of a composite particle could normally be
up to about 100 micrometers in diameter. The composite
material particles are heterogeneous throughout, having
film-forming binder particles therein, and hardened polymer
formed from the irradiation polymerisable liquid, and
possibly other particles such as opacifying particles. The
composite material particles have film-forming binder
particles contained therein and exposed at the surface of
the composite particles.
The process of the present invention by which the
composite material particles are made is to be distinguished
- 10 -
CA 02286362 1999-10-O1
WO 98/45378 PCT/US98/06292
from conventional emulsion polymerization processes. In
such conventional processes, an initiator dissolved in the
aqueous phase enters surfactant micelles in which some of
the water-insoluble monomer is ;aolubilized, and they
initiate the polymerization therein. By contrast, in the
irradiation process of the present invention, the
polymerization takes place within the monomer volumes
dispersed throughout the aqueou:a phase.
The composite material particles of the present
invention are used in aqueous correction fluids. For
example, they can be added to a previously formed correction
fluid to increase the solids loading thereof, or they can be
mixed with a compatible film forming latex material and/or
opacifier to form a correction fluid. Hy "compatible film
forming latex material", we mean one which is not of
opposite polarity to the film-forming binder particles used
in the composite material particles. Thus, the composite
material particles can be mixed with a non-ionic film
forming latex but they can only be mixed with an anionic
film forming latex when the composite material particles
have been made from a non-ionic or an anionic film forming
latex. Similarly, when the composite material particles
have been made from a cationic :Film forming latex, they
cannot be mixed with an anionic film-forming binder latex.
However, instead of using the composite material
particles of the invention in the above described ways, we
prefer to make the composite ma~:.erial particles in situ in a
correction fluid. This is effected by dispersing the
irradiation polymerisable liquid in the correction fluid,
and then irradiating. In this way, the film-forming binder
particles in the composite material are the same as the
film-forming binder particles o:f the correction fluid itself
and are thus compatible. When 'this is done, the resulting
- 11 -
CA 02286362 1999-10-O1
WO 98/45378 PCT/US98/06292
correction fluid will generally contain too little free
film-forming binder (by "free film-forming binder" we mean
particles of film-forming binder which have not become :art
of a composite material particle) to form a coherent filcr.
when coated on a substrate and dried. The coating tends to
crumble and fall off the paper. Even if the amount of
film-forming binder in the correction fluid is increased
above the normal level (for that fluid) before the composite
material particles are formed therein, the result is still
not very satisfactory. However, if the amount of
film-forming binder is increased after irradiation, such as
by adding more (untreated non-irradiated) correction fluid,
having compatible film-forming binder therein, the results
are extremely advantageous as will be discussed hereinafter.
It is preferred, in accordance with the invention,
that the film-forming binder used in the preparation of the
composite material particles of the invention be the same
as, or closely similar to, the film-forming binder of the
further correction fluid in which the suspension is mixed in
step (c). This ensures the desired compatibility to achieve
a stable dispersion and good film formation. Absolute
identity between the two film-forming binders is not
essential (though it is preferred): chemical similarity
will suffice provided the two compatible binders can
coalesce together satisfactorily at film formation.
In the method of making the composite material
particles of the invention, irradiation polymerisable liquid
is dispersed in an aqueous latex of at least one
film-forming binder, and then polymerized. The irradiation
polymerisable liquid is a liquid (at ambient temperature)
monomer or oligomer containing ethylenic unsaturation. Upon
exposure to radiation, the monomer or oligomer undergoes
addition polymerization via the ethylenic unsaturation.
- 12 -
CA 02286362 1999-10-O1
WO 98/45378 PCT/US98/06292
There. are many different irradiation polymerisable liquids
which can be used. We prefer t:o use acrylate and
methacrylate monomers, especially bisphenol A propyl
dimethacrylate (available as H:~-Tad from London Resin
Company, London, England). Other specific examples include
ethylene glycol dimethacrylate, triethylene glycol
dimethacrylate and tetraethylene glycol dimethacrylate.
More generally, members of the following groups of
irradiation polymerisable mononners or oligomers are
preferred, viz. bisphenol A aliphatic urethane
dimethacrylates, bisphenol A a7.iphatic epoxy
dimethacrylates, and bisphenol A aliphatic ester
dimethacrylates. Specific examples of suitable irradiation
polymerisable liquids are as follows. These are available
from Cray Valley, 92970 Paris La Defense, France, and the
catalogue designation is given in parentheses.
a) epoxy acrylate oligomers
standard bisphenol A epoxy diacrylate (CN104),
epoxidized soya bean oil diacrylate (CN111)
b) urethane acrylate oligomers
aliphatic urethane triacrylate (CN931 A60)
aromatic urethane triacrylate (CN970 A60)
c) olicro acrylates
tris (2-hydroxyethyl) isocyanurate triacrylate
(SR 368)
d) olicro ether acrvlates
polyethylene glycol 400 diacrylate (SR 344)
ethoxylated trimethylopropane triacrylate (SR
454 )
ethoxylated pentaerythritol tetra acrylate (SR
494 )
e) difunctional acrvlate monomers
tripropylene glycol diacrylate (SR 306)
- 1_i -
CA 02286362 1999-10-O1
WO 98/45378 PCT/US98/06292
f) trifunctional acrylate monomers
trimethylolpropane triacrylate (SR 351)
Other examples include dipentaerythritol
hexaacrylate and aromatic urethane diacrylate.
The amount of irradiation polymerisable liquid used
should not be so much that, when dispersed in the aqueous
latex of film-forming binder particles, the dispersion is
converted to a solid block on irradiation. Routine trial
and experiment in any particular case will reveal optimum
amounts. when the latex is in a correction fluid, we have
found that the amount of irradiation polymerisable liquid
used should generally be less than about 50% by weight of
the mixture of irradiation polymerisable liquid and the
other ingredients of the correction fluid. The amount of
irradiation polymerisable liquid will, of course, determine
the amount of composite material particles in the resulting
particulate suspension produced by the polymerization.
The irradiation polymerisable liquid is preferably
uniformly dispersed throughout the aqueous latex of
film-forming binder before polymerization. This can be
achieved by using standard mixers, such as a Dispermat
mixer. As described above, the discrete volumes of the
irradiation polymerisable liquid are not in globular form
but rather exist in a flattened laminar, e.g., disc-like or
pancake-like, shape in the aqueous latex of film-forming
binder.
In the method of making the composite material
particles of the invention, the irradiation polymerisable
liquid (monomer or oligomer) is polymerized by irradiation,
i.e., by exposure to light, electron beam, microwave or
gamma radiation. We prefer to use light (using a photo
initiator) or, more preferably, electron beam irradiation.
The radiation doses required to effect polymerization will,
- 14 -
CA 02286362 1999-10-O1
WO 98/45378 PCT/US98/06292
of course, depend on the particular monomer or oligomer
being polymerized and on the overall system, but electron
beam irradiation doses of up to about 10 MRad have been
found satisfactory for Hi-Tad and similar materials.
The correction fluids of the invention contain, in
addition to the composite mater~.al particles of the
invention, opacifying particles and film-forming binder
particles.
The opacifying particles used in the correction
fluids of the invention can be any pigment suitable for the
purpose, the most usual being titanium dioxide. Where a
color other than white is needed, other pigments or
colorants can be included to give the desired effect. In
general, the opacifying particles will have an average
particle size of less than 0.8 micrometer (to increase
dispersibility), and the particles may have been pre-treated
with alumina or a combination of: alumina and silica to
increase dispersibility.
The amount of opacifying particles in the correction
fluids of the invention will generally be from 20% to 70%,
preferably from 25% to 60%, and most preferably from 30% to
50%, by weight. Preferably, the average particle size will
be about 0.4 micrometers or lesss. Suitable titanium dioxide
pigments include commercially available rutile titanium
dioxide and anatase titanium dioxides, or blends or mixtures
of these which preferably have an average particle size from
approximately 0.2 micrometers to 0.4 micrometers and an
average oil absorption of about 16 g/100 g pigment.
One preferred titanium dioxide is Ti-Pure R-900
(DuPont, Wilmington, Delaware), which is rutile titanium
dioxide composition manufactured by the chloride process.
(Ti-Pure is a trademark.) This composition includes about
94% titanium dioxide, and 4.S% alumina. The particle size
- 15 -
CA 02286362 1999-10-O1
WO 98/45378 PCT/US98/06292
varies, but 80% of the particles are 0.4 micrometer or
smaller, and 90% of the particles are under 0.6 micrometer.
Other suitable titanium dioxide compositions include
Ti-Pure R-901, Ti-Pure R-902, Ti-Pure R-931, Tioxide R-XL
(Tioxide America, Inc., Columbia, Maryland) and Kronos 2131
(Kronos, Inc., Houston, Texas). Other opacifying particles
may be used either alone or, preferably, in combination with
titanium dioxide. Such pigments include zinc sulfide and
zinc oxide.
The film-forming binder used in the composite
material particles of the invention and in the correction
fluids of the invention can be any natural or synthetic
polymer which forms a continuous and cohesive film on loss
of aqueous suspending medium or solvent at ambient
temperature. The film-forming binder particles preferably
consist only of film-forming binder.
Examples of suitable film-forming binders include:
a) styrene acrylic copolymers, such as a copolymer
of 40% styrene/ 55% ethyl acrylate/ 5% divinyl
benzene.
b) styrene methacrylic copolymers, such as a
copolymer of 40% styrene /55 % butyl
methacrylate/ 5% divinyl benzene (and UCAR
materials referred to hereinafter).
c) ethylene vinyl acetate copolymers, such as a
copolymer of from 8 to 25% ethylene and from 92
to 75% vinyl acetate.
d) vinyl acrylic copolymers, such as a copolymer
of 60% vinyl acetate /40% styrene.
e) acrylic polymers, such as a copolymer of 70%
methylmethacrylate /28% ethyl acrylate /2%
divinyl benzene.
- 16 -
CA 02286362 1999-10-O1
WO 98/45378 PCT/US98/06292
f) vinyl acetate or vinyl alcohol polymers, such
as polyvinyl acetate, and copolymers.
g) polyurethanes (Witco polymers described
hereinafter) .
S h) cationic methacrylate copolymers, such as a
copolymer of 75% mE:thylmethacrylate/ 25%
dimethylaminoethylrnethacrylate.
The film-forming binder is used in the form of an
aqueous latex, that is to say a dispersion of particles of
the film-forming binder in an aqueous suspension medium, the
particles being small enough to remain suspended as a result
of Brownian motion thereof. Generally, the particles will
be no greater than about 10 micrometers (in diameter) and
more usually they will be less than 1 micron in diameter.
When composite material particles of the invention are made
by dispersing an irradiation-polymerisable liquid in an
aqueous latex of film-forming binder, and irradiating, at
least some and sometimes most of the film-forming binder
particles become included within the composite material
particles that are formed. Any film-forming binder
particles which do not become part of the composite
particles remain free in suspension.
The majority of water-soluble dyes used in aqueous
inks are anionically charged. The use of aqueous correction
2S fluids containing anionically a:nd/or non-ionically
stabilized film-forming binder dispersions and pa.gments can
lead to bleed of these water-soluble dyes into the
correction layer and formation of an unsatisfactory
correction. To prevent the bleeding of the dyes, a cationic
species which will interact with the anionic dye molecules
rendering them insoluble and unable to diffuse through the
correction can be included in the formulation as a non-bleed
agent. This non-bleed agent may be a cationic film-forming
- 17 -
CA 02286362 1999-10-O1
WO 98/45378 PCT/US98/06292
polymer, for example Worleecryl 8721 (Worlee Chemie
GmbH,Hamburg, Germany), a cationic non-film-forming polymer,
for example Rhoplex R PR-26 (Rohm & Haas, Philadelphia) or a
non-polymeric cationic species capable of forming complexes
with anionic dyes, rendering them insoluble. Examples of
materials which act in this way are diquaternary ammonium
compounds of the type described in British Patent
Application 9211760.5 (included by reference herein in its
entirety), for example
1,2-ethanediaminium-N,Nl-ditetradecyl-N,N,N1,N1-tetramethyl
dibromide.
Dispersions of film-forming binders in water are
stabilized by addition of surfactant. The surfactant may be
anionic, cationic or non-ionic in character. It is
important that the charge carried by the surfactant is
compatible with that on other species in the fluid to
prevent interaction which may lead to destabilization of the
polymer dispersion. The majority of available film-forming
polymer dispersions are anionically stabilized making them
unsuitable for use with cationic non-bleed agents.
Advantageously, the film-forming binder is stabilized with a
cationic surfactant. Examples are Vinamul 90045 (vinyl
acetate/dimethylamino ethyl methacrylate copolymer) (from
Vinamul Ltd., Carshalton, Surrey, U.K:), Witcobond W-2123,
(polyurethane from Witco Corporation, Chicago, II, U.S.A.),
Primal LE-1126 (or E-1179N or E-1242) (self-crosslinking
acrylic emulsion from Rohm and Haas Company, London,
England) and Vinac XX210 (or XX220, XX230 or XX240) (all
vinyl acetate homopolymer emulsions from Air Products,
Allentown, PA, U.S.A.). Examples of non-ionic systems
include Vinamul 6955 (vinyl acetate/Veova Shell Chemicals/
other olefins) and Vinamul 6975 (vinyl acetate/Veova).
- 18 -
CA 02286362 1999-10-O1
WO 98/45378 PCT/US98/06292
We describe in detail hereinafter certain correction
fluids of the invention which are very fast setting. In
these fluids, we prefer to use a soft film-forming binder to
give a flexible film, i.e., a l:ilm-forming binder latex in
which the film-forming binder has a glass transition
temperature of less than 12°C. However, in the case of
correction fluids of the invention which are not especially
fast setting, then it can be advantageous to include two or
more film-forming binders in the latex. Thus, for example,
the correction fluids can include a soft film-forming binder
and a hard film-forming binder (i.e., a binder having a
glass transition temperature greater than 12°C).
Alternatively, the correction fluid may include only one
film-forming binder if the binder has a glass transition
temperature of between 10°C and I7°C.
The preferred soft film-forming binders have a glass
transition temperature of less than 10°C. Film-forming
binders having a glass transition temperature of less than
12°C include styrene-acrylic copolymers, styrenemethacrylic
copolymers, ethylene-vinyl acetate copolymers, vinyl acrylic
polymers and acrylic polymers. Specific examples include
UCARR Latex 446 {Tg = 9°C) (Union Carbide, Cary, N.C.,
U . S . A . ) , UCARR 10 0 , 7 6 RES 417 0 ( f rom Rohrn and Haas
Company, Philadelphia, PA, U.S.A.), Airflex 426, {from Air
Products, Allentown, PA, U.S.A.), UCARR 354, and UCARR 415.
UCARR Latex 446 is a particularly preferred film. forming
binder with a low glass transition temperature. It is a
latex emulsion including synthetic acrylate/styrene polymers
that is non-ionically stabilized. UCARR 446 Latex includes
about 38% water and 62% copolymer of methyl methacrylate,
butyl acrylate, styrene, methacrylic acid, and 2--
hydroxyethyl acrylate. It also includes about 0.03%
formaldehyde and 0.02% ammonia. In general, the amount of
- 19 -
CA 02286362 1999-10-O1
WO 98!45378 PCT/US98/06292
soft film-forming binder needed in a correction fluid to
give the desired softness and flexibility to the film will
be between about 2% and about 12% (more preferably between
about 4% and about 9%) by weight.
Hard film-forming binders include, for example
styrene acrylic copolymers, styrene-methacrylic copolymers,
ethylene-vinyl acetate copolymers, vinyl acrylics, acrylics.
Examples include UCARR Latex 144 ((Union Carbide) Tg = 20°C)
UCARR 5 0 3 , UCARR 4 2 2 , and UCARR 51 . UCARR L a t a x 14 4 i s
particularly preferred. UCARR 144 includes about 52% water
and 48% copolymer of butylacrylate, styrene, methacrylic
acid. It also includes about 0.1% ammonia. The amount of
hard film-forming binder used is such as to provide the
hardness of the film as desired. Generally, the amount of
any hard film-forming binder present in a correction fluid
will be between about 3% and about 12% (more preferably
between about 4% and about 9%) by weight. The preferred
weight ratio (on a dry basis, i.e., in terms of the
respective solids contents) of hard to soft film-forming
binder is between about 70:30 and about 20:80, and more
preferably between about 40:60 and about 60:40.
The correction fluids of the invention preferably
have a viscosity of between about 50 and 500 mPa s, more
preferably between about 80 and 300 mPa s (as measured at 50
s-1 shear). The correction fluids to be applied by brush
will preferably have a total solids content of at least 60%,
and more preferably a total solids content of at least 65%.
The very fast setting fluids to be described hereinafter
will usually have solids contents of over 70%. Preferably,
correction fluids of the invention will also include one or
more dispersing agents and surfactants.
The overall ratio of opacifying particles (including
any extender pigments, colorants, etc.) to film-forming
- 20 -
CA 02286362 1999-10-O1
WO 98/45378 PCT/US98/06292
binder should preferably be betyreen about 5:1 and about 3:1,
and more preferably between about 4:1 and about 3.7:1.
The correction fluids of the invention may contain
acicular particles to dimensionally stabilize the film that
is formed. The preferred acicul.ar particles are less than
about O.lmm in length, and have an aspect ratio of between
about 15:1 and about 2:1, and more preferably have an aspect
ratio of between about 5:1 and about 10:1. (Aspect ratio is
the ratio of the major dimension to the minor dimension.)
-Particularly preferred acicular particles are composed of
calcium metasilicate and are sold under the trade name NYAD
400 (NYCO Minerals, Inc., Willsboro, NY, U.S.A.).
Preferably, the correction fluids include between about o.5%
and about 9% (more preferably beaween about 1% and 5%) of
the acicular particles by weight:.
The preferred pigment di,spersants in the above
anionically stabilized systems are sodium salts of
carboxylate polyelectrolytes such as NopcosperseR 44 (Henkel
Corp., Ambler, PA, U:S.A.) or preferably sodium salts of
malefic anhydride copolymers, e.c~., TAMOLR 731 SD (Rohm &
Haas, Philadelphia, PA, U.S.A.) or sodium salts of
poly-methacrylic acid. In a cationic system, a preferred
dispersant would be a fatty quaternary compound such as
Hipochem CGB (from High Point Chemical Corp., High Point,
NC, U.S.A.). The dispersant should be added in a sufficient
amount to ensure complete dispersion of the high~quantity of
pigment in the correction fluid at low viscosity and with
little or no foam generation. Other suitable
polyelectrolytic acrylate dispersants (for anionically
stabilized systems) include TAMOLR 850, or 950. Preferably,
the correction fluid includes 0.5% to 1.5%, and more
preferably 0.8% to 1.1%, of the dispersant by weight.
- 21 -
CA 02286362 1999-10-O1
WO 98/45378 PCT/US98/06292
The surfactant serves as a wetting agent. Any
suitable surfactant can be used. Preferred surfactants are
non-ionic and include acetylenic diols and alcohols such as
SurfynolR 104 (Air Products and Chemicals, Inc., Allentown,
PA, U.S.A.) which is 2,4,7,9-tetramethyl-5-decyne-4,7-diol.
Preferably, the correction fluid includes about 0.3% to
about 2.0%, and more preferably 0.44% to 1.5%, of the
surfactant by weight.
The correction fluids may contain extender pigments.
Preferred extender pigments include kaolin ("China") clay,
such as KaopaqueR 10-S (DryBranch Kaolin Co. Dry Branch, GA,
U.S.A.), which includes greater than 97% kaolin clay
(A1203.2Si02.2H20), less than 3% water, and 0.35% of a
sodium polyacrylate/soda ash dispersant. Other .suitable
kaolin clays are NcNamee Clay (R. T. Vanderbilt, Inc.,
Norwalk, Connecticut, U.S.A.) and Huber 40C {J. M. Huber
Corp., Macon, Georgia, U.S.A.). The extender pigment is
added in an amount that enhances the ability to write with
ballpoint pen ink over the corrected spot. Preferably, the
correction fluid should include 5% to 15%, and more
preferably 5% to 10%, of the extender pigment by weight.
To color match, e.g., lined notebook paper, the
titanium dioxide primary pigment can be tinted with other
pigments, such as black, burnt umber, and blue, using
standard color matching techniques. The preferred black
pigment is an aqueous dispersible carbon black such as Mars
Black. A preferred Burnt Umber Dizment is Burnt Umber
W-3247, AurasperseR (Engelhard Chemical., N.J., U.S.A.).
These colorants are used in amounts to color match the shade
of white of the paper on which the correction fluid is to be
used. Of course, colored correction fluid for colored paper
can also be made. Preferably, the correction fluid should
include less than 2.0% of the colorant pigments by weight.
- 22 -
CA 02286362 1999-10-O1
WO 98/45378 PCT/US98/06292
The correction fluid may contain a biocide. The
biocide is used to prevent bacteria from contaminating the
correction fluid. Bactena attack some polymers, and are
often found dormant in some of the ingredients, e.g.,
pigments, used to manufacture correction fluids. A
sufficient amount to prevent bacterial growth should be
used. Preferably the correction fluid includes about 0.05%
to 1.0% of the biocide by weight. The nature of the biocide
is not critical. Examples include fluorinated
sulphonamides, organic amides and fatty acid modified
amides. One particular example is
I,2-benzisothiazolin-3-one.
The correction fluid may contain a defoaming agent.
A preferred defoaming agent is I~opcoR 8034 (Henkel Corp. ,
Ambler, PA, U.S.A.), although any defoaming agent typically
used in correction fluids can be used. Such defoaming
agents are usually mineral oil derivatives mixed with
amorphous silica, and should be added in an amount
sufficient to ensure that the correction fluid is deaerated
and does not foam when shaken. Preferably, the correction
fluid includes 0.05% to 1.0%, and more preferably 0.21% to
0.4%, of the defoaming agent by weight.
The correction fluids of: the invention and the
aqueous suspensions of composite material particles of the
invention comprise solid particles (the composite particles)
dispersed in a suspending medium. The medium may be water
alone or it may, for example, be a mixture of water and one
or more other liquids such as, for example, alcohols, e.g.,
methanol, ethanol or propanol, or glycol ethers such as
2-methoxyethanol or 2-ethoxyethanol. The medium may contain
dissolved substances such as dispersant polymers, chelates
for dyes, soluble film-forming binder, or surfactants, for
example. The amount of aqueous suspending medium in the
- 23 -
CA 02286362 1999-10-O1
WO 98/45378 PCT/US98/06292
correction fluids of the invention is generally from less
than 15% to 40%, and preferably from about 15% to 30%.
The correction fluids of the invention can in
general be made in a number of ways. For example, the
individual components can be mixed in any order or
combination. As previously stated, however, we prefer to
make the composite material particles in situ in a
correction fluid, and then to add more film-forming binder
(e. g., further correction fluid containing compatible
film-forming binder). Also the invention should be
understood not to be limited to irradiation polymerisable
materials. Any method of forming composite material
particles is included within the scope of this invention,
though irradiation polymerization is the most preferred.
We have found, in accordance with the preferred
practice of the invention, that the polymerization of the
irradiation-polymerisable liquid in situ in a correction
fluid comprising an aqueous latex of at least one
film-forming binder and opacifying particles, surprisingly
enables the solids loading of the correction fluid to be
significantly increased without destabilization of the fluid
and without unacceptable increases in viscosity. This is
due in part, we believe, to the nature of the composite
material particles formed by the polymerization of the
liquid, and especially to the fact that the composite
material particles incorporate film-forming binder particles
and possibly other components of the system in which the
composite material particles are formed (bound by the
polymer formed by irradiation of the irradiation
polymerisable liquid), which makes them inherently
compatible with the system. In particular, in this way it
is possible to disperse the irradiation-polymerisable liquid
in a high solids loading dispersion containing a latex of a
- 24 -
CA 02286362 1999-10-O1
WO 98/45378 PCT/US98/06292
film-forming binder, and to irradiate to form the composite
material particles of the invention, the resulting
dispersion being stable and containing a much higher solids
loading than previously.
The invention thus provides a technique whereby, for
use in collection fluids, the solids loadings of suspensions
containing aqueous latexes of film-forming binders, can be
increased without loss of stability and without the increase
in viscosity which would be expected from such a solids
increase. The invention thus provides a way of providing in
correction fluids, higher solid, loadings than previously
possible by conventional techniques, without destabilization
and without unacceptable increases in viscosity. In
particular, whilst maximum solids loadings with prior known
aqueous correction fluids was about 72%, solids loadings of
up to about 85% or more can be achieved in accordance with
the present invention. While it is not known why the high
solids loading material still remains a useable fluid, one
possible explanation is that th.e generally spherical binder
particles adjacent the composite particles surfaces)
provide additional lubricity between the composite
particles. Also, the free binder particles are electrically
charged so that until compaction is applied to force them
into contact with each other and with the composite
particles, when the correction fluid is applied in use, they
tend to remain apart. Furthermore, the aqueous..phase 6 (see
Fig. 3 described hereinafter) between the composite
particles containing binder particles 2 also increases the
hubricity between the composite particles and maintains the
formulation in the fluid state even with an extraordinarily
high solids loading. These higher solids fluids of the
invention generally have a shorter writeover time than prior
- 25 -
CA 02286362 1999-10-O1
WO 98/453?8 PCT/US98/06292
known conventional correction fluids, and some can show a
nearly instantaneous hardening effect.
The aqueous correction fluids of the invention can
be formulated to have the normal writeover time for aqueous
correction fluids, e.g., a writeover time of about 40
seconds or more. However, by using higher solids loadings
and/or greater proportions of the composite material
particles of the invention, the writeover times can be very
significantly reduced. We prefer writeover times of less
than 30 seconds, more preferably less than 15 seconds and
most preferably less than 10 seconds. Indeed, according to
a very highly preferred feature of the invention, the fluids
can be so formulated that substantially no delay is
necessary between applying a correction fluid to paper or
another substrate, and applying writeover thereto. Such
fluids are unique and constitute, together with their method
of preparation, further aspects of the invention per se.
("Writeover" is understood in the art to mean that when
writing with a writing implement, no ploughing of the
writing tip in the correction fluid occurs, and that the
quality and legibility of the ink lay-down is equivalent to
the lay-down on paper. The writing implement, e.g., ball
pen, is clean and does not pick up soft or tacky correction
film. One test procedure for measuring writeover time is
described hereinafter with reference to the Examples.)
The invention thus includes a correction fluid which
comprises an aqueous suspending medium and opacifying
particles, film-forming binder particles and composite
material particles of the invention dispersed in said
medium, the correction fluid being such that when the fluid
is spread as a layer on a substrate, the layer is converted
to a solid substantially non-tacky layer within 10 seconds,
and most preferably substantially immediately.
- 26 -
CA 02286362 1999-10-O1
WO 98/45378 PCT/US98/06292
The actual time between laying down a layer (or
film) of the correction fluid on paper (or another
substrate) and its ability to accept writeover can be varied
from substantially nil (i.e., no delay whatever) to any
desired time. The actual time taken will depend on the
solids loading and the proportion of composite material
particles of the invention present, as well as on the other
components of the fluid. (In general, the greater the
solids loading and/or the greater the proportion of
composite material particles of the invention, the-shorter
the writeover time). By routine: trial and experiment, a
fluid formulation can be chosen to give the optimum
qualities in any particular situation. Since, in general,
it is preferred to have as short: a writeover time as
possible, further description of: the invention will be
directed to correction fluids of: the invention which provide
minimal writeover time, but it i.s to be understood that the
invention includes correction fluids of longer writeover
time.
The highly preferred fast setting correction fluids
of the present invention form a solid film immediately or
within a second or two, after they are spread on paper or
another substrate. Moreover, the entirety of the fluid
deposited on the paper becomes stolid upon spreading or
shortly thereafter. Thus, when a blob of the fluid is
deposited on a substrate, the material remains Fluid until
it is spread whereupon, substantially simultaneously with
the spreading, the material is a.ll converted to a
dimensionally stabilized material capable of accepting
writeover by a writing instrument. The material typically
hardens sufficiently to be considered a solid. During the
spreading of the fluid material on the substrate, the
particles of composite material therein are not broken but
- 27 -
CA 02286362 1999-10-O1
WO 98/45378 PCT/US98/06292
remain substantially intact: together with the other
particulate matter present, the composite material particles
pack together closely to form the hardened material.
In contrast to prior known aqueous correction
S fluids, the solidification or setting of the correction
fluids of the invention is not caused by loss of water but
by the application of shear during spreading. Indeed, the
immediately set dimensionally stabilized, i.e., "solid"
layer will normally contain water which will subsequently be
lost in the usual way by evaporation or otherwise.
As soon as solidification has taken place, the
dimensionally stabilized film can be written on, typed over
or otherwise accept so-called writeover. The fluids can be
so formulated that no delay at all after spreading is
required, or they can be formulated to need a few seconds or
longer delay after spreading, as desired. In some cases,
the solidified film will feel dry to the touch immediately
upon its formation, but with fluids containing a greater
amount of water, the.film may initially feel slightly damp.
Even so, writeover can be effected substantially immediately
and certainly within a very few seconds.
Another highly preferred feature of the invention is
in the provision of a correction fluid which can be
solidified without the loss of any liquid therefrom and can
immediately accept writeover. In another aspect, therefore,
the invention provides a correction fluid comprising an
aqueous suspending medium, particles of composite material
according to the invention, opacifying particles and a
film-forming binder latex, which fluid can be solidified to
a film without loss of any liquid therefrom, and which film
can immediately accept writeover.
These highly preferred correction fluids of the
present invention provide dimensionally stabilized, "solid"
- 28 -
CA 02286362 1999-10-O1
WO 98/45378 PCT/US98/06292
films in which the opacifying particles are very evenly
distributed and, usually, far more evenly distributed,
giving a more even opacity than in prior known aqueous
correction fluid films. This means that these highly
preferred fluids of the present invention can be deposited
in thinner layers than prior art correction fluids, without
sacrificing opacity, and the use of thinner layers is
generally advantageous in the correction fluid art.
The way in which the fasct setting correction fluids
of the invention are spread to :Form a smooth opaque film is
important. We have found that :it is usually preferred to
spread the fluid using edge shear, for example with a doctor
blade, knife edge or Bird bar to achieve instant hardening.
By "edge shear" we mean the shear generated in the fluid
between the substrate and a sti:Ef straight edge moved
parallel to the substrate, the degree of shear depending on
the separation of the edge from the substrate and its speed
of movement relative thereto. :3preading with a brush or
with a felt or foam spreader, for example, may not be
adequate or satisfactory to cause immediate hardening, but
is perfectly satisfactory where a delay of a few seconds is
acceptable. The special fast setting fluids of the
invention are thus preferably dispensed from containers
which include an edge shear member, e.g., a knife edge
spreader or other means of appl!,ring the appropriate shear.
In one preferred arrangement, tlZe fluids can be spread using
a feed nozzle which includes a :spreader edge. If spreading
is effected in some other way, the instant solidification
may not be obtained in which cage the time before writeover
will, of course, be longer.
As indicated, the degree of shear can be important
in that if too little shear is used, the fluid may not
instantly harden as desired. In general, routine trial and
- 29 -
CA 02286362 1999-10-O1
WO 98/45378 PCT/US98/06292
experiment with any particular fluid will reveal the optimum
requirement. It is a feature of the fast setting fluids of
the invention that they can be solidified by applying a
degree of shear easily available manually on a desk top. In
general, the degree of shear will be from about 50 to about
10000 s--1, for example about 2000 s.
The precise formulation of the fast setting
correction fluids of the invention can vary depending on the
nature of the components. In general, the solids content
will usually be above 75% by weight and can be up to about
85% or more by weight. These fluids will normally give
instant solidification and immediate writeover. As the
solids content is reduced, and/or the content of particles
of composite material of the invention is reduced, writeover
time will increase. Routine trial and experiment in any
particular case will indicate optimum conditions. The
amount of opacifying particles will usually be between about
20% and 40%. The amount of composite material of the
invention will usually be up to a maximum of about 50%, more
preferably from 8 to 30%, and most preferably from 12 to
20%.
As indicated previously, the preferred way of making
the fast setting correction fluids of the invention is by
polymerizing in situ the irradiation-polymerisable liquid
dispersed in an aqueous latex of a film-forming binder. In
this method, some of the film-forming binder tends to be
incorporated into the composite material particles formed as
the polymerisable liquid polymerizes. This can result in a
shortage of film-forming binder in the final product, so
that the films formed therefrom tend to be brittle and flake
- from the paper when dry. This can be dealt with simply by
mixing into the irradiated correction fluid some more (non--
irradiated) film-forming binder. One way of doing this is
- 30 -
CA 02286362 1999-10-O1
WO 98/45378 PCT/US98/06292
to add a conventional (non-irradiated) correction fluid
latex to the irradiated correction fluid of the invention.
Thus, a correction fluid of the invention can be made from a
conventional aqueous correction fluid by (a) dispersing the
irradiation polymerisable liquid therein and polymerizing,
and (b) mixing the irradiated product with further
conventional (non-irradiated) aqueous correction fluid. The
proportion of further (non-irradiated) fluid mixed with the
product of step (a) can be as high as about 90:10, but will
usually be from 50:50 to 70:30, most preferably about 60:40
by weight. It will be appreciated that, by this procedure,
the original product of step (a) receives not only further
film-former but~also extra opacifying particles. This can
result in greater covering power in use.
It has also been found that, when correction fluids
have particles of composite material of the invention added
to them, the shelf stability of the suspension can be
greatly improved. In such cases, it can be unnecessary to
shake a container even after storage for several months, in
order to re-disperse the solids.
It is a feature of the present invention that, if
desired, correction fluids with very high solids loadings,
e.g., above 80%, can be made without associated high
viscosity. Thus, the fluids do not become unwieldy pastes
and/or unstable dispersions. The fluids remain as fluids
and whilst they may become relatively viscous, e.g., 6000 Pa
s, they are easily spreadable manually, under 50 sec-1
shear for example, using a brush, giving a viscosity of
about 300 Pa s. This very high aolids loading without loss
of stability, etc., is novel and constitutes a further
aspect per se of the present invention.
- 31 -
CA 02286362 1999-10-O1
WO 98/45378 PCT/US98/06292
In a further aspect, the invention provides a method
of making a correction fluid of the invention which
comprises the steps of:
(a) providing composite material particles of the
invention; and
(b) mixing with said material at least one
compatible film forming latex material, and
particles of at least one opacifier.
The invention also includes the use of composite
l0 material particles of the invention, or aqueous suspensions
thereof, as a component of an aqueous correction fluid.
Further, the invention includes the use of composite
material particles of the invention or of an aqueous
suspension thereof, in forming a coating on a substrate.
I5 The invention also provides a coating on-a
substrate, which coating comprises composite material
particles of the invention, wherein the composite material
particles are bound together in said coating by a compatible
film-forming latex material.
In order that the invention may be more fully
understood, reference is made to the accompanying drawings
wherein Figures 1 to 5 are SEM photographs, in which
drawings:
Fig. 1 is a top plan view of a Latex II correction
fluid dried film which is not in accordance with the
invention;
Fig. 2 is a top plan view of a frozen and fractured
mixture described in Example 1;
Fig. 3 is a top plan view of a frozen and fractured
aqueous suspension of particulate material according to the
present invention, as described in Example 1;
- 32 -
CA 02286362 1999-10-O1
WO 98/45378 PCT/US98/06292
Fig. 4 is a top plan view of a dried film formed
from an aqueous suspension of particulate material according
to the present invention, as described in Example 1;
Fig. 5 is a top plan view of a film of a dried
correction fluid of the invention as described in Example 2;
Fig. 6 is a view of a composite particle formed in
the process of the invention by the polymerization of Hi-Tad
(Example 1);
Fig. 7 is a chart of particle size distribution in
Latex II (line A); in an aqueous suspension of particulate
material according to the invention (line B), (Example 1);
and in a correction fluid of th.e invention (line C);
(Example 2);
Figs . 8 (a) , 8 (b) and 8 (c:) illustrate one embodiment
of correction fluid applicator of the invention and its use.
Referring to the drawings, Fig. 7 shows particle
size distributions in three fluids. The measurements were
made on a Malvern Mastersizer instrument and the plots are
of % volume of the solid product occupied by particles of a
given diameter. Plot A is for Latex II which is a
correction fluid which is not in accordance with the
invention and whose composition is set out in Table I
hereafter. As can be seen (and is confirmed by other work).
most of the solids (the film-forming latex particles and the
titanium dioxide particles) are in the size range 0.2 to 2.0
micrometers. In particular, the particle size and volume
distribution are as follows:
size rancre volume %
0.125 - 0.313 9.6
0.313 - 0.576 23.4
0.576 - 0.781 16.0
0.781 - 1.06 15.3
- 33 -
CA 02286362 1999-10-O1
WO 98/45378 PCT/US98/06292
1.06 - 1.44 13.2
1.44 - 1.95 9.1
1.95 - 2.65 4.7
2.65 - 6.63 6.4
6.63 - 16.57 2.3
100.0
It can be seen that over 91% by volume of the particles lie
in the main peak area (i.e., up to 2.65 micrometers in
diameter) and only a few (less than 9%) are in the second
very minor peak area (i.e., greater tha n 2.65 micrometers).
The particles in the second peak area a re believed to be
mainly film forming binder particles. Furthermore, the mode
sizes of the particles in the two peaks are, respectively,
about 0.75 and 5.7 micrometers.
Plot B is of an aqueous suspension of composite
material (particles of the invention as made in Example 1
hereafter (i.e., an aqueous suspension which was an
irradiated mixture of Hi-Tad and Latex II). As can be seen,
plot H is markedly different from plot A in that plot B has
a new second peak of particles ranging in size from about
3
to about 70 micrometers. This peak is of the composite
material particles produced by the polymerization
reaction
and they comprise latex particles of film-forming
binder
fixed relative to each other by a matrix
of polymerized
Hi-Tad which also includes some filler opacifying particles
(e. g., titanium dioxide). The particle
size and volume
distribution are as follows:
size range volume %
0.05 - 0.092 28
0.092 - 0.170 6.9
0.170 - 0.313 10.1
0.313 - 0.576 99
0.576- 1.06 7.8
- 34 -
CA 02286362 1999-10-O1
WO 98/45378 PCT/US98/06292
1.06 - 2.65 g,l
2.65 - 3.60 1.6
3.60 - 9.00 6,g
9.00 - 16.57 16.3
16.57 - 30.53 21.8
30.53 - 76.32 7,g
1~00.~p
It can be seen that the smaller size peak (up to 2.65
micrometers diameter particles) contains about 47% volume
of the particles and the larger size peak (above 2.65
micrometer diameter particles) contains about 53~ volume ~
of the particles. Furthermore, the mode sizes of the
particles in the two peaks are, respectively, about 0.3 and
micrometers. A bimodal size pattern of this general type
15 is an important highly preferred feature of the invention,
since it is believed to contribute to the achievement of
very short writeover times. In particular, it enables the
smaller size particles to occupy interstitial spaces between
the larger particles, when the :suspension is applied as a
20 film on a substrate. This efficient close packing is also
facilitated by the generally spherical nature of the
composite particles and of the :latex particles.
Plot C is of a correction fluid of the invention
(see Example 2) formed by mixing the particulate suspension
of plot B with Latex II (50:50).. Comparing plots B and C,
it can be seen that there is an increase in the,size of the
left-hand peak (indicating an increase in the amount of free
film-forming binder particles and filler particles in the
suspension), but the plot retains the bimodal features of
plot B.
The particle size and volume distribution are as
follows:
- 35 -
CA 02286362 1999-10-O1
WO 98/45378 PCT/US98/06292
size range volume
0.05 - 0.230 7.7
0.230 - 0.424 13.1
0.424 - 0.781 17.7
0.781 - 1.44 15.1
1.44 - 2.65 7.9
2.65 - 3.60 2.0
3.60 - 9.00 7.2
9.00 - 12.21 4.2
12.21 - 22.49 11.1
22.49 - 56.23 12.5
56.23 - 76.32 1.5
100.0
The left hand peak (up to about 3.60 micrometers diameter)
contains about 63% by volume of the particles, and the right
hand peak (from 3.6 micrometers upwards) contains about 37%
by volume of the particles. The mode sizes of the particles
in the two peaks are, respectively, about 0.3 and 20
micrometers.
Fig. 8(a) is a schematic vertical sectional view of
one embodiment of correction fluid applicator (80) according
to the present invention. The applicator comprises a
flexible bottle-like container (81) (shown inverted) with a
cap (82) having an outlet passage (83) therethrough. A
valve may be provided (not shown) to control the ingress of
air and egress of correction fluid from the container (81).
Within the container is a correction fluid (84) of the
invention.
Externally of container (81} is a shear member {85)
connected to cap (82). Shear member (85) is of plate-like
shape and has two spaced feet (86) at its lower edge (87).
Between the feet (86) is a recessed shear edge (88) (see
Fig. 8(c)).
- 36 -
CA 02286362 1999-10-O1
WO 98/45378 PCT1US98/06292
Figs. 8(b) and 8(c) show the same applicator as Fig.
8(a), but in Fig. 8(c) the applicator is illustrated in the
direction of section line A-A in Fig. 8(a).
In use, the applicator (80) is disposed over a
substrate (90), e.g., a sheet of paper. A drop (91) or
larger quantity of correction fluid is dispensed on to the
substrate (90) by squeezing the flexible container (81) -
Fig. 8(a). The applicator is then tilted to bring shear
member (85) behind the drop (91). The applicator is moved
to the left (Fig. 8(b) - see arrow H) with the applicator
feet (86) bearing on the substrate (90). The fluid drop
(91) is thus spread as a thin film (92) by the shear edge
(88) of the shear member (85). During the spreading, the
shear edge (88) subjects the fluid to edge shear.
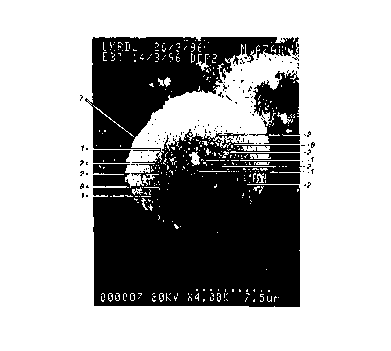
Fig. 1 to 6 are SEM photographs. Fig. h shows the
surface of a film formed of the aqueous correction fluid
herein called Latex II which is not in accordance with the
invention and whose constitution is given in Table I. The
film was formed by placing a drop of the correction fluid on
paper and then spreading it with a 50 micrometer Hird bar.
The film includes titanium dioxide particles (1) and
film-forming binder particles (2). The film is opaque and
can be flexed without cracking.
Figs. 2 to 6 will be described in relation to
Examples 1 and 2 below.
In the Examples, writeover times were determined as
follows. Using a ballpoint pen, handwriting was produced on
a paper sheet. Correction fluid was applied to the
handwriting and the time noted. After a measured interval,
a writeover correction was made on the correction fluid film
by ballpoint pen handwriting. If the writeover was not
accepted, the test was repeated. at longer intervals until
writeover was accepted. By "accepted", we mean that the
- 37 -
CA 02286362 1999-10-O1
WO 98/45378 PCT/US98/06292
handwriting should make a mark without ploughing or
fracturing the surface of the correction fluid film. The
shortest interval at which writeover was accepted was noted
as the writeover time. The ballpoint pen used was a
Papermate Flexgrip Ultra Medium, but other ballpoint pens
can be used.
The following Examples are given by way of
illustration only. Parts are by weight unless indicated
otherwise.
Example 1
Hi-Tad liquid monomer (40 parts) was placed in a
vessel, and Latex II (60 parts) added thereto. The two
components were then vigorously mixed using a Dispermat
mixer. A sample of the mixture so formed was frozen and
fractured and subjected to SEM as shown in Fig. 2 of the
accompanying drawings. It is clear from Fig. 2 that the
Hi-Tad monomer is not present as spherical globules dispersed
in the aqueous phase. Rather, the Hi-Tad is present in
discrete volumes (4) of a laminar or pancake-like shape.
These volumes (4) are surrounded by the aqueous suspension
(5) containing film-forming binder particles and titanium
dioxide particles.
Samples of the liquid mixture were sealed in
polyethylene tubes and subjected to a total electron beam
irradiation of 2 MRad (in 1 MRad increments at room
temperature). The resulting fluid which was an aqueous
suspension containing composite material particles of the
invention, was very viscous (above 50000 mPa s) but was
stable in that the solids did not settle out. A sample of
the fluid was frozen and fractured and subjected to SEM as
shown in Fig. 3 of the accompanying drawings. Fig. 3 shows
parts of two composite particles (3) which have been formed
by the polymerization. These composite particles have
- 38 -
CA 02286362 1999-10-O1
WO 98/45378 PCT/US98/06292
film-forming binder particles at their surface. Between the
composite particles (3) is the aqueous phase (6) containing
some film-forming binder particles (2) and some titanium
dioxide particles.
S Fig. 6 shows, on a larger scale, a composite
particle after separation from the fluid. The composite
material particle includes filrn-forming binder particles (2)
bound together by, and thus fiaced relative to each other by,
a matrix (8) of Hi-Tad polymer, the matrix also including
titanium dioxide particles (1)" The~whole composite material
particle will normally have a diameter of from 50 to 100
times that of the film-forming binder particles (2). The
film-forming binder particles (2) are present within the
composite particle and adj acent: its surface ( 7 ) .. The
particles of film-forming binder (2) and the opacifying
particles are very close, and adjacent particles can be
touching and thus bound to one another. The matrix (8) of
irradiation polymerized polymer- fills the interstices
between the particles.
After irradiation, a drop of the fluid was removed
from the tube onto paper and spread using a SO micrometer
Bird bar. The fluid immediately formed a smooth relatively
solid (dimensionally stabilized) opaque coating on the
paper. The coating was dry to the touch and could be
written over immediately. However, the coating had little
coherence and was crumbly and easily removed from the paper.
A sample of the coating was examined under an SEM and
photographed (Fig. 4). Referring to Fig. 4, the film
surface comprises composite particles (3) of the invention,
and titanium dioxide particles (1). There are very few free
film-forming binder particles.
The constitution and properties of the Latex II and
of the irradiated mixture were as in Table I:
_ 3c, _
CA 02286362 1999-10-O1
WO 98/45378 PCT/US98/06292
TABLE I
Example 1
Ingredient (solid %) LATEX II Irradiated mixture
(weight %) of Latex II/Hi-Tad
60/40 (weight %)
Water 15.60 9.36
Ti PURE 8900 (100% (1) 47.48 28.49
NYAD 400 (100%) (2) 3.75 2.25
Kaopaque lOS (3) 5.00 3.00
UCAR 466 (62%) (4) 11.34 6.80
UCAR 144 (48%) (5) 14.64 8.78
Nopcosperse 44 (35%) (6) 1.07 0.64
Nopco 8034 (35%) (7) 0.21 0.13
Magnet Black (100%) (8) 0.12 0.07
Rhoplex PR-26 (9) 0.81 0.49
Hi-Tad (10) - 40.00
Hi-Tad/Binder - 4.74:1
Pigment/Binder 4/1 4/1
Total Solids 71% 83% I
Writeover Time 18 sec immediate
Viscosity (mPa s) 76 X50,000
at 50 s-1
(1) Titanium dioxide from DuPont, Wilmington,
Delaware contains 94% titanium dioxide and
about 4.5% alumina Particle size 90% <0.6
micrometers, 80%<0.4 micrometers.
(2) Calcium metasilicate acicular particles from
NYCO Minerals Inc., Willsbro, NY.
(3) Extender pigment containing over 97% kaolin
clay and 0.35% sodium polyacrylate/soda ash
- 40 -
CA 02286362 1999-10-O1
WO 98/45378 PCT/US98/06292
dispersant. From Dry Branch Kaolin Co,., Dry
Branch, GA.
(4) Synthetic acrylate/styrene polymer latex
emulsion (62% solids) of methyl methacrylate,
butyl acrylate, styrene, methacrylic acid and
2-hydroxyethyl acrylate. Tg = 9°C from Union
Carbide, Cary, NC.
(5) Synthetic acrylate/styrene polymer latex
emulsion (48% solids) of butylacrylate, styrene
and methacrylic acid. From Union Carbide,
Cary, NC .
(6) Sodium salt of car:boxylate polyelectrolyte from
Henkel Corp., Ambler, PA.
(7) Mixture of mineral oil derivatives and silica
defoaming agent from Henkel Corp., Ambler, PA.
(8) Carbon black.
(9) Cationic non-film :forming acrylic polymer
antibleed agent. From Rohm & Haas, PA.
(10) Bisphenol A propyl dimethacrylate.
Example 2
The irradiated fluid of Example 1 (50 parts) was
mixed with a compatible film-farming latex fluid such as
Latex II (SO parts) and a drop of the resulting correction
fluid mixture was applied to paper. Upon spreading the
correction fluid with a 50 micrometer Bird bar, it
immediately set to form a dimensionally stabilised
relatively solid film on the paper. The film had excellent
opacity and good flexibility, a.nd could accept writeover
immediately. A sample was examined under an SEM and
photographed (Fig. 5). Fig. 5 shows the surface of a film
formed from a correction fluid of the invention. The
significantly larger particles (3) are the composite
material particles formed by the polymerization. These
- 41 -
CA 02286362 1999-10-O1
WO 98/45378 PCT/US98/06292
composite material particles and the titanium dioxide
particles (1) are bound together by the film-forming binder
particles (2). The even distribution of particles is
evident as is the close packing.
We have found that, in the correction fluids of the
invention, the composite particles, the opacifier and the
film-forming binder particles are extremely well dispersed.
This is evidenced by the fact that, even when subjected to
very high shear (200,OOOs-1), surprisingly they do not form
the expected Hofman bodies from breakdown of the dispersion.
Because the particles are well dispersed and because there
is a bimodal size distribution and the composite particles
and film-forming binder particles are generally spherical,
the effect of shear from the Bird bar (about 2000s-1) is to
cause the particles to close pack efficiently which then
results in coalescence of adjacent film-forming particles
(both free and on the surfaces of composite particles) to
form a quickly set relatively solid film.
The constitution and properties of the correction
fluid were as shown in Table II:
TABLE II
Example 2
Ingredient Irradiated Latex II/Hi-Tad
(solid %) (60:40} and Latex II in weight
ratio (50:50) (weight %)
Water 12.48
Ti PURE 8900 (100% 3799
NYAD 400 (100%) 3.00
~Kaopaque lOS 4.00
UCAR 466 (62%) 9-07
UCAR 144 (48%) 11.71
Nopcosperse 44 (35%) 0.85
- 42 -
CA 02286362 1999-10-O1
WO 98/45378 PCT/US98/06292
Example 2
Ingredient Irradiated Latex II/Hi-Tad
(solid %) (60:40) and Latex II in weight
ratio (50:50) (weight %)
Water 12.48
Nopco 8034 (35%) 0.17
Magnet Black (100%) 0.10
Rhoplex PR-26 0.68
Hi-Tad 20.00
Hi-Tad/Binder 1.8:1
Pigment/Binder 4/1
Total Solid ' 77.3%
Writeover Time (sec) 8 sec
Viscosity at 50 s-1 (mPa s) 364
Example 3 to 6
Example 1 was repeated but using different
proportions of Latex II and Hi-".Cad monomer. At 50:50
(Example 3), the irradiated product was solid. At 52:48
(Latex: Hi-Tad) (Example 4), the. product was a fluid having
properties very similar to those: of the irradiated product
of Example 1. With increasing proportions of Latex II the
resulting fluid was less viscous; and could be spread more
readily, but still stabilized dimensionally (solidified)
immediately on being subjected t:o shear. Over about 70:30
(Example 5), the writeover time began to rise.
The constitution and properties of the formulations
were as shown in Table III:
- 43 -
CA 02286362 1999-10-O1
WO 98/45378 PCT/US98/06292
TABLE III
Irradiated Latex II/Hi-Tad in weight ratios:
Example 3 Example 4 Example 5 Example 6
Ingredient 50:50 52:48 70:30 80:20
(solid %) (weight %) (weight %) (weight %) (weight %)
Water 7.80 8.11 10.92 12.48
Ti PURE 8900 23.74 24.69 33.23 37.98
(100%)
NYAD 400 1.875 1.95 2.62 3.00
(100%)
Kaopaque IOS 2.50 2.60 3.50 4.00
UCAR 446 5.67 5.89 7.94 9.07
(62%)
UCAR. 144 7.32 7.61 10.25 11.71
(48%)
Nopcosperse 0.535 0.556 0.75 0.856
44 (35%)
Nopco 8034 0.105 0.109 0.15 0.168
(35%)
Magnet Black 0.06 0.062 0.084 0.096
(100%)
Rhoplex PR-26 0.405 0.421 0.567 0.648
Hi-Tad 50 48 30 20
Hi-Tad/Binder 7.1:1 6.6:1 3:1 1.8:1
Pigment/ 4/1 4/1 4/1 4/1
Binder
Total Solid 85.5% 85.3% 80.1'% 77.3%~
Writeover solid immediate immediate 5-l0 sec
Time stick
Viscosity solid >50,000 --- ---
(mPa s) stick
- 44 -
CA 02286362 1999-10-O1
WO 98J45378 PCT/US98/06292
Examples 7 to 10
Example 2 was repeated using various proportions of
irradiated fluid and Latex II. Satisfactory correction
fluids were obtained with increasing amounts of Latex II up
to proportions of about 80:20 (Latex II: irradiated fluid)
(Example 9). With increasing amounts of Latex II, whilst
the fluids dimensionally stabilized sufficiently to
apparently set to a solid on the application of shear, the
material tended to be softer and to require a few seconds
before accepting writeover. The constitution and properties
of the formulations were as shown in Table IV.
In these Examples, it was found that with increasing
proportions of Latex II, films spread using a Bird bar
became of reduced thickness so that, although the films
could be overwritten very quickly, their opacity was poorer.
It was therefore impossible to make a fair comparison of
writeover time versus composition. Accordingly, the data in
the Table for writeover time were determined by a different
technique. The correction fluid was applied over a mark on
a test sheet using a brush until the mark had been
satisfactorily covered, and then the time was measured until
the correction could be successfully overwritten. For
comparison, the writeover time measured in this way for
Latex 11 was 26 seconds. The 'viscosity of Latex II at 50 s-
1 was 52 mPa s.
_ 4~~ _
CA 02286362 1999-10-O1
WO 98/45378 PCT/US98/06292
TABLE IV
Latex II and irradiated Latex II/Hi-Tad (60:40) in
weight ratio:
Example Example Example Example Example
2 7 8 9 10
Ingredient 50:50 60:40 70:30 80:20 90:10
(solid %)
Water 12.48 13.10 13.73 14.35 14.97
Ti PURE 8900 37.99 39.88 41.78 43.68 45.48
(100%)
NYAD 400 3.00 3.15 3.30 3.45 3.60
(100%)
Kaopaque IOS 4.00 4.20 4.40 4.60 4.80
UCAR 446 9.07 9.52 9.98 10.43 10.88
(62%)
UCAR 144 11.71 12.29 12.88 13.47 14.05
(48%)
Nopcosperse 0.85 0.90 0.94 0.98 1.03
44 (35%)
Nopco 8034 0.17 0.18 0.18 0.19 0.20
(35%)
Magnet Hlack 0.10 0.10 0.10 0.11 0.11
(1000
Rhoplex PR-26 0.68 0.68 0.71 0.74 0.78
Hi-Tad 20 16 12 8 4
Hi-Tad/Binder 1.8/1 1.4/1 1.0/1 .06/1 0.3/1
Pigment/ 4/1 4/1 4/1 4/1 4/1
Binder
Total Solid 77.3 76.2 75.0 73.9 72.8
Writeover 6 8 14 16 20
Time (sec)
Viscosity at 364 249 168 141 101
50 s'1 (mPa s)
- 46 -
CA 02286362 1999-10-O1
WO 98/45378 PCT/US98/06292
Example 11 and 12
Example 1 was repeated using Latex A instead of
Latex II. Latex A is~a non-bleed correction fluid. The
results obtained were essentially the same as in Example 1.
Thus, the irradiated product wars a very viscous 050,000 mPa
s) but stable fluid. When a quantity of the fluid was
spread using a 50 micrometer Bird bar, the fluid was
immediately converted to a smooth dimensionally stabilized
solid opaque coating which could be written over
immediately. A sample of the f:Luid was then mixed with
further Latex A in weight proportions of 50:50 (Example 11).
The resulting fluid could be spread with a 50 micrometer
Bird bar to form immediately a dimensionally stabilized
solid opaque film. The film accepted writeover immediately.
The constitution and properties of Latex A and the
formulations thereof were as shown in Table V.
TAHLE~ V
Example 11 Example 12
Ingredient Latex A Irradiated Latex A and
(Solid %) wt% Latex A: Hi-Tad irradiated
70:30 Latex A: Hi-Tad
(70:30) in wt
ratio 90:10
Water 8.47 5.92 6.18
Hipochem CGH (1) 1.08 0.75 0.79
Worlecryl 8?21 (2) 34.42 24.09 25.12
(30%)
Ti Pure 8931 (3) 37.16 26.01 27.13
Ti Pure 8902 (4) 12.39 8.68 9.05
VINAC XX210 (5) 6.26 4.38 4.57
(55%)
Nopco 8034 (6) 0.20 0.14 0.15
Harshaw Black (7) 0.02 0.01 0.01
- 47 -
CA 02286362 1999-10-O1
WO 98/45378 PCT/US98/06292
Hi-Tad (8) - 30.00 27.00
Hi-Tad/Hinder N/A 3.1/1 2.7/1
Pigment/Binder 3.6/1 3.6/1 3.6/1
Total Solids 64.6% 75.2% 74.2%
Writeover Time 36 4 14
(sec)
(1) Fatty quaternary dispersant from High Point
Chemical Corp., High Point, NC, USA.
(2) From Worlee Chemie GmbH, Hamburg, Germany
(3) Titanium dioxide from DuPont, Wilmington,
Delaware, U.S.A.
(4) Titanium dioxide from DuPont, Wilmington,
Delaware, U.S.A.
(5) Vinyl acetate homopolymer emulsion.
(6) Mineral oil mixed with amorphous silica,
defoaming agent from Henkel Corp., Ambler, PA.
(7) Aurasperse carbon black W-107 from Engelhard
Corp., Iselin, New Jersey.
(8) Bisphenol A propyl dimethacrylate.
Example 13
Latex II (60 parts) was placed in a vessel and
Hi-Tad monomer (40 parts) was slowly added thereto with
gentle mixing using a Dispermat mixer. After addition was
complete the resulting mixture was vigorously mixed using
the Dispermat. Samples of the liquid mixture were sealed in
polyethylene tubes and subjected to electron beam
irradiation of 2 MRad at room temperature. The resulting
viscous fluid behaved similarly to Example 1. When a drop
of the fluid was placed onto paper and spread using a 50
micrometer Bird bar, the fluid immediately formed a smooth
dimensionally stabilized solid opaque coating on the paper.
- 48 -
a
CA 02286362 1999-10-O1
WO 98/45378 PCT/US98/06292
The coating was dry to the touch and could be written over
immediately. However, the coating had little coherence and
was crumbly and easily removed from the paper. When the
fluid was diluted with an equal. weight of Latex II, there
resulted a fluid with comparable performance to that of
Example 2. When a drop of the resulting fluid was applied
to paper and spread with a 50 micrometer Hird bar, it
immediately set to form a solid film on the paper. The film
had excellent opacity and good flexibility, and could accept
writeover immediately.
- 49 -