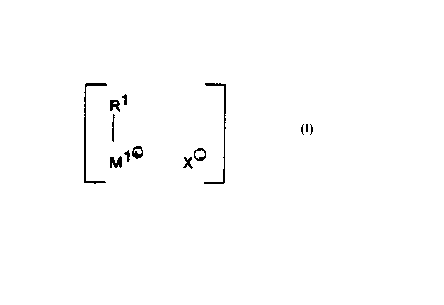Note: Descriptions are shown in the official language in which they were submitted.
CA 02286695 1999-10-08
WO 98/46614 PCT/EP98/02003
Catalyst and use of the catalyst for polymerization
The present invention relates to a novel catalyst and the use of the catalyst
for the polymerization of functionalized and nonfunctionalized cycloolefins.
The vinylic polymerization of norbornene gives a polymer which has a high
glass transition temperature, a high density and a high index of refraction,
cf. Macromol. Chem. Phys 1886, 197, 3435-3453. The introduction of
functional groups on the norbornene skeleton should make it possible to
vary the properties of the polymer.
A polynorbornene which is substituted by an ester group in the 2 position,
for example vinylically polymerized methyl 5-norbornene-2-carboxylate,
displays better solubility properties than unsubstituted polynorbornene
n
c oocH3
where n is a number > 1. In contrast to pol~norbornene, it is readily soluble
in tetrahydrofuran, methyl acetate, acetone and other solvents. The
polymer of the methyl ester is amorphous, has a glass transition
temperature above 250°C and has a higher density than polynorbornene.
Polymer-analogous reactions such as saponification and the preparation of
blends are possible.
The ester can, like many other functionalized norbornene derivatives, be
easily prepared by means of the Diets-Alder reaction. However, it can be
polymerized only very slowly using the palladium(II)-nitrite catalysts used
for the polymerization of norbornene. US-A-3,330,815 describes the
polymerization of various norbornene derivatives using Pd(II) chloro
complexes.
Macromolecules 1996, 29, 2755-2763 discloses ~3-allylpalladium(II) and
palladium(II)-nitrite catalysts for the addition polymerization of norbornene
compounds containing functional groups. Such catalysts produce polymers
CA 02286695 1999-10-08
WO 98/46614 - 2 - PCT/EP98/02003
having a molecular weight which is not high enough for industrial
applications.
It is an object of the invention to provide a novel catalyst for the
polymerization, which has a higher reactivity and produces polymers
having a higher molecular weight, and also a process for preparing
homopolymers and/or copolymers,
The object of the present invention is achieved by a catalyst comprising the
compound of the formula (I):
R1
(I).
M~~ XO
where
R~ is a monocyclic or polycyclic hydrocarbon,
M~+ is a transition metal of group Vlllb,
X is at least one noncoordinating or weakly coordinating anion.
In a preferred embodiment of the invention, the catalyst comprises a
compound of the formula (I) in which
R~ is a monocyclic or polycyclic hydrocarbon having at least one
unsaturated bond within or outside the ring,
M~+ is Rh, Ru, Pd, Co or Ni,
X is BF4_, PFg_, SbFg_, AsFg_, CI04_, BPh4_, where the phenyl groups
may be substituted by fluorine or trifluoromethyl, closo-boranes and
also carboranes and their halogenated derivatives or triflates.
In a particularly preferred embodiment of the invention, the catalyst
comprises a compound of the formula (I) in which
R~ is substituted or unsubstituted norbornene, cyclooctene,
tricyclodecene or exo-methylenecyclohexene,
M~+ is Pd,
X is BF4_, PFg_, SbFg_, AsFg_ or a carborane.
CA 02286695 1999-10-08
WO 98/46614 - 3 - PCT/EP98/02003
In a very particularly preferred embodiment of the invention, the catalyst
comprises a compound of the formula (1) in which
R~ is a compound of the formula (II)
RZ (~~).
where
R2 is a hydrogen atom, a C~-C2p-alkyl group, a Cg-C2p-aryl group,
ORS, SRS, OCOR3, R300CCHCOOR3 or R30CCHCOR3, where
R3 is a hydrogen atom, a C~-C2p-alkyl group or a Cg-C2p-aryl group,
-CN, -SCN, -NR32, N3 or a halogen atom.
According to the invention, the compound of the formula (I) is dissolved in
at least one halogenated hydrocarbon, aliphatic hydrocarbon or aromatic
hydrocarbon which may, if desired, contain a heteroatom such as halogen,
oxycten or nitrogen.
The compound of the formula (I) is preferably dissolved in methylene
chloride, chloroform, nitromethane, dimethylformamide, N-methyl-
pyrrolidone, dimethylethyleneurea, nitrobenzene or chlorobenzene.
The invention provides a compound of the formula (I) and a cycloolefin
which has at least one radical R4, where the ratio of the compound of the
formula (I) to the cycloolefin is from 1:1 to 1:10 and R4 is a COORS, -NC or
COR3 group.
The invention provides a process for preparing the compound of the
formula (I), which comprises reacting
M~+R~HaI with M2+X
in at least one solvent, where
Hal is F , CI , Br , I or a pseudohalogen such as CN or SCN and
M2+ is an alkali metal, an alkaline earth metal, Ag, TI or Cu.
The invention provides a process for preparing the compound of the
formula (I) and a cycloolefin, which comprises initially charging an amount
CA 02286695 1999-10-08
WO 98/46614 - 4 - PCT/EP98/02003
of from 1:1 to 1:10, based on the compound of the formula (I) and reacting
M~+R~HaI with M2+X in at least one solvent.
The invention provides a process for preparing homopolymers and/or
copolymers using at least one compound of the formula (I) as catalyst
system.
Here, the invention provides a process for preparing a homopolymer and/or
copolymer by polymerization of from 0.1 to 100% by weight, based on the
total amount of monomers, of at least one polycyclic olefin of the formula
III, IV, IV', V, VI, VII or VIII
/R5
C ' CH
R'Z.-C R8 (,III)
CH
H/ ~R6
C'CH' NCH
( CH
Rte' C R$ CH
HC CH
CH ~ \CH
2
HC' C~ /CH2
I H
R~ C R$ CH2 (IV')
hiC CH
CH ~ \CH
2
CA 02286695 1999-10-08
WO 98/46614 - 5 - PCT/EP98/02003
./~ C~ ,,~ CH .~, ~ RS
HC C~ CH
RZ~C Rg Rg C R1~ ~ M
H C~ CH
C~ ~ CH~ ~ R6
H 'CH~C~CH-._ CH- CH~CH~RS
8' '~ ( ~1~0.. 1t__ ~ _ R1'2
(~ R C-R ~ R C ~ R C ~ M)
CH ~ CH ~ CH
CH~ ~ C~ ~ Chli ~ R6
R9
CH ~ ~ CH -~.' ~ R5
aC~ CH CH
R7 C RS ~ (Vil)
H CH CH'
CH~ ~.. R6
CH
Rto
R9
'CH ~ ~Chl ~ RS
IH ~CH~
. CH CH
R~- C--Rg ~. ~ R11._ C-R12 ~ (VIII)
CH
CH CH
\ \ ~ R6
C 'CH ~ ~C~
1 10
where R9, R1~, R11, R12, R13, R14, R15 and R16 are identical or different
and are each a hydrogen atom or a hydrocarbon radical, where identical
radicals in the various formulae may have different meanings and the
polycyclic olefins are preferably at least monosubstituted carboxylic acid
CA 02286695 1999-10-08
WO 98/46614 - 6 - PCT/EP98/02003
and/or dicarboxylic acid derivatives, and from 0 to 99.9% by weight, based
on the total amount of monomers, of at least one monocyclic olefin of the
formula IX
CH CH
\ ~ (~X)
(CN2)q
where q is a number from 2 to 10, and from 0 to 99.9% by weight, based
on the total amount of monomers, of at least one acyclic 1-olefin of the
formula X
R13 R15
\ /
C (X)
t8
R14 R
where R~3, R14. R15 and R16 are identical or different and are each a
hydrogen atom or a hydrocarbon radical, preferably a Cg-Cep-aryl radical
or a C1-Cg-alkyl radical.
According to the invention, homopolymers and/or copolymers of
norbornenecarboxylic acid derivatives and norbornenedicarboxylic acid
derivatives are obtained. Here, homopolymers and/or copolymers of the
norbornene substituted by at least one carboxyl, ester, amide or nitrite
group or the norbornenecarboxylic anhydride or -imide are obtained.
Preferred examples of norbornenecarboxylic acid and
norbornenedicarboxylic acid derivatives are:
methyl bicyclo[2.2.1]hept-5-ene-2-carboxylate, ethyl bicyclo[2.2.1]hept-5-
ene-2-carboxylate, bicyclo[2.2.1]hept-5-ene-2-carboxylic acid,
bicyclo[2.2.1]hept-5-ene, methyl 2-methylbicyclo[2.2.1]hept-5-ene-2-
carboxylate, dimethyl bicyclo[2.2.1]hept-5-ene-2,3-dicarboxylate, diethyl
bicyclo[2.2.1]hept-5-ene-2,3-dicarboxylate, bicyclo[2.2.1]hept-5-ene-2,3-
dicarboxylic anhydride, N-p-tolylbicyclo[2.2.1]hept-5-ene-2,3-dicarboximide
and also further aliphatic and aromatic N-substituted norbornene-2,3-
dicarboximides.
The derivatives can also be substituted in the 7 position.
CA 02286695 1999-10-08
WO 98/46614 - 7 - PCT/EP98/02003
The invention provides for the use of a compound of the formula (I) as
catalyst system for preparing homopolymers and/or copolymers.
The invention is illustrated by the following examples.
Examples
General procedures
The analyses were carried out by the methods described below. The NMR
spectra were recorded on a Bruker AC 300 spectrometer at a frequency of -
300 MHz for ~H-NMR and 75 MHz for ~3C-NMR. The IR spectra were
recorded on a Perkin Elmer FT-IR spectrometer 1600. Solids were
measured as pressed KBr disks, liquids were measured between two
sodium chloride plates. The GPC (gel permeation chromatography)
analyses were carried out using a solution of 0.2 g of polymer in 10 ml of
tetrahydrofuran on two 10 mm polymer-mixed-gel columns (600 x 8 mm) of
PSS. A differential refractometer and differential viscornetsr from Knauer
served as detector. A Knauer pump was employed. The inherent
viscosities were determined at a temperature of 25°C on 0.5 percent
strength by weight polymer solutions in dichloromethane. The GC analyses
were carried out on a gas chromatograph model 5890 from Hewlett
Packard. Injector and detector had a temperature of 300°C. The
temperature program employed was: isothermal at 70°C for 4 minutes,
then heating to 280°C at a heating rate of 15°C/min. The
capillary column
HP-5 (crosslinked with 5% of PhMe silicone) from Hewlett Packard having
a film thickness of 25 mm, a length of 30 m and an internal diameter of
0.32 mm was used.
Abbreviations:
VN - viscosity number in cm3/g
GC - gas chromatograph
MW - weight average molar mass in g/mol
MW/M~ - molar mass distribution, determined by gel permeation
chromatography
CA 02286695 1999-10-08
WO 98/46614 - 8 - PCT/EP98/02003
Starting compounds
Example 1
Ethyl bicyclo[2.2.1]hept-5-ene-2-carboxylate (exo/endo=25/75)
In a 1 I three-necked flask, 342.53 g (3.42 mol) of ethyl acrylate were
added to 268 g (4.05 mol) of freshly distilled cyclopentadiene while cooling
in ice. The ice bath was removed and the mixture was stirred for 8 hours
on a water bath at 20°C and then heated at 40°C for 1 hour.
After
distillation (boiling point: 73°C/6 mbar), 454.4 g (80% of theory) of
the ethyl
ester were obtained. GC analysis indicated an exo-endo ratio of 20:80.
Elemental analysis found C 72.27, H 8.59 (calculated for C~pH1402, C
72.26, H 8.49).
~H-NMR (CDCI3): b = 1.2 (4H, m, exo/endo-Ct-13, 1/2 endo-CH2), 1.3
(2H, m, exo/endo-CH2), 1.4 (1 H, m, 1/2 exo-CHZ), 1.8
(1 H, m, 1/2 exo/endo-CH2), 2.1 (1 H, m, exo-CHCCO),
2.8 (2H,- m, exo/endo-CH, endo-CHCOO), 2.9 (1 H, m,
exo-CH), 3.1 (1 H, m, endo-CH), 4.0 (2H, m, exo/endo-
COOCH2), 5.8 (1 H, dd, endo = CH), 6.0 (2H, dd, exo =-
CH), 6.1 (1 H, dd, endo = CH) ppm
13C-NMR (CDCI3): exo: 8 = 14.1 (1 C, s, CH3), 30.1 (1 C, s, CH2), 41.5
(1C, s, (1C, 1s, =CH), 137.8 (1C, 1s, =CH), 175.9 (1C,
1 s, CO) ppm
endo: 8 = 14.1 (1 C, s, CH3), 29.0 (1 C, 1 s, CH2), 2.4
(1 C, 1 s, CH), 43.4 (1 C, 1 s, CHCOO), 45.5 (1 C, 1 s,
CH), 49.4 (1 C, 1 s, CH2) 59.8 (1 C, 1 s, OCHl), 132.2
(1C, 1s, =CH), 137.4 (1C, 1s, =CH), 174.4 (1C, 1s,
CO) ppm
IR (KBr): v = 3061 (m), 2978 (s), 2956 (s), 2903 (m), 2874 (m),
1733 (s), 1447 (m), 1370 (s), 1335 (s), 2171 (s), 1186
(s), 1110 (s), 1039 (s), 862 (m), 838 (m), 712 (s) cm ~
CA 02286695 1999-10-08
WO 98/46614 - 9 - PCT/EP98/02003
Example 2
Ethyl bicylco[2.2.1]hept-5-ene-2-carboxylate (exo/endo=60/40)
In a 200 ml flask, 1.65g (24.2 mmol) of sodium ethoxide were added to
80.51 g (0.485 mol) of ethyl 5-norbornene-2-carboxylate (exo/endo=25/75)
and the mixture was stirred for 20 hours at a temperature of 100°C. The
ester was subsequently distilled under reduced pressure in the presence of
the sodium ethoxide via a 60 cm Vigreux column at a reflux ratio of 1:20.
After the distillation (boiling point found: 86-88°C/16 mbar), 73.3 g
(91 % of
theory) of a colorless liquid were obtained. GC analysis indicated an
exo/endo ratio =20/80=60/40.
Example 3
Methyl bicyclo[2.2.1]hept-5-ene-2-carboxylate (exo/endo=70/30)
In a 1 I three-necked flask, 239.43 g (2.78 mol) of methyl acrylate .were
added to 218.91 g (3.31 mol) of freshly distilled cyclopentadiene while
cooling in ice. The ice bath was removed and the mixture was stirred for 8
hours at 20°C on a water bath and then heated at 40°C for 1
hour. After
distillation (boiling point: 73°C/14 mbar), 377.8 g (89% of theory) of
the
methyl ester were obtained. GC analysis indicated an exo/endo ratio of
20/80.
8.0 g (0.15 mmol) of sodium methoxide were added to 377.8 g (2.48 mol)
of methyl 5-norbornene-2-carboxylate (exo/endo=20/80) and the mixture
was stirred for 20 hours at a temperature of 105°C. The ester was
subsequently distilled under reduced pressure in the presence of the
sodium methoxide via a 100 cm packed column containing rolls of woven
wire mesh at a reflux ratio of 1:100. After the distillation (boiling point
found: 75°C-77°C/19 mbar), 280.9 g (74% of theory) of a
colorless liquid
were obtained. GC analysis indicated an exo/endo ratio of 70/30.
Elemental analysis found C 71.02, H 8.04 (calculated for CgH~202,
C 71.03, H 7.94).
CA 02286695 1999-10-08
WO 98/46614 - 10 - PCTIEP98/02003
Example 4
Bicyclo[2.2.1]heptadienepalladium chloride
In a 200 ml flask, 2.00 g (11.3 mmol) of palladium(II) chloride were
dissolved in 5 ml of concentrated hydrochloric acid by stirring at 50°C
for 1
hour. The solution was then cooled to room temperature. 150 ml of ethanol
were added to the brown solution, the solution was filtered and the residue
was washed with 20 ml of ethanol. 2.5 ml (2.27 g, 25 mmol) of
norbornadiene were added to the filtrate whilst stirring. A yellow precipitate
was formed and this was filtered off after 10 minutes and washed three
times with 30 ml each time of diethyl ether. The product was recrystallized
from dichloromethane. After drying, 2.28 g (77% of theory) of a yellow
crystalline solid were obtained. Elemental analysis found C 31.38, H 2.98
(calculated for C~HgPdCl2, C 31.20, H 2.99).
~ H-NMR (DMSO-d6): b = 1.87 (t, J = 1.6 Hz, 2H, CH2), 3.56 (quip, J =
1.8Hz,2H,CH),6.76(t,J=l.9Hz,4H,CH)ppm
~3C-NMR (DMSO-d6): a = 50.4 (CH), 74.8 (CH2), 143.1 (CH) ppm
IR (KBr): v = 3047 (s), 2956 (w), 2924 (w), 1407 (s), 1304
(s), 1226 (m), 1183 (m), 967 (m~, 939 (m), 898
(m), 828 (m), 792 (s), 769 (s) cm
Example 5
Di-~-chloro-bis(6-methoxybicyclo[2.2.1]hept-2-ene-endo-5a.2n)palla-
dium(II)
In a 100 ml nitrogen flask, 4 ml of methanol were added under nitrogen to
300 mg (1.11 mmol) of bicyclo[2.2.1]heptadienepalladium chloride and
101 mg (0.95 mmol) of sodium carbonate and the mixture was stirred at
room temperature. After one hour, a light-yellow, fine precipitate had
formed and this was filtered off and dried under an oil pump vacuum.
289 mg (98% of theory) of a light-yellow solid were obtained. Elemental
analysis found C 35.88, H 4.27 (calculated for C~gH22Pd2C12, C 36.25, H
4.18).
~ H-NMR (CDCI3): 8 = 6.09 (t, J = 3.87 Hz, =CH), 5.88 (t, J = 3.87
Hz, 1 H =CH), 4.12 (s, 1 H, CH) 3.24 (s, 3H,
CA 02286695 1999-10-08
WO 98/46614 - 11 - PCT/EP98/02003
OCH3), 3.20 (s, 1 H, CH), 2.90 (s, 1 H, CH), 1.87
(s, 1 H, CH), 1.88 (d, J = 10.19 Hz, 1 H, CH2), 1.61
(d, J = 9.85 Hz, 1 H, CH2)
Polymerization
Example 6
All monomers used were dried over CaH2, distilled under reduced pressure
and saturated with nitrogen. The preparation of the catalyst solutions was
carried out under nitrogen. All polymerization experiments were likewise
carried out in a nitrogen atmosphere with exclusion of moisture and
oxygen.
Example 7
Methyl bicyclo[2.2.1]kept-5-ene-2-carboxylate (exolendo=70/30)
Polymerization of the methyl ester using an rl3-allylpalladium(II)
tetrafluoroborate complex at room temperature, [Pdj:[M]=1:70
1n a 100 ml flask, 14.6 mg (0.064 mmol) of r~3-allylpalladium chloride dimer
were dissolved in 2 ml of dichloromethane. 24.0 mg (0.124 mmol) of
AgBF4 were added thereto and the mixture was stirred for 20 minutes at
room temperature. The yellow catalyst solution was filtered and the filtrate
was added to 0.6 ml (0.66 g, 4.3 mmol) of methyl 5-norbornene-2-
carboxylate. The reaction mixture was stirred at room temperature for 5
days. The polymer was precipitated from methanol, filtered off, washed
with methanol and dried at 100°C for 8 hours. The yield was 647 mg (98%
of theory). The polymer had the following properties:
Mn(GPC)=11,600, M~",(GPC)=22,100.
Example 8
Polymerization at room temperature, [Pdj:[Mj=1:250
In a 100 ml flask, 13.7 mg (0.0377 mmol) of r~3-allylpalladium chloride
dimer were dissolved in 2 ml of dichloromethane. 16.3 mg (0.0841 mmol)
CA 02286695 1999-10-08
WO 98/46614 - 12 - PCT/EP98/02003
of AgBF4 were added to the solution and the mixture was stirred at room
temperature for 20 minutes, forming a white precipitate. The yellow catalyst
solution was filtered and the filtrate was added to a solution of 2.56 g
(16.8 mmol) of methyl 5-norbornene-2-carboxylate in 8 ml of
dichloromethane. The reaction mixture was stirred at room temperature for
4 days. The polymer was precipitated from methanol, filtered off, washed
with methanol and dried at 100°C for 8 hours. 1.82 g (71 % of theory)
of a
white solid were obtained. The polymer had the following properties:
M~(GPC)=7200, MW(GPC)=79,000.
Example 9
Polymerization of the methyl ester using di-~-chloro-bis(6
methoxybicyclo[2.2.1]hept-2-ene-endo-5a,2n)palladium(II) at room
temperature, [Pd]:[M]=1:270
In a 100 ml nitrogen flask, 6.7 mg (0.015 mmol) of di-N-chloro-bis(6-
methoxybicyclo[2.2.1]hept-2-ene-endo-5a,2n)palladium(II) were dissolved
in 4 ml of dichloromethane. The solution was added to 1.09 g (7.17 mmol)
of methyl 5-norbornene-2-carboxylate and the mixture was stirred at room
øemperature for 5 days. The polymer was precipitated from methanol,
filtered off, washed with methanol and dried at 100°G for 8 hours. The
yield
was 0.43 g (40% of theory). The polymer had the following properties:
Mn(GPC)=4600 M""(GPC)=7800
Example 10
Polymerization of the methyl ester using di-~-chloro-bis(6-methoxy-
bicyclo[2.2.1]hept-2-ene-endo-5a,2n)palladium(II) and NaAsFg, at room
temperature, [Pd]:[M]=1:250
In a 100 ml flask, 6.5 mg (0.012 mmol of di-~-chloro-bis(6-
methoxybicyclo[2.2.1]hept-2-ene-endo-5a,2~)palladium(II) were dissolved
in 2 ml of dichloromethane. 11.2 mg (0.033 mmol) of NaAsFg were added
thereto and the mixture was stirred for 30 minutes at room temperature.
The yellow catalyst solution was filtered and the filtrate was added to 1.1 g
(7.2 mmol) of methyl 5-norbornene-2-carboxylate. The polymerization
solution became orange after half an hour. It was stirred at room
CA 02286695 1999-10-08
WO 98/46614 - 13 - PCT/EP98/02003
temperature for 20 hours. The polymer was precipitated in methanol,
filtered off, washed and dried at 100°C for 8 hours. 0.23 g (21 % of
theory)
of a white solid was obtained.
Example 11
Polymerization of the methyl ester (endo/exo=50150) using a water-
containing Pd catalyst, [Pd]:[M]=1:250
To prepare the catalyst solution, 318 mg (2.72 mmol) of NOBF4 were
added to a mixture of 143.9 mg (1.35 mmol) of Pd(0) powder, 25 ml of
nitromethane and 0.15 ml (150 mg, 8.3 mmol) of water in a 100 ml flask.
The gas formed was removed by evacuation. The solution was initially
yellow, then it became green and finally dark red. In a 100 ml flask, 2.19 g
(14.1 mmol) of methyl bicyclo[2.2.1]hept-5-ene-2-carboxylate were
dissolved in 10 ml of nitromethane. 1.0 ml of the catalyst solution was
added thereto. After a reaction tirne of 4 days, the mixture was precipitated
in methanol, the precipitate was filtered off, washed with methanol and
dried at 100°C for 8 hours. The yield was 1.77 g (81 % of theory). The
polymer had the following properties: M~(GPC)=17,000, MW(GPC)=24,000
Example 12
Polymerization of the methyl ester (endo/exo=70/30) using a 6-methoxy-
bicyclo[2.2.1]hept-2-ene-endo-56,2~)palladium(II) tetrafluoroborate
complex at room temperature, [Pd]:[M]=1:220
Preparation of the catalyst
In a 100 ml flask, 6.9 mg (0.013 mmol) of di-~-chloro-bis(6-
methoxybicyclo[2.2.1]hept-2-ene-endo-5a,2n)palladium(II) were dissolved
in 4 ml of dichloromethane. 8.0 mg (0.041 mmol) of AgBF4 were added
thereto and the mixture was stirred for 5 minutes at room temperature. The
yellow catalyst solution was filtered.
~ H-NMR (CDCI3): s = 6.33 (s, 1 H, =CH), 6.15 (s, 1 H, =CH), 4.12 (s,
1 H, CH), 3.36 (d, J = 4.18 Hz, 1 H, CH), 3.29 (s,
3H, OCH3), 3.04 (s, 1 H, CH), 2.95 (S, 1 H, CH),
1.96 (d, J =9.81 Hz, 1 H, CH2), 1.73 (d, J =9.61,
Hz, 1 H, CH2) ppm
CA 02286695 1999-10-08
WO 98/46614 - 14 - PCT/EP98/02003
Polymerization
The filtrate was added to 0.80 ml (0.88 g, 5.8 mmol) of methyl 5-
norbornene-2-carboxylate. The polmerization solution was stirred at room
temperature for 5 days. The polymer was precipitated in methanol, filtered
off, washed and dried at 100°C for 8 hours. 0.84 g (96% of theory) of a
white solid was obtained. The polymer had the following properties:
Mn(GPC)=102,000, M"i,(GPC)=131,000, r~inh=0.366 dl/g, [r~]=0.374 dl/g,
25°C, CH2C12.
Elemental analysis calc. for (CgH~202)g71: C 71.03% H 7.95%
found: C 70.67% H 7.98&
~ H-NMR (CDCI3): 8 = 0.9 - 3.2 (m, CH, CH2, maxima at 1.5, 1.8 and
2.3), 3.6 (m, COOCH3) ppm
~3C-NMR (CDCI3): 8 = 30 - 60 (m, CH, CH2, maxima at 34.1, 37.2,
39.2, 42.3, 45.2, 46.3 and 51.5) 176.1 (m, CO)
ppm
IR (KBr): v = 2953 (s), 2883 (m), 1732 (s), 1435 (s), 1361
(m), 1197 (s), 1173 (s), 1042 (m) cm
Example 13
Polymerization at room temperature, [Pd]:[M]=1:500
In a 100 ml flask, 20.0 mg (0.038 mmol) of di-~-chloro-bis-(6-
methoxybicyclo[2.2.1]hept-2-ene-endo-5a,2~)palladium(II) were dissolved
in 2 ml of dichloromethane. 21.9 mg (0.113 mmol) of AgBF4 were added
thereto and the mixture was stirred for 5 minutes at room temperature. The
yellow catalyst solution was filtered and the filtrate was added to a solution
of 6.32 g (42 mmol) of methyl 5-norbornene-2-carboxylate in 10 ml of
dichloromethane. 2 ml of this polymerization solution were removed after
0.5, 1, 2, 3.5 and 5.5 hours and the remainder of the solution was taken
after 8 hours, and these samples were precipitated in methanol, filtered off,
washed and dried at 100°C for 8 hours. The yield was 5% after 0.5 h, 5%
after 1 h, 17.4% after 2 h, 27% after 3.5 h, 41.8% after 5.5 h and 52.9%
after 8 h. The polymer had the following properties: Mn(GPC)=93,000
M~,(GPC)=130,000 after a polymerization time of 8 hours.
CA 02286695 1999-10-08
WO 98/46614 - 15 - PCT/EP98/02003
Example 14
Polymerization at room temperature, [Pd]:[M]=1:1000
In a 100 ml flask, 8.3 mg (0.0157 mmol) of di-N-chloro-bis(6-
methoxybicyclo[2.2.1]hept-2-ene-endo-56,2~)palladium(II) were dissolved
in 2.2 ml of dichloromethane. 9.2 mg (0.047 mmol) of AgBF4 were added
thereto and the mixture was stirred for 5 minutes at room temperature. The
yellow catalyst solution was filtered and added to a solution of 5.09 g
(33 mmol) of methyl 5-norbornene-2-carboxylate in 8 ml of chlorobenzene.
The polymerization solution was stirred at room temperature for 20 hours.
The polymer was precipitated in methanol, filtered off, washed and dried at
100°C for 8 hours. 1.21 g (24% of theory) of a white solid were
obtained.
The polymer had the following properties: M~(GPC)=95,000
MW(GPC)=203,000.
Example 15
Polymerization at 40°C, [Pd]:[M]=1:500
In a 100 ml flask, 4.4 mg (0.0083 mmol) of di-N-chloro-bis(6-
methoxybicyclo[2.2.1]hept-2-ene-endo-5a,2~)palladium(II) were dissolved
in 2 ml of dichloromethane. 110 mg (0.72 mmol) of methyl 5-norbornene-2-
carboxylate and 4.7 mg (0.024 mmol) of AgBF4 were added thereto. The
mixture was stirred at room temperature for 3 minutes, filtered and the
filtrate was added to 1.29 g (8.5 mmol) of methyl 5-norbornene-2-
carboxylate. The polymerization solution was stirred at room temperature
for half an hour. The polymer was precipitated in methanol, filtered off,
washed and dried at 100°C for 8 hours. 0.70 g (54% of theory) of a
white
solid was obtained. The polymer had the following properties:
Mn(GPC)=65,000 MN,(GPC)=98,000.
Example 16
Polymerization at 40°C, [Pd]:[M]=1:1000
In a 100 ml flask, 24.0 mg (0.0455 mmol) of di-N-chloro-bis(6-
methoxybicyclo[2.2.1]hept-2-ene-endo-5a,2~)palladium(II) were dissolved
CA 02286695 1999-10-08
WO 98/46614 - 16 - PCT/EP98/02003
in 2.4 ml of chlorobenzene. 26.5 mg (0.137 mmol) of AgBF4 were added
thereto and the mixture was stirred at room temperature for 3 minutes. The
yellow catalyst solution was filtered and the 0.33 ml of filtrate was added to
a solution of 2.2 g (14 mmol) of methyl 5-norbornene-2-carboxylate in 4 ml
of chlorobenzene. The polymerization solution was stirred at a temperature
of 40°C for 3 hours. The polymer was precipitated in methanol, filtered
off,
washed and dried at 100°C for 8 hours. 194.8 mg (8.9% of theory) of a
white solid were obtained. The polymer had the following properties:
Mn(GPC)=64,000 M""(GPC)=91,000
Example 17
Polymerization at 60°C, [Pd]:[M]=1:500
in a '100 ml flask, 24.0 mg (0.0455 mmol) of di-N-chloro-bis(6-
methoxybicyclo[2.2.1]hept-ene-endo-5a,2n)palladium(II) were dissolved in
2.4 ml of chlorobenzene. 26.5 mg (0.137 mmol) of AgBF4 were added
thereto and the mixture was stirred at room temperature for 3 minutes. The
yellow catalyst solution was filtered and 0.67 ml of the filtrate was added to
a solution of 2.2 g (14 mmol) of methyl 5-norbornene-2-carboxylate in
3.6 ml of chlorobenzene. The yellow polymerization solution was stirred at
a temperature of 60°C 'for half an hour. The polymer was precipitated
in
methanol, filtered off, washed and dried at 100°C for 8 hours. 251.6 mg
(11.4% of theory) of a white solid were obtained. The polymer had the
following properties: M~(GPC)=18,000 MW(GPC)=3600
Example 18
Polymerization of the methyl ester (endo/exo=70/30) using a 6-methoxy
bicyclo[2.2.1]hept-2-ene-endo-5a,2~)palladium(II) hexafluoroantimonate
complex
Polymerization at room temperature, [Pd]:[M]=1:600
In a 100 ml flask, 20.0 mg (0.038 mmol) of di-N-chloro-bis(6-methoxy-
bicyclo[2.2.1]hept-2-ene-endo-5a,2~)palladium(II) were dissolved in 2 ml of
dichloromethane. 38.7 mg (0.113 mmol) of AgSbFg were added thereto
and the mixture was stirred at room temperature for 3 minutes. The
CA 02286695 1999-10-08
WO 98/46614 - 17 - PCT/EP98/02003
orange-brown colored solution was filtered and 0.66 ml of the filtrate was
added to a solution of 2.28 g (15 mmol) of methyl 5-norbornene-2-
carboxylate in 3.3 ml of dichloromethane. The yellow polymerization
solution was stirred at room temperature for 5 days. The solution became
green after 10 minutes. The polymer was precipitated in methanol, filtered
off, washed and dried at 100°C for 8 hours. 0.43 g (18.8% of theory) of
a
white solid was obtained. The polymer had the following properties:
M~(GPC)=82,000 MW(GPC)=101,000.
Example 19
Homopolymerization of ethyl bicyclo[2.2.1]hept-5-ene-2-carboxylate using
a 6-methoxybicyclo[2.2.1]hept-2-ene-endo-5a,2~)palladium(II) tetrafluoro-
borate complex, (endo/exo=60/40), [Pd]:[M]=1:500
In a 100 ml flask, 8.9 mg (0.046 mmol) of AgBF4 were added to a solution
of 8.8 mg (0.017 mmol) of di-N-chloro-bis(6-methoxybicyclo[2.2.1]hept-2-
ene-endo-5a,2~)palladium(II) in 2.4 ml of dichloromethane and the mixture
was stirred at room temperature for 3 minutes. The yellow catalyst solution .
was filtered through a Millipore filter and the filtrate was added to a
solution
o. 3.'16 g (19.0 mmol) of ethyl 5-norbornene-2-carboxylata in 3.2 ml of
dichloromethane. The polymerization mixture was stirred at room
temperature for 8 hours. The polymer was precipitated from methanol,
filtered off and washed with methanol. The product was dried at 100°C
for
8 hours. 1.49 g (47% of theory) of polymer were obtained. Tlie polymer
had the following properties: Mn(GPC)=12,700 MW(GPC)=37,300.
Example 20
Polymerization of the ethyl ester (endo/exo=25/75), [Pd]:[M]=1:550
In a 100 ml flask, 10.5 mg (0.054 mmol) of AgBF4 were added to a solution
of 9.6 mg (0.018 mmol) of di-N-chloro-bis(6-methoxybicyclo[2.2.1]hept-2-
en-endo-5a,2~)palladium(II) in 2.6 ml of dichloromethane and the mixture
was stirred at room temperature for 3 minutes. The yellow catalyst solution
was filtered through a Millipore filter and the filtrate was added to 3.34 g
(22.0 mmol) of ethyl 5-norbornene-2-carboxylate in 3.6 ml of
dichloromethane. The yellow polymerization mixture was stirred at room
temperature for 20 hours. The polymer was precipitated from methanol,
CA 02286695 1999-10-08
WO 98/46614 - 18 - PCT/EP98/02003
filtered off and washed with methanol. The product was dried at 100°C
for
8 hours. 0.47 g (14% of theory) of polymer was obtained. The polymer had
the following properties: Mn(GPC)=9400 MW(GPC)=11,600.
Example 21
Polymerization of the ethyl ester (endo/exo = 60/40), [Pd]:[M]=1:500
In a 100 ml flask, 8.9 mg (0.046 mmol) of AgBF4 were added to a solution
of 8.8 mg (0.017 mmol) of di-N-chloro-bis(6-methoxybicyclo[2.2.1]hept-2-
ene-endo-5a,2~)palladium(II) in 2.4 ml of dichloromethane and the mixture
was stirred at room temperature for 3 minutes. The yellow catalyst solution
was filtered through a Millipore filter and the filtrate was added to a
solution
of 3.16 g (19.0 mmol) of ethyl 5-norbornene-2-carboxylate in 3.2 ml of
dichloromethane. The polymerization mixture was stirred at room
temperature for 8 hours. The polymer was precipitated from methanol,
filtered off and washed with methanol. The product was dried at 100°C
for
8 hours. 1.49 g (47% of theory) of polymer were obtained.
M~(GCP)=12,700 M""(GCP)=37,300
Elemental analysis calc. for (C~pH1402)76~ C 72.75% H 8.49%
found: C 71.80% H 8.59%
~H-NMR (CDCI3): 8 = 1.1-2.9 (m, CH, CH2, CH3; maxima at 1.2, 1.6 and
2.3), 4.1 (m, COOCH2) ppm
~3C-NMR (CDCI3): 8 = 14.2 (s, CH3), 30-60 (m, CH, CH2, maxima at 33.9,
36.8, 38.8, 42.2, 45.5, 46.9 and 50.7), 60.2 (s, OCH2),
175.7 (m, CO) ppm
IR (KBr): v = 2976 (s). 2954 (s), 2904 (s), 2878 (s), 1728 (s),
1449 (m), 1371 (m), 1347 (m), 1300 (m), '1182 (s),
'1043 (m) cm
Example 22
Homopolymerization of bicyclo[2.2.1]hept-5-ene using a 6-methoxy-
bicyclo[2.2.1]hept-2-ene-endo-5a,2~)palladium(II) tetrafluoroborate
complex, at room temperature, [Pd]:[M]=1:600
In a 100 ml flask, 24.0 mg (0.0455 mmol) of di-N-chloro-bis(6-methoxy-
bicyclo[2.2.1]hept-2-ene-endo-5a,2n)palladium(II) were dissolved in 2.4 ml
CA 02286695 1999-10-08
WO 98/46614 - 19 - PCT/EP98/02003
of chlorobenzene. 26.5 mg (0.137 mmol) of AgBF4 were added thereto and
the mixture was stirred at room temperature for 3 min. The yellow catalyst
solution was filtered and the 0.63 ml of filtrate was added to 1.35 g
(0.014 mol) of norbornene. The polymerization solution was stirred at room
temperature for 1 hour. The polymer was precipitated in methanol, filtered
off, washed and dried at 100°C for 8 hours. 20 mg (1.5% of theory) of a
white solid were obtained.