Note: Descriptions are shown in the official language in which they were submitted.
CA 02287648 1999-10-26
-1- 1033
AMORPHOUS METAL/METALLIC GLASS
ELECTRODES FOR ELECTROCHEMICAL PROCESSES
FIELD OF THE INVENTION
This invention relates to an improved electrode material for use in
electrochemical processes and particularly an amorphous metal/metallic glass
electrode
material intended for constituting the active surface of an electrode for use
in the
electrolysis of aqueous solutions and more particularly in the electrochemical
production of oxygen and hydrogen by said electrolysis.
BACKGROUND OF THE INVENTION
In electrolytic cells for the production of hydrogen and oxygen, such as those
of the bipolar and unipolar type, an aqueous caustic solution is electrolyzed
to produce
oxygen at the anode and hydrogen at the cathode with the overall reaction
being the
decomposition of water to yield hydrogen and oxygen. The products of the
electrolysis
are maintained separate by use of a membrane/separator. Use of amorphous
metalslmetallic glasses and nanocrystalline materials, as electrocatalysts for
the
hydrogen and oxygen evolution reaction are known. The terms "amorphous metal"
or
"metallic glass" are well understood in the art and define a material which
contains no
long range structural order but can contain short ratige structure and
chemical ordering.
Henceforth, in this specification and claims both terms will be used as being
synonymous and are interchangeable. The term "nanocrystalline" refers to a
material
that possesses a crystallite grain size of the order of a few nanometers; i.e.
the
crystalline components have a grain size of less than about 10 nanometers.
Further, the
term "metallic glass" embraces such nanocrystalline materials in this
specification and
claims.
In an electrolysis application, not all of the voltage that is passed through
the
cell during electrolysis is utilized in the production of hydrogen and oxygen.
This loss
CA 02287648 1999-10-26
-2- 1033
of efficiency of the cell is often referred to as the cell overpotential
required to allow the
reaction to proceed at the desired rate and is in excess of the reversible
thermodynamic
decomposition voltage. This cell overpotential can arise from: (i) reactions
occurring at
either the cathode or the anode, (ii) a potential drop because of the solution
ohmic drop
between the two electrodes, or (ifi) a potential drop due to the presence of a
membrane
/ separator material placed between the anode and cathode. The latter two
efficiencies
are fixed by the nature of the cell design while (i) is directly a result of
the activity of the
electrode material employed in the cell including any activation or pre-
treatment steps.
Performance of an electrode is then directly related to the overpotential
observed at
both the anode and cathode through measurement of the Tafel slope and the
exchange
current density (hereinafter explained).
Superior electrode performance for the electrolysis of water may be achieved
by the use of addition of metal salts to the electrolyte as "homogeneous"
catalysts that
function only in the liquid phase. These "homogeneous" catalysts suffer from
the
difficulty of having to add these additions to an operating cell to be
functional, along
with the toxicity of the metal salts in powder form and the disposal of
electrolyte
containing these additions. A desirable alternative would then be a base alloy
comprised
of Ni, and one or more of these metallic salt constituents which would still
provide the
same operating characteristics of a low voltage, high current cell behaviour
corresponding to the evolution of hydrogen or oxygen while being
electrochemically
stable in the alkaline solution.
United States Patent No. 5,429,725, issued July 04, 1995 to Thorpe, S.J. and
Kirk, D.W. describes the improved electrocatalytic behaviour of alloys made by
combinations of the two elements Mo and Co in a Ni-base metallic glass.
However, this is still a need for higher exchange current densities combined
with lower Tafel slopes in the (Cr, V)- containing alloys compared with the Mo-
containing alloys and, accordingly, a need for enhanced operating efficiency
of
electrocatalyst material for alkaline water electrolysis
CA 02287648 2005-07-08
'. '
-3- 1033
REF'ERENCE LIST
The present specification refers to the following publications,
PUBLICATIONS:
1. Lian, K. Kirk, D.W. and Thorpe, S.J., "Electrocatalytic Behaviour of Ni-
base
Amorphous Alloys", Electrochim. Acta, 36, p. 537-545, (1991)
2. Kreysa, G. and Hakansson, "Electrocatalysis by Amorphous Metals of
Hydrogen and Oxygen Evolution in Alkaline Solution", J. Electroanal. Chem.,
201, p. 61-83, (1986).
3. Podesta, J.J., Piatti, R.C.V., Arvia, A.J., Ekdunge, P., Juttner, K. and
Kreysa,
G., "The Behaviour of Ni-Co-P base Amorphous Alloys for Water Electrolysis
in Strongly Alkaline Solutions Prepared through Electroless Deposition", Int.
J.
Hydrogen Energy, 17, p. 9- 22, (1992).
4. Alemu, H. and Juttner, K., "Characterization of the Electrocatalytic
Properties
of Amorphous Metals for Oxygen and Hydrogen Evolution by Impedance
Measurements", Electrochim. Acta., 33, p. 1101-1109, (1988).
5. Huot, J.-Y., Trudeau, M., Brossard, L. and Schultz, R. "Electrochemical and
Electrocatalytic Behaviour of an Iron Base Amorphous Alloy in Alkaline
Solution at 70 C", J. Electrochem. Soc., 136, p. 2224-2230, (1989).
6. Vracar, Lj., and Conway, B.E., "Temperature Dependence of Electrocatalytic
Behaviour of Some Glassy Transition Metal Alloys for Cathodic Hydrogen
Evolution in Water Electrolysis", Int. J. Hydrogen Energy, 15, p. 701-713
(1990).
7. Wilde, B.E., Manohar, M., Chattoraj, I., Diegle, R.B. and Hays, A.K., "The
Effect of Amorphous Nickel Phosphorous Alloy Layers on the Absorption of
Hydrogen into Steel", Proc. Symp. Corrosion, Electrochemistry and Catalysis
of Metallic Glasses, 88-1, Ed. R.B. Diegle and K. Hashimoto, Electrochemical
Society, Pennington, p. 289-307 (1988).
8. Divisek, J., Schmitz, H. and Balej, "Ni and Mo Coatings as Hydrogen
Cathodes", J. Appl. Electrochem., 19, p. 519-530, (1989).
9. Huot, J.-Y. and Brossard, L., "In-situ Activation of Nickel Cathodes by
Sodium Molybdate during Alkaline Water Electrolysis at Constant Current", J.
Appl. Electrochem., 20, p. 281, (1990).
10. Huot, J.-Y. and Brossard, "In-situ Activation of Nickel Cathodes during
Alkaline Water Electrolysis by Dissolved Iron and Molybdenum Species", J.
Appl. Electrochem., 21, p. 508, (1991).
11. Raj, I.A. and Vasu, K.I., "Transition Metal-based Hydrogen Electrodes in
Alkaline Solution- Electrocatalysis on Nickel-based Binary Alloy Coatings",
Int. J. Hydrogen Energy, 20, p. 32, (1990).
12. Jaksic, M.M., Johansen, B., and Ristic, M., "Electrocatalytic In-situ
Activation
of Noble Metals for Hydrogen Evolution" in Hydrogen En= ProgLress VII I,
CA 02287648 1999-10-26
-4- 1033
T.N. Veziroglu and P.K. Takahashi, Eds., Pergamon Press, NY, p. 461,
(1990).
SITMMARY OF THE INVENTION
It is an object of this invention to provide an improved electrode having an
electrochemically active surface that can be used for the electrolysis of
water.
It is a further object of this invention to provide an improved electrode that
is
chemically stable in an alkaline environment for both static and dynamic
cycling
operations of the cell.
It is a further object of the present invention to provide an improved
electrode
material that is sufficiently active so as to reduce either or both the anodic
overpotential
for oxygen evolution or the cathodic overpotential for hydrogen evolution.
It is a further object to provide an electrode that contains relatively
inexpensive
elemental constituents compared to the platinum group metals.
It is a further object to provide an electrode whose total processing
operations
necessary to final electrode fabrication are minimized in comparison to
conventional
electrode materials.
It is a further object to provide an electrode which can be operated at
elevated
temperatures in an alkaline environment to provide enhanced performance since
the
overpotential required to produce either hydrogen or oxygen is reduced as the
operational temperature of the cell is increased.
Accordingly, the invention provides in one aspect a metallic glass of use in
electrochemical processes, said metallic glass consisting essentially of a
material of the
general nominal composition
(Ni,Co)loo-x_t Ax Zt
wherein:
A is a member selected from the group consisting of IVb, Vb, VIb VIIb and
VIII of the Periodic Table;
Z is a member selected from the group consisting of carbon and a metalloid
element selected from group IIIa, IVa, Va and VIa of the Periodic Table; and
wherein
CA 02287648 1999-10-26
-5- 1033
x, t and (100-x-t) are atomic percents.
Preferably, A is at least one metal selected from the group consisting of Ti,
V,
Cr, Mn, Fe, Zr, Nb, Mo, Tc, Hg, Ta, and W; and wherein x is selected from
about 1 to
20 atomic percent, more preferably x is selected from about 1-5 atomic
percent.
Preferably, Z is at least one member selected from the group consisting of
silicon, phosphorus, carbon, and boron; and wherein t is selected from about
15 to
25 atomic percent, more preferably t is about 20 atomic percent.
The metallic glass is most preferably in an elemental and homogenous state but
some degree of non-homogeneity in both anionic and cationic form can be
tolerated.
It will be understood that the general formula defined hereinabove represents
a
nominal composition and thus allows of some degree of variance from the exact
atoniic
ratios shown.
Preferred materials according to the invention have the nominal compositions
selected from NiSOCo25Cr5B2D,NiS0Co25V5B20 and Ni45Co25Cr5V5B20.
The alloys of the present invention are readily made into self-supporting
structures.
In a further aspect, the invention provides an electrode of use in an
electrochemical cell comprising a metallic glass consisting of a material as
hereinabove
deflned. The electrode may act as an anode, cathode or both as a working
electrode.
The materials of the invention may constitute a full electrode or a surface
coating on a
substrate such as a metal or other electrically conductive material.
In a yet further aspect, the invention provides an improved process for the
electrochemical production of oxygen and hydrogen from an aqueous solution in
an
electrochemical cell, said process comprising electrolysing said aqueous
solution with
electrodes, said improvement comprising one or more of said electrodes
comprising a
metallic glass consisting essentially of a material as hereinabove defined.
In the electrolytic production of oxygen and hydrogen, the aqueous solution is
alkaline.
Surprisingly, the metallic glasses according to the invention do not suffer
from
the loss of element "A" during use and retain electrolytic activity under
severe
CA 02287648 1999-10-26
-6- 1033
conditions of use. Thus, we have found that the presence of element "A" in the
alloys
of the invention, while providing the unexpected advantages hereindescribed,
surprisingly, does not result in dissolution of the element "A" under alkaline
electrolysis
conditions.
Thus, the invention provides a metallic glass / amorphous metal electrode
material for electrochemical processes produced by rapid solidification (i)
having a
structure that is either amorphous or nanocrystalline, (n) containing the
principal
alloying elements as Ni and Co, (iii) containing alloying additions such as
Cr, V, Ti,
Mn, Fe and the like in the range of 1 to 20 at. %, and when combined with Ni
and Co,
represent 0.75 to 0.85 of the atomic fraction of the alloy, and (iv)
containing metalloid
elements comprised preferably of one or more of the elements C, B, Si and P
either
singly or in combination to represent 0.15 to 0.25 atomic fraction of the
alloy. The
electrodes have excellent thermal stability, improved stability in an aqueous
electrolyte
and can provide improved current efficiency - anodic or cathodic overpotential
performance. They are of use in the electrolysis of aqueous electrolyte
solutions such
as mixtures of caustic (KOH, NaOH) and water in the production of oxygen and
hydrogen.
The electrodes are comprised of low cost transition metals in combination with
metalloid elements in specific ratios to permit the alloy composition to be
solidified into
an amorphous state. They offer improved current efficiencies via anodic or
cathodic
overpotential performance and offer improved stability in both static and
cyclic
exposures. They can be used in concentrated alkaline solutions and at elevated
temperatures for improved electrode performance. The electrodes are of use in
the
electrolysis of alkaline solutions resulting in the production of hydrogen and
oxygen via
the decomposition of water, and also additional uses in electrodes for fuel
cells, electro-
organic synthesis or environmental waste treatment.
Processing methodology of rapid solidification offers many cost advantages
compared to the preparation of conventional Raney Ni type electrodes. The
process is
a single step process from liquid metal to finished catalyst, which can be
fabricated in
the form of ribbons or wires for weaving into a mesh grid. The process can
also be
used to produce sheets, powders, flakes, etc. which can further be
consolidated into a
CA 02287648 1999-10-26
-7- 1033
desired shape or patterned. By comparison, conventional electrode fabrication
involves
the production of a billet or rod, wire drawing and annealing operations,
weaving to
form a wire mesh grid, surface treatment, powder deposition, powder
consolidation
and an activation step.
Table 1 summarizes the results of prior art investigations involving
transition
metal-metalloid glasses. The performance of an electrocatalyst in Table 1 has
been
summarized in terms of two principle parameters: (i) the Tafel slope, P., and
(ii) the
logarithm of the exchange current density, log io. The exchange current
density is
equivalent to the reversible rate of a reaction at equilibrium at the standard
half-cell or
redox potential. The Tafel slope refers to the slope of the line representing
the relation
between overpotential and the rate of a reaction reflected as current density
where there
exists linearity on a semilogarithmic plot of overpotential and current
density.
Table 1.0: Polarization Data of Ni-Co base Amorphous Metals for HER in
Alkaline Solutions
Amorphous Solution Temperature -log io Qr Referenc
Electrode (A/cmz (mV/decade e
) )
NisoCozs.SiisBio 1M KOH 30 5.7 110,178 1
30 6.5 90 2
50 10.6 93 2
70 7.6 127 2
90 7.9 113 2
Surface-treated 1M KOH 30 5.4 91,145 1
NisoCossSiiaBio 1M KOH 30 5.8 101,144 1
Surface-treated 1M KOH 30 5.4 111,166 1
CosoNissPisBio 1M KOH 30 5.4 124,174 1
Surface-treated 1M KOH 30 5.1 110,173 1
Thermally-treated and 1M KOH 30 4.0 100 3
anodically oxidized 50 3.2 120 3
Ni5.5Co9oP45 70 2.8 120 3
90 2.2 100 3
Nis8Co2oSi1oB12 1M KOH 30 5.0 140 2
50 4.7 146 2
70 4.7 155 2
90 4.3 145 2
Co2sNi1oFe5SinBi6 1M KOH 30 4.6 174 2
50 5.5 119 2
70 5.4 120 2
90 5.3 128 2
NiMMo2oSi5Bs 1M KOH 30 4.1 165 2
70 3.8 106 2
CA 02287648 1999-10-26
-8- 1033
Amorphous Solution Temperature -log io Referenc
Electrode (A/cm2 (mV/decade e
) )
90 3.6 276 2
Fe39Ni39Mo2Sii2B8 1M KOH 30 5.0 123 2
50 4.8 150 2
70 4.9 173 2
90 4.9 167 2
Ni7sSi8B14 1M KOH 25 6.0 140 4
30 6.1 102 2
50 4.3 150 4
50 4.4 144 2
70 4.9 130 2
75 3.8 125 4
90 4.4 148 2
Anodically oxidized 30% KOH 70 2.9 130 5
FeaoNi4oBzo 1M KOH 30 3.9 174 2
50 3.8 184 2
70 4.3 230 2
90 3.0 188 2
Ni66.5Mo23.5Bio 0.5M 25 5.6 120 6
NaOH
Ni5e.5Mo23sFe1oB1o 0.5M 25 5.3 100 6
NaOH
Ni5e.5Mo215CnoBio 0.5M 25 5.0 135 6
NaOH
NinP2oCio coating 1N NaOH 25 6.2-8.4 65-95 7
Ni75Cr5P2o 1M HC1* 30 3.5 - 8
Ni73CnP2o 1M HCI* 30 3.8 - 8
Ni7oCnoP2o 1M HC1* 30 4.0 - 8
* not for electrolysis in an alkaline media
CA 02287648 1999-10-26
- 9 - 1033
Table 2: Polarization Data of Ni-Co base Amorphous Metals for HER in Alkaline
Solution with
Homogeneous Catalyst additions
Substrate and Addition Solution Tem rature -log i Q Referenc
of Catalyst (ppm x 103) ~ 0 c
(A/cm2 (mV/decade e
) )
Substrate Co 7.6M KOH 70 3.9 79 9
Fe 3.9 80
Ni 3=7 95
Pt 4.2 75
Fe addition = 0.014
Substrate Co 7.6M KOH 70 3.4 151 10
Fe 3.1 154
Ni 2.8 182
Pt 3.1 163
Mo addition = 0.024
Fe addition = 0.024
Substrate mild steel 6.OM KOH 80 - 112 11
NiSO4 addition = 80
Na2MoO4 addition = 20
Substrate mild steel 6.OM KOH 80 - 112 11
NiSO4 addition = 80
Na2MoO4 addition = 20
Substrate mild steel 6.OM KOH 80 - 25 11
NiSO4 addition = 80
Na2WO4 addition = 20
Substrate mild steel 6.OM KOH 80 - 50 11
NiSOa addition = 80
ZnSO4 addition = 40
Substrate mild steel 6.OM KOH 80 - 25 11
NiSO4 addition = 80
FeSO4 addition = 20
Substrate mild steel 6.OM KOH 80 - 112 11
NiSO4 addition = 80
CoSO4 addition = 20
Substrate mild steel 6.OM KOH 80 - 150 11
NiSO4 addition = 80
CrO3 addition = 20
Substrate Pt 5.OM KOH 25 - 80 12
Molybdate addition
CA 02287648 1999-10-26
-10- 1033
The electrodes described in Table 1 contain various combinations of the
transition metals in combinat,ion with (Ni,Co) but: none of them incorporate
element
"A" in addition as described above. The electrodes described in Table 2 derive
activity
from the presence of the dissolved salts of element "A" as described above
when added
to the solution phase of the electrolytic cell, but not when incorporated
directly into the
substrate material.
BRIEF DESCRIPTION OF THE DRAWINGS
In order that the invention may be better understood, preferred embodiments
will now be described by way of example only, with reference to the
accompanying
drawings, wherein:
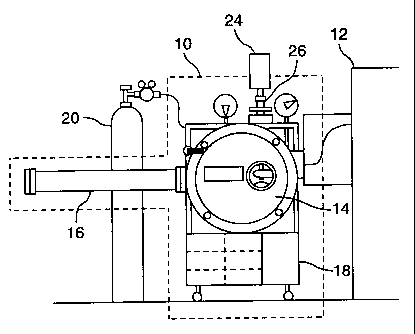
Fig. 1 is a schematic diagram of an apparatus for making a metallic glass
according to
the invention;
Fig. 2 is a schematic diagam detailing the interior of the vacuum chamber of
the
apparatus shown in Fig. 1;
Fig. 3 is a perspective representation of a boron nitride ceraniic crucible of
use in the
apparatus of Fig. 1;
Fig. 4 is a schematic diagram of a three component cell used in the evaluation
of the
electrochemical activity and stability of the materials according to the
invention;
Fig. 5 is a diagrammatic representation of the apparatus of use in obtaining
electrochemical measurements, and wherein the sanie numeral denotes like
parts.
DETAILED DESCRIPTTON OF 'THE PREFERRED
EMBODIlVIENTS OF THE INVENTION
The general methods for the preparation and testing of the materials
according to the invention followed those described in aforesaid United States
Patent No. 5,429,725.
EXPERIMENTAL
Electrode metallic glass materials were prepared as follows having the nominal
CA 02287648 2005-07-08
- 11 - 1033
composition:
EXAMPLE 1
This Example illustrates the preparation electrodes having a nominal
composition:
Ni50Co25 Cr5 B20
A series of processing trials were performed to fabricate amorphous alloy
ribbons by the melt-spinning technique. The process was divided into two
steps. The
first step was termed "pre-melting" where a powder mixture of pure materials,
i.e.,
nickel, cobalt, chromium, and boron, was charged onto a water cooled copper
hearth,
and melted via the use of vacuum arc melting. The second step employed a boron
nitride ceramic crucible, which enabled the pre-melted and crushed button to
be
remelted and superheated to a temperature higher than 1100 C in the vacuum
chamber.
A stream of molten metal was then blown through a thin slit of the ceramic
crucible on
to the peripheral surface of a massive copper wheel rotating at a high speed.
Rapid
quenching took place on the cold surface of the wheel, and the solidified
deposit was
produced in the form of thin ribbons. A concise description of amorphous metal
production is given in the following subsections.
Apparatus:
Melt-Spinner D-7400 Tubingen, Edmund BiihlerkGermany
3.3 x 10"2 Pascal High Vacuum Chamber
Induction Heater : TOCCOTRON 2EG103~The Ohio Crankshaft Co., U.S.A
Maximum output 10 kW, 450 kHz
Pyrometer : Model ROS-SU, Capintec Institute Inc., U.S.A
Fig. I illustrates the experimental apparatus consisting of a melt-spinner
shown
generally as 10 and an induction heating unit shown generally as 12. The melt-
spinner
assembly 10 comprised a high vacuum chamber 14, a ribbon collector tube 16,
and a
controller 18. The vacuum chamber 14 was connected to an argon cylinder 20
that
supplied argon gas for purging the chamber 14 and pressurizing a ceramic
crucible 22
(Fig. 2) in order to eject a molten mass of liquid material (not shown). The
temperature of the molten mass of liquid in ceramic crucible 22 is measured by
means
of an optical pyrometer 24 attached to a quartz window 26 located above vacuum
* Trade-mark
CA 02287648 1999-10-26
- 12 - 1033
chamber 14.
Induction heater unit 12 was comprised of an induction heater coil 28 (Fig. 2)
in vacuum chamber 14, a 3-stage step-up transformer and a closed-loop water
recirculator (not shown) which supplied cooling water through the induction
coil
during heating.
Fig. 2 shows the arrangement of a copper ivheel 30 (20 cm in diameter, 3.8 cm
in width), ceramic crucible 22 induction coil 28 in high vacuum chamber 14 and
ribbon
guide 32.
A: Premelting
The targeted chemical compositions exemplified are collectively expressed as
Ni50Co2Cr5%. Because the compositional range of the alloy is relatively small,
careful
sample preparation was required to ensure an effective comparison in
subsequent
electrochemical measurements. In order to achieve the targeted compositions
with
high accuracy, pure material powders were utilized to fabricate pre-melted
buttons first
by vacuum arc melting followed by mechanical crushing and remelting using
vacuum
induction melting. In the exemplified powders each mixture contained 50 atomic
%
nickel, 25 atomic % Co and 20 atomic % of boron. The remaining 5 atomic % was
made up with element A, in this example chromium. In an alternate embodiment
of this
invention, the boron was added in the form of an intermetallic compound like
nickel
boride which acted as a melting point depressant and enabled the whole powder
mixture to start melting at a relatively low temperature, ca.1035 C.
A batch of 20 - 50 g of the powder mixture was charged into a quartz
crucible (I.D. = 19.05 mm, O.D. = 22.2 mm, height = 130 mm, with round
bottom).
The quartz crucible was mounted in the vacuum chamber of the melt-spinner and
centered in the induction coil. The vacuum chamber was then purged three times
with
argon and evacuated to ca. 5 x 104 torr (7 x 10-' Pa) before heating. The
material
powder mixture was melted at greater than 1100 C in the quartz crucible. The
weight
loss ratio of materials through pre-melting was found to be < 1 weight % for
all
constituents.
CA 02287648 2005-07-08
-13- 1033
B: Melt Spinning
The melt spinner used in this work was an experimental sized model
manufactured by Edmund Buhlerv'GMBH capable of processing in batch mode 5 -
100
gram samples of alloy mixtures. The melt-spinner assembly comprised a high
vacuum
chamber, a ribbon collector tube, and a motor speed controller. The induction
heater
unit was comprised of an induction heater coil in the vacuum chamber, a 3-
stage step-
up transformer, and a closed-loop water recirculator, which supplied cooling
water
through the induction coil during heating. The vacuum chamber was connected to
an
argon cylinder that supplied gas for purging the chamber and pressurizing the
ceramic
crucible in order to eject a molten mass of liquid. The temperature of the
molten mass
of liquid in the ceramic crucible was measured by means of an optical
pyrometer that
was attached to a quartz window located above the vacuum chamber
One or two pre-melted buttons were charged into the BN ceramic
crucible. Boron nitride has the advantages of high hardness at elevated
temperatures
and good oxidation resistance that enabled the molten liquid to be superheated
to over
14000C without any chemical reaction with the crucible.
The crucible was mounted above the Cu wheel in the vacuum chamber.
The chamber was purged and evacuated in the same manner as that described
during
premelting. The pre-melted button(s) was superheated in the crucible by the
induction
coil until the liquid temperature reached a stable maximum temperature, which
was
dependent on the alloy composition. The molten mass of liquid was ejected by
argon
pressure on to the wheel through a fine slit nozzle (0.5 x 15 mm). Planar
amorphous
ribbons were formed on the surface of the wheel rotating counterclockwise and
driven
along the ribbon guides to the collector tube. This particular form of melt
spinning is
referred to as the planar flow casting technique. From the wheel rotation
speed, a
quenching rate was estimated to be ca. l 06 C/sec. One side of the ribbon was
free
from contact with the wheel and had a shiny appearance (shiny side) compared
with the
dull appearance for the other side in contact with the wheel (wheel side). To
minimize
surface imperfections on the dull side due to contact with the wheel, the
peripheral
surface of the wheel was thoroughly polished with diamond paste and degreased
with
* Trade-mark
CA 02287648 1999-10-26
-14- 1033
acetone before each run. Standard experimental parameters of the melt-spinning
operation are summarized in Table 3.
Table 3: S of Operational Parameters of Melt-S innin
Clearance between the bottom most edge of the 0.5 mm
crucible and the wheel surface
Point of impingement 12 degrees counterclockwise
from the top of the wheel
Pre-melt button weight 20-50 g
Vacuum chamber pressure 7 x 10 Pa or lower
Molten ejection pressure 40 kPa
Wheel rotation speed 1800-2900 rpm
LSuperheat temperature higher than 1100 C
The alloys of the invention so produced by planar flow casting were
submitted to the following further types of evaluation.
The first evaluation relates to the actual composition of the alloys
produced as poor recoveries during melting can produce substantial deviations
between
the nonzinal and actual composition of a given alloy.
The second evaluation relates to the structure of the alloys produced as
the processing method produces a metastable structure that is amorphous or
nanocrystalline in nature.
The third evaluation relates to the electrode perfomiance in relation to
the overvoltage necessary for hydrogen production for as-melt spun ribbons
under
conditions related to the electrolysis of an alkaline solution.
The fourth evaluation refers to the examination of the surface of the
,electrode materials used under both constant potential and conditions of
potential
cycling as described above.
The first test was performed in order to obtain reliable information on
the elemental composition of the amorphous alloys using inductively coupled
plasma
CA 02287648 1999-10-26
- 15 - 1033
spectroscopy (ICP). Although only a very small weight loss, less than 1 weight
%, was
found during the premelting operation, if the loss was due to a single
component,
inaccuracies in the targeted compositions would result. Additionally, there
was
concern about any compositional fluctuation in the longitudinal direction of
the
amorphous ribbon. For this reason, two positions designated as center and tail
were
taken from each ribbon and analyzed. ICP is a technique that provides a
quantitative
analysis of almost all elements with a high level of detectability.
The technique requires that the sainple to be analyzed be dissolved in
an aqueous solution because the sample is introduced to the inductively
coupled plasma
in the form of an aerosol. Each amorphous ribbon was dissolved into
concentrated
nitric acid and diluted with water and hydrochloric acid to complete the
designated
matrix solution which contained 4 weight % HNO3 and 4 weight % HCI. For
experimental error analysis, some standard solutions were prepared with pure
material
powders: The major elements analyzed were Ni, Co, Cr, V, and B. Expected
concentrations of Ni, Co, Cr, V, and B in the standard and sample solutions
are
summarized in Table 4.
CA 02287648 1999-10-26
- 16 - 1033
Table 4: Summary of Expected Concentrations of ICP Samples (ppm)
Serial Solute Ni Co Cr or V B
No.
#1 Blank (1) 0 0 0 0
#2 Standard (2) - metals 10.00 10.00 10.00 0
#3 Standard (3) - metals 100.00 100.00 100.00
#4 NiS4CoZCr1B20center 53.7 24.7 0.9 20.6
#5 Ni Co Cr B tail 54.0 24.8 1.0 20.1
#6 Standard 1~ 54.0 25.0 1.0 20.0
#7 Ni50Co25Cr5B20 center 50.6 24.6 5.0 19.9
#8 Ni Co Cr B~ tail 49.9 24.6 5.7 19.7
~ Standard 5 50.0 25.0 5.0 20.0
#10 N'i3SCo2,Cr~% center 35.6 25.1 20.2 19.1
#11 Ni35Co2CrMB20tail 35.7 25.1 20.2 18.9
#12 Standard 35.0 25.0 20.0 20.0
#13 Ni50Co25VSB20 center 50.8 25.3 4.6 19.3
#14 Ni Co V B tail 50.9 25.3 4.7 19.1
#15 Standard S~ 50.0 25.0 5.0 20.0
#16 Standard B 1 0 0 0 10
#17 Standard B2 0 0 0 25
#18 Standard B3 0 0 0 50
#19 Standard B4 0 0 0 100
The second test was performed using the technique of X-ray diffraction
in order to confirm the degree of crystallinity of the manufactured ribbons.
For
comparison, measurements were also carried out on crystallized fragments of
the
amorphous alloys as well as pure elemental nickel, cobalt, chromium, boron and
the
intermetallic nickel boride. The amorphous samples were prepared by cutting
ribbons
into 4 mm x 10 mm rectangular pieces. The samples were then degreased with
acetone, methanol and deionized water in sequence. The crystallized fragments
had the
same bulk composition as the corresponding amorphous alloy and were primarily
in the
form of brittle plate-like powder. To avoid preferential diffraction due to
the plate-like
surface of the fragments, the crystallized amorphous alloy was ground to form
a fine
, ...~ -..w...~
CA 02287648 2005-07-08
- 17 - 1033
powder in an agate mortar and dispersed on a slide glass before measurement.
Diffraction patterns were measured on a Siemens'tD5000 X-ray diffractometer
using 50
kV Cu-Ko radiation with a Ni filter in the range of 20 to 70 degree-20 at a
scan rate of
2 degree-20 per minute. The data was processed by Diffrac AT software.
The third test involved determining the electrochemical overpotential
for hydrogen evolution by determination of the Tafel slope and exchange
current
density for the alloys produced above. Working electrodes were prepared from
the Ni-
Co-Cr-B amorphous alloy ribbons of ca. 20-50 m thickness and 4 to 15 mm in
width.
The shiny side of the ribbon was ground, polished, and degreased. The as-
polished
ribbon was cut into approximately 10 mm x 10 mm pieces, and each piece was
joined
to an insulated copper lead. The joined area, unpolished wheel side, and
periphery of
the polished side were thoroughly coated three times at 24 hr intervals by
Amercoat
90 epoxy resin. This masking coat resists either alkaline or acidic
environments. The
exposed geometrical surface area of the fabricated electrodes was typically
0.03 0.01
2
cm.
The electrolytic cell shown in Fig. 4 generally as 40 had a three-
compartment structure consisting of a 300 ml capacity main body formed of
Teflon
containing a worldng electrode 42 of the ribbon of alloy of the invention, a
1/2" Teflori
tube 44 housing a counter electrode 46, and a 1/4" Teflon PTFE tube filled
with
mercury-mercuric oxide paste (Hg/HgO) 48. The compartments were separated by
electrolyte-permeable membranes 50 in the form of a diaphragm or frit. The
counter
electrode 46 was a 25 mm x 12.5 mm platinum gauze with a surface area of ca..
4.4
cm2. The Hg/HgO paste in aqueous I M KOH solution was used as a reference
electrode 52. The tip 54 of a Luggin caapillary of the reference electrode
compartment
was placed a distance of ca. 2 mm to the working electrode surface of the
alloys of the
invention. All potentials quoted herein are referred to the Hg/HgO electrode
in I M
KOH solution at 30 C. The electrolyte was aqueous 8 M potassium hydroxide
solution prepared with KOH and Type I water that had undergone pre-
electrolysis for a
minimum of 24 hours to remove any impurities in the KOH. The electrolyte was
replaced with fresh electrolyte and was deaerated by argon at a rate of 30
ml/min prior
* Trade-mark
CA 02287648 2005-07-08
- 18 - 1033
to each experiment. Argon bubbling was continued during the experiment. The
solution temperature was controlled at 700C in an 18 L water bath 56 (Fig. 5)
with an
immersion heater (Polystat Immersion Circulator, Cole-Palmer).
The apparatus used for electrochemical measurements comprises water
bath 56 in electrical contact with a potentiostat/galvanostat Hokuto Denko HA-
501 G
with a 200 MHz Pentium II personal computer 60, through a GPIB interface 62
and
arbitrary function generator (Hokuto Denko HA-105B) 66.
The electrocatalytic activity of the amorphous alloys for the hydrogen
evolution reaction (I ER) was studied by a quasi-steady-state polarization
technique.
In practice, polarization curves of the amorphous electrodes were measured
under
quasi-potentiostatic conditions at a very low sweep rate of 2 mV/min. This
potential
sweep rate was found to be the maximum sweep rate that provided reproducible
steady-state measurements. The as-polished working electrode was rinsed
ultrasonically with acetone, methanol, and Type I water in sequence prior to
testing.
The electrode was then placed in the cell with deaerated 1M KOH solution and
held at
a potential of -1.3 V vs. I4g/HgO for 3 hours to clean the electrode surface
electrochemically. The potential was swept over the range of -0.9 to -1.5 V
vs.
Hg/HgO for multiple cycles in order to assess the Tafel behaviour of the
electrode
response. Polarization curves were replicated at least three times for each
electrode
and analyzed for their reproducibility.
The fourth test was performed on amorphous alloy and crystalline
surfaces to compare the degree of surface roughening and hence electrode
degradation
by using optical and scanning electron microscopy prior to and post use as an
electrocatalyst in the cell. Optical investigation was achieved using a light
stereoscope
and light metallograph. Electron imaging was accomplished using a HitachrS-570
SEM equipped with a Link Analytical 10/85s x-ray analyzer. Nominal imaging
conditions were: accelerating voltage - 20kV, beam current - 100 A, sample
tilt - 150.
In the first test a quantitative composition analysis by Inductively
Coupled Plasma (ICP) Spectroscopy was performed. The average experimental
composition of each amorphous ribbon as determined by the ICP analysis is
listed in
* Trade-mark
CA 02287648 1999-10-26
-19- 1033
Table 5. All of the measured compositions of the amorphous ribbons were in
good
agreement with the targeted compositions. An average magnitude of the
deviation of
the actual from the nominal composition was < 1 atomic %. Variations of
principal
element concentrations were also measured at two different longitudinal
positions over
the ribbon such as center and tail. There was no significant difference in the
compositions at different positions. From these data, the amorphous ribbons
can be
regarded as homogeneous in the longitudinal direct"ron.
Table 5: Com osition of the Amo hous Ribbons atomic ercen e
Targeted Composition Measured Composition
Ni54CouCr1B20 Ni53.7Co24.8Cri.oB20.i
Ni50Co25Cr5B20 N149.9Co24.6Cr5.7B 19.7
Ni45Co25Cr1aB20 Ni45.iCo24.9Crio.oB20.0
Ni4OCouCr1SB20 Ni4O 3Co25Cr15.oB19.7
Ni3SCouCr2~B20 Ni35.7Co25.iCr2o.2Bi8.9
Ni50Co25V5B20 NiS0.9Cou 3V4..tB19.1
In the second test, the structure of the ribbon was assessed using x-ray
diffraction, as it is an integral part of the electrode performance
independent of the
exact composition of the electrode material. It is known that a typical X-ray
diffraction
(XRD) pattern of an amorphous material is a broad spectrum with no prominent
sharp
peaks relating to crystalline structure. Thus, qualitative confirmation of the
amorphous
nature of an alloy is demonstrated by a broad band peak in its XRD profile.
As additional information, an index, viz. effective crystallite dimension
was calculated to evaluate the largest potential size of crystal embryos in
the melt-spun
ribbons.
The effective crystallite dimension is expressed by the equation:
D = 0.91~
f3cos6
where D is the effective crystallite dimension in nm and k is wavelength of
the Cu-Ko
CA 02287648 1999-10-26
- 20 - 1033
radiation, i.e. 0.1542 nm. 13 denotes the full width of a given diffraction
peak in radians
at half the maximum intensity. 0 is the Bragg angle of the peak maximum. The
effective crystallite dimension was measured for all the melt-spun ribbons.
Results of
the calculations are summarized in Table 6. The melt-spun Ni-Co-Cr-B alloys
displayed very small values of the effective crystallite dimension determined
from their
broad band peak width in X-ray diffraction confirming the amorphous nature of
the
melt spun ribbons.
Table 6: Effective C stallite Dimension
Amorphous Peak Apparent Full Width Effective
Alloy Maximum Mean of Half the Crystallite
Composition Position d-Spacing Maximum Dimension
20(0) d(A) Intensity D (nm)
13 (rad)
Ni3SCo25Cr2OB20 45.1 1.993 0.138 1.1
Ni50Co25Cr5B20 45.7 2.015 0.126 1.2
Ni51.4Co25 3.6 2 Cr B0 46.3 2.015 0.136 1.1
In the third test, the electroca.talytic perfonmance of the various
amorphous electrodes was measured and compared to the behaviour of the
crystalline
elemental constituents. In the potential range of -0.9 to -1. 5 V vs. Hg/HgO,
the current
responses (polarization curves) of crystalline Ni, C;o, Cr, and the amorphous
Ni-Co-
(Cr,V)-B alloys varied from ca. 0.001 to 1000 mA/cm2. A linear correlation was
found
in the potential vs. logarithmic current plot (Tafel plot) which were analyzed
to obtain
Tafel parameters, ~ , and i , by a statistical regression method. The Tafel
slopes and
exchange current densities are summarized in Table 7.
CA 02287648 1999-10-26
-21- 1033
Table 7: Tafel Parameters of Electrodes for the HER in 1M KOH at 30 C
MATERIAL TAFEL PARAMETERS
...
-E= -togio ~ c
C stalline
Ni 1.25-1.56 3.2i-0.3 239f14
Co 1.25-1.44 4.0 0.1 178 4
Mo 1.20-1.40 6.6 0.2 90 4
Amorphous
Ni50Co25Cr5B20 1.01-1.50 3.15 161
Ni35Co25Cr2OB20 1.01-1.50 3.58 114
Ni5oCo25VSB20 1.00-1.50 3.96 100
Ni.nMo8B20 0.94-1.55 4.0 0.04 180 2
NVo2Mo6B20 1.00-1.50 5.1 0.07 142 3
Ni50Co4Mo4B20 1.00-1.50 5.1 0.03 148 2
* Potential range (V vs. Hg/HgO),
* * Exchange current density (A/cm2),
*** Tafel slope (mV/decade), high field
Appreciable differences in the current density values were clearly observed
as a function of the compositions of the amorphous alloys as shown in Table 7.
The following ranking of the electrocatalytic activity was found:
N150CO25V5B20 > Ni35Co25Cr2o$20 I Ni50Co25Cr5B20
This ranking order does not simply follow the order of magnitude of the Cr/V
content in the amorphous alloys, but is particular to the elemental form. The
highest electrocatalytic activity of Ni50Co25V5B20 amongst the amorphous
alloys
could possibly be attributed to the synergetic effect of Ni-Co-V that may
influence
the nature of the oxide film formed on this amorphous alloy.
The improvement of this invention compared with United States
Patent No. 5,429,725 is also evident from 'Table 7 by comparison of the
performance of the amorphous alloys. The invention shows higher exchange
CA 02287648 1999-10-26
- 22 - 1033
current densities combined with lower Tafel slopes in the (Cr,V)-containing
alloys
compared with the Mo-containing alloys; both features contribute to enhanced
operating efficiency of the material as an electrocatalyst for alkaline water
electrolysis.
In the fourth test, in order to obtain additional information on the
condition of the electrode surface after multiple cycles of operation,
specimens
were examined using optical and scanning electron microscopy (SEM). It was
found that the potential cycled crystalline Ni, Co and Mo electrodes had thick
corrosion product layers. Crystalline Ni electrodes after 200 and 600 cycles
showed a growth in the corrosion layer with potential cycling. The crystalline
Co
electrode showed a sign of crystallization / dissolution reactions by polygon-
plate-
like uniform deposits on the electrode surface. The crystalline Mo electrode
showed a severely corroded surface and a remaining skeleton structure that
indicated the active dissolution of Mo. All ciystalline electrodes showed much
higher roughness than their as-polished state.
In contrast, potential cycled amorphous electrodes showed very
smooth surfaces and no indication of corrosion. Only a slight surface layer
(probably Ni oxides) could be seen characterized by a dull transparent film
that
covered the very smooth surface of the amorphous alloys. No significant
difference was found between the amorphous electrodes pre and post cycling.
Hence, after exposure to severe potential cycling conditions, the amorphous
alloy
electrodes were more stable than the crystalline electrodes of the elements
Ni, Co
or Mo.
Although this disclosure had described and illustrated certain preferred
embodiments of the invention, it is to be understood that the invention is not
restricted to these particular embodiments. Rather, the invention includes all
embodiments that are functional or mechanical equivalents of the specific
embodiment and features that have been described and illustrated.