Note: Descriptions are shown in the official language in which they were submitted.
CA 02288607 1999-11-04
WO 98/53004 PCT/CA98/00499
In one of its aspects, the present invention relates to the
hydrophobicizing of particles, particularly mineral particles that are
hydrophilic
and have surface hydroxyl groups, for example silica, silicates, clay,
alumina,
titanium dioxide and the like. The invention also extends, however, to
treatment of non-mineral particles, for instance carbon black. In another of
its
aspects, the present invention also relates to the treated partices, ner sass.
The treated particles are useful particularly, but not exclusively, as a
filler in
polymers, especially rubber. In another of its aspects, the present invention,
also relates to a filled, particularly silica-filled, rubber masterbatch, and
to a
process for preparing it.
In recent years, there has developed a considerable interest in
silica reinforced tires, particularly since the appearance in 1992 of the
Groups
Michelin (G-M) patents (EP 05 01 227 A 1; AU-A-111 77 192) indicating that
tires made with tread formulations incorporating silica enjoy some important
performance advantages over those based on conventional carbon black
filler. Improvements are claimed for this "Green Tire" in the areas of (a)
lower
rolling resistance, (b) better traction on snow, and (c) lower noise
generation,
when compared with conventional tires filled with carbon black.
Rubber for tires is often supplied by a rubber producer to a tire
manufacturer in the form of a masterbatch containing an elastomer, which is
typically a hydrocarbon rubber, an oil extender and a filler. The conventional
filler has been carbon black in the form of fine particles. These particles
have
hydrophobic surface characteristics and will therefore disperse relatively
easily within the hydrophobic elastomer. In contrast, conventional silica has
a
relatively hydrophilic surface, and considerable difficulty has been
encountered in dispersing conventional silica in the hydrophobic rubber
elastomer.
In the past, efforts have been made to make masterbatches
CA 02288607 1999-11-04
WO 98/53004 PCTlCA98/00499
-2_
from elastomer dispersions and aqueous dispersions of silica pigment, such
as those referred to and attempted by Burke, in United States patent
3,700,690. Burke attempted to overcome the previously known difficulties of
incorporating fine particles of silica uniformly into a masterbatch. At the
time
of the Burke invention, there was no known elastomer-silica masterbatch
offered in the commercial market. Similarly today, to the Applicant's
knowledge, there are no commercially available in situ produced elastomer-
silica masterbatches in the market, despite the efforts of Burke (i.e.,
conventional elastomer-silica masterbatches are produced and available in
the dry state).
It is an object of the present invention to obviate or mitigate at
least one of the above-mentioned disadvantages of the prior art.
It is another object of the present invention to provide a novel,
relatively hydrophobic particulate material.
It is yet another object of the present invention to provide a
novel process for treating particulate material to render it relatively
hydrophobic.
It is yet another object of the present invention to provide a
novel masterbatch composition comprising an elastomer and a relatively
hydrophobic particulate material.
It is yet another object of the present invention to provide a
novel process for producing a masterbatch composition comprising an
elastomer and a relatively hydrophobic particulate material.
Accordingly, in one of its aspects, the present invention provides
a process for treating particles, particularly mineral particles, to render
them
hydrophobic, the process comprising the steps of:
(a) contacting the particles with a compound of Formula I:
CA 02288607 1999-11-04
WO 98/53004 PCT/CA98/00499
-3-
R ~ R1
Rg~N-R4-SI~R32 (I)
or an acid addition or quaternary ammonium salt thereof, in which:
at least one of R', R2 and R3, preferably two of R', Rz and R3
and most preferably R', R2 and R3 are hydroxyl or hydrolysable groups;
R4 is a divalent group that is resistant to hydrolysis at the Si-R4
bond;
R5 is selected from the group comprising: hydrogen; a C~~o
alkyl; a C2~o mono-, di- or tri-unsaturated alkenyl group; a C6 C4o aryl
group; a
group of the formula:
R13
-Cx HEN
R14
in which x is an integer from 2 to 10, R'3 and R'4, which may be the same or
different, are each hydrogen; C,_,8 alkyl; C2_,e mono-, di- or tri-unsaturated
alkenyl; phenyl; a group of formula:
-(CH2)b
CH=CH2
wherein b is an integer from 1 to 10; a group of formula:
R23
(CH2)c N~R22
CA 02288607 1999-11-04
WO 98/53004 PCT/CA98/00499
-4-
wherein c is an integer from 1 to 10 and R22 and Rz3 which may be the same
or different, are each hydrogen, C,_,o alkyl group or C2_,o alkenyl group,
provided that there is no double bond in the position alpha to the nitrogen
atom; a group of formula:
- ~ (CH2)rNH ]d- H
wherein r is an integer from 1 to 6 and d is an integer from 1 to 4;
R6 may be any of the groups defined for R5, or R5 and R6 may
together form a divalent group of formula:
(C H2)t ~
~(C H2)v
in which A is selected from the group comprising -CHR or -NR group in which
R is hydrogen or a C,~n alkyl or CZ~o alkenyl group, a Cs C4o aryl group, an
oxygen atom and a sulfur atom, and t and v are each independently 1, 2, 3 or
4; provided that the sum of t and v does not exceed 6, and is preferably 4;
and
(b) contacting the particles with a compound of the Formula II:
R15
R 12_Si~R 16 (II)
in which:
R'S, R'6 and R" have the same definitions as R', RZ and R3; and
R'2 is selected from the group comprising a CB~o alkyl group or a
Ce~o mono-, di- or tri-unsaturated alkenyl group, either of which can be
interrupted by one or more aryl groups, preferably phenyl groups; a group of
CA 02288607 1999-11-04
WO 98/53004 PCT/CA98/00499
-5-
formula:
R1 ~
R20~ N _ R 18-
or an acid addition or quaternary ammonium salt thereof in which R'e is a
divalent group resistant to hydrolysis at the SI-R'e bond, R'9 is selected
from
the group comprising hydrogen, a C,~° alkyl group, a C2~° mono-,
di- or tri-
unsaturated alkenyl group, a substituted aromatic group, for example the
phenylene group -(C6H4)-, the biphenylene group -(C6H4)-(CsH4)-, the
-(C6H4)-O-(CsH4)- group or the naphthylene group, -(C,°Hs)-, the
aromatic
group being unsubstitued or substituted by a C,_2° alkyl or
CZ_Z° mono-, di- or
tri-unsaturated alkenyl group; and R2° may be any of the groups defined
for
R'9, with the provisos that R'9 and R2° do not have a tertiary
carbon atom
adjacent to the nitrogen atom and that at least one of R'9 and R2° has
a
carbon chain at least 8 carbon atoms in length uninterrupted by any
heteroatoms.
Preferably, R'8 is a C,-C4° saturated or unsaturated group (e.g.,
alkenyl, aryl, cycloalkyl and the like).
In the present process, Steps (a) and (b) may be conducted
concurrently or sequentially. If Steps (a) and (b) are conducted sequentially,
it is preferred to conduct Step (a) followed by Step (b).
As will be apparent to those of skill in the art, there are
instances where Formulae I and li may be the same compound - e.g., when
R5=R'9= a CB,~° alkyl group or R5=R'9= a CB.~° mono-, di- or
tri-unsaturated
alkenyl group. Thus, in such cases where Formulae I and 11 are the same
compound, it will be clearly understood that the present process intentionally
embodies a single step process (i.e., where the compound of Formulae i and
II is added in a single step) and a multi-step process (i.e., where the
compound of Formulae I and II is added proportionally in two or more steps).
In another of its aspects, the present invention provides a
CA 02288607 1999-11-04
WO 98/530114 PCT/CA98/00499
-6-
treated particulate material comprising particles having bound thereto an
aminohydrocarbonsiloxane (e.g., an amino(alkyl)siloxane) moiety - i.e., a
hydrocarbon moiety comprising both silicon and nitrogen.
Preferably, the aminohydrocarbonsilane moiety has the formula
Ra
R~2 SI Rb
W Rc
in which:
Ra, Rb and R° are the same or different and each is selected
from -O- and -CPH2P , optionally substituted by one or more oxygen atoms and
wherein p is an integer of from 1 to 10; and
R'2 is a CB~o alkyl group; a Coo mono-, di- or tri-unsaturated
alkenyf group; a group of formula
R5
\N_R4-
6~
R
or an acid addition or quaternary ammonium salt thereof in which R4 is a
divalent group resistant to hydrolysis at the Si-R4 bond, R5 is hydrogen
C,_4o alkyl, C2~o mono-, di- or tri-unsaturated alkenyl; a group of formula
-ArCWH~"+,
in which Ar represents a divalent aromatic group and w is an integer from 1 to
20, and R6 may be any of the groups defined for R5, with the proviso that at
least one of R5 and Rs must have an uninterrupted carbon chain at least 8
carbon atoms in length.
In yet another of its aspects, the present invention provides a
CA 02288607 1999-11-04
WO 98/53004 PCT/CA98/00499
-7-
particulate material comprising particles having: (i) bound thereto an
aminohydrocarbonsiloxane (e.g., an amino(alkyl)siloxane) moiety (i.e., a
hydrocarbon moiety comprising both silicon and nitrogen), and {ii) a contact
angle of at least about 100°. Preferably, the aminohydrocarbonsilane
moiety
has the formula set out hereinabove. Preferably, the particles have a contact
angle of at least about 110°, more preferably in the range of from
about 115°
to about 160°, even more preferably in the range of from about
120° to about
150°, most preferably in the range of from about 120° to about
140°. In
contrast, the contact angle of silica particles which are not treated in
accordance with the present process is typically 75°.
The contact angle of the particles with water may be readily
determined according to the following procedure:
(i) double-sided tape is attached to a probe (e.g., a stirrup)
and coated with the particulate material by immersing the
tape in a sample of the particulate material;
(ii) excess powder is removed by gentle tapping and large
powder clusters are removed by careful wiping;
{iii) the probe coated with particulate material is immersed
into distilled water using a conventional contact angle
analyzer (e.g., a Cahn Dynamic Contact Angle Analyzer)
at a rate of 100 microns per second.
This procedure results in determination of the advancing contact angle of the
particles.
In yet another of its aspects, the present invention provides a
particulate material produced by contacting the particles with a compound of
Formula I:
R ~ R ~
Rs~N-R4-Si~R32 {I)
CA 02288607 1999-11-04
WO 98/53004 PCT/CA98/00499
_$-
or an acid addition or quaternary ammonium salt thereof, in which:
at least one of R', R2 and R3, preferably two of R', RZ and R3
and most preferably R', R2 and R3 are hydroxyl or hydrolysable groups;
R4 is a divalent group that is resistant to hydrolysis at the Si-R4
bond;
R5 is selected from the group comprising: hydrogen; a C,_4o
alkyl; a C2~o mono-, di- or tri-unsaturated alkenyl group; a C6 C4o aryl
group; a
group of the formula:
R13
-CXH~N
R14
in which x is an integer from 2 to 10, R'3 and R'4, which may be the same or
different, are each hydrogen; C,_,8 alkyl; C2_,8 mono-, di- or tri-unsaturated
alkenyl; phenyl; a group of formula:
-(CH2)b
CH=CH2
wherein b is an integer from 1 to 10; a group of formula:
~R23
-(CH2)~ N~R22
wherein c is an integer from 1 to 10 and R22 and R23which may be the same
or different, are each hydrogen, C,_,o alkyl group or CZ_,o alkenyl group,
provided that there is no double bond in the position alpha to the nitrogen
atom; a group of formula:
CA 02288607 1999-11-04
WO 98/53004 PCT/CA98/00499
_g-
~ (CH2)rNH ~d- H
wherein r is an integer from 1 to 6 and d is an integer from 1 to 4;
R6 may be any of the groups defined for R5, or R5 and Rs may
together form a divalent group of formula:
/(CH2)t~
A ~(C H2)v
in which A is selected from the group comprising -CHR or -NR group in which
R is hydrogen or a C,~o alkyl or C2~o alkenyl group, a Cs-C4o aryl group, an
oxygen atom and a sulfur atom, and t and v are each independently 1, 2, 3 or
4; provided that the sum of t and v does not exceed 6, and is preferably 4;
and a compound of the Formula I l:
R15
R 12-Si~R ~7 (II)
in which:
R'S, R'6 and R" have the same definitions as R', R2 and R3; and
R'2 is selected from the group comprising a Coo alkyl group or a
C8.~ mono-, di- or tri-unsaturated alkenyl group, either of which can be
interrupted by one or more aryl groups, preferably phenyl groups; a group of
formula:
R 1 ~
R20~N_R18-
CA 02288607 1999-11-04
WO 98/53004 PCT/CA98/00499
-10-
or an acid addition or quaternary ammonium salt thereof in which R'e is a
divalent group resistant to hydrolysis at the Si-R'$ bond, R'9 is selected
from
the group comprising hydrogen, a C,~° alkyl group, a C2,~° mono-
, di- or tri-
unsaturated alkenyl group, a substituted aromatic group, for example the
phenylene group -(C6H4)-, the biphenylene group -(C6H4)-(C6H4)-, the
-{C6H4)-O-(CgH4)- group or the naphthylene group, -(C,°H6)-, the
aromatic
group being unsubstitued or substituted by a C,_2° alkyl or C2_20 mono-
, di- or
tri-unsaturated alkenyl group; and RZ° may be any of the groups defined
for
R'9, with the provisos that R'9 and R2° do not have a tertiary
carbon atom
90 adjacent to the nitrogen atom and that at least one of R'9 and R2°
has a
carbon chain at least 8 carbon atoms in length uninterrupted by any
heteroatoms.
Preferably, the present process of treating a particulate material
is carried out in an aqueous solution, dispersion or slurry, so that the
product
of the process is an aqueous dispersion or slurry of hydrophobicized mineral
particles.
In one preferred embodiment, the dispersion or slurry resulting
from the present process, and containing the treated particles {preferably
mineral particles such as silica), is then mixed with a hydrocarbon solution
of
an elastomer, and then dried to form a silica-filled rubber masterbatch. Owing
to the hydrophobicized nature of the silica filler, it is well dispersed in
the
elastomer. This preferred embodiment results in the in situ production of a
masterbatch composition comprising the elastomer and the treated particles.
By "in situ production" is meant that treated particles are incorporated into
a
masterbatch composition without being isolated (i.e., separated from the
dispersion or slurry, and subsequently dried). This preferred embodiment is
believed to be the first in.~itu production of a masterbatch composition
comprising an elastomer and a treated particulate material such as silica.
Alternatively, the treated particulate material may be separated
from the dispersion or slurry, and subsequently dried for later use.
CA 02288607 1999-11-04
WO 98/53004 PCTlCA98/00499
-11 -
Embodiments of the present invention will be described with
reference to the accompanying drawings, in which:
Figures 1-4 illustrate a reaction pathway for a specific
embodiment of the present process;
Figure 5 illustrates a schematic of a system used to conduct the
present process in the Examples hereinbelow.
Throughout this specification, the invention is illustrated with
reference to silica as the particle having surface hydroxyl groups, but it
should
be appreciated that the invention applies to the use of other such minerals,
and the description understood accordingly. For example, the present
process may be applied to a particulate mineral material selected from the
group comprising silicates, silicas (particularly silica made by carbon
dioxide
precipitation of sodium silicate), clay, titanium dioxide, alumina, calcium
carbonate, zinc oxide and mixtures thereof. The present process may also be
applied to a particulate non-mineral material such as carbon black. Of
course, mixtures of particulate materials may be used.
In a preferred embodiment, the treatment is carried out in an
aqueous dispersion or slurry and the concentration of the aqueous dispersion
or slurry of silica particles may be between 1 and 30 percent by weight of
silica in water, preferably between 5 and 25 percent by weight of silica in
water and most preferably between 8 and 22 percent by weight of silica in
water. Dried amorphous silica suitable for use in accordance with the
invention may have a mean agglomerate particle size between 1 and 100
microns, preferably between 10 and 50 microns and most preferably between
10 and 25 microns. It is preferred that less than 10 percent by volume of the
agglomerate particles are below 5 microns or over 50 microns in size. A
suitable amorphous dried silica moreover has a BET surface area, measured
in accordance with DIN (Deutsche Industrie Norm) 66131, of between 50 and
450 square meters per gram and a DBP absorption, as measured in
CA 02288607 1999-11-04
WO 98/53004 PCT/CA98/00499
-12-
accordance with DIN 53601, of between 150 and 400 grams per 100 grams
of silica, and a drying loss, as measured according to DIN ISO 787/11, of from
0 to 10 percent by weight. If filter cake is used, it may be made by any known
means such as described in Ullmann's Encyclopedia of industrial Chemical
Vol A23 pages 642-643, VCH Publishers, ~1993. The filter cake has a
preferred solids content of between 5 and 30 percent by weight, most
preferably between 15 and 25 percent by weight, and it may be redispersed
in water in accordance with the present process to give a silica concentration
of between 5 and 20 percent by weight and most preferably between 8 and
12 percent by weight. It is preferred to use a filter cake.
If a never-filtered slurry prepared from the known reaction of a
solution of alkali metal silicate with either mineral acid or carbon dioxide
is
used, it is preferred that the solids content of the never-filtered slurry be
between 1 and 30, more preferably between 5 and 10, percent by weight of
silica. The slurry temperature may be between 0 and 100 degrees Celsius if
the process is conducted at atmospheric pressure or between 0 and 135
degrees Ceisius if the operation is conducted in a pressure vessel. Most
preferably, the process is conducted at atmospheric pressure in which case
the preferred temperature is between 30 and 95 degrees Celsius and most
preferably between 45 and 90 degrees Celsius.
It is desirable that, prior to the addition to the silica particles of
the compound of Formula I, the dispersion or slurry shall have a pH in the
range from 6 to about 8, more preferably from about 6.8 to about 7.2. If
necessary, the pH can be adjusted by addition of acid or alkali, for example
mineral acid, alkali metal hydroxide, alkaline earth hydroxide, ammonium
hydroxide and the like. These can be added as such or in aqueous solution.
In the compound of Formula I, it is preferred that all three of the
groups R', RZ and R3 are readily hydrolysable. Suitable groups R' include
hydroxyl groups and hydrolysable groups of formula OCpH2P+1, where p has a
value from 1 to 10. The alkyl chain can be interrupted by oxygen atoms, to
give groups, for example, of formula CH30CH20-, CH30CH20CH20-,
CH3(OCH2)40-, CH30CH2CH20-, CZH50CH20-, C2H50CH20CH20-, or
CA 02288607 1999-11-04
WO 98/53004 PCTlCA98/00499
-13-
CZH5OCH2CH2O-. Other suitable hydrolysable groups include phenoxy,
acetoxy, chloro, bromo, iodo, ONa, OLi, OK or amino or mono- or
dialkylamino, wherein the alkyl groups) have 1 to 30 carbon atoms.
R2 and R3 can take the same values as R', provided that only
one of R', RZ and R3 is chioro, bromo or iodo. Preferably, only one or two of
R', RZ and R3 is hydroxyl or ONa, OLi or OK.
Non-limiting examples of groups R2 and R3 that are not
hydrolysable include C,_,o alkyl, C2_,o mono- or diunsaturated alkenyl, and
phenyl. R2 and R3 can also each be a group -R'-NR5R6, discussed further
below. It is preferred that R', R2 and R3 are all the same and are CH30-,
C2H50- or C3H80-. Most preferably they are all CH30-.
The divalent group R4 is preferably such that N-R4-Si is of the
formula:
N-(CH2)p(O)o(CBH4)n(CH2)m(CH=CH)k SI
in which k, m, n, o and p are all whole numbers. The order of the moieties
between N and Si is not particularly restricted other than neither N or O
should be directly bound to Si. The value of k is 0 or 1, the value of m is
from
0 to 20 inclusive, the value of n is 0, 1 or 2, the value of o is 0 or 1 and
the
value of p is from 0 to 20 inclusive, with the provisos that the sum of the
values of k, m, n, o and p is at least 1 and not more than 20 and that if o is
1,
p is 1 or greater and the sum of k, m and n is 1 or greater, i.e. that the Si
atom is linked directly to a carbon atom. There should be no hydrolysable
bond between the silicon and nitrogen atoms. Preferably, m is 3 and I, n, o
and p are all 0, i.e., R4 is -CHZCH2CHz .
The group R~ is preferably a C$_ZO monounsaturated alkenyl
group, most preferably a C,&,e monounsaturated alkenyl group. Rs is
preferably hydrogen.
Suitable compounds of Formula I include, but are not limited to:
3-aminopropylmethyldiethoxysilane, N-2-(vinylbenzylamino)-ethyl-3-
aminopropyl-trimethoxysilane, N-(2-aminoethyl)-3-aminopropyftrimethoxy-
CA 02288607 1999-11-04
WO 98/53004 PCT/CA98100499
-14-
silane, trimethoxysilylpropyldiethylenetriamine, N-2-(aminoethyl)-3-
aminopropyltris(2-ethylhexoxy}silane, 3-aminopropyldiisopropylethoxysilane,
N-(6-aminohexyl)aminopropyltrimethoxysilane, 4-aminobutyltriethoxysilane, 4-
aminobutyldimethylmethoxysilane, triethoxysilylpropyl-diethylenetriamine, 3-
aminopropyltris(methoxyethoxyethoxy)silane, N-(2-aminoethyl~3-
aminopropyltrimethoxysilane, N-2-(aminoethyl)-3-aminopropyltris(2-
ethylhexoxy)silane, 3-aminopropyldiisopropylethoxysilane, N-(6-
aminohexyl)aminopropyltrimethoxysifane, 4-aminobutyltriethoxysilane, and
(cyclohexylaminomethyl)-methyldiethoxysilane.
Preferred compounds of Formula I include those in which R5 is
hydrogen and R6 is the alkenyl group from the following: Soya alkyl, tall oil
alkyl, stearyl, tallow alkyl, dihydrogenated tallow alkyl, cocoalkyl, rosin
alkyl,
and palmityl, it being understood that in this case the alkyl may include
unsaturation.
It is preferred that at least one of R4, R'3 and R'4 has a chain of
at least 8 carbon atoms, more preferably at least 10 carbon atoms,
uninterrupted by any heteroatom.
The compound of Formula I can be used as the free base, or in
the form of its acid addition or quaternary ammonium salt, i.e.
XO
R1
R \O
Rs~N-R4-Si~
R7 R
wherein R', R2, R3, R4, R5 and R6 are as defined above; R' is selected from
the group comprising hydrogen, a C,~o alkyl group or C2_ao mono-, di- or tri-
unsaturated alkenyl group, and X is an anion. X is suitably chlorine, bromine,
or sulphate, of which chlorine and bromine are preferred, and R' is preferably
hydrogen.
Non-limiting examples of suitable salts of compounds of
Formula I include N-oleyl-N-[(3-triethoxysilyl)propyl] ammonium chloride, N-3-
CA 02288607 1999-11-04
WO 98/53004 PCT/CA98/00499
- 15-
aminopropylmethyldiethoxy-silane hydrobromide, (aminoethylamino-
methyl)phenyltrimethoxysilane hydrochloride, N-[(3-trimethoxysilyl)propyl]-N-
methyl, N-N-diallylammonium chloride, N-tetradecyl-N,N-dimethyl-N-[(3-
trimethoxysilyl)propyl] ammonium bromide, 3[2-N-benzylaminoethyl-
aminopropyl]trimethoxysilane hydrochloride, N-octadecyl-N,N-dimethyl-N-[(3-
tri-methoxysilyl)propyl] ammonium bromide, N-[(trimethoxysilyl)propyl)-N-tri(n-
butyl) ammonium chloride, N-octadecyl-N-[3-triethoxysilyl)propyl] ammonium
chloride and N-2-(vinylbenzylamino)ethyl-3-aminopropyi-trimethoxysilane
hydrochloride.
It is preferred to use the compound of Formula I in salt form.
The most preferred compound is N-oleyl-N-[(3-trimethoxysilyl)propyl)
ammonium chloride.
The amount of the compound of Formula I may be between 0.1
and 20 percent by weight of the mineral particles in the slurry (dry basis)
and
preferably between 0.25 and 10 percent by weight and most preferably
between 0.5 and 2 percent by weight. Preferably, the amount of the
compound of Formula l used varies inversely with the mineral particle size.
The compound may be added to the slurry in its natural state, either as a
liquid or a solid. However, to facilitate dispersion, it is preferred where
possible to add the compound as a liquid. If the melting point of the
compound is below 95 degrees Celsius, it is preferred to add it to the slurry
in
a molten state at a temperature at least 5 degrees Celsius above the melting
point, provided the temperature of the compound in the liquified state does
not exceed 100 degrees Celsius and provided that the compound does not
decompose under these conditions. If the melting point exceeds 95 degrees
Celsius, it is most preferred to use a solvent. Prefer-ed solvents are water
and alcohols containing 1 to 5 carbon atoms and most preferably those
containing 1 to 3 carbon atoms, that is to say methanol, ethanol, n-propanol
or isopropanol. If the compound of Formula I is an alkoxysilane, then most
preferably the alkoxy group of the solvent alcohol will be the same as the
alkoxy group of the alkoxysiiane. For example, if the compound of Formula I
is a methoxysilane, the preferred solvent is methanol. The concentration of
CA 02288607 1999-11-04
WO 98/53004 PCT/CA98/00499
-16-
the compound in the solvent may be from 10 to 90 percent by weight and
more preferably between 25 and 75 percent by weight and most preferably 50
percent by weight. Preferably, the solution can be prepared and added to the
slurry at a temperature between a lower limit of 0 degrees Celsius and an
upper limit which is the lower of at least 10 degrees below the boiling point
of
the solvent and 95 degrees Celsius. The dispersion of the compound is
effected by mixing.
It is preferred that, for the specific compound of Formula I which
is added, the equivalent balance (EB) should be calculated. The EB is used
to determine whether mineral acid or alkali metal hydroxide, or solution
thereof, should be added. The equivalent balance (EB) may be determined
from the absolute value of the sum of the group values of X (if present), R',
RZ
and R3 and the magnitude of the sum of the group contributions of X (if
present), R', R2 and R3 together with the weight added and the molecular
weight of the compound of Formula I, according to the following scheme: The
group contribution of X for either X=CI or X=Br is -1, thus, if X is present,
it is
given a value of -1. The group contribution of each of R', RZ and R3 is
generally zero for all groups except as follows: if the group is CH3C00, CI or
Br, in which case it is -1, or if it is amine (including an imine), ONa, OK or
OLi
in which case it is +1. If the sum of the group contributions for X, R', R2
and
R3 is zero, no adjustment with mineral acid or alkali metal hydroxide (or
solutions thereof) is necessary. If the sum of the group values is a positive
integer, adjustment with mineral acid is desirable, and if it is negative,
adjustment with alkali metal hydroxide is desirable.
For example, where R'=OCH3, R2=CH3, R3=CI and X=Br, the
sum of the group values (g.v.) is:
~ _ (g.v. OCH3)+(g.v. CH3)+(g.v. CI)+(g.v. Br) _ (0)+(0)+(-1 )+(-1 ) _ -2
The negative sign in front of the sum indicates adjustment with
alkali metal hydroxide is required. The number of equivalents of alkali
required is given by the equivalent balance (EB) which includes the absolute
CA 02288607 1999-11-04
WO 98/53004 PCT/CA98/00499
-17-
value of the sum of the group contributions ( ~ ~ ~ ) as a scaling factor:
EB = ~~, ~ x weight in grams of the chemical added
molecular weight of the added chemical
In continuing the example, if a process according to the present
invention were scaled so as to require 6,000 grams of a chemical of Formula I
with a molecular weight of 350 grams and the sum of the group values gave
-2, EB would be calculated as follows:
EB = -2 x 6000/350 = -34.28 gram-equivalents
Thus, in this example, 34.28 gram-equivalents of alkali metal
hydroxide would be added. Sodium hydroxide is the preferred alkali metal
hydroxide. The weight of sodium hydroxide would be:
Weight = (EB) x (Equivalent Weight of NaOH) = 34.28 x 40.0 = 1371.2 grams
The preferred technique according to the invention is to dissolve
the alkali metal hydroxide or mineral acid in water so as to obtain a
concentration between 5 and 25% by weight and most preferably between 5
and 10% by weight prior to adding the solution to the slurry.
It is known to incorporate a coupling agent into rubber that is
intended to be vulcanized and used, for instance, in tires. Suitable coupling
agents include those described in United States patent 4,704,414, published
European patent application 0,670,347A1 and published German patent
application 4435311A1, the disclosures of each of which are incorporated by
reference. One suitable coupling agent is a mixture of bis[3-(triethoxy-
silyl)propyl]monosulfane, bis[3-(triethoxysilyl)propyl]disulfane, bis[3-
(triethoxy-
silyl)propyl]trisulfane and bis[3-(triethoxysilyl)propyl]tetrasulfane and
higher
sulfane homologues - for example, coupling agents available under the trade
names Si-69 (average sulfane 3.5), SilquestT"" A-1589 or Si-75 (average
sulfane 2.0). Another non-limiting examples of a suitable coupling agent is
CA 02288607 1999-11-04
WO 98/53004 PCT/CA98/00499
-18-
bis[2-(triethoxysilyl)ethyl)tetrasulfane, available under the trade name
Silquest
RC-2. In the past, achieving a good balance between the coupling agent and
particles, such as silica, without scorching or premature curing has proven
difficult. In accordance with the invention, if particles, particularly silica
particles, are being treated to render them hydrophobic for use in rubber
which is subsequently to be vulcanized, it is possible to include a step of
adding a coupling agent in the process of the invention, so that the coupling
agent becomes attached to the surface of the hydrophobicized mineral
particles and becomes dispersed in the rubber with the mineral particles.
Thus, in some preferred embodiments of the invention, a
coupting agent is added to the dispersion, more preferably after the addition
of the compound of Formula I but before the compound of Formula II is
added. As discussed above, in some cases, Formulae I and II may represent
the same compound. In these cases, it is preferred to add the coupling agent
between sequential additions of the compound of Formulae I and Il.
The coupling agent may be added after any addition of mineral
acid or alkali metal hydroxide that is indicated by the calculation of the EB.
Non-limiting examples of suitable coupling agents include compounds of
formula:
RsRsR,oMR"
in which at least one of R8, Rs and R'°, preferably two of R8, Rs and
R'° and
most preferably R8, Rs and R'°, are hydroxyl or hydrolysable groups.
The
groups R8, Rs and R'° are bound to the atom M, which is silicon,
titanium or
zirconium. The group R8 may be hydroxyl or OCPH2p+1 where p is from 1 to
10 and the carbon chain may be interrupted by oxygen atoms, to give groups,
for example, of formula CH30CHz0-, CH30CHZOCH20-, CH3(OCH2)40-,
CH30CH2CH20-, CZHSOCH20-, C2H$OCH20CH20- or C2H50CH2CH20-.
Aitemativefy R8 may be phenoxy. if M is titanium or zirconium, Re may be the
neopentyl(diallyl)oxy group, but not if M is silicon. The group R9 may be the
same as R8. If M is silicon, Rs may also be a C,-,o alkyl group, a phenyl
CA 02288607 1999-11-04
WO 98/53004 PCT/CA98/00499
-19-
group, or a CZ_,° mono- or diunsaturated alkenyl group. If M is
titanium or
zirconium, R9 may be the neopentyl(diallyl)oxy group, but not if M is silicon.
Further, R8 may be the same as the group R" described below.
R'° may be the same as R8, but it is preferred that R8, R9 and
R'° are not all hydroxyl. If M is silicon, R'° may also be
C,_,° alkyl, phenyl,
C2_,° mono- or diunsaturated alkenyl. If M is titanium or zirconium,
R'° may be
the neopentyl(diallyl)oxy group, but not if M is silicon. Further R'°
may be the
same as the group R" described below.
The group R" attached to M is such that it may participate in a
crosslinking reaction with unsaturated polymers by contributing to the
formation of crosslinks or by otherwise participating in crosslinking. In the
case where M is silicon, R" may have one of the following structures: R" may
represent the allyl group -H2CCH=CH2, the vinyl group -CH=CH2, the 5-
bicycloheptenyl group or the group described by
-(alk)Q(Ar}fS;(alk)°(Ar)hSiR8R9R'°
where R8, R9 and R'° are the same as previously defined, alk is a
divalent
straight hydrocarbon group having between 1 and 6 carbon atoms or a
branched hydrocarbon group having between 2 and 6 carbon atoms, Ar is
either a phenylene -CeH4 , biphenylene -C6H4 C6H4 or -C6H4 OC6H4 group
and e, f, g and h are either 0, 1 or 2 and i is an integer from 2 to 8
inclusive
with the provisos that the sum of a and f is always 1 or greater than 1 and
that
the sum of g and h is also always 1 or greater than 1. Alternately, R" may be
represented by the structures (alk)e(Ar~SH or (alk)e(Ar)fSCN where a and f are
as defined previously. Moreover, it is possible for R" to have the structure
-(CH=CH)k(CI"IZ}rt,(~%sl"la}~(O)o(CI"IZ}PR's
wherein k, m, n and o and p are all whole numbers and R'3 represents the
acryloxy CH2=CHCOO- or the methacryioxy CH2=CCH3C00-group. Further,
the value of k may be 0 or 1, m may be from 0 to 20 inclusive, n may be
CA 02288607 1999-11-04
WO 98/53004 PCT/CA98100499
-20-
between 0 and 2, o may be 0 or 1, and p may be from 0 to 20 inclusive, with
the provisos that the sum of k, m, n and o is at least 1 and not greater than
20, and that if n is 1 or 2 or o is 1, p is 1 or greater. It is most
preferable that
m=3 and k, n, o and p are all 0.
Preferably, R8, R9 and R'° are all either OCH3, OCzHS or OCHB
groups and most preferably all are OCH3 groups. It is most preferred that the
coupling agent is bis[3-(trimethoxysilyl)propyl]tetrasulfane (Si-168). The
amount of coupling agent to add is optional; levels between 2 and 10 percent
by weight of the silica in the slurry (dry basis) are preferred. The
dispersion of
the chemical may be effected by mixing.
Non-limiting illustrative examples of other coupling agents
include the following: bis[(trimethoxysiiyi}propyl)]disulfane (Si-166),
bis[(triethoxysilyl)propyl)]disulfane (Si-266), bis[2-(trimethoxysilyl)ethyl]-
tetra-
sulfane, bis[2-(triethoxysilyl)ethyl]trisulfane, bis[3-
(trimethoxysilyl)propyl]-
disulfane, 3-mercaptopropyltrimethoxysilane, 3-mercaptopropylmethyldi-
ethoxysilane, 3-mercaptoethylpropylethoxymethoxysilane, 1,3-bis(3-
acryloxypropyl)tetramethoxydisiloxane, acryloxypropylmethyldimethoxysilane,
3-methacryloxypropyl-trimethoxysilane, allyltrimethoxysilane,
diallyldiethoxysilane, 5-(bicycloheptenyl)triethoxysilane, 5-
(bicycloheptenyl)methylmethoxyethoxysilane, isopropoxytriacryltitanate,
diisopropyldimethacryltitanate, diethoxydi(3-mercaptopropoxy)zirconate,
triisopropoxy-(2-mercaptoethoxy)zirconate, and di[neopentyi(diallyl)oxy]-di{3-
mercaptopropoxy~zirconate.
Other preferred coupling agents include those disclosed in
published German patent application 44 35 311 A1. On pages 2 and 3, there
is disclosure of oligomers and polymers of sulphur containing
organooxysilanes of the general formula:
Sx_2-R ~-S
Si(OR2)~R3-~
m
CA 02288607 1999-11-04
WO 98/53004 PCT/CA98/00499
_21 _
in which R' is a saturated or unsaturated, branched or unbranched,
substituted or unsubstituted hydrocarbon group that is at least trivalent and
has from 2 to 20 carbon atoms, provided that there are at least two carbon-
sulphur bonds, R2 and R3, independently of each other, are saturated or
unsaturated, branched or unbranched, substituted or unsubstituted
hydrocarbon groups with 1 to 20 carbon atoms, halogen, hydroxy or
hydrogen, n is 1 to 3, m is 1 to 1000, p is 1 to 5, q is 1 to 3 and x is 1 to
8.
Preferred compounds are of the general formula
SX_1 S
C HZ-C H2-S i(O R2)3
x
wherein R2, m and x have the meanings given above, and RZ is preferably
methyl or ethyl. These compounds disclosed in German Patent Application
No. 44 35 311 A1 are preferred coupling agents for use in the present
invention.
Also preferred for use in this invention are coupling agents
disclosed in the abovementioned published European patent application
0,670,347A1, which discloses coupling agents of the general formula:
R'R2R3S'-X'-(-SX Y-)m (-Sx Xz-SIR'R2R3)~
in which R', RZ and R3 are the same or different and are C,~ alkyl, C,~
alkoxy,
phenyl or phenoxy, provided that at least one of R', Rz and R3 is an alkoxy or
phenoxy group. X' and X2 are the same or different and are divalent linear or
branched, optionally unsaturated C,_,Z alkyl groups, Y is a di-, tri- or
tetravalent linear, branched or cyclic C,_,8 alkyl group that is optionally
CA 02288607 1999-11-04
WO 98/53004 PCT/CA98/00499
-22-
unsaturated and is optionally substituted by C6_,2 aryl, C,_8 alkoxy or
hydroxy
groups and which can be interrupted by oxygen, sulphur or nitrogen atoms or
aromatic Cs_,2 aryl groups, or Y is a C6_,2 aryl or heteroaryl group, m is an
integer from 1 to 20, n is an integer from 1 to 6 and x is an integer from 1
to 6.
Particularly preferred coupling agents are those of the following
general formulae:
(RO)3SiCH2CH2CH2-tSX-CH2CH2-~SX-CH2CH2CH2Si(OR~
in which R = -CH3 or -C2H5, x=1-6 and n=1-10;
OH
I
(RO)3SiCH2CH2CH2 Sx-CH2-CH-CH2 Sx-CH2CHZCH2Si(OR~
n
in which R = -CH3 or -CZHS, x = 1-6 and n = 1-10;
(RO)gSiCH2CH2CH2~~-(CH2)g-~-nSX-CHZCHZCH2Si(ORy~
in which R = -CH3, -C2H5 or -C3H,, n = 1-10 and x = 1-6;
OR OR
I I
HgC-SiCH2CH2CH2~-(CHZ)g ~ Sx-CH2CH2CHZSi-CHg
OR OR
in which R = -CH3, -C2H5 or -C3H~, n = 1-10 and x = 1-6;
CA 02288607 1999-11-04
WO 98/53004 PCTlCA98/00499
-23-
OR OR
HgC-SICH2--fSX-(CH2)6 USX-CH2-Si-CHg
OR OR
in which R = -CH3, -C2H5 or -C3H,, n = 1-10 and x = 1-6;
(RO)3Si-CHZCH2CH2-f-SX-CHZCH20CH2CH2-~SX CH2CH2CHZSi(ORy3
in which R = -CH3, -C2H5, -C3H,, n = 1-10 and x = 1-6;
(RO)3St-CH2CH2CH2-E-SX-CH2 / \ CH2-~SX-CH2CH2CH2Si(OR~
in which R = -CH3, -C2H5 or C3H,, n = 1-10 and x = 1-6;
(RO)3Si-CH2CH2CH2-~Sx~N ~~Sx-CH2CH2CHZSi(ORy3
I
N \\ / N
~R1
in which R = -CH3, -C2H5, or -C3H,; R' _ -CH3, -C2H5, -C3H,, -CsH5, -OCH3, -
OC2H5, -OC3H~ or -OC6H5, n = 1-10 and x = 1-8; and
(RO)gSi-CH2CHZCH2-f-SX-(CH2) r~--SX-(CH2)grCh12CH2CH2Si(ORg)
in which R = -CH3, -C2H5 or -C3H,, r+p = 2-10 and x = 1-fi.
Especially preferred are coupling agents of the formulae:
CA 02288607 1999-11-04
WO 98/53004 PCT/CA98/00499
-24-
(RO)3SiCH2CH2CH2 f Sx'(CH2CH2)g~Sx-CH2CH2CH2-Si(OR~
OH
I
(RO)gSiCH2CH2CH2 Sx-CH2-CH-CH2 Sx-CH2CH2CH2Si(OR~
n
in which x is 1-6 and n is 1-4.
In Step (b) of the present process, the compound of Formula II
is added to the particulate filler material. Again, it is preferred that the
particulate filler material, more preferably a mineral filler, is in the form
of an
aqueous slurry or a dispersion, and the compound of Formula II is added to
the slurry or dispersion under intense mixing. In the compound of Formula II
the possible and preferred values for R'S, R'6 and R" are the same as the
possible and preferred values for R', Rz and R3 that are discussed above in
relation to Formula I. If R'2 is an amino group of formula -R'8-NR'9R2o,
preferred values for R'8 are such that N-R'8-Si includes groups of the
formula:
N'(CHZ)P(0)o(Csf.la)UCE"12)mUf"I=CI"I)k Si
in which k is 0 or 1, m is 0 to 20 inclusive, n is 0, 1 or 2, o is 0 or 1 and
p is 0
to 20 inclusive, provided that the sum of k, m, n, o and p is at least 1 and
not
greater than 20, and further provided that if o is 1, p is also 1 or greater,
and
the sum of k, m and n is 1 or greater. The order of the moieties between N
and Si is not particularly restricted other than neither N or O should be
directly
bound to Si. There should be no hydrolysable group between the silicon and
nitrogen atoms. Preferably k, n, o and p are all 0 and m is 3, i.e. R'8 is
-CH2CH2CH2.
R'Z may be a moiety containing at least one primary, secondary,
or tertiary amine nitrogen. In this case the amino group bonded to R'8- is
CA 02288607 1999-11-04
WO 98/53004 PCT/CA98/00499
-25-
given by the formula -NR'9R2°. R'g may be a H or a C,~o alkyl group or
a C2~o
mono-, di- or tri-unsaturated alkenyl group. R'9 may also be a C,_2o alkyl-
substituted or C2_~o alkenyl-substituted aromatic group. The aromatic group
may be, for example, the phenylene group -(CeH4)-, the biphenylene group
-(C6H4)-(CgH4)-, the -(CsH4)-O-(C8H4r group, or the naphthylene group
-(C,oHs)-. R2° may be one of the same groups as R'9 with the further
proviso
that at least one of R'9 and RZ° must contain a continuous carbon chain
of at
least 8 carbons in length, uninterrupted by any heteroatoms.
As stated above, if R'9 and RZ° are other than hydrogen, the
carbon atom attached to the nitrogen atom is not tertiary. Preferably the
carbon atom attached to the nitrogen atom is primary, i.e., -CH2 .
It is preferred that R'9 is a mono-unsaturated alkenyl group of
12-20 carbons in length and most preferable that R'9 is a monounsaturated
alkenyl group of 16 to 18 carbons in length. It is most preferable also that
R2°
is H.
Alternatively, R'2 may be a moiety which contains a mineral acid
salt or a quaternary ammonium salt of an amine. The formula of R'Z may thus
be described by the extended formula -R'$-NR'9R2°~R2'x wherein -R'8-,
R'9
and RZ° are as previously defined and RZ' may be a H, or a C,_~o alkyl
or C2.~o
mono-, di- or tri-unsaturated alkenyl group and X is an anion, preferably CI
or
Br, although sulphate can be used.
There is the further proviso that at least one of R'9 and R2° must
contain a continuous carbon chain of at least 8 carbons in length,
uninterrupted by any heteroatom. It is preferred to use an amine salt where
R'9 is a mono- or di-unsaturated alkenyl group of 12-20 carbons in length and
most preferably that R'9 is a mono- or di-unsaturated alkenyl group of 16 to
18 carbons in length. It is most preferable also that R2° is H and that
R2' is H
and X is chiorine. The preferred hydrophobicizing agent of Formula II is N-
oleyl-N-{3-trimethoxysilyl)propyl ammonium chloride.
Preferably, the amount of the hydrophobic compound of
Formula II to add is generally between 0.5 and 20 percent by weight of the
weight of the particles (preferably mineral particles such as silica) in the
slung
CA 02288607 1999-11-04
WO 98/53004 PCT/CA98/00499
-26-
(dry basis), and is inversely proportional to the particle size of the silica
particles. The compound may be added to the slurry in its natural state,
either as a liquid or a solid. However, to facilitate dispersion, it is
preferred,
where possible, to add the compound as a liquid. If the melting point of the
compound is below 95 degrees Celsius, it is preferred to add it to the slurry
in
a molten state at a temperature at least 5 degrees Celsius above the melting
point, provided the temperature of the compound in the liquified state does
not exceed 100 degrees Celsius and provided that the compound does not
decompose under these conditions. If the melting point exceeds 95 degrees
Celsius, it is most preferred to use a solvent. Suitable solvents are alcohols
containing 1 to 5 carbon atoms and most preferably those containing 1 to 3
carbon atoms, that is to say methanol, ethanol, n-propanol or isopropanol. If
the compound of Formula II is an alkoxysilane, most preferably the alkoxy
group of the solvent alcohol wilt be the same as the alkoxy group of the
alkoxysilane. For example, if the compound of Formula II is a methoxysilane,
the preferred solvent is methanol. The concentration of the compound in the
solvent may be from 10 to 90 percent by weight and most preferably between
and 75 percent by weight and most preferably 50 percent by weight.
Preferably, the solution is prepared and added to the slurry at a temperature
20 between a lower limit of 0 degrees Celsius and an upper limit which is the
lower of at feast 10 degrees below the boiling point of the solvent and 95
degrees Celsius.
After the addition of the hydrophobic compound of Formula II
which is added, the equivalent balance (EB) should be calculated to
25 determine how much, if any, mineral acid or alkali metal hydroxide (or
solutions thereof) to add. The equivalent balance (EB) may be determined
from the absolute value of the sum of the group values of X, R'S, R'6 and R"
and the weight added, and the molecular weight of the compound, according
to the following scheme: The group contribution of X for either X=CI or X=Br
is
-1, thus if X is present it is given a value of -1. The group contribution of
each
of R'S, R'6 and R" is generally zero for all groups except as follows: if the
group is CH3C00°, CI° or Br°, in which case it is -1, or
if it is amino, ONa,
CA 02288607 1999-11-04
WO 98/53004 PCT/CA98/00499
-27-
OK, or OLi in which case it is +1. If the sum of the group contributions for
X,
R'S, R's and R" is zero, no adjustment with mineral acid or alkali metal
hydroxide (or solutions thereof) is necessary. If the sum of the group values
is a positive integer, adjustment with mineral acid is desirable, and if it is
negative, adjustment with alkali hydroxide is desirable.
For example, where R'S=OCZHS, R'6=OCH3 R"=CH3 and X=CI,
the sum ~ of the group values {g.v.) is:
~ _ {g.v. OC2H~)+{g.v. OCH3)+(g.v. CH3)+{g.v. CI) ={0)+(0)+(0)+(-1 ) _ -1.
The negative sign in front of the sum indicates adjustment with
alkali metal hydroxide is required. The number of equivalents of alkali
required is given by the equivalent balance (EB) which includes the absolute
value of the sum of the group contributions ( ~ ~ ~ ) as a scaling factor.
EB = ,~S I x weight in grams of the compound added
molecular weight of the added chemical.
In continuing the example, if a process according to the present
invention were scaled so as to require 3450 grams of a compound of Formula
II with a molecular weight of 466 grams and the sum of the group values gave
-1, EB would be calculated as follows:
EB = ~-1 ~ x 3450/466 = 7.4 gram-equivalents.
Thus, in this example, 7.4 gram-equivalents of alkali metal
hydroxide would be added. Sodium hydroxide is the preferred alkali metal
hydroxide. The weight of sodium hydroxide added would be:
Weight = (EB)x(Equivalent Weight of NaOH) = 7.4 x 40.0 = 296 grams.
The preferred technique according to the invention is to dissolve
the alkali hydroxide or mineral acid in water so as to obtain a concentration
CA 02288607 1999-11-04
WO 98/53004 PCT/CA98/00499
- 28 -
between 5 and 25% by weight and most preferably between 5 and 10% by
weight prior to adding the solution to the slung. The temperature of the
solution may be from 0 degrees Celsius to 100 degrees Celsius under
atmospheric pressure, or if a pressure vessel is used for preparation of the
solution, it may be from 0 degrees Celsius to 130 degrees Celsius. It is
preferred that the temperature of the solution be within 10 degrees of the
solution of the slurry. The dispersion of the solution in the slurry is
effected by
mixing.
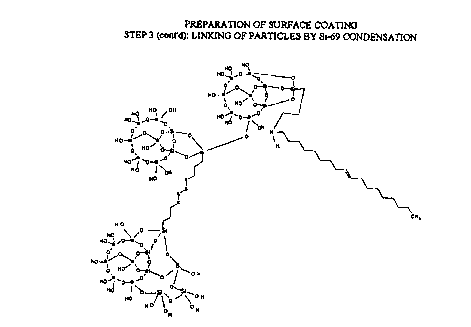
While not wishing to be bound by any particular theory or mode
of action, it is believed that the mechanism of the present process of
treating
particulate materials can be illustrated as shown in Figures 1-4. In this
illustrative reaction scheme, silica is shown as the particulate material
being
treated with a specific compound falling within Formulae I and II defined
hereinabove. Of course, Figures 1-4 are provided for illustrative purposes
only and should not be used to limited the scope of the invention.
With reference to Figure 1, there is illustrated an initial step in
the present process. As illustrated, an adduct of 3-CPTMS/oleylamine',
preferably in the form of a methanolic solution, is added to an aqueous slurry
of silica particles. The methoxy silane linkages in the adduct are readily
hydrolysed by water or by contact with the acidic surface silanol groups on
the silica resulting in condensation of the adduct on the surface of the
silica
particles.
With reference to Figure 2, a preferred step in the present
process is illustrated. Specifically, an active surface-bound catalyst is
prepared by neutralization of the hydrochloride salt with a base (NaOH is
shown). The strongly basic amine so produced is believed to further react
with surface silanols resulting in deprotonation of the latter, as shown.
With reference to Figure 3, another preferred step in the present
'N-(3-trimethoxysilylpropyi)-N (octadec-9-enyl) ammonium chloride, the
production of which is discussed in a copending International application
filed on
even date herewith in the name of the Applicant, the contents of which are
hereby
incorporated by reference.
CA 02288607 1999-11-04
WO 98/53004 PCT/CA98/00499
-29-
process is illustrated. Specifically, a coupling agent commercially available
under the tradename Si-692 is added to the active surface-bound catalyst
produced in Figure 2. Preferably, the coupling agent is added slowly (this
minimizes self condensation) and under high shear conditions (this facilitates
dispersion). The deprotonated silanol groups readily react with the ethoxy
moieties on the Si-69 releasing ethanol and regenerating the active catalyst
(not shown). The process continues until the Si-69 coupling agent has
reacted. The condensed Si-69 moieties may link several silica particles
together (see Figure 4 and discussion below) or, in the case of larger
particles
or agglomerates, substantially all condensed endgroups may be attached to
the same particle.
With reference to Figure 4, after sufficient time has been
allowed for the Si-69 coupling agent to react to completion, additional 3-
CPTMS/oleylamine adduct is added to react with most of the remaining
residual silanol groups (Figure 1 ). Preferably, this is again followed by
neutralization with a base (Figure 2). The long alkyl groups now attached to
the surface of the silica particle render the particle strongly hydrophobic
and
thus more compatible with, inter alia, a hydrocarbon polymer cement.
Further, the bulky alkyl groups attached to the silica particles serve to
sterically limit the interaction of water molecules with the surface.
The process described thus far provides an aqueous slurry or
dispersion of hydrophobicized silica (i.e., it has not yet been contacted with
an
elastomer or other substrate to be filled), which can be used as such or can
be filtered and dried. Hydrophobicized silica can be used as a filler in a
multitude of materials including, but not limited to, the following:
elastomers,
alkyd paints, as a component of antifoaming preparations or foam regulators
in laundry detergents, or as toners such as those used in photocopiers, and
rubber vulcanizates. Mention is made particularly of tire treads and of shoe
2While bis[3-(triethoxysilyl)propyl]tetrasulfane is shown in Figure 3, as
discussed hereinabove, those of skill in the art recognize that Si-69 is
mixture of
bis[3-{triethoxysilyl)propyl]monosulfane, bis[3-
(triethoxysilyl)propyl]disulfane, bis[3-
(triethoxysilyl)propyl]trisulfane and bis[3-
{triethoxysilyl)propyl]tetrasulfane (average
sulfane 3.5).
CA 02288607 1999-11-04
WO 98/53004 PCT/CA98/00499
-30-
soles.
In a preferred embodiment of this invention the hydrophobicized
silica, in the aqueous dispersion or slurry, is incorporated into a polymer,
for
example an elastomer to form a rubber masterbatch. It is particularly
preferred that the hydrophobicized silica shall have been treated with a
coupling agent, for example Si-69, Si-168 or Silquest RC-2, as discussed
above. The slurry is mixed with a hydrocarbon or other solution of the
elastomer. Preferably, the solvent in which the elastomer is dissolved is
immiscible with, or mostly immiscible with, water to form a preblend. This
elastomer solution may be made by dissolving the solid elastomer in a
solvent, or it may be the solution resulting from the polymerisation of
monomers in the solvent. The elastomer may be a hydrocarbon rubber, a
graft polymer or block polymer of monomers having at least one ethylenically
unsaturated bond and polymerizable through this unsaturation. Other
suitable polymers include, but are not limited to IIR, HIiR, IR, EPDM, SBR,
BR, NBR, HNBR, HSRE, natural rubber, polystyrene, polychloroprene,
epichlorohydrin (ECO), chlorinated polyethylene, silicone and ABS. Suitable
solvents include but are not limited to cyclohexane, hexane, benzene, toluene
and pentane. Optionally, processing oil and antioxidants may be added to
the hydrocarbon solution prior to mixing with the slurry, or they may be added
after mixing the slurry and the elastomer solution.
The viscosity of the final elastomer solution, sometimes referred
to as an elastomer cement, containing the optional ingredients is preferably
such that it closely matches the viscosity of the silica slurry and is
generally
between 1,000 and 50,000 centipoise. The temperature of the elastomer
solution is preferably the same as that of the slurry and the amount of cement
that is added is such that the final masterbatch may contain from 5 to 250
parts of silica per hundred parts of elastomer, preferably from 35 to 100
parts
of silica per hundred parts of elastomer, most preferably from 60 to 80 parts
of silica per hundred parts of elastomer.
The elastomer cement and, optionally, oil and antioxidants, is
mixed with the silica slurry until the mixture becomes homogeneous and the
CA 02288607 1999-11-04
WO 98/53004 PCT/CA98/00499
-31 -
milky colour of the silica slurry disappears to form a preblend. A small
amount of water may separate at this stage.
If not added previously, or if additional amounts are desired, oil
and antioxidants may be added next and the mixing continued further until the
oil and antioxidant become incorporated in the continuous phase.
Any water which separates from the preblend may be removed,
discarded or recycled for silica slurry make-up by stopping the agitator for a
suitable period and allowing the water phase to accumulate in the bottom of
the mixing tank from which it may be drained prior to proceeding with the next
step. Agitation is preferably restarted after the water layer is removed.
If antioxidants and processing oil were not previously added, or
if additional amounts are desired, they may be added at this stage and
stirring
continued until the preblend is again homogeneous.
The preblend is then added to water heated to a temperature
equal to, or preferably higher than the boiling point of the solvent used for
the
elastomer cement so as to remove the solvent and produce a masterbatch
coagulum in the form of a crumb suspended in water. The preferable
temperature of the water prior to addition of the preblend is between 50 and
100 degrees Celsius, most preferably between 90 and 95 degrees Celsius,
and the preblend is added at a rate so as to maintain a so-fixed or reasonably
so-axed water temperature throughout the coagulation. The agitation is set
sufficiently high so as to maintain the crumb in a suspended state within the
water but not so high as to cause the crumb to subdivide into particles
smaller
than approximately 5 millimeters.
The solvent may be recovered from the coagulator by
recondensing the vapours. The material containing the suspended crumb is
passed through a fitter screen sized so as to recover the wet masterbatch.
The material passing through the screen may be optionally recycled for
further silica slurry make-up.
The wet crumb is dried such as by using forced air or fluidized
bed or microwave drying techniques at a temperature between about 75 and
about 135 degrees Celsius, preferably between about 85 and about 120
CA 02288607 1999-11-04
WO 98/53004 PCT/CA98/00499
-32-
degrees Celsius, most preferably between about 85 and about 105 degrees
Celsius, until a suitably dry masterbatch crumb is obtained.
The dried crumb may be further processed according to industry
and customer requirements.
Preferred embodiments of the invention are illustrated by the
examples which follow. The examples can be interpreted with the aid of
Figure 5 which illustrates an arrangement for carrying out a masterbatch
process embodiment of the invention, according to Examples I and II. The
legend in Figure 5 is as follows:
R1: A balance-mounted portable paint pot of nominal capacity 120 litres.
The pot is equipped with a Strahman (piston) bottom valve (Vs), an
oversized air-operated motor, one 6-inch radial flow agitator (top) and
one 10-inch marine impeller (bottom) on a single shaft, and an external
steam coil (J) for heating. The lower impeller has approximately 2
inches of clearance from the bottom of R1; the top impeller is attached
at a point 9 inches higher. A valued chemical addition port (P1 ) is
available on the removable lid and the pot may be purged with nitrogen
through another port (N 1 ) when transfer of the contents is required. A
water line may be coupled to an additional port (W). A portable
exhaust snorkel (E) is available in the vicinity to remove fugitive
methanol and ethanol emissions. R1 is used for the silica slurry
makeup and as a vessel to carry out the described additions to
produce a hydrophobicized silica slurry.
T1: A nominal 500 USG glass-lined chemical reactor used for cement
make-up and storage and as a mixing vessel for the silica slurry and
polymer cement prior to coagulation. It is equipped with a 200 rpm
pneumatic drive, a marine impeller and heating jacket to speed
dissolution of rubbers. It has various addition ports including: M, a
small manhole for introducing rubber and oil, P2, for solvent (hexane)
addition, a nitrogen line port (N2) for pressure transfer of the contents
CA 02288607 1999-11-04
WO 98/53004 PCT/CA98/00499
-33-
through a large bottom drain with a valve (V2). The bottom valve is
located a short distance from the tank bottom in order to reduce dead
space in the piping.
H: Armoured flex hose, 2 inch diameter, for slurry and cement transfers.
V1: A 3-way valve to control the direction of flow.
T2: A steam coagulator of nominal capacity 400 litres. It is equipped with a
steam sparge port near the bottom and a connection to service water.
An overflow port (P3) and overflow channel are situated close to the
top to allow for product discharge. A large pipe at the top directs
solvent vapours to a condenser (C). The tank is stirred by means of an
air operated motor and an 8-inch diameter marine impeller. S: A 24-
inch diameter Sweco~ shaker screen (100 mesh).
C: A condenser for solvent recovery from coagulation. It is connected to
cold process water through a valve (V4).
T3: A solvent decanter, approx. 250 USG, for recycle solvent storage and
water separation. A valve (V3) allows for sampling and water
discharge.
T4: A 60 litre plastic tank for fines settling.
G: Perforated trays for product dewatering and drying.
Ex: A short (24" long, 3-inch diameter screw) dewatering extruder "Rocket"
powered by an explosion proof motor via a variable speed gearbox.
Embodiments of the present invention will be described with
reference to the following Examples which should not be used to limit the
CA 02288607 1999-11-04
WO 98/53004 PCT/CA98/00499
-34-
scope of the invention.
EXA~LF~t
1 l~ Cement Preparation: A hydrocarbon solution of polybutadiene (~ 16
wt%) was prepared by adding 66.9 kg of a high cis-polybutadiene rubber
(Taktene 1203), previously cut into small pieces, to 351 kg cyclohexane in T-
1. The mixture was stabilized by adding 0.5 kilos each of a hydroperoxide
scavenger (Polygard) and a hindered phenol antioxidant (Irganox 1076) and
dissolution was effected by heating to 60 degrees Celsius with stirring for 2
days. ft was then allowed to cool to ambient temperature in the absence of
agitation.
2) Hydrophobicized Silica Slurry Preparation: The recipes/procedures
used are shown in Table I, which follows.
The agitation rate on R1 was set at 250 rpm during all steps on
each of the four days.
TABLE 1: Preparation of Hydrophobicized Silica Slurry
ub-Step Parameters: DAY 1 DAY 2 DAY 3 DAY 4
a) Slurry preparation
Water k 53.5 53.5 53.5 53.5
Water Tem erature C 65 64 61 58
HiSll-233 silica k 13.4 13.4 13.4 13.4
Stirrin time mins 5 5 5 5
Final Tem erature C 58 57.8 56.2 52
b) Addition of compound of Formula I as the hydrochloride salt: (N-oleyl-
N-(3-trimethoxysilyl)propyl ammonium chloride at 50 wt% in methanol)
Added rams of 50% soln. 133.8 133.8 133.8 133.8
Addition eriod mins 5 5 5 5
Tem . at addition C 58 57.8 56.2 52
CA 02288607 1999-11-04
WO 98/53004 PCT/CA98/00499
-35-
(c) Addition of caustic after calculation of the equivalent balance (EB)
Caustic concentration 5.5/66 5.5/66 5.5/66 5.5/66
NaOH/H O, /
Addition eriod mins 5 5 5 5
Tem . at addition C 58 57.8 56.2 52
(d) Addition of the coupling agent, bis(triethoxysilylpropyl)tetrasulfane (Si-
69) to the silica surface
Si-69 wei ht, k s. 1.07 1.07 1.07 1.07
Addition eriod, mins 30 30 30 30
Reaction time hours 1.25 1.25 1.5 1.5
Initial Tem erature C 58 57 56 52
(e) Addition of compound of Formula II as the hydrochloride salt: (N-oleyl-
N-{3-trimethoxysilyl)propyl ammonium chloride at 50 wt% in methanol
Amt. Added k s 50% soln.~1.2 , 1.2 1.2 1.2
Addition eriod mins 5 5 ., 5
5
Reaction time mins 5 5 5 5
(f) Addition of caustic after calculation of Equivalent Balance
Caustic concentration 52.2/660 52.2/50052.2/66052.2/660
NaOH/H O, rams/ rams
Addition eriod mins 5 5 5 5
Reaction time mins 5 5 5 5
3)~ Cementl8ilica ~lypi m~: On day 1, the first batch prepared siuny
was added to the cement in T1 by vacuum transfer. Four litres of water were
CA 02288607 1999-11-04
WO 98/53004 PCT/CA98/00499
-36-
used to wash down the sides of R1 and the washings were similarly
transferred. No agitation was used on T1. On day 2 and day 3 the process
was repeated. On day 4 20.0 kg. of Sundex 8125 aromatic process oil was
added to T1 from a top manhole. The final batch of silica slurry was then
added and the agitator speed was increased to 200 rpm and heat was
applied to the jacket on T1. After a temperature of 50 degrees Celsius had
been attained, the heating and agitation were stopped and the reactor was
allowed to remain in a quiescent state for 30 minutes.
41 Coaguli ation: The mixture in T1 was pressurised by nitrogen directly
into T2 coagulation vessel maintained at 92-95 degrees Celsius by means of
low pressure steam. The air-driven agitator was started at ~50-60 rpm. The
low speed gave a crumb size of approximately 1 cm and provided sufficient
agitation to prevent the cement from forming a surface cake on the water.
Finishing: The crumb from the coagulation vessel T2 was passed over
a Sweco shaker screen for preliminary dewatering and then allowed to sit for
an hour on an open tray. The initial moisture level measured on the trayed
material averaged 54%. Two trays of wet product at ambient temperature
were passed once through the dewatering rocket. The feeding characteristics
of the material were excellent even at the highest screw speed and it required
two operators to manually feed the material to the extruder in order to keep
up with the discharge rate. The exit temperature was judged to be
approximately 35 degrees Celsius, and the one-pass material had a moisture
level of 31.1 %, or approximately 42% reduction in water content. The product
was reasonably cohesive with the appearance of long strands of spaghetti.
One half of this stranded product was segregated for drying tests and the
other half was passed two more times through the extruder to give a further
reduction in moisture to a final 16.3%. During the second and particularly
third passes, only a small amount of water was recovered from the rear
discharge but the product on exiting the extruder periodically squirted water
from within the material. The exit temperature of the product on the third
pass
was approximately 55 degrees Celsius. The 3-pass product had remarkable
green strength for a still-wet material and considerable force was required to
CA 02288607 1999-11-04
WO 98/53004 PCT/CA98/00499
-37-
break the strands manually.
The 20 full trays obtained were then stacked in the forced air
' dryers maintained at 85 degrees Celsius and dried for 6 hours. The
agglomerated dried product cake was passed through a Cumberland grinder
to homogenize it and then bagged. Yield was approximately 141 kg., 96.4%
of theoretical.
CharacterizationlTesting of Product
The moisture levels measured using a moisture balance set at
105 degrees Celsius on the dried unextruded product average 0.27%, on the
one-pass material 0.26%, but on the 3-pass material it was still 2.4%.
Ash levels on the finished product, were determined by calcining
at 700 degrees Celsius.
The results from the limited characterization of the product are
shown below in Table 2.
CA 02288607 1999-11-04
WO 98/53004 PCT/CA98/00499
-38-
Tra # Moisture % Ash % 700C
1 0.32 nla
2 n/a 33.34
3 0.29 n/a
4 n/a 33.69
5 0.23 n/a
6 n/a 32.1
7 0.18 nla
8 0.24 32.3
9 nla nla
10 0.16 nla
11 0.29 n/a
12 nla nla
13 0.32 n/a
14 n/a 30.9
15 0.22 n/a
16 0.27 n/a
17 n/a n/a
18 0.26 31.62
one-extruder
ass
AV E RAG E 0.25 32.3
1~~ Cement Pre rn~a ation: A solution of an oil extended SBR rubber was
prepared by adding Buna VSL 5025-1 (Buna VSL 1950 S25), previously cut
into small pieces, to hexane in T1. Dissolution was effected by heating to 60
degrees Celsius with stirring. At the time of the trial, T1 held the
equivalent of
62.84 kg of Buna VSL 5025-1 in 183.6 kg of hexane, giving a cement
containing 25.5 wt.% polymer plus oil. The cement was stabilized by adding
0.34 kilos each of Polygard and Irganox 1076.
CA 02288607 1999-11-04
WO 98/53004 PCT/CA98/00499
-39-
2,1~ HysiCQphobicized Silica Sf~mr Pre ar ~Qn_: Two batches of silica slurry
were prepared; this was done over a two day period: The recipes/procedures
' used are shown in Table 3 following. The agitation rate on R1 was set at
250 rpm during all steps on both days.
Table 3: Preparation of I- drophobicized Silica Sl~rrv
Sub-Step Parameters:/Date DAY 1 DAY 2
(a) Slurry preparation
Water k 85.34 60.71
Water Tem erature C 60 60
HiSil-233 silica k 21.37 15.2
Stirrin time mins 5 5
Final Tem erature C 52 52
{b) Addition of a compound of Formula I as the hydrochloride salt: (N-oleyl-N-
(3-trimethoxysilyl)propyl ammonium chloride at 50 wt% in methanol
Added rams of 50% soln. 213.6 151.9
Addition eriod mins 5 5
Tem . at addition C 52 52
(c) Addition of caustic after calculation of equivalent balance
Caustic concentration 8.55175 6.08/50
NaOH/H O , rams/ rams
Addition eriod mins 5 5
Tem . at addition C 52 52
CA 02288607 1999-11-04
WO 98/53004 PCTlCA98/00499
-40-
(d) Addition of coupling agent Si-69 to the silica surface:
Si-69 wei ht, k s. 1.71 1.21
Addition eriod, mins 30 30
Reaction time hours 1.25 1.25
Initial Tem erature C 52 52
Final Tem erature C 48.6 46.6
(e) Addition of compound of Formula II as the hydrochloride salt (N-oleo-N-(3-
trimethoxysilyl)propyl ammonium chloride at 50 wt% in methanol
Amt. Added k s 50% soln. 1.92 1.37
Addition eriod mins 5 5
Tem . at addition C 48.6 46.6
Reaction time mins 5 5
Final Tem erature C 47.2 46
(f) Addition of caustic after calculation of equivalent balance
Caustic concentration 83.1 /700 59.31500
NaOH/H O, rams/ rams
Addition eriod mins 5 5
Tem . at addition C 47.2 46
Reaction time mins 5 5
Final Tem erature C 47 45.4
3): Cement/Silica slurry mixing: On day 1 the first batch prepared slurry
was added to the 60 degrees Celsius cement in T1 by vacuum transfer. Four
litres of water were used to wash down the sides of R1 and the washings
were similarly transferred. T1 was stirred under mild agitation and 60
CA 02288607 1999-11-04
WO 98/53004 PCT/CA98/00499
-41 -
degrees Celsius thermostatting overnight. On day 2, the second batch of
slurry was added to T1, again by vacuum transfer. R1 was washed again
with 4 litres of water and the washings added to T1. The agitator speed was
increased to 200 rpm for 10 minutes after which agitation was stopped. After
standing for 15 minutes, some of the water layer from the bottom of T1 was
cautiously removed to ascertain the extent of transfer of the silica to the
cement (organic phase). The first 2 litres of the water phase contained an
estimated 150 grams of coated silica from the piping dead space in front of
the valve. The remainder of the water phase was clear although slightly
yellowish. Following the sampling, the vessel T1 was again put under mild
agitation in preparation for coagulation. Due to the minor amount of
untransferred silica, no corrections were made to the formulation.
4.)~ Coagulation: The mixture in T1 was pressurised by nitrogen directly
into T2 coagulation vessel maintained at 92-95 degrees Celsius by means of
low pressure steam. The agitator speed was adjusted downward to 100 rpm
and finally to -50-60 rpm. The low speed was found adequate to maintain a
crumb size of approximately 1 cm while still preventing the preblend from
forming a surface cake on the water. A flow rate of 1 kg/min of material from
T1 into the coagulator was found satisfactory to devolatilize the crumb. The
entire contents of T1 were coagulated in one pass.
5~,Finishing: The crumb from the coagulation vessel T2 was passed over
a Sweco shaker screen for preliminary dewatering and then allowed to sit for
an hour on an open tray. The initial moisture level measured on the trayed
material was 60-65%. The 18 full trays obtained were then stacked in the
forced air dryers maintained at 85-90 degrees Celsius and dried for 4-6 hours.
During this period the product was turned over once by hand to provide even
drying. The agglomerated dried product cake was passed through a
Cumberland grinder to homogenize it and then bagged. Yield was
approximately 99 kg., 95.3% of theoretical.
CharacterizationlTesting of Product
Final moisture levels on the dried product ranged from 0.2-
CA 02288607 1999-11-04
WO 98/53004 PCT/CA98/00499
-42-
0.5%, measured on a moisture balance set at 105 degrees Celsius. Thermo-
gravimetric analysis (TGA) on the finished product indicated an ash level of
31.72%.
A masterbatch of a silica-filled vinyl solution styrene butadiene
rubber (SSBR MB), the product of Example I, was converted to a vulcanized
rubber for use in tire treads. For comparison there was also tested a dry
blend of the same rubber, mixed with silica particles that had not been
hydrophobicized in accordance with the invention.
The masterbatch (228 parts), composed of 100 parts of rubber,
37.5 parts of aromatic oil extender, 80 parts of silica particles
hydrophobicized
in accordance with the invention by treatment with N-oleyl-N-(3-
trimethoxysilyl)propyl ammonium chloride, with
bis(triethoxysilylpropyl)sulfane
(Si-69) (6.4 parts} and again with N-oleyl-N-(3-trimethoxysilyl)propyl
ammonium chloride was placed in a Banbury mixer, BR-82 (Capacity 1.6
litres) under the following conditions:
Speed: 77 RPM
Start Temperature: 40°C
Ram Pressure: 30 psi
Mokon: Set at 25°C
To the masterbatch there were added stearic acid (1 part) and
zinc oxide (2.5 parts) as activators, and these were mixed for 180 seconds.
Any ingredients that had risen onto the surface and escaped from the mass in
the mixer were swept back into the mass and mixing continued for a further
60 seconds, after which time the mixture was dumped from the Banbury
mixer. To the mixture on a warm mill there were then added sulphur (1.4
parts), an accelerator Vulkacit CZ/EG-C(CBS) (1.7 parts) and a further
accelerator Vulkacit D/C (DPG) 2 parts. These ingredients were refined on
the mill (6 passes) to give a product whose specific gravity was 1.193. The
CA 02288607 1999-11-04
WO 98/53004 PCT/CA98100499
-43-
total mixing time taken, from commencing mixing in the Banbury mixer to the
completion of milt mixing, was 8 minutes.
For the dry mix, vinyl solution styrene butadiene rubber
containing 100 parts polymer and 37.5 parts aromatic oil extender were
placed in the Banbury mixer and mixed for 60 seconds. After 60 seconds,
there were added untreated silica particles (Hi-Sil 233, 40 parts) and Si-69
coupling agent (3.2 parts) and mixing continued for a further 60 seconds.
After 120 seconds, a further 40 parts of untreated silica particles and 3.2
parts of Si-69 were added and ingredients that had escaped from the mass
were swept back into the mass, and mixing continued for a further 60
seconds. After 180 seconds, the ram of the mixer was raised, escaped
ingredients were swept back into the mass, the ram lowered and mixing
continued for a further 60 seconds. After 240 seconds, escaped ingredients
were again swept back into the mass and stearic acid (1 part) and zinc oxide
(2.5 parts) were added. Mixing was resumed, and after 300 seconds
escaped ingredients were swept back into the mass, and mixing continued.
After 420 seconds, the mass was dumped out of the mixer and formed into a
sheet.
The mixer was allowed to cool to 40°C, then the mass was
returned to the mixer and mixing continued until the temperature of the ram
temperature probe reached 150°C. The mass was then dumped out. To the
mixture on a warm mill there were then added sulphur (1.4 parts). Vulkacit
CZ/EG-C (CBS) (1.7 parts) and Vulkacit D/C (DPG) (2 parts) and these
ingredients refined on the mill (6 passes) to give a product whose specific
gravity was 1.195. The total mixing time from commencement of mixing was
15.5 minutes.
The silica dry mix and the masterbatch of the invention were
then subjected to tests whose results are given below:
CA 02288607 1999-11-04
WO 98/53004 PCT/CA98/00499
- 44 -
VSBR Silica dry Mix vs Masterbatch
SILICA DRY MIX SSBR MB
COMPOUND MOONEY VISCOSITY
ML 1+4' @ 100°C (MU) 61.9 78.6
Mooney Relaxation:
Time to Decay 80% (min) 0.16 0.25
COMPOUND MOONEY SCORCH
Rotor Size:farge
t5 @ 138°C (min) >30 7.8
STRESS STRAIN (DUMBELLS)
Cure time (min) 25 18
Cure Temperature:166C
Stress @ 25% elongation (MPa) 1.1 0.95
Stress @ 50% elongation (MPa) 1.8 1.5
Stress @ 100% elongation (MPa) 3.8 3.1
Stress @ 200% elongation (MPa) 10.5 7.4
Stress @ 300% elongation (MPa) - 14.4
Tensile (MPa) 13.8 14.4
Elongation (%) 240 300
Hardness (A) 68 64
Tensile x Elongation/100 33.1 43.2
DIE C TEAR
Cure Time (min) 25 18
CA 02288607 1999-11-04
WO 98/53004 PCT/CA98/00499
-45-
Cure Temperature 1fi6°C
Tear Strength (kN/m) 26.9 42.6
DIN Abrasion
Volume Loss (mm3) 123 105
Comno~~t~1 Compound
#2
1950525 XQ209
SILICA DRY MIX SSBR MB
ZWICK REBOUND
Cure Time (min) 30 23
Cure Temperature:166C
Resilience @ 0C (%) 4.6 5.0
Resilience @ 23C (%} 13.1 13.0
Resilience @ 100C (%) 61.0 64.6
GOODRICH FLEXOMETER
Cure Time (min} 30 23
Cure Temperature: 166C
Ambient Temperature: 55C
Load on Beam:11 kg
Stroke (Compression): 17.5%
Heat Rise (C) 16.3 14.7
Permanent Set (%) 2.0 1.5
MER 1100 Dynamic Properties
Frequency: 20Hz @ 60C
Load:7% statict3% dynamic
Static Stiffness (kg/mm) 2.93 3.94
Dynamic Stiffness (kg/mm ) fi.33 6.49
Ratio-dynamicatatic 2.16 1.fi5
CA 02288607 1999-11-04
WO 98/53004 PCT/CA98/00499
-46-
Power Loss (g.m/sec) 1.25 1.28
Tan Delta 0.149 0.148
These results suggest that, when used in tire treads, the product
from the masterbatch will display lower rolling resistance, better abrasion
resistance and equal traction characteristics, when compared with the silica
dry mix.
A masterbatch of a high cis polybutadiene rubber, the product of
Example II, was converted to a vulcanized rubber for use in tire treads. For
comparison there was also tested a dry blend mix of the same rubber mixed
with silica particles that had not been hydrophobicized in accordance with the
invention.
The masterbatch (220.4 parts) composed of 100 parts of rubber,
30.0 parts aromatic extender oil, 80 parts of silica particles hydrophobicized
in
accordance with the invention by treatment with N-oleyl-N-(3-
trimethoxysilyl)propyl ammonium chloride, with Si-69 (6.4 parts) and again
with N-oleyl-N-{3-trimethoxysilyl)propyl ammonium chloride was placed in the
same Banbury mixer as used in Example III, and mixed with stearic acid (1
part) and zinc oxide (2.5 parts) under the same conditions as in Example I I
I.
Thereafter, the mixture was dumped from the Banbury mixer and mixed with
sulphur (1.4 parts), Vulkacit CZ/EG-C(CBS) (1.7 parts) and Vulkacit D/C
{DPG) (2 parts) on a warm mill. The ingredients were refined (6 passes) to
yield a product whose specific gravity was 1.193. The total mixing time was 8
minutes.
The dry mix of rubber (100 parts) untreated silica {80 parts) Si-
69 (6.4 parts) stearic acid (1 part) aromatic extender oil (30 parts) and zinc
oxide (2.5 parts) were mixed in the Banbury mixer under the same regime as
described in Example III. Thereafter the mixture was admixed on a warm mill
with sulphur (1.4 parts) Vulkacit CZIEG-C(CBS) (1.7 parts) and Vufkacit D/C
(DPG) (2 parts). The product had a specific gravity of 1.190. The total mixing
CA 02288607 1999-11-04
WO 98/53004 PCT/CA98/00499
- 47 -
time was 15.5 minutes.
The vulcanizates produced from the silica dry mix and the
masterbatch of the invention were then subjected to tests whose results are
given below:
BR Silica Masterbatchs
TAKTENE 1203
+ Silica Silica mb
COMPOUND MOONEY VISCOSITY
ML 1+4' @ 100°C (MU) 64.9 71.3
Mooney Relaxation:
Time to Decay 80% (min) 0.20 0.23
COMPOUND MOONEY SCORCH
Rotor Size:large
t5 @ 138°C (min) 22.75 5.86
STRESS STRAIN (DUMBELLS)
Cure time (min) 13 8
Cure Temperature:166C
Stress @ 25% elongation (MPa) 1.2 0.97
Stress @ 50% elongation (MPa) 1.7 1.4
Stress @ 100% elongation {MPa) 2.7 2.1
Stress @ 200% elongation {MPa) 5.9 5.0
Stress @ 300% elongation {MPa) 10.6 9.4
M300:M100 3.93 4.48
Tensile (MPa) 15.6 14.2
Elongation (%) 395 400
Hardness (A) 67 72
Tensile x Elongation/100 61.62 56.8
CA 02288607 1999-11-04
WO 98/53004 PCT/CA98/00499
-48-
DIE B TEAR
Cure Time (min) 13 8
Cure Temperature:166C
Tear Strength (kN/m) 63.0 75.1
DIE C TEAR
Cure Time (min) 13 8
Cure Temperature:166C
Tear Strength (kN/m} 34.5 32.7
DIN Abrasion
Volume Loss (mm3) 61 54
Compound #3 Comr~ound
#4
TAKTENE 1203 XQ 211
BR
+ Silica Silica
mb
ZWICK REBOUND
Cure Time (min) 18 13
Cure Temperature:166C
Resilience @ 0C {%) 53.0 53.0
Resilience @ 23C (%) 56.8 55.0
Resilience @ 100C (%) 61.4 65.0
GOODRICH t=LEXOMETER
Cure Time (min) 18 13
Cure Temperature: 166C
Ambient Temperature: 55C
Load on Beam: 11 kg
Stroke (Compression): 17.5%
Heat Rise (C) 25.3 22.0
Permanent Set (%) 2.4 2.3
CA 02288607 1999-11-04
WO 98/53004 PCT/CA98/00499
- 49 -
MER 1100 Dynamic Properties
Frequency:20Hz @ 60C
Load:7% statict3% dynamic
Static Stiffness (kglmm) 5.68 4.41
Dynamic Stiffness (kg/mm) 9.25 8.14
Ratio-dynamicatatic 1.63 1.85
Power Loss (g.m/sec) 1.56 1.29
Tan Delta 0.129 0.126
Again, the results suggest that a tire tread vulcanizate produced
from the masterbatch will display lower rolling resistance and better abrasion
resistance, with equal traction characteristics, when compared with a tire
tread vulcanizate produced from the dry mix.