Note: Descriptions are shown in the official language in which they were submitted.
CA 02288612 1999-11-04
WO 98/52951 PCT/CA98/00500
N-SUBSTITUTED-.OMEGA-(ALKOXYSILYL)ALKYLAMINES AND PROCESS FOR PRODUCTION
THEROF
The present invention relates to N-substituted-c~-
(alkoxysilyl)alkylamines and salts thereof, and to a process for production
thereof.
Certain silane compounds, including some amine-containing
silane compounds, are said to be useful for attaching organic groups to .
mineral fillers; see "Silane treatment of mineral fillers - practical
aspects", by
E.J. Sadier, Plastics, Rubber and Composites Processing and Applications
Vol. 24, No. 5, 1995, pages 271 to 275.
It is known to prepare some N-substituted-3-(trialkoxysilyl)-
propylamines by reacting a 3-chforopropyltrialkoxysilane with a primary amine
or reacting an alkyl chloride with a 3-aminopropyltrialkoxysilane, at reflux
in
an alcoholic solution, where the alcohol solvent corresponds to the alkyl
group of the alkoxy portion of the silane. This is largely unsatisfactory as
the
alcohols CH3 OH, C2H50H and C3H,OH boil at temperatures below 98°C,
which is lower than the temperature required to obtain good yields of the
desired products in a reasonable time. While higher boiling alcohols, for
example butanol, may be used to achieve higher reflux temperatures and
hence increased reaction rates, this can result in an exchange of alkoxy
groups of the alcohol with those of the silane and a much decreased yield of
the desired product. The reaction rates can be increased by using higher
temperatures and a pressure autoclave, but this still requires removal of the
solvent alcohol, which complicates the synthesis, and also requires
specialized equipment (autoclave), which is undesirable.
It is also known to prepare some N-substituted-3{trialkoxysilyl)-
propylamines by reacting 3-bromopropyltrialkoxysilane with a primary amine,
or reacting an alkyl bromide with a 3-aminopropyltrialkoxysilane at reflux in
alcohol solution. Again, it is desirable that the alkoxy group of the alcohol
is
the same as the alkoxy group of the silane. While in some cases satisfactory
CA 02288612 1999-11-04
WO 98/52954 PCT/CA98/00500
-2-
yields can be obtained, in others the temperature of the boiling solvent is
too
high, causing unwanted side reactions, for instance dehydrobromination of
the starting 3-bromopropyltrialkoxysilane. In any case, it is necessary to
remove the alcohol to obtain the required product, which is expensive and
disadvantageous, particularly if it is important to remove al! traces of the
solvents.
It is an object of the present invention to obviate or mitigate at
least one of the above-identified disadvantages of the prior art.
It is another object of the present invention to provide novel
N-substituted-cu-(alkoxysilyl)alkylamines and salts thereof.
It is yet another object of the present invention to provide a
novel process for producing N-substituted-c~-(alkoxysilyl)alkylamines and
salts
thereof.
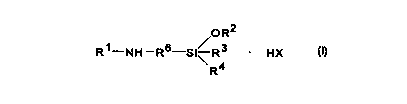
Accordingly, in one of its aspects, the present invention provides
a process for producing a compound of Formula I:
OR2
R~-NH -RS-Si~ R3 ~ HX (I)
R4
wherein R' is a Cs C4o alkyl or alkenyl group that is straight-chained or
branched, a C6 CQO aryl group, a C; C4o aralkyl group or a group R5A(CH2)p
wherein R5 is a C6 C3o alkyl or alkenyl group that is straight-chained or
branched, p is an integer from 2 to 6 and A is O or NH;
RZ is a C,-C,2 alkyl group (preferably a C,-C$ alkyl group) or a
C3 C,2 alkenyl group (preferably a C3 C5 alkenyl group);
- 30 R3 is a C,-C,2 alkyl group (preferably a C,-C5 alkyl group), a C,-
C,z alkoxy group (preferably a C,-C5 alkoxy group), a C2 C,2 alkenyl group
(preferably a C2 C5 alkenyi group) or a C3 C,2 alkenyloxy group (preferably a
CA 02288612 1999-11-04
WO 98/52954 PCT/CA98/00500
-3-
C3-C5 alkenyloxy group);
R4 has the same definition as R3 and may be the same as R4 or
d ifferent;
R6 is a divalent alkylene group having up to 10 carbon atoms
and is optionally interrupted one, two or three times by a phenylene group;
and
X is an anion;
the process comprising the step of:
(a) reacting a compound of the Formula II:
R'-NH2 {II)
wherein R' is as defined above, with a compound of Formula III:
OR2
X-RS-Si.~ R3 (III)
R4
wherein R2, R3, R', R6 and X are as defined above, in the absence of a
solvent; or
(b) reacting a compound of the Formula IV:
R'-X (IV)
wherein R' and X are as defined above, with a compound of Formula V:
ORZ
t 30 H2N-RS-Si~ R3 C v)
R4
CA 02288612 1999-11-04
WO 98/52954 PCT/CA98/00500
-4-
wherein RZ, R3, R4 and R6 are as defined above, in the absence of a solvent.
It is surprising that the N-substituted-c~-(alkoxysilyl)alkylamines
of Formula 1 can be obtained in good yields, within reasonable reaction times,
without contamination by alcoholic solvent and without necessity for removal
of alcoholic solvent.
In another of its aspects, the present invention provides a
compound of Formula !:
OR2
R~-NH-RS-Si~R3 ~ HX (I)
R4
wherein R' is a C,2 C4o alkyl or alkenyl group that is straight-chained or
branched, a C6-C4o aryl group, a C~ C4o aralkyl group or a group R5A(CH2)p
wherein R5 is a Cs C3o alkyl or alkenyl group that is straight-chained or
branched, p is an integer from 2 to 6 and A is O or NH;
R2 is a C,-C,2 alkyl group (preferably a C,-C5 alkyl group) or a
C3 C,2 alkenyl group (preferably a C3 C5 alkenyl group);
R3 is a C,-C,2 alkyl group (preferably a C,-C5 alkyl group), a C,-
C,2 alkoxy group (preferably a C,-C5 alkoxy group), a CZ C,2 alkenyl group
(preferably a C2-C5 alkenyl group) or a C3-C,2 alkenyloxy group (preferably a
C3 C5 alkenyloxy group);
R4 has the same definition as R3 and may be the same as R4 or
different;
R6 is a divalent alkylene group having up to 10 carbon atoms
and is optionally interrupted one, two or three times by a phenylene group;
and
X is an anion.
In another of its aspects, the present invention provides a
compound of Formula:
CA 02288612 1999-11-04
WO 98/52954 PCT/CA98/00500
-5-
OR2
R 1-NH -R6-Si\ R3
R4
wherein R' is a C,2 C4o alkyl or alkenyl group that is straight-chained or
branched, a C6-C4o aryl group, a C; C4o aralkyl group or a group R5A(CH2)p
wherein R5 is a Cs C~ alkyl or alkenyi group that is straight-chained or
branched, p is an integer from 2 to 6 and A is O or NH;
R2 is a C,-C,2 alkyl group (preferably a C,-C5 alkyl group) or a
C3 C,2 alkenyl group (preferably a C3 C~ alkenyl group);
R3 is a C,-C,2 alkyl group (preferably a C,-C5 alkyl group), a C,-
C,2 alkoxy group (preferably a C,-C5 alkoxy group), a C2 C,2 alkenyl group
(preferably a CZ C5 alkenyl group) or a C3 C,2 alkenyloxy group {preferably a
C3 C5 alkenyloxy group);
R4 has the same defiriition as R3 and may be the same as R4 or
different; and
R6 is a divalent alkyiene group having up to 10 carbon atoms
and is optionally interrupted one, two or three times by a phenylene group.
The anion X is suitably a chloride, bromide or iodide anion, of
which the chloride and the bromide are preferred. The preferred reaction
conditions vary, depending upon whether X is chlorine or bromine. If X is
chlorine, it is preferred to carry out the reaction at a temperature at least
about 100°C, more preferably in the range of from about 130°C to
about
185°C. The reaction may take anywhere from about 30 minutes to about 4
hours. If X is bromine, then it is preferred to operate at a lower
temperature,
preferably from about 30°C to about 75°C. With bromine the
reaction
proceeds more slowly and reaction times of from about 8 to 24 hours are not
unusual.
CA 02288612 1999-11-04
WO 98/52954 PCT/CA98/00500
-6-
In one embodiment of the present process, an amine compound
of Formula II is reacted with an alkoxysilane compound of Formula Ill.
Non-limiting examples of suitable amines of Formula II may be
selected from the group comprising hexylamine, octylamine, nonylamine,
decylamine, octadecylamine, octadec-9-enylamine and mixtures thereof.
Indeed, mixed amines are commercially available and these are conveniently
suitable for use. Non-limited examples of such amines may be selected from
the group comprising soya amine, tall oil amine, stearyl amine, tallow amine,
dihydrogenated tallow amine, cocoamine, rosin amine, palmitylamine and
mixtures thereof. These amines may be use in distilled or undistilled form.
When R' is alkenyl, it may contain one, two or more double-bonds and when
it contains two or more double bonds, they may be conjugated or
unconjugated.
In another embodiment of the present process, an amine
compound of Formula IV is reacted with an alkoxysilane compound of
Formula V.
Non-limiting examples of suitable alkyl or alkenyl halides of
Formula IV may be selected from the group comprising 1-bromooctane, 1-
chlorononane, 1-bromononane, 1-bromodecane, 1-chlorododecane, 1-
bromooctadecane, 1-bromooctadec-9-ene and mixtures thereof. Indeed,
mixed alkyl/alkenyl halides are commercially available and these are
conveniently suitable for use. Non-limited examples of such alkyUalkenyl
halides may be selected from the stearyl chloride, stearyl bromide, oleyl
chloride and mixtures thereof. These materials may be use in distilled or
undistilled form. Again, R' is alkenyl, it may contain one, two or more double
bonds and when it contains two or more double bonds, they may be
conjugated or unconjugated.
In the compound of Formula III or V, as stated R2 is preferably
C,-C5 alkyl or C3 C5 alkenyl, but more preferably it is C,-C3 alkyl, i.e.,
methyl,
ethyl, propyl or isopropyl. It is preferred that R3 and R4 are C,-CS alkoxy,
particularly methoxy, ethoxy, propoxy or isopropoxy. It is preferred that OR2,
R3 and R' are all the same and are methoxy. If OR2, R3 and R4 are not the
CA 02288612 1999-11-04
WO 98/52954 PCT/CA98/00500
-7-
same it is preferred that R3 and R4 are alkyl or alkenyl. Preferably R2, R3
and
R4 are unsubstituted, but the use of substituted groups is not excluded,
provided that the substituent does not interfere with the course of the
reaction. These remarks also apply in respect of R' and R6.
- 5 The reactions may be carried out under inert atmosphere, for
example by flushing the reactor with an inert gas such as argon or nitrogen.
This is not essential, however.
The reaction of the compound of Formula II with the compound
of Formula III, or the compound of Formula IV with the compound of Formula
V, results in release of the acid HX. Generally, the product of reaction will
therefore be in the form of its acid addition salt with HX. For many purposes
the compounds of Formula I can be used in the form of their salts.
Alternatively, they can be converted from the salt to the free base, if
needed.
This is best done by reacting the compound with an alkali metal alkoxide in
which the alkyl moiety is R2, as defined above. For instance, if R2 is methyl,
it
is preferred to use sodium methoxide to convert the salt to the free base.
Particularly, if the compounds of Formula I are to be used to
render a material hydrophobic, it is preferred that R' is a longer chain alkyl
or
alkenyl group that preferably has ten carbon atoms, more preferably twelve
carbon atoms, and more, and it is preferred that the chain is not branched at
the atom connecting R' to the nitrogen.
It is believed that compounds of Formula I, prepared from mixed
amines of more than 12 carbon atoms are novel. Thus, these compounds,
in the acid addition salt form or free base form, are another aspect of the
invention.
The compounds of Formula I are useful for treating mineral
fillers to alter the properties of the mineral fillers. They can be used, for
example, to treat silica, silicate mineral powders, clays, calcium carbonate,
pigments such as titanium dioxide and other materials to render their surfaces
hydrophobic. They can also be used as intermediates in the preparation of
silica-bound catalysts and intermediates or ion-exchangers, or as coupling
agents to improve the bond between paint and a wood or metal substrate, or
CA 02288612 1999-11-04
WO 98/52954 PCT/CA98/00500
_$_
as a waterproofing agent for wood or concrete.
Embodiments of the invention will be illustrated with reference to
the following Examples which should to be used to construe or limit the scope
of the invention.
F~ple 1Oleyl amine:(3-chloroRo_Qylltrim~ysiiane 1:1 Adduct N-
~y)~(~-trimethox~r-siL~~~R~rlamine (R' = oleyl)
To a 1000 ml Erlenmeyer flask were added 267.5 grams of
distilled oleylamine (Witco Kemamine 989D) and 198.8 grams of (3-
chloropropyl)trimethoxysilane (Aldrich}. A magnetic stirring bar and
thermometer were inserted and the headspace above the liquid was flushed
with Argon gas. The neck of the flask was then loosely plugged with tissue to
hold the thermometer in place. The flask was placed on a hot plate and
slowly heated to 160°C over a period of 90 minutes with stirring. On
cooling,
the title compound, in the form of a yellow waxy solid (464.2 grams, m.p. 68-
74°C) was obtained. The material was readily soluble in methanol.
Example 2 - Palmityl amine:~(3-chloro~p~~)~trimethoxysilane 1.1 Adduct N-
Palmit~~-N-~~3-trimethoxysilyj)~Rylamine (R = ap Imityl)~
To a 250 ml Erlenmeyer flask were added 60.5 grams of distilled
palmitylamine (Akzo-Nobel Armeen 16D) and 50.4 grams of (3-
chloropropyl)trimethoxysilane (Aldrich). A magnetic stirring bar and
thermometer were inserted and the headspace above the slurry was flushed
with Argon gas. The neck of the flask was then loosely plugged with tissue to
hold the thermometer in place. The flask was placed on a hot plate and
slowly heated with stirring to 50°C to dissolve the amine. The heat was
then
increased and the contents were heated to 170°C over a period of 30
minutes
with stirring and then held at that temperature for 10 minutes. The heat was
turned off and the flask was allowed to cool to room temperature over a one
hour period. At 110°C, a yellow solid began to separate. The final
yield was
107.1 grams of the title compound in the form of a yellow waxy solid (m.p.
106-110°C) which dissolved readily in methanol.
CA 02288612 1999-11-04
WO 98/52954 PCT/CA98/00500
-9-
N;Ste~l.~L~('~-t~mPthoxy~,j)~~pylamine ~(R' = steayl)
To a 250 ml Erlenmeyer flask were added 76.7 grams of distilled
stearylamine (Akzo-Nobel Armeen 18D) and 57.4 grams of (3-
chloropropyl)trimethoxysilane (Aldrich). A magnetic stirring bar and
thermometer were inserted. The neck of the flask was loosely plugged with
tissue to hold the thermometer in place. The flask was placed on a hot plate
and heated rapidly with stirring to 198°C at which point the heat was
shut off.
The reaction exothermicity kept the temperature at 190°C for 10
minutes after
which the material cooled quickly to room temperature. At 130°C, a
yellow .
solid began to separate. The final yield was 131,.4 grams of the title
compound in the form of a yellow waxy solid (m.p. 120-135°C), easily
soluble
in methanol.
Example 4 - Dodecyl amine:~~3-chloro~r_o~,rl)trimethoxysilane 1.1 Adduct
N-Dodecyrl-N-(3-trimethoxysil~~~QyJamine ~(R' = dodecvll
To a 250 ml Erlenmeyer flask were added 22.9 grams of
dodecylamine (Aldrich) and 24.8 grams of (3-chloropropyl)trimethoxysilane
(Aldrich). A magnetic stirring bar and thermometer were inserted. The neck
of the flask was then loosely plugged with tissue to hold the thermometer in
place. The flask was placed on a hot plate and heated slowly to 185°C
with
stirring, at which point the heat was reduced and the contents kept at 175-
180°C for 20 minutes. The heat was then shut off and the flask contents
were allowed to cool. Solidification of the contents to an off white solid
began
at around 50°C. The final product (47.3 grams) dissolved quickly in
methanol
with gentle stirring.
methox~silane 1:1 Adduct (R' = C-18 to is rid a
To a 1000 ml Erlenmeyer flask were added 162.5 grams of C-18
tertiary alkyl primary amine (Primene JM-T, Rohm and Haas) and 100.4
grams of (3-chloropropyl)trimethoxysilane (Aldrich). A magnetic stirring bar
CA 02288612 1999-11-04
WO 98/52954 PCT/CA98/00500
-10-
and thermometer were inserted. The neck of the flask was then loosely
plugged with tissue to hold the thermometer in place. The flask was placed
on a hot plate and heated slowly over a period of 95 minutes to 180°C
with
stirring, at which point the heat was shut off and the flask contents were
allowed to cool. No solidification occurred. 258.9 grams of a viscous amber
liquid were recovered. The liquid was miscible with methanol in all
proportions.
F_~nlP - Tallow amines(3-chloro~Ryl~itrimethoxvsilane 1:1 Adduct N-
Tallow-al yl~L(3-trimethoxysil~~oroRylamine ~(R' = tallow alkyl)
To a 1000 ml Erlenmeyer flask were added 134 grams of
distilled tallow amine (Armeen TD, Akzo-Nobel) and 100.4 grams of (3-
chloropropyl)trimethoxysilane (Aldrich). A magnetic stirring bar and
thermometer were inserted. The neck of the flask was then loosely plugged
with tissue to hold the thermometer in place, a needle was inserted and the
headspace was flushed with Argon to remove air. The flask was placed on a
hot plate and heated over a period ~of one hour to 180°C with stirring,
at which
point the heat was shut off. The heat of reaction continued to raise the
temperature to 192°C after which the material cooled to room
temperature
over one hour. Solidification of the contents began at 65°C and the
material
was completely solid by 55°C. The yield was 234 grams of a yellowish
pasty
solid. This material dissolved easily in methanol to give a clear solution.
F_~mple 7 - OctyJ amine:(3-chlor~p~~lltrimethoxvsilane 1.1 Adduct
N-Ochrl-N-~(3-trimethoxysil~~nroRyrlamine ~(R' = oc ily
To a 500 ml 2-neck round bottom flask equipped
with a drying tube and magnetic stin-er were added 99.5 grams of octylamine
(Aldrich) and 154.5 grams of (3-chforopropyl)trimethoxysilane (Aldrich). A
thermometer was inserted through a side arm. The flask was placed in a
mantle equipped with a Variac controller and the heat was energized. Over a
period of 85 minutes, the temperature climbed to 190°C and then fell to
180°C within 25 minutes at which point the heat was shut off.
Solidification of
CA 02288612 1999-11-04
WO 98/52954 PCT/CA98/00500
-11-
the contents began at 60°C and the material was completely solid by
50°c.
The yield was 253.6 grams of the title compound in the form of a yellow solid.
This material dissolved readily in methanol to give a clear yellow solution.
EXample 8 - Cocoamine:~(3-chlorQOrop~~trimethoxysilane 1:1 Adduct,~R' _
cocoa alkvll
To a 500 ml Erlenmeyer flask were added 100 grams of distilled
cocoamine (Armeen CD, Akzo-Nobel) and 100 grams of (3-chloropropyl~
trimethoxysilane (Aldrich). A magnetic stirring bar and thermometer were
inserted and the neck of the flask was then loosely plugged with tissue to
hold
the thermometer in place. The flask was placed on a hot plate and heated
over a period of 45 minutes to 185°C with stirring, at which point the
heat was
turned off. The heat of reaction continued to raise the temperature to
200°C
over 5 minutes after which it fell to 125°C 20 minutes later. 198.4
grams of
product were isolated. At room temperature, the material was a yellow waxy
solid which dissolved easily in methanol on gentle shaking.
Example 9 - 1-Chfor~2 ecane:~(3-amin~nrop)~)~trimethoxvsilane 1:1 Adduct N
Dec)rl-N-y'i-trirnethyx),rsilyrl)orop) I~ am_in~(R' = dec~~
To a 500 ml Erlenmeyer flask were added 95 grams of 1-
chlorodecane (Aldrich) and 96.4 grams of (3-aminopropyl)trimethoxysilane
(Petrarch/UCT). A magnetic stirring bar and thermometer were inserted and-
the neck of the flask was then loosely plugged with tissue to hold the
thermometer in place. The flask was placed on a hot plate and heated with
stirring. After 90 minutes the temperature reached 184°C and then fell
to
175°C 20 minutes later at which point the heat was turned off and the
material removed from the hot plate. When cooled, the material was a waxy
solid, and was recovered which dissolved easily in methanol.
Example 10- 1-Bromododecane:~~, -amino~p,~~~trimethoxysilane 1:1 Adduct
N-Dodecyl-N-~(~3-trimethoxysil~~nropylamine~R' = dodec~
To a 100 ml Erlenmeyer flask were added 24.9 grams of 1-
CA 02288612 1999-11-04
WO 98/52954 PCT/CA98/00500
-12-
bromododecane (Aldrich) and 17.9 grams of (3-aminopropyl)trimethoxysilane
{Petrarch/UCT). The flask was stoppered and shaken to effect mixing. The
material was allowed to stand undisturbed at room temperature (23°C)
for 5
hours. Examination of the mixture after this period revealed an upper phase
consisting of a clear liquid and a lower phase of feathery white crystals. The
flask was transferred to a hot plate previously equilibrated to give a surface
temperature of 45 and left for 16 hours. At the end of this time all the
material had reacted to give 41.2 grams of pale yellow crystals. An NMR
spectrum was consistent with that expected from N-dodecyl-N-(3-
trimethoxysilyl)propyl ammonium bromide. The material was freely soluble in
methanol with a distinct endothermic effect.
Exarnnle 11- Soya aminev(3-chloro~r_op~rl)trimethoxysilane 1:1 Adduct ~(R' _
To a 1000 ml Erlenmeyer flask were added 263.6 grams of
distilled soya amine (Akzo-Nobel Armeen SD) and 198.7 grams of (3-
chloropropyl)trimethoxysilane (Aldrich}. A magnetic stirring bar and
thermometer were inserted. The neck of the flask was loosely plugged with
tissue to hold the thermometer in place. The flask was placed on a hot plate
and heated slowly to 45 in order to effect solution of the soya amine. It was
then heated to 160°C over two hours and then held at 147-160°C
for a further
two hours after which it was allowed to cool slowly to room temperature.
Yellow crystals began to separate from the mother liquid at 75°C and
at
room temperature the contents of the flask comprised a yellow solid mass.
The yield was 460.0 grams. The product was easily soluble in methanol to
give a clear yellowish solution.