Note: Descriptions are shown in the official language in which they were submitted.
., I 1 , . . ..
CA 02289711 2005-04-20
TITLE
IMPROVED METHOD FOR PREPARING LOW-
CONCENTRATION POL.YAL.UMINOSIL.ICATE MICROC.ELS
BACKGROUND QF THE I1yVENTION
The present invention relates to an improved method and apparatus for
preparing low-concentration polysilicate microgels, i.e., aqueous solutions
having an
active silica concentration of generally less than about 1.0 wt.%, which are
formed by the
partial gelation of an allcali metal silicate or a polysilicate, such as
sodium polysilicate,
having in its most common form one part Na20 to 3.3 parts Si02 by weight. The
microgels, which are referred to as "active" silica in contrast to commercial
colloidal
silim, comprise solutions of from 1 to 2 nm diameter linked silica particles
which have a
surface area of at least about 1000 m2/g. The particles are linked together
during
preparation, i.e., during partial gelation, to form aggregates which are
arranged into three-
dimensional networks and chains. The polysilicate microgels can be further
modified by
the incorporation of aluminum oxide into their structure. Such alumina
modified
polysilicates are classified as polyaluminosilicate microgels and are readily
produced by a
modification of the basic method for polysilicate microgels. A critical aspect
of the
invention is the ability to produce the microgels within a reasonable time
period, i.e., not
longer than about 15 minutes until the microgel is ready for use, without the
risk of
solidification and with minimum formation of undesirable silica deposits
within the
processing equipment. In this connection, the incorporation of alumina into
the
polysilicate microgel has been found beneficial in that it increases the rate
of microgel
formation. Polysilicate microgels produced according to the invention are
particularly
useful in combinations with water soluble cationic polymers as a drainage and
retention
aid in papermaking. At low pH values, below pH of 5, these products are more
appropriately referred to as polysilicic acid microgels. As the pH value is
raised, these
products can contain mixtures of polysilicic acid and polysilicate microgels;
the ratio
CA 02289711 1999-11-12
WO 98/55398 PCTIUS97/09674
2
being pH-dependent. For sake of convenience, these products hereinafter will
be referred
to as polysilicate microgels.
SUMMARY OF THE INVENTION
The present invention is an improved method and apparatus for
continuously preparing a low-concentration polysilicate rnicrogeI which
comprises:
(a) simultaneously introducing a first stream comprising a water soluble
silicate solution
and a second stream comprising a strong acid having a pKa less than 6 into a
mixing zonc
where the streams converge at an angle of not less than 30 degrees and at a
rate sufficient
to produce a Reynolds number of at least about 4000 and a resulting
silicate/acid mixture
having a silica concentration in the range of from about 1.0 to 6.0 wt.% and a
pH in the
range of from 2 to 10.5; (b) aging the silicate/acid mixture for a period of
time sufficient
to achieve a desired level of partial gelation (i.e., forming the microgel);
usually for at
least 10 seconds but not more than about 15 minutes; and (c) diluting the aged
mixture to
a silica concentration of not greater than about 2.0 wt.% whereby gelation is
stabilized.
To produce polyaluminosilicate microgels, a water soluble aluminum salt is
added first to
the acid stream prior to mixing it with the silicate stream.
For best results, the silica concentration of the water soluble silicate
starting solution is in the range of from 2 to 10 wt.% silica, and the
concentration of the
strong acid (e.g., sulfuric acid) is in the range of from 1 to 20 wt.% acid as
the two
streams are being introduced into the mixing zone. The preferred conditions in
the
mixing zone are a Reynolds number greater than 6000, a silica concentration in
the range
of 1.5 to 3.5 wt.% and a pH in the range of 7 to 10. The most preferred
conditions are a
Reynolds number greater than 6000, silica concentration of 2 wt.% and a pH of
9. The
preparation of alumina modified microgel is best conducted by adding a soluble
aluminum salt to the acid stream in an amount ranging from about 0.1 wt.% up
to the
solubility limit of the aluminum salt. The most useful polyaluminosilicate
microgels are
those prepared with an A12O3/SiO2 mole ratio ranging from 1:1500 to 1:25 and,
preferably, from 1:1250 to 1:50.
The apparatus according to the invention comprises:
(a) a first reservoir for containing a water soluble silicate solution; (b) a
second reservoir
for containing a strong acid having a pKa of less than 6; (c) a mixing device
having a
first inlet which communicates with said first reservoir, a second inlet
arranged at an
angle of at least 30 degrees with respect to said first inlet which
communicates with said
seQond reservoir, and an exit; (d) a first pumping means located between said
first
reservoir and said mixing device for pumping a stream of silicate solution
from said first
reservoir into said first inlet, and first control means for controlling the
concentration of
, ,.r
CA 02289711 1999-11-12
WO 98/55398 PCT/US97/09674
3
silica in said silicate solutiorr while said solution is being pumped such
that the silica
concentration in the exit solution from the mixing device is in the range of 1
to 6 wt.%;
(e) a second pumping means located between said second reservoir and said
mixing
device for pumping a stream of acid from said second reservoir into said
second inlet at a
rate relative to the rate of said first pumping means sufficient to produce a
Reynolds
number within said mixing device of at least 4000 in the region where the
streams
converge whereby said silicate and said acid are thoroughly mixed; (f) mixture
control
means located within said exit and responsive to the flow rate of said acid
into said
mixing device for controlling the pH of the silicate/acid mixture in the range
of from 2 to
10.5; (g) a receiving tank; (h) an elongated transfer loop which conununicates
with the
exit of said mixing device and said receiving tank for transferring said
mixture
therebetween; (i) a dilution means for diluting the silicate/acid mixture-in
the receiving
tank to a silica concentration of not more than 1.0 wt.%; (j) a fourth
reservoir for
containing a water soluble aluminum salt; (k) a fourth pumping device for
introducing the
aluminum salt into the acid stream; and (1) a control valve responsive to the
aluminum
salt flow and linked in parallel with the silicate control valve, and located
between the
fourth pumping device and the point of introduction of the aluminum salt into
the acid
stream.
In an alternate embodiment, the apparatus of the invention includes a
NaOH reservoir and means for periodically flushing the production system with
warm
NaOH which has been heated to a temperature of from 40 to 60 C whereby
deposits of
silica can be solubilized and removed.
In a further embodiment of the invention, an agitating gas stream such as a
stream of air or nitrogen or other inert gas can be introduced into the mixing
device
described by means of an additional inlet located at or near the mixing
junction. Gas
agitation provides an important industrial benefit in that it permits low
silicate flow rates
to be employed while maintaining the required turbulence and Reynolds number
in the
mixing zone.
In yet a furfher embodiment of this invention, mixing of the acid,
aluminum salt and the water soluble silicate solution can be accomplished in
an annular
mixing device. This device can be an intemal pipe or tube which protrudes into
and
subsequently discharges inside of a larger pipe or tube. The internal pipe
discharge point
is usually, but not necessarily, concentrically located inside the external
pipe. One of the
two fluids to be mixed is fed into the intemal pipe. The second fluid is fed
into the
external pipe and flows around the outside of the internal pipe. Mixing of the
two fluids
occurs where the first fluid exits the internal pipe and combines with the
second fluid in
CA 02289711 1999-11-12
WO 98/55398 PCT/US97/09674
4
the larger external pipe. Usually, the acid and the aluminum salt solution are
premixed
prior to being fed into one of the pipes.
For the purpose of mixing the two liquids, the water soluble silicate
solution and the acid can be fed to either the internal or the external pipes
at rates
sufficient such that when the two streams are combined, a Reynolds number of
greater
than 4000 is produced in the mixing zone. An agitating gas stream can also be
optionally
employed to aid in the mixing of the two streams.
As a fin-ther embodiment to this invention, mixing of the acid and water
soluble silicate solution can be accomplished in a vessel equipped with
mechanical means
to create the necessary turbulence, such that mixing of the two streams is
accomplished at
a Reynolds number of greater than 4000. The vessel can optionally be equipped
with
baffles. The acid and water soluble silicate solution can be but do not have
to be fed to
the vessel simultaneously.
To produce polyaluminosilicate microgels, a concentrated solution of an
aluminum salt, preferably aluminum sulfate, is pumped from an additional
reservoir and
mixed into the diluted acid stream at a point before that at which the diluted
acid and
silicate streams are mixed and reacted. By the addition of the aluminum salt
to the acid
stream, the rate of formation of microgel is increased and a
polyaluminosilicate microgel
is formed having aluminum moieties incorporated throughout the microgel
structure.
The method and apparatus of the invention are capable of producing stable
polysilicate and polyaluminosilicate microgels resulting in reduced silica
deposition
within a convenient time frame of not more than about 15 - 16 minutes, but
usually
within 30 to 90 seconds, without the risk of solidification and with minimum
formation
of undesirable silica deposits within the processing equipment. Temperature of
operation
is usually within the range of 0-50 C.
Silica deposition in production apparatus is undesirable because it coats all
internal surfaces of the apparatus and can impede the functioning of vital
moving parts and
instrumentation. For example, silica deposition can build to the point where
valves can no
longer function and can re trict fluid flow through pipes and tubing.
Deposition of silica is
also undesirable on the pH sensing electrode as it prevents monitoring the
process pH, a
critical quality control parameter for silica microgel production.
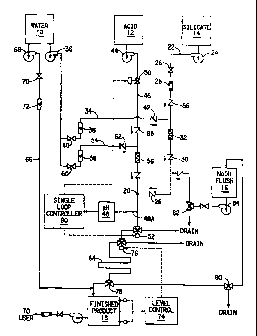
BRIEF DESCRIPTION OF THE DRAW[NCS
Fig. 1 is a schematic diagram of the process which includes a NaOH
reservoir and means for periodically flushing the production system.
= I 11 1114 CA 02289711 2005-04-20
Fig. 2 is a schematic diagram of a dual line polysilicate microgel
production system which provides for uninterrupted microgel production.
Fig. 3 is a schematic diagram of the process of the invention for the
production of polyaluminosilicate microgels which includes an aluminum salt
reservoir
5 and means for introducing said salt into the dilute acid stream.
DETAIL.ED DESGIZIPTION OF THE INVENTInN
Active silica is a specific form of microparticulate silica comprising very
small 1-2 nm diameter particles which are linked together in chains or
networks to form
three-dimensional siructures known as "microgels". The surface area of the
active silica
microparticulates, i.e., the microgels, is at least about 1000 m2/g. General
methods for
preparing polysilicate microgels are described in U.S. Patent 4,954,220.
Of the methods described therein, the
acidification of a dilute aqueous solution of an alkali metal silicate. with
an inorganic acid
or organic acid, i.e., a strong acid having a pKa of less than 6, is the
method to which this
invention is particularly applicable. The present invention provides for the
reliable and
continuous preparation of low-concentration polysilicate and
polyaluminosilicate
microgels at the site of intended consumption without formation of undesirable
silica
deposits within the processing equipment and at very reasonable aging times
generally
less than 15 minutes, and preferably between from 10 to 90 seconds.
The method of the invention is carried out by simultaneously introducing a
stream of a water soluble silicate solution and a stream of strong acid having
a pKa less
than 6, along with an aluminum salt, into a mixing zone or mixing junction
such that the
streams converge at an angle of generally not less than 30 degrees. with
respect to each
other and at a rate which is sufficient to produce a Reynolds number in the
region where
the two streams converge of at least 4000, and preferably in the range of
about 6000 and
above. Reynolds number is a dimensionless number used in engineering to
describe
liquid flow conditions within a tube or pipe. Numbers below 2000 represent
laminar flow
(poor mixing environment)'and numbers of 4000 and above represent turbulent
flow
(good mixing environment). As a general rule, the larger the Reynolds number
the better
the mixing. Reynolds number, (Re) for flow in a pipe or tube, is determined
from the
equation
Re= Qxd
Dxu
Where: Q Flow in cubic feet per second
d Density in pounds per cubic foot
CA 02289711 1999-11-12
WO 98/55398 6 PCT/US97/09674
D Pipe diameter in feet
u= Viscosity in pounds per foot second
Reynolds number for impeller-stirred vessels is determined from the equation
Re= (D2xNxp)/u
Where: D = Impeller diameter in cm
N = Rotational velocity in revolutions per second
p = Fluid density in grams per cm3
u= Viscosity in grams per (second)(centimeter)
The concentrations of the converging silicate solution and the
acid/aluminum salt streams are controlled so that the resulting silicate/acid
mixture thus
produced has a silica concentration in the range of I to 6 wt.% and a pH in
the range of 2
to 10.5. More preferably the silica concentration is in the range of 1.5 to
3.5 wt.% and
the pH is in the range of 7 to 10. The most preferred operating conditions are
with a
Reynolds number larger than 6000, a silica concentration of 2 wt.% and a pH of
9.
Aging is generally accomplished in from 10 up to about 90 seconds by
passing the silicate/acid mixture through an elongated transfer loop in route
to a finished
product receiving tank in which the mixture is immediately diluted and
thereafter
maintained at an active silica concentration of not greater than 2.0 wt.% and,
preferably,
not greater than 1.0 wt.%. Partial gelation which produces the three-
dimensional
aggregate networks and chains of high surface area active silica particles is
achieved
during aging. Dilution of the silicate/acid mixture to low concentration
operates to halt
the gelation process and stabilize the microgel for subsequent consumption.
The method of the invention and an apparatus for carrying it out will now
be discussed in greater detail in reference to the drawings in which Fig. 1 is
a schematic
diagram of the process in its simplest form to prepare polysilicate microgels.
The sizes,
capacities and rates described herein can be varied over wide ranges depending
primarily
on the quantities of polysilicate microgel required and the expected rate of
consumption.
The sizes and capacities described in reference to the drawings relate to a
system for
producing, i.e., generating, polysilicate microgel on a generally continuous
basis for
consumption as a drainage and retention aid in a papermaking process in which
the
consumption rate ranges from about 10 to 4000 lbs. microgel per hour.
There is shown in Fig. 1 a dilution water reservoir 1~, an acid reservoir JL2,
and a silicate reservoir 14. The reservoirs, i.e., tanks, are conveniently
made of
CA 02289711 1999-11-12
WO 98/55398 PCTIUS97/09674
7
polyethylene, with the water reservoir having a capacity of 500 gallons, the
acid reservoir
having a capacity of 100 gallons, and the silicate reservoir having a capacity
of 300
gallons. Other vessels shown in Fig. 1 are NaOH flush tank 1.~ and finished
product
receiving tank 1$. The NaOH flush tank is made of a non-corrosive material,
such as, for
example, 316 stainless steel; it has a capacity of 20 gallons and is heated
with an
electrical resistance drum heater wrapped around it (Cole-Palmer, 2000 watts,
115 volts).
The finished product receiving tank has a capacity of 1000 gallons and is made
of
polyethylene.
A critical element of the process is mixing junction 20 which defines a
mixing zone in which a stream of acid and a stream of water soluble silicate
are
introduced along individual paths which converge within the mixing zone at an
angle
generally not less than 30 degrees. A mixing "T" or "Y" junction is suitable
for
practicing the invention and may readily be constructed from an appropriately
sized 316
stainless steel "Swagelok" compression coupling fitted with stainless steel
tubing. A"T"
junction is generally preferred.
The rates at which the two streams enter, i.e. are pumped into, the mixing
zone are selected to produce a Reynolds number therewithin of at least 4000
and
preferably up to 6000 or higher which results in practically instantaneous and
thorough
mixing of the acid and silicate such that the resulting mixture has a silica
concentration in
the range of from 1.5 to 3.5 wt.% and a pH of from 7 to 10. Any convenient
commercial
source of water soluble silicate can be employed, such as, for example, "PQ
(N)" sodium
silicate (41 Baume, Si02:Na20 = 3.22:1 by weight, 28.7 wt.% Si02) marketed by
the PQ
corporation. The commercial silicate is maintained undiluted in reservoir 14,
usually at a
concentration of 24 to 36 wt.% as supplied by the manufacturer, until it is
needed. It is
supplied to the mixing junction 20 via suitable tubing 22 (316 SS, 1/4 inch
OD) by means
of a low flow rate gear or micropump 24 (e.g., Micropump Corp., model 140,
max. flow
1.7 gpm). Non-corrosive materials of construction, e.g., 316 stainless steel,
are preferred
to avoid any risk of corrosion and subsequent contamination. The silicate
supply line
also includes flow control valve 26 (Whitey, 316 SS, 1/4 inch needle),
magnetic flow
meter 28 (Fisher Porter, 316 SS, 1/10 inch size) and check valve 86 (Whitey,
316 SS, 1/4
inch diameter) for controlling and monitoring the amount and direction of
silicate flow.
In operation, dilution water is introduced into the silicate supply line 22 at
a convenient
location upstream of the silicate/acid mixing junction 20 so as to adjust the
silica
concentration to a value in the range of from 2 to 10 wt.%. To insure complete
mixing of
silicate and water an in-line static mixer 32 (Cole-Palmer, 316 SS, 1/2 inch
tubing, 15
elements) is provided followed by a check valve 30 (Whitey, 316 SS, 1/2 inch
diameter).
CA 02289711 1999-11-12
WO 98/55398 8 PCT/US97/09674
The dilution water is supplied via line 34 (1/2 inch OD, 316 SS) by
centrifugal pump 36
(Eastern Pump, 1 HP, max. flow 54 gpm), and a rotameter 38 (Brooks, Brass
Ball, 3.06
gpm max.). Control valve 40 (Whitey, 316 SS, 1/2 inch NE needle) and check
valve 42
(Whitey, 316 SS, 1/2 inch diameter) can be employed to the control flow rate
and
direction.
Although a wide range of acidic materials, such as, for example, mineral
acids, organic acids, acid salts and gases, ion-exchange resins and the salts
of strong acids
with weak bases, have been described for use in preparing active silica, the
simplest and
most convenient means of acidification is with a strong acid having a pKa less
than 6.
The preferred acid is sulfuric acid. Commercial grades manufactured by DuPont
and
others are generally suitable. In operation, a stock solution of acid is
maintained at a
concentration in the range of from 5 to 100 wt.% in acid reservoir 12. The
acid is
pumped using a gear or similar micropump 44 (e.g., Micropump mode1040, 1/4HP,
max.
flow 0.83 gpm) to junction mixer 20 through line 46 (316 SS, 1/4 inch OD) and
check
valve 88 (Whitey, 316 SS, 1/4 inch diameter). A single loop controller 90
(Moore, Model
352E) is combined with pH transmitter 48 (Great Lakes Instruments, Model
672P3FICON) and pH Probe 48A (Great Lakes Instruments, Type 6028P0) to
regulate
the flow of acid to junction mixer 20 via automatic flow control valve 50
(Research
Controls, K Trim, 1/4 inch OD, 316 SS) in response to the pH of the
silicate/acid mixture
measured at the exit of the junction mixer. An automatic three-way valve 52
(Whitey,
316 SS, 1/2 inch diameter) is also employed within the control system to allow
for the
possibility of having to divert off-spec. silicate/acid mixture to the sewer.
Dilution water
from water reservoir A is provided via line 54 (316 SS, 1/2 inch OD) to dilute
the acid
supply upstream ofjunction mixer 20 to a predetermined concentration in the
range of
from I to 20 wt.%. A static mixer 56 (Cole-Palmer, 316 SS, 1/2 inch diameter,
15 turns)
is provided downstream of the point where dilution water is introduced into
the acid
supply line to insure complete mixing and dilution of the acid. A rotameter 58
(Brooks,
Brass Ball, 1.09 gpm. maximum), control valve 60 (Whitey, 316 SS, 1/2 inch
needle) and
check valve 62 (Whitey, 316 SS, 1/2 inch diameter) are used to control flow
rate and flow
direction of the dilution water.
The silicate/acid mixture which exits junction mixer 20 has preferably a
Si02 concentration in the range of from 1.5 to 3.5 wt.% and a pH in the range
of from 7
to 10. Most preferably the silica concentration is maintained at 2 wt.% and
the pH at 9.
The mixture is passed through an elongated transfer line 64 (1-1/2 inch
schedule 40 PVC
pipe, 75 feet in length) in route to finished product receiving t.ank 1$. The
length of the
transfer line is selected to insure that the transfer will take at least 10
seconds, but
CA 02289711 1999-11-12
WO 98/55398 9 PCT/US97/09674
preferably from about 30 seconds to 90 seconds, during which time "aging" or
partial
gelation of the mixture takes place. Transfer time can be as long as 15-16
minutes at very
low flow rates and still produce satisfactory results. Dilution water from
reservoir 1.Q is
added via line 66 (316 SS, 1/2 inch OD) to the mixture just prior to its entry
into finished
product receiving tank 1$ or at any other convenient location so long as the
silicate/acid
mixture is diluted to an Si02 concentration of less than 1.0 wt.% which
stabilizes the
gelation process. Dilution water is supplied with centrifugal pump 68
(Eastern, 316 SS, I
HP, 54 gpm maximum), and flow control is accomplished at a predetermined rate
with
control valve 70 (Whitey, 316 SS, 1/2 inch needle) and rotameter 72 (Brooks,
SS Ball,
12.46 gpm maximum). The finished product receiving tank 1$ is provided with a
level
control system 74 (Sensall, Model 502) which operates in conjunction with an
automatic
three-way valve 76 (Whitey, 316 SS, 1/2 inch diameter) to divert flow of the
silicate/acid
mixture to the sewer if the level of finished product becomes too high.
After a period of continuous operation, which depends on the amount of
active silica produced, it may be desirable to cease generation of the active
silica and
flush the mixing junction 20 and that portion of the system which is
downstream, i.e.,
piping, valves, transfer lines, etc., which have been in contact with the
silicate/acid
mixture, with water and warm NaOH. Flushing the system removes any undesirable
silica deposits which may have accumulated in parts of the apparatus where the
required
turbulent flow conditions could not have been maintained due to design
restrictions, as
for example in the region of pH measurement. The flushing procedure helps
maintain the
system free of silica deposition and is begun by first shutting off dilution
pump 68, acid
pump 44 and silicate pump 24. Dilution water from pump 36 is then circulated
through
the downstream portion of the system for about 5 minutes, after which pump 36
is shut
off, and the dilution water reservoir is isolated by closing valves 40, 60 and
70. Three-
way automatic valves 52 and 76, and manual valves 78, 80 and 82 (all Whitey,
316 SS,
1/2 inch OD) are then activated along with centrifugal circulating pump 84
(Eastem, 316
SS, 1.5HP, 15 gpm maximum) to allow NaOH, maintained at a concentration of 20
wt.%
and a temperature in the range of from 40 to 60 C, to circulate through the
downstream
portion of the system for generally not longer than about 20-30 minutes. The
NaOH
circulating pump 84 and the flush tank 1-6 are then isolated from the system
by again
activating three-way valves 80 and 82, and dilution water is again flushed
through the
downstream system and released to the sewer. Having completed the
cleaning/flushing
procedure, the production of active silica can be resumed.
Referring now to Fig. 2, there is shown a schematic diagram of a dual line
production system for active silica, whereby one line can be operational at
all times while
CA 02289711 1999-11-12
WO 98/55398 PCTIUS97/09674
the other line is being flushed or being maintained in a stand-by condition.
The
component parts are numbered in accordance with Fig. 1. A commercial system
according to either of Figs. 1 or 2, will generally be constructed of
stainless steel or
polyvinyl chloride tubing of generally one inch diameter or less, depending on
the
5 requirement for active silica. When stainless steel tubing is used,
connections of the
various instruments, fittings, valves, and sections can be conveniently made
with
"Swagelok" compression joints.
Fig. 3 is a schematic diagram showing a modification of the basic
apparatus of Fig. 1 suitable for the production of polyaluminosilicate
microgels. From
10 the reservoir 100, a concentrated solution of an aluminum salt, preferably
aluminum
sulfate, cari be pumped through tubing (1/4 inch diameter 316 stainless steel)
by means of
a diaphragm metering pump 102 (Pulsatron Model LPR 2-MA.PTC 1, glass filled
polypropylene, Teflon diaphragm, max. flow 12.5 ml/min). The metering pump
102
can be linked electronicaliy to the controller 90 and can move in parallel
with silicate
usage. After passing through check valve 104 (Whitey, 316SS, 1/4 inch
diameter), the
aluminum salt solution can be introduced into the diluted acid line at the
point 106 by
means of a 316 SS "T" junction. Thorough mixing of the aluminum salt with the
diluted
acid can be completed by the in-line mixer 56 before reaction with the
silicate, to produce
polyaluminosilicate microgels, occurs at "T" junction 20. A preferred aluminum
salt
solution for use in the method is a commercial solution of aluminum sulfate
such as
liquid alum solution A12(S04)3,14H20 containing 8.3 wt.% A1203 supplied by the
American Cyanamid Company.
Periodically, it is necessary to flush the polyaluminosilicate apparatus free
from silica deposits by means of warm caustic soda solution as described
above.
It should be understood that a dual line apparatus for the continuous
production of polyaluminosilicate microgels can be constructed by the
appropriate
modifications of the dual line apparatus of Fig. 2.
Example 1- Demonstratink the effect of turbulence in reducinQ silica depo i
ion
A laboratory generator for producing polysilicate microgels was
constructed according to the principles described in Fig. 1. The silicate and
sulfuric acid
feeds, before dilution and mixing, contained 15 wt.% silica and 20 wt.% acid
respectively. The critical junction mixer was constructed from a 1/4 inch, 316
stainless
steel "Swagelok" T-compression fitting fitted with 6 inch arms of 1/4 inch OD
316 SS
tubing. The internal diameter of the fitting was 0.409 cm. For the tests in
which a gas
was introduced into the mixing junction a similar "Swagelok" X-compression
coupling
CA 02289711 1999-11-12
WO 98/55398 PCT/US97/09674
I1
was used with the fourth arm of the X as the gas inlet. An in-line filter
comprised of 1
inch diameter 60 mesh stainless steel screen was placed about 12 inches from
the
acid/silicate junction to trap particulate silica. The screen was weighed at
the beginning
of each test and again at the end of each test, after washing and drying, so
as to give a
measure of silica deposition. All tests were run so as to maintain conditions
of 2 wt.%
silica and pH 9 at the point of silicate acidification and each test was run
for sufficient
time to produce a total amount of 1,590 gms. of polysiiicate microgel. The
results of the
tests are given in Table 1 below. Liquid flow represents the total liquid
flow, that is, the
flow of the combined silicate/acid mixture in the exit tube. In the tests
where a gas was
introduced to enhance liquid flow and turbulence, the Reynolds number was
calculated
on the basis of the increased flow rate of the liquid portion alone, assuming
that liquid
density and viscosity did not change appreciably. This method of calculation
was
adopted because there is no ready formula for calculating the Reynolds number
of
liquid/gas mixtures.
Table I
Silica Deposition As A Function Of Reynolds Number
Test Reynolds Run Time Liquid Flow Gas Flow Silica
N-Q. No. mins. ml/m ml/rrm de osn ited=
gms,
1 1,036 330 250 none 0.339
2 2,072 165 499 none 0.135
3 4,144 83 999 none 0.009
4 6,217 55 1,498 none 0.007
5 10,362 33 2,497 none 0.002
6 12,433 27 2,996 none 0.008
7 12,260 120 694 Air, 2,260 0.008
8 9,064 120 694 Air, 1,490 0.005
9 5,375 120 694 Air, 601 0.004
10 5,375 120 694 N2,601 0.014
A comparison of the results of Tests 1& 2 with the results of Tests 3-10
clearly demonstrate the beneficial effect of turbulent liquid flow (Reynolds
number above
4,000) in reducing the amount of silica deposition observed. Under turbulent
flow
conditions of the present invention, the average silica deposition of 0.007 g
represented
only 0.0004% of the total amount of silica processed. When the Reynolds number
was
CA 02289711 1999-11-12
WO 98/55398 12 PCT/US97/09674
below the minimum of 4,000 required by the instant invention, undesirable
silica
deposition was at least approximately 15-fold increased. Once the minimum
Reynolds
number required by the process of this invention was reached, increasing the
Reynolds
number above 4,000, for example from 4,144 to 6,217 to 10,362, etc. did not
appreciabiy
reduce silica deposition further.
Example 2 - Apparatus
A commercial sized apparatus for preparing active silica microgels was
assembled according to the schematic design shown in Fig. 1 and installed in a
commercial paper mill. The apparatus, except for the raw material supply
reservoirs, was
rigidly mounted on steel framework on two skids each measuring approximately
six feet
by eight feet. On skid 1 was mounted inlets for connection to commercial
supplies of
sodium silicate and sulfuric acid and an inlet for city water which was used
for dilution
purposes. Also on skid I was mounted the dilution and flow control means, the
silicate/acid mixing junction, pH measurement and pH controller, sodium
hydroxide flush
reservoir, required pumps and valves and the electrical controls. On skid 2
was mounted
the aging loop, finished product reservoir, level controller and required
pumps and
valves. Overall height of each skid was about seven feet. The manufacturers
supply
containers were used as reservoirs for the silicate and sulfuric acid and
these were
connected directly to the appropriate inlets on skid 1.
The apparatus was operated continuously for six (6) days during which 0.5
wt.% active silica was produced at a rate which varied between 3 and 4.8
gallons per
minute. At a production rate of 3 gpm, a Reynolds number of 4250 was
calculated for the
mixing zone employed. No silica deposition was observed within the junction
mixer 20,
although some silica deposition was observed in the proximity of the pH probe
located
immediately downstream from the junction mixer exit after 12 hours of
continuous
operation. To alleviate this situation, a water/NaOH/water flush sequence was
conducted,
which took less than 30 minutes, and the system was then returned to normal
production.
Over the entire six day period, the apparatus operated without fault and
produced active
silica of excellent quality which was utilized by the mill for the production
of a range of
papers with different basis weights.
P.xamole 3 - Preparation of Polyaiuminosilicate Microgel
A commercial-sized apparatus for preparing polyaluminosilicate microgel
solution was assembled according to the principles shown in Figure 3. The
apparatus,
except for the raw material supply reservoirs, was rigidly mounted on steel
framework on
. . . , . I . i.
CA 02289711 1999-11-12
WO 98/55398 13 PCT/US97/09674
two skids each measuring approximately eight feet by eight feet. On skid I
were
mounted inlets for connection to supplies of sodium silicate, sulfuric acid,
sodium
hydroxide and papermaker's alum and an inlet for city water which was used for
dilution
= purposes. Also mounted on skid 1 were the required pumps for each chemical
and a
reservoir for containing the finished polyaluminosilicate microgel solution.
On skid 2
were mounted flow control valves for sodium silicate, acid, and the dilution
water, the
silicate/acid mixing junction, pH measurement means and pH controller, an
aging loop,
and a sodium hydroxide flush reservoir. Flow of the papermaker's alum was
controlled
by a diaphragm pump at rate proportional to the silicate flow. The
papermaker's alum
was introduced into the diluted acid stream prior to the silicate/acid mixing
junction. The
resulting polyaluminosilicate microgel solution had an A1203/Si02 molar ratio
of
approximately 1/1250.
The apparatus was used to produce 6000 gallons of 0.5 wt%
polyaluminosilicate microgel solution at a rate of 20 gallons per minute. A
Reynolds
number of 22,700 was calculated for the mixing zone. Only minor silica
deposition was
noted on the pH electrode after 5 hours of operation. To remove the silica
deposits, a
NaOH flush was conducted, which took less than 30 minutes, and the system was
then
returned to normal production. The polyaluminosilicate microgel solution was
utilized
by a paper mill for the production of liquid packaging board with excellent
results.