Note: Descriptions are shown in the official language in which they were submitted.
CA 02291066 1999-11-22
WO 99!48536 PCT/US99/06344
-1-
LOCAL DELIVERY OF LONG LASTING THERAPEUTIC AGENTS
FIELD OF THE INVENTION
This invention relates to the field of therapeutic agents in
medicine. In particular, this invention relates to the field of localized
delivery of therapeutic agents wherein the agents are capable of
covalently bonding to a site of interest in vivo, to provide increased
tissue retention and pharmacodynamic duration of therapeutic
benefit for the given drug.
BACKGROUND OF THE INVENTION
The technology of local delivery of a therapeutic using drug
delivery catheters or devices is well established. Under ideal
circumstances, the therapeutic agent will remain near the site of
administration for increased effectiveness. While useful, the main
drawback of this technology is the rate at which the therapeutic
agent is washed away from the site of application. For example,
according to imanishi et al. (J Cardio, 1996, 27, 267-271 ), the
concentration of residual argatroban introduced through pressure
balloon catheter is decreased by three folds in the first five minutes
after deflation of the balloon. This phenomenon is reported
repeatedly in the literature for other therapeutic agents resulting in a
general deficiency that drugs from a diverse therapeutic areas have
limited utility following localized delivery due to their inability to
maintain adequate concentrations within hours after delivery
(Circulation, 1994, 89 (41, 1518-1524; Circulation, 1997, 96 (1),
154-165). As a result, repeated dosings of locally administered
therapeutic agents are required in order to avoid rapid reduction of
SUBSTITUTE SHEET (RULE 26)
CA 02291066 1999-11-22
WO 99/48536 PCT/US99/06344
drug levels and sub-optimal performance or sub-effective
responsiveness. This results in increased costs and unnecessary
patient exposure to excessive amounts of therapeutic agents.
Thus, there is a need to provide therapeutic agents to localized
sites such that the therapeutic agents have increased retention at the
desired site and prolonged duration of action. These therapeutic
agents are not as easily washed away from the site of administration
so that reduced amounts of the agents can be supplied. In
particular, there is a need to provide therapeutic agents capable of
forming covalent bonds to localized sites such that the therapeutic
agents have increased effective presence for therapeutic benefits.
SUMMARY OF THE INVENTION
In order to meet these needs, the present invention is directed
to therapeutic agents capable of forming covalent bonds to localized
sites such that the therapeutic agents have increased tissue retention
and half-lifes.
A first aspect of this invention relates to the modification of
drugs at a site not involved in the pharmacophore receptor
interaction.
A second aspect of this invention relates to novel chemistry
involved in the non-specific formation of covalent bonds using homo
and heterobifunctionai cross-linking reagents.
In addition, the invention includes the non-specific labeling of
fixed blood proteins and tissues with N-Hydroxy-Succinimide (NHS)-
drugs and sulfo-NHS drugs, although other reactive groups, which
are functional in an aqueous medium such as blood, may also be
employed. In some cases, special reagents find use, such as azido,
diazo, carbodiimide anhydride, hydrazine, dialdehydes, thiol groups,
or amines to form amides, esters, imines, thioethers, disulfides,
SUBSTITUTE SHEET (RULE 26)
CA 02291066 1999-11-22
WO 99/48536 PCT/US99/06344
-3-
substituted amines, or the like. Usually, the covalent bond which is
formed should be able to be maintained during the lifetime of the
blood component, unless it is intended to be a release site. A major
advantage of this technology is the small amount of drug necessary
to provide an effective therapeutic window when compared to
systemic administration. The reasons for this advantage are
explained by the targeting of the delivery, the high yield of reaction
between reactive entity and reactive functionality and the irreversible
nature of the bond formed after reaction. The therapeutic agent is
localized in the vicinity of the therapeutic target and is not allowed to
circulate in the blood stream like any other free drug. Another
advantage of the technology is its limited side effects that is a
consequence of the advantage described above. Once bound to the
membrane or tissue the therapeutic agent is not susceptible to liver
metabolism, kidney filtration and excretion, and may even be
protected from protease (inclusive of endopeptidase) activity which
usually leads to loss of activity and accelerated elimination.
Another aspect of this invention relates to novel chemically
reactive derivatives of radiolabeled molecules which can react with
available functionalities on fixed blood proteins and on tissues to
form covalent linkages, and in which the resulting covalently bound
conjugates have radioactive properties.
As compared with free radiolabeled drugs the conjugated
molecules have extended lifetimes in the bloodstream and are,
therefore, capable of maintaining the radioactivity for extended
periods of time as compared to the unconjugated parent drug, and
provide such activity with reduced centrally mediated side effects.
In addition, the invention includes the non-specific labeling of
fixed blood proteins and tissues with NHS-radiolabeled drugs and
sulfo-NHS radiolabeled drugs although other reactive groups, which
SUBSTITUTE SHEET (RULE 26)
CA 02291066 1999-11-22
WO 99/48536 PCT/US99/06344
-4-
are functional in an aqueous medium such as blood, may also be
employed. In some cases, special reagents find use, such as azido,
diazo, carbodiimide, anhydride, hydrazine, dialdehydes, thiol groups,
or amines to form amides, esters, imines, thioethers, disulfides,
substituted amines, or the like. Usually, the covalent bond which is
formed should be able to be maintained during the lifetime of the
blood or tissue component, unless it is intended to be a release site.
The invention further includes the conjugates of the
radiolabeled derivatives with fixed blood components and tissues and
methods for providing radioactivity in vivo comprising administering
to a mammalian host the novel radiolabeled derivatives through the
use of percutaneous catheter technology, endarterectomy or direct
tissue incubation.
Another aspect of this invention relates to novel chemically
reactive derivatives of RGD-containing peptides which can react with
available functionalities on fixed blood proteins and on tissues to
form covalent linkages, and in which the resulting covalently bound
conjugates have RGD peptide activity.
As compared with RGD peptide drugs the conjugated
molecules have extended lifetimes in the bloodstream or tissues and
are, therefore, capable of maintaining RGD peptide activity for
extended periods of time as compared to the unconjugated parent
drug. Such activity is provided with reduced centrally mediated side
effects due to the high efficiency of localized delivery, the irreversible
covalent bond between therapeutic agent and membrane protein and
the lower therapeutic doses used with this invention.
In addition, the invention includes the non-specific labeling of
fixed blood proteins and tissues with NHS-RGD peptides and sulfo-
NHS peptides although other reactive groups, which are functional in
an aqueous medium such as blood, may also be employed. In some
SUBSTITUTE SHEET (RULE 26)
*rB
CA 02291066 1999-11-22
WO 99/48536 PCT/US99/06344
-5-
cases, special reagents find use, such as azido, diazo, carbodiimide
anhydride, hydrazine, dialdehydes, thiol groups, or amines to form
amides, esters, imines, thioethers, disulfides, substituted amines, or
the like. Usually, the covalent bond which is formed should be able
to be maintained during the lifetime of the blood or tissue
component, unless it is intended to be a release site.
The invention further includes the conjugates of the RGD
peptide derivatives with fixed blood components and tissues and
methods for providing RGD peptide activity in vivo comprising
administering to a mammalian host the novel RGD peptide
derivatives through the use of percutaneous catheter technology,
endarterectomy, pericardial, advantitial, intracardiac or direct tissue
incubation.
The invention further includes the method of use for
conjugates of RGD derivatives with fixed blood components and
tissues for providing RGD peptide to perform wound heating through
platelet immobilization generated by the binding of the peptide onto
glycoprotein 1Ibl111a receptors.
The invention further includes the method of use for
conjugates of RGD derivatives with fixed blood components and
tissues for providing RGD peptide to produce anti-restenosis
properties through direct interaction with known integrin receptors
(alpha V beta III, 1Ibl111a, alpha 5 beta III etc. ) to modulate the
stimulation of platelets and inflammatory responses including
induction of proliferation, migration, cell adhesion and tissue
remodeling.
The invention further includes the method of use for
conjugates of RGD derivatives with fixed blood components and
tissues for providing RGD peptide to produce anti-angiogenic and
antimetastatic properties through modification of adhesion and
SUBSTITUTE SHEET (RULE 26)
CA 02291066 1999-11-22
WO 99/48536 PCT/US99/06344
-6-
migration mediated by integrin receptor interaction with cell adhesion
and cell to cell coupling as well as matrix degradation associated
with cellular immigration and emigration.
The invention further includes a local delivery agent
comprising a compound of the formula: X-Y-Z wherein X is selected
from the group of wound healing agents, antibiotics, anti-
inflammatories, antioxidants, anti-proliferatives, anti-restenosis, anti-
angiogenic, immunosuppressants, anti-infectives and anti-cancer
agents. In the formula, Y is a linking group consisting of 0-30 atoms
and Z is a chemically reactive entity capable of reaction with a
reactive functionality on fixed blood components to form covalent
bonds therewith.
in one format, Z may be selected from N-hydroxysuccinimide,
N-hydroxy sulfosuccinimide, maleimide-benzoyl-succinimide, gamma-
maleimido-butyryloxy succinimide ester, maieimidopropionic acid,
isocyanate, thiolester, thionocarboxyiic acid ester, imino ester,
carbodiimide anhydride and carbonate ester.
In another format, X may be selected from peptides, organic
molecules and radioactive molecules. In another format, X may be an
RGD containing peptide having wound healing properties. The RGD
peptide may have the sequence Ac-RIARGDFPDDRK(EGSI-NH2 where
EGS is ethylene glycol-bis(succinimidylsuccinatel.
The invention further includes a method of increasing the
retention time of a therapeutic agent locally administered to a site by
delivering a compound of the formula: X-Y-Z wherein X is selected
from the group of wound healing agents, antibiotics, anti-
inflammatories, antioxidants, anti-proliferatives, anti-restenosis, anti-
angiogenic, immunosuppressants, anti-infectives and anti-cancer
agents. In the formula, Y is a linking group consisting of 0-30 atoms
and Z is a chemically reactive entity capable of reaction with a
SUBSTITUTE SHEET (RULE 26)
CA 02291066 1999-11-22
WO 99/48536 PCT/US99/06344
-7_
reactive functionality on fixed blood components to form covalent
bonds therewith.
The invention further includes a method of promoting wound
healing at a wound site, comprising administering a compound of the
formula: X-Y-Z wherein X is a wound healing agent, Y is a linking
group of 0-30 atoms and Z is a chemically reactive entity capable of
reaction with a reactive functionality on fixed blood components to
form covalent bonds therewith.
The invention further includes a method of treating a tumor
comprising a compound of the formula X-Y-Z wherein X is an anti-
cancer agent, Y is a linking group of 0-30 atoms and Z is a
chemically reactive entity capable of reaction with a reactive
functionality on fixed blood components to form covalent bonds
therewith.
i5
BRIEF DESCRIPTION OF THE DRAWINGS
The invention will be better understood by reference to the
figures, in which:
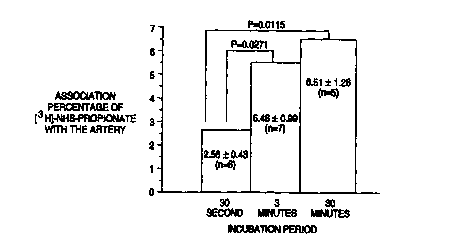
Figure 1 represents the association efficiency of (3H )-NHS-
propionate with the damaged rabbit carotid arteries following local
incubation.
Figure 2 represents the retention efficiency of (3H ]-NHS
propionate following a 3 minutes incubation period in damaged rabbit
carotid arteries.
Figure 3 represents the 3 days retention efficiency of [3H ]-
NHS-propionate and (3H ]-propionate following a 3 minutes
incubation period with damaged rabbit carotid arteries.
SUBSTITUTE SHEET (RULE 26)
CA 02291066 1999-11-22
WO 99/48536 PCT/US99/06344
_$_
DETAILED DESCRIPTION OF THE INVENTION
To ensure a complete understanding of the invention the
following definitions are provided:
Local Delivery Agent: Local delivery agents are agents that
rnay be delivered to a local site of interest. Such agents include
therapeutic agents. Local delivery includes topical application to
both internal and external sites requiring therapeutic treatment.
Therapeutic Agents: Therapeutic agents are agents that have a
therapeutic effect. Therapeutic agents include wound healing
agents, antibiotics, anti-infectives, anti-oxidants, chemotherapeutic
agents, anti-cancer agents, anti-inflammatory agents, and
antiproliferative drugs.
Fixed blood components: Fixed blood components are non-
mobile blood components and include tissues, membrane receptors,
interstitial proteins, fibrin proteins, collagens, platelets, endothelial
cells, epithelial cells and their associated membrane and
membraneous receptors, somatic body cells, skeletal and smooth
muscle cells, neuronal components, osteocytes and osteoclasts and
all body tissues especially those associated with the circulatory and
lymphatic systems.
Mobile blood components: Mobile blood components are blood
components that do not have a fixed situs for any extended period of
time, generally not exceeding 5, more usually one minute. Mobile
blood components include soluble blood proteins such as
immunoglobulins, serum albumin, ferritin, transferrin and the like.
SUBSTITUTE SHEET (RULE 26)
CA 02291066 1999-11-22
WO 99/48536 PCT/US99/06344
_g_
Wound Heating Agents: Wound healing agents are agents that
promote wound healing. Wound healing agents include integrins, cell
adhesion molecules such as 1CAM, ECAM, ELAM and the like,
antibiotics, growth factors such as EGF, PDGF, IGF, bFGF, aFGF and
KGF, fibrin, thrombin, RGD peptides and the like.
Antiprofiferatives: Antiproliferatives include antimetabolites,
topoisomerase inhibitors, folic acid antagonists like methotrexate,
purine antagonists like mercaptopurine, azathioprine, and pyrimidine
antagonists like fluorouracil, cytarabine and the like.
Antioxidants: Antioxidants are agents that prevents oxidative
damage to tissue and include aspartate, orotate, tacophenol
derivative (vitamin E), and free radical scavengers such as SOD,
glutathione and the like.
Mammalian cells are continuously exposed to activated oxygen
species such as superoxide, hydrogen peroxide, hydroxyl radical, and
singlet oxygen. These reactive oxygen intermediates are generated
in vivo by cells in response to aerobic metabolism, catabolism of
drugs and other xenobiotics, ultraviolet and x-ray radiation, and the
respiratory burst of phagocytic cells (such as white blood cells) to kill
invading bacteria such as those introduced through wounds.
Hydrogen peroxide, for example, is produced during respiration of
most living organisms especially by stressed and injured cells.
Active oxygen species can injure cells. An important example
of such damage is lipid peroxidation which involves the oxidative
degradation of unsaturated lipids. Lipid peroxidation is highly
detrimental to membrane structure and function and can cause
numerous cytopathological effects. Cells defend against lipid
SUBSTITUTE SHEET (RULE 26)
CA 02291066 1999-11-22
WO 99/48536 PCT/US99/06344
-10-
peroxidation by producing radical scavengers such as superoxide
dismutase, catalase, and peroxidase. Injured cells have a decreased
ability to produce radical scavengers. Excess hydrogen peroxide can
react with DNA to cause backbone breakage, produce mutations,
and alter and liberate bases. Hydrogen peroxide can also react with
pyrimidines to open the 5,6-double bond, which reaction inhibits the
ability of pyrimidines to hydrogen bond to complementary bases,
Hallaender et al. (1971 ). Such oxidative biochemical injury can
result in the loss of cellular membrane integrity, reduced enzyme
activity, changes in transport kinetics, changes in membrane lipid
content, and leakage of potassium ions, amino acids, and other
cellular material.
Antioxidants have been shown to inhibit damage associated
with active oxygen species. For example, pyruvate and other alpha-
ketoacids have been reported to react rapidly and stoichiometrically
with hydrogen peroxide to protect cells from cytolytic effects,
0'Donnell-Tormey et al., J. Exp. Med., 165, pp. 500-514 (1987).
Anti-lnfective Agents: Anti-infective agents are agents that
inhibit infection and include anti-viral agents, anti-fungal agents and
antibiotics.
Anti-Viral Agents: Anti-viral agents are agents that inhibit virus
and include vidarabine, acyclovir and trifluorothymidine.
Anti-Fungal Agents: Anti-fungal agents are agents that inhibit
fungal growth. Anti-fungal agents include anphoterecin B,
myconazole, terconazole, econazole, isoconazole, thioconazole,
biphonazole, clotrimazole, ketoconazole, butaconazole, itraconazole,
oxiconazole, phenticonazole, nystatin, naphthyphene, zinoconazole,
SUBSTITUTE SHEET (RULE 26)
CA 02291066 1999-11-22
WO 99/48536 PCT/US99/06344
-1 1-
cyclopyroxofamine and fluconazole.
Antibiotics: Antibiotics are natural chemical substances of
relatively low molecular weight produced by various species of
microorganisms, such as bacteria (including Bacillus species),
actinomycetes (including Streptomyces) and fungi, that inhibit
growth of or destroy other microorganisms. Substances of similar
structure and mode of action may be synthesized chemically, or
natural compounds may be modified to produce semi-synthetic
antibiotics. These biosynthetic and semi-synthetic derivatives are
also effective as antibiotics. The major classes of antibiotics are
(1 ) the beta-lactams, including the penicillins, cephalosporins and
monobactams; (2) the aminoglycosides, e.g. gentamicin, tobramycin,
netilmycin, and amikacin; (3) the tetracyclines; (4) the sulfonamides
and trimethoprim; (5) the fluoroquinolones, e.g. ciprofloxacin,
norfloxacin, and ofloxacin; (61 vancomycin; (7) the macrolides, which
include for example, erythromycin, azithromycin, and clarithromycin;
and (8) other antibiotics, e.g., the polymyxins, chloramphenicol and
the lincosamides.
Antibiotics accomplish their anti-bacterial effect through
several mechanisms of action which can be generally grouped as
follows: (1 ) agents acting on the bacterial cell wall such as
bacitracin, the cephalosporins, cycloserine, fosfomycin, the
penicillins, ristocetin, and vancomycin; (2) agents affecting the cell
membrane or exerting a detergent effect, such as colistin, novobiocin
and polymyxins; (3) agents affecting cellular mechanisms of
replication, information transfer, and protein synthesis by their
effects on ribosomes, e.g., the aminoglycosides, the tetracyclines,
chloramphenicol, clindamycin, cycloheximide, fucidin, lincomycin,
puromycin, rifampicin, other streptomycins, and the macrolide
SUBSTITUTE SHEET (RULE 26)
CA 02291066 1999-11-22
WO 99/48536 PCT/US99/06344
-12-
antibiotics such as erythromycin and oleandomycin; (4) agents
affecting nucleic acid metabolism, e.g., the fluoroquinolones,
actinomycin, ethambutol, 5-fluorocytosine, griseofulvin, rifamycins;
and (5) drugs affecting intermediary metabolism, such as the
sulfonamides, trimethoprim, and the tuberculostatic agents isoniazid
and para-aminosalicylic acid. Some agents may have more than one
primary mechanism of action, especially at high concentrations. In
addition, secondary changes in the structure or metabolism of the
bacterial cell often occur after the primary effect of the antimicrobial
drug.
Anti-Cancer Agents: Anti-cancer agents are natural or
synthetic molecules which are effective against one or more forms of
cancer. This definition includes molecules which by their mechanism
of action are cytotoxic (anti-cancer chemotherapeutic agents), those
which stimulate the immune system (immune stimulators) and
modulators of angiogenesis. The outcome in either case is the
slowing of the growth of cancer cells.
Anti-cancer therapy include radioactive isotopes such as 32P
used in the treatment of polycythemia vera and in chronic leukemia.
Radioactive phosphorus has a biological half-life of about 8 days in
humans. It emits beta rays that exert a destructive effect on the
rapidly multiplying cells. 32P is usually administered in doses of
about 1 me daily for 5 days. Either the oral or intravenous route may
be used and the doses are not greatly different. Radioactive iodine
'3'1, radioactive gold 'ssAu, and other isotopes are not as useful as
szP. Nevertheless, '3'I has some limited applications in metastatic
thyroid carcinoma. Other radioactive isotopes can be used with our
technology either as complexes of radioactive metal such as 5' Cr,
s2Mn ~ szMg~ s~ Ni~ ssCo and SsP, sSFe , ,osPd~ ,s2lr~ s4Cu and s'Cu or
SUBSTITUTE SHEET (RULE 26)
CA 02291066 1999-11-22
WO 99/48536 PCT/US99/06344
-13-
as chelates of these metals using bifunctional chelating agents like
(BFCs), 6-(p-(bromoacetamido)benzyl]-1,4,8,11-
tetraazacyclotetradecane-1,4,8,11-tetraacetic acid
(BAT), 6-(p-(isothiocyanato)benzyl]-1,4,8,11-
tetraazacyclotetradecane-1,4,8,11-tetraacetic acid (SCN-TETA),
4-(( 1,4,8,1 1-tetraazacyclotetradec-1-yl)methyl]benzoic acid (CPTA),
and 1-((1,4,7,10,13-pentaazacyclopentadec-1-yl)methyl]benzoic acid
(PCBA1.
Numerous drugs fall into the category of chemotherapeutic
agents useful in the treatment of neoplastic disease that are
amenable to the embodiment of this application for focal drug
delivery and retention of the modified drug substance at the tumor
site. Such agents derivitized with this technology can include anti-
metabolites such as metotrexate (folic acid derivatives), fluoroaucil,
cytarabine, mercaptopurine, thioguanine, petostatin (pyrimidine and
purine analogs or inhibitors), a variety of natural products such as
vincristine and vinblastine (vinca alkaloid), etoposide and teniposide,
various antibiotics such as miotomycin, plicamycin, bleomycin,
doxorubicin, danorubicin, dactomycin; a variety of biological
response modifiers including interferon-alpha; a variety of
miscellaneous agents and hormonal modulators including cisplatin,
hydroxyurea, mitoxantorne, procarbozine, aminogultethimide,
prednisone, progestins, estrogens, antiestorgens such as tamoxifen,
androgenic steroids, antiadrogenic agents such as flutamide,
gonadotropin releasing hormones analogs such as leuprolide, the
matrix metalloprotease inhibitors (MMPIsI as well as anti-cancer
agents including Taxol (paclitaxel) and related molecules collectively
termed taxoids, taxines or taxanes.
Included within the definition of "taxoids" are various
modifications and attachments to the basic ring structure (taxoid
SUBSTITUTE SHEET (RULE 26)
CA 02291066 1999-11-22
WO 99/48536 PCTNS99/06344
-14-
nucleus) as may be shown to be efficacious for reducing cancer cell
growth and which can be constructed by organic chemical
techniques known to those skilled in the art.
Chemotherapeutics include podophyllotoxins and their
derivatives and analogues. Another important class of
chemotherapeutics useful in this invention are camptothecins.
Another preferred class of chemotherapeutics useful in this
invention are the anthracyclines (adriamycin and daunorubicin).
Another important class of chemotherapeutics are compounds
which are drawn from the following list: Taxotere, Amonafide, Illudin
S, 6-hydroxymethylacylfulvene Bryostatin 1, 26-succinylbryostatin 1,
Palmitoyl Rhizoxin, DUP 941, Mitomycin B, Mitomycin C,
Penclomedine, angiogenesis inhibitor compounds, Cisplatin
hydrophobic complexes such as 2-hydrazino-4,5-dihydro-1 H-
imidazole with platinum chloride and 5-hydrazino-3,4-dihydro-2H-
pyrrole with platinum chloride, vitamin A, vitamin E and its
derivatives, particularly tocopherol succinate.
Other compounds useful in the invention include: 1,3-bis(2-
chioroethyl)-1-nitrosurea ("carmustine" or "BCNU"), 5-fluorouracil,
doxorubicin ("adriamycin"), epirubicin, aclarubicin, Bisantrene (bis(2-
imidazolen-2-ylhydrazone)-9,10-anthracenedicarboxaldehyde,
mitoxantrone, methotrexate, edatrexate, muramyl tripeptide,
muramyl dipeptide, lipopolysaccharides, vidarabine and its 2-fluoro
derivative, resveratrol, retinoic acid and retinol, carotenoids, and
tamoxifen.
Other chemotherapeutic agents useful in the application of this
invention include: Decarbazine, Lonidamine, Piroxantrone,
Anthrapyrazoles, Etoposide, Camptothecin, 9-aminocamptothecin, 9-
nitrocamptothecin, camptothecin-11 ("Irinotecan'), Topotecan,
Bleomycin, the Vinca alkaloids and their analogs [Vincristine,
SUBSTITUTE SHEET (RULE 26)
CA 02291066 1999-11-22
WO 99/48536 PCTNS99/06344
-15-
Vinorelbine, Vindesine, Vintripol, Vinxaltine, Ancitabine], 6-
aminochrysene, and Navelbine.
Other compounds useful in the application of the invention are
mimetics of taxol, eieutherobins, sarcodictyins, discodermolides and
epothiolones.
RGD Peptides: The RGD peptide for conjugation to tissues or
fixed endogenous proteins in accordance with the present invention
includes a sequence of amino acids, preferably naturally occurring
L-amino acids and glycine, having the following formula:
R,-Arg-Gly-Asp-R2
In this formula, R, and R2 represent an amino acid or a
sequence of more than one amino acid or a derivatized or chemically
modified amino acid or more than one derivatized or chemically
modified amino acids.
Delivery Devices: Delivery devices are devices useful for local
delivery of therapeutic agents. Delivery devices include catheters,
syringes, trocars and endoscopes.
Reactive Entities: Reactive entities are entities capable of
forming a covalent bond. Such reactive agents are coupled or
bonded to a therapeutic agent of interest. Reactive entities will
generally be stable in an aqueous environment and will usually be
carboxy, phosphoryl, or convenient acyl group, either as an ester or
an anhydride, or an imidate, thereby capable of forming a covalent
bond with an amino group at the target site to form an amide or
amide derivative. For the most part, the esters will involve phenolic
compounds, or be thiol esters, alkyl esters, phosphate esters, or the
like.
SUBSTITUTE SHEET (RULE 26)
CA 02291066 1999-11-22
WO 99/48536 PCTIUS99l06344
-16
While the reactive entity is usually chosen to react with an
amino group at the target site, other reactive functionalities at the
target site may be exploited. For example, the reactive functionality
may comprise various phosphinyl or phosphonyl derivatives for the
bonding to available hydroxyl functions at the target site or may
comprise an imine, thioimine or disulfide for bonding to thiol
residues.
Reactive Functionalities: The reactive functionalities available
on vascular proteins for covalent bond formation with the reactive
group are primarily amino, carboxyl and thiol groups. While any of
these may be used as the target for the reactive entity, for the most
part, bonds to amino groups will be employed, particularly with the
formation of amide bonds.
To form amide bonds, one may employ a wide variety of
active carboxyl groups as the reactive functional group of the
bifunctional molecule, particularly esters, where the hydroxyl group
is physiologically acceptable at the levels required. While a number
of different hydroxyl groups may be employed, the most convenient
wilt be N-hydroxysuccinimide and N-hydroxy sulfosuccinimide,
although other atcohols, which are functional in the vascular
environment may also be employed. In some cases, special reagents
find use such as diazo, azido, carbodiimide, anhydride, hydrazine, or
thiol groups, depending on whether the reaction is in vivo or in vitro,
the target, the specificity of the reactive entity, and the like.
IC50: Concentration of an enzyme inhibitor at which
50% of the enzymatic activity is inhibited.
Protective Groups: Protective groups are chemical
SUBSTITUTE SHEET (RULE 26)
CA 02291066 1999-11-22
WO 99/48536 PCT/US99/06344
-17-
moieties utilized to protect reactive entities from reacting with
themselves. Various protective groups are disclosed in U.S.
5,493,007 which is hereby incorporated by reference. Such
protective groups include acetyl, fluorenylmethyloxycarbonyl
(FMOC), t-butyloxy carbonyl (BOC), benzyloxycarbonyl (CBZ), and
the like.
Linking Groups: Linking groups are chemical moieties
that link or connect reactive entities to therapeutic agents. Linking
groups may comprise one or more alkylene, alkyleneoxy, alkenylene,
alkynylene or amino group substituted by alkyl groups; cycloalkylene
groups, polycyclic groups, aryl groups, polyaryl groups, substituted
aryl groups, heterocyclic groups, and substituted heterocyclic
groups. Linking group will have from 2-100, more usually from 2-
18, preferably from 6-12 atoms in the chain, particularly carbon,
oxygen, phosphorous and nitrogen, more particularly carbon and
oxygen.
DETAILED DESCRIPTION
Taking into account these definitions, in its first aspect, the
invention is directed to the local delivery of therapeutic agents which
have been modified with reactive entities so that they will covalently
react and bond in vivo with reactive functionalities onto a fixed blood
component and provide increased half lifes for the therapeutic
agents.
The derivatized therapeutic agent of the present invention will, for the
most part, have the following formula: X-Y-Z wherein:
X is selected from the group consisting of wound healing
agents, antibiotics, anti-inflammatories, antioxidants,
SUBSTITUTE SHEET (RULE 26)
CA 02291066 1999-11-22
WO 99/48536 PCT/US99/06344
-18-
antiproliferatives, immunosuppressants, anti-infective and
chemotherapeutic agents;
In the formula, Y is a linking group of from 0-30, more usually
of from 2-12, preferably of from 4-12 atoms, particularly carbon,
oxygen, phosphorous and nitrogen, more particularly carbon and
oxygen, where the oxygen is preferably present as oxy ether, where Y
may be alkylene, oxyalkylene, or polyoxyalkylene, where the
oxyalkylene group has from 2-3 carbon atoms, and the like. A linking
group of 0 atoms is preferred when it is desired to place X as close to
Z as possible;
In the formula, Z is a reactive entity, such as carboxy, carboxy
ester, where the ester group is of 1-8, more usually 1-6 carbon atoms,
particularly a physiologically acceptable leaving group which activates
the carboxy carbonyl for reaction with amino groups in an aqueous
system, e.g. N-hydroxysuccinimide (NHS), N-hydroxysulfosuccinimide,
(sulfo-NHS), maieimide-benzoyl-succinimide (MBS), gamma-maleimido-
butyryloxy succinimide ester (GMBS) and maleimidopropionic acid
(MPA), N-hydroxysuccinimide isocyanate, isothiocyanate, thiolester,
thionocarboxylic acid ester, imino ester, mixed anhydride, e.g.
carbodiimide anhydride, carbonate ester, etc. and the like.
The reactive functionalities which are available on proteins for
covalently bonding to the chemically reactive entity of the derivatized
therapeutic agent are primarily amino groups, carboxyl groups and
thiol groups. While any of these may be used as the target of the
chemically reactive entity on the therapeutic agent, for the most part,
bonds to amino groups will be employed, particularly with formation of
amide bonds. To form amide bonds, one may use as a chemically
reactive group a wide-variety of active carboxyl groups, particularly
esters, where the hydroxyl moiety is physiologically acceptable at the
levels required. While a number of different hydroxyl groups may be
SUBSTITUTE SHEET (RULE 26)
CA 02291066 1999-11-22
WO 99/48536 PCT/US99/06344
-19_
employed, the most convenient will be N-hydroxysuccinimide, (NHS)
and N-hydroxy sulfosuccinimide, (sulfo-NHS), maleimide-benzoyl-
succinimide (MBS), gamma-maleimido-butyryloxy succinimide ester
(GMBS) and maleimidopropionic acid (MPA), although other alcohols,
which are functional in an aqueous medium such as blood, may also
be employed. In some cases, special reagents find use, such as azido,
diazo, carbodiimide, anhydride, hydrazine, dialdehydes, thiol groups, or
amines to form amides, esters, imines, thioethers, disulfides,
substituted amines, or the like. Usually, the covalent bond which is
formed should be able to be maintained during the lifetime of the
blood or tissue component, unless it is intended to be a release site.
In the preferred embodiments of this invention, the functional group on
this protein will be a thiol group and the chemically reactive group will
be a maleimido-containing group such as GMBA or MPA. GMBA
stands for gamma-maleimide-butyrylamide.
The manner of producing the derivatized therapeutic agents of
the present invention will vary widely, depending upon the nature of
the various elements comprising the molecule. The synthetic
procedures will be selected so as to be simple, provide for high
yields, and allow for a highly purified product. Normally, the
chemically reactive group will be created as the last stage, for
example, with a carboxyl group, esterification to form an active ester
will be the fast step of the synthesis. Methods for the production of
derivatized therapeutic agents of the present invention are described
in examples 1-6. Each therapeutic agent selected to undergo the
derivatization with a linker and a reactive agent will be modified
according to the following criteria: if a carboxylic group, not critical
for the retention of pharmacological activity is available on the
original molecule and no other reactive functionality is present on the
molecule, then the carboxylic acid will be chosen as attachment
SUBSTITUTE SHEET (RULE 26)
CA 02291066 1999-11-22
WO 99/48536 PCTNS99/06344
-20-
point for the linker-reactive entity modification. If no carboxylic
acids are available, then any other functionalities not critical for the
retention of pharmacological activity will be selected as attachment
point for the linker-reactive entity modification. If several
functionalities are available on a therapeutic agent, a combination of
protecting groups will be used in such a way that after addition of
the linker/reactive entity and deprotection of all the protected
functional groups, retention of pharmacological activity is still
obtained. If no reactive functionalities are available on the
therapeutic agent, synthetic efforts will allow for a modification of
the original parent drug in such a way that retention of biological
activity and retention of receptor or target specificity is obtained.
The chemically reactive entity is at a site, so that when the
therapeutic agent is bonded to the fixed blood component, the
therapeutic agent retains a substantial proportion of the parent
compound's inhibitor activity.
The derivatized therapeutic agent of the present invention will
generally have substantially lower ICSO's generally in the range of
about 0.5-0.01 of the ICSO of the parent molecule. Desirably, the
ICSO should be not less than 0.05, preferably not less than about 0.1.
In view of the varying ICSO's, the amount of the derivatized
therapeutic agent administered will also vary.
The determination of the nature and length of the linker will be
performed through an empirical optimization phase and will be
measured by the retention or the loss of biological activity. For
instance, with a given inhibitor enzyme interactions, an iteration of
the modification of the nature and the length of the linker and a
measure of the biological enzymatic activity may be necessary to
determine the most favored linker length and nature. Preferably a
SUBSTITUTE SHEET (RULE 26)
CA 02291066 1999-11-22
WO 99/48536 PCT/US99/06344
-21-
short hydrophilic 4-12 atom linker easily synthesized will be favored
to start the iteration process.
In the case of radiolabeled therapeutic agents, a minimum
distance from the target has to be respected based on the nature of
the isotope and its penetration. The length and nature of the linkers
are not as important as they are for an enzyme inhibitor combination.
For instance an isotope that emits a beta rays like 32P should be
positioned within 5 mm from the target to have maximum efficiency
(99%) with limited or no effect coming from a small change on the
nature and length of the linker.
The reactive entities have to be chosen in such a way that
most of the reactive entity reacts with the reactive functionality in
the shortest amount of time for an in vivo application. Some
surgical applications require a maximum incubation time of three
minutes, due to biological constrains (interruption of arterial blood
flow for instance). Some other surgical intervention like vascular
grafting which is performed ex vivo may take several minutes or
hours if the graft tissue is kept under appropriate conditions of
conservation. The reactive entities may thus be different for the
type of application based on their rate of reaction with the reactive
functionalities. Preferably, 1-30 % of the molecule added will bond
to the target reactive functionality in vivo within a 3 minutes
incubation window. More preferably 10-90% of the reactive entity
will bond to the reactive functionality in vivo within a 3 minute
window. Most preferably, the reaction will be over within 5 minutes.
For an ex vivo loading, 10% of the molecule will add to the reactive
functionality within a 3 minute window. Preferably 10-50% of the
reactive entity will add to the reactive functionality within a 3 minute
window. Most preferably, the reaction will be over within 15
minutes.
SUBSTITUTE SHEET (RULE 26)
CA 02291066 1999-11-22
WO 99/48536 PCT/US99/06344
-22-
Modified therapeutic agents are infused either via standard
balloon catheters, using pressure or iontophoresis to produce an
efficient distribution of the infused drug substance, or introduced via
standard techniques of infusion, lavage or topical application so that
the drug is locally delivered to the site of interest. Drugs are
modified with appropriate connectors to retain activity when bound
to exposed proteins in the vessel wall. !n vivo covalent attachment is
achieved through the use of either non-specific NHS or other
crosslinking agents or through the use of site specific covalent
affinity probes to specific proteins. Once bound, the modified drugs
retain biological activity. The covalent attachment of the therapeutic
agents provides a long lasting effect superior in time to existing
methods or local drug delivery.
The technology described herein finds application to a broad
range of therapeutic uses including the following:
1.RGD peptides
A. RGD peptides for Improved Wound Healing
RGD (Arg-Gly-Asp)-containing peptides and proteins such as
Osteopontin have been recognized to accelerate wound healing in
animal models. The mechanism by which these peptides are thought
to work is through stimulation of fibroblast and epithelial cell
migration and adhesion into the wound. The RGD containing
peptides bind to a defined receptor, the alpha(v)beta3 integrin
receptor on these cells which stimulates attachment and migration.
RGD containing peptides have failed to improve wound healing, i.e.
acceleration or increased strength, in human clinical trials. These
peptides most likely failed to work because the residence time in the
dermal ulceriwound was too brief and/or because of lack of
SUBSTITUTE SHEET (RULE 26)
CA 02291066 1999-11-22
WO 99/48536 PCT/US99/06344
-23-
attachment to fixed structures in the wound substratum.
An example of an RGD containing peptide useful in the present
invention is the following:
HN~,NHZ
NH 'TFA HN NHy
~TFA
O H O CH3 H OII H OII
- O O
H~~H N H~N~H~N~H N [ C~H I' H II
H3 ~~H O H N~H N~NH2
H3 ~ ~C H
NH
HN~NH TFA D
2
~/N'O O~O NH
Ao-RfARGDFPDDRK(EGS)-NHZ O ~ ~O
An RGD containing peptide, such as those that are the subject
of the current application, could be covalently coupled to the wound
substratum using reactive chemistry and, in combination with the
appropriate spacer/linker, be available for a more prolonged but
correct presentation to wound healing elements than are
conventionally applied RGD containing peptides. Binding and
attachment to cellular wound healing elements such as fibroblasts
and epithelial cells through the alpha/v)beta3 receptor is not altered
by the chemistry employed in the current application.
The drug product described here could be applied topically to
skin wounds and incisions including burns, dermal ulcers, trophic
ulcers, diabetic ulcers, surgical incisions, skin graft donor and
acceptor sites, surgical flap repairs, excision biopsy sites, fistulae,
fissures, as well as sites of gastrointestinal ulceration in the
stomach, duodenum, colon or rectum. The agent could also be
SUBSTITUTE SHEET (RULE 26)
CA 02291066 1999-11-22
WO 99/48536 PCT/US99/06344
-24-
applied to sites of intestinal or other visceral anastamosis, or
peritoneal, pleural, or mucosal surfaces to stimulated repair and
healing. The agent would be applied to the raw surface after
adequate debridment either physically be irrigation or cleaning, or
following enzymatic preparation and cleaning with agents such as
hyaluronidase, papain, etc.
The drug could be applied to a cleaned and slightly moist
wound surface either in an aqueous solution, in DMSO, as a powder,
an aerosolized mist, ointment, lotion, emulsion, or as a film or as a
component of a bandage or wrap. Unreacted drug may be removed
after a period of time such as 5-20 mins of application. The wound
should be infection free and kept clean and most likely bandaged
with wet to dry dressings post application.
B. RGD peptides as anti-Angiogenic agents
Tumor metastasis is characterized by a series of steps
involving interaction of various host cells (endothelial cells, platelets,
lymphocytes) and an extracellular matrix such as collagens I and IV,
fibronectin, laminin and sulfated glycosaminoglycans. The
interactions between cells and components of the extracellular
matrix are regulated by cell surface receptors called integrins. Some
synthetic peptides derived from adhesion molecules that are present
in the extracellular matrix have been shown to modulate the
mechanism involved in the metastasizing function of tumor cells. For
instance several integrins recognize the amino acid sequence RGD
which mediates the adhesion of normal and tumor cells to
components of the extracellular matrix.
Angiogenesis is a multistep process involving matrix
degradation, cellular proliferation and migration and recolonization in
which capillary endothelial cells sever their normal cell cell
SUBSTITUTE SHEET (RULE 26)
CA 02291066 1999-11-22
WO 99/48536 PCT/US99I06344
-25-
attachment, migrate through the extracellular matrix and reform cell
cell attachment to create new capillary.
C: RGD peptides as anti-restenosis agents
Restenosis is the result of a complex vascular wound healing
that occurs in most of the patients who undergo angiopiasty therapy.
Recent studies suggest that a cellular proliferative process leading
to intimal hyperplasia and the remodeling of the vessel are
responsible for the phenomenon of restenosis also described as the
renarrowing of a vessel after angioplasty . Srivatsa et al. (Cardio Res
1997 36:408-428) demonstrated that RGD peptidomimetics limits
neointimal hyperplasia and lumen stenosis following deep porcine
coronary arterial injury. They also noticed that sustained
pharmacologic blockade of alpha(v)beta(3) beyond 14 days post
injury is required to achieve maximal anti-restenosis efficacy.
Spelian, et al. (Circulation 1998 97:1818-27) showed that local
delivery of cyclic RGD peptides to the adventitial surface of balloon-
injured rat carotid arteries led to a significant reduction in smooth
muscle cells migration and reduction in neointimal thickening at 14
days after balloon injury. An RGD containing peptide, such as those
that are the subject of the current application, could be covalently
coupled to the site where the angioplasty was performed and the
sustained pharmalogic blockade of the alphalv) beta(3) receptor
should be achieved beyond 14 days thus allowing maximal anti-
restenosis efficacy.
The RGD peptide for conjugation to tissues or fixed
endogenous proteins in accordance with the present invention
includes a sequence of amino acids, preferably naturally occurring
L-amino acids and giycine, having the following formula:
R,-Arg-Gly-Asp-R2
SUBSTITUTE SHEET (RULE 26)
CA 02291066 1999-11-22
WO 99/48536 PCT/US99/06344
-26
In this formula, R, and R2 represent an amino acid or a
sequence of more than one amino acid or a derivatized or chemically
modified amino acid or more than one derivatized or chemically
modified amino acids.
In a specific embodiment, R, represents XY(Z)", in which X, Y
and Z independently represent an amino acid; and n represents 0 or
1; R2 represents OH or NH2; or any amino acid; or a sequence of
more than one amino acid or a derivatized or chemically modified
amino acid. In a specific embodiment, R2 represents an amino acid
other than serine, threonine or cysteine or the amide thereof wherein
the amino acid is rendered a carboxyamide. In another specific
embodiment, RZ is more than one amino acid, the first amino acid in
the sequence, which is attached to aspartic acid, being other than
serine, threonine or cysteine, or the amide of any free carboxyl
groups wherein R2 includes a derivatized or chemically modified
amino acid.
In a preferred embodiment, RZ includes a linking group having
a chemically reactive group which covalently bonds to reactive
functionalities or proteins and R, includes a protective group to
prevent the chemically reactive group of R2 from reacting with R,. In
another embodiment, R, includes a linking group having a chemically
reactive group which covalently bonds to reactive functionalities on
proteins and R2 includes a protective group to prevent the chemically
reactive group of R, from reacting with R2.
In yet another embodiment, both R, and RZ include a linking
group having a chemically reactive entity which covalently bonds to
functionalities on fixed proteins. In this embodiment, the linking
groups may be similar or different.
In the RGD peptide of this invention, I, R, and R2 may include
any amino acid or sequence thereof. The amino acids are preferably
SUBSTITUTE SHEET (RULE 26)
CA 02291066 1999-11-22
WO 99/48536 PCT/US99/06344
-27-
naturally occurring. The most common naturally-occurring amino
acids are shown in Table I:
TABLE I
NATURAL AMINO ACIDS AND THEIR ABBREVIATIONS
3-Letter 1-Letter
Name Abbreviation Abbreviation
Alanine Ala A
Arginine Arg R
Asparagine Asn N
Aspartic acid Asp D
Cysteine Cys C
Glutamic acid Glu E
Glutamine Gin Q
Glycine Gly G
Histidine His H
Isoleucine Ile I
Leucine Leu L
Lysine Lys K
Methionine Met M
Phenylalanine Phe F
Proline Pro P
Serine Ser S
Threonine Thr T
Tryptophan Try W
Tyrosine Tyr Y
Valine Val V
However, R, and R2 in the RGD peptide of this invention are
not limited to the 20 natural-amino acids. In other embodiments, R,
and R2 can be D-amino acids, non-classical amino acids or cyclic
peptides or peptidomimetics (chemical peptide analogs). Non-
classical amino acids include but are not limited to the D-isomers of
the common amino acids, a-amino isobutyric acid, 4-aminobutyric
acid, hydroxyproline, sarcosine, citrulline, cysteic acid, t-butylglycine,
t-butylalanine, phenylglycine, cyclohexylalanine, ~i-alanine, designer
amino acids such as ~i-methyl amino acids, Ca-methyl amino acids,
Na-methyl amino acids, and amino acid analogs in general.
SUBSTITUTE SHEET (RULE 2G)
CA 02291066 1999-11-22
WO 99/48536 PCT/US99106344
_28_
Furthermore, the Arg and/or Asp in the RGD sequence can be
the D fdextrarotaryl or L (levorotary) amino acid.
When R, and/or R2 are a sequence of amino acids, there is no
necessary limitation on the number of amino acids in the
sequence(s). Accordingly, the polypeptide for conjugation to fixed
blood proteins can be any size, and encompasses what might
otherwise be called an oligopeptide, a protein, an organic molecule or
a polymer such as polyethylene glycol. Preferably, the polypeptide
will have no more than about 1,000 amino acids.
The polypeptide may be prepared by methods that are known
in the art. For example, in brief, solid phase peptide synthesis
consists of coupling the carboxyl group of the C-terminal amino acid
to a resin and successively adding N-alpha protected amino acids.
The protecting groups may be any known in the art. Before each
new amino acid is added to the growing chain, the protecting group
of the previous amino acid added to the chain is removed. The
coupling of amino acids to appropriate resins is described by Rivier et
al., U.S. Pat. No. 4,244,946. Such solid phase syntheses have been
described, for example, by Merrifield, 1964, J. Am. Chem. Soc.
85:2149; Vale et al., 1981, Science 213:1394-1397; Marki et al.,
1981, J. Am. Chem. Soc. 103:3178 and in U.S. Pat. Nos.
4,305,872 and 4,316,891.
Derivatives of RGD peptides and their analogs which can
conjugate with proteins and other fixed blood proteins are prepared
as is known in the art by the use of linking groups having chemically
reactive groups which covalently bond to reactive functionalities on
proteins.
SUBSTITUTE SHEET (RULE 26)
CA 02291066 1999-11-22
WO 99/48536 PCTNS99/06344
-29-
2. Gastrointestinal Ulcer Treatment
Integrins, growth factors and cell adhesion molecules may be
modified using the NHS/linker technology disclosed here so that they
will covalently bond to the base of an esophageal, gastric or
duodenal ulcer when applied topically to the lesion via an endoscope.
Continued high levels of topically applied growth factors such as,
but not limited to, GF,PDGF, IGF, bFGF, aFGF and KGF, and integrins
and cell adhesion molecules such as those mentioned above will
stimulate reepithelialization and ulcer healing. NSAIDS, steroids and
other antiinflammatory drugs could also be modified by our
technology to produce ulcer healing.
In addition, antibacterials or bacteriostatic agents such as
bismuth sulphate designed to kill or inactivate Heiiobacter pylorii
could be applied to the base of peptic ulcers in order to achieve
persistent and high local concentrations of these therapies in the
region of the ulcer to aid healing. H. pylorii is recognized as an
etiological factor in peptic ulcers and its continued presence in the
ulcer is recognized to interfere with healing.
3. Intraoaerative Administration to Prevent Adhesions
Intraabdominal and intrathoracic surgery is often complicated
by adhesions which develop within weeks to months
postoperatively. These adhesions may remain undetected and of no
consequence; however, often months to years postoperatively they
may cause problem with the function of an organ or viscus, such as
the intestines, or cause intractable pain requiring reoperation. Using
antiinflammatory peptides and drugs such as inhibitors of cell and
matrix adhesion molecules coupled via spacers to NHS, topically
applied during surgery to key sites that are prone to adhesions such
SUBSTITUTE SHEET (RULE 26)
CA 02291066 1999-11-22
WO 99/48536 PCT/US99/06344
-30-
as small or large intestinal anastomoses, adhesions can be
prevented.
4. Intraooerative Administration to Prevent Bleeding
Fibrin, thrombin or tissue factor peptides, and fragments of
these and other procoagulant proteins and drugs can be attached to
sites of bleeding in the body using NHS/linker technology to effect
hemostasis to prevent bleeding. These agents can be topically
administered to sites of bleeding during open or iaparascopic or other
minimally invasive surgery, including but not limited to arthroscopy,
thorascopic, or culdoscopic and endoscopic surgery. These agents
could also be used to prevent bleeding post arteriotomy, or locally at
sites following arterial puncture with catheters such as angiographic,
angioplasty or hemodialysis or hemoperfusion catheters to reduce the
risk of post puncture hemorrhage or intrauterine for post partum
hemmorhage or dysfunctional uterine bleed. These agents could also
be of great value to prevent potentially serious hemorrhage
intrathoracically post cardiac surgery, intracranialiy following
neurosurgery, or after repair of an aneurysm either intracraniaily,
intrathoracically, or intraabdominally.
5. Onthalmic Suraerv
The technology of this invention may be used to affect
retention and the local effect of antiproliferatives to prevent and/or
minimize adhesions in opthalmic surgery. In particular, it could be
used during anterior chamber surgery for glaucoma to prevent
postoperative growth of a proliferative tissue in the region of the
trabecular meshwork or anterior chamber resorptive apparatus. In
addition topical use of protease inhibitors derivatized with NHS
esters could be utilized to inhibit bacterial damage to the sclera as
SUBSTITUTE SHEET (RULE 26)
CA 02291066 1999-11-22
WO 99/48536 PCT/US99/06344
-31
well as directly injected into the retinal to inhibit angiogenesis and
macula degeneration.
6. Uroloav
In urological procedures, postoperative hemorrhage is common
and the procoagulants mentioned above when coupled, via linker
technology, to reactive NHS esters can be applied topically via a
cystoscope or in an open operative field to sites of potential
hemorrhage. The technology of the invention may be applied
postoperatively for the prevention of hemorrhage following prostate
surgery including but not limited to TURP or open prostatectomy,
polyp removal, bladder cancer removat or partial bladder resection.
The technology can be used to apply bacteriostatic or bactericidal
agents perioperatively via a cystoscope or postoperatively via a Mey
catheter and bladder irrigation to prevent or treat infection. Anti-
inflammatory agents can be similarly applied to prevent postoperative
urethral stricture formation.
7. Endoscopv
The same technology could be used in the prevention and
treatment of esophageal stricture formation following esophageal
surgery or due to benign causes such as Barrett's esophagus or
severe reflux esophagitis. Endoscopy application of
phosphodiesterase inhibitors such as methylxanthines (pentoxifyiine,
aminophyHine, theophylline and related derivatives) as well as the
local covalent linkage of selected anti-inflammatory agents may be
useful in managing patients in acute asthmatic attack or cystic
fibrosis.
SUBSTITUTE SHEET (RULE 26)
CA 02291066 1999-11-22
WO 99/48536 PCT/US99/06344
-32-
8. Biliarv Su~~ery
NHS/linker coupled anti-inflammatory agents, or
bacteriostatic/bactericidal agents such as those described above can
be applied topically to sites of anastomosis/resection during open or
laparoscopic biliary surgery to prevent adhesions, strictures and
infection.
9. Colonoscoaic Use
The technology may be utilized coionoscopically in the
management of inflammatory bowel disease (IBD) to apply and
sustain high local concentrations of select small molecule anti-
inflammatory drugs such as steroids, NSAIDS and aspirin. Selectins
and inhibitors of cell adhesion and antiproliferatives can be applied
topically to the inflamed section/sections of the colon affected by
Crohn's disease or Ulcerative Colitis in order to accelerate healing,
reduce inflammation, and prevent stricture formation.
10. Suinal Cord and Peripheral Nerves
Topical application of TRH or a neurotrophic growth factor
such as NGF, BNDF, CNTF, PTN, MK, or NTII coupled via a linker to
NHS, during open surgical repair of either a transected or injured
spinal cord, or damaged or transected peripheral nerve can be
undertaken using this technology to improve nerve regeneration.
11. Pulmonary
The technology can be used to apply anti-inflammatory and
antibacterial agents to sites of biopsy or stricture in the airway when
identified and visualized endobronchially. Similarly, hemostasis can
be achieved or aided by the topical application post-biopsy of
procoagulant factors/peptides attached via linkers to NHS esters.
SUBSTITUTE SHEET (RULE 26)
CA 02291066 1999-11-22
WO 99/48536 PCTIUS99/06344
-33-
Topical application of selected thrombin inhibitors attached via
linkers to NHS esters may aid in the inhibition of small cell lung
carcinoma and act as a an adjunctive therapy to surgical resection
procedures.
12. ~ental
Procoagulant and antibacterial factors can be applied to tooth
sockets postextraction to prevent bleeding and infection. Local
application of selected antibiotics such as tetracyclin can result in the
inhibition of proteases that are associated with gingivitis and gum
deterioration by permanently adhering the drug for prolonged periods
of time.
13. ENT Surg~erv
In a similar fashion these procoagulant and antibacterial drugs
coupled to NHS via linkers can be applied topically to head and neck
sites during surgery such as tumor resection to prevent bleeding and
infection. The procoagulant coupled NHS agents can be applied
topically to nosebleeds, especially severe posterior septal bleeds
which are prone to recurrence and are difficult to access and control.
14. Intratumor
Slow and sustained intratumor release of anticancer agents is
possible using this NHS/linker technology in order to achieve
sustained killing of tumor cells over a period of days to weeks. This
can be achieved by intratumor injection of antiproliferative drugs
coupled via Pinker technology to reactive NHS esters. The NHS
coupled drugs will react following injection with stomal and cellular
elements of the tumor and can be released slowly into the tumor
from these anchored sites by breakdown of the attached site.
SUBSTITUTE SHEET (RULE 26)
CA 02291066 1999-11-22
WO 99/48536 PCT/US99/06344
-34-
15. Catheter-bas~d Radiotheranv
As a replacement for radioactive implants or as a substitute
for radioactive injection of liquid for multiple applications. 'sZlr and
32P are known to reduce coronary restenosis in patients with
previous restenosis. The intracoronary radiotherapy has been shown
to reduce the intimal hyperpfasia that is part of restenosis. '3'Cs, BsSr
and s°Sr, 32P, Iodine '251 and '3' I, 's2lr, s°Y, soY~Sr,
bismuth, radium
are used internal therapy of various forms of cancers including those
in the mouth, lip, breast, anus, vagina, thyroid, bone marrow, lungs
and prostate. In most cancer cases, the radiotherapy has been
shown to shrink the tumor or reduce the risk of spreading before and
during surgery. Internal radiotherapy is also given to kill off any tiny
amounts of the tumor that may have been left after surgery.
All these isotopes could be covalently attached to the tumor or
in the vicinity of the tumor itself for either palliative, curative and
radical treatments or as an adjuvant to other therapies.
For instance 3zP can be covalently attached to tissue or
membrane proteins through the use of an NHS phosphodiester or
triester.
Other radioisotopes can be used with our technology either as
complexes of radioactive metal such as 5'Cr, 52Mn , 52Mg, 5' Ni,
ssCo and 56P, 55Fe , or as chelates of these metals. Preferred
radioactive isotopes are beta ray and gamma ray emitters.
16. Imrrmno-suppressant activity
A variety of immuno-suppressant agents such as cyclosporin
and derivatives, corticosteroids, sulfasalazine, thalidomide,
methotrexate, OKT3, peptide-T, or agents that inhibit T-cell
activation or adhesion would be useful to locally apply to organs
SUBSTITUTE SHEET (RULE 26)
CA 02291066 1999-11-22
WO 99/48536 PCT/US99/06344
-35
prior to transplantation to mask immune responsiveness and organ
rejection. Such agents could be applied locally at the time of tissue
harvest (e.g. heart, lung, liver harvest) or immediately prior to
restitution of blood flow in the recipient. Such immuno-suppressant
agents would prevent the recognition of foreign antigen from the
donor tissue that would facilitate short term acceptance and
facilitate longer term ability for the host to accommodate the
transplanted organ.
17. lntra-cardiac delivery
Slow and sustained presence of selected drugs into the heart
is possible using this NHS/linker technology in order to achieve
sustained presence of selected drugs over a period of days to weeks.
Such agents could be delivered to the heart by pericardial delivery
catheter, percutaneous delivery catheter or by direct injection into
the epicardium, myocardium or endocardium during open chest or
open heart surgical procedures. Such drugs delivered in this matter
coutd include NHS derivatives of anti-arrhythmic agents such as
disopyramide, quinidine, amiodarone to control cardiac rate and
rhythm disturbances; antibiotics or anti inflammatory agents such as
corticosteroids into the pericardial space to control pericardititis;
growth factors such as VEGF or FGF to induced topical
revascularization, or inotropic agents such as methylxanthines,
digitalis or phosphodiesterase inhibitors to improve contractility and
ejection fraction in the failing heart.
18. Intra-medullarv
Slow and sustained presence of selected drugs into the bone
marrow is possible using this technology in order to achieve
sustained presence of selected drugs over a period of days to weeks.
SUBSTITUTE SHEET (RULE 26)
CA 02291066 1999-11-22
WO 99/48536 PCT/US99/06344
-36
Such agents could be delivered into the vascular space of long
bones to provide chemotherapeutics to treat leukemia or growth
factors to facilitate production of progenitor blood cells. Such
growth factors could include granulocyte colony stimulating factor
(G-CSF), granulocyte/monocyte colony stimulating factor (GM-CSF),
erythropoetin, thrombopoetin, interleukin-3.
19. Intra-vascular delivery
Slow and sustained presence of selected drugs into
cardiovascular structures (arteries/veins) via (avage or catheter
delivery is possible using this NHS/linker technology in order to
achieve sustained presence of selected drugs over a period of days
to weeks. Agents used for such purposes could include
antithrombotics, antiproliferative agents (methotrexate, colchicine,
angiopeptin, or heparin derivatives etc) to limit restenosis, protease
inhibitors to limit vascular breakdown associated with aneurism and
restenosis, radioactive therapy or DNA delivery to limit proliferation,
angiostatic agents such as NHS derivatives of endostatin or
angiostatin to limit vascular development or oligonucleotides, cDNA
or naked DNA, and growth factors VEGF, FGF or to encourage
vascular regeneration. Agents such as nitroso donors such as
nitroprusside or other nitric oxide donors to restore normal vascular
function in injured tissue would prove to be beneficial. Other agents
such as oligosaccharides, cyclodextrans or mannose derivatives
could be attached covalently to vasculature lumenal interface via
NHS derivatization for the purpose of inhibiting leukocyte cell
adhesion and rolling to limit vascular damage and inflammation in
response to injury such as reperfusion, angioplasty or stent
placement. Such agents would benefit from the derivatization with
the reactive functionalities in order to facilitate placement and
SUBSTITUTE SHEET (RULE 26)
CA 02291066 1999-11-22
WO 99/48536 PCT/US99/06344
-37-
covalent adherence and enhance cellular loading and uptake to
improve tissue retention and drug delivery.
Packaging
The therapeutic compound of the invention will be packaged in
vials as a sterile liquid (preferably for room temperature storage), or as
a lyophilisate in a vial for reconstitution. The solution (or diluent in the
case of the lyophilisate) may contain a low concentration of some type
of organic solvent in order to ensure solubility and stability. The liquid
or post-reconstitution solution will contain as high a concentration as
possible of the therapeutic compound. Each vial of therapeutic
compound will contain an overage of therapeutic material required to
treat each of the indications for the compound or to diagnose
indications for the disease or condition.
Delivery Options
There are several delivery options for localized delivery of
therapeutic agents.
a) Ooen suraical field lavaqe: There are a number of
indications for local therapeutic compounds which would entail
administration of the therapeutic compound as an adjunct to open
surgery. In these cases, the therapeutic compound would either be
lavaged in the surgical site (or a portion of that site) prior to closure, or
the therapeutic compound would be incubated for a short time in a
confined space (e.g., the interior of a section of an artery following an
endarterectomy procedure or a portion of GI tract during resection)
and the excess fluid subsequently evacuated.
b) Incubation of tissue , rafts: Tissue grafts such as
autologous and xenobiotic vein/artery and valve grafts as well as
organ grafts can be pretreated with therapeutic compounds that have
SUBSTITUTE SHEET (RULE 26)
CA 02291066 1999-11-22
WO 99/48536 PCT/US99/06344
-38-
been modified to permit covalent bond formation by either incubating
them in a therapeutic solution and/or perfusing them with such a
solution.
c) Catheter delivery: A catheter is used to deliver the
therapeutic compound either as part of an endoscopic procedure into
the interior of an organ (e.g., bladder, GI tract, vagina/uterus) or
adjunctive to a cardiovascular catheter procedure such as a balloon
angioplasty. Standard catheters as well as newer drug delivery and
iontophoretic catheters can be utilized.
d) Direct infection: For certain poorly vascularized spaces
such as intra-articular joint spaces, a direct injection of a therapeutic
compound may be able to bioconjugate to surface tissues and achieve
a desirable duration of drug effect. Other applications could include
intra medullary, intratumor, intravaginal, intrauterine, intra intestinal,
intra eustachian tube, intrathecal, subcutaneous, intrarticular,
intraperitoneal or intraocular injections as weei as via bronchoscope,
via nasogastiric tube and via nophrostomy.
Patient Dosin4
The compounds of this invention may be administered to a
mammal, preferably a human. Because the delivery of a local
therapeutic compound is targeted to achieve a local instead of a
systemic effect, dosing as a function of patient weight or body
surface area is not appropriate. For some therapeutic compounds,
there is no significant risk of a local area safety or toxicity effect of
the compound as a result of an overdosage. In such cases the
therapeutic compound concentration formulation is optimized to
deliver the highest possible density of conjugative bonding to the cells
and proteins lining the targeted local area in order to retain therapeutic
levels of localized drug activity at the site for as long a period of time
SUBSTITUTE SHEET (RULE 26)
CA 02291066 1999-11-22
WO 99/48536 PCT/US99/06344
-39-
as possible. However, some therapeutic compounds may have an
optimal dose level (usually calculated based on conjugation density per
surface area). In such cases, the density of conjugation per surface
area is controlled as a function of a) surface site preparation, b)
reaction time and conditions (e.g., temperature), c) therapeutic
compound concentration of solution, and d) solution pH and buffers.
Administration Issues
The key factors in localized delivery of therapeutic agents are as
follows: a) maintain the consistency of conjugation bonding density to
the target tissues; b) minimize any local tissue irritation or other
adverse effects of the therapeutic agent administration and
c) minimize the amount of local therapeutic compound which goes
systemic. To achieve these goals, it is important to prepare the site to
eliminate as much of the surface "debris" (e.g., blood cells, loose
proteins, etc.) as possible. This ensures that as much of the
bioconjugated compound remains as close to the local site as possible,
while ensuring uniformity of conjugation bonding density. Optimizing
the compound solution, pH and buffers results in maximizing the level
of conjugation bonding to the site surface. Removal of any residual
liquid solution from an open operating field minimizes the amount of
the therapeutic compound that may go systemic.
The invention can be more clearly illustrated by the following
non-limiting examples.
EXAMPLES
Example 1
Synthesis of a NHS derivative from a carboxylic acid in absence of
other sensitive functionalities in the molecule
To a solution of compound drug to be modified ( 1 mmol)
SUBSTITUTE SHEET (RULE 26)
CA 02291066 1999-11-22
WO 99/48536 PCTNS99/06344
-40-
containing a carboxylic acid in absence of other sensitive
functionalities in the molecule and N-hydroxysuccinimide ( 1.1 mmol)
in anhydrous CH2 C12 (x mL) is added EDC ( 2.2 mmol). The solution
is stirred at room temperature for 20 hours or until complete. The
reaction is then washed with water, saturated sodium chloride, dried
with anhydrous sodium sulfate, filtered and concentrated in vacuo.
The residue is dissolved in minimum amount of solvent and purified
by chromatography or recrystailized from the appropriate solvent
system to give the NHS derivative.
Example 2
Synthesis of an NHS derivative from a molecule containing an amino
and/or a thiol functionality and a carboxylic acid
When a free amino or thiol group is present in the molecule, it
is preferable to protect these functional groups prior to perform the
addition of the NHS derivative. For instance, if the molecule
contains a free amino group, a transformation of the amine into a
Fmoc or preferably into a tBoc protected amine is necessary prior to
perform the chemistry described in example 1. The amine
functionality will not be deprotected after preparation of the NHS
derivative. Therefore this method applies only to a compound whose
amine group is not required to be freed to induce a pharmacological
desired effect. If the amino group needs to be freed to retain the
original biological properties of the molecule, then another type of
chemistry described in example 3-6 has to be performed.
SUBSTITUTE SHEET (RULE 26)
*rB
CA 02291066 1999-11-22
WO 99/48536 PCT/US99/06344
-41-
Example 3
Synthesis of an NHS derivative from a molecule containing an amino
or a thiol functionality and no carboxylic acid.
When the selected molecule contains no carboxylic acid, an
array of bifunctional linker can be used to convert the molecule into a
reactive NHS derivative. For instance, ethylene glycol-
bis(succinimydylsuccinate) (EGS) and triethylamine dissolved in DMF
and added to the free amino containing molecule (with a ratio of
10:1 in favor of EGS) will produce the mono NHS derivative.
To produce an NHS derivative from a thiol derivatized molecule, one
can use N-[y-maleimidobutyryloxy)succinimide ester (GMBS) and
triethylamine in DMF. The maleimido group will react with the free
thiol and the NHS derivative will be purified from the reaction
mixture by chromatography on silica or by HPLC.
Example 4
Synthesis of a NHS derivative from a molecule containing multiple
chemical functionalities
Each case will have to be analyzed and solved in a different
manner. However, thanks to the large array of protecting groups and
bifunctional linkers that are commercially available, this invention is
applicable to any molecule with preferably one chemical step only to
derivatize the molecule (as described in example 1 or 3) or two steps
(as described in example 2 and involving prior protection of a
sensitive group) or three steps (protection, activation and
deprotection). Under exceptional circumstances only, would we
require to use multiple steps (beyond three steps) synthesis to
transform a molecule into an active NHS or maleimide derivative.
SUBSTITUTE SHEET (RULE 26)
CA 02291066 1999-11-22
WO 99/48536 PCT/US99/06344
-42-
Example 5
Synthesis of a maleimide derivative from a molecule containing a free
amino group andlor a free carboxylic acid
To produce an maleimide derivative from a amino derivatized
molecule, one can use N-[y-maleimidobutyryloxy]succinimide ester
(GMBS) and triethylamine in DMF. The succinimide ester group will
react with the free amino and the maleimide derivative will be
purified from the reaction mixture by crystallization or by
chromatography on silica or by HPLC.
Example 6
Synthesis of a maleimide derivative from a molecule containing
multiple other functionafities and no free carboxylic acid
When the selected molecule contains no carboxylic acid, an
array of bifunctional crosslinking reagentsl can be used to convert
the molecule into a reactive NHS derivative. For instance
maleimidopropionic acid (MPA) can be coupled to the free amine to
produce a maleimide derivative through reaction of the free amine
with the carboxylic group of MPA using HBTU/HOBt/DIEA activation
in DMF.
Many other commercially available heterobifunctional
crosslinking reagents can alternatively be used when needed.
Example 7
Preparation of rhodamine NHS ester
Rhodamine GreenT""-X, succinimidyl ester, hydrochloride mixed
isomers is commercially available from Molecular Probes (Eugene
Oregon) as illustrated below:
SUBSTITUTE SKEET (RULE 26)
CA 02291066 1999-11-22
WO 99/48536 PCT/US99/06344
-43-
Example 8
In vivo addition of NHS-rhodamine
New Zealand rabbits (2 Kg), male or female, were
intramuscularly anesthetized with Xylazine (20 mg/kg), Ketamine (50
mg/kg) and Acepromazine (0.75 mg/kg) prior to surgical exposure of
left carotid artery. Both carotid arteries were isolated and blood
flows were measured. A catheter (22G) was inserted in the arterial
segment and rinsed with 0.9% sodium chloride via catheter until
there was no more visible evidence of blood in the segment.
A 1-cm incubation chamber was created by ligatures in the
segment area. The incubation chamber was flushed three times with
1 mL of 0.9% sodium chloride. A solution of 100p1 of 500pM NHS-
Rhodamine was prepared and incubated in the incubation chamber
for 3 minutes. The excess of rhodamine was withdrawn with a 1
mL syringue. The incubation chamber was washed once again with
3 times 100 mL of 0.9% sodium chloride. The incubation chamber
was then removed from the rabbit, cut in three pieces and dipped in
10% formalin for further evaluation. The NHS-Rhodamine treated
arteries exhibited dramatic levels of fluorescence whereas those
arteries treated solely with Rhodamine exhibited little fluorescence
over background. These results demonstrate that Rhodamine was
SUBSTITUTE SHEET (RULE 26)
CA 02291066 1999-11-22
WO 99/48536 PCT/US99/06344
-44-
covalently bonded to a local delivery site.
Example 9
Preparation of [3H )-NHS-propionate
[3H )-NHS-propionate is available from Amersham Canada Ltd.
(Oakville, Ontario, Canada) and can be prepared from the tritiated
propionic acid through known to the art condensation of N-
hydrosuccinimide in presence of EDC in DMF or methylene chloride.
Example 10
In vivo pharmacokinetics studies of [3H )-NHS-propionate
New Zealand rabbits (2 kg), male or female, were
intramuscularly anesthetized with Xylazine (20 mg/kg), Ketamine (50
mg/kg) et Acepromazine (0.75 mg/kg) prior to surgical exposure of
left carotid artery. Segments of 10 mm of carotids, were transiently
isolated by temporary ligatures and rinsed with C.9% sodium
chloride via a cannula until there was no more visible evidence of
blood components.
A catheter (18G) was inserted in the arterial segment and
served to introduce the angioplasty balloon (2.5 mm of diameter,
over the wire/Boston Scientific Inc.). A vascular damage
(angioplasty) was performed on the isolated segment in order to
eliminate the layer of endothelial cells. The angioplasty balloon was
serially inflated at different atmospheres (4, 6, 8 and 10) during 1
minute, with 45 seconds of delay between inflations. At 4
atmospheres a balloon traction was performed 5 times and 1000
U/kg of heparin were infused in the blood circulation.
The angioplasty balloon was then retrieved from the artery and
the catheter was reintroduced. The arterial segment was rinsed 3
times with saline, and 100 pM of [3H )-NHS-propionate was
SUBSTITUTE SHEET (RULE 26)
CA 02291066 1999-11-22
WO 99/48536 PCT/US99/06344
-45
incubated within the isolated segment of the artery for either 30
seconds, 3 minutes or 30 minutes. At the end, the excess of
incubation liquid was withdrawn from the artery, and the segment
was rinsed 5 times with saline. The treated artery was immediately
harvested, and incorporation of [3H] -labeled compounds within the
artery was evaluated by scintillation counting (see figure 1 ?. After
30 seconds of incubation, we recorded an association efficiency of
2.55%. At 3 min and 30 min, we recorded an association efficiency
of 5.5 and 6.5%, respectively. We decided that a 3 min incubation
time was sufficient to treat the artery in an efficient way.
When evaluating the retention levels, 100 ~,M of [3H ]-NHS-
propionate or [3H ]-propionate were incubated with the artery for a
period of 3 minutes, after which the segment has been rinsed 5
times with saline. The catheter was then removed and the
arteriotomy site was closed with microsutures, thus reestablishing
the blood flow within the carotid. Finally, the neck wound was
closed with sutures, and animals are allowed to recuperate. Three
days following the treatment, the animals are sacrificed with an
overdose of sodium pentobarbital, the carotid segments are removed
and examined for compound's presence by scintillation counting. As
shown on figure 2, 10.94% retention of [3H ]-NHS-propionate was
monitored after three days following a 3 minute incubation period
based on residual radioactivity in the artery. Figure 3 shows the
difference in retention efficiency between covalently and non
covalently bound propionate after a 3 minutes incubation period. An
outstanding 12 fold enhancement in retention was recorded (0.6%
of total amount incubated against 0.046% for the non covalently
bound) in favor of the NHS-propionate. This indicates that the tissue
association of a compound is dramatically enhanced by the covalent
attachment in vivo. Subsequent restitution of blood flow
SUBSTITUTE SHEET (RULE 26)
CA 02291066 1999-11-22
WO 99/48536 PC'T/US99/06344
-4fi-
demonstrated retention (3H )-NHS-propionate of approximately 10%
of the material 72 hours after injury (figure 2) . This represents
excessive tissue retention using the embodied technology of agents
markedly beyond that seen with all local drug delivery technologies
as exemplified in the literature for standard non covalent agents
(Circulation 1994 89 (4) 1518-1524).
Example11
Synthesis of (32P) NHS derivative
To a solution of protected R and R' (both R and R' can be
O
32 ~.~~
~~~/IOR
X/ OR'
alkyl, phenyl or aikoxy groups, and X is either 0 or S, alkoxy, alkyl
and any other functionality stable under these conditions)
phosphodiester (0.1 mmol) and N-hydroxysuccinimide (0.2 mmol) is
added diisopropylethylamine 0.11 mmol), followed by addition of
HBTU (0.22 mmol). The reaction mixture is stirred at room
temperature for 36 hours. DMF is removed by vacuum distillation
and the residue is dissolved in MeOH (10 mL). The MeOH solution is
filtered to remove the insolubles, the filtrate is concentrated in
vacuo, and the residue is dissolved in a minimum amount of MeOH.
Water is then added to induce precipitation and the precipitate is
dried on vacuum to give the desired compound
The yield of the reaction can usually be improved by using
EDC as the coupling reagent, as exemplified below. To a solution of
R and R' phosphodiester (0.054 mmol) and N-hydroxysuccinimide
SUBSTITUTE SHEET (RULE 26)
CA 02291066 1999-11-22
WO 99/48536 PCT/US99/06344
-47-
(0.115 mmol) in anhydrous DMF (3 mL), is added EDC (31 mg,
0.162 mmoll. The solution is stirred at room temperature for 24
hours. DMF is removed by vacuum distillation and the residue is
further dried on high vacuum. The residue is then dissolved in a
minimum amount of MeOH (0.12 mL) and H20 (3.2 mL) is added to
induce precipitation. The precipitates are washed with H20 (3 x 0.8
mU and dried on vacuum to give a solid product.
Any protected phosphonate derivatives may undergo similar
transformation.
Example 12
New Zealand rabbits (2 kg), male or female, were anesthetized
with xylazine (20 mg/kg), ketamine (50 mg/kg) and acepromazine
(0.75 mg/kg) intramuscularly prior to surgical exposure of left carotid
artery. Carotid arteries were surgically dissected and segments of
approximately 10 mm length were isolated. The vessels were
cannulated and rinsed with 0.9% sodium chloride until there was no
more visible evidence of blood components.
A catheter ( 18G) was inserted in the arterial segment and
served to introduce the angioplasty balloon (2.5 mm of diameter,
over the wire/Boston Scientific Inc.). Vascular damage (angiopiasty)
was performed on the isolated segment in order to eliminate the
layer of endothelial cells. The angioplasty balloon was serially inflated
at different atmospheres (4, 6, 8 and 10) for 1 minute, with 45
seconds of delay between inflations. At 4 atmospheres a balloon
traction was performed 5 times and 1000 U/kg of heparin were
infused in the blood circulation.
The angioplasty balloon was then retrieved from the artery and
the catheter was reintroduced. The arterial segment was rinsed 3
times with saline, and 100 NM of (32P)- NHS-(linker] was incubated
SUBSTITUTE SHEET (RULE 26)
CA 02291066 1999-11-22
WO 99/48536 PCT/US99/06344
-48-
within the isolated segment of the artery for 3 minutes. At the end,
the excess of incubation liquid was withdrawn from the artery, and
the segment was rinsed 5 times with saline. The vessel was sutured
closed, blood flow restored and surgical wounds repaired. Animals
were returned to the vivarium for periods up to four weeks. Tissue
retention of [32P]-NHS-(Sinker] was evaluating using whole animal
radiography at selected periods of time after injury. Tissue response
to this therapy can be evaluated using standard histomophometric
analysis quantifying if the extent of tissue proliferation and neoitimal
formation in the treated versus control animals to determine if this
form of brachotherapy can limit the response to vascular injury and
hyperproliferative overgrowth classical observed under these
conditions.
Example 13
Synthesis of ["'I]- NHS derivative
To a solution of protected amino protected ['3' I]-iodotyrosine
10.1 mmol) and N-hydroxysuccinimide (0.2 mmol) is added
diisopropylethylamine (0.11 mmol), followed by addition of HBTU
(0.22 mmol). The reaction mixture is stirred at room temperature for
12 hours. DMF is removed by vacuum distillation and the residue is
dissolved in MeOH (10 mL). The MeOH solution is filtered to remove
the insolubles, the filtrate is concentrated in vacuo, and the residue
is dissolved in a minimum amount of MeOH. Water is then added to
induce precipitation and the precipitate is dried on vacuum to give
the desired compound
The yield of the reaction can usually be improved by using EDC as
the coupling reagent, as exemplified below. To a solution of ['3' I]-
iodotyrosine (0.054 mmol) and N-hydroxysuccinimide (0.1 15 mmoi)
in anhydrous DMF (3 mL), is added EDC (31 mg, 0.162 mmol). The
SUBSTITUTE SHEET (RULE 26)
CA 02291066 1999-11-22
WO 99/48536 PCT/US99/06344
-49
solution is stirred at room temperature for 24 hours. DMF is
removed by vacuum distillation and the residue is further dried on
high vacuum. The residue is then dissolved in a minimum amount of
MeOH (0.12 mL) and water (3.2 mL) is added to induce
precipitation. The precipitates are washed with H20 (3 x 0.8 mL)
and dried on vacuum to give a solid product.
,"
Example 14
In vivo pharmacology of ' 3' I derivative
New Zealand rabbits (2 Kg), male or female, were anesthetized
with xylazine (20 mg/kg), ketamine (50 mg/kg) and acepromazine
(0.75 mg/kg) intramuscularly prior to surgical exposure of left carotid
artery. Carotid arteries were surgically dissected and segments of
approximately 10 mm length were isolated. The vessels were
cannulated and rinsed with 0.9% sodium chloride until there was no
more visible evidence of blood components.
A catheter ( 18G) was inserted in the arteriat segment and
served to introduce the angioplasty balloon (2.5 mm of diameter,
over the wire/Boston Scientific Inc.). Vascular damage (angioptasty)
was performed on the isolated segment in order to eliminate the
SUBSTITUTE SHEET (RULE 26)
CA 02291066 1999-11-22
WO 99/48536 PCTlUS99/06344
-50-
layer of endothelial cells. The angioplasty balloon was serially inflated
at different atmospheres (4, 6, 8 and 10) for 1 minute, with 45
seconds of delay between inflations. At 4 atmospheres ~a balloon
traction was performed 5 times and 1000 U/kg of heparin were
infused in the blood circulation.
The angioplasty balloon was then retrieved from the artery and
the catheter was reintroduced. The arterial segment was rinsed 3
times with saline, and 100 NM of ['3'I]-NHS-[linker] was incubated
within the isolated segment of the artery for 3 minutes. At the end,
the excess of incubation liquid was withdrawn from the artery, and
the segment was rinsed 5 times with saline. The vessel was sutured
closed, blood flow restored and surgical wounds repaired. Animals
were returned to the vivarium for periods up to four weeks. Tissue
retention of ('3'I]-NHS-(linker] was evaluated using whole animal
radiography at selected periods of time after injury. Tissue response
to this therapy can be evaluated using standard histomophometric
analysis quantifying if the extent of tissue proliferation and neoitimal
formation in the treated versus control animals to determine if this
form of brachotherapy can limit the response to vascular injury and
hyperproliferative overgrowth classical observed under these
conditions.
Example 15
General
Products from the following examples were purified by
preparative reversed phase HPLC using a Varian (Rainin) preparative
binary HPLC system: gradient elution of 5-60% B (0.045% TFA in
Hz0 (A) and 0.045% TFA in CH3CN (B)) at 9.5 mL/min using a
Dynamax C,B, 60~, 8,um, 21 mm x 25 cm column equipped with a
Dynamax C,B, 601, 8,um guard module and a UV detector (Varian
SUBSTITUTE SHEET (RULE 26)
CA 02291066 1999-11-22
WO 99!48536 PCT/US99/06344
-51-
Dynamax UVD II) detecting at x,214 and 254 nm. Analytical HPLC
were performed using a Varian (Rainin) binary HPLC system: gradient
elution of 5-60% B (0.045% TFA in H20 (A) and 0.045% TFA in
CH3CN (B)) at 0.5 mL/min using a Dynamax C,B, 601, 8 gum, 4.6 mm
x 25 cm column equipped with a Dynamax C,B, 60~, 8 ,um guard
module and an UV detector (Varian Dynamax UVD II) detecting at
x,214 and 254 nm. Mass spectrometry was performed on a PE Sciex
API III electro-spray Biomolecular Mass Analyzer.
Example 16
Synthesis of Ac-RIARGDFPDDRK-NH2 ~ 4 TFA
Syntheses of Ac-RIARGDFPDDRK-NH2 peptide was performed
on an ABI 433A Peptide Synthesizer using 510 mg of 0.49 mmol/g
of Fmoc protected Rink Amide MBHA resin (NovaBiocheml, 4 eq. of
Fmoc protected amino acids, 4 eq. of a 0.45 M 0-benzotriazol-1-yl-
N, N, N', N'-tetramethyl-uronium hexafluorophosphate (HBTU) and 1-
hydroxybenzotriazole (HOBt) in N,N dimethylformamide solution as
activation with 4 eq. of 2 M N,N,-diisopropylethylamine (DIEA) in 1-
methyl-2-pyrrolidinone (NMP), and piperidine deprotection of Fmoc
groups. Upon completion of the sequence, the resin was dried to
afford 990 mg of a tan resin (91 %).
The peptide was removed from the resin by shaking 609 mg
of Ac-RIARGDFPDDRK-MBHA-Resin with two-5 mL portions of a
cleavage cocktail (comprised of: 10 mL of trifluoroacetic acid (TFA);
0.75 g of phenol; 0.25 g of thioanisoie; 0.5 mL of ethanedithiol
(EDTI; and 0.5 mL of water) for 2 h each. The filtrates were each
collected and combined and combined with the filtrates from
washing the resin with 5 mL of TFA and 5 mL of CH2C12. The
filtrates were then concentrated to approximately 10 mL and the
product was precipitated out by the addition of 40 mL of dry-ice cold
SUBSTITUTE SKEET (RULE 26)
CA 02291066 1999-11-22
WO 99/48536 PCT/US99/06344
-52-
EtzO. The resulting precipitate was collected by centrifugation and
re-suspended in 40 mL of dry-ice cold Et20, centrifuged and process
repeated to afford 163 mg of the crude peptide as a white solid
(0.084 mmol, 60%). Analytical HPLC showed purity to be
approximately 72%.
Example 17
Synthesis of Ac-RIARGDFPDDRK(EGS)-NH2 ~ 3 TFA
fn a 15-mL centrifuge tube, 46.2 mg of ethylene giycol-
bis(succinimidylsuccinate) (EGS) (0.071 mmol) and 8.50 ,uL of
triethylamine (0.061 mmol) was dissolved in 500 ,uL of DMF. To
this vortexing solution was added dropwise over 30 sec a solution of
12.2 mg of Ac-RIARGDFPDDRK-NHZ ~ 4 TFA (0.006 mmol)
(Example 2) in 100 NL of DMF and following addition the reaction
was allowed to stand at RT for 1.5 h. To this was added 9.88 ,uL of
TFA (0.122 mmol), vortexed and product precipitated out by the
addition of 1.5-mL of dry-ice cold Et20. The precipitate was collected
by centrifugation and solid taken up in 1 mL of 0.045% TFA in
CH3CN and 1 mL 0.045% TFA in water and deposited on prep HPLC
and desired fractions coI(ected and lyophilized to afford 6.80 mg of
product as a white solid (0.003 mmol, 50%). Analytical HPLC
indicated product to be > 70% pure with Rt = 38.17 min (product)
and 37.21 min (hydrolysis product) ESI-MS m/z for C"H"9Nz4028
(MH+), calcd. 1827.9, found MH2+ 914.8. Hydrolysis product ESI-
MS m/z for C,3H"sN23O28 (MH+), calcd. 1731.9, found MHZ+ 866.2.
Example 18
Cell culture
Human umbilical vein endothelial cells (HUVEC) from ATCC
are grown to confluence in Medium 199 containing 2.2 mg/mL of
SUBSTTTUTE SHEET (RULE 26)
CA 02291066 1999-11-22
WO 99/48536 PCT/US99/06344
-53-
sodium bicarbonate supplemented with 20% heat-inactivated FBS,
ECGS 150~g/mL, penicillin 100 Ui/mL, streptomycin 100 p.g/mL.
Cells are grown in 75 cm2 flask at 37° C under 5% C02 and the
medium is replaced on the first day of seeding and every two days
there after.
The cells are used between second and fourth passages. Selected
RGD containing agents are evaluated for their ability to inhibit the
growth and proliferation of these cells under normal conditions as
well as in confluent cultures that are injured mechanically by
scraping a lesion into the confluence and measuring the rate of
wound healing or trough repopulation.
Example 19
In vitro cell adhesion assay
Standard 48-well cell culture plates (Costar) are coated with
100 ~,L fibronectin (5 and 10 ~g/mL), vitronectin (5-10 p.g/mL) or
HSA 1 in PBS overnight and air dried. Endothelial and somatic cells
are harvested by treating with trypsin (0.25%, w/v)/EDTA (1mM) (5
mL/25cmz of surface areal, washed twice in PBS and suspended at
5x105 ceIIs/mL in PBS containing 10 pg/mL of fluorescein
isothiocyanate (FITC) for 30 minutes at 37° C.
Labeled cells are washed and re suspended at 5x105 ceIIs/mL
in incubation medium M 199, then incubated with different
concentrations of the HSA-RGD peptide for 30 minutes at 37° C.
Control and pretreated cells are applied into ECM-coated plates
at a density of 5x104 cells/well and incubate for 90 minutes at 37° C
allowing adhesion to occur. After washing twice with PBS, non
adherent cells are removed by aspiration, and plates are subjected to
quantification of fluorescence density using the SpectroMax.
Following detachment by trypsin, the adherent cells are also counted
SUBSTITUTE SHEET (RULE 26)
CA 02291066 1999-11-22
WO 99/48536 PCT/US99/06344
-54-
by hemacytometer and the percentage of cells adhering to ECM is
calculated. The direct effect of HSA-RGD on cell adhesion in this
assay is a toot to define effects not only in wound healing but in
inhibiting platelet and leukocyte adhesion and diapedesis following
adhesion.
Example 20
In vitro cell migration assay
Cells are harvested by treating with trypsin/EDTA (5mL/25
cm2 of surface areas, washed twice in PBS and suspended at 5x 105
cells/mL in PBS containing different concentrations of the HSA-RGD
peptide. The isolated cells are Incubate for 30 minutes at 37° C and
then added to the upper chamber of the Transwell (Costar 8.0 uml
chamber that had been precoated with a type I collagen as a
chemotractant.After 6-18 hours of incubation at 37° C, 5% C02 the
membrane insert was recovered and cells remove with Q-tip cotton
swab and placed into 1 % crystal violet in 20 % methanol 80%
water. The cells were stained for 20 minutes and extracted with
10% acetic acid and absorbance measured at 600 nm. The number
of migrated cells are calculated from a standard curve.
Example 21
Matrigel-induced capillary formation
Endothelial cells spontaneously form capillary tube when
seeded on natural ECM. Basement membrane matrix (Sigma) is
diluted at 4 mg/mL with cold PBS and added to 24-well plates
(Costarl in a total volume of 200 pL in each well. Plates are left at
37° C for 30 minutes to farm a gel layer into which HUVEC cells
2x105 in a medium with 20% FBS supplemented with
concentrations of peptides are applied to each well and incubated
SUBSTTTUTE SHEET (RULE 26)
CA 02291066 1999-11-22
WO 99/48536 PCT/US99/06344
-55-
at 37° C for 24 hours with 5% C02. After incubation, cells are
washed, fixed in 2% giutaldehyde for 10 minutes and subjected to
inverted contrast-phase microscopy. The effect of the RGD peptides
on tube forming sprouts are visually measured to determine the
effects on capillary formation.
Example 22
Chicken CAM (chorioallantoic membrane) assay
The CAM in the chick embryo is a classical in ovo assay to
quantitate drug effect on vascular angiogenesis. Ten days old
chicken embryos are incubated at 37° C with 60% humidity. A 1
cm2 window is made to access the underlying CAM. Angiogenesis
is induced by injecting 200 pL of TNF a , 5ng/mL on the CAM. After
24 hours, test RGD peptides are applied in a volume of 50 pL. The
window is covered with sterile cellophane tape and the embryos are
incubated for a further 48 hours at 37° C with 60% humidity. After
incubation, CAM tissue is resected and angiogenesis is visualized on
microscope. The inhibition of vascular formation is semi quantitively
scored to assess the effects of the RGD peptides in inhibiting
angiogenesis.
Example 23
Corneal neovascularization bioassay
Rats are anesthetized and small pocket is made on the cornea
to inserted material to induce blood vessel formation. Pellets of a
slow releasing polymer (Hydron) containing angiogenic factor (VEGF)
and different concentrations of RGD peptides are implanted into the
pocket. (See preparation mode of mixture in Hydron in D'Amato
technique. Angiogenesis 1996). After 3 to 5 days, the animals will
be killed and corneal vessel is photographed. The density of blood
SUBSTITUTE SHEET (RULE 26)
CA 02291066 1999-11-22
WO 99/48536 PCT/US99/06344
-56
vessel growth is scored and activity of test RGD peptides defined as
reflected by the extent of inhibiting blood vessel formation.
Example 24
In vivo wound healing activity
Nude mice are anesthetized with sodium pentobarbital (25
mg/kg ip) and skin lesions produced with 0.5 mm silver nitrate
cautery device. The lesion produced heals in 3-5 days. The rate of
cellular infiltrate and the limitation of scarring is assessed in animals
treated with RGD peptides to facilitate wound healing. In advanced
animal models RGD peptides for surgical wound healing will be
evaluated in models of gastrointestinal surgery. Under surgical
anesthesia rats with undergo a laparotomy and a complete
transection of the duodenum. AT the time of surgery the cut and
adjacent ends are treated with RGD peptides and resutured. The
time to healing is assessed in comparison to controls based upon the
rate of histopathologicai healing as well as restitutioning GI function
as assessed by charcoal transit times evaluated in these animals 24
- 72 hours after surgical injury. The ability of the RGD peptides to
heal the cut ends of the GI tract will be characterized over a several
week period of time to assess the enhancement of the healing
process.
Example 25
In vivo angiogenesic and anti-metastatic activity
The anti-metastatic activity of such RGD peptides is evaluated
in nude mice inoculated with different human cancer cell lines. fn
this assays the tumor are established to a defined mass greater than
2 cm using defined growth and mass curves. Animals are injected
with the NHS RGD peptides directly into the established human
SUBSTITUTE SHEET (RULE 26)
CA 02291066 1999-11-22
WO 99/48536 PCT/US99/06344
-57-
tumor and the effects of the local application of the RGD peptide on
the progression of tumor size and mass is determined in comparison
to vehicle treated animals. The anti-proliferative activity and effects
on angiogenesis is determined by direct quantification of capillary
formation, cell number, tumor density and blood flow defined into
the tumor after treatment.
Example 26
In vivo anti restenosis activity
New Zealand rabbits (2 Kg), male or female, were
intramuscularly anesthetized with Xylazine (20 mg/kg), Ketamine (50
mg/kg) et Acepromazine (0.75 mg/kg) prior to surgical exposure of
left carotid artery. Segments of 10 mm of carotids, were transiently
isolated by temporary ligatures and rinsed with C.9% sodium
chloride via a cannula until there was no more visible evidence of
blood components. The carotid artery is injured by standard balloon
angioplasty and RGD applied at the time of injury. The surgical
incisions are repaired and the animals returned to the vivarium for
periods of time up to one month after injury.
At the time of terminal sacrifice the injured vessel is isolated
and inspected with perfusion fixation and harvesting. The response
to injury is assessed histomorphologically using computer imaging of
the cross sectional areas and calculating the intimal to medial ratios
as well as evaluating using BrDU the extent of cellular proliferation in
this in vivo assay. The effects of local RGD peptides on preventing
the hyperproliferative response in this model reveals drug interactions
in stabilizing the injured vessel.
SUBSTITUTE SHEET (RULE 26)
CA 02291066 1999-11-22
WO 99/48536 PCT/US99/06344
-58-
Example 27
Ex vivo vascular grafting
New Zealand rabbits (2 kg), mate or female, were
intramuscularly anesthetized with Xylazine (20 mg/kg), Ketamine (50
mg/kg) et Acepromazine (0.75 mg/kg) prior to surgical exposure of
left jugular vein. Segments of 20 mm of carotids, were transiently
isolated by temporary ligatures and rinsed with 0.9% sodium
chloride via a cannula until there was no more visible evidence of
blood components. The vessels are removed and surgical
transplanted into the descending aorta. Twenty four hours after
surgery the animals are reanesthetized and blood flow measured
using Transonic flow probes to determine if thrombosis has occurred.
The application of NHS RGD to the vascular graft can act as a
procoagulant or an anticoagulant. The local application of the RGD
will be measured in different uses to prevent bleeding at the suture
sites as well as to define the effects on platelet deposition and
arterialization at various time points in this model.
The invention now being fully described, it will be apparent to
one of ordinary skill in the art that many changes and modifications
can be made thereto without departing from the spirit or scope of
the appended claims.
SUBSTITUTE SHEET (RULE 26)