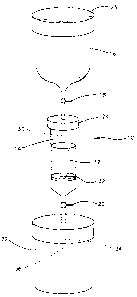Note: Descriptions are shown in the official language in which they were submitted.
CA 02291706 1999-11-22
WO 98/00174 PCT/LTS97/16514
Method of Preparing a Composite Bone Graft
Background of the Invention
Bone grafting is widely used to treat fractures, non
unions and to induce arthrodeses. Autogenous cancellous
bone, which is taken from one site in the graftee and
implanted in another site in the graftee, is currently the
most effective bone graft. Autogenous cancellous bone
provides the scaffolding to support the distribution of
the bone healing response. Autogenous cancellous bone also
provides the connective tissue progenitor cells which form
new cartilage or bone. However, the harvest of autogenous
bone results in significant cost and morbidity, including
scars, blood loss, pain, prolonged operative and
rehabilitation time and risk of infection. Furthermore, in
some clinical settings, the volume of the graft site can
exceed the volume of the available autograft . Accordingly,
alternatives to autografts have been developed in an
attempt to reduce the morbidity and cost of bone grafting
procedures.
1
CA 02291706 1999-11-22
WO 98/00174 PCTIUS97/16514
Several purified or synthetic materials, including
ceramics, polymers, processed allograft bone and collagen-
based matrices have been investigated or developed to serve
as substitutes for autografts. The FDA has approved a
porous coral derived synthetic hydroxyapatite ceramic for
use in contained bone defects. A purified collagen/ceramic
composite material is also approved for use in acute long
bone fractures. Although these materials avoid the
morbidity involved in harvesting autografts from the
graftee and eliminate problems associated with a limited
amount of available autograft, the clinical effectiveness
of the synthetic materials remains generally inferior to
autografts.
The synthetic graft materials have also been used as
carriers for bone marrow cells. When such composite
materials have been implanted into skeletal defects, the
connective tissue progenitor cells differentiated into
skeletal tissue. In some instances, the composite implants
were made by soaking the synthetic graft material in a cell
suspension obtained from a bone marrow plug. However, the
connective tissue progenitor cells, which have the capacity
to differentiate into cartilage, bone and other connective
tissue such as fat, muscle, and fibrous tissue are present
in the bone marrow in very minute amounts. The numbers of
such cells present in 1 ml of bone marrow varies widely
from subject to subject from about 100 cells to 20,000
cells. This represents a mean of about one in 20,000 to
one in 40,000 of the nucleated cells in bone marrow. Thus,
a composite implant made by soaking a given volume of
2
CA 02291706 1999-11-22
WO 98/00174 PCT/US97/16514 -
synthetic carrier graft material in a comparable volume of
fresh bone marrow contains relatively few connective tissue
progenitor cells.
Accordingly, a technique has been previously
developed to increase the relative concentration of
connective tissue progenitor cells in composite implants.
This technique involves plating a suspension of bone marrow
cells onto tissue culture dishes, culturing the cells in
a select medium for one or more days until the number of
connective tissue progenitor cells in the culture
increases, and then detaching the cells from the tissue
culture dishes to provide a cell suspension containing a
culturally-expanded population of connective tissue
progenitor cells. Composite implants are then made by
soaking synthetic ceramic carriers in this suspension of
culturally-expanded cells. Unfortunately, this method of
preparing composite implants is very time consuming.
Moreover, if the culturally-expanded cells used in this
method are derived from bone marrow aspirates obtained from
the graftee, the graftee must undergo multiple invasive
procedures, one to remove his or her bone marrow and one
at a later date to implant the composite implant. In
addition, the graftee may be exposed to anaesthesia more
than once.
Accordingly it is desirable to have a new method of
preparing a composite bone marrow graft which can be
performed intraoperatively, i.e., at the same time bone
marrow is being taken from the graftee. An intraoperative
method of preparing a composite bone marrow graft which
3
CA 02291706 1999-11-22
WO 98/00174 PCT/US97/16514
uses bone marrow aspirate as the source of the connective
tissue progenitor cells and which results in the formation
of a composite bone graft containing an enriched population
of connective tissue progenitor cells is especially
desirable.
Summary Of The Invention
The present invention provides a new and improved
method for preparing a composite bone graft. As used
hereinafter the term "bone graft" refers to a graft which
comprises connective tissue progenitor cells and is,
therefore, capable of differentiating into cartilage or
bone. The method comprises providing a bone marrow
aspirate suspension and passing the bone marrow aspirate
suspension through a porous, biocompatible, implantable
substrate to provide a composite bone graft having an
enriched population of connective tissue progenitor cells.
Because the method is preferably performed intraoperatively
using a bone marrow aspirate from the graftee, it reduces
the time and expense required for graft preparation and
also the number of times the graftee must return to the
operating room to undergo invasive procedures. The
improved composite bone graft prepared by the present
method contains an enriched population of connective tissue
progenitor cells and a greater number of connective tissue
progenitor cells per unit volume than that found in the
original bone marrow aspirate.
The present invention also relates to the composite
bone marrow graft prepared according to the present method
4
CA 02291706 1999-11-22
WO 98/00174 PCT/US97/16514
and a kit comprising the apparatus for preparing the
composite bone graft.
Brief Description of the Figures
Figure 1 is a representation, somewhat schematic, of
an apparatus used to prepare a composite bone graft in
accordance with the present invention.
Figure 2a is a graph showing the effect of increasing
the concentration of nucleated cells in the bone marrow
aspirate suspension on the number of nucleated cells
retained on a composite bone graft comprising a
hydroxyapatite substrate.
Figure 2b is a graph showing the effect of increasing
the concentration of nucleated cells in the bone marrow
aspirate suspension on the number of connective tissue
progenitor cells retained on a composite bone graft
comprising a hydroxyapatite substrate.
Figure 3a is a graph showing the effect of increasing
the concentration of nucleated cells in the bone marrow
aspirate suspension on the concentration of nucleated cells
retained on a composite bone graft comprising a
demineralized human cancellous bone matrix substrate.
Figure 3b is a graph showing the effect of increasing
the concentration of nucleated cells in the bone marrow
aspirate suspension on the number of connective progenitor
cells retained on a composite bone graft comprising a
demineralized human cancellous bone matrix substrate.
Detailed Description Of The Invention
5
CA 02291706 1999-11-22
WO 98/00174 PCT/US97/16514 -
The present invention provides a new and improved
method for preparing a composite bone graft. The method
comprises collecting a bone marrow aspirate from a donor,
preferably in the presence of an anti-coagulant to provide
a bone marrow aspirate suspension, and passing the bone
marrow aspirate suspension through a porous, biocompatible,
implantable substrate. Preferably, the method is performed
intraoperatively using a bone marrow aspirate preferably
from the graftee.
Preparinq_A Bone Marrow Aspirate Suspension
Bone marrow aspirate contains plasma, nucleated
connective tissue progenitor cells, other nucleated cells
of hematopoietic origin, nucleated endothelial cells, and
cells derived from contaminating peripheral blood. Since
bone marrow aspirate also contains peripheral blood, it is
preferred that the bone marrow be collected in a syringe
containing an anti-coagulant. Suitable anti-coagulants
include, for example, heparin, sodium citrate, EDTA and
dextran. Preferably, the bone marrow aspirate is mixed
with a sterile isotonic solution to provide a concentration
in the range of from about 10 million to about 300 million
nucleated cells/ml, preferably from about 20 million to
about 250 million nucleated cells/ml, more preferably from
about 50 million to about 200 million nucleated cells/ml.
Suitable isotonic solutions include, for example, isotonic
buffered salt solutions, such as Hank's Balanced Salt
Solution and phosphate buffered saline, and tissue culture
medium such as minimal essential medium. As used herein,
6
CA 02291706 1999-11-22
WO 98/00174 PCT/US97/16514
the term "bone marrow aspirate suspension" refers to a bone
marrow aspirate that has not been mixed with an isotonic
solution and to a bone marrow aspirate that has been mixed
with an isotonic solution.
Substrate
The substrate is made from a biocompatible,
implantable graft material. Preferably, the material has
a charged surface. Examples of biocompatible, implantable
graft materials having a charged surface include synthetic
ceramics comprising calcium phosphate, some polymers,
demineralized bone matrix, or mineralized bone matrix.
More preferably, cell adhesion molecules are bound to
the surface of the substrate. The term "cell adhesion
molecules" refers collectively to laminins, fibronectin,
vitronectin, vascular cell adhesion molecules (V-CAM) and
intercellular adhesion molecules (I-CAM) and collagen.
Preferably, the substrate has a sufficient number of
pores or passageways so that the total surface area of the
substrate is at least five times greater than a solid
object having the same external dimensions. Thus, the
preferred total surface area can be achieved by using a
substrate which comprises a mass of powder, a mass of
granules , a mass of f fibers , or a highly porous block of
substrate material. Preferably, the size of the pores in
the substrate is greater that 20 ~, more preferably greater
than 40 ~., most preferably greater than 100 ~..
Particularly suitable graft materials include, for
example, isolated mineralized cancellous bone sections,
7
CA 02291706 1999-11-22
WO 98/00174 PCT/US97/16514 -
powders or granules of mineralized bone, demineralized
cancellous bone sections, powders or granules of
demineralized bone, guanidine-HC1 extracted demineralized
bone matrix, sintered cortical or cancellous bone,
coralline hydroxyapatite sold by Interpore under the trade
name Interpore 500, and granular ceramics such as that
incorporated into the bone graft substitute Collagraft
sold by Zimmer, or filamentous sponges such as those made
from collagen by Orquest.
Substrate Container
Preferably, the substrate is disposed in a container
configured to retain the substrate in the container and to
allow fluid and bone marrow cells to flow through the
container. This is accomplished by using a container
having two openings at either end thereof and comprising a
member having one or more pores disposed between the
substrate and one of the openings. Preferably, the pores
of the member have a diameter of sufficient size to allow
fluid and cells to flow therethrough and to retain the
substrate in the container. Preferably, the length of the
container is greater than the width of the container to
increase residence time of the suspension in the substrate .
Preferably, the container is made of a material which
is biocompatible and pyrogen-free. Suitable container
materials include for example glass, plastic or metal.
Although the container may comprise two fluid flow
restrictors blocking the openings at either end of the
container, preferably, a fluid flow regulator is attached
8
CA 02291706 1999-11-22
WO 98100174 PCT/US97116514
to at least one end of the container to regulate flow of
the bone marrow aspirate suspension through the substrate.
Conditions
Preferably, the bone marrow aspirate suspension is
permitted to flow through the substrate under hydrostatic
pressure which may be generated by external forces or the
force of gravity. Preferably, the linear elution rate of
the suspension through the substrate is between 2 and 500
mm/minute, more preferably between 5 and 200 mm/minute,
most preferably between 10 and 100 mm/minute.
Optionally, the effluent is collected sterilely in
an effluent collector and recycled through the substrate
one or more times to increase the number of connective
tissue progenitor cells in the composite bone graft.
Optionally, a wash solution is passed through the
substrate after the original bone marrow aspirate
suspension and any effluents have been passed through the
substrate. Preferably, the wash solution comprises a
sterile, isotonic, buffered solution having a pH range of
7.3 to 7.5. Suitable wash solutions include, for example,
phosphate-buffered saline, Hank's balanced salt solution,
and minimal essential medium.
Optionally, growth factors or additional cells which
secrete growth factors are added to the composite bone
graft prior to use. Growth factors which may be added
include for example, fibroblast growth factor, epithelial
growth factor, transforming growth factor Beta, insulin-
like growth factor, and bone morphogenic protein.
Preferably, growth factors are added by passing a solution
9
CA 02291706 1999-11-22
WO 98/00174 PCT/US97/16514
containing the growth factors through the substrate after
all previous suspensions and solutions have been passed
through the substrate. Alternatively, grow factors are
added by incorporation into the wash solution.
The following examples of methods of preparing a
composite bone graft are intended to illustrate but not to
limit the present invention:
Example 1
The present method for preparing a composite bone
graft may be more readily understood by reference to Figure
1 which depicts a preferred embodiment of the apparatus for
performing the method. The apparatus, shown generally as
10, comprises a porous, biocompatible, implantable
substrate 12, a container 14, for holding substrate 12, a
reservoir 16 for holding the bone marrow aspirate
suspension, a first fluid flow regulator 18, a second fluid
flow regulator 20, and an effluent collector 22. Prior to
preparation of the composite bone graft, all of the
components of the apparatus are sterilized. Following
removal of top 23, the bone marrow aspirate suspension is
introduced into reservoir 16. Then fluid flow regulator 18
is opened to allow the bone marrow aspirate suspension to
flow out of reservoir 16 and into opening 30 in removable
top 24 of container 14 and onto substrate 12.
As the suspension enters substrate 12, fluid flow
regulator 20 which is attached to tip 34 of container 14 is
opened to permit the effluent of the bone marrow aspirate
suspension to flow through porous member 32, through
opening 36 of container 14 and into effluent collector 22.
CA 02291706 1999-11-22
WO 98!00174 PCT/US97/16514
Reservoir 16 and removable top 24 are then detached
from container 14 and the improved composite bone marrow
graft is then removed from container 14. The improved
composite bone graft, which comprises substrate 12, an
enriched population of connective progenitor cells and a
heterogenous population of other nucleated bone marrow
cells is ready to use as an implant or in vitro.
Example 2
Nine cylindrical disks of coralline hydroxyapatite
(HA) measuring 13 mm in diameter and 5 mm in thickness were
obtained from Interpore, Inc., Irvine, California. Each
disk was placed in the tip of a vertically mounted 10 cc
syringe barrel f fitted with a stopcock . Marrow samples were
taken from the anterior iliac crest of nine volunteer human
subjects by aspiration. Samples were collected using a
Lee-Lok bone marrow aspiration needle and a 10 cc syringe
containing 1 ml of normal saline and 1000 units of Sodium-
Heparin. Two ml of bone marrow were aspirated from each
site. Marrow samples were suspended in a-MEM to prepare a
suspension of marrow cells containing 50 million nucleated
cells per ml. 2 ml of the marrow cell suspension were
introduced in to the top of the syringe and the stopcock
was adjusted to allow the marrow cell suspension to elute
through the disk at 2 ml/minute. Each sample of effluent
was recycled through the disk three times. After the
effluent was collected, the disk was washed with 6 ml
phosphate buffered saline at an elution rate of 2 ml/min,
to remove loosely adherent cells and to produce the
composite bone graft.
11
CA 02291706 1999-11-22
WO 98/00174 PCT/US97/16514
The number of nucleated cells in the initial
suspension, the effluents, and the washes were counted
using a hemocytometer to determine the number of nucleated
cells retained in the resulting composite bone grafts. To
determine the number of connective tissue progenitors
retained in the resulting composite bone grafts, the number
of connective tissue progenitors in the initial
suspensions, the effluent, and the washes were assayed by
colony counting on tissue culture plastic. For colony
counting, 500,000 nucleated cells from the original
suspension, the effluents and the wash were plated in
separate 35 mm diameter tissue culture wells and cultured
in a-MEM containing dexamethasone (10-e M) and ascorbate (50
mg/ml) for 9 days. The cultured cells were then stained for
alkaline phosphatase activity using a N', N', dimethyl
naphthol M-X phosphate as a substrate and Texas Fast Red as
a counter-stain. Alkaline phosphatase activity is a marker
of osteoblastic differentiation. Thus, the number of
colonies which stain positively for alkaline phosphatase
activity reflect the number of connective tissue
progenitors present in the original suspension, the
effluents and the wash.
The number of nucleated cells and connective tissue
progenitor cells which were retained on the substrate
following each step were calculated by subtracting the
number of nucleated cells and connective progenitor cells
found in the effluents or wash from the number of nucleated
cells and connective tissue progenitor cells in the initial
suspension. The average number of nucleated cells and
12
CA 02291706 1999-11-22
WO 98/00174 PCT/US97/16514
connective tissue progenitor cells retained in the nine
composite bone grafts and the percentage of nucleated cells
and connective tissue progenitor cells retained in the
composite bone.grafts are shown in Table 1.
Example 2
Composite bone grafts were prepared as described in
Example 1 except that bone marrow samples were taken from
the anterior iliac crest of three different volunteer human
subjects and the substrates used were cylindrical disks of
demineralized human cancellous bone matrix obtained from
Life Net, Virginia Beach, Virginia.
The number of nucleated cells and connective tissue
progenitor cells retained in the composite grafts were
determined as described above in example 1. The average
number of nucleated cells and connective tissue progenitor
cells retained in the composite bone grafts and the
percentage of nucleated cells and connective tissue
progenitor cells retained in the composite bone grafts are
shown in Table 1.
13
CA 02291706 1999-11-22
WO 98/00174 PCT/US97/16514
TABLE 1
Retention of Cells in Composite Bone Grafts
made using disks of hydroxyapatite or
demineralized human cancellous bone
HA Gancellous
Disks Bone
Nucleated Cells in 100 x 100 x 106
Original Suspension 10~
Nucleated Cells Retained 56.45 40.00 x 106
x
before Wash 106
Nucleated Cells Removed 9.12 x 15.78 x 106
with Wash 106
Nucleated Cells Retained 47.33 24.22 x 106
x
after Wash 106
CTPC in Original 7800 11100
Suspension
CTPC Retained After Wash 5162 4950
Percent of all Nucleated 470 240
Cells Retained
Percent of all CTPC 660 440
Retained
Ratio of CTPC to Nucleated 1.4 1.8
cells
Concentration of CTPC in 2.8 1.3
Composite Bone Graft vs I
Concentration of CTPC in
Original Suspension
CTPC = Connective ~1W ssue Progenitor yells
14
CA 02291706 1999-11-22
WO 98/00174 PCT/US97/16514
As shown in Table 1, composite grafts made with a
substrate of hydroxyapatite or demineralized human
cancellous bone retained a significant percentage of the
nucleated cells (47% and 24%, respectively) and an even
greater percentage of the connective tissue progenitor
cells (66% and 440, respectively) in the original
suspension. As also shown in Table 1, washing substrates of
cancellous bone or coralline hydroxyapatite resulted in
removal of a mean of 16.2% (range 10% - 33%) of the
nucleated cells which are initially retained in a coralline
HA substrates and 39.450 (range 33 -864) of the cells
retained in a demineralized cancellous bone matrix
substrates.
As shown in table 1, the composite grafts made with
either the hydroxyapatite or the demineralized human
cancellous bones selectively retained the connective tissue
progenitor cells as compared to other marrow derived
nucleated cells. This selective retention is illustrated
by the ratio (>1) of o connective tissue progenitor cells
retained vs o nucleated cells retained on the substrate.
Thus, the composite bone grafts prepared with either the
hydroxyapatite disks or the demineralized human cancellous
bone disks comprise an enriched population of connective
progenitor cells.
Concentration of connective tissue progenitor cells
above that found in the original bone marrow sample is
illustrated by dividing the number of connective tissue
progenitor cells retained by the volume of the disks (.63
cm3). As shown in Table 1, the mean concentration of
CA 02291706 1999-11-22
WO 98/OOI74 PCT/US97/16514
connective tissue progenitor cells retained in the
composite bone grafts comprising HA disks was 2.8 times
greater than the concentration in the original marrow
sample. Similarly, the mean concentration of connective
tissue progenitor cells retained in the composite bone
grafts comprising demineralized cancellous bone matrix was
1.3 times greater than in the original marrow sample.
Example 3
Forty-five composite bone grafts were prepared as
described in Example 1 except that the concentration of
nucleated cells in the marrow suspension was varied between
5, 10, 20, 40, and 50 million cells/ml from each of the
nine human donors. The number of nucleated cells and
connective tissue progenitor cells retained on cells
retained on each of the resulting composite bone grafts
were determined as described in Example 1. The results are
shown in Figures 2a and 2b.
As shown in figures 2a and b, the number of nucleated
cells and the number of connective tissue progenitor cells
retained in the composite bone grafts increased in an
essentially linear fashion as the number of marrow cells
passed through the hydroxyapatite substrate was increased,
indicating that saturation of the hydroxyapatite substrate
with marrow derived cells did not occur over the range of
cells to substrate volume evaluated.
Example 4
Fifteen composite bone grafts were prepared using
disks of demineralized cancellous bone matrix as described
in Example 2 except that the concentration of nucleated
16
CA 02291706 1999-11-22
WO 98/00174 PCT/US97/16514
cells in the marrow suspension was varied between 5, 10,
20, 40, and 50 million cells/ml from each of the three
human donors. Data reflecting the number of nucleated
cells and the number of connective tissue progenitor
colonies retained in the resulting composite bone grafts is
presented in Figure 3a and 3b.
As shown in figures 3a and 3b, the number of
nucleated cells and the number of connective tissue
progenitor cells retained in the composite bone grafts
increased in an essentially linear fashion as the number of
marrow cells passed through the demineralized cancellous
bone matrix substrate increased, indicating that saturation
of the substrate with marrow derived cells did not occur
over the range cells to substrate volume evaluated.
Example 5
A composite bone graft was prepared as described in
Example 1 using a 2 cc marrow suspension containing 5
million nucleated cells/ml except that the substrate was
not washed with 6 ml of phosphate buffered saline after
loading. Compared to an identical disk loaded in an
identical manner which was washed as in example 1, the
unwashed disk retained the same number of connective tissue
progenitors (1000 in the case shown) and a greater number
of marrow derived nucleated cells (2.2 million vs 1.2
million in the washed example). After culture for 24 days
in vitro, the presence of these additional cells resulted
in greater proliferation and differentiation of the
connective tissue progenitors. This was manifest by a
greater surface area covered by cells that expressed
17
CA 02291706 1999-11-22
WO 98/00174 PCT/US97/16514
alkaline phosphatase activity, which is a marker of
osteoblastic differentiation.
Example 6
A composite bone graft was prepared as in example 1
except that the bone marrow suspension was recycled over
the hydroxyapatite disk only once, rather than three times .
This reduced the number of cells and connective tissue
progenitors which remained attached to the disk of
coralline hydroxyapatite.
Example 7
Three composite bone grafts were prepared as in
example 1 except that the concentration of nucleated cells
in the marrow suspension was increased from 100 to 150
million nucleated cells per ml. This increase in the
number of cells passed through the hydroxyapatite disks
increased the number of nucleated cells and connective
tissue progenitor cells retained in the composite bone
grafts by a mean of 66.840 and 52.00, respectively. These
highly cellular suspensions exhibited increased viscosity
and slower elution flow rates.
These methods of preparing composite bone marrow
grafts typically required less than sixty minutes to
complete. Thus, these methods can be performed while the
bone marrow donor/graftee is in the operating room.
Accordingly, the number of occasions the graftee must
undergo invasive procedures to receive a composite bone
graft can be reduced by using these methods.
18
CA 02291706 1999-11-22
WO 98100174 PCT/US97/16514
The improved composite bone grafts prepared according
to these methods comprised a biocompatible, implantable
substrate and an enriched population of connective tissue
progenitor cells. As used herein the term "enriched
population of connective tissue progenitor cells" means
that the percentage of connective tissue progenitor cells
as compared to all nucleated bone marrow cells is greater
in the composite bone marrow graft than in the original
bone marrow aspirate. In addition, the concentration of
the connective tissue progenitor cells in the improved
composite bone marrow grafts was about two times greater
than the concentration of these cells in the original
aspirate.
The improved composite bone grafts also comprised a
population of nucleated cells other than connective tissue
progenitor cells, including endothelial cells and
hematopoietic cells derived from bone marrow, and a
population of platelets derived from peripheral blood. The
red blood cells and plasma in the bone marrow aspirate
suspension are not selectively retained in the composite
bone grafts and, thus, the improved composite bone grafts
typically contain less than five o of the red blood cells
in the original suspension.
The improved composite bone graft is suitable for
implantation into the bone marrow aspirate donor or into an
immunologically compatible host. The improved composite
bone graft is also useful for assessing the effect of
cytokines, hormones and other biochemical molecules on the
19
CA 02291706 1999-11-22
WO 98/00174 PCT/US97/16514
proliferation and differentiation of connective tissue
progenitor cells in vitro.
The present invention also provides a method for
increasing the concentration of connective tissue
progenitor cells in an isolated population of bone marrow
cells . The method comprises passing a bone marrow aspirate
suspension through a porous biocompatible, implantable
graft material to provide a matrix with nucleated bone
marrow cells chemically bonded thereto, disassociating the
nucleated bone marrow cells from the matrix with a solution
capable of disrupting the chemical bonds between the matrix
and the nucleated bone marrow cells, and then collecting
the disassociated cells. Suitable solutions for
disassociating the nucleated bone marrow cells from the
matrix include for example, medium containing trypsin,
growth medium containing a chelator such as for example
EGTA. To further increase the relative concentration of
connective tissue progenitor cells in the isolated
population of nucleated bone marrow cells, a wash solution
is passed through the matrix before the cells are
disassociated therefrom.