Note: Descriptions are shown in the official language in which they were submitted.
CA 02291983 1999-11-19
1: K
LIGAND=MEDIATED 1MMUNOFUNCTIONAL HORMONE
BINDING PROTEIN ASSAY METHOD
BACKGROUND OF THE INVENTION
Ffefd of the fnventfon
A novel ligand-mediated immunofunctional assay (LIFA) method is described
for detecting the presence and quantitating the amount of a polypeptide
hormone
binding protein in a biological fluid and/or determining the amount of the
ligand
1 0 polypeptide hormone specifically bound to the hormone binding protein.
This
modified immunometric assay for a hormone binding protein uses: 1) a first
solid
phase bound antibody to capture the hormone binding protein; 2) a saturating
amount
of ligand hormone; and, 3) a labeled second antibody specific for the ligand
hormone.
The LIFA method is exemplified by a growth hormone binding protein assay.
1 5 Descriotion of the Background Art
A hormone binding protein (HBP) is a carrier protein found in biological
fluids which has binding specificity for a ligand polypeptide hormone.
Examples of
such HBP's are growth hormone binding protein (GHBP), epidermal growth factor
(EGF) binding protein, insulin-like growth factor 1 and 2 (IGF-1, IGF-2)
binding
2 0 proteins (six of them), platelet derived growth factor (PDGFj binding
protein, nerve
growth factor (NGF) binding protein, insulin binding protein, corticotropin
releasing
factor (CRF) binding protein, transforming growth factor beta (TGF-8) binding
protein and activin binding protein (Follistatin).
One of the best characterized polypeptide hormone binding proteins is the
2 5 GHBP. The GHBP discussed in this invention is the extracellular domain of
the GH
receptor which circulates in blood and functions as a GHBP in several species
(Ymer,
S.1, and Herington, A.C., Mol. Cell. Endocrinol. 4J.:153 11985]; Smith, W.C.
and
Talamantes, F., Endocrinology JU:1489-94 (1988]; Emtner, M., and Roos, P.,
Acta
Endocrinologica ]Copenhagen] 1=.296-302 (1990]), including man (Baumann, G.
3 0 et al.,' J. Clin. Endocrinol. Metab. fiZ:134-141 [1986]; Herington, A.C.
et al., J. Clin.
Invest. LL:1817-1823 (1986]). Little is known about the fate of the GHBP or
its
regulation in various physiological and pathological conditions.
Hormone binding proteins have been assayed by antibody based precipitation
methods where the ligand is labeled and the antibody is specific for the
binding
3 5 protein itself. Monoclonal antibodies specific for human growth hormone
binding
protein (hGHBP) were used by Barnard et al., J. Endocrinol, M(2):327-
3211989]; for rabbit GHBP by Ymer et al., Endocrinology, 12512):993-9(1989];
and mouse by Smith et al., Endocrinology 123(3):1489-94(1988]. Currently
available methods for estimating GHBP levels in blood are based on incubation
of the
CA 02291983 1999-11-19
.. ;
2
sample with radiolabeled GH, followed by separation of bound and free GH
(Baumann,
G. et al., Acta Endocrinologica (Copenhagen) 1J.1: 529-34 119881; Amit, T. et
al., J.
Clin. Endo. Metab. 71:474-479 [19901). The results obtained by these assays
are
difficult to interpret due to interference by endogenous GH (Baumann, G. et
al., J.
Clin. Endocrinol. Metab. fiZ:134-141 (1986)). Others who have used labeled
growth
hormone to detect GHBP are: Emtner et al., Acta Endocrinol =(3):296-302,
(1990); Silbergeld et al., Clin. Endocrinol. 11(3):295-303 (1989); Daughaday e
t
al., J. Clin. Endocrinol Metab., M5):1072-4, (1987); Herington et al., J.
Clin.
Invest. u,(6):1817-23, (1986); and Laron et al., Acta Endocrinol. 121( 4):60 3-
8
1 0 (1989). These assays for GHBP in blood have serious problems. They are
laborious,
requiring separation of complexed GHBP-GH by size-exclusion chromatography or
antibody precipitation, and they may not give consistent results from one
laboratory
to another. In addition, they generate results that are arbitrary (i.e. not
calibrated to
a common protein standard) and influenced by endogenous growth hormone.
1 5 Therefore, there is a need for an improved assay method which will allow
detection of
all the polypeptide hormone binding proteins, including those bound to
endogenous
polypeptide hormone.
Methods for the production of monoclonal antibody-producing hybridomas are
disclosed by Kipps and Herzenberg in Clinical Endocrinology and Metabolism,
Vol 62,
2 0 No. 1, page 108.1- 108.9 (1986)
A monoclonal antibody-based immunoradiometric assay for IGF binding
protein was described by Pekonen et al, J. Immunoassay 10:325-37 (1989).
Immunometric or sandwich immunoassays using high affinity monoclonal
antibodies
were taught in David et al., U.S. Pat. 4,486,530. Such sandwich assays have an
2 5 antigen with two or more epitopes sandwiched between two antibodies.
Circulating
proteins that bind non-polypeptide hormone ligands, such as the thyroxine
binding
protein, have been measured using fluorescent labeled tracer (U.S. Pat.
4,476,228)
in order to determine the number of binding protein sites not occupied by
thyroxine.
lodothyronine immunoassays in a biological fluid using blocking agents and
thyroxine
3 0 binding globulin were described in Gordon et a!, U.S. Pat. 4,622,293.
Specific binding pairs (SBP) are discussed in reference to antigen-antibody
reactions, ligand-receptor, hormone-receptor and lectin-oligosaccharide (U.S.
Pat.
4,956,302). Zuk el al., (U.S. Pat. 4,594,327) describes assays of whole blood
to
detect members of such SBPs wherein one member of the SBP must be attached to
the
3 5 solid phase prior to contacting the blood. The other second member of the
SBP is
detected using labeled second SBP member in competitive reactions. Similarly,
Weng
et al. (U.S. Pat. 4,737,456) describes a method of reducing interfering
substances in
assays of a SBP member wherein the individual member of the SBP is labeled.
The
receptor is used in a competitive assay to capture both labeled and unlabeled
ligand,
CA 02291983 1999-11-19
3
not to analyze for the presence of and quantify receptor as in the present
invention. A
method for the determination of an antigen using two antibodies is disclosed
in U.S.
Pat. 4,343,896.
Problems in Previous Polypectide Hormone Binding Protein Assays
Problems in detecting HBP can be best illustrated by the problems
encountered in detecting GHBP. Previous methods for determining the presence
of
GHBP in biological fluids were not as accurate as desired and frequently
required the
use of radioactive materials. The present invention avoids the problems of the
previous assay methods in that it (a) does not require the use of radiolabeled
GH; (b)
1 0 does not require the removal of endogenous GH from the GHBP; (c) does not
require
any form of size separation of GH from the GH-GHBP complex; and, (d) measures
the
actual mass or absolute amount of GHBP rather than a relative amount reported
in
arbitrary units. The present invention has the added advantage that it
measures the
binding capacity of the circulating GHBP and is able to measure the degree of
1 5 saturation of the GHBP with respect to GH. Moreover, the present invention
is
specific for GHBP by substantially reducing background assay noise that causes
imprecision. The assay of the present invention avoids the problems in
standard
immuno-metric assays by using a first antibody to capture the GHBP and a
second
detectably labeled antibody to measure the presence of bound GH. The use of a
second
2 0 antibody specific for another epitope on the GHBP would not determine
whether the
GHBP was functional, and in addition, it could increase the background due to
other
serum proteins which bound both antibodies.
In order to study the function of the endocrine system it is essential to have
access to reliable methods for quantitation of all parts of the system, i.e.
the hormone,
2 5 its binding protein and the hormone-binding protein complex. These
measurements
have not been previously achieved because of interference in the assays by the
different components and the fact that both the hormone and the BP can be
present in
free and complexed forms. It is even more complicated when there are several
different binding proteins for the same hormone, as for the IGF-1 system.
However,
3 0 the present invention teaches how the use of monoclonal antibodies
directed at a
specific binding protein can measure the amount and degree of saturation of
that
specific binding protein. In the case of GH, for which a second binding
protein with
lower affinity has been described (Baumann, G. and Shaw, M.A., J. Clin.
Endocrinol.
Metab. 70:680-686 [1990]), this binding protein appears to be structurally
3 5 unrelated to the GH receptor and should not be detected in our assay.
However, this
other GH binding protein may also be detected in the present invention's assay
method
once the other binding protein is isolated and appropriate antibodies are
raised.
Currently, the standard method for quantitation of carrier proteins for
peptide hormones is incubation of serum with the radiolabeled ligand, followed
by
CA 02291983 1999-11-19
r--
~
4
chromatography or precipitation to separate the bound and free hormone. These
procedures are laborious and the results often difficult to interpret because
of the
interference by endogenous hormone in the sample. These assay methods give an
estimate of the binding capacity of serum proteins of a certain size but the
activities
of different binding proteins of similar size are not distinguished and the
relative
proportion of free and complexed BP cannot be determined. Another disadvantage
is
that the results are expressed in relation to reference serum pools, which
makes it
difficult to compare the results in different studies. In the present
invention, we
developed an assay for the GHBP by choosing a new approach. This assay was
1 0 surprisingly able to precisely detect individual hormone binding proteins
in a way
that has not been previously demonstrated.
The LIFA, which is the preferred method used in the present invention, is
simple to use and has the advantage that only functional binding protein is
detected.
When the assay method is applied to GHBP, both total and endogenously
complexed
1 5 GHBP are measured, and the assay does not require removal of endogenous GH
from the
GHBP or procedures to separate free GH from the GHBP complex. In contrast to
previous methods, endogenous GH does not interfere in the assay; instead,
bound GH,
either endogenous or exogenously added, is used to detect the total and
complexed
GHBP. In fact, *one cannot use the GH as the first member bound. to the solid
phase
2 0 since GH cannot complex with binding protein that is already complexed
with
endogenous GH. Therefore, one requirement of the present assay method is for a
solid
phase coat antibody which recognizes the binding protein both in a free and
complexed
form. The assay method taught in this invention can also be used to measure
the total
binding capacity and the saturation of other polypeptide hormone-binding
proteins
2 5 with respect to the ligands that they bind.
Therefore, the present invention describes the development of a novel,
sensitive and specific enzyme-linked immunosorbent assay (ELISA) for
quantitation
of biologically active HBP in biological fluids. The assay can also be used to
measure
the concentration of the ligand-hormone binding protein complex. The method of
the
3 0 present invention provides a number of advantages relating to ease of
analysis,
sensitivity, precision and reliability which will be more apparent as the
details of
the method are discussed.
SUMMARY OF THE INVENTION
A LIFA method is described for detecting the presence and the concentration of
3 5 a polypeptide hormone binding protein and/or the degree of saturation of
the hormone
binding protein with its specific ligand hormone. Functional binding between
the
hormone binding protein and the ligand hormone is required in the assay. As
illustrated in Figure 1, steps 1-3, a monoclonal or polyclonal antibody which
binds
one or more hormone binding protein epitopes, which are exposed in both the
free and
CA 02291983 1999-11-19
the ligand hormone-associated binding protein, is used to capture the hormone
binding protein on a solid matrix. In step 3, the captured hormone binding
protein is
incubated with the ligand hormone to saturate the hormone binding protein
sites
specific for the ligand hormone. In one option, the hormone binding protein is
not
5 saturated with the ligand hormone prior to the step 4 incubation with
hormone
specific labeled antibody. This allows determination of the level of
endogenous ligand
hormone associated with the hormone binding protein prior to incubation with
added
exogenous ligand hormone. The hormone binding protein and the figand hormone
may
be simultaneously incubated together and with the solid phase capture antibody
or
1 0 they may be sequentially incubated with the capture antibody. In step 4, a
detectably
labeled monoclonal or polyclonal antibody, that binds to one or more epitopes
on the
ligand hormone at a site that is different from where the hormone binding
protein
binds, stably binds to the ligand hormone-hormone binding protein complex.
When
the hormone binding protein is saturated with added hormone (step 3) the total
1 5 amount of detected hormone is a measure of the amount of binding protein
present.
When the hormone binding protein is not saturated with added hormone, the
amount of
detected hormone is a measure of endogenous ligand hormone associated with the
hormone binding protein. A comparison of the two values allows a determination
of
both the amount of hormone binding protein present and the relative saturation
with
2 0 endogenous Iigand hormone.
The therapeutic use of GHBP alone and in combination with GH to stimulate
growth hormone responsive tissues is shown. The use of a GHBP-GH therapeutic
composition is shown to result in greater stimulation of GH responsive tissues
with
the use of less GH. Furthermore, the GHBP-GH composition is longer lasting
2 5 following administration thereby permitting less frequent administration
than with
GH alone.
BRIEF DESCRIPTION OF THE DRAWINGS
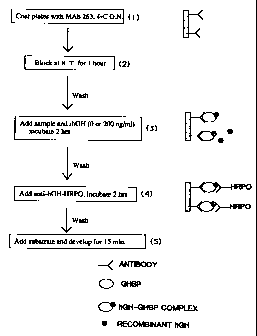
Figure 1. Schematic of Assay Procedure for detecting human growth hormone
binding protein.
3 0 Figure 2. Coat MAb selection. % bound GH-11 25 by MAb 5, 43 and 263 were
ploted
vs the three different incubation configurations. The immobilized MAbs were
evaluated in three different experiments. In the first experiment, using
sequential
incubation steps, GHBP was first incubated with MAb coated on the well,
followed by
addition of radiolabelled GH (GH-1125). In the second experiment the reaction
of
3 5 GHBP and GH-1125 was carried out simultaneously in MAb coated wells. In
the third
experiment, GHBP was pre-incubated with hGH-I1 25 overnight at 4 C and then
added
to the MAb coated well.
Figure 3. Comparison of two standard curves generated with and without
preincubation with GH. One set of standards were incubated with GH (final
CA 02291983 1999-11-19
= ~.r-,~ ,
6
concentration 200 ng/ml) over night at +4'C, the control standards were
incubated
with assay buffer. The samples so generated were then assayed in the LIFA.
Figure 4. Standard curves of hGHBP using 22 kD hGH as ligand, hGHBP using 20
kD
as ligand, and hGHBP using a combination of hGH 22kD and 20 kD.
Figure 5. Plot of theoretical hGHBP concentration based on dilution of three
different serum samples vs. hGHBP concentration in the serum samples diluted
in
assay buffer and measured by LIFA.
Figure 6. Total- and hGH-bound- hGHBP levels in random serum samples from 16
healthy adults (sample f<1-16) and two patients with Laron type dwarfism
(sample
1 0 l117 and 18).
Figure 7. Cross correlation of GH and GHBP (A) Twenty-four hour plasma
profiles
of GH (top panel), GH/GHBP-complex (middle panel) and total GHBP concentration
jbottom panel) in samples from a a 15 year old boy (profile No 15, Table 4);
(B)
statistical cross correlation analysis of data from (A).
1 5 Figure 8. GH binding protein levels in National Cooperative Growth Study
(NCGS)
patients indicating the log concentration of GHBP vs the age of patient. The
crossbars
represent mean values; solid vertical lines are plus or minus 1 SDs; dotted
vertical
lines are plus or minus two standard deviations (SDs). The separate black dots
each
represent one patient. (A) Idiopathic growth hormone deficiency (GHD) for
males;
2 0 (B) Idiopathic GHD for females; (C) Idiopathic short stature. (ISS) for
males; (D)
ISS for females; (E) Turner Syndrome.
DESCRIPTION OF THE PREFERRED EMBODIMENTS
Polvneotide Hormone Binding Protein Assay
Assaying for the presence of polypeptide hormone binding protein uses: (1) a
2 5 first capture monoclonal antibody specific for the binding protein; (2)
ligand
polypeptide hormone and (3) a second monoclonal antibody specific for the
ligand
polypeptide hormone. The LIFA we have developed is a simple and sensitive
method
which allows quantitation of the total concentration of functional hormone
binding
protein in a solution. The assay's binding protein detection range may vary
from
3 0 about 4 to 20,000 pmol/L, more preferably from about 10 to 4000 pmol/L,
and
most preferably from about 20 to 2000 pmol/L. The assay can also be modified
to
measure the concentration of the circulating ligand hormone-binding protein
complex. A monoclonal antibody directed against the binding protein, which
recognizes both free and hormone bound binding protein is used to capture the
binding
35 protein on a solid phase, such as a microtiter plate. The samples are
incubated with
the ligand hormone to saturate all binding sites and an anti-hormone antibody
is used
to detect the amount of hormone (endogenous and exogenous) which has bound to
the
binding protein. The same procedure, but without incubation with ligand
hormone,
allows quantitation of the levels of circulating hormone-binding protein
complex.
8 U eS 114 s~ ~T 1E , "S. A, hE E T
CA 02291983 1999-11-19
' .-.. .-.,.
7
This LIFA may be used for any polypeptide hormone binding protein. It offers
the ability to accurately detect the concentration of functional binding
protein present
in any biological fluid and to measure the endogenous level of saturation of
functional
binding protein with its ligand polypeptide hormone. For the first time this
assay
method permits the measurement of functional HBP and the catibration of a HBP
assay
to a verifiable mass unit as opposed to serum units, etc. While this can be
accomplished by using naturally produced HBP and ligand hormone (if
available), it
is usually accomplished by conducting the assay with recombinant polypeptide
hormones and creating a standard curve based upon recombinant HBP.
Surprisingly,
1 0 the present LIFA method may be used to detect both the total HBP and the
amount of
HBP complexed with endogenous polypeptide hormone. Therefore, for the first
time
there is an accurate method of determining the amount of functional HBP which
is not
influenced by ambient polypeptide hormone.
In one modification of the present immunofunctional HBP assay method, the
1 5 added ligand hormone contains a second detectable marker which does not
hinder the
binding of the detection antibody. This second marker on the ligand hormone
and the
first marker on the detection antibody are not the same. By separately
measuring the
amount of added ligand hormone bound and the amount of bound antibody, the
percent
saturation of the HBP with ligand hormone may be determined. The use of two
distinct
2 0 markers, one on the ligand hormone and one on the detection antibody,
permits a
simultaneous determination of the amount of bound exogenous ligand hormone and
total
HBP.
The assay of the present invention may also be used to quantitate the amount
of
ligand hormone in an unknown sample. Recombinant HBP, or HBP stripped of
2 5 endogenous ligand hormone, is bound to the solid phase as in the assay
method. First,
the unknown sample containing the ligand hormone is contacted with the
antibody
bound HBP. Second, the ligand hormone specific labeled antibody is added and
the
amount bound is determined. This application of the LIFA permits the use of
HBP in
smaller amounts than would be used by directly coupling the HBP to the solid
phase.
3 0 The use of antibody to bind the HBP assures that the orientation is such
that the HBP
binding sites for the ligand hormone are not blocked.
Human Growth Hormone 8lnding Protein Assay
Assaying for the presence of hGHBP uses: 1) a first capture monoclonal
antibody specific for the binding protein; 2) growth hormone; and 3) a second
3 5 monoclonal antibody specific for growth hormone (Figure 1). The LIFA we
have
developed is a simple and sensitive method which allows quantitation of the
total
concentration of functional growth hormone binding protein in biological
fluids. The
assay can also be modified to measure the concentration of the circulating
ligand-
binding protein complex. A monoclonal antibody directed against the growth
CA 02291983 1999-11-19
8
hormone-binding protein, which recognizes both free and GH bound binding
protein is
used to capture the binding protein on a microtiter plate (Figure 1, step 1).
The
samples are incubated with human growth hormone to saturate all binding sites
(Figure 1, step 3) and an anti-hGH antibody is used to detect the amount of
hGH
(Figure 1, step 4) (endogenous and exogenous) which has bound to the binding
protein. The same procedure, but without incubation with added hGH, allows
quantitation of serum levels of circulating hGH-binding protein complex.
A preferred assay herein is a monoclonal antibody (MAb)-based sandwich
ELISA for quantitation of GHBP in biological fluids such as human serum or
plasma.
1 0 The assay, which only detects functional GHBP, can be used to measure both
total and
complexed GHBP, and the degree of saturation of the GHBP with its ligand can
therefore be calculated. In an assay developed for hGHBP, the 263 (coat) MAb
binds
free GHBP as well as GHBP bound to hGH, and is used to capture the GHBP on
microtiter plates.= The conjugated MAb MCB, which is directed against hGH, is
used to
1 5 detect the hGH that is bound to the immobilized GHBP. The total amount of
GHBP is
measured by saturating the binding sites with hGH, followed by detection of
the total
(endogenous and exogenous GH) bound to the GHBP. The concentration of the
endogenous GH-GHBP complex is obtained by incubating the samples with the
conjugated antibody without previous saturation of the GHBP with GH. The assay
is
2 0 sensitive and appears to cover the physiological range since random
samples from 16
normal adults all had clearly detectable GHBP levels. Spike recovery
experiments
show that the assay is useful for measuring GHBP levels in both serum and
plasma,
with recoveries ranging from 89.1 to 113.6 %.
One requirement of the present assay method is for a coat antibody which
2 5 recognizes the antigen both in a free and complexed form, which for hGHBP
is the MAb
263. Using the techniques described herein for generating such antibodies, we
believe that the principle behind our GHBP LIFA could be used to measure the
total
binding capacity and the saturation of other polypeptide hormone-binding
proteins.
The difference in % bound observed when MAb 263 was tested in three different
assay
3 0 formats (Figure 2) is due to the assay kinetics rather than the MAb
binding
characteristics. In the overnight pre-incubation experiment, the GHBP had the
longest incubation with GH-1125 and hence the highest % bound. Even though the
reaction time (4 h) was the same in the simultaneous and the sequential assay,
the
complexing of GH-1125 to GHBP would be faster for a homogeneous system
3 5 (simultaneous) vs. a heterogeneous system (sequential), thus the % bound
would be
higher.
The GH used to saturate the GHBP may be a GH analog wherein there is a
binding site specific for the GHBP and one or more exposed epitopes for
binding the GH
specific antibody.
CA 02291983 1999-11-19
9
ARpficatlons of the GHBP Assay
The results of the assay for GHBP and the degree of saturation of the GHBP
with GH may be used for the diagnosis and treatment of growth deficiency. This
assay
is anticipated to provide an integrated index of the exposure of an organism
to GH. It
provides a more precise determination of total and free GH, which will better
identify
patients who are relatively GH-deficient and will benefit from GH replacement
therapy. In general, the assay would be of value in any situation where GH is
administered therapeutically for treating a clinical condition or where GH is
present
in excessive amounts and involved in causing or reflecting a clinical
pathophysiology.
1 0 The results provide an improved method for assessing compliance with GH
replacement therapy and titrating dose and individualizing GH therapy for
individual
patients or those with particular clinical manifestations of GH deficiency or
excess.
Use of these assay results facilitates diagnosing resistance to GH action due
to
decreased amounts or decreased binding activity of the GHBP or of the GH
receptor,
1 5 such as in hereditary or acquired syndromes. Hereditary syndromes,
including Laron
dwarfism and other syndromes of short stature such as ideopathic short stature
are
revealed when abnormalities of the GHBP are shown to exist. The invention also
assists in the identification and treatment of acquired syndromes including
disease
states where there is diminished or excessive expression of GHBP, such as in
liver
2 0 disease. Other examples of the use of the present assay invention include
detection of
diseases of ovarian function, diseases of joints or bone, or hypothalamic or
pituitary
diseases causing excess GH or GHBP production. Clinical use of GHBP, or the
GHBP/GH complexes as a therapeutic agent, are aided by the present invention
because the measurement of GHBP or GHBP+GH after their administration assists
2 5 clinical practice by allowing a determination of the presence of
therapeutically
effective or therapeutically ineffective amounts in bodily fluids.
All parts of the growth promoting system are interrelated and it is expected
that administering growth hormone releasing factor, growth hormone inhibitory
factor (somatostatin), IGF-1, or IGF-1 binding proteins, may perturb GHBP
3 0 concentrations. The measurement of GHBP assists clinical practice in
assessing the
suitability of patients for treatment with these proteins and the efficacy of
such
treatments.
Iin[cal AQpfications of GHBP Assay
Progress in our understanding of GHBP requires a quick, convenient and
3 5 accurate assay for GHBP. The initial paper describing a GHBP was published
in 1964
(Hadden et al., Nature Z.Q3-:1342 11964]). The serum GH-binding protein was
better characterized in the mouse in 1977 (Peeters, S. and Friesen, H.G.,
Endocrinology =.1164 (1977]), and in 1986 two groups further characterized
the existence of a human serum-binding protein for GH (Baumann, G. et at., J.
Clin.
CA 02291983 1999-11-19
Endocrinol. Metab. fil:134-141 11986]; Herington, A.C. et al., J. Clin.
Invest.
71,:1817-1823 11986]). When the GHBP was purified and characterized in the
rabbit its amino-terminal 37 residues were shown to be identical to those of
the
extracellular part of the rabbit liver GH receptor (Spencer et al., J. Biol.
Chem.
5 M:7862-7867 (1988]). It has been suggested that the GHBP derives from the
membrane receptor by proteolytic cleavage (Trivedi and Daughaday,
Endocrinology
=:2201 11988]) or that it is produced from a separate mRNA derived from the
same gene as the full-length GH receptor (Smith et a/., Mol. Endo, p.984
[1989];
Baumbach et al., Genes and Dev. X.1199 (1989]). Studies of the ontogeny of the
1 0 GHBP activity in man (Daughaday et al., J. Clin. Endocrinol. Metab. U:1072-
74
[1987]) and the changes in the concentrations of the GHBP and hepatic GH
receptors
in pregnant mice (Smith and Talamantes, Endocrinology M:1489-94 [1988])
suggest that the serum GHBP levels could be a peripheral indicator of GH
receptor
activity.
1 5 This idea that GHBP levels reflect GH receptor activity is supported by
the
finding that patients with Laron-type dwarfism, a syndrome caused by the lack
of
functional GH receptors, also lack GH binding activity in serum (Daughaday and
Trivedi, Proc. Natl. Acad. Sci. USA $4:4636-40 (1987]; Baumann et al., J.
Clin.
Endocrinol. Metabol 11:814-816 (1987]; Laron et al., Acta Endocrinologica
2 0 (Copenhagen) 12~L:603-608 (1989]). There are indications that in some
patients
with Laron type dwarfism the abnormality is caused by partial deletion of the
GH
receptor gene (Godowski et al., Proc. Natl. Acad. Sci. USA B&:8083-8087
(1989];
Anselem et al., New England J. Med. =.989-95 [1989]), which could result in
growth failure due to inability to bind GH. For this type of abnormality our
assay
2 5 would be particularly useful since it, in contrast to some immunoassays,
would not
detect the inactive protein. The serum GH binding capacity is reduced in Laron-
dwarfism heterozygotes, and it has been suggested that measurement of the
hGHBP
levels in serum could be of help for genetic counseling (Laron et al., supral.
Little is known about the physiological role of the GHBP, however, recent
3 0 studies have shown that the binding protein can modify the effects of
growth hormone.
It has been demonstrated that the GHBP alters the distribution and half-life
of GH
(Baumann et al., Metabolism 31:330-333 (1989]), and there is also evidence to
suggest that the GHBP affects the interaction of GH with its receptor on the
target
cells (Lim et al., Endocrinology 1276:1287 11990]). It has recently been shown
3 5 that recombinant hGHBP produced in F. coli enhances the growth promoting
effects of
hGH when given to GH deficient rats, indicating that the GHBP may play an
important
role in the regulation of body growth in humans (see Example 5).
The LIFA of the present invention is used to monitor the concentration of GHBP
in the biological fluids of a patient to detect aberrant concentrations. In
Examples 5,
CA 02291983 1999-11-19
11
6 and 7 numerous applications of the assay are used to monitor and evaluate
the
activity of human GHBP alone and complexed with hGH. These pharmacological
applications include purification, dosage, frequency of administration, and
duration in
circulation. The LIFA is also used to monitor the activity of hGHBP from
different
sources, such as E. coli, 293 cells or from natural tissue sources.
The LIFA has application in the pharmacologic evaluation of hGHBP action in
primates. The hGHBP with, and without, complexed hGH can be monitored in
primates
to improve the dosage and frequency of administration.
In Primates
1 0 The ability of the GHBP to allow one to give infrequent GH injections,
using
similar GH doses to those used currently, yet maintain growth responses, is of
clinical significance. Experiments in the monkey, using the LIFA demonstrate
that
clearance of a GHBP + GH complex (in one of the forms described above or in a
modified form), is delayed in primates. The clearance of injected GH bound to
the
1 5 GHBP is slowed to a degree similar to that which we have seen in the rat.
This
demonstrates in primates the improved growth promoting activities of
administering
hGH complexed to the hGHBP.
In primates, including humans, the GHBP-complex is able to be given at
infrequent intervals, greater than every 2 days, more preferably at greater
than
2 0 every 7 days, without a loss of efficacy compared to injections of GH
alone every 1 or
2 days or daily for a week or more. In addition, the GHBP complexed with GH or
alone
will be given at lower total weekly doses compared to GH alone. The
undesirable side
effects of GH treatment, for example the diabetogenic and fluid retaining
properties of
GH, will be reduced with the use of the GHBP. There are other beneficial
effects of
2 5 using GH-GHBP complex including a heightened IGF-1 response and the
ability of the
GHBP to direct GH preferentially to bone. This allows the GH-GHBP complex to
be
used for the treatment of bone disorders, including the prevention and
treatment of
osteoporosis. In each situation the LIFA is used to monitor the progress of
the
reaction. The dosages, formulations and methods of using and making hGHBP are
3 0 described in U.S. Pat. 5,057,417.
The LIFA will be used to define groups of patients who have aberrant amounts
of the GHBP complex. For example, a sub-set of poorly growing children, who
are
relatively resistant to the growth promoting activity of GH, will be found to
be
deficient in the GHBP. Such children include patients with Turner's Syndrome,
3 5 kidney disease, as well as a class of binding protein deficient patients
who were
previously described as having iodiopathic short stature. Pharmacokinetic
studies
delivering the GHBP or GHBP-GH complex subcutaneously, and assaying the blood
levels of GHBP-GH complex using the LIFA will be performed in man to establish
suitable temporal dosing regimes. Doses of GH-GHBP complex sufficient to
stimulate
CA 02291983 1999-11-19
, - ~
12
rises in IGF-1 concentrations in blood will be determined and these doses will
indicate the doses of GHBP-GH complex necessary to induce body growth.
Subsequently, long-term studies in the GH-resistant children will be initiated
to
demonstrate the ability of the GHBP-GH complex to stimulate whole body growth,
Including bone growth. The LIFA will be used to monitor blood levels of the
GHBP.
A primary application of this invention is to use the GHBP LIFA to monitor
endogenous levels of GHBP before and during treatment for GH or GHBP
deficiency.
The assay of this invention serves to direct the treatment that a patient
undergoes. If
there is ~o detectable GHBP in the blood, a Laron-type syndrome may be present
and
1 0 IGF-1 treatment indicated. If there is a low level of GHBP in the blood,
additional GH
or GHBP with or without IGF-1 may be indicated. Whatever treatment regime (GH
or
GH + IGF-1, or GH-GHBP complex treatment) instituted the LIFA will be most
valuable to determine the GHBP response to treatment. Blood GHBP
concentrations
will rapidly reflect the efficacy of GH treatment much more so than
measurement of
1 5 traditional endpoints. A lack of response of blood GHBP levels will be
used as a rapid
diagnostic for considering alternative strategies for treatment.
Another use of the LIFA is to detect the biological activity of endogenous
blood
GH. This assay uses the addition of a constant amount of GHBP followed by the
addition
of sample without saturating with GH. It is anticipated that patients will be
detected
2 0 who possess high immuno-reactive but low immunofunctional concentrations
of GH.
A similar assay format can be used to measure the amount of bioactive GH in
any
sample, especially to test batches of recombinant GH for their biological
activity.
The GHBP assay can be reversed to assay for GH. The captive antibody binds
GH, GHBP is complexed and the indicator antibody is specific for the bound
GHBP.
2 5 This permits assay of GH alone or complexed with native GHBP.
Modes of Carrying Out the Invention
The present invention may be used to measure any known polypeptide hormone
binding protein found in biological fluids. Similarly, it may be used with new
hormone binding proteins as they are discovered. Among the preferred binding
3 0 protein targets of the assay method of the present invention are the
following: any
growth hormone binding protein, epidermal growth factor binding protein,
insulin-
like growth factor binding proteins, insulin binding protein, corticotropin
releasing
factor binding protein, nerve growth factor binding protein, transforming
growth
factor beta binding protein and activin binding protein. Detection of each of
these
3 5 binding proteins using the assay method of the present invention requires
the use of
their respective ligand polypeptide hormone to saturate the hormone binding
sites on
the binding protein. Polypeptide hormones may be purified from natural
sources,
produced by solid phase protein synthesis or produced by recombinant means.
Among
the preferred ligand polypeptide hormones used are: growth hormone, epidermal
CA 02291983 1999-11-19
13
growth factor, insulin-like growth factor-1, insulin-like growth factor-2,
nerve
growth factor, insulin, corticotropin releasing hormone, transforming growth
factor
beta and activin. The preferred binding proteins and polypeptide hormones may
be
from any animal having such proteins in their biological fluids. The
biological fluids
may be any aqueous liquid such as the following: serum, plasma, lymph fluid,
synovial fluid, follicular fluid, seminal fluid, amniotic fluid, milk, whole
blood,
urine, spinal fluid, saliva, sputum, tears, perspiration, mucus, tissue
culture
medium, tissue extracts and cellular extracts.
The pH of the medium will usually be in the range of about 4-11, more
1 0 usually in the range of about 5-10, and preferably in the range of about
6.4-9.5.
The pH is chosen so as to maintain a significant level of specific binding by
the HBP
and the polypeptide hormone, the binding of the antibodies and the
requirements of the
detectable label. In some instances, a compromise will be made among these
three
considerations. = Various buffers may be used to achieve the desired pH and
maintain
1 5 the pH during the assay determination. Illustrative buffers include
borate,
phosphate, carbonate, Tris, barbital and the like. The particular buffer
employed is
not critical to this invention, but in individual assays one buffer may be
preferred
over another.
Moderate temperatures are normally employed for carrying out the assay
2 0 method and usually constant temperatures are maintained during the period
of the
assay. The temperatures for the determination will generally range from about
4 to
50 C, more usually from about 15 to 40 C.
The concentration of HBP which may be assayed will generally vary from
about 10-4 -10-15, more usually from about 10-6 - 10-13 M. While the
2 5 concentrations of the various reagents will generally be determined by the
concentration range of interest of the HBP, the final concentration of each of
the
reagents will normally be determined empirically to optimize the sensitivity
of the
assay over the range of interest.
The LIFA may be used to monitor clinical administration of hGHBP, either
3 0 complexed with or without hGH. A preferred use of the assay method is in a
method of
promoting mammalian growth and anabolism comprising: (a) determining the
optimal
amount of a growth hormone binding protein required to promote a growth
hormone
induced response; (b) measuring by LIFA the amount of growth hormone binding
protein present in the biological fluids of a person suspected of being
deficient in said
3 5 induced response; and, (c) comparing (a) with (b), and if (a) is greater
than (b),
administering growth hormone binding protein in an amount sufficient to
increase the
level of growth hormone binding protein to said optimal amount. Another
preferred
clinical application of the LIFA method is to monitor a growth hormone induced
response such as weight gain, bone growth, muscle growth and function, organ
growth
CA 02291983 1999-11-19
14
and function, skin growth, and the level of IGF-1. The organs whose growth and
function are stimulated may be thymus, kidney, liver, spleen, brain heart,
lungs or
gonads. Yet another application of the present LIFA method is for decreasing
the
frequency of injecting a growth promoting amount of a growth hormone binding
protein-growth hormone complex comprising: (a) determining the minimum
necessary serum level of growth hormone-growth hormone binding protein complex
required to maintain optimal growth; (b) measuring by LIFA the level of growth
hormone binding protein present in a patient suspected of being deficient in
growth
hormone binding protein-growth hormone complex; and, if the complex level in
(b)
1 0 is less than the level in (a), then, (c) administering an amount of growth
hormone
binding protein-growth hormone complex sufficient to maintain the level of
complex
for a period greater than two days. The period may be two to fourteen days,
more
preferably two to eight days, and most preferably three to seven days. The
optimal
amount of growi,h hormone binding protein is defined as that equal to or
greater than
1 5 90% of the average level found in healthy individuals.
Antlbodies
While polyclonal antibodies may be used, the preferred antibody is a
monoclonal antibody. Monoclonal antibodies are highly specific, being directed
against a single antigenic site. The antibody used in the present invention
must be
2 0 specific for epitopes which do not interfere or block the binding of the
polypeptide
hormone with the hormone binding protein. Therefore, the capture or coat
antibody
must bind to epitopcs on the binding protein which leave the hormone binding
site
available for hormone binding. Similarly, the detection antibody must be
specific for
those polypeptide hormone epitopes which remain exposed following binding of
the
2 5 polypeptide hormone to the hormone binding protein. The antibodies may be
made by
methods commonly available to those of ordinary skill in the art. Methods for
the
production of polyclonal antibodies are described in numerous immunology
texts,
such as Microbiology, 3rd Edition, by Davis et al., Harper & Row, New York,
1980.
Monoclonal antibodies may be produced by the method first described by
3 0 Kohler and Milstein, Eur. J. Immunot., fi:511 (1976). While the invention
is
demonstrated using mouse monoclonal antibodies, the invention is not so
limited;
monocionats from any animal species will also function in the assay method of
the
present invention. In fact, in the present assay method, chimeric antibodies
will also
function. Chimeric antibodies are described in U.S. Pat. 4,816,567, Morrison
et al.,
3 5 Proc. Natl. Acad Sci. USA, JL:6851 (1984); Neuberger et al., Nature
alz:604
(1984); Takeda et al., Nature a]A,:452(1985). Chimeric antibodies are made by
splicing the genes from a mouse antibody molecule of appropriate antigen
specificity
together with genes from another animal encoding a second antibody. Similarly,
monoclonal antibodies, or the antigen binding region of a monoclonal antibody,
such as
CA 02291983 1999-11-19
Fab or (Fab)2 fragments, may be produced by recombinant methods. Both chimeric
and recombinant antibodies or antibody fragments may be used in the assay
method of
the present invention.
The monoclonal antibodies can belong to any of the classes or subclasses of
5 antibodies, including IgA, IgD, IgE, IgG, and IgM. Actively binding
fragments of
antibodies can also be employed, such as Fab, Fv, (Fab)2, or the like. The
monoclonal
antibodies can be prepared by any convenient means which provides
immortalization
of the B-lymphocyte genes expressing the antibody sub-units, such as fusion
between
sensitized lymphocytes and a myeloid fusion partner; transformation, e.g.,
with
1 0 Epstein-Barr virus (EBV); or other immortalization techniques.
Alternatively, the
genes can be isolated from a lymphocytic host expressing the antibodies and
transferred to a more convenient host for expression in accordance with known
genetic engineering techniques.
The antibodies may be obtained from any convenient vertebrate source, such
1 5 as murine, primate, lagomorpha, bovine, ovine, equine, canine, feline or
porcine.
The antibodies are often prepared by fusing spleen cells from a host
sensitized to the
antigen with myeloma cell in accordance with known techniques or by
transforming
the spleen cells with an appropriate transforming vector to immortalize the
cells.
The cells can be cultured in a selective medium, cloned, and screened to
select
2 0 monoclonal antibodies that bind the designated antigens.
The methods used to produce the antibodies for the capture antibody and for
the
detection antibody may be conveniently made by administering the respective
immunogen, either binding protein or polypeptide hormone, and eliciting
antibody.
The preferred antibody is monoclonal antibody. The antibody produced is then
2 5 screened to determine the preferred antibody which does not hinder the
binding
reaction between the polypeptide hormone and the binding protein. The
preferred
monoclonal antibody for detecting the hGH when bound to hGHBP was produced by
a
mouse hybridoma (Cunningham et al., Science (1989) M:1330-1336). The anti-
hGHBP monoclonal antibody used as the capture or coat antibody is commercially
3 0 available (Agen Inc., 90 East Halsey Road, Parsippanyu NJ 07054) or can be
made
using the hGHBP as immunogen and screening the antibody as discussed above.
Because of the relative ease with which antibodies can now be prepared against
antigens, preferred embodiments of the present invention use monoclonal or
polyclonal antibodies attached to the solid phase to capture the HBP.
Techniques for
3 5 attaching specific HBP to various solid phase substrates, such as filter,
plastic etc.
are well known and need not be described here in detail. See, for example,
U.S. Pat.
4,376,110 and the references cited therein. Among the more preferred common
solid phase supports are: small sheets, plastic beads, assay plates or test
tubes made
from polyethylene, polystyrene, polypropylene or other suitable material. Also
CA 02291983 1999-11-19
, ---,~
16
useful are particulate materials such as papers, agarose, cross-linked
dextran, and
other polysaccharides.
Interference by Heteroohitlc Antibodies
When results from GHBP assays using HRPO-conjugated MCB and HRPO-
conjugated mouse polyclonal antibody were compared, one human serum sample
showed much higher GHBP levels when detected with the MCB-conjugate. To test
if
the discrepancy could be due to heterophilic antibodies, purified mouse IgG
(mlgG) at
0.5 mg/mi, was included in the sample buffer. The results showed that mlgG
reduced
the signal in sample #6 from 384 pmol/L to 215 pmol/L when assayed with the
1 0 MAb-conjugate, while the GHBP concentrations were unchanged in two control
samples (323 vs 338, 92 vs 111 pmol/L when assayed with and without mlgG
respectively). These results indicate that the two different conjugated
antibodies
were substantially the same if the unspecific binding was blocked by the mlgG.
Sandwich type immunometric assays are subject to positive interference by
1 5 heterophilic antibodies in the sample. This is caused by human anti-mouse
antibodies
in the human serum sample. Such heterophilic antibodies crosslink the coat and
the
conjugate mouse antibodies. Interference by heterophilic antibodies can be
diminished or eliminated if immunoglobulins from a nonimmunized animal (here
to
HBP or ligand hormone) are added to the assay to block the heterophilic
antibodies in
2 0 the sample. In fact, the discrepancy in the GHBP concentration was
eliminated when
mouse IgG was included in the assay buffer. This indicated that the unspecific
signal
which was detected when the conjugated MAb was used was due to the presence of
anti-
mouse IgG antibodies in the serum from this subject. Since one doesn't know
which
samples will show this type of interference it is best to afways include mouse
IgG in
2 5 the first step of the assay.
Deposit of Hybridoma
The hybridoma cell line producing this anti-hGH antibody is HGH-B which was
deposited with the American Type Culture Collection (ATCC) 12301 Parklawn
Drive,
Rockville, MD, USA. on November 9, 1990, and has ATTC Registration number HB-
3 0 10596. This deposit was made under the provisions of the Budapest Treaty
on the
International Recognition of the Deposit of Microorganisms for the Purpose of
Patent
Procedure and the Regulations thereunder (Budapest Treaty). This assures
maintenance of the viable cultures for 30 years from the date of the deposit.
The cells
will be made available by the ATCC under the terms of the Budapest Treaty, and
3 5 subject to an agreement between Genentech, Inc. and the ATCC, which
assures
permanent and unrestricted availability of the progeny of the cultures to the
public
upon issuance of the pertinent U.S. patent or upon laying open to the public
of any U.S.
or foreign patent application, whichever comes first, and assures availability
of the
progeny to one determined by the U.S. Commissioner of Patents and Trademarks
to be
CA 02291983 1999-11-19
, - .--,
17
entitled thereto according to 35 USC 122 and the Commissioner's rules pursuant
thereto (including 37 CFR 1.12 with particular reference to 886 OG 638).
The assignee of the present application has agreed that if the cultures on
deposit should die or be lost or destroyed when cultivated under suitable
conditions,
they will be promptly replaced on notification with a viable specimen of the
same
culture. Availability of the deposited strain is not to be construed as a
license to
practice the invention in contravention of the rights granted under the
authority of
any government in accordance with its patent laws.
Detectable Markers
1 0 Detectable markers or labels on the antibodies that may be covalently
attached
include a suitable indicator such as an enzyme, radioactive isotope,
fluorescer, or
other measurable tag as described in U.S. Pat. 4,275,149, bridging columns 19
to 28
and U.S. Pat. 4,318,980, columns 10-14. Of particular interest are enzymes
which
involve the production of hydrogen peroxide and the use of the hydrogen
peroxide to
1 5 oxidize a dye precursor to a dye. Particular combinations include
saccharide
oxidases, e.g., glucose and galactose oxidase, or heterocyclic oxidases, such
as uricase
and xanthine oxidase, coupled with an enzyme which employs the hydrogen
peroxide to
oxidize a dye precursor, that is, a peroxidase such as horse radish
peroxidase,
lactoperoxidase, or microperoxidase. Among the preferred enzymes are the
following:
2 0 horseradish peroxidase, glucoamylase, alkaline phosphatase, glucose
oxidase, and
beta-D-galactosidase.
Additional enzyme combinations may be found in the subject matter disclosed
in the cited references. When a single enzyme is used as a label, other
enzymes may
find use such as hydrolases, transferases, and oxidoreductases, preferably
2 5 hyudrolases such as alkaline phosphatase and beta-galactosidase.
Alternatively
luciferases may be used such as firefly luciferase and bacterial luciferase
(U.S. Pat.
4,737,456).
Following the use of antibody specific for the ligand hormone, the amount of
bound antibody is determined by measuring the activity of the attached
indicator. In
3 0 the case of enzymes, the amount of color developed and measured will be a
direct
measurement of the amount of hormone or hormone binding protein present. The
conjugation of such tags, including the enzymes, to an antibody as described
herein is
a standard manipulative procedure for one skilled in immunoassay techniques.
See
for example, O'Sullivan et al., (1981) Methods for the Preparation of Enzyme-
3 5 Antibody Conjugates for use in Enzyme lmmunoassay, in Methods in
Enzymology (ed
J.J. Langone & H. Van Vunakis), Academic press, New York, Vol. ZI, pp 147-166.
Kits
As a matter of convenience, the assay method of the present invention can be
provided as a kit, i.e., a packaged combination with other reagents for
combination
CA 02291983 1999-11-19
= , 1
, = ~--.
16
with a sample or samples in assaying for a polypeptide hormone binding protein
andlor to determine relative saturation with ligand polypeptide hormone. The
components of the kit will be provided in predetermined ratios. The kit will
contain
the specific carrier solid phase for the capture antibody, capture antibody
separate or
bound to the carrier solid phase, the ligand hormone (preferably recombinant),
and
the detection antibody containing a detectable label. Where the detectable
label is an
enzyme, the kit will include substrates and cofactors required by the enzyme,
a dye
precursor, which provides the detectable chromophore of fluorophore. In
addition,
other additives may be included such as stabilizers. buffers and the like. The
relative
1 0 amounts of the various reanents may be varied widely to provide for
concentrations in
solution of the reagents which substantially optimize the sensitivity of the
assay.
Particularly, the reagents may be provided as dry powders, usually
lyophilized,
including excipients, which on dissolution will provide for a reagent solution
having
the appropriate concentration for combining with the sample to be tested.
1 5 Deffnittons and Abbrevlations:
Capture (coat) antibody: Antibody specific for hormone binding protein epitope
such that binding of antibody does not hinder the binding of the ligand
polypeptide hormone to the hormone binding protein. Antibody is attached to a
solid phase and it is used to selectively bind to the binding protein and
2 0 facilitate removal of the binding protein from a solution.
Detection antibody: Antibody which is labeled with detectable marker specific
for an
epitope on the polypeptide hormone and which is the detectable marker.
HBP: hormone binding protein; the carrier protein found in biological fluids
that has
affinity for a ligand polypeptide hormone and acts as a carrier for the bound
2 5 ligand polypeptide hormone.
hGH: human growth hormone, including multiple naturally occurring forms such
as
22kd, 20kd, placental variant (GHyJ, and variants produced by recombinant
methods (Cunningham et al., Science (1989) 2AI:1330-1336).
GHBP: growth hormone binding protein.
3 0 hGHBP: human growth hormone binding protein.
GH: growth hormone.
MAb: monoclonal antibody.
MCB: Monoclonal B produced by the mouse hybridoma HGH-B.
3 5 HRPO: horse radish peroxidase.
BSA: Bovine serum albumin.
LIFA: Ligand-mediated immunofunctional assay.
~~ ~~~
CA 02291983 1999-11-19
' .-=. ~ ; ~
l = !
19
Purification of GHBP
The purification of the GHBP used is monitored using a conventional ELISA
(Fuh, G. et al., J. Biol. Chem. 2fLL 3111-3115 (1990]). The LIFA of the
present
invention provides a better assay for detecting the presence and assaying the
amount
of functional binding protein during such a purification. The LIFA ensures
that
functional active binding protein rather than immunologically active binding
protein
is purified. During hGHBP storage the LIFA is used to follow the amount of
functional
GHBP that remains in an active form with time. This property of the assay
greatly
aids in satisfying regulatory requirements concerning the stability of the
GHBP
1 0 during prolonged storage.
Materials and Methods
Selection of coat MAb
Four monoclonal antibodies to the rabbit liver growth hormone receptor,
MAbs 1, 2, 5 and 7 were provided by Dr. M. Waters (University of Queensland,
1 5 Queensland, Australia), and an additional two MAbs (43 and 263) were
purified from
mouse ascites fluid purchased from Agen, Australia. Immulon II removawell
(Dynatech Laboratories, Inc., Alexandria,Va.) were coated by incubating them
overnight at 4'C with antibody, 100 L per well at 5 g/mL in 50mmol/fiter
carbonate buffer, pH 9.6 (coating buffer). After removing the coating
solution,
2 0 nonspecific binding sites on the coated plates were blocked with 150 L of
phosphate-
buffered isotonic saline pH 7.2 (PBS) containing 5g of bovine serum albumin
(BSA)
per liter (blocking buffer), for 1h at room temperature, followed by :hree
washes
with wash buffer (0.5mL of Tween 20 and 0.1g of Thimerosal per liter of PBS).
The
immobilized MAbs were evaluated in three different experiments. In the first
2 5 experiment, using sequential incubation steps, GHBP was first incubated
with MAb
coated on the well, followed by addition of radiolabelled GH (GH-1125). In the
second
experiment the reaction of GHBP and GH-1125 was carried out simultaneously in
MAb coated wells. In the third experiment, GHBP was pre-incubated with hGH-11
2 5
overnight at 4 C and then added to the MAb coated well. In all three
experiments, the
3 0 nonspecific binding of tracer was determined by substituting the GHBP
solution in the
reaction mixture with incubation buffer (PBS containing per liter, 5g of BSA,
0.5mL
of Tween 20 and 0.1mL of Thimerosol).
Seauentiat Assay
In the sequential incubation experiment, 100 L of GHBP (2.5ng/100 L) was
3 5 allowed to react with immobilized MAb for 2h, washed with wash buffer, and
incubated for 4h at room temperature with GH-1125 (20,000cpm/100 L). The
wells were washed 6 times in wash buffer, blotted thoroughly on adsorbent
paper and
counted in a LKB series 1277 gamma counter (Pharmacia LKB Nuclear Inc.,
Gaithersburg, Md.) for 1 minute (Figure 6).
CA 02291983 1999-11-19
Simultaneous Assay
In the simultaneous incubation experiment, 50 L of GHBP at 2.5ng/50 L in
incubation buffer and 50 L of GH-1125 at 20,000 cpm150 L in incubation
buffer
were incubated in each well for 4h at room temperature. The wells were washed,
5 blotted and counted as described above.
Preincubation Assay
In the pre-incubation experiment, 150 L of GHBP at 2.5ng/50 L in
incubation buffer and 150 L of GH-1125 at 20,000cpm150 L in incubation
buffer
were incubated in test tube overnight at 4 C. The reaction mixture was then
added
1 0 (100 L per well) to the MAb coated well and incubated for 4h at room
temperature.
The wells were washed, blotted and counted as previously described.
Enzyme-con[uaated Antl-hGH Antibodies
The anti-hGH detection MAb was selected for conjugation because it did not
give detectable displacement of the GHBP and contains no overlapping
determinants
1 5 with the GHBP (Cunningham et al., Science Z~:1330-1336 [1989]). The
antibodies were purified from ascites fluid using protein A-Sepharose
(Repligen
Corp., Cambridge MA.) following established procedures (Ey et al., Immunochem.
15:429 [1978]; Goding, J.W., J. lmmunol. Meth. 2Q:241 (1978]) and stored
sterile
in 0.01 M sodium phosphate, 0.15M sodium chloride, pH 7.2 (PBS) at 4 C.
Purified
2 0 MAbs were conjugated to horseradish peroxidase (Nakane and Kawaoi, J.
Histochem.
Cytochem. ZZ:1084 (19741) and stored at -20 C in 50% glycerol.
LIFA assay standards
The recombinant human growth hormone binding protein (GHBP) was purified
from a mammalian cell line following the procedure outlined by Spencer et al.
(J.
2 5 Biol. Chem. LU:7862-7867 119881). The purified GHBP amino acid composition
as determined by quantitative amino acid analysis matched what was
theoretically
expected for the cloned gene product. 'The purified GHBP was homogeneous based
on
analysis of SDS-gel electrophoresis. The concentration of GHBP in the purified
preparation was established by Scatchard analysis (Scatchard, G., Annals of
the New
3 0 York Academy of Sciences 11:660-672 (1949]), and dilutions of this sample
in PBS
containing per liter, 5g of BSA, 5.0mM EDTA, 0.5ml of Tween 20 and 0.1g of
Thimerosal (assay buffer) were then used as standards in the LIFA. GHBP
produced in
E. coli was also used, but surprisingly found to give non-parallel dilution
curves in
the assay. Therefore, the preferred GHBP is either natural GHBP or GHBP
produce by
3 5 cells which produce GHBP in a native configuration, that is, glycosylated.
Recombinant human GH
Recombinant human growth hormone (GH) was supplied by Genentech Inc.,
South San Francisco, California, USA.
CA 02291983 1999-11-19
21
Serum and plasma samples
Serum and plasma (EDTA, citrate and heparin as anticoagulants) samples were
obtained from healthy adult volunteers (9 men and 7 women, 26 to 43 years
old).
The samples were centrifuged and stored at -70'C until assayed.
Therapeutic Treatment Following GHBP Evaluation
For the various purposes of this invention, the COMPOSITIONS (GHBP+GH,
GH, 1GF-I, IGF-1+binding protein) administered to the mammal or avian by any
suitable technique, including parenterally, and can be administered locally or
systemically.
1 0 The COMPOSITIONS are directly administered to the mammal by any suitable
technique, including parenterally, intranasally, or orally. The specific route
of
administration will depend, e.g., on the medical history of the patient,
including any
perceived or anticipated side or reduced anabolic effects using hGH or IGF-I
alone, and
the growth defect to be corrected. Examples of parenteral administration
include
1 5 subcutaneous, intramuscular, intravenous, intraarterial, and
intraperitoneal
administration. Most preferably, the administration is by continuous infusion
(using, e.g., minipumps such as osmotic pumps), or by injection using, e.g.,
intravenous or subcutaneous means. Preferably, the COMPOSITIONS administration
is subcutaneous. - The administration may also be as a single bolus or by slow-
release
2 0 depot formulation. Most preferably, the IGF-I or IGF-1 plus binding
protein is
administered continuously by infusion, most preferably subcutaneously; GHBP +
GH
or GH alone is administered daily subcutaneously by injection. Most
preferably, the
GHBP + GH is administered intermittently every 2 or more days, weekly,
biweekly,
or monthly.
2 5 The IGF-I is suitably administered together with its binding protein, for
example, BP53, which is described in WO 89/09268 published October 5, 1989 and
by Martin and Baxter, J. Biol. Chem., ZJL. 8754-8760 (1986), the disclosures
of
which are incorporated herein by reference. This protein is an acid-stable
component of about 53 Kd on a non-reducing SDS-PAGE gel of a 125-150 Kd
3 0 glycoprotein complex found in human plasma that carries most of the
endogenous IGFs
and is also regulated by GH. The IGF-I is also suitably coupled to a receptor
or
antibody or antibody fragment for administration. Similarly, the GH can be
delivered
coupled to another agent such as an antibody, an antibody fragment, or one of
its
binding proteins.
3 5 The COMPOSITIONS to be used in the therapy will be formulated and dosed in
a
fashion consistent with good medical practice, taking into account the
clinical
condition of the individual patient (especially the side effects of treatment
with hGH
or IGF-I alone or growth retardation after continuous GH treatment), the site
of
delivery of the COMPOSITIONS, the method of administration, the scheduling of
CA 02291983 1999-11-19
~ ,_ ~
, ~.
22
administration, and other factors known to practitioners. The 'effective
amounts' of
each component for purposes herein are thus determined by such considerations
and
must be amounts that enhance the anbolic growth of the treated patient.
As a general proposition, the total pharmaceutically effective amount of each
of the COMPOSITIONS administered parenterally per dose will be in the range of
about
1 g/kg/day to 50 mg/kg/day of patient body weight, although, as noted above,
this
will be subject to a great deal of therapeutic discretion. More preferably,
this dose is
at least 2 g/kg/day, and most preferably at least 5 g/kglday for each
hormone. If
given continuously, the IGF-I, IGF-1 + binding protein, GHBP + GH and GH are
each
1 0 typically administered at a dose rate of about 1 g/kg/hour to about 100
g/kg/hour, either by 1-4 injections per day or by continuous subcutaneous
infusions, for example, using a minipump. An intravenous bag solution may also
be
employed. The key factor in selecting an appropriate dose is the anabolic
result
obtained, as measured by increases in body weight gain, lean body mass, or
statutory
1 5 growth approxiniating the normal range, or by other criteria for measuring
anabolic
activity as defined herein as are deemed appropriate by the practitioner.
The COMPOSITIONS are also suitably administered by sustained-release
systems. Suitable examples of sustained-release compositions include semi-
permeable polymer matrices in the form of shaped articles, e.g., films, or
2 0 microcapsules. Sustained-release matrices include polylactides (U.S. Pat.
3,773,919, EP 58,481), copolymers of L-glutamic acid and gamma-ethyl-L-
glutamate (U. Sidman et al., Biopolymers, 21, 547-556 (1983)), poly(2-
hydroxyethyl methacrylate) (R. Langer et a/., J. Biomed. Mater. Res., jj: 167-
277
(1981), and R. Langer, Chem. Tech., 1,2,: 98-105 (1982)), ethylene vinyl
acetate
2 5 (R. Langer et al., ld.) or poly-D- (-)-3-hydroxybutyric acid (EP 133,988).
Sustained-release compositions also include liposomally entrapped
COMPOSITIONS.
Liposomes containing COMPOSITIONS are prepared by methods known per se: DE
3,218,121; Epstein et al., Proc. Nati. Acad. Sci. U.S.A., $l: 3688-3692
(1985);
Hwang et al., Proc. Nati. Acad. Sci. U.S.A., 77: 4030-4034 (1980); EP 52,322;
EP
3 0 36,676; EP 88,046; EP 143,949; EP 142,641; Japanese Pat. Appln. 83-118008;
U.S. Pat. Nos. 4,485,045 and 4,544,545; and EP 102,324. Ordinarily, the
liposomes are of the small (about 200-800 Angstroms) unilamellar type in which
the lipid content is greater than about 30 mol. percent cholesterol, the
selected
proportion being adjusted for the optimal COMPOSITIONS therapy.
3 5 For parenteral administration, in one embodiment, the IGF-I and GH are
formulated generally by mixing each at the desired degree of purity, in a unit
dosage
injectable form (solution, suspension, or emulsion), with a pharmaceutically
acceptable carrier, i.e., one that is non-toxic to recipients at the dosages
and
concentrations employed and is compatible with other ingredients of the
formulation.
CA 02291983 1999-11-19
23
For example, the formulation preferably does not include oxidizing agents and
other
compounds that are known to be deleterious to polypeptides.
Generally, the formulations are prepared by contacting the COMPOSITIONS
each uniformly and intimately with liquid carriers or finely divided solid
carriers or
both. Then, if necessary, the product is shaped into the desired formulation.
Preferably the carrier is a parenteral carrier, more preferably a solution
that is
isotonic with the blood of the recipient. Examples of such carrier vehicles
include
water, saline, Ringer's solution, and dextrose solution. Non-aqueous vehicles
such as
fixed oils and ethyl oleate are also useful herein, as well as liposomes.
1 0 The carrier suitably contains minor amounts of additives such as
substances
that enhance isotonicity and chemical stability. Such materials are non-toxic
to
recipients at the dosages and concentrations employed, and include buffers
such as
phosphate, citrate, succinate, acetic acid, and other organic acids or their
salts;
antioxidants such as ascorbic acid; low molecular weight (less than about ten
1 5 residues) polypeptides, e.g., polyarginine or tripeptides; proteins, such
as serum
albumin, gelatin, or immunoglobulins; hydrophilic polymers such as
polyvinylpyrrolidone; amino acids, such as glycine, glutamic acid, aspartic
acid, or
arginine,; monosaccharides, disaccharides, and other carbohydrates including
cellulose or its derivatives, glucose, mannose, or dextrins; chelating agents
such as
2 0 EDTA; sugar alcohols such as mannitol or sorbitol; counterions such as
sodium,
andlor nonionic surfactants such as polysorbates, poloxamers, or PEG.
The COMPOSITIONS are each typically formulated individually in such vehicles
at a concentration of about 0.1 mg/mI to 100 mg/mI, preferably 1-10 mg/mI, at
a
pH of about 4.5 to 8. Full-length IGF- I is generally stable at a pH of no
more than
2 5 about 6; des(1-3)- IGF-I is stable at about 3.2 to 5; hGH and GHBP are
stable at a
higher pH of, e.g., 6.0-7.8. It will be understood that use of certain of the
foregoing
excipients, carriers, or stabilizers will result in the formation of IGF-I or
GH salts.
In addition, the COMPOSITIONS preferably the full-length IGF- t, are suitably
formulated together in a suitable carrier vehicle to form a pharmaceutical
3 0 composition that does not contain cells. In one embodiment, the buffer
used for
formulation will depend on whether the composition will be employed
immediately
upon mixing or stored for later use. If employed immediately after mixing,
theCOMPOSITIONS can be formulated in mannitol, glycine, and phosphate, pH 7.4.
If
this mixture is to be stored, it is formulated in a buffer at a pH of about 6,
such as
3 5 citrate, with a surfactant that increases the solubility of the GH at this
pH, such as
0.1% polysorbate 20 or poloxamer 188. The final preparation may be a stable
liquid
or lyophilized solid.
The COMPOSITIONS to be used for therapeutic administration must be sterile.
Sterility is readily accomplished by filtration through sterile filtration
membranes
CA 02291983 1999-11-19
24
(e.g., 0.2 micron membranes). Therapeutic COMPOSITIONS (IGF-I, IGF-1 + binding
protein, GHBP + GH and GH compositions) generally are placed into a container
having a sterile access port, for example, an intravenous solution bag or vial
having a
stopper pierceable by a hypodermic injection needle.
COMPOSITIONS ordinarily will be stored in unit or multi-dose containers, for
example, sealed ampoules or vials, as an aqueous solution or as a lyophilized
formulation for reconstitution. As an example of a lyophilized formulation, 10-
m1
vials are filled with 5 ml of sterile-filtered 1% (w/v) aqueous GH solution,
and the
resulting mixture is lyophilized. The infusion solution is prepared by
reconstituting
1 0 the lyophilized GH using bacteriostatic Water-for-Injection.
GHBP plus GH ordinarily will be stored in unit or multi-dose containers, for
example, sealed ampoules or vials, as an aqueous solution or as a lyophilized
formulation for reconstitution. As an example of a lyophilized formulation, 10-
mi
vials are filled . with 5 ml of sterile-filtered 1% (w/v) aqueous IGF-I
solution, and
1 5 the resulting mixture is lyophilized. The infusion solution is prepared by
reconstituting the lyophilized GHBP plus GH using bacteriostatic Water-for-
Injection. The GHBP+GH formulation may further contain mannitol, glycine, a
buffer, and a non-ionic surfactant. The formulation of the subject invention
may
optionally include one of several types of non-ionic surfactants, such as the
2 0 polysorbates (e.g. polysorbate 20, 80, etc.) and the poloxamers (e.g.
poloxamer
188). When polysorbate 80 is used the molar ratio of GHBP+GH:polysorbate 80 is
1:0.07-30, advantageously 1:0.1-10, and most advantageously 1:3. On a weight
to
volume basis, polysorbate 80 is added in amounts of about 0.001 to about 2%
(w/v),
in order to enhance further the stability of the hGH. Polysorbate 80, in
2 5 concentrations above 0.01% (wlv) reduces the amount of aggregation forming
upon
lyophilization. In addition to improved shell life, the surfactant containing
formulation of the subject invention inhibits the formation of protein
aggregates
when the reconstituted formulation is shaken.
When GHBP+GH is administered, it must contain one or more of its binding
3 0 proteins. A well characterized such binding protein is the high-affinity
growth
hormone binding protein (GHBP) constituting the extracellular domain of the GH
receptor that circulates in blood and functions as a GHBP in several species
(Ymer and
Herington, Mol. Cell. Endocrino., 41-:153 (1985); Smith and Talamantes,
Endocrinology, M: 1489-1494 (1988); Emtner and Roos, Acta Endocrinologica
3 5 (Copenh.), I-LL: 296-302 (1990)], including man (Baumann et al., J. Clin.
Endocrinol. Metab., fiZ: 134-141 (1986); EP 366,710 published 9 May 1990;
Herington et al., J. Clin. Invest., jL: 1817-1823 (1986); Leung et al.,
Nature,
M: 537-543 (1987)). A second BP with lower affinity for GH has also been
CA 02291983 1999-11-19
described that appears to be structurally unrelated to the GH receptor
(Baumann and
Shaw, J. Clin. Endocrinol. Metab., ZQ: 680-686 (1990)).
Novel formulations of zinc, GHBP and GH result in a stable composition
suitable for prolonged storage, and for therapeutic administration.
Therapeutic
5 formulations containing the Zn2+ ion are stable allowing therapeutic
administration
of the formulation. The formulation aspect of the present invention is thus
directed to
such formulations, and to all associated formulations and as a means for
effectively
stabilizing GHBP+GH. The formulation contains zinc, and substantially pure
GHBP
and GH free of contaminating proteins or infectious agents found in humans.
1 0 Formulations of the presei-,; invention may additionally contain a
pharmaceutically
acceptable buffer, amino acid, bulking agent and/or non-ionic surfactant.
These
include, for example, buffers, chelating agents, antioxidants, preservatives,
cosolvents, and the like; specific examples of these could include,
trimethylamaine
salts ('Tris buffer'), and disodium edetate.
1 5 hGH Comcositions
As used herein, the terms 'human growth hormone' or 'hGH' denote human
growth hormone produced, for example, from natural source extraction and
purification, and by recombinant cell culture systems. The native sequence of
hGH
and its characteristics are set forth, for example, in Hormone Drugs,
Gueriguigan et
2 0 al., U.S.P. Convention, Rockville, MD (1982) The terms likewise cover
biologically
active human growth hormone equivalents; e.g., differing in one or more amino
acid(s) in the overall sequence. Further, the terms as used in this
application are
intended to cover substitution, deletion and insertion amino acid variants of
hGH, or
post translational modificationsExamples of such variants are described in PCT
Pub.
2 5 W090/04788 published 3 May 1990. The hGH used in the formulations of the
present invention is generally produced by recombinant means as previously
discussed. The formulation of recombinant GHBP+GH is substantially pure, free
of
other human proteins, free of infectious agents such as the human
immunodeficiency
virus (HIV) and it is soluble. 'Substantially pure' GHBP+GH means GHBP and GH
3 0 that is free of proteins with which it ordinarily is associated in bodily
fluids such as
blood, plasma and serum. Ordinarily, substantially pure means GHBP and GH
which
is greater than about 95% pure by weight of total protein, and preferably
greater
than - 98% pure by weight.
Formulation Amino aclds
3 5 In an alternative formulation embodiment, a pharmaceutically acceptable
amino acid, for example glycine, is added to the GHBP and GH:zinc ion
formulation.
When glycine is present, the molar ratio of GHBP+GH:glycine is 1:5-600 In
addition
to glycine, amino acids such as alanine, glutamine, asparagine, arginine or
lysine or
derivatives of such amino acids may be used in the subject formulation. Such
amino
CA 02291983 1999-11-19
26
acids are particularly advantageous when lyophilizing the formulation to
create a
sufficient mass to form a stable, dry caked formulation.
Non-Ionic Surfactant
In another embodiment a non-ionic surfactant is added to the GHBP+GH
formulation. The formulation of the subject invention may optionally include
one of
several types of non-ionic surfactants, such as the polysorbates (e.G.
polysorbate
20, 80 etc.) and the poloxamers (e.g. poloxamer 188). Advantageously
polysorbate
80 is used, and the molar ratio of hGH:polysorbate 80 may be 1:0.03-60. On a
weight
to volume basis, polysorbate 80 is added in amounts of about 0.001 to about 2%
1 0 (w/v), in order to enhance further the stability of the GHBP and GH.
Polysorbate
80, in concentrations above 0.01% (wlv) may reduce the amount of inactive
aggregates forming upon lyophilization and reconstitution. The use of non-
ionic
surfactants improves formulation stability when exposed to shear and surface
stresses without, causing denaturing of the protein. Further, such surfactant
1 5 containing GHBP and GH formulations, may be employed in aerosol devices
such as
those used in a pulmonary dosing, and needleless jet injector guns. Such
delivery
formulations may be improved by the addition of non-ionic surfactants in the
range of
0.1-5% (w/v).
As used herein, the expression mammal refers to any mammal but especially
2 0 primates, bovine, ovine, canine, feline, equine and rodentia. Specifically
it includes
human, cows, horses, rats, mice, rabbits, monkeys, cats, dogs and pigs. The
term
avain refers to any bird, particularly chicken, turkey, duck and gonse.
The foregoing written specification is considered to be sufficient to enable
one
skilled in the art to practice the invention. The present invention is not to
be limited
2 5 in scope by the constructs deposited, since the deposited embodiment is
intended to
illustrate only certain aspects of the invention and any constructs that are
functionally equivalent are within the scope of this invention. The deposit of
material
herein does not constitute an admission that the written description herein
contained
is inadequate to enable the practice of any aspect of the invention, including
the best
3 0 made thereof, nor is it to be construed as limiting the scope of the
claims to the
specific illustrations that they represent. Indeed, various modifications of
the
invention in addition to those shown and described herein will become apparent
to
those skilled in the art from the foregoing description and fall within the
scope of the
appended claims. The following examples are intended to illustrate the best
mode now
3 5 known for practicing the invention, but the invention is not to be
considered limited
to these examples.
CA 02291983 1999-11-19
27
EXAMPLE 1
SELECTION OF GHBP ASSAY ANTIBODY
Two distinct antibodies are required for the LIFA method. The first antibody
is
a capture antibody which coats the solid phase and is used to selectively
remove the
HBP from the biological sample being assayed. This antibody must be specific
for
epitopes which do not hinder the binding of the ligand hormone. The second
detection
antibody is specific for an epitope on the ligand hormone. This second
detection
antibody must bind the ligand hormone at a site that does not hinder its
ability to
complex with the HBP. In the case of GHBP, known commercially available
1 0 monoclonal antibodies were screened for the desired binding properties.
The detection
monoclonal antibody specific for hGH was newly created in a mouse hybridoma
system.
Coat MAb selection
For assaying for the presence of GHBP a capture antibody which binds GHBP is
1 5 needed to coat the solid phase, in this case a microtiter plate well, for
binding the
GHBP. Five mouse anti-GHBP MAbs (1, 5, 7, 43, and 263) were evaluated at their
optimal coat concentration, first to determine their binding sites on GHBP
relative to
GHBP-GH i.e. sequential assay and their capacity to bind to GHBP in the
presence of
circulating GH-1125 (simultaneous incubation format). They were then examined
20 based on their capacity to bind to GHBP-GH-1125 complex (pre-incubation
experiment). Since both MAbs 1 and 7 show very weak binding in the sequential
and
simultaneous assay format, only MAbs 5, 43 and 263 were tested in the pre-
incubation experiment. Figure 2 shows the percent bound for each MAb under
three
different assay configurations. The data show that MAb 263, which gave the
highest
2 5 bound in all three conditions is the most suitable MAb as coat since it is
able to bind to
free GHBP as well as GHBP in complex with GH. More importantly, the sequential
incubation experiment showed that the MAb 263 did not interfere with the
present
hGHBP-hGH binding site.
Figure 3 shows a comparison of two standard curves generated with and
3 0 without preincubation with hGH. One set of standards were incubated with
hGH (final
concentration 200ng/ml) over night at 4 C, the control standards were
incubated
with assay buffer. The samples so generated were then assayed in the LIFA
according
to the standard protocol i.e. all samples were exposed to hGH on the
microtiter plates.
As seen in Figure 3, similar results are obtained whether or not the GHBP is
3 5 preincubated with hGH. The binding of the GHBP to the coat antibody and
the
saturation of the GHBP with it's ligand can be carried our simultaneously by
coincubating the samples and the hGH in the microtiter wells. The simplified
LIFA
gave a standard curve similar to that obtained with two separate steps but
required
only half the incubation time (2h vs 4h, Fig 1, third box down).
CA 02291983 1999-11-19
~-. ~ .
. , ~
28
The MCB, see below (Detection MAb Selection), which was used for HRPO-
conjugation, binds to the 22kDa-GH with high affinity, but has very low
affinity for
the 20kDa and the possible interference by 20k0a-GH in the assay was
consequently
tested. Figure 4 shows that the addition of 200ng/ml of the 20kDa-hGH to the
200ng/ml hGH solution results in a standard curve similar to that obtained by
incubation with 200ng/ml of hGH. This shows that the 20kDa-GH does not
interfere
in the present GHBP assay to any substantial degree.
Detection MAb Selection
For assaying for the presence of hGH, a detection antibody is needed with high
1 0 affinity for an epitope on the hGH which will not hinder the binding of
the hGH to the
GHBP. Monoclonal antibody was made in a mouse system using recombinant hGH and
screened for the required specificity. The best mouse monoclonal antibody was
produced by a hybridoma designated HGH-B. This hybridoma was deposited with
the
ATCC as previously discussed.
1 5 EXAMPLE 2
LIFA ASSAY PROCEDURE FOR GHBP
The LIFA assay procedure developed for measuring the GHBP is as follows.
Ninety-six-well microtiter plates (Corning Glass Works, Corning, New York)
were
coated with MAb 263 by incubating overnight at 4' C with 100 Ilwell of
antibody at
2 0 20 g/mi in 50mmol/liter of sodium carbonate buffer, pH 9.6 (coat buffer)
(see
Figure 1, step 1). After removal of the coating solution, the coated plates
were
blocked with 150 i per well of 5g/liter of BSA in PBS for lh at room
temperature
(Figure 1, step 2), and washed six times with 0.5g/liter of Tween 20 in PBS
(wash
buffer).
2 5 Standards diluted in assay buffer or samples (50 M serum or plasma and
50 M assay buffer) were dispensed onto the coated wells (100 l/well) (Figure
1,
step 3). Plates were sealed and incubated at room temperature for 2h with
gentle
agitation. Plates were washed six times with wash buffer. Recombinant hGH at
200ng/ml or assay buffer was then added (100 l/well) and incubated at room
3 0 temperature for 2h. Plates were washed six times with wash buffer before
addition
of horseradish peroxidase (HRPO) labelled MAb MCB (100 i/well) Figure 1, step
4). After further incubation for 2h at room temperature, the plates were
washed six
times with wash buffer. Freshly prepared substrate solution (0.4g of
o-phenylenediamine dihydrochloride in one liter of PBS plus 0.4 ml of 30%
hydrogen
3 5 peroxide) was added to the plates (100 l per well) and incubation carried
out in the
dark for 15 min at room temperature Figure 1, step 5. The reaction was stopped
by
the addition of 100 I of 2.25mol/L sulfuric acid and absorbance at 490nm
determined on a Vmax plate reader (Molecular Devices, Menlo Park, CA). A
standard
curve was generated by plotting absorbance vs. log of GHBP concentration,
using a 4-
CA 02291983 1999-11-19
.~.
29
parameter nonlinear regression curve fitting program. Sample concentrations
were
obtained by interpolation of their absorbance on the standard curve.
EXAMPLE 3
PROPERTIES OF THE GHBP ASSAY
The GHBP assay was evaluated and the range, sensitivity, specificity, and
precision of the assay determined.
The specificity of this assay was tested by substituting the GHBP with four
other soluble receptors (rCD4, rHER2 ECD, EGF-receptor and rPRL-receptor) and
an unrelated protein produced in CHO cells (HIV envelope protein, gp120). All
four
1 0 proteins were obtained from Genentech Inc. The results (Table 1) showed
that the
assay has less than 0.01% cross-reactivity with these proteins. In addition,
cross-
reactivity with human placental lactogen (HPL) and human prolactin (PRL) was
tested. Substituting HPL and PRL for hGH at the same concentration (200nglml)
resulted in values undistinguishable from the blanks when when tested together
with
1 5 the GHBP or PRL-receptor.
TABLE I
CROSS-REACTIVITY
--------------------------------------------------
Protein Tested Concentration Cross-reactivitya
20 ---------------------
Tested Measured %
( g/mI) (ng/mI)b
----------------------------------------------------
rHER2 ECD 10.0 <0.6 <0.01
2 5 rCD4 10.0 <0.6 <0.01
rgp120 10.0 <0.6 <0.01
EGF-receptor 10.0 <0.6 <0.01
rPRL-receptor 10.0 <0.6 <0.01
---------------------------------------------------
3 0 a Percent cross-reactivity was calculated as concentration
measured / amount tested x 100.
b The concentration is claculated by using 26 kD as the molecular
weight of the rhGHBP
Assay precision
3 5 Serum samples with low, medium, or high GHBP concentrations were
analyzed in 24 replicates for the assessment of intra-assay precision (Table
IIA).
Interassay precision was determined by measuring samples of low, medium or
high
GHBP concentrations in ten separate experiments (Table 2B). The coefficients
of
CA 02291983 1999-11-19
. ' ~
intra-assay variation at all three levels ranged from 6.3 to 8.9% while the
coefficients of interassay variation ranged from 9.7 to 12.9%.
TABLE 2
PRECISION OF THE LIFA
5
A. tntra-assay Precision
Sample 1 Sample 2 Sample 3
1 0 Replicates 2 4 2 4 2 4
Mean (pmol/L) 138 268 61 4
S.D. (pmol/L) 9.2 16.9 54.6
C.V. (%) 6.6 6.3 8.9
B. Interassay = Precision
1 5 Sample 1 Sample 2 Sample 3
Replicates 1 0 1 0 1 0
Mean (pmol/L) 136 293 674
S.D. (pmol/L) 17.6 33.1 65.0
2 0 C.V. (%) 12.9 11.3 9.6
Linearity of the assay
The linearity of the assay was determined by making serial dilutions of serum
samples in assay buffer and measuring the concentration of GHBP. Results were
2 5 examined by correlating the observed concentration determined in the LIFA
with the
calculated concentration obtained by multiplying the dilution factor with the
concentration of the undiluted sample determined in the LIFA. Linear
regression
analysis of the samples resulted in correlation coefficients of 0.99 or
greater (Figure
5), indicating that the assay is linear.
3 0 EXAMPLE 4
DETERMINING GHBP IN BIOLOGICAL FLUIDS
Spike recovery
Purified GHBP at three different concentrations in assay buffer were added in
equal volume to four serum samples and four plasma samples. The generated
samples
3 5 were assayed in the LIFA. The theoretical concentration was calculated for
each mixed
sample and was used to calculate percent recovery. The data in Table 3A and
Table 3B
show an average recovery of 106.7 and 99.5 for serum and plasma respectively,
with
a range of 89.1 to 115.9%, demonstrating the accuracy of the assay.
CA 02291983 1999-11-19
C)
31
Saturation of the GH-BP with GH
Figure 3 shows a comparison of two standard curves generated with and
without preincubation with GH. One set of standards were incubated with GH
(final
concentration 200ng/ml) overnight at 4'C, the control standards were incubated
with assay buffer. The samples so generated were then assayed in the LIFA
according
to the standard protocol, i.e. all samples were exposed to GH on the
microtiter plates.
As seen in Figure 2, similar results are obtained whether or not the GHBP is
preincubated with GH.
Application of the LIFA method
1 0 Total and GH-bound GHBP levels in random serum samples from 16 healthy
adults and two patients with Laron type dwarfism are shown in Figure 6. GH-BP
levels were detectable in samples from all normal subjects (Figure 6; patients
1-
16). In contrast, GHBP concentrations were undetectable (< 30 pmol/L) in both
patients with Laron-type dwarfism (Figure 6: patients #17, #18).
CA 02291983 1999-11-19
~t )
32
TABLE 3A
ACCURACYa In serum
Spiked Sampleb Serum Sampleb Expectedc Observed % Recovery
mole/L mole/L mole/L mole/L
204 177 192 212 110.4
238 219 254 115.9
465 225 362 105.9
1138 671 735 109.5
658 177 419 412 97.6
238 446 500 111.1
465 562 577 101.8
1138 898 962 107.1
1427 177 802 762 95.0
238 832 931 112.0
465 946 965 102.1
1138 1282 1438 111.9
Average 106.7
Rarxje 95.0-115.9
a Equal volumn of three purified rGHBP (column #1) were each added to serum
samples
(column #2) and assayed in the LIFA.
b GHBP concentrations had been determined previously by LIFA.
C (spiked + serum)/2
CA 02291983 1999-11-19
33
TABLE 3B
ACCURACYa In lasma
Spiked Sampleb Plasma Sampieb Expectedc Observed % Recovery
moie/L moie/L mole/L mofe/L
142 100 121 112 92.6
150 146 154 105.5
300 221 231 104.5
569 355 335 94.4
435 100 267 254 95.1
150 292 323 110.6
300 367 327 89.1
569 502 500 99.6
835 100 467 458 98.1
150 492 538 109.3
300 567 531 93.6
569 702 715 101.9
Average 99.5
F-- Range 89.1-1 10.6
a Equal volumn of thred purified rGHBP (column #1) were each added to plasma
samples
(column #2) and assayed in the LIFA.
b GHBP concentrations had been determined previously by LIFA.
c (spiked + plasma)/2
EXAMPLE 5
MONITORING OF GHBP IN GROWTH PROMOTION
The LIFA of the present invention may be used to monitor the concentration of
GHBP in the biological fluids of a patient, and if the level is inadequate for
the desired
rate of growth, additional GHBP administered. An example of low GHBP levels is
in
Laron dwarfism; whereas a high GHBP levels may be present in patients with
excess
GH secretion. If the level GHBP is insufficient for the desired rate of
growth,
additional GHBP alone or complexed to hGH may be administered. The optimal
level of
GHBP may be determined by the methods discussed above, in combination with
CA 02291983 1999-11-19
34
measuring the level of GHBP in a se(es of normal healthy individuals. GHBP may
be
evaluated in any mammalian system, preferably in rodents and primates.
In Rodents
Two rodent models of GH deficiency are used: 1) rats where the gland
producing GH, the pituitary, is surgically removed (hypophy-sectomized rats)
and
2) animals genetically deficient in growth hormone (dwarf rats, Chariton, H.W.
e t
al., J. Endo.119:51-58 (19881). These rats are treated with human GH (hGH)
alone or hGH coupled to human GHBP which is produced recombinantly in E. coli
or
alternatively in mammalian 293 cells. Several indices of growth are measured
to
assess the effect of the binding protein on GH-induced body growth. Monitoring
of the
level of GHBP and GH is required to determine the metabolic fate of
administered
1 0 GHBP and complexed GH.
bycoohysectomized Rats
Recombinant hGHBP and hGH are given either alone or in combination to
hypophysectomized rats, a recognized model for measuring GH bioactivity
(Thorngren, K-G. & Hansson L.I. Acta. Endo. 11:653-668 (19771). Human GH
1 5 (0.03, 0.1 and 0.3 mg/kg, as 7 daily injections) induces a dose-related
weight gain
while injections of E. coli-derived hGHBP at these same 3 doses produces no
effect by
itself. However, co-administration of 0.3mg/kg hGHBP with 0.1mg/kg hGH not
only
gives greater weight gain than 0.1mg/kg hGH alone (p<0.01), but also induces
greater weight gain than three times more hGH (22.0 _ 3.6 vs 17.1 . 2.tg
2 0 respectively; mean _ s.d., p<0.01). Longitudinal bone growth parallels
body weight
gain. Thus, co-administration of 0.3mg/kg hGHBP and 0.1mg/kg hGH gives greater
bone growth than 0.3mg/kg hGH (102 . 14 vs 84 _ 17 microns/day; p<0.05), and
0.1mg/kg hGHBP plus hGH gives greater bone growth than hGH alone (99 f 6 vs 72
_ 17 microns/day; p<0.01).
2 5 The liver, spleen and kidney are all significantly larger following co-
adminstration of hGHBP (0.3mg/kg) with hGH (0.1mg/kg) than with 0.1mg/kg hGH
alone: liver (5.5 _ 0.4 vs 4.6 ;L 0.6g; p<0.01), spleen (292 _+_ 46 vs 240 :L
34mg;
p<0.05), and kidney (836 ;L 60 vs 716 . 57mg; p<0.05). The weights of liver,
spleen and kidney in excipient treated rats are 4.5 ;L 0.2g, 193 f. 32mg, and
687 ;L
3 0 58mg, respectively. These responses to hGH are at least doubled by hGHBP.
The
serum concentrations of IGF-1 and hGH 24h after the last injection are
markedly
elevated by co-administration of the two highest doses of hGHBP with hGH,
while
hGHBP causes as much as 20-fold more hGH to be present after 24h.
An ELISA (Fuh, G. et al., J. Biol. Chem. 265:3111-3115 (19901) for hGHBP
3 5 adapted for use in serum shows the reason for the persistence of the GH in
the blood
24 hours after the seventh subcutaneous bolus injection was given to the rats.
The
hGHBP is only detectable in the animals co-administered hGHBP and hGH. When
the
CA 02291983 1999-11-19
GHBP is given alone it disappears from the blood more rapidly than when the
GHBP is
given complexed to GH. These findings from measuring the GHBP in blood suggest
that
it is the persistence of the GH + GHBP complex in the blood of the rats that
causes
many or all of the above improved activities of GH. The LIFA method of the
present
5 invention is therefore used to follow GHBP during its preparation, storage,
use and in
body fluids following GHBP administration.
Dwarf Rat System
We also compared hGH and hGHBP in a dwarf rat which has a pituitary GH
content 5-10% of normal, grows slowly, and responds to GH treatment (Charlton,
1 0 H.W. et al., J. Endo. JJ.J: 51-58 (19881) Co-administration of 0.27mg/kg
of
hGHBP with 0.27mg/kg hGH increases weight gain compared to 0.27mg/kg hGH alone
(11.1 j. 4.2 vs 7.5 f 1.7g; p<0.05). Co-administration of all three doses of
hGHBP
with 0.27mg/kg of hGH significantly increasing bone growth compared to
0.27mg/kg
hGH alone (low 33.5 f 5.8, medium 38.6 t 8.6, high 35.5 ;L 5.0, vs 26.0 :L 4.1
1 5 microns/day; p<0.05). Serum IGF-1 concentrations are elevated by co-
administration even compared to 0.81mg/kg hGH alone (high hGHBP 136 :L 45 vs
90. _ 16ng per ml; p<0.05) as were hGH concentrations (high hGHBP, 609 t 240
vs
73 _ 22 pg per ml; p<0.0001).
GHBP From Mammalian 293 Cells
2 0 In marked contrast, hGHBP produced in human 293 cells completely inhibits
GH responses in hypophysectomized rats. Weight gains after 10 daily s.c.
injections
of hGH alone are 11.3 f 2.5, 16.4 , 2.1 and 21.1 _+_ 2.1g at 0.03, 0.1, and
0.3mg/kg/day respectively. When hGH at 0.03 and 0.1mg/kg/day is co-
administered with a 2-fold molar excess of 293-derived hGHBP this weight gain
is
2 5 abolished (3.0 _ 2.1 and 3.0 f 1.6g, respectively) compared to the hGH
and
excipient (2.3 :L 1.6g) groups. This difference between the growth responses
induced using these 2 forms of hGHBP may be due to a difference in hGH
clearance
from the circulation which is reduced about 10 fold for GHBP derived from E.
coli
(Moore, J.A. et a!, Proc. US Endocr. Soc. J~., Abstract #1652 (1989J).or
purified
3 0 from natural sources (Baumann, G. & Shaw, M.A., J. Clin Endocrinol.
Metab.,
j-L680-686 119901; Baumann, G., Shaw, M.A. & Buchanan, T.A., Metabolism.
=.330-333[1989).
The clearance (ml/min/kg) of hGH in normal male rats, following an i.v.
bolus of hGH alone is 18.6 :L 3.4, for hGH co-administered with GHBP from
rabbit
3 5 sera is 2.1 t_ 0.2, for GHBP from E. coli 1.9 :L 0.4, or from 293 cells
41.3 :L 16.7.
Therefore, for the 293-derived hGHBP the clearance of hGH is increased two
fold,
suggesting a correlation between in vivo potency and hGH clearance. The
decreased
clearance of hGH complexed to the E. coli -derived hGHBP may be due to the
complex
being of sufficient size (Mr>40 kDa) to escape filtration by the kidney.
Proteins
CA 02291983 1999-11-19
36
produced in 293 cells can have heterogenous carbohydrate patterns, possibly
due to
incomplete glycosylation, causing them to be rapidly cleared by the liver,
which may
explain the rapid clearance of 293-derived hGHBP.
In this instance the 293-derived GHBP therefore acts as an inhibitor of GH
action, compared to the enhancing activities of the E. coli-derived protein.
This
difference in activity appears to be due to the differing clearances of the
two
molecules from the blood. The present invention aids in similarly
discriminating
inhibitory and stimulatory binding proteins on the basis of their clearance
from the
blood.
1 0 Persistence of GHBP
This series of experiments shows the value of the knowledge gained from a
GHBP assay, and naturally follows from the above rat experiments. On the basis
of
the prolonged half-life of the E. coli-derived GH plus GHBP complex in blood,
the
GHBP allows GH to persist in the blood for sufficient time to allow less
frequent GH
1 5 injections when the GH is coupled to GHBP.
In 2 separate studies we inject hGH by itself, or combined and co-injected
with hGHBP, in GH deficient dwarf rats. In the first study hGH or hGH plus
hGHBP
are given daily or every 2 or 4 days for 8 days. The second study is designed
so that
hGH or hGH plus hGHBP are given daily or every 3 or 6 days for 18 days. In
both
2 0 studies hGHBP gives greater growth responses than hGH alone no matter the
injection
interval. These studies show that when hGH is injected with hGHBP the
injection
frequency can be greatly reduced, to once or twice a week, without reducing
the size
of the growth response.
Female dwarf rats (Study A, 12-15 weeks of age, 110-130g; Study B, 50
2 5 -70 days of age, 95-110g) are randomized into groups of 8 (Study A) or 7
(Study
B), and injected i.p. with tetracycline as an intravital marker of bone
growth. All
injections of GH or GHBP are given subcutaneously in a volume of 100
microlitres of
solution. In each study, all rats are given a daily injection of either
excipient or test
compounds and weighed daily.
3 0 The hGH used is rhGH (Genentech Lot N9267AX, G042A) dissolved in sterile
water. The GHBP used is produced in E. coli and purified. In this case the
GHBP has
an altered sequence to the GHBP used above, the molecule being produced by
removing
the exon 3 coding domain giving a 1-5, 27-238, peptide sequence.
In the first experiment the hGH and hGHBP are prepared to be injected in a
3 5 volume of 0.1 mI as:
a) hGH 0.25mg/ml or
b) hGH 1mg/ml
c) hGH 1mg/ml) + hGHBP (2mg/ml)
CA 02291983 1999-11-19
37
The 8 regimes of injection are:
1) Daily injections of a), b), and c),
2) Injections every 2 days of b) and c)
3) Injections every 4 days of b) and c)
4) Injections of excipient every day.
Therefore in this design the animals injected s.c. every 2nd day receive only
half the GH dose of the animals given daily injections and the rats injected
every 4th
day receive only a quarter of the cumuiative GH dose.
In the second experiment the hGH is prepared as:
1 0 a) 0.33mg/mi,
b) 1mg/ml, or
c) 2mg/ml.
The hGH + hGHBP solutions are 0.33, 1 or 2mg/mi of hGH combined with 2-
fold more hGHBP (0.66, 2 or 4mg/mi, respectively). The 9 regimes all injected
in
1 5 0.1mi s.c. are:.
1) Excipient control
2) Daily hGH injections 33 micrograms/day
3) Daily hG.H injections 100 micrograms/day
4) Daily hGH injections 33 micrograms/day + 66 g of GHBP
2 0 5) Daily hGH injections 100 micrograms/day + 200 g of GHBP
6) Every 3rd day hGH injections 100 micrograms/shot
7) Every 6th day hGH injections 200 micrograms/shot
8) Every 3rd day injections 100 micrograms hGH + 200 g GHBP/shot
9) Every 6th day injections 200 micrograms hGH + 400 g GHBP/shot
2 5 Therefore, in this design the animals injected s.c. every 3rd or 6th day
receive the
same cumulative GH dose (0.33mg/kg/day) as those injected s.c. with the low
dose of
GH.
The response to hGH is increased at all frequencies of injection by combining
hGHBP with the hGH. It is surprising that in study A despite decreasing the
injection
3 0 frequency from daily to every 2 days, and thereby reducing the cumulative
hGH dose
by half, the weight gain response GHBP + GH injections is the same. For hGH
injections the weight gain response is markedly reduced when the injections
are
given every 2 or 4 days. The weight gain in response to eight daily injections
of
0.25mg/kg hGH is identical to that for only 2 injections of the same
cumulative dose
3 5 of hGH given every 4 days.
In the second Experiment, two doses of hGH are given daily with or without a
2-fold excess of hGHBP. The response to hGH is greatly increased by combining
hGHBP with the hGH (see below). (In a subsequent study in the dwarf rat the
maximal weight gain response to hGH was increased when the hGH was complex
with
CA 02291983 1999-11-19
. ~ .~
38
and injected daily s.c. with hGHBP.) As in the previous experiments GHBP
improved
the IGF-1 response to the GH. This appears to be due to the GHBP-GH complex
causing a preferential and disproportionate growth of the liver, an activity
lacking
when GH is delivered alone. Giving the same total dose of hGH at 3 or 6 day
intervals
gives a poor growth response (compared to hGH given daily, Group 2). The
weight
gain response to combined treatment with GH + GHBP is much greater. The effect
of
daily hGH alone is directly compared with hGH + hGHBP given every 3 or 6 days.
Infrequent injections of the hGHBP-hGH combination are as effective as daily
injections of hGH. These data clearly show that the co-administration of hGH +
1 0 hGHBP allows the growth response to the hGH to be maintained with
infrequent
injection regimes. Co-administration of GH + GHBP allows the interval between
injections to be extended to 6 days (weekly) without a loss of activity on
longitudinal
bone growth (measured by the tetracycline labelling technique) compared to
injections of the, same dose of GH injected daily. The co-administration of GH
+ GHBP
1 5 also allows a smaller dose of GH to be given less frequently for an
equivalent growth
response (injections every 2 or 3 days at 1/2 to 1/3 the dose in the rat).
STUDY B: Bone Growth and Weight Gain in 18 days
----------------------------------------------------------------
Group Bone Growth (microns) Weight Gain (g)
2 0 &SD &SD
1) Excipient 34.0 8.0 10.3 3.6
2) low GH 53.8 8.3 27.8 3.3
3) Hi GH 62.1 8.4 34.0 5.4
2 5 4) Low GH + GHBP 73.4 9.7 28.1 4.9
5) Hi GH + GHBP 95.1 6.5 47.0 7.4
6) GH / 3 days 37.7 10.3 15.2 3.7
7) GH / 6 days 36.5 1 1. 7 16.8 6.2
8) GH + GHBP / 3 days 56.9 15.8 28.0 6.4
3 0 9) GH + GHBP / 6 days 47.0 4.6 18.5 4.8
----------------------------------------------------------------
In Primates
In normal juvenile Rhesus monkeys GHBP was monitored after administration
of GHBP + hGH or after hGH alone. The somatogenic and anabolic response was
3 5 determined by measuring IGF-1 concentrations. The monkeys received either
hGH
daily or hGHBP+ hGH weekly, and there was an excipient injected control group.
The
results, primarily from blood IGF-1 concentrations, showed that GHBP enhances
the
biologically activity of GH in primates. The dose of hGH (administered with
twice the
molar ratio of hGHBP) was 0.35 mg/kg injected weekly, the doses of hGH
injected
4 0 daily were 0.05mg/kg or 0.35mg/kg. Serum analyses indicated that the
maximum
IGF-1 response to hGH + hGHBP is greater than for hGH alone at either dosage.
As
measured, the hGH serum life when administered in combination with hGHBP was
CA 02291983 1999-11-19
39
increased 2.2 times over that of hGH administered alone. The administration of
0.35mg/kg hGH daily stimulated serum IGF-1 less than a single weekly
administration of hGH (0.35mg/kg) complexed with hGHBP at a 2:1 molar ratio.
Weekly administration of hGH+hGHBP (1:2 molar ratio, given subcutaneously as a
bolus), resulted in a physiological response greater than daily hGH
administration of
the same hGH dose, or a seven-fold greater total dose. Therefore, lesser
amounts of
hGH can be administered and less frequent injections given if hGH is complexed
with
hGHBP. In summary, in the rat and in the monkey, GHBP enhances GH activity, so
that in humans a similar enchancement is expected.
1 0 The mechanism of the above effects of the GHBP on body growth could be
explained by the greatly delayed absorption of the GH-GHBP complex from s.c.
injections and then the delayed clearance from the blood of the GH-GHBP
complex.
Both these effects are demonstrated. The magnitude of this effect is quite
surprising
as the absorption of free GHBP was similar to that for GH alone. These
1 5 pharmacokinetic mechanisms explain a large part of the increased activity,
and the
ability to give less frequent injections of the GHBP + GH complex. These
discoveries
are surprising as there is no prior art showing that increasing the half-life
of GH in
the blood would inevitably lead to an increased activity. If a molecule is
retained in
the blood its access to tissues will be limited, yet degradation of the
molecule in the
2 0 blood will continue. If the GH is bound to the GHBP access of the GH to a
cellular GH
receptor would be expected to be modified. It is clear that for the molecule
to be
active on tissues it must pass from the bloodstream into the tissues, so that
there are
limits to the degree of delayed clearance that is desirable.
EXAMPLE 6
2 5 Anatysis of 24-hour Plasma Profiles of GHBP, GH/GH-GHBP-complex
and GH In Healthy Children
We have used the LIFA to measure GHBP levels in plasma profiles from
healthy children. GH was measured by IRMA. Fifteen 24h plasma profiles from 12
healthy children (3 girls and 9 boys) of different ages (6-17 years), heights
(-3.7
3 0 to +3.5 SDS) and pubertal stages (1 to 4) were examined. Blood was
withdrawn
continuously for 24h and collected in 20 min fractions. Time series for GH,
GHBP and
GH/GHBP-complex were analyzed by cross-correlation and Fourier analysis. GH
was
secreted in a pulsatile fashion in all subjects. The concentration of the
GH/GHBP-
complex - varied during the sampling period, and the changes correlated
significantly
3 5 with the GH pulses with correlation coefficients reaching maximum at zero
time lag.
In contrast, the changes in the total GHBP concentration were minor (CV-10%),
and
not correlated to GH pulses. Fourier analysis showed similar spectral power
patterns
for GH and GH/GHBP-complex, suggesting a diurnal rhythm (12-24h periods) as
well as components of higher frequencies (around 4 h periods). In spite of the
subtle
CA 02291983 1999-11-19
fluctuations in the total GHBP concentration, Fourier transformation revealed
a
marked diumal rhythm, while components of higher frequencies were much less
abundant. We conclude that the variations in total GHBP during a 24h sampling
period
are small and that the levels can be estimated from a single random blood
sample.
5 Materlats and Methods
Subjects
Twelve children, 3 girls and 9 boys, of different ages (6-15 years old),
heights (-3.7 to +3.5 SDS) and pubertal stages (stage 1-4), were investigated
at the
Children's Hospital, Gbteborg, Sweden. Two of the subjects were studied on
more than
1 0 one occasion (Table 4). Height at the time of the study was expressed in
SD scores
compared to normal Swedish children (Karlberg P, Taranger J, Engstrdm 1,
Lichtenstein H, Svennberg-Redegren 1. The somatic development of children in a
Swedish urban community. Acta Pediatrica Scand. 1976; Suppl. 258:1). All
children
were healthy and well nourished, and had normal thyroid, liver and kidney
function.
1 5 Coeliac disease was excluded. Children with classical GH deficiency were
not included
in the study. The testicular volume was measured by orchidometer, and the
pubertal
stages were classified according to Tanner (Tanner JM, Whitehouse RH. Clinical
longitudinal standards for height velocity, weight velocity and stages of
puberty.
(Arch. Dis. Child. a:170-17911976)).
CA 02291983 1999-11-19
.... ~õ
41
TABLE 4
SUBJECT CHARACTERISTICS
Profile No Subic& &a AM Briglu Wei~ht $I&M
iim Lsm wLa_mh
1 A Female 60/12 +2.5 +2.0 1 1
2 B Female 12 8/12 -1.9 -2.0 1 1
3 C Female 13 3/12 -1.0 0 3 2
4 D Male 7 11/12 -0.3 +0.5 1 ml 1
E Male 11 0/12 -2.3 -2.0 2 ml 1
6 F Male 11 2/12 -2.0 -1.5 3 ml 1
7 G Male 11 3/12 +3.0 +2 2 ml I
8 H Male 13 0/12 +1.0 +1.0 15 ml 3
9 H Male 13 9/12 +0.8 +0.8 15 ml 4
I Male 12 5/12 +0.7 +0.1 4 ml 1
11 I Male 12 6/12 +0.7 0 4 ml 1
12 I Male 13 3/12 +0.4 -0.4 5 ml 1
13 J Male 14 0/12 -1.8 -1.8 6 ml 1
14 K Male 14 7/12 -2.5 -1.5 12 ml 3
L Male 14 8/12 -3.7 -3.2 5 ml 2
aBreast development (B), testicular volume (T). bPubic hair (PH).
Study crotocol
5 The Children stayed at the hospital for at least 2 days. They were given a
normal diet, with breakfast at 08.OOh, lunch at 12.OOh, dinner at 17.OOh, and
were allowed normal activity and sleep. A heparinized needle (Carmeda,
Stockholm,
Sweden) was inserted on the first evening. The following morning, at 08.OOh-
09.OOh, blood withdrawal began through a thrombogenic catheter (Carmeda)
inserted
1 0 through the needle and connected to a constant withdrawal pump (Swemed AB,
G6teborg, Sweden). The rate of withdrawal was 0.5-2 mI/h and the volume of the
tubing was 0.1-0.2 ml. The heparinized reservoir tubes were changed every 20
min
for 24h, thus giving 72 samples. The blood samples were kept at room
temperature
and centrifuged within 24h. After centrifugation the plasma was frozen until
assayed.
1 5 GH measurements
Plasma GH concentration was determined in duplicate using a polyclonal
antibody-based IRMA (Pharmacia, Sweden) and the WHO First International
CA 02291983 1999-11-19
. , +
42
Reference Preparation hGH 66217 as standard. lntra-assay variation was 3.2 to
3.5
% at GH levels between 2-100 mU/L. Interassay variation was 5.0 % and 2.7 % at
GH concentrations of 10 mU/L and 40 mU/L, respectively. When appropriate, a
conversion factor of 2.7U/mg, and a molecular weight of 22 000 was used to
express
GH concentrations in pmol/L.
GHBP measurements
Total GHBP was measured by LIFA as previously discussed in detail . Briefly,
ninety-six-well microtiter plates (Corning Glass Works, Corning, New York)
were
coated with a monoclonal antibody directed against GHBP (MAb 263, Agen,
Australia)
1 0 by incubating overnight at 4 C with 100 LAwell of antibody at 10 g/m L
in coat
buffer. The coated wells were blocked and washed. Standards (recombinant
hGHBP,
Genentech Inc.) or samples (50 L per well) were dispensed into the coated
wells
containing 50 p I/ well of 200 ng/ml rhGH. Plates were sealed, incubated at
room
temperature for 2 h with gentle agitation, then washed before addition of a
monoclonal
1 5 anti-hGH antibody (MAb MCB, Genentech Inc) conjugated to horseradish
peroxidase
(100 l/well). After further incubation for 2 h at room temperature, the
plates
were washed six times with wash buffer. Freshly prepared substrate solution
(0.4 g
of o-phenylenediamine dihydrochloride in one liter of PBS plus 0.4 mL of 30%
hydrogen peroxide) was added to the plates (100 I per well) and the
incubation
2 0 carried out in the dark for 15 min at room temperature. The reaction was
stopped by
the addition of 100 l of 2.25 mol/L sulfuric acid and the absorbance at 490
nm
determined. The same procedure, without the addition of rhGH to the samples,
was
used to measure the plasma concentration of the GH/GHBP-complex. The detection
range in the LIFA was 15.6 to 1000 pmol/L. The intra- and interassay
coefficients of
2 5 variation were 7.3 % and 11.3 %, respectively.
Statistical ana(vS11
The rhythmicity of the 24h profiles was analyzed by Fourier transformation.
The original GH, GHBP and GH/GHBP-complex concentration time series were
smoothed with a 3-point moving average (weights w-1= w+ 1=1 /4, wo =1 /2) in
30 order to reduce the influence of high frequency components. The smoothed
series
were analyzed as Fourier expansions(Chatfield C. The analysis of time series.
Chapman and Hall, London. 1989). The results are expressed as a power
spectrum,
where the amplitude is plotted as a function of frequency. To analyze the data
for
correlations between GH, GHBP and GH/GHBP-complex, cross-correlation followed
3 5 by Box-Jenkins autoregressive modeling (Haugh L, Box GEP. Identification
of
dynamic regression (distributed lag) models connecting two time series.
Journal of
the American Statistics Association. 1977; 72:121-130) was used.
CA 02291983 1999-11-19
43
Results
Plasma crofiles of GH GHlGHBP-comolex and total GHBP:
Twenty-four hour plasma profiles of GH, GHlGHBP-complex and total GHBP
from one representative subject (profile t- 15) are shown in Fig. 7. Plasma
concentrations of both GH (top panel) and GH/GHBP-complex (middle panel)
varied
over a wide range during the sampling period, and the changes appeared to be
synchronized in time. In contrast, the total concentration of GHBP (lower
panel) was
much less variable, and did not seem to be influenced by the changes in GH and
GH/GHBP-complex concentration.
1 0 Plasma concentrations of GH, GHBP and GH/GHBP-complex
Plasma concentrations of GH, GHBP and GH/GHBP-complex are shown in Table
5. The values given are the mean and coefficients of variation (CV) for each
individual 24h profile as well as the group average. The mean concentration of
GHBP,
GH/GHBP-complex and GH varied among different individuals (range 109-
1 5 247pmol/L, 13-109 pmol/L and 32-215 pmol/L for GHBP, GH/GHBP-complex and
GH, respectively). The highly pulsatile nature of GH and GH/GHBP-complex
plasma
profiles were reflected in their high CV (156.0% and 47.2%, respectively). The
percentage of GH that was bound to GHBP in high GH peaks (250 pmol/L) was
14.6%
The concentration of total GHBP was much less variable during the sampling
period,
2 0 as illustrated by the low coefficient of variation (9.6 %) (Table 5). The
complete
24h profiles of total GHBP concentration from all subjects illustrated that
levels
vary among individuals and that the concentration for each subject is
relatively
constant throughout the day.
CA 02291983 1999-11-19
}44
Table 5
Mean plasma concentration of total GHBP, GH/GHBP-complex and GH.
Total GHBP GH/GHBP -complex GH P
Profile No Mean CV Mean CV Mean CV
(pmol/L) M (Pmol/I-) (%) (lxnol/f-) (%)
1 109.3 9.4 34.8 25.6 73.1 129.6
2 235.2 9.5 12.9 113.9 83.3 178.5
3 179.0 12.7 26.2 111.0 99.8 128.9
4 148.5 8.1 24.1 25.8 40.4 107.7
198.9 7.8 47.3 51.0 167.9 176.7
6 160.1 8.6 25.7 28.6 36.6 160.7
7 215.0 5.5 39.8 22.5 31.6 151.0
8 247.4 7.7 108.8 15.3 215.2 121.6
9 217.5 9.3 80.1 17.2 189.1 133.7
126.2 11.8 28.2 46.4 163.4 151.3
11 120.4 13.8 20.7 37.0 101.9 125.7
12 168.7 9.2 34.3 43.4 99.8 119.4
13 130.0 11.1 29.5 41.5 140.9 154.9
14 191.1 11.8 19.8 107.5 103.7 99.1
.U 122.8 ZiSZ 4.~.4 2L2 32a 15315
Mean: 176.5 9.6_+0.6 38.5 47.2+9.2 110.0 139 6.3
Discussion
5 The objective in monitoring GIiBP was to investigate the possible diurnal
variations in the plasma concentration of GHBP in healthy children, and to
determine
if fluctuations in GHBP concentrations were correlated with the episodic
release of
GH. The subjects included in our study differed in age, sex, pubertal stage,
height and
GH levels. From a practical point of view we believe that a most useful
discovery
1 0 from the data is that total GHBP concentration shows only minor variations
during a
24h sampling period, implying that a single blood sample should give a good
estimation of the total GHBP level. Nevertheless, rigorous analysis of the
data
revealed that the small (CV-10 %) fluctuations in total GHBP plasma
concentration
account for a significant circadian rhythm. No significant cross-correlation
between
1 5 GH pulses and changes in total GHBP concentration was found. In contrast,
the plasma
concentration of GH/GHBP-complex showed rapid fluctuations, which were highly
correlated with the changes in GH concentration. Fourier analysis showed that
the
CA 02291983 1999-11-19
plasma patterns of both GH and GH/GHBP-complex follow a diumai rhythm but also
possess components of higher frequencies (around 4h periods).
The described LIFA, which was used for the GHBP measurements has the
advantages that only functional GHBP is detected and that endogenous GH, which
5 fluctuates rapidly over a wide range, does not affect the measurement of
total GHBP
concentrations. The assay can also measure the concentration of the GH/GHBP-
complex. It was recently reported that when the ratio of GHBP to GH exceeds
1:1,
trimers can form consisting of one GH molecule and two GHBP molecules. The
trimer
can not be bound by the capture antibody. so the GH/GHBP-complex that is
measured
1 0 in the LIFA is the heterodimer formed by one GH molecule and one GHBP
molecule.
When GH concentration increases,(e.g. when rhGH is added to the sample in the
LIFA
or during endogenous GH peaks), the trimer (GH-(GHBP)2] dissociates and dimers
IGH-GHBPI are formed, which can be bound by the MAb 263. This implies that all
the GHBP, including GHBP molecules in endogenously formed trimers, are
detectable
1 5 in the assay for the total GHBP concentration.
We found that about 15 % of GH in high peaks (>250 pmol/L) appear to be
bound in the GH/GHBP-complex, but the total bound fraction of GH may be higher
since some GH may have formed trimers with the GHBP.
The variation in the plasma concentration of GHBP in serial samples has
2 0 previously been addressed in two studies (Snow, KJ, Shaw MA, Winer LM,
Baumann
G. Diurnal pattern of plasma growth harmone-binding protein in man. J Clin
Endocrinol Metab. ;ZL:417-420 (1990); Hochberg Z, Amit T, Zadik Z. Twenty-
four-hour profile of plasma growth hormone-binding protein. J. Clin Endocrinol
Metab. U:236-239 (1991)). The study by Snow et al., which was carried out in
2 5 adults with low GH levels, agrees with our results that there is no major
variation in
total GHBP levels during the sampling period. However, since GH pulses were
absent
or very low in the study by Snow et al., the possibility that there could be
GH induced
variation in GHBP levels in subjects with pulsatile GH secretion could not be
excluded. In another study, by Hochberg et al., GH and GHBP levels were
measured in
3 0 samples obtained from normal children with pulsatile GH secretion and it
was
concluded that within 30 min the majority of the GH pulses were accompanied by
GHBP pulses. This is in contrast to the present results, where only minor
changes in
the total GHBP concentration were detected and they were not correlated with
the GH
pulses. The reason for the discrepancy between the two studies is npt clear,
but may
3 5 be due to differences between the GHBP assays. The LIFA directly measures
total
GHBP (i.e. the sum of free GHBP and GH-bound GHBP), while the assay used by
Hochberg et al. is based on the binding of radiolabeled hGH to the GHBP and
the values
are then corrected for interference by endogenous GH. Since our data regarding
the
GH/GHBP-complex indicate that the complex is formed and cleared rapidly, it is
CA 02291983 1999-11-19
. -, ~-..
46
possible that the apparent GHBP pulses, which Hochberg et a! observed 30 min
after
a GH pulse may reflect the desaturation of the GHBP, thereby allowing more
labeled
GH to be bound.
The total GHBP levels varied over a wide range in different subjects (109-
247 pmol/L) and it is probably that these levels are correlated to differences
in age,
sex, pubertal stage, growth velocity, etc. We conclude that GHBP levels are
relatively constant throughout the day and a single or pooled blood sample
should be
sufficient to estimate total GHBP concentration. This finding should
facilitate
comparisons with larger populations and illustrates the value of GHBP
measurement
1 0 as a diagnostic tool.
EXAMPLE 7
GHBP DETERMINATIONS OF NORMAL AND SHORT STATURE CHILDREN
Growth hormone binding protein was assayed using the LIFA assay for human
GHBP. The results are described in Tables 6, 7, 8 and 9 below. These tables
contain
1 5 summary statistics on GHBP levels in normal children (Table 6) as well as
in
children with short stature due to three different etiologies: idiopathic
growth
hormone deficiency (GHD) (Table 7), (ISS) (Table 8) and Turner syndrome (Table
9). Normal data were obtained from samples in Genentech's control and from
collaborations with outside investigators. The samples from children with
short
2 0 stature were obtained as part of an ongoing post-marketing surveillance
project from
Protropin human growth hormone, the Genentech National Cooperative Growth
Study (NCGS). . Now ISS children are now longer ideopathic in that the GHBP
deficiency likely reflects an underlying growth hormone receptior deficiency.
The summary statistics for normal children are presented by sex and age and
2 5 represent our best estimate of a normal range for GHBP. These statistics
consist of
the mean and mean plus or minus 2 standard deviations (SD) and were determined
from the logged (base 10) values of the GHBP levels, then converted back to
the
original units. Sample sizes used in the estimates are included with the
summary
statistics. Note that there were sufficient data to perform these calculations
for male
3 0 and female children aged, 3, 4 and 6 through 15 only. Data from children
of other
ages and from adults were too sparse to allow a good estimate of the mean.
The summary statistics listed for each of the etiologies of short stature are
the
sample size, mean and mean plus or minus one SD. These values were computed in
the
same manner as that described for the normals. The statistics are printed by
age and
3 5 sex for all available data regardless of sample size. The units of GHBP
are in
pmole/liter.
CA 02291983 1999-11-19
, ~. ~.
47
TABLE 6
Growth Hormone Bindinq Protein Norms
(GHBP Normal Range)
Sex Age N Mean Mean Mean
-2 SD +2 SD
Male 3 2 0 57.4 127.3 282.5
Male 4 21 64.6 120.2 223.5
1 0 Male 6 31 56.5 111.6 220.6
Male 7 31 78.2 143.0 261.7
Male 8 3 4 62.7 178.5 507.7
Male 9 3 6 64.9 198.1 604.7
Male 1 0 3 7 62.5 226.9 822.8
1 5 Male 1 1 4 0 70.7 234.6 779.0
Male 1 2 4 8 79 4 238.0 713.3
Male 1 3 3 3 72.5 231.7 739.9
Male 1 4 3 7 67.6 97.7 578.4
Male 1 5 3 3 51.7 173.4 581.8
2 0 Female 3 1 5 77.4 149.3 288.0
Female 4 1 7 62.0 179.3 518.6
Female 6 3 3 57.8 142.7 351.9
Female 7 3 2 73.5 175.2 417.7
Female 8 3 3 93.7 230.9 568.8
2 5 Female 9 3 6 90.8 215.7 512.4
Female 1 0 3 2 71.2 244.6 841.0
Female 1 1 3 3 97.5 285.8 838.3
Female t 2 3 6 87.7 228.7 596.8
Female 1 3 3 6 1 1 3.0 305.9 827.8
3 0 Female 1 4 3 5 99.7 260.2 678.7
Female 1 5 2 8 122.4 345.8 976.5
CA 02291983 1999-11-19
48
TABLE 7
Growth Hormone Bindlnq Protein Norms
(Idiopathic GHD: GHBP Levels)
Sex Age N Mean Mean Mean
-1 SD +1 SD
Male 1 1 - 73.5
Male 3 3 40.3 63.7 100.7
1 0 Male 4 5 56.3 95.4 161.7
Male 5 3 79.5 96.5 117.1
Male 6 7 76.9 97.3 123.1
Male 7 1 - 59.4 -
Male 8 4 87.3 127.8 187.1
1 5 Male 9 5 81.9 128.9 203.0
Male 1 0 1 2 73.5 150.3 307.2
Male 1 1 9 108.5 177.5 290.2
Male 1 2 1 4 106.8 193.5 350.4
Male 1 3 1 6 102.1 164.4 264.6
2 0 Male 1 4 1 7 83.0 149.8 270.4
Male 1 5 1 7 122.4 191.1 298.3
Male 1 6 5 132.1 231.4 405.3
Male 1 7 4 136.0 165.0 200.2
Male 1 8 1 - 772.5 -
2 5 Female 3 1 - 55.8 -
Female 5 2 77.4 82.5 87.9
Female 6 3 1 17.8 1 5 7.6 210.8
Female 8 2 200.3 241.3 290.7
Female 9 2 43.8 59.7 81.3
3 0 Female 1 0 3 83.4 253.9 772.7
Female 1 1 0 91.0 162.5 290.3
Female 2 7 89.0 189.9 405.2
Female 1 3 2 86.6 154.5 275.9
Female 5 2 294.0 398.6 540.4
TABLE 8
Growth Hormone Bind(n9 Protetn Norms
4 0 (Turner Syndrome: GHBP Levels)
Sex Age N Mean Mean Mean
-1 SD +1 SD
4 5 Female 4 2 93.2 118.1 149.5
Female 5 4 84.1 108.3 139.4
Female 6 7 65.3 135.8 282.2
Female 7 4 97.5 146.1 218.9
Female 8 5 116.3 194.5 325.4
5 0 Female 9 1 0 107.9 199.0 367.2
Female 1 0 1 1 1 0 5. 8 1 8 2. 7 31 5. 7
Female 1 1 8 181.0 241.9 323.2
Female 1 2 8 139.4 286.4 588.2
Female 3 9 133.3 241.8 438.7
5 5 Female 1 4 1 5 222.2 321.4 464.8
Female 1 5 6 101.0 189.0 353.5
Female 1 6 6 147.4 237.6 383.0
Female 1 7 2 1 1 7. 6 1 3 6. 0 1 5 7. 4
. e . CA 02291983 1999-11-19
- . ~ .
- ~ .
49
TABLE 9
Growth Hormone Blndlno Proteln Norms
(Idiopathic Short Stature: GHBP Levels)
Sex Age N Mean Mean Mean
-1 SD +1 SD
Male 2 1 - 37.3 -
1 0 Male 3 8 46.0 100.2 218.5
Male 4 1 4 68.6 96.3 135.4
Male 5 2 4 61.0 86.1 121.6
Male 6 1 7 47.9 65.1 88.4
Male 7 3 5 52.9 83.0 130.0
1 5 Male 8 2 9 61.1 90.8 135.0
Male 9 2 5 64.7 110.7 189.3
Male 1 0 4 5 69.5 108.3 168.6
Male 1 1 4 3 67.0 106.1 168.0
Male 1 2 7 7 70.5 109.5 170.2
2 0 Male 1 3 7 3 73.5 117.9 189.3
Male 1 4 7 1 70.8 110.4 172.0
Male 1 5 51 64.7 113.8 200.3
Male 1 6 1 2 55.9 102.9 189.4
Male 1 7 5 80.3 112.7 158.2
2 5 Male 1 8 2 64.5 195.0 589.6
Male 1 9 1 - 93.8 -
Male 2 1 1 - 184.3 -
Male 2 3 1 36.9 -
Female 1 1 - 63.3 -
3 0 Female 2 2 56.8 57.4 58.0
Female 3 2 45.3 88.2 171.4
Female 4 2 87.1 88.0 89.0
Female 5 4 50.8 91.4 164.5
Female 6 5 45.6 84.7 157.4
3 5 Female 7 6 76.6 109.2 155.6
Female 8 9 75.4 116.0 178.6
Female 9 9 89.8 120.7 162.2
Female 1 0 1 9 102.1 174.9 299.7
Female 1 1 3 1 84.3 139.3 230.1
4 0 Female 1 2 2 0 89.4 150.1 252.2
Female 1 3 1 7 110.1 146.3 1 9 4.3
Female 1 4 9 102.1 160.9 253.7
Female 1 5 4 56.5 110.7 216.8
Female 1 6 2 148.1 182.2 224.1
---------------------------------------
Figure 8 graphically illustrates the difference between normal GHBP and
various etiologies. Plotted are GH binding protein levels from the National
Cooperative Growth Study patients (log concentration of GHBP vs the age of
patient).
The crossbars represent mean values; solid vertical lines are plus or minus I
SDs;
5 0 dotted vertical lines are plus or minus two SDs. The separate black dots
each
represent one patient. (A) Idiopathic GHD for males; (B) Idiopathic GHD for
females;
(C) Idiopathic short stature for males; (D) Idiopathic short stature for
females; (E)
Turner Syndrome.
~U 8 S T jil'"~~ ~ ~~E T
CA 02291983 1999-11-19
The clinical utility of the LIFA assay for distinguishing between normals and
various etiologies can be seen in Figure 8(A-E). This is particularly
pronounced in
figure 8 C and D where the idiopathic short stature patients are predominantly
below
the mean for normal in both males and females. This provides a clinically
valuable
5 diagnostic test for evaluating the level of GHBP, and indirectly a
presumptive
determination of growth hormone receptor.
ideopathic short stature is no longer ideopathic in that it can now be viewed
as
a state of relative GH resistance. Based upon the relative lack of GHBP this
resistance
is likely due to a deficiency in GH receptors that are capable of responding
to GH.
1 0 Current therapy of ISS involves daily hGH injections. One approach to the
deficiency
in GH receptors is to administer higher doses of GH to stimulate those
receptors that
are present. A dosage of from 1.1 to 10 times that now used is expected to
increase
the GH response. Alternatively, now that the underlying basis of ISS is
revealed, new
treatment options different from that currently used are suggested. In GH
deficiency
1 5 and Turner's syndrome, situations of normal GHBP and presumably normal GH
receptor responsiveness, GHBP + GH is the logical treatment. In ISS patients,
GHBP
+GH may also be used to maximize the response by those GH receptors present.
However, in ISS, IGF-1 therapeutic treatment is also indicated. IGF-1 is given
alone
or with IGF binding protein it may be coadministered with GH, and/or with
GHBP.
2 0 Since ISS patients have reduced GHBP, the GHBP + GH combination elevates
the
response by those GH receptors present. Administration of therapeutic amounts
of
IGF-1 or IGF-1 plus IGF BP elevates the effective serum IGF-1, thus partially
circumventing the defective GH response and stimulating IGF-1 dependent
responses.