Note: Descriptions are shown in the official language in which they were submitted.
CA 02292591 1999-11-30
CVO 98/54369 PCT/US98/11528
METHOD AND ARTICLE FOR INTRODUCING
DENITROGENIZING FLUX INTO MOLTEN METAL
BACKGROUND OF THE INVENTION
1. Field of the Invention
This invention relates in general to the field of metallurgical processing,
and more
specifically to processes for adding materials, such as fluxes, into molten
metal such as liquid
steel.
2. Description of the Prior Art
In the production of metals such as steel, it is sometimes desirable to remove
unwanted
trace elements from the liquid metal by reacting one or more flux materials
with the liquid
metal.
For example, nitrogen is generally considered to be an unwanted element in
steel.
Nitrogen enters into liquid steel from the air and from contaminants, such as
oil, that may find
their way into the raw and recycled material from which steel is made. The
nitrogen changes
the mechanical properties of steel, making it harder and less ductile. It can
also chemically
combine with aluminum, or other elements, to form inclusions, affecting the
quality of the
product. Combined compounds can also migrate to grain boundaries in the
steel's
microstructure, weakening the steel. at elevated temperatures, giving rise to
inter-granular
cracks.
Nitrogen levels are particularly a problem in steel that is produced by the so-
called
"mini-mills," which generally use electric arc furnaces to melt the steel and
that also tend to
use a relatively high level of metal scrap as source material. It is not
uncommon to see steels
that are produced at such facilities as having a nitrogen content that is
within the range of
about 60 parts per million (ppm) to about 120 ppm. Steels that are made in
mills having a
basic oxygen furnace, on the other hand, have a nitrogen content that is
commonly within the
range of about 30 ppm to about 50 ppm. Some specialty applications, such as
for the
automotive body, however, require nitrogen levels that are as low as 20 ppm.
Some facilities
CA 02292591 1999-11-30
CVO 98/54369 PCT/US98/11528
use vacuum degassing equipment, which essentially exposes the liquid steel to
near vacuum
conditions to decarburize the steel. Some degree of nitrogen removal may be
achieved as a
by-product of this process. Unfortunately, this process is expensive and is
not able to extract
nitrogen that has already chemically combined with other elements, such as
aluminum.
More recently, it has been proposed to use fluxes to remove nitrogen from
molten steel
by adding a synthetic ladle slag of appropriate composition to the top surface
of the molten
steel within a ladle. The top slag process, which is also in common practice
for desulfurizing
steel, involves heating the steel within the ladle for an extended period of
time and to circulate
the steel, thereby exposing all the molten metal over time to the liquid metal-
slag reaction
interface. While denitrogenization with top ladle slag is promising in the
sense that it permits
reduction of nitrogen to levels otherwise not achievable by other processes,
it has several
practical limitations and consequently it is not in wide practice at this
point. The top ladle slag
denitrogenizing treatment would require skimming of carried over furnace slag
from the ladle
and introduction of a synthetic denitrogenizing ladle flux of a specific
composition on top of
liquid steel. Adding of such flux to the Ladle already containing other slag
would not be
desired and effective due to the dilution erect by the other slag. Effects of
nitrogen removal
by doing so would be questionable due to variability of composition of diluted
ladle slag. The
skimming operations, which are not uncommon in some practices such as special
desulfurization processes, are very time consuming and not energy efficient.
Temperature loss
of steel in the ladle not covered with slag can amount to 100-150 degrees F
depending on the
type of operation. Addition of solid slag fluxing mix requires extended
heating to melt and
bring the mix into solution. This requires a great deal of time and energy,
both of which are
expensive factors in the overall cost of production.
Denitrogenization using fluxes is being explored in several universities on
experimental
scale. The removal of nitrogen from steel appears to take place both in acidic
and basic fluxes.
The nitride capacity of fluxes has a V-shaped dependency on the optical
basicity. The nitride
capacity is high at low optical basicity; as the optical basicity is increased
it reaches a minimum
and starts to increase later. This behavior is explained by Sommerville et.al.
to be related to the
structural effects; the nitrogen which substitutes for oxygens in the network
shows an inverse
relationship with basicity whereas that replacing "free" oxygens is directly
related to basicity.
While the knowledge of denitrogenization with fluxes is improving, the
techniques used in
2
CA 02292591 1999-11-30
CVO 98/54369 PCT/US98/11528
these studies are on a laboratory scale and have employed the top slag method.
As discussed
above this technique has several practical limitations for routine technical
uses.
Articles addressing flux denitrogenization, the details of which are
incorporated into
this document as if set forth fully herein, are as follows: Studies on Slags
for Nitrogen
Removal from Steel, J.P. Ferreira et al., [75th Steelmaking Conference, Iron
&Steel Society,
April 5-8, 1992, Toronto, Ontario, Canada - Abstracts], pp. 216-217; Studies
of Nitrogen in
Steel in a Plasma Induction Reactor with a Ba0-Ti02 Slag, L.B. McFeaters et
al., [75th
Steelmaking Conference, Iron &Steel Society, April 5-8, 1992, Toronto,
Ontario, Canada -
Abstracts], pp. 218-219; and The Behavior of Nitrogen During Plasma Enhanced
Refining,
M. Takahashi et al., [75th Steelmaking Conference, Iron Steel Society, April 5-
8, 1992,
Toronto, Ontario, Canada - Abstracts], pp. 220-221; and Synthetic Slags for
Nitrogen
Removal, J.P. Ferreira, LD. Sommerville, and a. Mclean, [ Iron and Steelmaker,
May 1992],
pp. 43-49; and The use and Misuse of Capacities in Slags, LD. Sommerville, A.
Mclean and
Y.D. Young [Proceedings International Conference on Molten Slags, Fluxes and
Saits, 1997
Conference], pp. 375-383; and Solubility of Nitrogen in Cao-Sioz--CaF2 Slag
Systems, H. S.
Song, D.S. Kim, D.J. Min and P.C. Rhee [Proceedings International Conference
on Molten
Stags, Fluxes and Salts, 1997 Conference], pp. 583-587; and Nitride Capacities
in Stags,
H. Suito, K. Tomioka, and J. Tanabe, [Proceedings of 4th International
Conference on Molten
Stags and Fluxes, 1992, Sendai], pp. 161-166.
A need exists for an improved system and process for introducing a
denitrogenizing
flux to a quantity of molten metal, such as steel, in a manner that is less
time consuming and
less wasteful of energy than methods of flux addition and mixing that are in
conventional use.
SUMMARY OF THE INVENTION
Accordingly, it is an object of the invention to provide an improved system
and process
for introducing a denitrogenizing flux to a quantity of molten metal, such as
steel, in a manner
that is less time consuming and less wasteful of energy than methods of flux
addition and
mixing that are in conventional use. In order to achieve the above and other
objects of the
invention, a method of introducing a denitrogenizing flux to an amount of
molten metal,
includes, according to a first aspect of the invention steps of (a) encasing
the denitrogenizing
CA 02292591 1999-11-30
CVO 98/54369 PCT/US98/11528
flux with an outer layer of a metallic material of equal of lower melting
point in comparison to
the liquid metal; and (b) introducing the flux so encased into the molten
metal, whereby the
outer layer will melt, thereby introducing the flux into the molten metal.
According to a second aspect of the invention, an article for introducing a
denitrogenizing flux to an amount of molten metal includes an outer layer of a
metallic
material that has a melting point that is beneath the anticipated temperature
of the amount of
molten metal; and a denitrogenizing flux that is encased within the molten
metal, whereby the
outer layer will melt after the article has been introduced into the molten
metal for a
predetermined period of time, thereby permitting introduction of the
denitrogenizing flux into
the molten metal at a depth below the top surface of the molten metal.
According to a third aspect of the invention, a method of denitrogenizing an
amount of molten
metal includes steps of (a) providing an amount of molten metal; and (b)
introducing a
denitrogenizing flux into the molten metal in such a way that the flux becomes
exposed to the
molten metal at a location that is at a depth that is substantially below the
top surface of the
molten metal, thereby promoting more eiI'lcient mixing of the flux into the
molten metal.
A method of introducing a denitrogenizing flux to an amount of molten metal,
includes, according to a fourth aspect of the invention, steps of: (a)
supplying an amount of
denitrogenizing flux into a lance assembly of the type that includes a nozzle
that is constructed
and arranged to be immersed in molten metal; and {b) using the lance assembly
to introduce
the flux into the molten metal.
These and various other advantages and features of novelty which characterize
the
invention are pointed out with particularity in the claims annexed hereto and
forming a part
hereof. However, for a better understanding of the invention, its advantages,
and the objects
obtained by its use, reference should be made to the drawings which form a
further part
hereof, and to the accompanying descriptive matter, in which there is
illustrated and described
a preferred embodiment of the invention.
BRIEF DESCRIPTION OF THE DRAWINGS
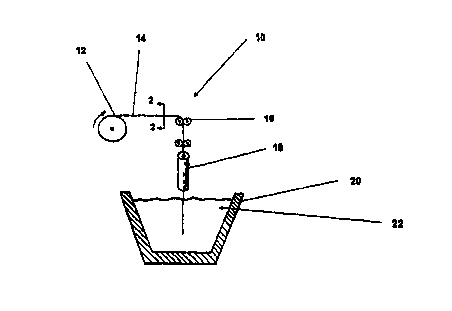
FIGURE 1 is a schematic depiction of a conventional wire feed machine, which
is
shown in operation according to the invention;
FIGURE 2 is a cross-sectional view taken along lines 2-2 in FIGURE 1;
4
CA 02292591 1999-11-30
-'WO 98/54369 PCT/US98/11528
FIGURE 3 is a schematic depiction of a system constructed according to an
alternative
embodiment of the invention; and
FIGURE 4 is a schematic control diagram.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS)
Referring now to the drawings, wherein like reference numerals designate .
corresponding structure throughout the views, and referring in particular to
FIGURE 1, an
improved system 10 for producing steel that has a low nitrogen content
includes a source 12
of a wire vector 14 that is constructed and arranged to introduce a
denitrogenizing flux into
molten metal such as steel. System 10 utilizes a conventional wire feed
machine of the type
that includes feeding structure 16 for feeding the wire vector into a guide
chute 18 at a
controlled velocity so as to cause the wire vector 14 to penetrate into the
molten steel 22 at a
predetermined speed and direction.
As may be seen in FIGURE 2, the wire vector 14 includes an outer layer 24 of a
material, such as steel, that has a melting point that is at or beneath the
temperature of the
molten metal 22. Preferably, the outer layer 24 is fabricated from steel a
material with equal
or lower melting point than the liquid melt, preferably the outer layer can be
made of steel or
aluminum. Outer layer 24 thus encases the nonmetallic substance in an
elongated, tube-like
hollow cladding of metallic material that is designed to melt after being
introduced into the
molten metal 22.
Wire vector 14 further includes an inner body of a powdered denitrogenizing
flux
material 26, which includes calcium oxide (Ca0) and at least one compound
selected from the
group consisting of oxides, silicates, carbonates of alkali and alkaline earth
metals and oxides,
fluorides, silicates and carbonates of metals selected from the group
consisting of Calcium
(Ca), Silicon (Si), Magnesium (Mg), Boron (B), Titanium (Ti), Barium (Ba) and
Aluminum
(Al). The most preferred flux materials are Ca0-Ba0-TiOz-(A1z03), Ca0-TiOz-
(A1z03 ) and
Calcium - Boron oxide bearing fluxes. Alternatively, any other flux that is
capable of
achieving the desired denitrogenization could be substituted.
A process according to one embodiment of the invention involves encasing the
denitrogenizing flux 26 with the outer layer of metallic material 24 and
introducing the flux 26
CA 02292591 1999-11-30
CVO 98/54369 PCT/US98/11528
so encased into the molten metal 22, whereby the outer layer will melt,
thereby introducing the
flux into the molten metal.
Another embodiment of the invention is depicted in FIGURES 3 and 4. Referring
in
particular to FIGURE 3, a system 30 for introducing a denitrogenizing flux 20
to an amount of
molten metal 32 that is constructed according to a preferred embodiment of the
invention
includes a container 34, such as a ladle, for holding an amount of molten
metal 32 such as.
liquefied steel. System 30 filrther includes a lance assembly 36 that is
preferably inclusive of a
container or hopper 38 of a supply of denitrogenizing flux 40, and a lance 42
for introducing
the flux 40 into the molten metal 32.
Preferably, the flux material 40 is a powdered denitrogenizing flux material
which
includes calcium oxide (Ca0) and at least one compound selected from the group
consisting of
oxides, silicates, carbonates of alkali and alkaline earth metals and oxides,
fluorides, silicates
and carbonates of metals selected from the group consisting of Calcium (Ca),
Silicon (Si),
Magnesium (Mg), Boron (B), Titanium (Ti), Barium (Ba) and Aluminum (AI). The
most
preferred flux materials are Ca0-Ba0-Ti02-(A1203), Ca0-Ti02-(A1203 ) and
Calcium - Boron
oxide bearing fluxes. Alternatively, any other flux that is capable of
achieving the desired
denitrogenization could be substituted.
A pressure source 44 of an inert gas, preferably argon, is communicated with a
first
end of the lance 42, and a control valve 46 is interposed between the pressure
source 44 and
the lance 42 in order to control the flow of the inert gas through the lance
42. A second end
of the lance 42 terminates in a nozzle 48, which during operation of the
system 30 is immersed
in the molten metal 32. The portion of the lance 42 that is expected to be
immersed in the
molten metal 32 during operation is encased in a protective refractory sleeve
54, as is shown in
FIGURE 3.
A conveyor 50 that is powered by a motor 52 is positioned to supply flux
material
from the hopper 38 into the lance 42 at a location that is between the valve
46 and the nozzle
48. As may be seen in FIGURE 4, System 30 includes a control system having a
CPU 56 that
controls operation of the motor 52 and the valve 56.
In operation, system 30 is operated to introduce the denitrogenizing flux 40
into the
molten metal 32 by CPU 56 instructing motor 52 to cause conveyor 50 to move
flux into the
lance 42, and by opening valve 46, thus causing the flux 40 to become
entrained in the flow of
6
CA 02292591 1999-11-30
CVO 98/54369 PCT/US98/11528
inert gas that is provided by the pressure source 44. The flux is then
injected into the molten
metal 32 at a preselected depth and velocity that is chosen to promote fast,
eiI'lcient mixing of
the flux 40 with the molten metal 32. Accordingly, the invention adds
denitrogenizing flux in
a manner that is less time consuming and less wasteful of energy than methods
of flux addition
and mixing that are in conventional use.
It is to be understood, however, that even though numerous characteristics and
advantages of the present invention have been set forth in the foregoing
description, together
with details of the structure and function of the invention, the disclosure is
illustrative only,
and changes may be made in detail, especially in matters of shape, size and
arrangement of
parts within the principles of the invention to the full extent indicated by
the broad general
meaning of the terms in which the appended claims are expressed.
7