Note: Descriptions are shown in the official language in which they were submitted.
CA 02293737 1999-12-09
WO 99/04014 PCT/US98/14935
TITLE
TRANSFORMED YEAST STRAINS AND THEIR USE FOR
THE PRODUCTION OF MONOTERMINAL AND
DITERMINAL ALIPHATIC CARBOXYLATES
FIELD OF THE INVENTION
The present invention is a bioprocess for the conversion of aliphatic
compounds, of the form CH3(CH2)"CH3 where n=4 to 20, to monoterminal and
diterminal carboxylates by genetically-engineered organisms. This invention
also
relates to yeast strains with enhanced alkane hydroxylating activity and/or
gene
disruptions in the (3-oxidation pathway for the production of carboxylates.
BACKGROUND OF THE INVENTION
Diterminal carboxylates of aliphatic compounds of the form
HO(O)C(CH2)nC(O)OH where n=7 to 16 are useful as polymer intermediates
(U.S. 4,767,828) and as anticorrosion compounds (JP 08113771 ). Monoterminal
carboxylates of aliphatic compounds of the form CH3(CH2)nC(O)OH where n=7
to 16 can serve as intermediates for surfactants (US 4,863,619). These
compounds can be produced from natural plant sources (Dale et al., J. Sci.
Food
Agr., 6:162, (1955)), but purification of the compound generally results in a
great
deal of by-product waste. Synthetic routes to enriched forms of such compounds
with reduced waste by-products would be commercially advantageous.
A number of yeasts are known to grow by metabolizing aliphatic
compounds of the form CH3(CH2)"CH3 where n=4 to 20. See, for example, Klug
et al. (Adv. in Microbial Physiology, 5: I -43, ( 1971 )). In addition,
Mauersberger
et al. (Non-conventional Yeasts in Biotechnolo~y. A Handbook, Klause Wolf
(ed.), Springer-Verlag, Berlin (1996)) note that Candida maltosa can grow on
aliphatic compounds of chain length C6 to C4p. In all cases examined to date,
growth on aliphatic compounds depends on specific enzymatic steps which
convert one or both terminal methyl groups to the carboxylate form. The first
step
in such transformations is the hydroxylation of the terminal methyl group by
the
yeast cytochrome P450 hydroxylating systems:
2CH3(CH2)"CH3 + 02 + NADPH -~ 2CH3(CH2)"CH20H + NAD+,
where n=7 to 16.
Such hydroxylating systems include at least three biological components:
cytochrome P450 monooxygenase, cytochrome P450-NADPH reductase, and
NADPH. The cytochrome P450-NADPH reductase transfers electrons from
NADPH (or NADPH) to the cytochrome P450 monooxygenase, activating it. In
the presence of oxygen and the aliphatic substrate, the activated cytochrome
P450
catalyses the reaction between oxygen and the aliphatic substrate to form the
corresponding alcohol. The necessity of electron transfer between the
reductase
CA 02293737 1999-12-09
WO 99/04014 PCT/US98/14935
and the cytochrome P450 monooxygenase requires proper structural orientation
of
the two components. In addition, the stoichiometric requirement for NADPH
means that hydroxylating activity requires a continuous supply of NADPH. This
NADPH supply is generally obtained from central metabolic pools in a living
cell.
In general, the hydroxylated compound is further metabolized to the
corresponding mono- or diterminal carboxyIate which can then provide energy
and carbon for yeast growth (Klug et al., Adv. in Microbial Physiology, 5:1-
43,
( I 971 )). The diploid yeast, Candida maltosa, can grow on alkanes as a sole
carbon source by deriving its carbon and energy through the ø-oxidation
pathway.
This pathway is so efficient that wild-type strains normally do not produce di-
carboxylic acids via c~-oxidation during growth on alkanes. However, in some
cases, the rate of carboxylate production exceeds the growth needs of the
organism. Under the proper conditions, this excess carboxylate production is
released into the growth medium. The resulting net production of carboxylates
from aliphatic starting materials has been exploited for industrial production
of
enriched carboxylate liquors from which the desired carboxylate compounds can
be easily separated.
The use of yeast for the production of mono- and diterminal carboxylates
is known in the art. A variety of native ("wild type") strains have been
exploited
for diterminal acid production. US 4,275,158 discloses the use of Debaryomyces
vanrijiae ATCC 20588 for the production of C ~ p to C 1 g diterminal
carboxylates
from aliphatic hydrocarbons or fatty acids. US 4,220,720 reports the use of
Debaryomyces pha~ ATCC 20499 for a similar purpose. The use of other native
strains is also reported for such carboxylate production including production
of
diterminal carboxylates from Cg to C ~ g aliphatic hydrocarbons by Pichia
polymorpha (JP 70024392) and production of carboxylates by Candida cloacae
(JP 76006750).
The diterminal carboxylates produced through fermentation by most
yeasts, including Candida maltosa, are most often shorter than the original
aliphatic substrate by one or more pairs of carbon atoms and mixtures are
common
(Ogino et al., Agr. Biol. Chem. 29:1009-1015 (1965); Shiio et al., Arg. Biol.
Chem. 35:2033-2012 ( 1971 ); Hill et al., Appl. Microbiol. Biotechnol.
24:168-174(1986)). Chain shortening is due to the degradation of the substrate
and product, after activation to their corresponding acyl-CoA ester, by the
peroxisomal ø-oxidation pathway. The initial step in the ø-oxidation (fatty
acid)
pathway involves oxidation of the acyl-CoA ester to its enoyl-CoA, and is
catalyzed by acyl-CoA oxidase. The enoyl-CoA is further metabolized to the
ø-ketoacyl-CoA by the action of enoyl-CoA hydratase and ø-hydroxyacyl-CoA
dehydrogenase. The fourth and last step of the ø-oxidation pathway is
catalyzed
2
CA 02293737 1999-12-09
WO 99/04014 PCT/US98/14935
by acyl-CoA acetyltransferase (more commonly called acyl-CoA thiolase), which
promotes reaction of the (3-ketoacyl-CoA with a molecule of free coenzyme A to
hydrolyze the carboxy-terminal two carbon fragment of the original fatty acid
as
acetyl-CoA.
Genetic mutations causing partial blockage of these latter reactions result
in the formation of unsaturated or hydroxylated byproducts (Meussdoerffer et
al.,
Proc. - World Conf. Biotechnol. Fats Oils Ind , 142-147 (1988)). These
undesirable byproducts are often associated with biological production of
diterminal carboxylates. Mutants produced by classical mutagenisis or by
genetic
engineering that enhance carboxylate production in excess of growth needs have
also been reported in the art. Mutants of Candida lipolytica (EP 229252,
DE 3929337, DE 4019166), Candida tropicalis (DE 3929337, DE 4019166,
EP 296506, EP 316072, US 5,254,466), Pichia carbofelas (JP 57129694),
Torulopsis candida {JP 52018885) and Torulopsis bombicola (US 3,796,630)
have been described. Enhancement of excess carboxylate production has been
achieved in these cases by decreasing the ability of the yeast to consume the
desired carboxylate as part of its normal metabolism. Often, mutants partially
defective in their ability to grow on alkane, fatty acid or di-carboxylic acid
substrates demonstrate enhanced di-carboxylic acid yields. However, most
mutants have not been characterized beyond their reduced ability to use these
compounds as a carbon source for growth. In all likelihood, their ability to
produce diterminal carboxylates is enhanced by a partial blockage of the
~i-oxidation pathway. Furthermore, compounds known to inhibit (3-oxidation
(i.e.,
acrylate) also result in increased diterminal carboxylate yields.
In regards to a biocatalyst for producing carboxylates, it would be
desirable to have an effective block of the ~3-oxidation pathway at its first
reaction,
catalyzed by aryl-CoA oxidase. A complete block at this step, would result in
enhanced yields of diterminal carboxylates by redirecting the substrate toward
the
c~-oxidation pathway while preventing reutilization of the diterminal
carboxylate
products through the ~i-oxidation pathway. In addition, the use of such a
mutant
would prevent the undesirable chain modifications associated with the (3-
oxidation
pathway, such as unsaturation, hydroxylation, or chain shortening. With
Candida
maltosa, the ~3-oxidation pathway may be functionally blocked by inactivation
of
both POX4 genes, which encode acyl-CoA oxidase (Masuda et al., Gene,
167:157-161 (1995), in order to redirect the metabolic flux to the microsomal
c~-oxidation pathway and thereby increase the yield of desired carboxylates.
A method for targeted gene disruption in yeast of the genus Pichia has
been disclosed in EP 226752. In addition, US 5,254,466 claims a method of
complete blockage of carboxylate consumption through genetic engineering of
3
CA 02293737 1999-12-09
WO 99/04014 PCT/US98/14935
Candida tropicalis. There is a great deal of scientific evidence to support
the vast
difference between Candida tropicalis and Candida maltosa as a commercial
biocatalyst, infra. Furthermore, a number of strains of Candida maltosa that
metabolize aliphatic hydrocarbons for growth have been described (Bos et al.,
Antoni van Leeuwenhoek, 39:99-107, (1973)). However, the prior art does not
report the use of Candida maltosa for production of mono- or diterminal
carboxylates.
In addition to blockage of the (3-oxidation pathway, recently another
strategy has been reported as a possible route to enhancement of excess
carboxylate production in yeasts. Rather than inhibiting consumption, attempts
have been made to enhance carboxylate production through enhancement of
cytochrome P450 hydroxylating activity. DE 19507546 discloses expression of
alkane hydroxylating cytochrome P450 systems in Saccharomyces cerevisiae, a
yeast normally not capable of aliphatic hydrocarbon or fatty acid
hydroxylation.
Enhanced alkane cytochrome P450 monooxygenase activity in this yeast naturally
results in carboxylate accumulation where the normal pathways for rapid
carboxylate consumption are lacking. However, cytochrome P450
monooxygenase activity in this strain appears unusually sensitive to poisoning
by
oxygen (Zimmer et al., DNA c~c Cell Biology, 14:619-628, (1995)), perhaps
indicating that the necessary structural integrity is lacking in this
genetically-
engineered strain.
WO 9114781 recites methods for the amplification of cytochrome P450
hydroxylating systems through genetic engineering in Candida tropicalis.
Although some enhancement of carboxylate production was observed, the
cytochrome P450 enzyme was poorly expressed and improvements in activity
were not completely successful (Picataggio et al., BiolTechnology, 10:894-898,
(1992)). In addition, German patent DE 3929337 describes the limited success
of
selection of mutants with improved cytochrome P450 monooxygenase activity
and dicarboxylate production through the use of the selective agent, 1-
dodecyne.
Wild-type Candida maltosa strains IAM12247 and ATCC 28140 are
equivalent organisms. They are available from the Institute of Applied
Microbiology (The University of Tokyo, Tokyo, Japan} and the American Type
Culture Collection (Manassas, VA, USA), respectively. Strains ATCC 90625 and
90677 are derived from IAM12247 and contain the nutritional marker mutations
adel, hiss (90625) and adel, hiss, ura3 (90677). Both of these strains are
available from the American Type Culture Collection, 1995 Yeast Reference
Guide, 19'~ ed.
Recent reports have described DNA sequence information for a number of
alkane cytochrome P450 monooxygenase as well as for the cytochrome P450
4
CA 02293737 1999-12-09
WO 99/04014 PCT/US98/14935
reductase from Candida maltosa IAM12247 / ATCC 28140 (Ohkuma et al., DNA
& Cell Biology, 14:163-173, (1995); Kargel et al., Yeast, 12:333-348, (1996}).
At
least eight structurally distinct cytochrome P450s with different substrate
specificities have been identified for Candida maltosa. Each of these integral
membrane proteins requires electron transfer from NADPH via a cytochrome
P450-NADPH reductase to catalyze monooxygenase reactions.
Mutated marker strains such as these are commonly used for genetic
transformations. However, in light of the limited success reported to date for
homologous expression of P450 monooxygenase systems in Candida tropicalis, it
has been uncertain whether such biocatalysts can be developed in Candida
maltosa.
There is a great deal of evidence that a biocataiyst for dodecanedioic acid
(DDDA) production based on Candida maltosa will be distinctly different than
one based on Candida tropicalis. These are two distinct species in the field
of
yeast taxonomy and significant differences exist between the two species at
the
molecular and biochemical level (Meyer et al., Arch. Microbiol., 104:225-231
(1975)). These distinctions, besides their taxonomic importance, have
practical
implications.
Candida maltosa cannot grow on starch. Candida tropicalis can grow on
starch. Candida maltosa is generally resistant to high concentrations of
cyclohexamide while Candida tropicalis is not. Candida tropicalis is often
associated with human disease while Candida maltosa is not.
These differences affect the utility of the organisms as a biocatalyst in
industrial processes. Starch is an inexpensive source for slow glucose release
and
a promising co-substrate for DDDA production by Candida tropicalis. Starch is
not a co-substrate option for Candida maltosa. Candida maltosa insensitivity
to
cyclohexamide eliminates use of one of the few antibiotic selection techniques
available for yeast genetic engineering.
Molecular comparisons also distinguish the two species from one another.
One widely accepted approach to evaluate evolutionary distances between
species
is based on DNA sequence comparisons for the small ribosomal RNA subunit
(18S). To date, comparisons between Candida maltosa and Candida tropicalis
have shown high similarities in these sequences, i.e., >94% (Ohkuma et al.,
Biosci. Biotech. Biochem., 57:1793-1794 (1993)); Pesole et al., Genetics,
141:903-907 (1995); Cai et al., Internat. J. Sys. Bacteriol., 46:542-549
(1996)).
However, comparisons of GenBank sequences for key enzymes in the alkane
oxidation process (cytochrome P450 monooxygenases and cytochrome P450
reductase) show greater dissimilarities for these genes than for the 18S RNA
gene
comparisons. For cytochrome P450 reductase, DNA sequences similarity equals
5
CA 02293737 1999-12-09
WO 99/04014 PCT/US98/14935
only 83%. For cytochromes P450 monooxygenase, maximum DNA sequence
similarity in a 7 gene by 7 gene comparison is only 77%. The majority of
cytochromes P450 monooxygenase sequence comparisons are below 70%. This
suggests that the genes important to the alkane oxidation process are under
selective pressure and have evolved separately in these two distinct species.
In
fact, Meyer et al. (Arch. Microbiol., 104:225-231 (1975)) have noted that the
two
species appear to occupy distinct ecological niches. Candida maltosa is only
found in hydrocarbon-contaminated environments. Candida tropicalis is most
often found associated with warm blooded animals, although it can grow in
hydrocarbon-contaminated environments. Finally, total genomic DNA
reassociation experiments show that Candida maltosa and Candida tropicalis
share <40% total DNA similarity (Meyer et al., Arch. Microbiol., 104:225-231
(1975)). Such differences in the molecular biology of Candida maltosa and
Candida tropicalis make it uncertain whether genes from the two organisms will
behave in a similar manner. Thus, limited success in causing enhanced P450
system activity in Candida tropicalis does not assure success in enhancing
activity
in Candida maltosa. Enhanced homologous expression of some P450 genes has
been demonstrated (Ohkuma et al., Biochim. Biophys. Acta., 1236:163-169
(1995}), but there have been no reports of enhanced P450 monooxygenase
activity
in Candida maltosa.
Successful expression of active P450 monooxygenase systems in
genetically-engineered Candida maltosa could lead to useful biocatalysts for
carboxylate production. To date, no report of a Candida maltosa transformant
capable of a combined expression of alkane P450 monooxygenase, fatty acid
monooxygenase and cytochrome P450-NADPH reductase expression is known in
the art.
SUMMARY OF THE INVENTION
The present invention relates to a process for the bioproduction of C6 to
C22 mono- and di-carboxylic acids by contacting, under aerobic conditions,
transformed Pichia pastoris characterized by a genetically engineered enhanced
alkane hydroxylating activity with at /east one C6 to C22 straight chain
hydrocarbon in the form CH3(CH2)nCH3 wherein n=4 to 20.
Another embodiment of the invention is a process for bioproduction of C6
to C22 mono- and di-carboxylic acids by contacting, under aerobic conditions,
transformed Candida maltosa characterized by a genetically engineered enhanced
alkane hydroxylating activity with at least one C6 to C~2 straight chain
hydrocarbon in the form CH3(CH2)nCH3 where n=4 to 20.
A fizrther embodiment of the invention is a transformed Pichia pastoris
comprising at least one foreign gene encoding a cytochrome P450
6
CA 02293737 1999-12-09
WO 99/04014 PCT/US98/14935
monooxygenase and at least one foreign gene encoding a cytochrome P450
reductase, each gene operably linked to suitable regulatory elements such that
alkane hydroxylating activity is enhanced. The genes encoding cytochrome P450s
are selected from the group consisting of P450 Alkl-A (D12475), Alk2-A
(X5881), Alk3-A (X55881), AIk4-A (D12716}, AIkS-A (D12717), Alk6-A
(D12718), Alk7 (D12719) and Alk8 (D12719) or genes substantially similar
thereto.
An additional embodiment of the invention is a transformed Candida
maltosa comprising at least one additional copy of genes encoding cytochrome
P450 monooxygenases and/or at least one additional copy of genes encoding
cytochrome P450 reductase, wherein the genes are operably linked to suitable
regulatory elements, such that alkane hydroxylating activity is enhanced.
Additionally, the instant invention describes the construction of expression
cassettes designed to deregulate expression of the major alkane monooxygenase
(P450A1k1-A), fatty acid monooxygenase (P450A1k3-A) and cytochrome
f450-NADPH reductase by precise fusion to the Candida maltosa
phosphoglycerol kinase (PGK) promoter and terminator.
The instant invention relates to a process for bioproduction of C6 to C22
mono- and diterminal carboxylates by contacting, under aerobic conditions,
transformed Candida maltosa characterized by a genetically-engineered, blocked
~3-oxidation pathway with at least one C6 to C22 straight chain hydrocarbon in
the
form CH3(CH2)nCH3 where n=4 to 20.
A further embodiment to the invention relates to a process for
bioproduction of C6 to C22 mono- and diterminal carboxylates by contacting,
under aerobic conditions, transformed Candida maltosa characterized by a
genetically-engineered, blocked (3-oxidation pathway and enhanced alkane
hydroxylating activity with at least one C6 to C~~ straight chain hydrocarbon
in
the form CH3(CHZ)nCH3 where n=4 to 20.
An additional embodiment of the invention is genetically-engineered
Candida maltosa strains that have enhanced cytochrome P450 activity and/or
gene
disruptions in the ~i-oxidation pathway.
A further embodiment of the invention is in novel DNA fragments. These
fragments comprise (a) a first Candida maltosa promoter operably linked to a
DNA encoding at least one polypeptide from Candida maltosa and (b) a second
Candida maltosa promoter operably linked to a DNA encoding at least one
polypeptide from Candida maltosa. The gene linked to the first Candida maltosa
promoter encodes cytochrome P450 monooxygenase and the gene linked to the
second Candida maltosa promoter encodes cytochrome P450 reductase. More
preferably, the first Candida maltosa promoter is PGK, the gene encoding
7
CA 02293737 1999-12-09
WO 99/04014 PCT/US98/14935
cytochrome P450 monooxygenase is Alkl-A (D12475), Alk2-A (X55881),
Alk3-A (X55881), Alk4-A (D12716), AlkS-A (D12717), Alk6-A (D12718), Alk7
(D 12719), and AIk8 (D 12719).
BRIEF DESCRIPTION OF THE SEQUENCE DESCRIPTIONS
BIOLOGICAL DEPOSITS AND FIGURES
The invention can be more fully understood from the following detailed
description, the biological deposits, sequence descriptions, and Figures which
form a part of this application.
Applicants have provided sequence listings in conformity with
37 C.F.R. ~1.821-1.825 ("Requirements for Patent Applications Containing
Nucleotide Sequences and/or Amino Acid Sequence Disclosures - the Sequence
Rules") and consistent with World Intellectual Property Organization (WIPO)
Standard ST.25 (1998) and the PCT and EPO sequence listing requirements.
SEQ ID NO: l represents the sense primer for the cytochrome
P450-NADPH reductase.
SEQ ID N0:2 represents the antisense primer for the cytochrome
P450-NADPH reductase.
SEQ ID N0:3 represents the sense primer for the cytochrome P450Alkl-A
gene.
SEQ ID N0:4 represents the antisense primer for the cytochrome
P450Alkl-A gene.
SEQ ID NO:S represents the sense primer for the cytochrome P450A1k3-A
gene.
SEQ ID N0:6 represents the antisense primer for the cytochrome
P450A1k3-A gene.
SEQ ID N0:7 represents the sense primer for the PGK promoter.
SEQ ID N0:8 represents the antisense primer for fusion of the PGK
promoter to the P450AIkI-A gene.
SEQ ID N0:9 represents the sense primer for the 5' end of the
P450A1k1-A gene.
SEQ ID NO:10 represents the antisense primer for the 5' end of the
P450AIk1-A gene.
SEQ ID NO:11 represents the sense primer for the 3' end of the
P450A1k 1-A gene.
SEQ ID N0:12 represents the antisense primer for the 3' end of the
P450A1k1-A gene.
SEQ ID N0:13 represents the sense primer for fusion of the PGK
terminator to the P450Alkl-A gene.
SEQ ID N0:14 represents the antisense primer for the PGK terminator.
8
CA 02293737 1999-12-09
WO 99/04014 PCTNS98/14935
SEQ ID N0:15 represents the antisense primer the fusion of for the PGK
promoter to the P450A1k3-A gene.
SEQ ID N0:16 represents the sense primer for the 5' end of the
P450A1k3-A gene.
SEQ ID N0:17 represents the antisense primer for the 5' end of the
P450A1k3-A gene.
SEQ ID N0:18 represents the sense primer for the 3' end of the
P450A1k3-A gene.
SEQ ID N0:19 represents the antisense primer for the 3' end of the
P450A1k3-A gene.
SEQ ID N0:20 represents the sense primer for fusion of the PGK
terminator to the P450A1k3-A gene.
SEQ ID N0:21 represents the antisense primer for fusion of the PGK
promoter to the cytochrome P450-ADPH reductase gene.
SEQ ID N0:22 represents the sense primer for the 5' end of the
cytochrome P450-NADPH reductase gene.
SEQ ID N0:23 represents the antisense primer for the 5' end of the
cytochrome P450-NADPH reductase gene.
SEQ ID N0:24 represents the sense primer for the 3' end of the
cytochrome P450-NADPH reductase gene.
SEQ ID N0:25 represents the antisense primer for the 3' end of the
cytochrome P450-NADPH reductase gene.
SEQ ID N0:26 represents the sense primer for fusion of the PGK
terminator to the cytochrome P450-NADPH reductase gene.
SEQ ID N0:27 represents the sense primer to the Candida maltosa POX4
gene.
SEQ ID N0:28 represents the antisense primer to the Candida maltosa
POX4 gene.
gene.
SEQ ID N0:29 represents the sense primer to the Candida maltosa URA3
SEQ ID N0:30 represents the antisense primer to the Candida maltosa
URA3 gene.
gene.
SEQ ID N0:31 represents the sense primer to the Candida maltosa ADE1
SEQ ID N0:32 represents the antisense primer to the Candida maltosa
ADE1 gene.
SEQ ID N0:33 represents the sense primer to the Candida maltosa HISS
gene.
9
CA 02293737 1999-12-09
WO 99/04014 PCT/US98/14935
SEQ ID N0:34 represents the antisense primer to the Candida maltosa
HISS gene.
Applicants have made the following biological deposits under the terms of
the Budapest Treaty on the International Recognition of the Deposit of Micro-
organisms for the Purposes of Patent Procedure. As used herein, "ATCC" refers
to the American Type Culture Collection International Depository located at
10801 University Boulevard, Manassas, VA 20110-2209 U.S.A. The
"ATCC No." is the accession number to cultures on deposit with the ATCC.
Depositor Identification International Depository
Reference Designation Date of Deposit
Pichia pastoris SW64/65 ATCC 74409 3 April 1997
Candida maltosa SW81/82 ATCC 74431 10 December 1997
Candida maltosa SW84/87.2 ATCC 74430 10 December 1997
Pichia pastoris SW64/65 is characterized as a Pichia pastoris strain with
the unusual ability that when induced by the presence of methanol is capable
of
producing active alkane cytochrome P450s which will convert C6 to C2~ alkanes
to the corresponding mono and diacids.
Candida maltosa SW81/82 is characterized as a Candida maltosa that is
unusual in its inability to grow on C6 to C22 alkanes or monofatty acids and
also
is unusual in its ability to produce diacids from C6 to C22 monoacids or
alkanes in
the presence of suitable carbon and energy sources such as glycerol. This
strain
contains disrupted POX4 genes and has other auxotrophic markers removed. This
strain is ~3-oxidation blocked.
Candida maltosa SW 84/87.2 is characterized as a Candida maltosa that is
unusual in its inability to grow on C6 to C~2 alkanes or monofatty acids and
also
is unusual in its ability to produce diacids from C6 to C22 monoacids or
alkanes in
the presence of suitable carbon and energy sources such as glycerol. In
addition,
SW84/87.2 is unusual in its ability to oxidize C6 to C2~ alkanes or monoacids
to
diacids in the presence of glucose at greater than 5 g/L concentration. This
strain
expresses enchanced alkane hydroxylating activity and contains disrupted POX4
genes.
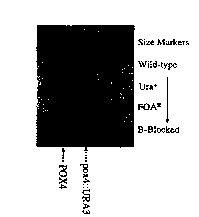
Figure 1 shows the strain lineage of ~3-oxidation-blocked Candida maltosa
via the Southern blot of XmnI-digested genomic DNA probed with the POX4
gene.
Figure 2 is a restriction map of pSW83.
Figure 3 is a restriction map of pSW84.
CA 02293737 1999-12-09
WO 99/04014 PCT/US98/14935
Figure 4 is a restriction map of pSW85.
Figure 5 is a restriction map of pSW87.
DETAILED DESCRIPTION OF THE INVENTION
The present invention comprises a process for the bioconversion of
aliphatic compounds, of the form CH3(CH2)"CH3 where n=4 to 20, to
monoterminal and ditenminal carboxylates using genetically-engineered
organisms. The present invention describes for the first time transfonmed
Candida
maltosa strains that have enhanced cytochrome P450 activity (including
combined, simultaneous expression of alkane P450 monooxygenase, fatty acid
monooxygenase and cytochrome P450-NADPH reductase expression) and/or gene
disruptions in the (3-oxidation pathway. Based on growth and alkane
utilization
rates of the wild-type strain, further improvements in volumetric productivity
(g
product/L/hr) of either the P450 enhanced or ~i-blocked-strain would be
required
for an economical bioprocess. Hence, the combination of these two concepts
provides a superior biocatalyst for the production of mono- and diterminal
carboxylates from aliphatic substrates. The present invention gives the
desired
carboxylates in quantities and conversion efficiencies sufficient to be
commercially viable.
One recombinant organism expresses enhanced alkane hydroxylating
activity. The alkane hydroxylating activity is responsible for the
hydroxylation of
a terminal methyl group. The enhanced hydroxylating activity may be due to
enhanced alkane monooxygenase, fatty acid monooxygenase or cytochrome P450
reductase separately or in various combinations. Additional enzymatic steps
are
required for its further oxidation to the carboxylate form. Two further
oxidation
steps, catalyzed by alcohol oxidase (Kemp et al., Appl. Microbiol. and
Biotechnol., 28:370 (1988)) and alcohol dehydrogenase, lead to the
corresponding
carboxylate.
Another recombinant organism has gene disruptions in the (3-oxidation
pathway. The diploid yeast, Candida maltosa, grows on alkanes as a sole carbon
source by deriving its carbon and energy through the (3-oxidation pathway.
This
pathway is so efficient that wild-type strains normally do not produce di-
carboxylic acids via w-oxidation during growth on alkanes. The ~i-oxidation
pathway was blocked in order to increase the metabolic flux to the co-
oxidation
pathway and thereby increase the yield and selectivity of a bioprocess for
conversion of alkanes to mono- and diterminal carboxylates.
A third recombinant organism has both enhanced alkane hydroxylating
activity and gene disruptions in the (3-oxidation pathway. The enhanced
hydroxylating activity may be due to enhanced alkane monooxygenase, fatty acid
monooxygenase or cytochrome P450 reductase separately or in various
11
CA 02293737 1999-12-09
WO 99/04014
PCT/US98/14935
combinations. The products of the present invention are useful as
intermediates in
the production of anticorrosive compounds and surfactants. More particularly,
the
methods and materials of the invention are useful for the bioproduction of
dodecanedioic acid. The bioprocess provides improved flexibility in
manufacturing and marketing of intermediates relative to the current chemical
route to polymer-grade and chemical-grade dodecanedioic acid. Specifically,
high
yields with good selectivity can be obtained. Further, the commercial
bioprocess
is expected to effect the environment more favorably than does the current
chemical process.
Terms and abbreviations used in this disclosure are defined as follows:
"Reduced nicotinamide-adenine dinucleotide" is abbreviated as NADPH.
"Reduced nicotinamide-adenine dinucleotide phosphate" is abbreviated as
NADPH.
"Candida maltosa IAM12247 cytochrome P450A1kI-A gene" is
abbreviated as Alk 1-A.
"Candida maltosa IAM12247 cytochrome P450A1k3-A gene" is
abbreviated as Alk3-A.
"Candida maltosa cytochrome P450-NADPH reductase gene" is
abbreviated as P450 reductase or CPR.
"Candida maltosa acyl CoA gene" is abbreviated as POX4.
"Candida maltosa IAM12247 URA3 gene codes for the enzyme orotidine-
5'-monophosphate decarboxylase" is abbreviated as URA3.
"Phosphoglycerol kinase" is abbreviated PGK.
"Alcohol oxidase I" is abbreviated as AOX 1.
"Gas chromatography" is abbreviated as GC.
"Polymerase chain reaction" is abbreviated as PCR.
"Autonomously replicating sequences" is abbreviated as ARS.
"Dodecanedioic acid" is abbreviated as DDDA.
The term "genetically-engineered" refers to the formation of new
combinations of heritable material by the insertion of nucleic acid molecules,
produced or derived by whatever means outside the cell, into any virus,
bacterial
plasmid or other vector system so as to allow their incorporation into a host
organism in which they are propagated and expressed to alter the phenotype of
the
host organism.
The term "transformation" refers to genetic engineering in which a nucleic
acid fragment is transferred into the genome of a host organism, resulting in
genetically stable inheritance. Host organisms containing the transferred
nucleic
acid fragments are referred to as "transgenic" or "transformed" organisms or
transformants.
12
CA 02293737 1999-12-09
WO 99/04014 PCT/US98/14935
The term "nucleic acid" refers to complex compounds of high molecular
weight occurring in living cells, the fundamental units of which are
nucleotides
linked together with phosphate bridges. Nucleic acids are subdivided into two
types: ribonucleic acid (RNA) and deoxyribonucleic acid (DNA).
An "isolated nucleic acid fragment" is a polymer of RNA or DNA that is
single- or double-stranded, optionally containing synthetic, non-natural or
altered
nucleotide bases. An isolated nucleic acid fragment in the form of a polymer
of
DNA may be comprised of one or more segments of cDNA, genomic DNA or
synthetic DNA.
The term "cytochrome P450" refers to a widely distributed
monooxygenase, active in many different biological hydroxylation reactions and
one component of the cytochrome P450 hydroxylating system.
The term "cytochrome P450 reductase" refers to a widely distributed
reductase, active in many different biological hydroxylation reactions and one
component of the cytochrome P450 hydroxylating system.
The terms "blocked J3-oxidation pathway" and "[3-blocked" refer to gene
disruptions that effectively eliminate acyl-CoA oxidase, the first enzyme in
the
~i-oxidation pathway of a wild-type.
"Altered levels" refers to the production of gene products) in organisms
in amounts or proportions that differ from that of normal, wild-type, or non-
transformed organisms. Production may be more specifically described as
"enhanced" or "decreased" relative to that of normal, wild-type, non-
transformed
organisms.
The term "enhanced" refers to an improvement or increase over an original
observation or function. Enhanced alkane hydroxylating activity is associated
with at least one additional copy of genes (relative to the wildtype) encoding
cytochromes P450 monooxygenase and/or cytochrome P450-NADPH reductase.
The terms "cassette" and "gene cassette" refer to a number of nucleotide
sequences which have been deliberately joined or combined in-vitro into a
unique
construction. An "expression cassette" specifically includes a promoter
fragment,
a DNA sequence for a selected gene product and a transcription terminator.
The terms "plasmid" and "cloning vector" refer to an extra chromosomal
element usually in the form of circular double-stranded DNA molecules and
often
carrying genes which are not part of the central metabolism of the cell. Such
elements may be autonomously replicating sequences, genome integrating
sequences, phage sequences, linear or circular, of a single- or double-
stranded
DNA or RNA, derived from any source. The term "autonomously replicating
sequence" refers to chromosomal sequences with the ability to allow autonomous
replication of plasmids in yeasts.
13
CA 02293737 1999-12-09
WO 99/04014 PCT/US98/14935
The term "expression" refers to the transcription and stable accumulation
of sense (mRNA) or antisense RNA derived from the nucleic acid fragment of the
invention. Expression may also refer to translation of mRNA into a
polypeptide.
"Overexpression" refers to the production of a gene product in transgenic
organisms that exceeds levels of production in normal or non-transformed
organisms. "Co-suppression" refers to the production of sense RNA transcripts
capable of suppressing the expression of identical or substantially similar
foreign
or endogenous genes (L1.S. 5,231,020).
The term "mutation" refers to a chemical change in the DNA of an
organism leading to a change in the genetic character of the organism. A
strain
exhibiting such a changed characteristic is termed a "mutant".
The term "oligonucleotide primer" refers to a short oligonucleotide that
base-pairs to a region of single-stranded template oligonucleotide. Primers
are
necessary to form the starting point for DNA poiymerase to produce
complementary-stranded synthesis with single-stranded DNA.
The terms "restriction enzyme" and "restriction endonuclease" refer to an
enzyme which catalyzes hydrolytic cleavage within a specific nucleotide
sequence
in double-stranded DNA.
The term "straight chain hydrocarbon" refers to aliphatic hydrocarbons,
fatty acids, and esters of fatty acids of carbon number C6 to C22 containing
0, 1 or
2 double bonds in the carbon backbone. In addition, the term includes any of
the
straight chain compounds described above where one of the terminal carbons has
been replaced by a phenyl group. Specific preferred hydrocarbons are nonane,
decane, undecane, dodecane, tridecane, tetradecane, pentadecane, hexadecane,
heptadecane, octadecane or any of the respective mono-carboxylic acids.
Preferred are C12-C14 alkanes. Dodecane is especially preferred.
The term "alkane hydroxylating activity" refers to the ability of an
organism, such as a yeast, to enzymatically hydroxylate the terminal methyl
group
of a straight-chain hydrocarbon using a cytochrome P450 hydroxylating system.
The term "cytochrome P450 hydroxylating system" refers to a hydroxylating
system composed of at least the following three biological components:
1 ) cytochrome P450 monooxygenase, 2) cytochrome P450-NADPH reductase and
3) reduced nicotinamide-adenine dinucleotide (NADPH) or reduced nicotinamide-
adenine dinucleotide phosphate (NADPH).
"Gene" refers to a nucleic acid fragment that encodes a specific protein,
including regulatory sequences preceding (5' non-coding sequences) and
following (3' non-coding sequences) the coding sequence. "Native gene" refers
to
a gene as found in nature with its own regulatory sequences. "Chimeric gene"
refers to any gene that is not a native gene, comprising regulatory and coding
14
CA 02293737 1999-12-09
WO 99/04014 PCT/US98/14935
sequences that are not found together in nature. Accordingly, a chimeric gene
may comprise regulatory sequences and coding sequences that are derived from
different sources, or regulatory sequences and coding sequences derived from
the
same source, but arranged in a manner different than that found in nature.
''Endogenous gene" refers to a native gene in its natural location in the
genome of
an organism. A "foreign" gene refers to a gene not normally found in the host
organism, but that is introduced into the host organism by gene transfer.
Foreign
genes can comprise native genes inserted into a non-native organism, or
chimeric
genes. A "transgene" is a gene that has been introduced into the genome by a
transformation procedure.
"Coding sequence" refers to a DNA sequence that codes for a specific
amino acid sequence. "Suitable regulatory sequences" refer to nucleotide
sequences located upstream (S' non-coding sequences), within, or downstream
(3' non-coding sequences) of a coding sequence, and which influence the
transcription, RNA processing or stability, or translation of the associated
coding
sequence. Regulatory sequences may include promoters, translation leader
sequences, introns, and polyadenylation recognition sequences.
"Promoter" refers to a DNA sequence capable of controlling the
expression of a coding sequence or functional RNA. In general, a coding
sequence is located 3' to a promoter sequence. The promoter sequence consists
of
proximal and more distal upstream elements, the latter elements often referred
to
as enhancers. An "enhancer" is a DNA sequence which can stimulate promoter
activity and may be an innate element of the promoter or a heterologous
element
inserted to enhance the level or tissue-specificity of a promoter. Promoters
may
be derived in their entirety from a native gene, or be composed of different
elements derived from different promoters found in nature, or even comprise
synthetic DNA segments. It is understood by those skilled in the art that
different
promoters may direct the expression of a gene in different tissues or cell
types, or
at different stages of development, or in response to different environmental
conditions. Promoters which cause a gene to be expressed under most growth
conditions at most times are commonly referred to as "constitutive promoters".
New promoters of various types useful in plant cells are constantly being
discovered; numerous examples may be found in the compilation by Okamuro
and Goldberg, (Biochemistry of Plants 15:1-82 (1989)). It is further
recognized
that since in most cases the exact boundaries of regulatory sequences have not
been completely defined, DNA fragments of different lengths may have identical
promoter activity.
The term "operably linked" refers to the association of nucleic acid
sequences on a single nucleic acid fragment so that the function of one is
affected
CA 02293737 1999-12-09
WO 99/04014 PCT/US98/14935
by the other. For example, a promoter is operably linked with a coding
sequence
when it affects the expression of that coding sequence (i.e., that the coding
sequence is under the transcriptional control of the promoter). Coding
sequences
can be operably linked to regulatory sequences in sense or antisense
orientation.
"Mature" protein refers to a post-translationally processed polypeptide;
i.e., one from which any pre- or propeptides present in the primary
translation
product have been removed. "Precursor" protein refers to the primary product
of
translation of mRNA; i.e., with pre- and propeptides still present. Pre- and
propeptides may be but are not limited to intracellular localization signals.
Construction of Recombinant Pichia pastoris:
Another embodiment of this invention relates to the genetic engineering of
Pichia pastoris to achieve expression of active P450 systems derived from a
heterologous source. Expression cassettes are constructed to include a
promoter,
such as, but not limited to, the strong, methanol-inducible promoter of
alcohol
oxidase I (AOX 1 ) fused to the Alk 1-A gene (or alternatively to the Alk3-A
or
P450 reductase genes) followed by a transcriptional terminator (such as from
AOXI). The expression cassettes are subcloned into vectors containing suitable
transformation markers, such as, but not limited to, HIS4, ARG4, SUC2 or the
sh
ble gene which encodes Zeocin resistance (Invitrogen, San Diego, CA, USA).
Sequential transformations of an appropriate strain of Pichia pastoris by
established methods (U.S. 4,855,231 ) results in the integration of expression
cassettes for genes into the Pichia pastoris genome. Transformants harboring
multiple copies of the expression cassettes can be identified by a variety of
methods such as, but not limited to, PCR and Southern blot analysis.
An alternative embodiment of engineering Pichia pastoris for expression
of active P450 systems derived from a heterologous source entails subcloning
multiple expression cassettes onto one or two plasmids. For example, the
expression cassettes for Alkl-A and Alk3-A genes may be subcloned on one
plasmid and the expression cassette for P450 reductase gene may be subcloned
on
a second plasmid; or expression cassettes for Alk 1-A and P450 reductase genes
may be subcloned on one plasmid and the expression cassette for Alk3-A gene
may be subcloned on a second plasmid; or the expression cassettes for AIk3-A
and
P450 reductase genes may be subloned on one plasmid and the expression
cassette
for Alkl-A gene may be subcloned on a second plasmid; or the expression
cassettes for Alkl-A and Alk3-A and P450 reductase genes may be subcloned on
one plasmid. The plasmids are then used to sequentially or simultaneously
transform a suitable Pichia pastoris host. Transformants harboring multiple
copies of the expression cassettes can be identified by a variety of methods
such
as, but not limited to, PCR and Southern blot analysis.
16
CA 02293737 1999-12-09
WO 99/04014 PCT/US98/14935
A further embodiment of engineering Pichia pastoris for expression of
active P450 systems derived from a heterologous source entails subcloning
expression cassettes for Alkl-A, Alk3-A and P450 reductase genes on to
replicating plasmids, individually or in multiple copies as described above
for the
integration plasmids. The replicating plasmids are then used to sequentially
or
simultaneously transform a suitable Pichia pastoris host. Transformants
harboring multiple copies of the expression cassettes can be identified by a
variety
of methods such as, but not limited to, PCR and Southern blot analysis.
Engineered Pichia pastoris cells containing multiple copies of expression
cassettes for Alkl-A, Alk3-A and P450 reductase genes are grown to saturation
in
minimal medium containing glycerol (or glucose) as the carbon source, followed
by induction of AOX1 promoter by methanol. This results in high Ievel
production of the P450 system components and high hydroxylating activity.
Aliphatic substrate may be added before, at the beginning of, or any time
during
induction, and after a suitable time, the medium is analyzed for carboxylates
as
described above.
PCR Amplification of Genomic DNA from Candida maltosa:
Oligonucleotide primers are prepared based on sequences available from
GenBank (National Center for Biotechnology Information, Bethesda, MD, USA)
for the Candida maltosa IAM12247 cytochromes P450 Alkl-A and Alk3-A, and
cytochrome P450 reductase genes, accession numbers D12475, X55881, and
D25327, respectively. Appropriate, unique restriction sites are designed into
the
primers to allow convenient ligation into a cloning vector as well as
construction
of a gene expression cassette (See, for example, Sambrook et al., Molecular
Clonine: A Laboratory Manual, Second Edition, Cold Spring Harbor Laboratory
Press, (1989)). In a similar manner, oligonucleotide primers are designed for
the
Candida maltosa IAM12247 URA3 gene. Using polymerase chain reaction
{PCR) U.S. Patent 4,683,202 (1987, Mullis et al.), and U.S. Patent 4,683,195
(1986, Mullis et al.), appropriate DNA sequences are amplified from genomic
DNA obtained from Candida maltosa IAM12247 which corresponds to
ATCC 90677. Similar protocols and appropriate primers would also allow PCR
amplification of other Candida maltosa IAM12247 sequences available from
GenBank including, but not limited to, cytochromes P450 Alk2-A (X5881),
Alk4-A (D12716), AlkS-A (D12717), Alk6-A (D12718), Alk7 (D12719) and
Alk8 (D 12719).
Construction of Recombinant Candida maltosa - Chromosomal Integration:
The descriptions that follow are embodiments of the invention that use
integrative transfer of the genes of interest in the transformed host.
17
CA 02293737 1999-12-09
WO 99/04014 PCT/US98/14935
The DNA fragments synthesized by PCR are sequentially inserted into a
convenient cloning vector such as pUC 18 or lambda Zap (Invitrogen, San Diego,
CA, USA) producing a vector which includes the gene cassette of the form
Alkl-A/Alk3-A/P450 reductase/CTRA3/Alkl-A. After cloning of the vector
containing the gene cassette in E. coli, the cassette fragment is linearized
by
cutting with appropriate restriction enzymes. Candida maltosa IAM12247
(corresponding to ATCC 28140) is transformed using techniques known in the art
(Sambrook et al., supra) and transformants which have gained functional copies
of
the URA3 gene are selected by growth on minimal medium supplemented with
histidine and adenine sulfate. Genomic DNA is isolated from the transformed
strains using techniques known in the art. The genomic DNA is cut using
appropriate restriction enzymes followed by probing using the Southern blot
method. In this way, clones that have the maximum number of gene copies
inserted into the chromosome are determined. Higher gene copy number
generally results in higher levels of enzyme activity.
A further embodiment of the invention is the sequential addition of the
P450 system genes to the Candida maltosa chromosome. Insertion into the host
genome of any cassette of the form X/URA3/X, where X = Alkl-A, Alk3-A, P450
reductase genes or other P450 system genes described above is accomplished by
following a similar protocol of PCR amplification, cloning, linearization,
transformation, minimal medium selection, and Southern blot screening to
produce clones containing at least one additional copy of gene X for each
original
copy in the chromosome. Since different cytochrome P450 enzymes may have
different substrate specificities, the insertion of the genes Alkl-A, Alk3-A
and
P450 reductase in any combination, or alternative insertions of one or more
genes
results in a set of biocatalysts useful for producing mono- or diterminal
carboxylates from any appropriate substrate with a carbon number of 9 through
18.
The copy number of multiple genes is increased through successive
integrative transformations, by inserting a recoverable marker gene along with
the
gene of interest during each transformation. In one embodiment of the
invention,
the URA3 gene is used repetitively. The ura3- genotype is regenerated by
selective growth on 5-fluoroorotic acid after each transformation, allowing
the
same marker gene to be used for the next transformation. This process is
repeated
for each additional transformation. In another embodiment of the invention the
hiss (GenBank Accession No. X17310) or adel (GenBank Accession
No. D00855) marker genes are used as the marker gene. Since Candida maltosa
strain ATCC 90677 is auxotropic for three different marker genes (URA3, HISS
18
CA 02293737 1999-12-09
WO 99/04014 PCT/US98/14935
and ADE1), up to three genes of interest can be inserted before it is
necessary to
regenerate an auxotrophic mutation.
Construction of Recombinant Candida maltosa - Autonomous Replication:
In another embodiment of the invention an autonomously replicating
sequence (ARS) is added to the vector containing a cassette having the genes
encoding a cytochrome P450 system. The host Candida maltosa is transformed
with this construct. The vector is stabily maintained in the host as a result
of the
ARS and selection pressure on a medium lacking uracil. As a result of the
extra
copies of the genes of interest carried by the vector, expression of active
P450
systems is increased resulting in greater carboxylate production. However, the
invention should not be considered limited by the use of genes Alkl-A, Alk3-A,
P450 reductase and URA3 in this example. Any of the P450 system genes
identified in this strain of Candida maltosa could be included alone or in
combination in a replicative plasmid construct and transformed into Candida
maltosa for the creation of a useful biocatalyst. Of particular use in the
present
invention are the genes Alkl-A, Alk3-A and P450 reductase. As a result of
increased expression levels of appropriate P450 system genes, higher levels of
carboxylate are produced.
Reaction Conditions for Candida maltosa:
Clones containing the highest levels of cytochrome P450 hydroxylating
activity are grown for 2-3 days on suitable medium, optionally containing
effective amounts of aliphatic substrate. At the end of this period,
additional
substrate is added and the cells are incubated for another 1-2 days. Cells are
removed and the supernatant is acidified resulting in the precipitation of the
monoterminal and diterminal carboxylates. The precipitate and any dissolved
carboxylates are extracted from the supernatant into methyl tertiary butyl
ether
(MTBE) and recovered in a substantially pure form after evaporation of the
MTBE solvent.
Substrates for Reactions:
The use of dodecane as a substrate to produce carboxylates is included for
illustrative purposes and should not be considered as limiting the scope of
the
invention. Alternative suitable substrates for carboxylate production include
straight chain hydrocarbons of carbon number C6 to C22, alone or in
combination.
Fatty acids with carbon number C6 to C22 also serve as substrates for
diterminal
carboxylate production. Furthermore, aliphatic hydrocarbons or fatty acids
containing 1 or 2 double bonds in the carbon backbone can serve as substrates
for
the production of carboxylates where one or two additional terminal
carboxylate
groups appear in the products. Any of the straight chain compounds described
19
CA 02293737 1999-12-09
WO 99/04014 PCT/US98/14935
above where one of the terminal carbons has been replaced by a phenyl group
are
also useful for carboxylate production.
Cell Strains and Growth Conditions:
Candida maltosa strains ATCC 90625 and ATCC 90677 (see ATCC
Catalogue of Yeasts) are used for transformation and expression of alkane
hydroxylating activity. Pichia pastoris strain GTS 115 is obtained from
Invitrogen
(San Diego, CA, USA). These strains are routinely grown in YEPD medium
(yeast extract, 10 g/L; peptone, 20 g/L; glucose, 20 g/L) at 30 °C with
shaking at
250 rpm. Transformants of Candida maltosa ATCC 90677 with additional
functional copies of the URA3 gene are selected by growth on minimal medium
supplemented with histidine and adenine sulfate. The minimal medium is YNB
(DIFCO Laboratories, Detroit, MI, USA), with amino acids + 50 mg/L histidine
and 20 mg/L adenosine sulfate + 10 g/L glucose.
GC Conditions:
The concentration of DDDA was determined by gas chromatography of
the MSTFA+1% TMCS derivatives using a SE 54 capillary column (15 m x
0.53 mm), 1.2 p.m coating with a temperature program of 1.5 min at 150
°C,
5 °C/min to 200 °C, 5 min at 200 °C; injector: 310
°C; detector: 320 °C; FID
detection.
EXAMPLES
The present invention is further defined in the following Examples, in
which all parts and percentages are by weight and degrees are Celsius, unless
otherwise stated. It should be understood that these Examples, while
indicating
preferred embodiments of the invention, are given by way of illustration only.
From the above discussion and these Examples, one skilled in the art can
ascertain
the essential characteristics of this invention, and without departing from
the spirit
and scope thereof, can make various changes and modifications of the invention
to
adapt it to various usage and conditions.
GENERAL METHODS
Procedures for enzymatic digestion of DNA with restriction
endonucleases, phosphorylations, legations, and transformations are well known
in
the art. Standard recombinant DNA and molecular cloning techniques used herein
are well known in the art and are described more fully in Sambrook, J.,
Fritsch,
E.F. and Maniatis, T. Molecular Cloning: A Laboratory Manual; Cold Spring
Harbor Laboratory Press: Cold Spring Harbor (1989), and by Silhavy, T.J.,
Bennan, M.L., and Enquist, L.W. Experiments with Gene Fusions, Cold Spring
Harbor Press: Cold Spring Harbor (1984), and by Ausubel, F.M. et al., Current
Protocols in Molecular Biolo~v, Greene Publishing Assac. and Wiley-
Interscience
(1987).
CA 02293737 1999-12-09
WO 99/04014 PCT/US98/14935
Materials and methods suitable for the maintenance and growth of
bacterial and yeast cultures are well known in the art. Techniques suitable
for use
in the following examples may be found in Manual of Methods for General
Bacteriolo~y (Phillipp Gerhardt, R. G. E. Murray, Ralph N. Costilow, Eugene W.
Nester, Willis A. Wood, Noel R. Krieg and G. Briggs Phillips, eds), American
Society for Microbiology, Washington, DC. (1994) or Thomas D. Brock in
Biotechnology: A Textbook of Industrial Microbiolo~y, Second Edition (1989)
Sinauer Associates, Inc., Sunderland, MA. All reagents and materials used for
the
growth and maintenance of bacterial cells were obtained from Aldrich Chemicals
(Milwaukee, WI, USA), DIFCO Laboratories (Detroit, MI, USA), GibcoBRL
(Gaithersburg, MD, USA), or Sigma Chemical Company {St. Louis, MO, USA)
unless otherwise specified.
The meaning of abbreviations is as follows: "h" means hour(s), "min"
means minute(s), "sec" means second(s), "d" means day(s), "mL" means
milliliters, "L" means liters, "pL" means microliter, and "mm" means
millimeter(s).
EXAMPLE 1
Construction of Pichia pastoris strain expressing
alkane hydroxylating activity
Cytochrome P450-NADPH reductase was PCR-amplified from Candida
maltosa ATCC 90677 using primers 1 (SEQ ID NO:1) and 2 (SEQ ID N0:2)
which incorporate terminal BamHI and AvrII sites (indicated in lower case
letters), respectively:
Primer 1 - (SEQ ID NO:1 ):
5'-AggatccATGGCATTAGATAAATTAG-3'
Primer 2 - (SEQ ID N0:2):
5'-AcctaggCTACCAAACATCTTCTTG-3'
This DNA fragment was subcloned between the BamHI and AvrII sites of the
vector pPIC3K (Invitrogen, San Diego, CA, USA) generating in pSW64 in which
the AOXI promoter drives expression of the cytochrome P450-NADPH reductase
gene. Cytochrome P450A1k1-A was PCR-amplified from Candida maltosa
ATCC 90677 using primers 3 (SEQ ID N0:3) and 4 (SEQ ID N0:4) which
incorporate terminal KpnI and ApaI sites (indicated in lower case letters),
respectively.
Primer 3 - (SEQ ID N0:3):
5'-CggtaccATGGCTATAGAACAAATTA-3'
Primer 4 - (SEQ ID N0:4):
5'-AgggcccTTTAGCAGAAATAAACAC-3'
21
CA 02293737 1999-12-09
WO 99/04014 PCT/US98/14935
This DNA fragment was subcloned between the KpnI and ApaI sites of the vector
pPICZA (Invitrogen, San Diego, CA, USA), generating pSW65 in which the
AOXI promoter drives expression of the cytochrome P450A1k1-A gene.
Cytochrome P450A1k3-A was PCR-amplified from Candida maltosa
ATCC 90677 using primers 5 (SEQ ID N0:5) and 6 (SEQ ID N0:6) which
incorporate terminal XhoI and ApaI sites (indicated in lower case letters),
respectively.
Primer 5 - (SEQ ID NO:S):
5'-ActcgagATGCCGGTTTCCTTTGTTC-3'
Primer 6 - (SEQ ID N0:6):
5'-AgggcccGTACATTTGGATATTGG-3'
This DNA fragment was subcloned between the XhoI and ApaI sites of the vector
pPICZA (Invitrogen, San Diego, CA, USA), generating pSW72 in which the
AOXI promoter drives expression of the cytochrome P450 Alk3-A gene. The
BgIII/BamHI fragment from pSW72 containing the AIk3-A expression cassette
was subcloned into the BamHI site of pSW65 which contains the Alkl-A
expression cassette, generating pSW73 which contains expression cassettes for
the Alkl-A and Alk3-A genes.
Pichia pastoris GTS 115 (his4) was transformed with pSW64 to HIS
prototrophy by the spheroplast method (Cregg et al., Mol. Cell Biol., 5:3376-
3385,
(1985)) a step that integrates the plasmid into the genome. A high copy number
transformant, designated SW64, was selected by growth in high concentration
(>1 mg/mL) of 6418 as described (Scorer et al., BiolTechnology, 12:181-184,
(1994)). Strain SW64 was re-transformed with pSW65 to zeocin resistance by the
electroporation method (Invitrogen, San Diego, CA, USA), a step that
integrates
the plasmid into the genome. PCR analysis verified the integration of
expression
cassettes for both P450 reductase and P450 Alkl-A genes into the genome of a
double transformant, designated SW64/65 and identified by ATCC Accession
No. 74409.
Pichia pastoris double transformant SW64/65 (ATCC 74409) was grown
to saturation (48 h) in 20 mL MGY (1.34% yeast nitrogen base without amino
acids, 1% glycerol, 0.00004% biotin) with shaking at 30 °C. Following
centrifugation, cells were induced by resuspension in 20 mL MM+Fe (1.34%
yeast nitrogen base without amino acids, 0.5% methanol, 0.00004% biotin, 1mM
Fe+3) and incubated with shaking at 30 °C for up to 48 h. Cells were
washed
twice in PBS (Sambrook et al., supra), once in sucrose buffer (0.25 M sucrose,
0.05 M Tris-HCl pH 7.5, 1 mM EDTA, 1 mM DTT), and resuspended in 2 mL
sucrose buffer supplemented with 0.3% BSA (Sambrook et al., supra). Extract
was prepared by vortexing cells with 1/2 volume of 0.5 mm glass beads (approx.
22
CA 02293737 1999-12-09
WO 99/04014 PCT/US98/14935
1 mL) for a total of 4 min in increments of 1 min, followed by 1 min on ice.
The
semi-clear lysate obtained by centrifugation (3000xg) of glass beads and cell
debris was centrifuged at 12000xg. The resulting supernatant was centrifuged
at
25000xg. The resulting supernatant was centrifuged at 45000xg, and the
microsomal pellet was resuspended in 1 mL sucrose buffer supplemented with
0.3% BSA.
An equal volume ( 1 mL) of NADPH/NADPH mix (0.5 mM NADPH and
0.5 mM NADPH in sucrose buffer) was added to a microsome preparation.
One ~L of 14C-lauric acid (SOmCi/mmole; ICN, Costa Mesa, CA, USA) was
added and the mix incubated at 30 °C with shaking at 150 rpm for 0-60
min. The
reaction was stopped by the addition of 0.1 mL H2S04, and then extracted 3
times
with 5 mL ether and pooled. The sample was air dried, resuspended in 0.3 mL
ether, and 2 ~L counted by liquid scintillation. A TLC plate (Kodak,
Rochester,
NY, USA) was loaded with 200,000 dpm, and TLC was run in an enclosed jar
with toluene:acetic acid (9:1) for approximately 2.5 hr. The plate was exposed
to
X-ray film overnight. Comparison to laboratory standards (Aldrich Chemical
Co.,
Milwaukee, WI, USA) confirmed conversion of lauric acid to 12-hydroxylauric
acid and to DDDA from engineered Pichia pastoris strain SW64/65
(ATCC 74409). No conversion to DDDA from control Pichia pastoris was
observed.
EXAMPLE 2
Construction of Candida maltosa P450 Alkl-A Expression Cassette
The major alkane monooxygenase (P450Alkl-A) gene was isolated
following PCR amplification and precisely fused to the Candida maltosa PGK
promoter and terminator by PCR-mediated overlap extension. This technique
allowed precise fusion of the PGK promoter and terminator to the translational
start and stop codons, respectively, of the P450A1k1-A structural gene without
any
DNA sequence alterations that might alter PGK-mediated expression. The PGK
promoter, comprising 766 by of 5'-flanking DNA sequence upstream of the PGK
structural gene (pos 56-756, not including primers), was amplified from 100 ng
Candida maltosa ATCC 90677 [adel, hiss, ura3/ura3] genomic DNA using
primers 7 (SEQ ID N0:7) and 8 (SEQ ID N0:8) to introduce a SpeI restriction
site (indicated in lower case letters) necessary for subsequent subcloning and
a 15
by DNA sequence corresponding to the 5'-end of the P450Alkl-A gene (the
indicated nucleotides are underlined):
Primer 7 - (SEQ ID N0:7):
S'-AactagtGGTAGAGCGATGGTTACATACGAC-3'
Primer 8 - (SEQ ID N0:8):
S'-TTGTTCTATAGCCATTCTAGTTAAGGCAATTGAT-3'
23
CA 02293737 1999-12-09
WO 99/04014 PCT/US98/14935
A 998 by DNA fragment corresponding to the 5'-end of the P450A1k1-A
gene (pos 47-977) was amplified from ~20 ng pGEM-Alk1-A DNA, containing
the Candida maltosa P450A1k1-A gene, using primers 9 (SEQ ID N0:9) and 10
(SEQ ID NO:10) to introduce a 15 by DNA sequence corresponding to the 3'-end
of the PGK promoter (the indicated nucleotides are underlined):
Primer 9 - (SEQ ID N0:9):
5'-GCCTTAACTAGAATGGCTATAGAACAAATTATTGAAGAA-3'
Primer 10 - (SEQ ID NO:10):
S'-TAAACCTGCAGTGGTATCTCTACCGGCA-3'
A 663 by DNA fragment corresponding to the 3'-end of the P450A1k1-A
gene (pos 1004-1596) was amplified from ~20 ng pGEM-Alkl-A DNA using
primers 11 (SEQ ID NO:11 ) and 12 (SEQ ID N0:12) to introduce a I S by DNA
sequence corresponding to the 5'-end of the PGK terminator (the indicated
nucleotides are underlined):
Primer 11 - (SEQ ID NO:11 ):
5'-TGCCGGTAGAGATACCACTGCAGGTTTA-3'
Primer 12 - (SEQ ID N0:12):
5'-CATAAAAAATCAATTCTATTTAGCAGAAATAAAAACACC-3'
The PGK terminator, comprising 588 by of 3'-flanking DNA sequence
downstream of the PGK structural gene (pos 2050-2571 ) was amplified from
100 ng Candida. maltosa ATCC 90677 genomic DNA using the primers 13
(SEQ ID N0:13) and 14 (SEQ ID N0:14) to introduce a NheI restriction site
(indicated in lower case letters) necessary for subsequent subcloning and a 15
by
DNA sequence corresponding to the 3'-end of the P450A1k1-A gene (the indicated
nucleotides are underlined):
Primer 13 - (SEQ ID N0:13):
5'-ATTTCTGCTAAATAGAATTGATTTTTTATGACACTTG-3'
Primer 14 - (SEQ ID N0:14):
S'-AAAGCTAGCTTTGAAACAATCTGTGGTTG-3'
These PCRs were performed in a 50 pL volume using a Perkin Elmer Amplitaq
kit. Amplification was carried out in a Perkin Elmer GeneAmp PCR System 9600
for 35 cycles, each comprising 1 min at 94 °C, 1 min at SO °C
and 2 min at 72 °C.
Following the last cycle, there was a 5-min extension period at 72 °C,
after which
the samples were held at 4 °C prior to analysis by gel electrophoresis.
The
expected DNA fragments were isolated following preparative gel electrophoresis
and purified using a Gene Clean kit (Bio101, Vista, CA).
The 766 by DNA fragment comprising the PGK promoter and the 998 by
DNA fragment corresponding to the 5'-end of the P450A1k1-A gene were
combined in a second PCR in which the complementary 3' end of the PGK
24
CA 02293737 1999-12-09
WO 99/04014 PCT/US98/14935
promoter and the 5' end of the P450A1k1-A gene were annealed. Addition of the
5'-PGK and 3'-P450Alkl-A primers, primers 7 and 10, respectively, allowed
amplification of a 1749 by DNA fragment comprising a precise fusion of the PGK
promoter to the 5' end of the P450A1k1-A gene. The 663 by DNA fragment
corresponding to the 3'-end of the P450ALK1A gene and the 588 by DNA
fragment comprising the PGK terminator and were combined in a second PCR in
which the complementary 3' end of the P450A1k1-A gene and the S' end of the
PGK terminator were annealed. Addition of the 5'-P450A1k1-A and 3'-PGK
primers, primers 11 and 14, respectively, allowed amplification of a 1236 by
DNA
fragment comprising a precise fusion of the 3' end of the P450A1k1-A gene to
the
PGK terminator. These PCRs were performed in a 50 uL volume using a Perkin
Elmer Amplitaq kit. Amplification was carried out in a Perkin Elmer GeneAmp
PCR System 9600 for 35 cycles, each comprising 1 min at 94 °C, 1 min
at 45 °C
and 2 min at 72 °C. Following the last cycle, there was a 5-min
extension period
at 72 °C, after which the samples were held at 4 °C prior to
analysis by gel
electrophoresis. The expected DNA fragments were isolated following
preparative gel electrophoresis and purified using a Gene Clean kit (Bio 101
).
The 1749 by DNA fragment comprising a precise fusion of the PGK
promoter to the 5' end of the P450A1k1-A gene was digested with SpeI and PstI
and ligated to similarly digested pLitmus 38 (New England Biolabs, Beverly,
MA). The ligated DNA was used to transform E. toll DHSa (GibcoBRL,
Gaithersberg, MD) and analysis of the plasmid DNA from ampicillin-resistant
transformants demonstrating white colony color in LB media (1% (wlv) tryptone;
1% (w/v) NaCI and 0.5% (w/v) yeast extract (Difco, Detroit, MI) containing X-
gaI
(40 pg/mL) confirmed the presence of the expected plasmid, which was
designated pLPAI. The 1236 by DNA fragment comprising a precise fusion of
the 3' end of the P450ALK1 A gene to the PGK terminator was digested with PstI
and NheI and ligated to similarly digested pLitmus 38. The ligated DNA was
used to transform E. toll DHSa and analysis of the plasmid DNA from ampicillin-
resistant transformants demonstrating white colony color in LB media
containing
X-gal confirmed the presence of the expected plasmid, which was designated
pLAIT. Next, pLPAl was linearized by digestion with PstI and NheI and ligated
to the 1236 by PstI/NheI DNA fragment from pLAl T. The ligated DNA was used
to transform E. toll DHSa and analysis of the plasmid DNA from ampicillin-
resistant transformants confirmed the presence of the expected plasmid, which
was designated pLPAIT. Digestion of this plasmid with SpeI and NheI generates
a 2985 by expression cassette containing the Alki-A gene precisely fused to
the
PGK promoter and terminator.
CA 02293737 1999-12-09
WO 99/04014 PCT/US98/14935
EXAMPLE 3
Construction of Candida maltosa P450 Alk3-A Expression Cassette
The major fatty acid monooxygenase (P450AIk3-A) gene was also isolated
and precisely fused to the Candida maltosa PGK promoter and terminator by
PCR-mediated overlap extension. The 766 by PGK promoter (pos 56-756, not
including primers) was amplified from 100 ng Candida maltosa ATCC 90677
genomic DNA using primers 7 (SEQ ID N0:7) and 15 (SEQ ID NO:15) to
introduce a SpeI restriction site (indicated in lower case letters) necessary
for
subsequent subcloning and a 15 by DNA sequence corresponding to the 5'-end of
the P450A1k3-A gene (the indicated nucleotides are underlined):
Primer 7 - (SEQ ID N0:7):
5'-AactagtGGTAGAGCGATGGTTACATACGAC-3'
Primer 15 - (SEQ ID NO:15):
5'-AAAGGAAACCGACATTCTAGTTAAGGCAATTGAT-3'
A 628 by DNA fragment corresponding to the 5'-end of the P450AIk3-A gene
(pos 62-655) was amplified from ~20 ng pGEM-Alk3-A DNA, containing the
Candida maltosa P450AIk3-A gene, using primers 16 (SEQ ID N0:16) and 17
(SEQ ID N0:17) to introduce a 15 by DNA sequence corresponding to the 3'-end
of the PGK promoter (the indicated nucleotides are underlined):
Primer 16 - (SEQ ID N0:16):
5'-GCCTTAACTAGAATGTCGGTTTCCTTTGTTCACAACGTT-3'
Primer 17 - (SEQ ID N0:17):
5'-TCTTGGATATCGAAAGTTTTACCTTGAC-3'
A 1058 by DNA fragment corresponding to the 3'-end of the P450A1k3-A gene
(pos 652-1632) was amplified from ~20 ng pGEM-AIk3-A DNA using the
primers 18 (SEQ ID N0:18) and 19 (SEQ ID N0:19) to introduce a 15 by DNA
sequence corresponding to the 5'-end of the PGK terminator (the indicated
nucleotides are underlined):
Primer 18 - (SEQ ID N0:18):
5'-GTCAAGGTAAAACTTTCGATATCCAAGA-3'
Primer 19 - (SEQ ID N0:19):
5'-CATAAAAAATCAATTTTAGTACATTTGGATATTGGCACC-3'
The 588 by PGK terminator (pos 2050-2571 ) was amplified from 100 ng
Candida maltosa ATCC 90677 genomic DNA using the primers 20 (SEQ ID
N0:20) and 14 (SEQ ID N0:14) to introduce a NheI restriction site (indicated
in
lower case letters) necessary for subsequent subcloning and a 15 by DNA
sequence corresponding to the 3'-end of the P450AIk3-A gene (the indicated
nucleotides are underlined):
Primer 20 - (SEQ ID N0:20):
26
CA 02293737 1999-12-09
WO 99/04014 PCT/US98/14935
5'-ATCCAAATGTACTAAAATTGATTTTTTATGACACTTG-3'
Primer 14 - (SEQ ID N0:14):
5'-AAAgctagcTTTGAAACAATCTGTGGTTG-3'
These PCRs were performed in a 50 uL volume using a Perkin Elmer Amplitaq
kit. Amplification was carned out in a Perkin Elmer GeneAmp PCR System 9600
for 35 cycles, each comprising 1 min at 94 °C, 1 min at 50 °C
and 2 min at 72 °C.
Following the last cycle, there was a 5-min extension period at 72 °C,
after which
the samples were held at 4 °C prior to analysis by gel electrophoresis.
The
expected DNA fragments were purified using a Gene Clean kit (Bio101).
The 766 by DNA fragment comprising the PGK promoter and the 628 by
DNA fragment corresponding to the 5'-end of the P450A1k3-A gene were
combined in a second PCR in which the complementary 3' end of the PGK
promoter and the 5' end of the P450A1k3-A gene were annealed. Addition of the
5'-PGK and 3'-P450A1k3-A primers, primers 7 and 17, respectively, allowed
amplification of a 1379 by DNA fragment comprising a precise fusion of the PGK
promoter to the 5' end of the P450A1k3-A gene. The 1058 by DNA fragment
corresponding to the 3'-end of the P450A1k3-A gene and the 588 by DNA
fragment comprising the PGK terminator and were combined in a second PCR in
which the complementary 3' end of the P450A1k3-A gene and the 5' end of the
PGK terminator were annealed. Addition of the 5'-P450A1k3-A and 3'-PGK
primers, primers 18 and 14, respectively, allowed amplification of a 1631 by
DNA
fragment comprising a precise fusion of the 3' end of the P450A1k3-A gene to
the
PGK terminator. These PCRs were performed in a 50 ~L volume using a Perkin
Elmer Amplitaq kit. Amplification was carried out in a Perkin Elmer GeneAmp
PCR System 9600 for 35 cycles, each comprising 1 min at 94 °C, 1 min
at 45 °C
and 2 min at 72 °C. Following the last cycle, there was a 5-min
extension period
at 72 °C, after which the samples were held at 4 °C prior to
analysis by gel
electrophoresis. The expected DNA fragments were isolated following
preparative gel electrophoresis and purified using a Gene Clean kit (Bio 1 O1
).
The 1379 by DNA fragment comprising a precise fusion of the PGK
promoter to the 5' end of the P450A1k3-A gene was digested with SpeI and
EcoRV and ligated to similarly digested pLitmus 38. The Iigated DNA was used
to transform E. coli DHSa and analysis of the plasmid DNA from ampicillin-
resistant transformants demonstrating white colony color in LB media
containing
X-gal confirmed the presence of the expected plasmid, which was designated
pLPA3. The 1631 by DNA fragment comprising a precise fusion of the 3' end of
the P450A1k3-A gene to the PGK terminator was digested with EcoRV and NheI
and ligated to similarly digested pLitmus 38. The ligated DNA was used to
transform E. coli DHSa and analysis of the plasmid DNA from ampicillin-
27
CA 02293737 1999-12-09
WO 99/04014 PCT/US98/14935
resistant transformants demonstrating white colony color in media containing
X-gal confirmed the presence of the expected plasmid, which was designated
pLA3T. Next, pLPA3 was linearized by digestion with EcoRV and NheI and was
ligated to the 1631 by EcoRV/NheI DNA fragment from pLA 1 T. The ligated
DNA was used to transform E. coli DHSa and analysis of the plasmid DNA from
ampicillin-resistant transformants confirmed the presence of the expected
plasmid,
which was designated pLPA3T. Digestion of this plasmid with SpeI and NheI
generates a 3010 by expression cassette containing the Alk3-A gene precisely
fused to the PGK promoter and terminator.
EXAMPLE 4
Construction of Candida maltosa Cytochrome P450-NADPH
Reductase Expression Cassette
The cytochrome P450-NADPH reductase (CPR) gene was also isolated
and precisely fused to the Candida maltosa PGK promoter and terminator by
PCR-mediated overlap extension. The 766 by PGK promoter (pos 56-756, not
including primers) was amplified from 100 ng Candida maltosa ATCC 90677
genomic DNA using primers 7 (SEQ ID N0:7) and 21 (SEQ ID N0:21) to
introduce a SpeI restriction site (indicated in lower case letters) necessary
for
subsequent subcloning and a 15 by DNA sequence corresponding to the 5'-end of
the CPR gene (the indicated nucleotides are underlined):
Primer 7 - (SEQ ID N0:7):
5'-AactagtGGTAGAGCGATGGTTACATACGAC-3'
Primer 21 - (SEQ ID N0:21 ):
5'-TTTATCTAATGCCATTCTAGTTAAGGCAATTGAT-3'
A 1038 by DNA fragment corresponding to the 5'-end of the CPR gene
(pos 88-1065) was amplified from ~20 ng pGEM-CPR DNA, containing the
Candida maltosa CPR gene, using primers 22 (SEQ ID N0:22) and 23 (SEQ ID
N0:23) to introduce a 15 by DNA sequence corresponding to the 3'-end of the
PGK promoter (the indicated nucleotides are underlined):
Primer 22 - (SEQ ID N0:22):
5'-GCCTTAACTAGAATGGCATTAGATAAATTAGATTT-3'
Primer 23 - (SEQ ID N0:23):
5'-AAGTGGAATCTAAAGCTTTTAATTCG-3'
A 1062 by DNA fragment corresponding to the 3'-end of the CPR gene
(pos 1090-2089) was amplified from ~20 ng pGEM-CPR DNA using primers 24
(SEQ ID N0:24) and 25 (SEQ ID N0:25) to introduce a 15 by DNA sequence
corresponding to the 5'-end of the PGK terminator (the indicated nucleotides
are
underlined):
Primer 24 - (SEQ ID N0:24):
28
CA 02293737 1999-12-09
WO 99/04014 PCT/US98/14935
5'-CGAATTAAAAGCTTTAGATTCCACTT-3'
Primer 25 - (SEQ ID N0:25):
5'-CATAAAAAATCAATTCTACCAAACATCTTCTTGGTA-3'
The 588 by PGK terminator (pos 2050-2571) was amplified from 100 ng
Candida maltosa ATCC 90677 genomic DNA using primers 26 (SEQ ID N0:26)
and 14 (SEQ ID N0:14) to introduce a NheI restriction site (indicated in lower
case letters) necessary for subsequent subcloning and a 15 by DNA sequence
corresponding to the 3'-end of the CPR gene (the indicated nucleotides are
underlined):
Primer 26 - (SEQ ID N0:26):
5'-GAAGATGTTTGGTAGAATTGATTTTTTATGACACTTG-3'
Primer 14 - (SEQ ID N0:14):
5'-AA.AgctagcTTTGAAACAATCTGTGGTTG-3'
PCRs were performed in a 50 pL volume using a Perkin Elmer Amplitaq~ kit.
Amplification was carried out in a Perkin Elmer GeneAmp~ PCR System 9600
for 35 cycles, each comprising 1 min at 94 °C, I min at 50 °C
and 2 min at 72 °C.
Following the last cycle, there was a 5-min extension period at 72 °C,
after which
the samples were held at 4 °C prior to analysis by gel electrophoresis.
The
expected DNA fragments were purified using a Gene Clean kit (Bio 1 O 1 ).
The 766 by DNA fragment comprising the PGK promoter and the 1038 by
DNA fragment corresponding to the 5'-end of the CPR gene were combined in a
second PCR in which the complementary 3' end of the PGK promoter and the 5'
end of the CPR gene were annealed. Addition of the 5'-PGK and 3'-CPR primers,
primers 7 and 23, respectively, allowed amplification of a 1789 by DNA
fragment
comprising a precise fusion of the PGK promoter to the 5' end of the CPR gene.
The 1062 by DNA fragment corresponding to the 3'-end of the CPR gene and the
588 by DNA fragment comprising the PGK terminator and were combined in a
second PCR in which the complementary 3' end of the CPR gene and the 5' end of
the PGK terminator were annealed. Addition of the 5'-CPR and 3'-PGK primers,
primers 24 and 14, respectively, allowed amplification of a 1635 by DNA
fragment comprising a precise fusion of the 3' end of the CPR gene to the PGK
terminator. PCRs were performed in a 50 p.L volume using a Perkin Elmer
Amplitaq~ kit. Amplification was carried out in a Perkin Elmer GeneAmp~ PCR
System 9600 for 35 cycles, each comprising 1 min at 94 °C, 1 min at 45
°C and
2 min at 72 °C. Following the last cycle, there was a 5-min extension
period at
72 °C, after which the samples were held at 4 °C prior to
analysis by gel
electrophoresis. The expected DNA fragments were isolated following
preparative gel electrophoresis and purified using a Gene Clean kit (Bio101).
29
CA 02293737 1999-12-09
WO 99/04014 PCT/US98/14935
The 1789 by DNA fragment comprising a precise fusion of the PGK
promoter to the 5' end of the CPR gene was digested with SpeI and HindIII and
ligated to similarly digested pLitmus 38. The ligated DNA was used to
transform
E coli DHSa and analysis of the plasmid DNA from ampicillin-resistant
transformants demonstrating white colony color in LB media containing X-gal
confirmed the presence of the expected plasmid, which was designated pLPR.
The 1635 by DNA fragment comprising a precise fusion of the 3' end of the CPR
gene to the PGK terminator was digested with HindIII and NheI and ligated to
similarly digested pLitmus 38. The Iigated DNA was used to transform E coli
DHSa and analysis of the plasmid DNA from ampicillin-resistant transformants
demonstrating white colony color in LB media containing X-gal confirmed the
presence of the expected plasmid, which was designated pLRT. Next, pLPR was
linearized by digestion with HindIII and NheI and was Iigated to the 1635 by
HindIII/NheI DNA fragment from pLRT. The ligated DNA was used to transform
E. coli DHSa and analysis of the plasmid DNA from ampicillin-resistant
transformants confirmed the presence of the expected plasmid, which was
designated pLPRT. Digestion of this plasmid with SpeI and NheI generates a
3424 by expression cassette containing the CPR gene precisely fused to the PGK
promoter and terminator.
EXAMPLE 5
Construction of Candida maltosa Strain
Expressing Enhanced Alkane Hydroxylatin~ Activity
Cytochrome P450 reductase is PCR-amplified from Candida maltosa
ATCC 28140 using two primer sets. One set incorporates a BamHI site at the 5'
end and a PstI site at the 3' end and amplifies a DNA fragment extending
2616 bases from a site 340 bases upstream of the reductase start codon. The
other
set incorporates a PstI site at the S' and a SphI site at the 3' end with an
XhoI site
immediately upstream of the 3' SphI site and amplifies the same DNA fragment.
The first DNA fragment containing the P450 reductase gene is then cloned
between the BamHI and PstI sites in the pUC 18 cloning vector (GibcoBRL,
Baltimore, MD, USA) resulting in plasmid pRDF 1. The later DNA fragment
containing the P450 reductase gene is subcloned between the Pstl and SphI
sites
resulting in plasmid RDF2. The adel gene is then amplified from Candida
maltosa ATCC 28140 using primers that incorporate an XhoI site at the 5' end
and
a SphI site at the 3' end. These primers amplify a DNA fragment extending
1396 bases from a site 284 bases upstream of the phosphoribosylamidoimidazole-
succinocarboxamide synthetase start codon. The XhoI-adel-SphI DNA fragment
is cloned into plasmid pRDF2 between the Xhol site and the Sphl sites
resulting
in plasmid pRDF3. The same DNA segment containing the adel gene is then
CA 02293737 1999-12-09
WO 99/04014 PCT/US98/14935
again amplified from Candida maltosa ATCC 28140 using primers that
incorporate a SmaI site at the S' end and a BamHI site at the 3' end. This DNA
fragment is cloned into pRDF3 between the Smal and BamHI sites resulting in
plasmid pRDF4.
A second plasmid construct is made by first amplifying the URA3 gene by
PCR from Candida maltosa ATCC 28140 using primers that incorporate a BamHI
site at the 5' end and a XbaI site at the 3' end. These primers amplify a DNA
fragment extending 1171 bases from a site 285 bases upstream of the orotidine-
5'-
phosphate decarboxylase start codon. In addition, another set of primers that
incorporate a SphI site at the 5' end and a HindIII site at the 3' end is used
to
amplify the same DNA fragment containing the URA3 gene. The BamHI - URA3
gene - XbaI fragment is cloned into the pUC 18 cloning vector (GibcoBRL,
Baltimore, MD, USA) between the BamHI and XbaI sites resulting in pRDFS.
The later DNA fragment containing the URA3 gene is subcloned between the
SphI and HindIII sites in pRDFS resulting in pRDF6. The Alkl-A gene is then
amplified from Candida maltosa ATCC 28140 using primers that incorporate a
SaII site at the 5' end and a SphI site at the 3' end of the DNA fragment.
These
primers amplify a DNA fragment extending 1958 bases from a site 291 bases
upstream of the P450A1k1-A start codon. The SaII - P450A1k1-A - SphI fragment
is cloned into plasmid pRDF6 between the SaII site and the Sph 1 sites
resulting in
plasmid pRDF7. The Alk3-A gene is then amplified from Candida maltosa
ATCC 28140 using primers that incorporate a XbaI site at the 5' end and a SaII
site at the 3' end. These primers amplify a DNA fragment extending 2063 bases
from a site 276 bases upstream of the P450A1k3-A start codon. This DNA
fragment is cloned into pRDF6 between the XbaI and SaII sites resulting in
plasmid pRDFB.
Plasmid RDF4 is used to transform Candida maltosa ATCC 90677 [ade 1,
hiss) to adenine prototrophy by spheroplast method (Cregg et al., Mol. Cell
Biol.,
5:3376-3385, (1985)) resulting in plasmid integration into the genome. High
copy
number transformants are selected by screening of transformants using the
Southern blot method. Clones yielding the strongest signal contain the highest
number of integrated copies of the P450 reductase gene. A high copy number
transformant is retransformed to uracil prototrophy with plasmid RDF8
resulting
in plasmid integration into the genome. Transformants are selected for growth
on
dodecane in the presence of increasing amounts of I-dodecyne. Clones
expressing the highest levels of P450 activity are able to grow at the highest
1-dodecyne concentrations. In addition, PCR and/or Southern blot analysis is
used to verify the integration of expression cassettes for Alkl-A, Alk3-A and
P450 reductase genes into the genome of a double transformant.
31
CA 02293737 1999-12-09
WO 99/04014 PCT/US98/14935
Double-transformed Candida maltosa ATCC 90677 strains are grown to
late log phase (.~ 48 h) in YEP (10 g/L yeast extract + 20 g/L peptone, pH 8)
+
0.05% Tween 80 + 10 g/L dodecane at 30 °C with shaking at 250 rpm.
Cells are
centrifuged and washed once in 10% YEP, pH 8. Cells are resuspended in 10%
YEP, pH 8 + 0.05% Tween 80 + 10 g/L dodecane at 30 °C and shaken at
250 rpm
for 24 h. The loss of dodecane and the production of dodecanedioic acid are
measured followed extraction by GC analysis. The production of dodecanedioic
acid is found to be enhanced in the doubly-transformed strains as compared to
the
original ATCC 90677 strain.
EXAMPLE 6
Construction of a POX4 Disruption Cassette
Gene specific primers were used to amplify the Candida maltosa POX4
gene from genomic DNA, while adding unique restriction sites to their flanking
regions for subsequent ligation into plasmids. A 1567 by Candida maltosa POX4
gene fragment (pos 908-2412) was PCR-amplified from 100 ng Candida maltosa
ATCC 90677 [adel, hiss, ura3/ura3] genomic DNA in 50 ~L of a standard PCR
mix using a Perkin Elmer Amplitaq kit and primers 27 (SEQ ID N0:27) and 28
(SEQ ID N0:28) to introduce the BamHI cleavage sites (indicated in lower case
letters) necessary for subsequent subcloning:
Primer 27 - (SEQ ID N0:27):
5'-GGGTCACggatccAATGTTGCTGG-3'
Primer 28 - (SEQ ID N0:28):
5'-GCAGCAGTGTATggatccTTAGTGTTCTTTGGTGGG-3'
Amplification was carried out in a Perkin Elmer GeneAmp PCR System 9600 for
35 cycles, each comprising 1 min at 94 °C, 1 min at 55 °C and 2
min at 72 °C.
Following the last cycle, there was a 5-min extension period at 72 °C,
after which
the samples were held at 4 °C prior to analysis by gel electrophoresis.
The
reactions containing the expected 1567 by DNA fragment were extracted with
phenol:chloroform:isoamyl alcohol (25:24:1 v/v), and the DNA was precipitated
with ethanol and resuspended in TE buffer ( 10 mM Tris pH 7.5, 1 mM EDTA).
The 1567 by DNA fragment was digested with BamHI and ligated to BamHI-
digested pBR322 (New England Biolabs, Beverly, MA). The ligated DNA was
used to transform E. coli DMl (GibcoBRL, Gaithersberg, MD) and analysis of the
plasmid DNA from ampicillin-resistant, tetracycline-sensitive transformants
confirmed the presence of the expected plasmid, which was designated
pBR-CMPOX4.
A 1184 by DNA fragment containing the Candida maltosa URA3 gene
(pos 8-1192) was PCR-amplified from 100 ng Candida maltosa ATCC 90625
[adel, hiss, ura3/ura3] genomic DNA in 50 q,L of a standard PCR mixture using
a
32
CA 02293737 1999-12-09
WO 99/04014 PCT/US98/14935
Perkin Elmer Amplitaq kit and primers 29 (SEQ ID N0:29) and 30 (SEQ ID
N0:30) to introduce the BcII cleavage sites (indicated in lower case letters)
necessary for subsequent subcloning:
Primer 29 - (SEQ ID N0:29):
5'-GACTTtgatcaATTTTGGTACCAT-3'
Primer 30 - (SEQ ID N0:30):
5'-AGGGTACCATGAAGTTTTAGACTCTtgatcaCT-3'
Amplification was carried out in a Perkin Elmer GeneAmp PCR System 9600 for
35 cycles, each comprising 1 min at 94 °C, 1 min at SO °C and 2
min at 72 °C.
Following the last cycle, there was a 5-min extension period at 72 °C,
after which
the samples were held at 4 °C prior to analysis by gel electrophoresis.
The
reactions containing the expected 1184 by DNA fragment were extracted with
phenol:chloroform:isoamyl alcohol (25:24:1 v/v), and the DNA was precipitated
with ethanol and resuspended in TE buffer (10 mM Tris pH 7.5, 1 mM EDTA).
The 1184 by PCR fragment containing the URA3 selectable marker was
digested with BcII and ligated to pBR-CMPOX4 which had been digested with
BcIII and treated with calf intestinal phosphatase. The ligated DNA was used
to
transform E. coli DHSoc competent cells (GibcoBRL, Gaithersberg, MD) and
analysis of the plasmid DNA from ampicillin-resistant confirmed the presence
of
the expected plasmid, which was designated pBR-pox4::URA3. Digestion of this
plasmid with BamHI released a 2.8 kb linear POX4 disruption cassette
containing
the URA3 selectable marker flanked by 770 by of 5'- and 734 by of 3'-homology
to the POX4 target gene.
EXAMPLE 7
Construction of a Candida maltose
Strain with Disrupted POX4 Genes
A (3-oxidation-blocked strain of Candida maltose was developed by
sequential disruption of both POX4 genes encoding acyl-CoA oxidase, which
catalyzes the first reaction in the pathway. Candida maltose ATCC 90677 lacks
the URA3 gene product, orotidine-5'-monophosphate decarboxylase, and requires
uracil for growth. The 2.8 kb linear POX4 disruption cassette derived from
plasmid pBR-pox4:URA3 was used to transform Candida maltose ATCC 90677
to uracil prototrophy as described by Gietz and Woods in Molecular Genetics of
Yeast: A Practical Approach (Johnson, J.R., ed.) pp. 121-134, Oxford
University
Press ( 1994). Ura+ transformants were selected in a supplemented minimal
media
containing 0.67 g/L Yeast Nitrogen Base (Difco, Detroit, MI), 2% (w/v)
glucose,
2% Bacto-agar (Difco) and 20 mg/L each of adenine sulfate and L-histidine.
Southern hybridization of XmnI-digested genomic DNA from 20
independent Ura+ transformants to a POX4 probe showed each to contain the
33
CA 02293737 1999-12-09
WO 99/04014 PCT/US98/14935
expected disruption of a single copy of POX4 (see Figure l, lane marked Ura+).
These results indicate that the ends of linear DNA are highly recombinagenic
and
dictate the precise site of integration into the Candida maltosa genome. Since
Candida maltosa is a diploid yeast, these transformants also contained a
second
functional copy of the POX4 gene that had to be disrupted in order to
inactivate
the (3-oxidation pathway. To sequentially disrupt both copies of the POX4 gene
in
a single strain, uracil-requiring revertants were first counter-selected in
supplemented minimal media also containing 2 mg/mL 5-fluoroorotic acid
(5-FOA), a toxic analogue of a uracil biosynthesis pathway intermediate that
is
incorporated only into Urat cells. Thus, only Ura cells survive and grow in
the
combined presence of 5-FOA and uracil. Several FOA-resistant, uracil-requiring
derivatives were isolated and one which retained the original POX4 disntption
and
also showed low reversion frequency to uracil prototrophy (See Figure I, lane
marked FOAR) was transformed a second time to uracil prototrophy using the
same pox4::URA3 disruption cassette derived from plasmid pBR-pox4:URA3.
Following transformation, about half of the resulting Ura+ transformants were
unable to grow on dodecane as the sole carbon source, suggesting that their
[3-oxidation pathway had been blocked. Analysis of these transformants by
Southern hybridization confirmed that both genomic copies of the POX4 gene
were disrupted (See Figure 1, lane marked (3-blocked). Subsequent analyses
have
confirmed the absence of any remaining acyl-CoA oxidase activity in these
transformants and their ability to convert dodecane to DDDA. Each
URA3-mediated gene disruption conveniently provides a distinct integration
target for subsequent amplification of the cytochrome P450 monooxygenase and
cytochrome P450-NADPI-I reductase genes.
EXAMPLE 8
Construction of Candida maltosa Strain with Disrupted POX4 Genes and with
Other Auxotrophic Markers Removed
The Candida maltosa ADE1 gene was isolated by PCR, using primers 31
(SEQ ID N0:31 ) and 32 (SEQ ID N0:32), which incorporate NdeI sites
(indicated in lower case letters), and subcloned into the NdeI site of pUCl8m
(a
derivative of pUC 18, in which SpeI and NheI restriction sites have been
inserted
between SaII and XbaI in the polylinker region), to generate pSW8l:
Primer 31 - (SEQ ID N0:31 ):
5'-CTTCTTCAAACCTTcatatgACATTGTTTCG-3'
Primer 32 - (SEQ ID N0:32):
5'-CTAATGGTCAAGcatatgTTGCATTATC-3'
34
CA 02293737 1999-12-09
WO 99/04014 PCT/US98/14935
The Candida maltosa HISS gene was isolated by PCR, using primers 33 (SEQ ID
N0:33) and 34 (SEQ ID N0:34), which incorporate NdeI sites (indicated in lower
case letters), and subcioned into the NdeI site of pUCl8m, to generate pSW82:
Primer 33 - (SEQ ID N0:33):
5'-TTTGGTTGACTcatatgTGAGCGCGGTAAAG-3'
Primer 34 - (SEQ ID N0:34):
5'-GTTTTGTCTGGCcatatgTTGAACTGGATGG-3'
One (3-blocked Candida maltosa transformant described in Example 7
(designated Candida maltosa 11-11) was further modified to eliminate the two
remaining auxotrophic requirements, adenine and histidine, which derive from
ATCC 90677. This removal was accomplished by co-transforming Candida
maltosa 11-11 with pSW81 and pSW82, by lithium chloride transformation
method essentially as described (Gietz et al., Methods Mol. Cell. Biol., 5:255-
269,
( 1996)), and selecting at 30 °C on minimal plates ( 1.34% Yeast
Nitrogen Base
without amino acids, 2% (w/v) glucose} without adenine or histidine
supplements.
The resulting strain is designated Candida maltosa SW81/82, and is identified
by
ATCC Accession No. 74431.
EXAMPLE 9
Construction of Candida maltosa Strain Expressing Enhanced
Alkane Hydroxylatin~ Activity and Disrupted POX4 Genes
The Candida maltosa cytochrome P450 Alkl-A expression cassette, as
described in Example 2, was subcloned into pSW81 (as described in Example 8)
between Spel and NheI, to generate pSW83 (see Figure 2). The Candida maltosa
cytochrome P450-NADPH reductase expression cassette, as described in
Example 4, was subcloned into pSW83 NheI, to generate pSW84, which contains
expression cassettes for both cytochrome P450A1k1-A and cytochrome
P450-NADPH reductase, plus the ade 1 selectable marker (see Figure 4). The
Candida maltosa cytochrome P450A1k3-A expression cassette, as described in
Example 3, was subcloned into pSW82 between SpeI and NheI, to generate
pSW85. The Candida maltosa cytochrome P450-NADPH reductase expression
cassette, as described in Example 4, was subcloned into pSW85 Nhel, to
generate
pSW87, which contains expression cassettes for both cytochrome P450A1k3-A
and cytochrome P450-NADPH reductase, plus the hiss selectable marker.
The Candida maltosa (3-blocked strain designated 11-11 (as described in
Example 7) was co-transformed with pSW84 (see Figure 4) and pSW87 (see
Figure 5), by lithium chloride transformation method essentially as described
(Gietz et al., Methods Mol. Cell. Biol., 5:255-269, (1996)), and selected at
30 °C
on minimal plates (1.34% Yeast Nitrogen Base without amino acids, 2% (w/v)
glucose) supplemented with adenine, or supplemented with histidine, or without
CA 02293737 1999-12-09
WO 99/04014 PCT/US98/14935
supplements. PCR and/or Southern analyses confirmed chromosomal integration
of expression cassettes for cytochrome P450A1k1-A and cytochrome
P450-NADPH reductase (strain designated Candida maltosa SW84), or
cytochrome P450A1k3-A and cytochrome P450-NADPH reductase (strain
designated Candida maltosa SW87), or cytochrome P450Alkl-A, cytochrome
P450A1k3-A, and cytochrome P450-NADPH reductase {strain designated
Candida maltosa SW84/87). One Candida maltosa SW84/87 double
transformant, designated Candida maltosa SW84/87.2 is identified by ATCC
Accession No. 74430. After growing Candida maltosa SW84, Candida maltosa
SW87, and Candida maltosa SW84/87 at 30 °C in YEPD {1% yeast
extract, 2%
peptone, 2% glucose) to saturation (24 h), cells were harvested by
centrifugation,
broken with glass beads to produce a semi-clear lysate as described in Example
1,
and assayed for hydroxylation activity as described in Example 1. Candida
maltosa SW84, Candida maltosa SW87 and Candida maltosa 84/87 each
demonstrate conversion of lauric acid to DDDA.
EXAMPLE 10
Production of Dodecanedioic Acid (DDDA) from
Dodecane by Candida maltosa Strain SW81/82 (ATCC 74431)
A 5 mL seed inoculum of Candida maltosa SW81/82 (ATCC 74431) was
grown for 24 h at 30 °C with shaking at 250 rpm in YEPD medium (10 g/L
yeast
extract, 20 g/L peptone and 20 g/L glucose). The resulting mixture was
inoculated into 350 mL of pH 5 yeast minimal medium (3 g/L (NH4)2SO4, 6.6 g!L
KH2P04, 0.4 g/L K2HP04, 0.6 g/L anhydrous MgS04, 4 g/L yeast extract, 75 g/L
glucose, 100 ~g/L biotin, 13 mg/L FeS04-7H20, 2 mg/L CuS04-SHOO, 20 mg/L
ZnS04-7H20, 6 mg/L MnS04-H20, 2 mg/L Co(N03)2-6H20, 3 mg/L
NaMo04~2H20 and 1.6 mg/L KI) and grown for 24 h at 30 °C with
shaking at
250 rpm. A fermenter (Braun) containing 7 L of pH 5 yeast minimal medium was
inoculated with the overnight culture. The fermenter was maintained at minimal
airflow and agitation until dissolved oxygen dropped to 20% of atmospheric.
The
dissolved oxygen was then raised to approximately 80% of atmospheric and
maintained through fermenter control of aeration up to 2 win and agitation up
to
1400 rpm at 30 °C. The addition of 10% w/v NH40H provided nitrogen for
cell
growth and also maintained the medium at pH 5. After approximately 14 h,
glucose concentration dropped to near zero. Dodecane was then added to a final
concentration of approximately 20 g/L. The pH of the medium was adjusted to
7.5 through the addition of 20% w/v KOH. Further additions of 20% w/v KOH to
the medium maintained the pH at 7.5 for the remainder of the fermentation.
Dodecane concentrations were monitored periodically and maintained above
3 g/L. In addition, glucose was fed at a slow rate in the range of 0.2 to 0.8
g
36
CA 02293737 1999-12-09
WO 99/04014 PCT/US98/14935
glucose/min and glucose concentration was monitored. The slow rate of glucose
feed was used to maintain the glucose concentration below 1 g glucose/L.
Approximately 69 h after dodecane addition, material from the fermenter was
harvested and analyzed for DDDA.
DDDA was recovered from the whole fermenter liquor (cells and
supernatant) by acidifying the liquor to pH 2 with 2M phosphoric acid and
extracting the precipitated material into 3 x 5 mL methyl-tertiary butyl
ether. A
portion of the ether extract was evaporated to dryness and the recovered DDDA
was reacted with MSTFA (N-methyl-N-trimethylsilyltrifluoroacetamide) to form
a derivative detectable by GC under the standard conditions specified above.
DDDA was present at 28.8 g/L or a total yield of 187 g from the fermenter.
The mean production rate is 2.7 g DDDA/h.
EXAMPLE 11
Production of Dodecanedioic Acid (DDDA) from Dodecane
by Candida maltosa 84/87.2 (ATCC 74430
A 10 mL seed inoculum of Candida maltosa strain 84/87.2 (ATCC 74430)
was grown for 24 h at 30 °C with shaking at 250 rpm in YEPD medium (10
g/L
yeast extract, 20 g/L peptone and 20 g/L glucose). The resulting mixture was
inoculated into 2 x 350 mL of pH 5 yeast minimal medium (3 g/L (NH4)2504,
6.6 g/L KH2P04, 0.4 g/L K2HP04, 0.6 g/L anhydrous MgS04, 4 g/L yeast
extract, 75 g/L glucose, 100 ~g/L biotin, 13 mg/L FeS04~7H20, 2 mg/L
CuS04~5H20, 20 mg/L ZnS04~7H20, 6 mg/L MnS04~H20, 2 mg/L
Co(N03)2~6H20, 3 mg/L NaMo04~2H20 and 1.6 mg/L KI) and grown for 24 h at
°C with shaking at 250 rpm. A fermenter (Braun) containing 7 L of pH 5
yeast
25 minimal medium was inoculated with 525 mL of overnight culture. The
fermenter was maintained at minimal airflow and agitation until dissolved
oxygen
dropped to 20% of atmospheric. The dissolved oxygen was then raised to
approximately 80% of atmospheric and maintained through fermenter control of
aeration up to 2 vvm and agitation up to 1400 rpm at 30 °C. The
addition of 10%
30 w/v NH40H provided nitrogen for cell growth and also maintained the pH of
the
medium at 5. After approximately 18 h, glucose concentration dropped to near
zero. Dodecane was then added to a final concentration of approximately 20
g/L.
The pH of the medium was adjusted to 7.5 through the addition of 20% w/v KOH.
Further addition of 20% w/v KOH maintained pH of the medium at 7.5 for the
remainder of the fermentation. Dodecane concentrations were monitored
periodically and maintained above 3 g/L. In addition, glucose was fed at a
slow
rate in the range of 0.2 to 0.8 g glucose/min and glucose concentration was
monitored. The slow rate of glucose feed was used to maintain the glucose
37
CA 02293737 1999-12-09
WO 99/04014 PCT/US98/14935
concentration below 1 g glucose/L. Approximately 51 h after dodecane addition,
material from the fermenter was harvested and analyzed for DDDA.
DDDA was recovered from the whole fermenter liquor (cells and
supernatant) by acidifying the liquor to pH 2 with 2M phosphoric acid and
extracting the precipitated material into 3 x 5 mL methyl-tertiary butyl
ether. A
portion of the ether extract was evaporated to dryness and the recovered DDDA
was reacted with MSTFA (N-methyl-N-trimethylsilyltrifluoroacetamide) +1 %
TMCS (trimethylchlorosilane) to form a derivative detectable by GC under
standard conditions specified above.
DDDA was present at 21.6 g/L or a total yield of 173 g from the fermenter.
The mean production rate for Candida maltosa SW84/87.2 was 3.4 g DDDA/h, a
20% improvement over the production rate for Candida maltosa SW81/82.
EXAMPLE 12
Production of Dodecanedioic Acid (DDDA) from Lauric Acid Methyl Ester
by Candida maltosa Strain 84/87-2 (ATCC 774301
A 10 mL seed inoculum of strain 84/87-2 (ATCC 77430) is grown for 24 h
at 30 °C and 250 rpm in YEPD medium (10 g/L yeast extract, 20 g/L
peptone and
g/L glucose). This seed is inoculated into 2 x 350 mL of pH 5 yeast minimal
medium (3 g/L (NH4)2504, 6.6 g/L KH2P04, 0.4 g/L K2HP04, 0.6 g/L
20 anhydrous MgS04, 4 g/L yeast extract, 75 g/L glucose, 100 pg/L biotin, 13
mg/L
FeS04-7H20, 2 mg/L CuS04-SH20, 20 mg/L Zn S04-7 H20, 6 mg/L
MnS04~H20, 2 mg/L Co(N03)2-6 H20, 3 mg/L NaMo04-2 H20 and 1.6 mg/L
KI) and grown for 24 h at 30 °C and 250 rpm. A fermenter (Braun)
containing
7 L of pH 5 yeast minimal medium is inoculated with 525 mL of the overnight
culture. The fermenter is maintained at minimal airflow and agitation until
dissolved oxygen drops to 20% of atmospheric. The dissolved oxygen is then
raised to approximately 80% of atmospheric and maintained through fermenter
control of aeration up to 2 wm and agitation up to 1400 rpm at 30 °C.
The
addition of 10% w/v NH40H provides nitrogen for cell growth and also maintains
pH of the medium at 5. When the glucose concentration in the fermenter drops
to
< 1 g/L, lauric acid methyl ester is added to a final concentration of
approximately
5 g/L. The pH of the medium is adjusted to 7.5 through the addition of 20% w/v
KOH. Further addition of 20% w/v KOH maintains pH 7.5 of the medium for the
remainder of the fermentation. Lauric acid methyl ester concentrations are
monitored periodically and the concentration is maintained above 3 g/L. In
addition, glucose is fed at a slow rate in the range of 0.2 to 0.8 g
glucose/min and
glucose concentration is monitored. The slow rate of glucose feed is used to
maintain the glucose concentration below 1 g glucose/L. After 48 h lauric acid
methyl ester addition is stopped and the reaction allowed to procede until
lauric
38
CA 02293737 1999-12-09
WO 99/04014 PCT/US98/14935
acid methyl ester is no longer detectable. Material from the fermenter is
harvested
and DDDA is recovered.
39