Note: Descriptions are shown in the official language in which they were submitted.
r CA 02295249 2000-01-11
Attorney Docket: 203-2089
FIBRIN GLUE APPLICATOR SYSTEM
BACKGROUND
1. Technical Field
The disclosure relates generally to a fibrin glue
applicator system for dispensing a first and a second
component of a tissue sealant based on human or animal
proteins and, more particularly, to an applicator system for
dispensing a first and a second protein solution to be
applied to tissues or organs to form a fibrin sealant for
sealing wounds, stopping bleeding and the like.
2. Description of Related Art
A fibrin sealant is a biological adhesive formed
by mixing two protein components, namely, fibrinogen and
thrombin. Each protein component is derived from human
plasma and is subjected to virus elimination procedures.
- 15 The components are typically individually dehydrated and
stored in separate vials as sterile freeze-dried powders.
It is known that purified fibrinogen and thrombin,
together with a variety of known adjuvants, can be combined
in vitro to produce a polymer having great potential
benefit, both as a hemostatic agent and as a tissue
adhesive. Because of the rapid polymerization upon intimate
interaction of fibrinogen and thrombin, it is important to
maintain these two blood proteins separate until applied at
the application site. These protein solutions are generally
~~_
CA 02295249 2007-06-12
mixed and dispensed by devices such as a dual syringe
apparatus.
One dual syringe apparatus for applying a
fibrinogen-based tissue adhesive is disclosed in U.S. Pat.
No. 4,359,049 to Redl et al. This reference discloses a
mechanism in which two standardized one-way syringes are
held in a support having a common actuating means. The
dispensing end of each syringe is inserted into a
collection manifold where the two components are mixed.
The components are then dispensed through a common needle
capable of covering a limited area of the application
site.
Typical devices for mixing and dispensing
solutions of fibrinogen and thrombin require the addition
1~ of these proteins in powdered form to the body of the
syringe. This makes the proteins susceptible to
contamination by impurities which may enter the syringe
body. Further still, the use of the syringe body to mix
the proteins with water to create the protein solutions
can cause the solutions to leak out from either the
dispensing end of each syringe or the proximal end of the
syringe body.
A dual syringe apparatus for the application of
fibrinogen and thrombin solutions to an application site
generally contains several parts, such as a syringe
plunger, a "Y" manifold connector, a dispensing needle, a
syringe holder, syringe needles, and conduits for
transporting the solutions to the dispensing needle.
Therefore, fibrin sealant applicators, such as disclosed
in U.S. Patent No. 4,359,049 to Redl et al discussed
above, and in U.S. Patent Nos. 4,874,368 to Miller et al
and 4,979,942 to Wolf et al are difficult to reuse. The
replenishment of the protein components typically requires
2
CA 02295249 2007-06-12
removing a clip which couples the syringe plunger,
removing the syringe plunger, detaching the syringes from
the "Y" connector, removing the syringes from the holder,
inserting new syringes, affixing the syringes to the "Y"
connector, adding fibrinogen to one syringe and thrombin
to another syringe, adding sterile water to each syringe,
replacing the syringe plunger, replacing the plunger clip,
and mixing the solutions. In an application where time
may be of the essence, such a lengthy replenishing process
is impractical and cumbersome.
SUMMARY
In accordance with an embodiment of the present
invention there is provided an applicator system for
dispensing from respective dispensing orifices at least
two components for forming a multicomponent biological
adhesive, the applicator system comprising: a housing
configured to receive a loading unit having at least two
piston assemblies, each of the at least two piston
assemblies having a piston matingly engaging the proximal
end of a cylinder configured for retaining one of the at
least two components, and for dispensing the one component
through a distal end of the cylinder; and an activator
assembly provided on the housing having an activator
movable from a first position to a second position to
compress each of the at least two pistons to dispense the
at least two components through the at least two conduits
in the dispensing unit; and characterized by: a dispensing
unit having at least two conduits which in use are in
fluid communication with the respective distal ends of the
cylinder of the at least two piston assemblies and with
respective ones of the dispensing orifices, the dispensing
unit including a coupler interface having at least two
piercers.
3
CA 02295249 2007-06-12
In accordance with another embodiment of the
present invention there is provided a manually-operated
applicator for dispensing a first and second component of
a biological adhesive, the applicator comprising: a
conduit assembly having a first pair of conduits and a
first valve movable from a closed position to an open
position; a piston assembly having a first cylinder
containing the first component and a second cylinder
containing the second component, the first cylinder being
in communication with a first of the first pair of
conduits and the second cylinder being in communication
with a second of the first pair of conduits; an activator
assembly having an activator for imparting distal pressure
to the first and second cylinders to effect dispensing of
the first and second components to the first pair of
conduits and move the first valve from the closed position
to the open position; and a coupler interface in
engagement with the conduit assembly and configured to
receive, in use, at least two vials storing the first and
second components for replenishing the first and second
cylinders.
Yet another embodiment of the present invention
provides a kit for dispensing from respective vials a
first and second component of a biological adhesive, the
kit comprising: a loading unit having at least two piston
assemblies, each of the at least two piston assemblies
having a piston matingly engaging a cylinder configured
for retaining one of the first and second components;
a housing configured to receive the loading unit; a
dispensing unit having at least two conduits in fluid
communication with the cylinders; a coupler interface
configured for receiving the respective vials storing the
first and second components therein, the coupler interface
4
CA 02295249 2007-06-12
having at least two piercers in fluid communication with
the cylinders; the housing having an'activator movable
from a first position to a second position to compress the
cylinders to dispense the first and sec;ond components to
the at least two conduits, and the loading unit having a
loading lever movable from a first position to a second
position to transfer the first and second components from
the vials to the cylinders to replenish the first and
second components within the cylinders.
A fibrin glue applicator system is provided, in
accordance with preferred embodiments, for dispensing a
first and a second protein solution. The first and second
protein solutions form a biological adhesive when inter-
mixed on an application site. The fibrin glue applicator
system includes a coupler interface, a disposable loading
unit (DLU), and a housing having a handle portion which
includes a trigger operatively associated with a slide bar
for dispensing the solutions from the DLU. The coupler
interface is removably mounted to the DLU and the DLU is
removably mounted to the housing.
The DLU includes two piston assemblies for
storing the solutions therein before the solutions are
dispensed. Each piston assembly includes a cylinder which
matingly engages a piston having a rod which translates
proximally and distally within the cylinder. The piston
assemblies are connected to one another by a loading lever
at a proximal end and a dispensing unit at a distal end
for holding the assemblies substantially parallel to one
another.
The coupler interface includes two mounting
holes each having a piercer therein for receiving a vial
having the first protein solution and a vial having the
second protein solution. The protective seal on each vial
is
4a
CA 02295249 2000-01-11
Attorney Docket: 203-2089
pierced by the piercer within.each hole when the vials are
inserted therein. Each piercer is in fluid communication
with one of the cylinders of the DLU via a conduit connected
to a main conduit. A first one-way valve is included within
each conduit to open and close a path leading from the
piercer to the main conduit. The first one-way valves are
forced open by air pressure created when the loading lever
connected to the two pistons of the DLU is translated
proximally causing the protein solutions to be transferred
to their corresponding cylinder. -
When the protein solutions have been transferred
from the vials to the cylinders of the DLU, the vials and
the coupler interface can be removed from the DLU. The DLU
is then mounted to the housing for dispensing the protein
solutions from the cylinders to the application site.
The solutions are dispensed via two dispensing
orifices each in fluid communication with one of the main
conduits. A second one-way valve is fixed within each main
conduit in proximity to each dispensing orifice to open and
close a path leading from each cylinder to its corresponding
dispensing orifice. The second one-way valves open when the
solutions are forcibly transferred from the cylinders to the
main conduits. The solutions are forcibly transferred when
the pistons are translated distally by pressing the trigger
of the handle portion to translate the slide bar distally
which causes the loading lever to translate distally which
in turn causes the pistons to translate distally thus
creating pressure within the cylinders for dispensing the
solutions within the main conduits. The first and second
protein solutions are preferably fibrinogen and thrombin
5
- --_ _ --- ------ . ,
~ CA 02295249 2000-01-11
Attorney Docket: 203-2089
solutions which intermix on the application site to form a
fibrin sealant.
BRIEF DESCRIPTION OF THE DRAWINGS
Various embodiments are described herein with
reference to the drawings, wherein:
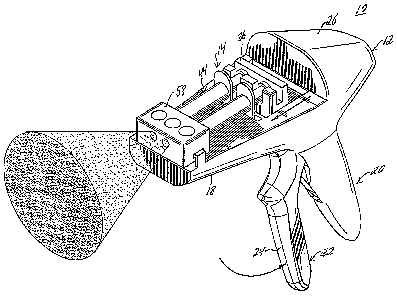
FIG. 1 is a perspective view of a preferred
embodiment of a fibrin glue applicator system;
FIG. 2 is a perspective view of the housing
showing the elongated body portion.and handle portion;
FIG. 3 is a perspective view of a disposable
loading unit (DLU) having a coupler interface attached
thereto;
FIG. 4 is an enlarged cross-sectional view of the
DLU and coupler interface with the one-way dispensing and
one-way loading valves being in the closed position;
FIG. 4A is enlarged view of the one-way loading
valve shown in FIG. 4;
FIG. 4B is an enlarged cross-sectional view of the
DLU and coupler interface with the dispensing valve in the
closed position and the loading valve in the open position;
FIG. 4C is an enlarged view of the valves shown in
FIG. 4B;
FIG. 5 is an enlarged cross-sectional view of the
distal end of the applicator system showing the dispensing
valve in the open position and the loading valve in the
closed position for dispensing the protein solution; and
FIG. 6 is a perspective view showing the protein
solutions being dispensed from the fibrin glue applicator
system of FIG. 1 as the trigger is depressed.
6
.-..... 1
CA 02295249 2000-01-11
C
Attorney Docket: 203-2089
DETAILED DESCRIPTION OF PREFERRED EMBODIMENTS
Referring to FIGS. 1 through 4A, a fibrin glue
applicator system and corresponding parts thereof according
to a preferred embodiment of the present disclosure are
shown. The applicator system, designated generally by
numeral 10, includes a longitudinal housing 12, a disposable
loading unit (DLU) 14, and a coupler interface 16. The
housing includes an elongated body portion 18 defining a
longitudinal axis and a handle portion 20.
The handle portion 20 includes a trigger 22
configured to pivot with respect to the handle portion 20.
The trigger includes a grip portion 24 extending downwardly
and adapted to be pressed by the fingers of a user's hand to
actuate the applicator system 10 to dispense protein
solutions within the DLU 14 as further described below.
The elongated body portion 18 includes a rounded
proximal end 26 and a flat surface 28 extending from above
the handle portion 20 to a distal end 30. A pair of flanges
32 extend perpendicular to the longitudinal axis at the
distal end 30 and a slot 34 is formed at the mid-point of
the elongated body portion 18.
Elongated body portion 18 further includes a slide
bar 36 having two apertures 38 and a slot 40 adjacent
thereto. The slide bar 36 is operatively associated with
the trigger 22 for distal and proximal translation of the
slide bar 36 along a chamber 42 of the elongated body
portion 18 as the trigger 22 is moved proximally and
distally, respectively. The trigger 22 may be operatively
associated with the slide bar 36 by any conventional
mechanism, such as a mechanism having rotatable gears and/or
movable rods. It is contemplated to provide a ratchet
7
' ~~ t
CA 02295249 2000-01-11
Attorney Docket: 203-2089
mechanism having notches on an.upper portion of the trigger
22 to control the amount of movement of the trigger 22 and
hence the slide bar 36.
The DLU 14 includes two piston assemblies 44 each
having a cylinder 46, a piston 48 having a rod 50 connected
to a stopper 52 at a proximal end. As shown by FIG. 3, a
loading lever 54 connects each stopper 52 to one another to
effect simultaneous movement of each rod 50 when the loading
lever 54 is translated proximally to transfer the solutions
from the vials 56 to the cylinders 46 as described below.
The DLU 14 further includes a dispensing unit 58
having two main conduits 60 therein each in fluid
communication with a corresponding cylinder 46 at a proximal
end and a dispensing orifice 62 at a distal end as shown by
the cross-sectional view of FIG. 4. The dispensing unit 58
further includes two conduits 64 perpendicular to the two
main conduits 60. The two conduits 64 are in fluid
communication with a corresponding piercer 66 and a
corresponding main conduit 60.
With reference to FIG. 3, the coupler interface 16
includes two holes 80 for receiving the vials 56 containing
solutions of fibr'inogen and thrombin which form a fibrin
_ sealant when intermixed on an application site. Each
piercer 66 of the dispensing unit 58 aligns with a
corresponding hole 80 for piercing the protective seal 81 on
each vial 56 when the vial 56 is inserted within the hole
80. As noted above each piercer 66 is in fluid
communication with a conduit 64 connected to one of the main
conduits 60 within the dispensing unit 58 for transferring
the fibrinogen and thrombin solutions to a corresponding
cylinder 46.
8
CA 02295249 2000-01-11
Attorney Docket: 203-2089
A one-way loading valve 68 is included within each
conduit 64 which opens when the loading lever 54 is moved
proximally as described below with reference to FIGS. 4B and
4C. A one-way dispensing valve 70 is included within each
main cdnduit 60 which opens when the trigger 22 is pressed
as described below with reference to FIGS. 5 and 6.
As shown by FIGS. 4 and 4A, each one-way valve
includes a pointed tip 69 attached to a spring 71. The
pointed tip 69 engages a mouth 73 of a conduit to prevent
fluid flow in a first position and.the pointed tip 69
disengages the mouth 73 of the conduit to permit fluid flow
in a second position. The pointed tip 69 disengages the
mouth 73 of the conduit when pressure is applied within the
conduit by either translating the pistons 48 proximally to
open the loading one-way valves 68 or distally to open the
dispensing one-way valves 70 as described below with
reference to FIGS. 4B to 5. The loading and dispensing one-
way valves are both closed when the loading lever 54 is
stationary or the trigger 22 is in the inactivated position.
Two notches 72 are included on two opposing faces
74 of the dispensing unit 58 which are used to snap-fit the
dispensing unit 58 with the flanges 32 of the elongated body
_ portion 18 to secure the DLU 14 to the housing 12. The DLU
14 is further secured to the housing 12 by two tabs 76 which
are fitted around each piston rod 50 and are adapted to be
press-fitted to the slot 34 on the flat surface 28 of the
elongated body portion 18. In addition, a portion of the
rods 50 and stoppers 52 of the pistons 48 are positioned
within the two apertures 38 and slot 40 of the slide bar 36
to further secure the DLU 14 to the housing 12.
9
l
CA 02295249 2000-01-11
Attorney Docket: 203-2089
As shown by the cross-sectional view of FIG. 4, a
spring mechanism 82 is included in proximity to each hole 80
of the coupler interface 16. The spring mechanism 82
includes two springs 84 and detents 88 attached thereto at
opposing sides of the coupler interface 16 and equidistant
from the piercer 66 for engaging the neck 86 of the vial 56
for locking and holding the vial 56 in position. It is
contemplated to provide a safety door mechanism in proximity
to each piercer 66 which would include safety doors which
close above each piercer 66 when the vials 56 are removed
from the coupler interface 16 to prevent the user from being
accidentally pierced by the piercers 66.
The operation of the applicator system 10 will now
be described with reference to FIGS. 4B to 6. As shown by
FIG. 4B, the vial 56 is placed within the hole 80 to pierce
the protective seal 81 by the piercer 66. The piston 48 is
then translated proximally to create proximal air pressure
from the tip of the piercer 66 to the interior of the
cylinder 46, thereby disengaging the pointed tip 69 of the
loading one-way valve 68 from the mouth 73 of the conduit 64
to transfer the protein solution within the cylinder 46.
The,enlarged view of FIG. 4C illustrates the disengagement
_ of the pointed tip 69 of the loading one-way valve 68 from
the mouth 73 of the conduit 64 as air pressure is created
within the conduit 64. It is noted that both protein
solutions are simultaneously transferred from their
respective vial 56 to a corresponding cylinder 46 as the
pistons 48 are simultaneously translated proximally.
Once the protein solutions have been transferred
to their corresponding cylinders 46, the loading lever 54,
the vials 56, and the coupler interface 16 can be removed
..~~
CA 02295249 2000-01-11
~..
Attorney Docket: 203-2089
from the DLU 14. The DLU 14 is then secured to the housing
12 as discussed above. The user can then bring the housing
12 with the DLU 14 attached thereto in proximity to a
surgery site or a wound for dispensing the solutions to form
a fibrin sealant over the surgery site or wound.
The protein solutions are dispensed simultaneously
as the trigger 22 is pressed by the user's fingers to cause
the slide bar 36 to translate distally thereby forcing the
pistons 48 to translate within the cylinders 46 to force the
solutions to be dispensed within the main-conduits 60. As
each solution is dispensed within one of the main conduits
60, pressure is created upon the pointed tip 69 of each
dispensing one-way valve 70 causing the pointed tip 69 to be
disengaged from the mouth 73 of each main conduit 60 as
shown by FIG. 5 to permit each solution to be dispensed from
its respective dispensing orifice 62 as shown by FIG. 6.
It is contemplated that the present invention may
be packaged as a kit for applying a solution of fibrinogen
and a solution of thrombin on a wound to stop bleeding or
the like. The kit may include the housing 12, the DLU 14,
the coupler interface 16, and the dispensing unit 58.
It is understood that various modifications may be
made to the embodiments disclosed herein. Also, besides
applying solutions of fibrinogen and thrombin to form a
fibrin sealant, the fibrin glue applicator system can be
used to perform human or veterinary surgical procedures
including applying antiseptics, medication and other similar
procedures. Therefore, the above description should not be
construed as limiting, but merely as exemplifications of
preferred embodiments.
11