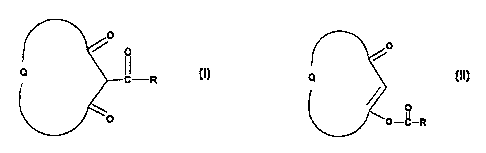Note: Descriptions are shown in the official language in which they were submitted.
CA 02295892 2000-01-04
WO 99/28282 PCT/GB98/03458
PROCESS FOR THE PREPARATION OF ACYLATED CYCLIC 1,3-DICARBONYL COMPOUNDS
The present invention relates to the production of acylated cyclical 1,3-
dicarbonyl
compounds and in particular to the production of benzoyl-1,3-cyclohexanediones
and
cycloalkyl- 1,3-cyclohexanediones.
The compounds produced by the process are known as herbicides and plant growth
regulators. 2-(Substituted benzoyl)-1,3-cyclohexanediones are known as
herbicides from, for
example, US Patent No. 4,780,127, US Patent No. 4,806,146, US Patent No.
4,946,981, US
Patent No. 5,006,158, WO 9408988 and WO 9404524. Cyclopropylcarbonyl-
l o cyclohexanediones are known as plant growth regulators from, for example,
EP126713.
One method of producing these compounds is by re-arrangement of an enol ester.
This
method is described in US Patent No. 4,695,673. This process provides a means
to obtain
the desired compounds but the process also requires the use of a cyanide
source as a catalyst.
In WO 9622957 it was shown that in certain solvents the rearrangement of a
cyclohexanedione enol ester would proceed in the absence of a cyanide
catalyst. However
the reactions proceeded much more slowly and produced a lower yield. There is
therefore a
continuing need for a rearrangement process which produces acceptable yields
but which
does not use a cyanide catalyst. It has surprisingly been found that azoles
may be used in a
cyanide-free rearrangement process.
According to the present invention there is provided a process for preparing a
compound of Formula (I)
0
o
Q LR
O
where Q completes an optionally substituted 5- or 6-member saturated
carbocyclic ring and
R is optionally substituted phenyl or optionally substituted C3-C6 cycloalkyl
which process
comprises the rearrangement of a compound of Formula (II)
CA 02295892 2000-01-04
WO 99/28282 PCT/GB98/03458 -
- 2 -
0
Q
OfI
O-C-R
(~~)
where Q and R are as defined in relation to Formula (I) in a polar aprotic,
dipolar aprotic or
aromatic hydrocarbon solvent in the presence of a moderate base and an azole.
The compounds of formula (I) may exist as one or more of the structural
formulae
shown below because of tautomerism.
O
O
C-R
D
O
II-R O
OH
4 _C-R
The values of Q and R are as defined above.
The term "azole" refers to a five membered nitrogen-containing ring which is
optionally substituted and which may be fused to other rings.
*rB
CA 02295892 2007-05-11
30584-57
- 3 -
Optional substituents for the carbocyclic ring formed by Q include C,r, alkyl,
C,,
haloalkyl, C,, alkoxy, C2_5 alkylene (in which case the compounds have a spiro
structure)
COC,, alkyl, COOH, COOC,_, alkyl, phenyl, halophenyl, C,, haloa.lkylphenyl,
phenoxy
halophenoxy, Cõ haloalkylphenoxy, or heterocyclic groups such as pyridyl or
pyrimidinyl.
Optional substituents for the phenyl and cycloalkyl rings R include halogen,
cyano,
NO2, C, alkyl, C,-4 haloalkyl, C,-0 alkoxy, phenoxy, halogen substituted
phenoxy, C,,
haloalkyl substituted phenoxy, RbS(O)n Om in which m is 0 or 1, n is 0, 1 or 2
and Rb is C,.,
alkyl, C,, haloalkyl, phenyl or benzyl, NHCOR' in which R' is C,-4 alkyl,
NRdR' in which R'
and R' independently are hydrogen or C,, alkyl; RC(O)- in which R'is hydrogen,
C,, alkyl,
C, 4 haloalkyl or C,, alkoxy; SO,NRsRh in which Rs and Rh independently are
hydrogen or
Cõ alkyl; or any two adjacent substituents together with the carbon atoms to
which they are
attached form a 5 or 6 membered heterocyclic ring containing up to three
heteroatoms
selected from 0, N or S and which may be optionally substituted by C,, alkyl,
C,., haloalkyl,
C,, alkoxy, =NOC1 , alkyl or halogen.
As used herein the term "alkyl", refers to straight or branched chains. The
term
"haloalkyl" refers to an alkyl group substituted by at least one halogen.
Similarly the term
"haloalkoxy" refers to an alkoxy group substituted by at least one halogen. As
used herein
the term "halogen" refers to fluozine, chlorine, bromine and iodine.
As used herein the term "aryl" refers to aromatic carbocyclic ring systems
such as
phenyl or naphthyl, especially phenyl.
A preferred carbocyclic ring formed by Q is an optionally substituted
cyclohexanedione.
CA 02295892 2007-05-11
30584-57
- 3a -
According to one aspect of the present invention,
there is provided a process for preparing a compound of
formula (I):
O O
II
Q C-R
(I)
or a salt thereof, wherein Q completes an optionally
substituted 5- or 6-member saturated carbocyclic ring and R
is optionally substituted phenyl or optionally substituted
C3-C6 cycloalkyl, which process comprises the rearrangement
of a compound of formula (II)
0
Q ~ O
11
O-C- R
(II)
wherein Q and R are as defined in relation to formula (I),
in a polar aprotic, dipolar aprotic or aromatic hydrocarbon
solvent in the presence of a molar excess of a moderate base
with respect to the enol ester of formula (II) and an azole
compound of formula (III)
CA 02295892 2007-05-11
30584-57
- 3b -
H
B-N
~ jA (III)
R21 N~
in which A is N or CR2z; B is N or CR23 and R21, R22 and R23 are
independently H, alkyl, or aryl or when B is CR23, R21 and R23
together with the carbon atoms to which they are attached
form a 6-membered carbocyclic ring.
According to another aspect of the present
invention, there is provided a compound of formula (VII)
Ri o
R9
Y-C ~
11 _ Rs
O
Z
R7
(VII)
wherein Y is a 1,2,4-triazolyl or a 1,2,3-benzotriazolyl
group and R7, R8, R9 and R10 are as defined in relation to
formula (IA) as described herein, provided that when Y
is 1, 2, 4-triazolyl and R' is halo, C1_4 alkyl, C1_4 haloalkyl,
C1_4 alkoxy, nitro or cyano, then none of Ra, R9 or R10 may be
halo, C1_4 alkyl, C1_4 haloalkyl, C1-4 alkoxy, nitro or cyano
at the 6-position of the phenyl ring.
One class of compounds of formula (I) is
cyclohexanediones of formula (IA)
2 Ri0
R1 R p
R9
3 0 / R
R 8
4
\ _
R 5
R6 p R7
(IA)
CA 02295892 2007-05-11
30584-57
- 3c -
in which Rl, R2, R3, R4, R5 and R6 are independently hydrogen
or C1_6 alkyl; R' is hydrogen, halogen, cyano, NO2, C1_4 alkyl,
C1_9 haloalkyl, C1-4 alkoxy or RaS in which Ra is C1-4 alkyl;
CA 02295892 2000-01-04
WO 99/28282 PCT/GB98/03458 -
- 4 -
Rg, R9 and R10 independently are hydrogen, halogen, C,4 alkyl, C,4 alkoxy, CW
haloalkyl,
C,4 haloalkoxy, CN, NO2, phenoxy, phenoxy, halophenoxy or C,.,
haloalkylphenoxy;
R'S(O)n Om in which m is 0 or 1, n is 0, 1 or 2 and Rb is C,, alkyl, C,.,
haloalkyl, phenyl or
benzyl, NHCOR in which R is C,4 alkyl, NRdR' in which Rd and R'
independently are
hydrogen or C,4 alkyl; WC(O)- in which Rf is hydrogen, C,4 alkyl, C,4
haloalkyl or C, 4
alkoxy; SOZNRgRh in which Rg and Rh independently are hydrogen or C,4 alkyl;
or any two
of R8, R9 and R10 together with the carbon atoms to which they are attached
form a 5 or 6
membered heterocyclic ring containing up to three heteroatoms selected from 0,
N or S and
which may be optionally substituted by CW alkyl, C,, haloalkyl, C,-4 alkoxy,
=NOC1-4 alkyl
or halogen which are prepared from compounds of formula (IIA)
RZ
R, 0
R3
R10
R4 / Rs
R5 ~
O C
Rg O~ Re
R (IIA)
where R', R2, R3, R4, R5, R6, R', Ra, R9 and R10 are as defined in relation to
Formula (IA).
A preferred group of compounds of Formula (IA) are those where R', RZ, R3, R',
RS
and R6 are independently hydrogen or C!_6 alkyl; R' is halogen, cyano, NOZ,
C,4 alkyl, C,4
haloalkyl, C,., alkoxy or R S in which Ra is C,, alkyl; R8, R9 and R10
independently are
hydrogen, halogen, C,, alkyl, C,., alkoxy, C,, haloalkyl, C,., haloalkoxy, CN,
NO2, phenoxy
or substituted phenoxy; RbS(O)n Om in which m is 0 or 1, n is 0, 1 or 2 and Rb
is C,4 alkyl,
C,., haloalkyl, phenyl or benzyl, NHCOR in which R is C,4 alkyl, NRdR in
which Rd and
R independently are hydrogen or C,4 alkyl; RfC(O)- in which Rf is hydrogen,
C,4 alkyl, C,4
haloalkyl or C,, alkoxy; or S02NRgR'' in which Rg and Rh independently are
hydrogen or C,,
alkyl.
Preferably R', R2, R3, R4, RS and R6 are independently hydrogen or C,4 alkyl.
More preferably R', R2, RS and R6 are hydrogen and R3 and R4 are independently
hydrogen or
methyl, especially hydrogen.
R' is preferably halogen or NO2. A preferred value for Rg is hydrogen.
CA 02295892 2000-01-04
WO 99/28282 PCT/GB98/03458 -
- 5 -
R9 is preferably hydrogen or C,-4 alkoxy, especially ethoxy. Most preferably
R9 is hydrogen.
Preferably R10 is a group RbS(O)nOm where Rb, n and m are as defined above.
More
preferably m is zero, n is 2 and Rb is CH3 or C2H5. Most preferably R10 is a
group CH3SO2
attached to the benzoyl group at the 4- position.
The most preferred compounds of Formula (IA) are 2-(2-chloro-4-
methanesulphonylbenzoyl)-1,3-cyclohexanedione and 2-(2-nitro-4-
methanesulphonylbenzoyl)-1,3 -cyclohexanedione
Another class of compounds of formula (I) are compounds of formula (IB)
O
Q O
11
C-R''
(IB)
where Q is as defined in relation to Formula (I) and R' is C3-6 cycloalkyl
optionally
substituted by one more groups RZ where RZ is as defmed for R' above. A
preferred group R''
is optionally substituted cyclopropyl. A preferred compound of Formula (IB) is
trinexepac
ethyl (ethyl4-cyclopropyl(hydroxy)methylene-3,5-
dioxocyclohexanedionecarboxylate).
Preferred azoles are compounds of Formula (III)
H
B-N
/J-_' A
R21 N (III)
in which A is N or CRZZ; B is N or CR23; R2', R22 and R23 are independently H,
alkyl, or aryl
or when B is CR23, RZ' and R23 together with the carbon atoms to which they
are attached
form a 6-membered carbocyclic ring; and salts thereof.
A is preferably N or CH.
Preferably B is N and R2' is H or B is CR 23 and RZ' and R23 together with the
carbon
atoms to which they are attached form a 6-membered unsaturated carbocyclic
ring.
*rB
hCV. vo% ta'n vi C:\CiI[_\ uE;~~ 20-10-CA 02295892 2000-01-04 CCI"l:r c~3: 4-
40 ;;;) _:3;1:~44(;:~:N 4
' - 6 -
particularly preferred compound.s of Formula (III) are 1H-),2.4-triazolY and
1H-1,2,3-benzotriazole.
Suitable salts of azoles may be. for example the potassium salt or the
tetrabutylammonium salt.
The azole is used in an arnotmt up to about 50 mole percent based on the enol
ester.
Generally aboUe 1-10 n ole % of the azole is preferred.
The process is conductcd with a molar excess, with respect to the enol ester
compound of Formula (II), of a nDoderate base. By the term "moderate base" is
mc::nt a
substance which acts as a base yet whose strength of activity as a base lies
betwecn that of
1o strong bases such as bydroxides (which could cause hydrolysis of the enol
ester) and that of
weak bases such as N,N-dimethylaniline (which would not ftanction
effecalvely). Moderate
bases suitable for use in this ernbodiment include both orga_nic bases such as
trialkylamines
and inorgaziic bases such as alkali metal carbonates and phosphates. The
triaIkylamines are
preierably tri(lower alkyl) amines having from 1 to 6, preferably 1 to 4
carbon atoms per
a!icy? a oup. A particularly preferable amine is triethylan:.ine. Suitable
inorganic bases
iasclude sodium carbonate, potassium carbonate and trisodium phosphate. Even a
bicarbonate such as potassium bicarbonate will iunction effectively in this
reaction when
used in combxnat;oa with a dipolar aprotic solvent such as dirnethylformamide.
The base is
used in an amount of from about 1 to about 4 moles per mole of enol ester,
preferably about
,~!D 2 moles per mole. A preferred base is an inorganic base especially
potassium carbonate.
A number of different solvents may be usable in this process, dependino on the
narurY
of the reactants. Suitable solvents are polar aprotic solvents (such as
aeetonitrile, cyclic
ethers such as tetrrairydrofuran, linear ethers such as 1,2-di.methoxyethane,
ketones such as
methyl Lsobutyl ketone or esters such as al",l acetates for example ethyl
acetate); dipolar
2; aprotic solvents (,such as d.unethylformamide, dimethylsulphoxide and dmac)
and i'.rom::tie
hydiocarbons (including ai.kylatcd hydrocarbons such as toluene, xylene,
cumeae, and
cymene and halogenated hydrocarbons such as chlorobenzene). Pre.ferred
solvents are polar
aprotic solvents, dipolar aprotic solvents and aromatic hydrocarbons,
especially polar aprotic
or dipola.r solvents. A particularl_y preferred solvent is acetonitrile.
30 Depending on the choice of reactants and in particular the choice of
solvents a phase
catalyst may also be employed. The selection of a suitable phase transfer
catalyst can be
AMENDED SHEET
CA 02295892 2000-01-04
WO 99/28282 PCT/GB98/03458 -
- 7 -
determined by routine procedures well known to the skilled chemist. Known
phase transfer
catalysts include tetralkyl ammonium halides and phosphonium salts. Preferred
catalysts are
tetralkyl ammonium halides, especially tetrabutyl ammonium bromide, tetrabutyl
ammonium
chloride, cetyltrimethyl ammonium bromide or cetyltrimethyl ammonium chloride.
The
phase transfer catalyst is generally used at 1-10 mol%.
If the choice of reaction conditions require the use of a phase-transfer
catalyst, the
catalyst may still be omitted by use of a suitable salt of the azole e.g. the
tetrabutyl
ammonium salt.
In one embodiment the process is carried out in a non-polar solvent, in the
presence
of a moderate inorganic base and a phase transfer catalyst.
In general, depending on the nature of the reactants and the azole, the
rearrangements may be conducted at temperatures from -10 C, up to about 100 C,
preferably 0-60 C, most preferably 20-40 C. In some cases, for instance when
there is a
possible problem of excessive by-product formation (for instance, when using
an orthonitro
benzoyl halide) the temperature should be kept at about 40 C maximum.
Depending on the nature of the reactants and more especially the nature of the
solvent
used in the reaction, water may be added to the reaction medium. In general it
has been
found that the amount of water should not exceed 0.2 w/w% of the whole system
or 0.1
mol/mol based on the substrate.
The process may be carried out using the enol ester as the starting material,
or with
generation of the enol ester in situ, for instance for the preparation of
compounds of Formula
(IA) by reaction of a compound of Formula (IV)
R' RZ O
R3
R4
(IV)
RS
s O
R
where R', R2, R3, RS and R6 are as defined in relation to Formula (IA) with a
compound
of Formula (V)
CA 02295892 2000-01-04
WO 99/28282 PCT/GB98/03458 -
- 8 -
R10
R9
ZC / (V)
OI - Ra
R~
where R7, R8, R9 and R10 are as defined in relation to Formula (IA) and Z is a
halo, preferably
chloro.
When the enol ester is utilised as a starting material it may be prepared by
any of a
number of known means. For example when preparing compounds of formula (IA),
the
appropriate enol ester is formed by acylation of a compound of Formula (IV)
with, a
compound of Formula (V).
The enol ester may be isolated from the resulting product mix by known
techniques,
for instance washing the resultant solution with acid and base, and with
saturated sodium
chloride solution, and drying. Such a technique is advantageous when a
different solvent is
preferred for the rearrangement of the enol ester to the compound of Formula
(I). The dried
enol ester may be mixed with an appropriate solvent such as acetonitrile, 1,2-
dichloroethane,
or toluene and contacted with the appropriate amounts of azole, base and,
optionally, phase
transfer catalyst and, if required, heated to the desired temperature, to
produce the final
product.
The production of compounds of Formula (I) according to the invention maybe
advantageously carried out starting with compounds such as those of Formula
(IV) and
Formula (V) and may be carried out without isolation of the intermediate enol
ester (II).
Thus the compound of Formula (IV) and the compound of Formula (V) are reacted
in the
presence of a base such as an alkali or alkaline earth metal carbonate.
The rearrangement reaction proceeds via an intermediate of Formula (VI)
O
11
Y-C-R
(VI)
where R is as defined in relation to Formula (I) and Y is the residue formed
with the azole.
When compounds of Formula (IA) are being prepared the rearrangement reaction
proceeds
via an intermediate of Formula (VIA)
CA 02295892 2000-01-04
WO 99/28282 PCT/GB98/03458 -
_ 9 _
R10
R9
Y -C /
11 - Rs
O
R'
(VIA)
where R7, R8, R9 and R10 are as defined in relation to Formula (IA) and Y is
the residue
formed with the azole.
The compounds of Formula (VI) may be isolated by standard techniques such as
filtration or extraction into a solvent such as dichloromethane and removal of
the solvent by
evaporation. To prepare compounds of formula (IA) compounds of Formula (VIA)
may then
be reacted with compounds of Formula (IV)
Z
R' O
R3
R4
(IV)
Rs
s O
R
in the presence of a solvent and a base to yield the final product.
Certain compounds of Formula (VI) are novel and as such form a further aspect
of the
invention. In particular novel compounds of formula (VII) are compounds of
formula (VIA)
where Y is al,2,4-triazolyl or a 1,2,3-benzotriazolyl group and R', R8, R9 and
R10 are as
defined in relation to Formula (IA) provided that when Y is 1,2,4-triazolyl
and R7 is halo,
CW alkyl, C,., haloalkyl, C,4 alkoxy, nitro or cyano, then none of Rg, R9 or
Rt0 may be
halo, C,.d alkyl, C,.q haloalkyl, Cõ alkoxy, nitro or cyano at the 6-position
of the phenyl ring.
In a preferred alternative the enol ester of Formula (II) may be retained in
the reaction
mass formed from a reaction of a compound of Formula (IV) with a compound of
Formula
(V) by adding an azole, water and additional base if necessary and then
retaining the
intermediate of Formula (VI) in the reaction mass and continuing the reaction
to produce the
compound of Formula (IA). Most preferably all stages are carried out using the
same
solvent.
CA 02295892 2000-01-04
WO 99/28282 PCT/GB98/03458 -
- 10 -
Comparable yields can be obtained either with or without isolation of the enol
ester
of Formula (II) and/or the isolation of compounds of Formula (VI) .
The compound of Formula (I) is obtained from this reaction in the form of its
salt.
The desired acylated compound of Formula (I) may be obtained with
acidification
and extraction with an appropriate solvent.
Compounds of formula (II), (III), (IV) and (V) are known compounds or may be
produced from known compounds by known methods.
The process of the invention is illustrated by the following examples.
EXAMPLE I
2-benzoyl-1,3-cyclohexanedione from the acid chloride.
A mixture of 1,3-Cyclohexanedione (2.31g), potassium carbonate (1.5g) and
acetonitrile (20
ml) were stirred at 35 C for 3 hrs. To the resulting suspension was added
benzoylchloride
(1.5g) over a few minutes and the mixture was stirred for 30 minutes.
Potassium carbonate
(2g) and 1,2,4-triazole (0.035g) were then added and the mixture was stirred
at 35 C for 16
hrs. After this time the reaction mixture was evaporated under reduced
pressure, the mixture
dissolved in water and acidified with HCl to precipitate the product.
Extraction into
chloroform and evaporation gave a 90% yield of 2-benzoyl-1,3-cyclohexanedione.
EXAMPLE 2
2-(2-Chloro-4-methanesulphonylbenzoyl)-1,3-cyclohexanedione from the acid
chloride.
2-Chloro-4-methanesulphonylbenzoyl chloride (5g) was prepared by the reaction
of 2-
chloro-4-methanesulphonylbenzoic acid with thionyl chloride. The acid chloride
was
dissolved in acetonitrile (40ml). A mixture of 1,3-cyclohexanedione (2.24g),
potassium
carbonate (6.9g) and acetonitrile (40m1) was stirred at room temperature for
4hours. To this
solution was added the acid chloride solution over 10min and allowed to stir 1
hour. 1,2,4-
Triazole (0.07g) was then added and the mixture allowed to stir 16 hours at
room
temperature. The solvent was removed and the residue was dissolved in water
and acidified.
The product was extracted into dichloromethane and the solvent was dried then
evaporated to
give the desired product in 83.6% yield.
EXAMPLE 3
2-benzoyl-1,3-cyclohexanedione from the enol ester.
CA 02295892 2000-01-04
WO 99/28282 PCT/GB98/03458 -
- 11 -
3-(benzoyloxy)-2-cyclohexen-l-one (2.32g), potassium carbonate (1.99g), 1,2,4-
triazole
(0.034g) and acetonitrile (20m1) were placed in a 50m1 round-bottomed flask
containing a
magnetic follower. The flask was stoppered and placed in a 35 C thermostatted
bath. The
white suspension/solution in the flask was then stirred rapidly and
periodically the stirring
was stopped for HPLC analysis samples to be taken. After 2 hours the reaction
mixture had
turned yellow and 45% of the enol ester had reacted to form 2-benzoyl-l,3-
cyclohexanedione
and benzoyl triazole. The reaction mixture had also thickened slightly, but
was not
immobile. After 6 hours the reaction was complete by HPLC with all enol ester
and benzoyl
triazole used up.
The solvent was removed on a rotary evaporator to leave a yellow solid which
was
dissolved in water (100m1) and the solution acidified with aqueous HCl to pH
2.8 (31 ml of
IM HCl) to precipitate 2-benzoyl-l,3-cyclohexanedione. The suspension was then
extracted
with chloroform (2 x 50m1), the chloroform extracts dried (magnesium
sulphate), and the
solvent removed to leave the desired product. Yield 2.07g, 89.1%.
EXAMPLE 4
2-(2-nitro-4-methanesulphonylbenzoyl)-1, 3-cyclohexanedione
3-(2-nitro-4-methanesulphonylbenzoyloxy)-2-cyclohexen-l-one (2.0g), potassium
carbonate
(1.22g), solvent (20m1), 1,2,4-triazole (0.02g) and a phase transfer catalyst
(5 mol%) were
placed in a reaction tube. The mixture was stirred at 57 C and the amount of
product (2-(2-
nitro-4-methanesulphonylbenzoyl)-1,3-cyclohexanedione) determined over a
period of time.
The results are set out in the Table below.
Solvent PTC Time Yields (% theory)
Triketone Enol Ester Hydrolysis
Toluene - 6 hours 1.1 98 -
Toluene TBAB 5 hours 69.0 10 13
Toluene CTAB 5 hours 71 2 22
MiBk - 5 hours 70 0 25
Triketone = 2-(2-nitro-4-methanesulphonylbenzoyl)-1,3-cyclohexanedione
Enol ester = 3-(2-nitro-4-methanesulphonylbenzoyloxy)-2-cyclohexen- 1 -one
CA 02295892 2000-01-04
WO 99/28282 PCT/GB98/03458 _
- 12 -
PTC = phase transfer catalyst
TBAB = tetrabutylammonium bromide
CTAB = cetyltrimethylammonium bromide
CHD = 1,3-cyclohexanedione
MiBk = methyl isobutyl ketone
EXAMPLE 5
2-(2-nitro-4-methanesulphonylbenzoyl)-1,3-cyclohexanedione
3-(2-nitro-4-methanesulphonylbenzoyloxy)-2-cylohexen-l-one (1 g), potassiiun
carbonate
(0.61g), 1,2,4-triazole (0.01 g), tetrabutylammonium bromide (see below) and
acetonitrile
(l Oml) were stirred 20 C. After the reaction period, the solvent was removed
under reduced
pressure and the residue was dissolved in water. Acidification with HCI,
extraction into
diethyl ether and evaporation gave 2-(2-nitro-4-methanesulphonylbenzoyl) 1,3-
cyclohexanedione.
With 30 mol% tetrabutylammonium bromide, the reaction gave a 93% yield after
2.5
hours. Without tetrabutylammonium bromide, the reaction gave 85% yield and 15%
hydrolysis after 12 hours.
EXAMPLE 6
2-Chloro-4-methanesulphonyl benzoyltriazolamide
CI O
N
~ N)
CH3SO O
Z
2-Chloro-4-methanesulphonylbenzoic acid (2.355g) was suspended in toluene
(150m1) and
dimethylfonnamide (0.lm1) was added via a syringe. The suspension was heated
to 75 C,
then thionyl chloride (0.8m1) in toluene (10m1) added over 30 minutes. The
suspension was
heated for 1 hour to effect reaction, refluxed for 1 hour to remove acidic
gases, then added
via a cannula to a stirred suspension of 1,2,4-triazole (1.390g) in toluene.
The resultant
suspension was stirred at room temperature for 48 hours then filtered. The
precipitate was
extracted with dichloromethane, and the solvent removed under reduced pressure
to afford
the title compound as a colourless powder (1.635g, 57.4%). Recrystalisation
from ethyl
acetate produced the compound as colourless spines.
CA 02295892 2000-01-04
WO 99/28282 PCT/GB98/03458 _
- 13 -
1 H NMR (200 MHz, CDC13 ): 9.14 (1 H,s,NCH), 8.12 (1 H, d, J 1.5Hz. H3). 8.09
(1 H, s,
NCH), 8.02 (1H.dd, J 8.I.J' 1.6Hz, HS), 7.79 (1H,d,J 8.1 Hz, H6), 3.16
(3H,s,Me).
13C NMR (200MHz, CDC13): 163.82, 154.04, 144.65, 144.57, 136.32, 134.54,
130.78,
129.20, 125.77, 44.33.
EXAMPLE 7
2-Nitro-4-methanesulphonyl benzoyltriazolamide
N02 O
Q ~=N
CH3SO2
2-Nitro-4-methanesulphonylbenzoic acid (5.12g), was suspended in 50/50 w/w
xylene/acetonitrile (300m1) and dimethylformamide (0.lml), added via a
syringe. The
suspension was heated to 75 C, then thionyl chloride (1.7m1), in xylene (l
Oml) added over
30 minutes. The suspension was heated for 6 hours to effect reaction, then
added via a
cannula to a stirred suspension of 1,2,4-triazole (2.918g), in 50/50 w/w
xylene/acetonitrile
(l 00m1). The resultant suspension was stirred at room temperature for 15
hours, filtered,
and the solvent removed under reduced pressure to afford the title compound as
a pale
yellow powder (3.53g, 56.8%). Recystallisation from ethyl acetate yielded the
compound as
colourless plates.
1 H MNR (200 MHz, d6.acetone) : 9.40 (1 H,s,NCH) , 8.83 (1 H, d, J 1.5 Hz,
H3), 8.59 (1H,
dd, J8.1, J' 1.7Hz, H5) 8.26 (1H, d,J 7.9 HzH6), 8.14 (1H,s,NCH), 3.41
(3H,s,Me):
13C NMR (200MHz, d6. acetone) : 163.57, 154.87, 147.77, 145.97, 145.63,
134.47, 133.46,
132.37, 124.51, 43.94.
EXAMPLE 8
2-(2-Chloro-4-methanesulphonyl benzoyl)cyclohexan-1,3-dione,
CI O O
0
CH3So2 O
1-(2-chloro-4-methanesulphonylbenzoyl)-1,2,4-triazole (388mg), cyclohexan-1,3-
dione
(161mg) and potassium carbonate (259mg) were suspended in acetonitrile (30m1)
and stirred
CA 02295892 2000-01-04
WO 99/28282 PCT/GB98/03458 _
- 14 -
overnight. The acetonitrile was removed under reduced pressure, the residue
dissolved in
water ( l 00m1) and acidified with 1 M hydrogen chloride solution to pH 1.5.
The solution was
extracted with dichloromethane, dried (magnesium sulfate), filtered and the
solvent removed
under reduced pressure to afford the title compound as a pale yellow solid
(0.263g, 55.6%).
'H NMR (200 Mhz, CDC13); 7.88-8.01 (2H,m,Ph), 7.36-7.40 (1H, m, Ph), 3.11
(3H,s,Me),
2.68-2.76(2H,m,CH2C=O), 2.37-2.47 (2H,m,CH2C=O), 1.97-2.18(2H,m,CH2-CH2).
EXAMPLE 9
2-(2-nitro-4-methanesulphonyl benzoyl)cyclohexan-1,3-dione
No2 o O
O
CH3SO O
l0 1-(2-Nitro-4-methanesulphonylbenzoyl)-1,2,4-triazole (666mg), cyclohexan-
1,3-dione
(254mg) and potassium carbonate (419mg, 1.8mmol, 1.4 equiv) were suspended in
acetonitrile (30m1) and stirred overnight. The acetonitrile was removed under
reduced
pressure, the residue dissolved in water ( l 00m1) and acidified with 1 M
hydrogen chloride
solution to pH 1.5. The solution was extracted with dichloromethane, dried
(magnesium
sulfate), filtered and the solvent removed under reduced pressure to afford
the title
compound as a pale yellow solid (0.789g, quant).
'H NMR (200Mhz, CDC13); 8.74 (1H,s,H6); 8.26(1H,d, J 8.1 Hz, Ph); 7.46 (1H, d,
J 7.86 Hz,
Ph), 3.16 (3H, s, Me , 2.83 (2H,t, J 6.3Hz, CH2-C=0), 2.36 (2H,t, J 6.5Hz, CH2-
C=0), 2.00-
2.12 (2H,m, CH CHZ).
13C NMR (200 Mhz, CDC13); 195.86; 194.26; 145.66; 142.04; 141.17; 132.76;
128.26;
123.23; 112.69; 44.37; 37.24; 31.63; 19.11.