Note: Descriptions are shown in the official language in which they were submitted.
CA 02296089 2000-O1-14
WO 99/04010 PCT/CA98100697
TITLE OF INVENTION
NUChEIC ACID VACCINES ENCODING G PROTEIN OF
RESPIRATORY SYNCYTIAL VIRUS
FIELD OF INVENTION
The present invention is related to the field of
respiratory syncytial virus (RSV) vaccines and is
particularly concerned with vaccines comprising nucleic
acid sequences encoding the attachment (G) protein of
RSV.
BACKGROUND OF INVENTION
Respiratory syncytial virus (RSV), a negative
strand RNA virus belonging to the Paramyxoviridae family
of viruses, is the major viral pathogen responsible for
bronchiolitis and pneumonia in infants and young
children (ref. 1 - Throughout this application, various
references are referred to in parenthesis to more fully
describe the state of the art to which this invention
pertains. Full bibliographic information for each
citation is found at the end of the specification,
immediately preceding the claims. The disclosures of
these references are hereby incorporated by reference
into the present disclosure). Acute respiratory tract
infections caused by RSV result in approximately 90,000
hospitalizations and 4,500 deaths per year in the United
States (ref. 2). Medical care costs due to RSV
infection are greater than $340 M annually in the United
States alone (ref. 3). There is currently no licensed
vaccine against RSV. The main approaches for developing
an RSV vaccine have included inactivated virus, live-
attenuated viruses and subunit vaccines.
A protective immune response against RSV is thought
- to require the induction of neutralizing antibodies
against the surface fusion (F) and attachment (G)
glycoproteins (ref. 4). In addition, cytotoxic T
lymphocytes (CTL) responses are involved in viral
clearance. The F protein is conserved amongst the RSV A
SUBSTITUTE SHEET (RULE 26)
CA 02296089 2000-O1-14
WO 99!04010 PCT/CA98/00697
2
and B subgroups.
The G protein (33 kDa) of RSV is heavily O-
glycosylated giving rise to a glycoprotein of apparent
molecular weight of 90 kDa (ref. 5). Two broad subtypes ,
of RS virus have been defined: A and B (ref. 6) . The
major antigenic differences between these subtypes are
found in the G glycoprotein (refs. 3, 7).
The use of RSV proteins as vaccines may have
obstacles. Parenterally administered vaccine candidates
have so far proven to be poorly immunogenic with regard
to the induction of neutralizing antibodies in
seronegative chimpanzees. The serum antibody response
induced by these antigens may be further diminished in
the presence of passively acquired antibodies, such as
the transplacentally acquired maternal antibodies which
most young infants possess. A subunit vaccine candidate
for RSV consisting of purified fusion (F) glycoprotein
from RSV infected cell cultures and purified by
immunoaffinity or ion-exchange chromatography has been
described (ref. 8). Parenteral immunization of
seronegative or seropositive chimpanzees with this
preparation was performed and three doses of 50 ~g were
required in seronegative animals to induce an RSV serum
neutralizing titre of approximately 1:50. Upon
subsequent challenge of these animals with wild-type
RSV, no effect of immunization on virus shedding or
clinical disease could be detected in the upper
respiratory tract. The effect of immunization with this
vaccine on virus shedding in the lower respiratory tract
was not investigated, although this is the site where
the serum antibody induced by parenteral immunization .
may be expected to have its greatest effect. Safety and
immunogenicity studies have been performed in a small .
number of seropositive individuals. The vaccine was
found to be safe in seropositive children and in three
SUBSTITUTE SHEET (RULE 26)
CA 02296089 2000-O1-14
WO 99/04010 PCT/CA98100697
3
seronegative children (all > 2.4 years of age). The
effects of immunization on lower respiratory tract
disease could not be determined because of the small
number of children immunized. One immunizing dose in
' S seropositive children induced a 4-fold increase in virus
neutralizing antibody titres in 40 to 60% of the
vaccinees. Thus, insufficient information is available
from these small studies to evaluate the efficacy of
this vaccine against RSV-induced disease. A further
l0 problem facing subunit RSV vaccines is the possibility
that inoculation of seronegative subjects with
immunogenic preparations might result in disease
enhancement. In the 1960's, vaccination of infants with
a formalin-inactivated RSV preparation (FI-RSV) resulted
15 in enhanced lung disease upon subsequent exposure to
live virus, also referred to as immunopotentiation
(refs. 9, 10). These vaccinees developed strong
serological responses, but were not protected against
infection and some developed severe, occasionally fatal
20 respiratory tract disease upon natural infection.
Although precise mechanisms remain unknown, it has been
suggested that this form of immune enhancement might
reflect either structural alterations of RSV antigens
(ref. 11), residual serum and/or cellular contaminants
25 (ref. 12), a specific property of the viral attachment
(G) protein (refs. 13,14) or an imbalanced cell-mediated
immune response (refs. 13,15). It has been demonstrated
that the FI-RSV vaccine induced a TH2-type immune
response in mice whereas immunization with live RSV,
30 which does not cause immunopotentiation, elicits a TH1
response (ref . I5) .
In some studies, the immune response to
immunization with a synthetic RSV FG fusion protein
' resulted in disease enhancement in rodents resembling
35 that induced by a formalin-inactivated RSV vaccine.
SUBSTITUTE SHEET (RULE 26)
CA 02296089 2000-O1-14
WO 99/04010 PCT/CA98/00697
4
Immunization of mice with a recombinant vaccinia virus
expressing the RSV G protein resulted in G-specific T
cell responses in the lungs which are exclusively
recruited from the CD4+T cell sublineage and are .
strongly Th2-biased. G-specific T cells induce lung
haemmorrage, pulmonary neutrophil recruitment (shock
lung), intense pulmonary eosinophilia, and sometimes
death in the adoptively transferred murine recipients
(ref. 14). The association of immunization with disease
enhancement using certain vaccine preparations including
non-replicating antigens suggests caution in their use
as vaccines in seronegative humans.
Live attenuated vaccines against disease caused by
RSV may be promising for two main reasons. Firstly,
infection by a live vaccine virus induces a balanced
immune response comprising mucosal and serum antibodies
and cytotoxic T-lymphocytes. Secondly, infection of
infants with live attenuated vaccine candidates or
naturally acquired wild-type virus is not associated
with enhanced disease upon subsequent natural
reinfection. It will be challenging to produce live
attenuated vaccines that are immunogenic for younger
infants who possess maternal virus-neutralizing
antibodies and yet are attenuated for seronegative
infants greater than or equal to 6 months of age.
Attenuated live virus vaccines also have the risks of
residual virulence and genetic instability.
Injection of plasmid DNA containing sequences
encoding a foreign protein has been shown to result in
expression of the foreign protein and the induction of
antibody and cytotoxic T-lymphocyte (CTL) responses to
the antigen in a number of studies (see, for example,
refs. 16, 17, 18). The use of plasmid DNA inoculation
to express viral proteins for the purpose of
immunization may offer several advantages over the
SUBSTITUTE SHEET (RULE 26)
CA 02296089 2000-O1-14
WO 99104010 PCT/CA98/00697
strategies summarized above. Firstly, DNA encoding a
viral antigen can be introduced in the presence of
' antibody to the virus itself, without loss of potency
due to neutralization of virus by the antibodies.
5 Secondly, the antigen expressed in vivo should exhibit a
native conformation and the appropriate glycosylation.
Therefore, the antigen should induce an antibody
response similar to that induced by the antigen present
in the wild-type virus infection. In contrast, some
processes used in purification of proteins can induce
conformational changes which may result in the loss of
immunogenicity of protective epitopes and possibly
immunopotentiation. Thirdly, the expression of proteins
from injected plasmid DNAs can be detected in vivo for a
considerably longer period of time than that in virus
infected cells, and this has the theoretical advantage
of prolonged cytotoxic T-cell induction and enhanced
antibody responses. Fourthly, in vivo expression of
antigen may provide protection without the need for an
2 0 extrinsic adj uvant .
The ability to immunize against disease caused by
RSV by administration of a DNA molecule encoding an RSV
G protein was unknown before the present invention. In
particular, the efficacy of immunization against RSV
induced disease using a gene encoding a secreted form of
the RSV G protein was unknown. Infection with RSV leads
to serious disease. It would be useful and desirable to
provide isolated genes encoding RSV G protein and non-
replicating vectors, including plasmid vectors, for in
vivo administration and for use in immunogenic
preparations, including vaccines, for protection against
disease caused by RSV and for the generation of
diagnostic reagents and kits. In particular, it would
" be desirable to provide vaccines that are immunogenic
and protective in humans, including seronegative
SUBSTITUTE SHEET (RULE 26)
CA 02296089 2000-O1-14
WO 99/04010 PCT/CA98/00697
6
infants, that do not cause disease enhancement
(immunopotentiation) .
SUMMARY OF INVENTION
The present invention relates to a method of
immunizing a host against disease caused by respiratory
syncytial virus, to non-replicating vectors containing
nucleic acid molecules used in immunogenic compositions
for such purpose, and to diagnostic procedures utilizing
the vectors and nucleic acid molecules. In particular,
the present invention is directed towards the provision
of nucleic acid vaccines encoding the G protein of
respiratory syncytial virus.
In accordance with one aspect of the invention,
there is provided an immunogenic composition for in vivo
administration to a host for the generation in the host
of protective antibodies to respiratory syncytial virus
(RSV) G protein, comprising a non-replicating vector
comprising:
a first nucleotide sequence encoding a RSV G
protein or a RSV G protein fragment that generates
antibodies that specifically react with RSV G protein,
a promoter sequence operatively coupled to said
first nucleotide sequence for expression of said RSV G
protein in the host, and
a second nucleotide sequence located between said
first nucleotide sequence and said promoter sequence to
increase expression of said RSV G protein in vivo from
said vector in the host, and
a pharmaceutically-acceptable carrier therefor.
The first nucleotide sequence may be that which
encodes a full-length RSV G protein. The first
nucleotide sequence may comprise the nucleotide sequence
shown in Figure 2 {SEQ. ID No: 1) or encode a full
length RSV G protein having the amino acid sequence
shown in Figure 2 (SEQ. ID no: 2?.
SUBSTITUTE SHEET (RULE 26)
CA 02296089 2000-O1-14
WO 99/04010 PCTlCA98100697
7
Alternatively, the first nucleotide sequence may be
that which encodes an RSV G protein from which the
transmembrane coding sequence and sequences upstream
thereof are absent. The first nucleotide sequence
encoding the truncated RSV G protein may comprise the
nucleotide sequence shown in Figure 3 (SEQ. ID no: 3) or
may comprise a nucleotide sequence encoding the
truncated RSV G protein having the amino acid sequence
shown in Figure 3 (SEQ ID no: 4). The lack of
expression of the transmembrane region results in a
secreted form of the RSV G protein.
The non-replicating vector may further comprise a
heterologous signal peptide encoding nucleotide sequence
immediately upstream of the 5'-terminus of the first
nucleotide sequence. The signal peptide encoding
sequence may encode the signal peptide of human tissue
plasminogen activator.
The promoter sequence may be an immediate early
cytomegalovirus (CMV) promoter. The second nucleotide
sequence may comprise the human cytomegalovirus Intron
A.
The non-replicating vector generally is a plasmid
vector. Plasmid vectors encoding the G protein and
included in the immunogenic composition provided by this
aspect of the invention may specifically be pXLS or
pXL6, constructed and having their characterizinQ_
elements, as seen in Figures 4 or 5, respectively.
In accordance with a further aspect of the present
invention, there is provided a method of immunizing a
host against disease caused by infection with
respiratory syncytial virus (RSV), which comprises
administering to the host an effective amount of a non-
replicating vector comprising:
' a first nucleotide sequence encoding an RSV G
protein or a RSV G protein fragment that generates
SUBSTITUTE SHEET (RULE 26)
CA 02296089 2000-O1-14
WO 99/04010 PCT/CA98/00697
8
antibodies that specifically react with RSV G protein,
a promoter sequence operatively coupled to said
first nucleotide sequence for expression of said RSV G
protein in the host, and
a second nucleotide sequence located between said
first nucleotide sequence and said promoter sequence to
increase expression of said RSV G protein in vivo from
said vector in the host.
The immunization method may be effected to induce a
balanced Thl/Th2 immune response.
The present invention also includes a novel method
of using a gene encoding respiratory syncytial virus
(RSV) G protein or a RSV G protein fragment that
generates antibodies that specifically react with RSV G
protein, to protect a host against disease caused by
infection with respiratory syncytial virus, which
comprises:
isolating the gene;
operatively linking the gene to at least one
control sequence to produce a non-replicating. vector,
said control sequence directing expression of the RSV G
protein when said vector is introduced into a host to
produce an immune response to the RSV G protein, and
introducing the vector into the host.
The procedure provided in accordance with this aspect of
the invention may further include the step of:
operatively linking the gene to an immunoprotection
enhancing sequence to produce an enhanced
immunoprotection by the RSV G protein in the host,
preferably by introducing the immunoprotection enhancing
sequence between the control sequence and the gene,
including introducing immunostimulatory CpG sequences in
the vector.
In addition, the present invention includes a
method of producing a vaccine for protection of a host
SUBSTITUTE SHEET (RULE 26)
CA 02296089 2000-O1-14
WO 99/04010 PCTlCA98/00697
9
against disease caused by infection with respiratory
syncytial virus (RSV), which comprises:
isolating a first nucleotide sequence encoding an
RSV G protein or a RSV G protein fragment that generates
antibodies that specifically react with RSV G protein,
operatively linking the first nucleotide sequence
to at least one control sequence to produce a non-
replicating vector, the control sequence directing
expression of the RSV G protein when introduced into a
host to produce an immune response to the RSV G protein
when expressed in vivo from the vector in a host,
operatively linking the first nucleotide sequence
to a second nucleotide sequence to increase expression
of the RSV G protein in vivo from the vector in a host,
and
formulating the vector as a vaccine for in vivo
administration.
The vector may be a plasmid vector selected from
pXL5 and pXL6. The invention further includes a vaccine
for administration to a host, including a human host,
produced by this method.
As noted previously, the vectors provided herein
are useful in diagnostic applications. In a further
aspect of the invention, therefore, there is provided a
method of determining the presence of a respiratory
syncytial virus (RSV) G protein in a sample, comprising
the steps of:
(a) immunizing a host with a non-replicating
vector to produce antibodies specific for the RSV G
protein, the non-replicating vector comprising a
first nucleotide sequence encoding an RSV G protein
or an RSV G protein fragment that generates
antibodies that specifically react with RSV G
protein, a promoter sequence operatively coupled to
the first nucleotide sequence for expression of the
SUBSTITUTE SHEET (RULE 26)
CA 02296089 2000-O1-14
WO 99/04010 PCTlCA98/00697
RSV G protein in the host and a second nucleotide
sequence located between the first nucleotide
sequence and the promoter sequence to increase
expression of the RSV G protein in vivo from the
5 vector in the host;
(b) isolating the RSV G protein-specific
antibodies;
(c) contacting the sample with the isolated
antibodies to produce complexes comprising any RSV
10 G protein present in the sample and the RSV G
protein-specific antibodies; and
(d) determining production of the complexes.
The non-replicating vector employed to elicit the
antibodies may be a plasmid vector pXL5 or pXL6.
The invention also includes a diagnostic kit for
detecting the presence of a respiratory syncytial virus
(RSV) G protein in a sample, comprising:
(a) a non-replicating vector capable of
generating antibodies specific for the RSV G
protein when administered to a host, said non
replicating vector comprises a first nucleotide
sequence encoding an RSV G protein or an RSV G
protein fragment that generates antibodies that
specifically react with RSV G protein, a promoter
sequence operatively coupled to the first
nucleotide sequence for expression of the RSV G
protein in a host, and a second nucleotide sequence
located between the first nucleotide sequence and
the promoter sequence to increase expression of the
RSV G protein is vivo from the vector in the host;
(b) isolation means to isolate the RSV G protein
specific antibodies;
(c) contacting means to contact the isolated RSV
G protein-specific antibodies with the sample to
produce a complex comprising any RSV G protein
SUBSTITUTE SHEET (MULE 26)
CA 02296089 2000-O1-14
WO 99/04010 PCT/CA98/00697
11
present in the sample and RSV G protein specific
antibodies; and
(d) identifying means to determine production of
the complex.
The present invention further is directed to a
method for producing antibodies specific for a G protein
of a respiratory syncytial virus (RSV) comprising:
(a) immunizing a host with an effective amount of a
non-replicating vector to produce RSV G-specific
antibodies, said non-replicating vector
comprising:
a first nucleotide sequence encoding a RSV G
protein or a RSV G protein fragment that generates
antibodies that specifically react with RSV G
protein,
a promoter sequence operatively coupled to
said first nucleotide sequence for expression of
said RSV G protein in the host, and
a second nucleotide sequence located between
2fl said first nucleotide sequence and said promoter
sequence to increase expression of said RSV G
protein in vivo from said vector in the host; and
(b) isolating the RSV G specific antibodies from
the host.
The present invention is also directed to a method
for producing monoclonal antibodies specific for a G
protein of respiratory syncytial virus (RSV), comprising
the steps of
(a) constructing a vector comprising a first
nucleotide sequence encoding a RSV G protein or a
RSV G protein fragment that generates antibodies
that specifically react with RSV G protein, a
promoter sequence operatively coupled to the first
' nucleotide sequence for expression of the RSV G
protein in the host and a second nucleotide
SUBSTITUTE SHEET (RULE 26)
CA 02296089 2000-O1-14
WO 99/04010 PCT/CA98/00697
12
sequence located between the first nucleotide
sequence and the promoter sequence to increase
expression of the RSV G protein when in vivo from
the vector in a host;
(b) administering the vector to at least one
mouse to produce at least one immunized mouse;
(c) removing B-lymphocytes from the at least one
immunized mouse;
(d) fusing the B-lymphocytes from the at least
one immunized mouse with myeloma cells, thereby
producing hybridomas;
(e) cloning the hybridomas;
(f) selecting clones which produce anti-RSV G
protein antibody;
(g) culturing the anti-RSV G protein antibody-
producing clones; and
(h) isolating anti-RSV G protein monoclonal
antibodies.
Such,monoclonal antibodies may be used to purify RSV G
protein from virus.
In this application, the term "RSV G protein" is
used to define a full-length RSV G protein, such
proteins having variations in their amino acid sequences
including those naturally occurring in various strains
of RSV, a secreted form of RSV G protein lacking a
transmembrane region, as well as functional analogs of
the RSV G protein. In this application, a first protein
is a "functional analog" of a second protein if the
first protein is immunologically related to and/or has
the same function as the second protein. The functional
analog may be, for example, an immunologically-active
fragment of the protein or an immunologically-active
substitution, addition or deletion mutant thereof.
SUBSTITUTE SHEET (RULE 2fi)
CA 02296089 2000-O1-14
WO 99!04010 PCT/CA98/00697
13
BRIEF DESCRIPTION OF THE FIGURES
The present invention will be further understood
from the following General Description and Examples with
reference to the Figures of the accompanying drawings,
in which:
Figure 1 illustrates a restriction map of the gene
encoding a G protein of respiratory syncytial virus
(RSV);
Figure 2 illustrates the nucleotide sequence of a
gene encoding a membrane bound form of the G protein of
respiratory syncytial virus (SEQ ID No: 1) as well as
the amino acid sequence of the RSV G protein encoded
thereby (SEQ ID No: 2);
Figure 3 illustrates the nucleotide sequence of a
gene encoding the secreted form of the RSV G protein
lacking the transmembrane domain (SEQ ID No: 3) as well
as the amino acid sequence of a truncated RSV G protein
lacking the transmembrane domain encoded thereby (SEQ ID
No: 4);
Figure 4 shows the construction of plasmid pXL5
containing a gene encoding a full-length membrane
attached form of the RSV G protein and containing the
CMV Intron A sequence;
Figure 5 shows the construction of plasmid pXL6
containing a gene encoding a secreted form of the RSV G
protein lacking the transmembrane domain and containing
the CMV Intron A sequence as well as a nucleotide
sequence encoding a signal peptide of the human tissue
plasminogen activator (TPA);
Figure 6 shows the nucleotide sequence for the
plasmid VR-1012 (SEQ ID No. 5);
Figure 7 shows the nucleotide sequence for the 5'
untranslated region and the signal peptide of the human
tissue plasminogen activator (TPA)(SEQ. ID no: 6) and
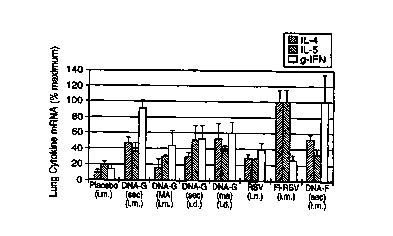
Figure 8 shows the lung cytokine expression profile
SUBSTITUTE SHEET (RULE 26)
CA 02296089 2000-O1-14
WO 99/04010 PCTICA98/00697
14
in DNA immunized mice after RSV challenge.
GENERAL DESCRIPTION OF INVENTION
As described above, the present invention relates
generally to polynucleotide, including DNA, immunization
to obtain protection against infection by respiratory
syncytial virus (RSV) and to diagnostic procedures using
particular non-replicating vectors. In the present
invention, several recombinant plasmid vectors were
constructed to contain a nucleotide sequence encoding an
RSV G protein.
The nucleotide sequence of the full length RSV G
gene is shown in Figure 2 (SEQ ID No: 1). Certain
constructs provided herein include the nucleotide
sequence encoding the full-length RSV G (SEQ ID No: 2)
protein while others include an RSV G gene modified by
deletion of the transmembrane coding sequence and
nucleotides upstream thereof (see Figure 3, SEQ ID No:
3), to produce a secreted or truncated RSV G protein
lacking the transmembrane domain (SEQ ID No. 4).
The nucleotide sequence encoding the RSV G protein
is operatively coupled to a promoter sequence for
expression of the encoded RSV G protein in vivo. The
promoter sequence may be the human immediately early
cytomegalovirus (CMV) promoter. This promoter is
described in ref. 19. Any other convenient promoter may
be used, including constitutive promoters, such as, the
Rous Sarcoma Virus LTRs, and inducible promoters, such
as the metallothionin promoter, and tissue specific
promoters.
The non-replicating vectors provided herein, when
administered to an animal in the form of an immunogenic
composition with a pharmaceutically-acceptable carrier,
effect in vivo RSV G protein expression, as demonstrated
by an antibody response in the animal to which it is
administered. Such antibodies may be used herein in the
SUBSTITUTE SHEET (RULE 26)
CA 02296089 2000-O1-14
WO 99104010 PCTlCA98/00697
1$
detection of RSV protein in a sample, as described in
more detail below. The administration of the non-
replicating vectors, specifically plasmids pXL5 and
pXL6, produced anti-G antibodies, virus neutralizing
antibodies, a balanced Thl/Th2 response in the lungs
post viral challenge and conferred protection in mice
against live RSV infection, as seen from the Examples
below.
The recombinant vector also may include a second
nucleotide sequence located adjacent the RSV G protein
encoding nucleotide sequence to enhance the
immunoprotective ability of the RSV G protein when
expressed in vivo in a host. Such enhancement may be
provided by increased in vivo expression, for example,
by increased mRNA stability, enhanced transcription
and/or translation. This additional sequence generally
is located between the promoter sequence and the RSV G
protein-encoding sequence. This enhancement sequence
may comprise the immediate early cytomegalovirus Intron
A sequence.
The non-replicating vector provided herein may also
comprise an additional nucleotide sequence encoding a
further antigen from RSV, an antigen from at least one
other pathogen or at least one immunomodulating agent,
such as a cytokine. Such vector may contain the
additional nucleotide sequence in a chimeric or a
bicistronic structure. Alternatively, vectors
containing the additional nucleotide sequence may be
separately constructed and coadministered to a host,
along with the non-replicating vectors provided herein.
The non-replicating vector may further comprise a
' nucleotide sequence encoding a heterologous viral or
eukaryotic signal peptide, such as the human tissue
plasminogen activator (TPA) signal peptide, in place of
the endogenous signal peptide for the truncated RSV G
SUBSTITUTE SHEET (RULE 2fi)
CA 02296089 2000-O1-14
WO 99/04010 PCT/CA98/00697
16
protein. Such nucleotide sequence may be located
immediately upstream of the RSV G encoding sequence in
the vector.
The immunogenicity of the non-replicating DNA
vectors may be enhanced by inserting immunostimulatory
CpG sequences in the vector.
It is clearly apparent to one skilled in the art,
that the various embodiments of the present invention
have many applications in the fields of vaccination,
diagnosis and treatment of RSV infections. A further
non-limiting discussion of such uses is further
presented below.
1. Vaccine Preparation and Use
Immunogenic compositions, suitable to be used as
vaccines, may be prepared from the RSV G genes and
vectors as disclosed herein. The vaccine elicits an
immune response in an animal which includes the
production of anti-RSV G antibodies. Immunogenic
compositions, including vaccines, containing the nucleic
acid may be prepared as injectables, in physiologically-
acceptable liquid solutions or emulsions for
polynucleotide administration. The nucleic acid may be
associated with Iiposomes, such as lecithin liposomes or
other liposomes known in the art, as a nucleic acid
liposome (for example, as described in WO 9324640, ref.
20) or the nucleic acid may be associated with an
adjuvant, as described in more detail below. Liposomes
comprising cationic lipids interact spontaneously and
rapidly with polyanions, such as DNA and RNA, resulting
in liposome/nucleic acid complexes that capture up to
100% of the polynucleotide. In addition, the
polycationic complexes fuse with cell membranes,
resulting in an intracellular delivery of polynucleotide
that bypasses the degradative enzymes of the lysosomal
compartment. Published PCT application WO 94/27435
SUBSTITUTE SHEET (RULE 26)
CA 02296089 2000-O1-14
WO 99104010 PCT/CA98100697
17
describes compositions for genetic immunization
comprising cationic lipids and polynucleotides. Agents
which assist in the cellular uptake of nucleic acid,
such as calcium ions, viral proteins and other
transfection facilitating agents, may advantageously be
used.
Polynucleotide immunogenic preparations may also be
formulated as microcapsules, including biodegradable
time-release particles. Thus, U.S. Patent 5,151,264
describes a particulate carrier of a
phospholipid/glycolipid/polysaccharide nature that has
been termed Bio Vecteurs Supra Moleculaires (BVSM). The
particulate carriers are intended to transport a variety
of molecules having biological activity in one of the
layers thereof.
U.S. Patent 5,075,109 describes encapsulation of
the antigens trinitrophenylated keyhole limpet
hemocyanin and staphylococcal enterotoxin B in 50:50
poly (DL-lactideco-glycolide). Other polymers for
encapsulation are suggested, such as poly(glycolide),
poly(DL-lactide-co- glycolide), copolyoxalates,
polycaprolactone, poly(lactide-co-caprolactone),
poly(esteramides), polyorthoesters and poly(8-
hydroxybutyric acid), and polyanhydrides.
Published PCT application WO 91/06282 describes a
delivery vehicle comprising a plurality of bioadhesive
microspheres and antigens. The microspheres being of
starch, gelatin, dextran, collagen or albumin. This
delivery vehicle is particularly intended for the uptake
of vaccine across the nasal mucosae. The delivery
vehicle may additionally contain an absorption enhancer.
The RSV G gene containing non-replicating vectors
may be mixed with pharmaceutically acceptable excipients
which are compatible therewith. Such excipients may
include, water, saline, dextrose, glycerol, ethanol, and
SUBSTITUTE SHEET (RULE 26)
CA 02296089 2000-O1-14
WO 99/04010 PCT/CA98/00697
18
combinations thereof. The immunogenic compositions and
vaccines may further contain auxiliary substances, such
as wetting or emulsifying agents, pH buffering agents,
or adjuvants to enhance the effectiveness thereof.
Immunogenic compositions and vaccines may be
administered parenterally, by injection subcutaneously,
intravenously, intradermally or intramuscularly,
possibly following pretreatment of the injection site
with a local anesthetic. Alternatively, the immunogenic
compositions formed according to the present invention,
may be formulated and delivered in a manner to evoke an
immune response at mucosal surfaces. Thus, the
immunogenic composition may be administered to mucosal
surfaces by, for example, the nasal or oral
(intragastric) routes. Alternatively, other modes of
administration including suppositories and oral
formulations may be desirable. For suppositories,
binders and carriers may include, for example,
polyalkylene glycols or triglycerides. Oral
formulations may include normally employed incipients,
such as, for example, pharmaceutical grades of
saccharine, cellulose and magnesium carbonate.
The immunogenic preparations and vaccines are
administered in a manner compatible with the dosage
formulation, and in such amount as will be
therapeutically effective, protective and immunogenic.
The quantity to be administered depends on the subject
to be treated, including, for example, the capacity of
the individual's immune system to synthesize the RSV G
protein and antibodies thereto, and if needed, to
produce a cell-mediated immune response. Precise
amounts of active ingredient required to be administered
depend on the judgment of the practitioner. However,
suitable dosage ranges are readily determinable by one
skilled in the art and may be of the order of about 1 ~tg
SUBSTITUTE SHEET (RULE 26)
CA 02296089 2000-O1-14
WO 99104010 PCT/CA98/00697
19
to about 2 mg of the RSV G gene-containing vectors.
Suitable regimes for initial administration and booster
' doses are also variable, but may include an initial
administration followed by subsequent administrations.
S The dosage may also depend on the route of
administration and will vary according to the size of
the host. A vaccine which protects against only one
pathogen is a monovalent vaccine. Vaccines which
contain antigenic material of several pathogens are
l0 combined vaccines and also belong to the present
invention. Such combined vaccines contain, for example,
material from various pathogens or from various strains
of the same pathogen, or from combinations of various
pathogens.
15 Immunogenicity can be significantly improved if the
vectors are co-administered with adjuvants, commonly
used as 0.05 to 0.1 percent solution in phosphate-
buffered saline. Adjuvants enhance the immunogenicity
of an antigen but are not necessarily immunogenic
20 themselves. Adjuvants may act by retaining the antigen
locally near the site of administration to produce a
depot effect facilitating a slow, sustained release of
antigen to cells of the immune system. Adjuvants can
also attract cells of the immune system to an antigen
25 depot and stimulate such cells to elicit immune
responses.
Immunostimulatory agents or adjuvants have been
used for many years to improve the host immune responses
to, for example, vaccines. Thus, adjuvants have been
30 identified that enhance the immune response to antigens.
Some of these adjuvants are toxic, however, and can
cause undesirable side-effects, making them unsuitable
for use in humans and many animals. Indeed, only
aluminum hydroxide and aluminum phosphate (collectively
35 commonly referred to as alum) are routinely used as
SUBSTITUTE SHEET (RULE 26)
CA 02296089 2000-O1-14
WO 99/04010 PCT/CA98100697
adjuvants in human and veterinary vaccines.
A wide range of extrinsic adjuvants and other
immunomodulating material can provoke potent immune
responses to antigens. These include saponins complexed
5 to membrane protein antigens to produce immune
stimulating complexes (ISCOMS), pluronic polymers with
mineral oil, killed mycobacteria in mineral oil,
Freund's complete adjuvant, bacterial products, such as
muramyl dipeptide (MDP) and lipopolysaccharide (LPS), as
10 well as monophoryl lipid A, QS 21 and polyphosphazene.
In particular embodiments of the present invention,
the non-replicating vector comprising a first nucleotide
sequence encoding an G protein of RSV may be delivered
in conjunction with a targeting molecule to target the
15 vector to selected cells including cells of the immune
system.
The immunogenicity of the non-replicating vector
may be enhanced by coadministering plasmid DNA vectors
expressing cytokines or chemokines or by coexpressing
20 such molecules in a bis-cistronic or fusion construct.
The non-replicating vector may be delivered to the
host by a variety of procedures, for example, Tang et
al. (ref. 21) disclosed that introduction of gold
microprojectiles coated with DNA encoding bovine growth
hormone (BGH) into the skin of mice resulted in
production of anti-BGH antibodies in the mice, while
Furth et al. (ref. 22) showed that a jet injector could
be used to transfect skin, muscle, fat and mammary
tissues of living animals.
3 0 2 . Iuanunoassays
The RSV G genes and vectors of the present
invention are useful as immunogens for the generation of
anti-G antibodies for use in immunoassays, including
enzyme-linked immunosorbent assays (ELISA), RIAs and
other non-enzyme linked antibody binding assays or
SUBSTITUTE SHEET (RULE 26)
CA 02296089 2000-O1-14
WO 99/04010 PCTICA98100697
21
procedures known in the art. In ELISA assays, the non-
replicating vector first is administered to a host to
generate antibodies specific to the RSV G protein.
These RSV G-specific antibodies are immobilized onto a
selected surface, for example, a surface capable of
binding the antibodies, such as the wells of a
polystyrene microtiter plate. After washing to remove
unadsorbed antibodies, a non-specific protein, such as a
solution of bovine serum albumin (BSA) that is known to
be antigenically neutral with regard to the test sample,
may be bound to the selected surface. This allows for
blocking of non-specific adsorption sites on the
immobilizing surface and thus reduces the background
caused by nonspecific bindings of antisera onto the
surf ace .
The immobilizing surface is then contacted with a
sample, such as clinical or biological materials, to be
tested in a manner conducive to immune complex
(antigen/antibody) formation. This procedure may
include diluting the sample with diluents, such as
solutions of BSA, bovine gamma globulin (BGG) and/or
phosphate buffered saline (PBS)/Tween. The sample is
then allowed to incubate for from about 2 to 4 hours, at
temperatures such as of the order of about 20° to 37°C.
Following incubation, the sample-contacted surface is
washed to remove non-immunocomplexed material. The
washing procedure may include washing with a solution,
such as PBS/Tween or a borate buffer. Following
formation of specific immunocomplexes between the test
sample and the bound RSV G specific antibodies, and
subsequent washing, the occurrence, and even amount, of
immunocomplex formation may be determined.
BIOLOGICAL MATERIALS
Certain plasmids that contain the gene encoding the
RSV G protein and referred to herein have been deposited
SUBSTITUTE SHEET (RULE 26)
CA 02296089 2000-O1-14
WO 99/04010 PCT/CA98/00697
22
with the American Type Culture Collection (ATCC) located
at 12301 Parklawn Drive, Rockville, Maryland, 20852,
U.S.A., pursuant to the Budapest Treaty and prior to the
filing of this application.
Samples of the deposited plasmids will become
available to the public upon grant of a patent based
upon this United States patent application and all
restrictions on access to the deposits will be removed
at that time. Samples of the deposited plasmids will be
replaced if the depository is unable to dispense viable
samples. The invention described and claimed herein is
not to be limited in scope by plasmids deposited, since
the deposited embodiment is intended only as an
illustration of the invention. Any equivalent or
similar plasmids that encode similar or equivalent
antigens as described in this application are within the
scope of the invention.
Plasmid ATCC Designation Date Deposited
pXL5 209143 July 16, 1997
pXL6 209144 July 16, 1997
EXAMPLES
The above disclosure generally describes the
present invention. A more complete understanding can be
obtained by reference to the following specific
Examples. These Examples are described solely for
purposes of illustration and are not intended to limit
the scope of the invention. Changes in form and
substitution of equivalents are contemplated as
circumstances may suggest or render expedient. Although
specific terms have been employed herein, such terms are
intended in a descriptive sense and not for purposes of
limitations.
Methods of molecular genetics, protein
biochemistry, and immunology used but not explicitly
described in this disclosure and these Examples are
SUBSTITUTE SHEET (RULE 26)
CA 02296089 2000-O1-14
WO 99104010 PCTlCA98/00697
23
amply reported in the scientific literature and are well
within the ability of those skilled in the art.
Example 1
This Example describes the construction of vectors
containing the RSV G gene.
Figure 1 shows a restriction map of the gene
encoding the G protein of respiratory syncytial virus
and Figure 2 shows the nucleotide sequence of the gene
encoding the full-length RSV G protein (SEQ ID No: 1)
and the deduced amino acid sequence (SEQ ID No: 2).
Figure 3 shows the gene encoding the secreted RSV G
protein (SEQ ID No: 3) and the deduced amino acid
sequence (SEQ ID No: 4).
Plasmid pXL5 (Figure 4) was prepared for the
expression of the full-length RSV G protein as follows:
A recombinant Bluescript plasmid (RSV G12)
containing the cDNA encoding the full-length G protein
of a clinical RSV isolate (subgroup A) was used to
construct vectors for RSV DNA-G immunization. RSV G12
was digested with AfIIII and EcoRI and filled-in with
the Klenow subunit of DNA polymerase. The resulting
1.23 kb fragment containing the coding sequence for the
full-length G protein was gel-purified and ligated to
VR-1012 (Vical) (Figure 6) previously linearized with
EcoRV. This procedure placed the RSV G cDNA downstream
of the immediate-early cytomegalovirus (CMV) promoter
and Intron A sequences of human cytomegalovirus (CMV)
and upstream of the bovine growth hormone (BGH) poly-A
site. The junctions of the cDNA fragments in the plasmid
construct were confirmed by sequencing analysis. The
resulting plasmid was designated pXL5.
Plasmid pXL6 (Figure 5) was prepared for the
expression of a secretory RSV G protein as follows:
RSV G12 was digested with EcoRI, filled-in with
Klenow and digested again with BamHI. The BamHI
SUBSTITUTE SHEET (RULE 26)
CA 02296089 2000-O1-14
WO 99/04010 PCT/CA98100697
24
cleavage resulted in the generation of a cDNA fragment
encoding a RSV G protein with N-terminal truncation.
This DNA segment was gel-purified and ligated in the
presence of a pair of 11 mer oligodeoxynucleotides
(5'GATCCACTCAG 3') (SEQ ID no: 7)
3' GTGAGTCCTAG 5' (SEQ ID no: 8)
to VR-1020 (Vical) previously digested with BglII,
filled in with Klenow, digested again with BamHI and
gel-purified. This procedure placed the truncated RSV G
cDNA (lacking the coding region for the N-terminal 91
amino acid residues including the transmembrane domain)
downstream of the immediate-early CMV promoter and
Intron A sequences of human CMV and upstream of the BGH
poly-A site. In addition, there was the introduction of
approximately 100 by of 5' untranslated region and the
coding sequence for the signal peptide of human
plasminogen activator protein (Figure 7) fused in frame
to the N-terminus of the RSV G protein coding sequence
downstream of the CMV promoter/Intron A sequences. The
junctions of the cDNA fragments in the plasmid construct
were confirmed by sequencing analysis. The resulting
plasmid was designated pXL6.
Examble 2
This Example describes the immunization of mice.
Mice are susceptible to infection by RSV as described in
ref . 24 .
Plasmid DNA was purified through double CsCl
centrifugations. For intramuscular (i.m.) immunization,
tibialis anterior muscles of BALB/c mice (male, 6 to 8
week old) (Jackson Lab., Bar Harbor, ME, USA) were
bilaterally injected with 2 x 50~g (l~g/~L in PBS) of
either pXL5, pXL6 or V-1012. Five days prior to DNA
injection, the muscles were treated with 2 x 50~.L (10~M
in PBS) of cardiotoxin (Latoxan, France) to increase DNA
uptake and enhance immune responses, as reported by
SUBSTITUTE SHEET (RULE 26)
CA 02296089 2000-O1-14
WO 99104010 PCT/CA98100697
Davis et al (ref. 23). The animals were boosted with the
same dose of plasmid DNA 6 weeks and 13 weeks later,
respectively. For intradermal (i.d.) immunization,
100~Cg of the plasmid DNA (2~.g/~L in PBS) of were
5 injected at the base of the tail and boosted 6 weeks and
13 weeks later, respectively. Mice in the positive
control group were immunized intranasally (i.n.) with
106 plaque forming units (pfu) of a clinical RSV strain
of the A2 subtype grown in Hep2 cells kindly provided by
10 Dr. B. Graham (ref. 24).
Four weeks after the third immunization, mice were
challenged intranasally with 106 pfu of the RSV A2
strain. Lungs were asceptically removed 4 days later,
weighed and homogenized in 2 mL of complete culture
15 medium (ref. 25). The number of pfu in lung homogenates
was determined in duplicate as previously described
(ref. 26) using vaccine-quality Vero cells.
Example 3
This Example describes the immunogenicity and
20 protection by polynucleotide immunization.
Antisera obtained from immunized mice were analyzed
for anti-RSV G IgG antibody titres using specific
enzyme-linked immunosorbent assay (ELISA) and for RSV-
specific plaque-reduction titres. ELISAs were performed
25 using 96-well plates coated with immunoaffinity-purified
RSV G protein (50 ng/mL)and 2-fold serial dilutions of
immune sera. A goat anti-mouse IgG antibody conjugated
to alkaline phosphatase (Jackson ImmunoRes.,
Mississauga, Ontario, Canada) was used as secondary
antibody. Plaque reduction titres were determined
according to Prince et al (ref. 26) using vaccine-
quality Vero cells. Four-fold serial dilutions of immune
sera were incubated with 50 pfu of the RSV Long strain
(ATCC) in culture medium at 37°C for 1 hr in the
presence of 5% COz and the mixtures were used to infect
SUBSTITUTE SHEET (RULE 26)
CA 02296089 2000-O1-14
WO 99/04010 PCT/CA98/00697
26
Vero cells. Plaques were fixed with 80% methanol and
developed 5 days later using a mouse anti-RSV F
monoclonal IgGl antibody and donkey anti-mouse IgG
antibody conjugated to peroxidase (Jackson ImmunoRes.,
Mississauga, Ontario, Canada). The RSV-specific plaque
reduction titre was defined as the dilution of serum
sample yielding 60% reduction in plaque number. Both
ELISA and plaque reduction assays were performed in
duplicate and data are expressed as the means of two
determinations.
The results obtained are reproduced in Tables I and
II below:
SUBSTITUTE SHEET (RULE 2fi)
CA 02296089 2000-O1-14
WO 99104010 PCT/CA98/00697
27
Table 1. Immunog enicity ofDNA-G is BALBlc Mice
Immunogen Anti RSV RSV Specific
G IgG
Titre
lL o~ 2/titrel100) Plaque Reduction
Titre
6 weeks 1 D weeks 17 weeks (Log Z titre)
17 weeks
VR-1012 (i.m.)0.00 + 0.00 + 0.000.00 + 0.000.00 + 0.00
0.00
pXLS (i.m.) 3.10 _+ 9.70 _+ 8.60 _+ 5.40 _+ 1.65
2.77 1.06 1.17
pXL6 (i.m.) 5.78 + 9.30 + 0.828.89 + 1.547.26 + 0.82
1.20
pXLS (i.d.) 1.50 _+ 8.60 _+ 8.30 _+ 7.92 _+ 0.59
1.27 1.43 1.25
pXL6 (i.d.) 3.70 + 10.30 + 9.44 + 1.246.92 + 0.94
1.25 1.06
RSV (i.n.) 6.83 + 9.67 + 0.529.83 + 0.41I 1.80 + 0.08
0.41
SUBSTITUTE SHEET (RULE 26)
CA 02296089 2000-O1-14
WO 99!04010 PCT/CA98/0069'7
28
Table II. Immunoprotective Ability ofDNA-Gtr BALBlc Mice
Mean Yirus Lung
Immunogen No. Mice Tytre* (pfulglung) No. FuDy
(Log 10 f SD) Protected Mice
VR-1012 (i.m.)6 4.81 + 0.01 0
pXLS (i.m.) 6 0.29 0.90 S
pXL6 (i.m.) 6 0.40 1.20 S
pXLS (i.d.) 6 0.30 _+ 1.10 5
pXL6 (i.d.) 6 0.29 + 0.90 5
RSV (i.n.). 6 0.00 + 0.00 6
*Sensitivity of the assay: 10'-96 pfu/g lung.
# The term, fully protected mice, refers to animals with no detectable RSV in
the lungs 4
days post viral challenge.
SUBSTITUTE SHEET (RULE 26)
CA 02296089 2000-O1-14
WO 99104010 PCTlCA98/00697
29
As seen in Table I, plasmids pXL5 and pXL6 were
found to be immunogenic following either i.m, or i.d.
immunization producing anti-G antibodies and virus
neutralizing antibodies. In addition, as seen in Table
II, the plasmids pXL5 and pXL6 protected immunized mice
against primary RSV infection of the lower respiratory
tract. The control vector produced no immune response
and did not confer protection.
Example 4
This Example describes the determination of the
local lung cytokine expression profile in mice immunized
with pXL5 and pXL6 after RSV challenge.
BALB/c mice were immunized at 0 and 6 weeks with
100~g of pXLS and 6, prepared as described in Example 1,
and challenged with RSV i.n. at 10 weeks. Control
animals were immunized with placebo PI-RSV and live RSV
and challenged with RSV according to the same protocol.
In addition, animals were immunized with pXL2, as
described in copending United States Patent Application
no. 08/476,397 filed June 7, 1995 (WO 96/40945) and
challenged with RSV, also following the same protocol.
Four days post viral challenge, lungs were removed from
immunized mice and immediately frozen in liquid
nitrogen. Total RNA was prepared from lungs homogenized
in TRIzol/~3-mercaptoethanol by chloroform extraction and
isopropanol precipitation. Reverse transcriptase-
polymerase chain reaction (RT-PCR) was then carried out
on the RNA samples using either IL-4, IL-5 or IFN-y
specific primers from CloneTech. The amplified products
were then liquid-hybridized to cytokine-specific 32P-
labeled probes from CloneTech, resolved on 5~
polyacrylamide gels and quantitated by scanning of the
radioactive signals in the gels. Three mouse lungs were
removed from each treatment group and analyzed for lung
cytokine expression for a minimum of two times. The
SUBSTITUTE SHEET (RULE 26)
CA 02296089 2000-O1-14
WO 99/04010 PCT/CA98100697
data is presented in Figure 8 and represents the means
and standard deviations of these determinations.
As may be seen from the data presented in Figure
8:
5 1. Immunization with live RSV intranasally (i.n.)
resulted in a balanced cytokine profile (IFN-y, IL-
4 and IL-5), whereas that with FI-RSV
intramuscularly (i.m.) resulted in a Th2
predominance (elevated IL-4 and IL-5). These
10 results are similar to those reported in the
literature.
2. Immunization with pXL5 or pXL6 via either the
i.m. or intradermal (i.d.) route gave rise to a
balanced cytokine profile similar to that with live
15 RSV immunization.
3. The magnitude of the cytokine responses with
i.m. pXL6 (RSV G) and pXL2 (RSV F) immunization
using the construct expressing a secretory form of
the protein (SEC) is significantly higher than that
20 with live RSV immunization.
4. The magnitude of the cytokine response with
pXLS immunization using constructs expressing a
full-length membrane-associated RSV G protein (MA)
and i.d. pXL6 was somewhat higher than that with
25 live RSV immunization.
5. The balanced local cytokine response observed
with DNA-G immunization contrasts with that
reported by Openshaw et al (ref. 13). Using a
recombinant vaccinia virus expressing the G
30 protein, these investigators reported a local Th2
response by analysis of bronchoalveolar lavage.
The results herein, which were obtained through a
monogenic approach, indicate that the Th2 response
is not necessarily an intrinsic property of the G
protein.
SUBSTITUTE SHEET (RULE 26)
CA 02296089 2000-O1-14
WO 99/04010 PCT/CA98/00697
31
SUMMARY OF THE DISCLOSURE
In summary of this disclosure, the present
invention provides certain novel non-replicating vectors
containing genes encoding RSV G proteins, methods of
immunization using such vectors and methods of diagnosis
using such vectors. Modifications are possible within
the scope of this invention.
SU8ST1TUTE SHEET (RULE 26)
CA 02296089 2000-O1-14
WO 99/04010 PCT/CA98/00697
32
REFERENCES
1. McIntosh K., Canock, R.M. In: Fields B.N, Knipe,
DM, editors. Virology. New York: Raven Press: 1990:
1045-1072
2. Heilman, C.A., J. Infect. Dis. 1990, 161: 402 tc
406.
3. Wertz GW, Sullender WM., Biotechnology 1992; 20:
151-176
4. Murphy, B. R. et al, 1994, Virus Res. 32: 13-36.
5. Levine, S., Kleiber-France, R., and Paradiso, P.R.
(1987) J. Gen. Virol. 69, 2521-2524.
6. Anderson, L.J., Hierholzer, J.C., Tsou, C., Hendry,
R.M., Fernie, B.F., Stone, Y. and McIntosh, K.
(1985) J. Infect. Dis. 151, 626-633.
7. Johnson et al., J. Virol 1987, 61: 3163-3166
8. Crowe, J.E., Vaccine 1995, 13: 415-421
9. Kapikian, A.Z. et al 1969, Am. J. Epidemiol. 89:
405-421.
10. Kim, H.W., et al 1969 Am. J. Epidemiol. 89: 422-
434.
11. Murphy, B.R. et al 1986 J. Clin. Microbiol. 24:
19'7-202.
12. Vaux-Peretz, F. et al 1992 Vaccine 10: lI3-118.
13. Openshaw, P.J. 1995 Springer-Semin Immunopathol.
17: 187-201.
14. Alwan et al 1994 J. Exp. Med. 179:81-89.
15. Graham, B.S. 1995 Am. J. Respir. Crit. Care Med.
152:563-6
16. WO 90/11092
I7. WO 94/21797
18. Ulmer, Current Opinion, Invest Drugs, 1993, 2: 983-
989
19. Chapman, B.S.; Thayer, R.M.; Vincent, K.A. and
Haigwood, N.L., Nucl. Acids. Res. 1991, 19: 3979-
3986.
SUBSTITUTE SHEET (RULE 26)
CA 02296089 2000-O1-14
WO 99/04010 PCT/CA98/00697
33
20. Nabel, G.J. 1993, Proc. Natl. Acad. Sci. USA 90:
11307-11311.
21. Tang et al., Nature 1992, 356: 152-154
22. Furth Analytical Biochemistry, 1992, 205:
et al.
365-368
23. Davis al., Vaccine 1994, 12: 1503-1509
et
24. Graham, B.S. ; Perkins M.D.; Wright, P.F. and
Karzon, D.T. J. Mod. Virol. 1988 26: 153-162.
25. Du, R.P et . 1994., Bio Technology 12: 813-818.
al
26. Prince, G.A. et al, 1978. Ame. J. Pathol. 93: 771-
790.
SUBSTITUTE SHEET (RULE 25)