Note: Descriptions are shown in the official language in which they were submitted.
CA 02297295 2000-O1-19
WO 99/04708 PCT/US98/15139
APPARATUS AND METIiOD FOR TRANSMYOCARDIAL
REVASCULARIZATION BY LASER ABLATION
BACKGROUND
Technical Field
The present disclosure relates to improved
apparatus and methods for transmyocardial revascularization
(TMR) by laser ablation with a lasing device.
2. Background of the Related Art
TMR is a procedure for treating heart disease,
wherein multiple channels of small diameter are created in
the heart wall, extending into the ventricle. Such channels
facilitate delivery of blood directly from the ventricle to
oxygen starved areas of the heart. TMR is typically used on
patients with ischemic heart disease, particularly those who
are not candidates for coronary artery bypass or
percutaneous transluminal angioplasty.
During a typical TMR procedure, dozens of channels
are created from the epicardium, through the myocardium and
endocardium and into the ventricle, with each channel being
of sufficiently small diameter such that the end portions of
the channels at the epicardium can be closed by blood
clotting. The channels can be created by employing either a
mechanical coring apparatus or a lasing device. With either
technique, an objective is to produce channels that remain
internally patent in the long term and which do not close up
due to fibrosis and/or scarring.
In early laser myocardial revascularization, a C02
laser was used to produce holes in the heart wall by
transmitting laser energy from the laser to the heart wall.
Typical C02 lasers used for TMR are externally located and
have an articulated support arm for aiming and directing
laser energy through a series of mirrors that reflect the
energy onto the heart wall. Thus, some surgical opening of
_.__._____ _ _-___ ___
CA 02297295 2000-O1-19
WO 99/04708 PCT/US98/15139
the chest wall is required to access the heart muscle. The
entrance wound in the heart can be closed by relatively
brief external pressure while the endocardial and myocardial
layers remain open to permit blood.flow from the ventricle
to the heart muscle.
Less traumatic approaches to laser myocardial
revasculari2ation have been disclosed. These methods
include the use of optical fibers introduced either through
a patient's vasculature or alternatively, directly into the
patient's chest cavity. The intravascular method involves
the direction of laser energy from inside the heart to form
a bore in the heart wall while the other method involves
introduction. of the lasing apparatus through a relatively
small iincision in the patient's chest to access the outer
wall of the heart.
U.S. Patent No. 4,658,817 to Hardy discloses a
method and apparatus for TMR using a laser wherein a hollow
needle having a sharp distal tip is inserted into the
epicardium and a laser beam is focussed through the needle
to create channels. It is stated in the Hardy patent that
this technique eliminates surface bleeding and the need for
suture. However, there is no laser ablation member (e. g.,
optical fiber) that advances through the needle and into the
myocardium contemporaneously with laser energy being
generated.
With current TNfR procedures wherein channels are
formed from the outer heart wall, the technique for stopping
the bleeding from each channel at the epicardium after
channel formation typically entails applying pressure to the
3o opening of~the just-fonr~ed channel. Pressure is typically
applied by the finger of the surgeon or assistant during
open heart surgery, or with a laparoscopic instrument when
the procedure is performed laparoscopically. In either
case, because pressure is applied to each channel opening
for at least several seconds, and it is impractical to begin
forming another channel until the bleeding is stopped from
_2_
CA 02297295 2000-O1-19
WO 99/04708 PC'T/US98/15139
the previous channel, the overall TMR procedure can be
undesirably prolonged by the time expended on applying
pressure to each channel.
Accordingly, a need exists for a TMR procedure
wherein the time spent to stop the blood flow from each of
the individual transmyocardial channels is reduced or
eliminated, thereby increasing the likelihood of success of
each operation.
The present disclosure is directed to methods for
performing transmyocardial revascularization employing a
laser device having a laser ablation member, e.g., one or
more optical fibers. One preferred method includes the
steps of: advancing the laser ablation member a
predetermined distance within the patient's heart tissue to
mechanically pierce the epicardium; then outputting laser
energy from the laser ablation member to ablate heart tissue
and create a patent channel extending into the patient's
2o ventricle; and, withdrawing the laser ablation member from
the heart tissue, whereby epicardial tissue pushed aside
during the initial advancing step substantiall}~ returns to a
position coinciding with the channel and acts as a channel
cap to reduce bleeding from the channel. In an alternative
method, the distal end of the laser fiber is advanced to or
maintained at a position such that it extends distally from
a laser handpiece held by the surgeon; the fiber is caused
to press against the epicardium prior to laser firing,
thereby causing the exterior tissue to "tent". The fiber is
then advanced at a desired rate as the laser fires to form
the channel. In each of the above methods, it is preferable
to advance the laser ablation member at a rate coordinated
with the magnitude of laser energy generated in order to
precisely form each channel.
In another alternative method, the laser fiber can
be placed against the epicardium and initially advanced at a
-3-
CA 02297295 2000-O1-19
WO 99/04708 PCT/US98/15139
rate faster than the tissue ablation rate of the laser. In
this method, the fiber during this stage, will mechanically
pass through the heart tissue. After the fiber travels
though at least a portion of the epicardium, the fiber
advancement rate can be decreased to such a rate that the
laser energy alone ablates the tissue to form the channel.
In yet another alternate method, the fiber can be
advanced at a constant rate while the pulse rate of the
laser is varied to allow mechanical passage of the fiber
through at least a portion of the epicardium. More
specifically, the amount of energy delivered by the laser
fiber can be low, e.g., fewer pulses per second, while
passing through a portion of the epicardium, and increased
thereafter to complete the channel.
Advantageously, with the present methods, the
fiber creates a flap or "channel cape in the epicardial
surface. The interface between the channel cap and the
adjacent epicardial/myocardial tissue defines a very narrow
opening such that blood clotting can occur rapidly at the
interface. Thus, the time expended for applying 'pressure to
the channel opening at the epicardium following each
channel's formation is substantially reduced as compared to
prior art methods. The present method may even allow the
pressure-applying step to be eliminated entirely. In
addition, the pressure of the channel cap may permit larger
channels to be formed, as compared to channels without the
cap, due to the channel cap's ability to moderate or prevent
the flow of blood from the channel.
ERIEF DESCRIPTION OF THE DRAWINGS
Various preferred embodiments are described herein
with reference to the drawings, wherein:
FIG. 1 illustrates a laser ablation device used to
create TNR channels;
-4-
CA 02297295 2000-O1-19 ;
~yG ~~pl7~g PCTIUS98115139
FIG. 2 is a perspective view of the laser ablation
device;
FIG. 3 is a perspective view of the hand piece of
the laser ablation device;
FIG. 4 is an exploded view showing the various
components of the hand piece;
FIG. 5 is a side view of the hand piece having a
fiber extended in proximity to the epicardium;
FIG. 6 is a side view showing piercing of the
epicardium;
FIG. 7 is a side view showing the fiber being
advanced through the myocardium and endocardium;
FIG. 8 is a side view showing-withdrawal of the
fiber from the heart tissue to reveal the channel created
therein;
FIG. 9A is a cross-sectional view of a completed
transmyocardial channel;
FIG. 9B is an end view of the epicardium having a
channel capped by a flap;
FIGS. 10-13 illustrate an alternate method for
performing TMR disclosed herein, where:
FIG. 10 is a side view of the hand piece in
proximity to the epicardium;
FIG. 11 is a side view showing piercing of the
epicardium;
FIG. 12 is a side view showing the fiber being
advanced through the myocardium and endocardium;
FIG. 13 is a side view showing withdrawal of the
fiber from the heart tissue to reveal the channel created
therein;
FIGS. 14-16 illustrate an alternative method for
performing TMR disclosed herein; where:
FIG. 14 is a side view showing piercing of the
epicardium;
FIG. 15 is a side view showing the fiber being
advanced through the myocardium and endocardium;
-5-
CA 02297295 2000-O1-19
WO 99/04708 PCT/US98/15139
FIG. 16 is a side view showing withdrawal of the
fiber from the heart tissue to reveal the channel created
therein;
FIGS. 17-19 illustrate an alternative method for
performing TMR disclosed herein, where:
FIG. 17 is a side view showing piercing of the
epicardium;
FIG. 18 is a side view showing the fiber being
advanced through the myocardium and endocardium; and
l0 FIG. 19 is a side view showing withdrawal of the
fiber from the heart tissue to reveal the channel created
therein.
DETAILED DESCRIPTION OF PREFERRED EI~ODIMENTS
Preferred embodiments of TMR methods will now be
described in detail with reference to the drawings, in which
like reference numerals designate identical or corresponding
elements.
Referring to FIGS. 1 and 2, a laser ablation
device, designated generally as 10, is employed to practice
a TMR procedure in accordance with the present disclosure.
Device 10 is capable of advancing a laser ablation member 18
through heart tissue while concomitantly outputting laser
energy, where the advancement rate is coordinated with the
magnitude of laser energy generated and with the pulsing
frequency of the laser source. This coordination enables
highly patent and precise TMR channels to be created.
Lasing device 10 is similar to lasing devices disclosed in
copending, commonly assigned U.S. Patent Application Serial
No. 08/648,638 to Pacala et al., filed May 13, 1996, the
subject matter of which is incorporated herein by reference.
Laser ablation device 10 includes a hand piece 11,
an optical fiber advancing mechanism 12, a laser generator
14, a foot operated actuator 16, and a control module 17.
The optical fiber advancing mechanism 12 is of the type
capable of precisely transmitting longitudinal motion to
-6-
CA 02297295 2000-O1-19
WO 99/04708 PCT/US98/15139
laser ablation member 18, e.g., an optical fiber, optical
fiber bundle or other laser energy transmission mechanism.
The controlled longitudinal motion can be provided by one or
more motors and preferably by one or more commercially
available stepper motors. The laser generator 14 may be
either a continuous wave laser or a pulsed, high energy
laser, such as, for example, an excimer, C02, Yag or an
alexandrite laser.
The optical fiber advancing mechanism 12 and the
to laser generator 14 are operably connected to foot actuator
16. By depressing foot actuator 16, laser energy is
transmitted through the optical fiber 18 by laser generator
14 while fiber advancing mechanism 12 contemporaneously
advances optical fiber 18 relative to hand piece 11.
Alternately, foot actuator 16 can cause at least partial
advancement of the fiber without transmission of laser
energy, as will be described in greater detail. An
electrical signal from foot actuator 16 actuates control
module 17 which communicates with fiber advancing mechanism
12. Control module 17 is programmable and controls the
. motors or other suitable advancing structure in advancing
mechanism 12 upon actuation of foot actuator 16. Control
module 17 is shown with a receptacle 19 adapted to engage a
terminal of a programmable computer to interface control
module 17 with the computer. As such, instructions required
to operate advancing mechanism 12 can then be stored in
memory within control module 17. A toggle switch 15 may be
provided on the control module 17 to switch from an
operation mode to a test mode. In a particular test mode,
when the foot actuator 16 is acted upon, the flexible
optical fiber is moved sequentially from a retracted
position, to a predetermined extended position, and back to
the retracted position.
Fiber advancing mechanism 12 can be equipped with
two internal limit switches Inot shown). The first limit
switch is activated when the optical fiber 18 is at a
CA 02297295 2000-O1-19
WO 99104708 PCT/US98/15I39
desired retracted position (i.e., a "home" position),
wherein the mechanism that is retracting the fiber is caused
to stop. Optical fiber 18 is in the retracted position
unless foot actuator 16 is depressed or the test mode is
activated. The exact retracted position is selectable by
means of selector 23, e.g., a rotatable knob. One way of
implementing a TMR procedure in accordance with the present
disclosure using laser ablation device 10 is to select the
retracted position as a position in which the distal end of
optical fiber 18 protrudes from the distal end of hand piece
11, the purpose of which is discussed in greater detail,
below.
The second limit switch within unit 12
limits/controls the maximum distance that the optical fiber
can extend from hand piece 11. This limit switch is an
indexes which includes external selector 21. Selector 21 is
provided so that the operator can select the desired maximum
extension of the distal end of the optical fiber from the
handpiece. For example, selector 21 can be in the form of a
rotatable knob that can be set at selectable positions,
wherein each position corresponds to a predetermined maximum
longitudinal position of the optical fiber. When the fiber
reaches the selected maximum position, a limit switch
automatically terninates the fiber's advancement. By way of
example, the operator can select maximum fiber extension
positions so that the distal end of the fiber extends from
the distal end of hand piece 11 from between about 0.5 cm
and about 5.0 cm, with the ability to select in increments
of about 0.25 cm to about 0.5 cm. The maximum extension
position is preferably chosen to be slightly longer than the
heart wall thickness for the particular patient such that
fiber 18 will penetrate into the patient's ventricle. Once
the maximum extended position is reached, output of laser
energy is automatically suspended.
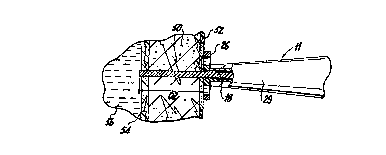
FIG. 3 illustrates a perspective view of the hand
piece ll of laser ablation device 10. Briefly, hand piece
_g_
CA 02297295 2000-O1-19
WU 99/04708 PCT/US98l15139
11 includes housing 20 formed from molded housing half-
sections 20a and 20b. Housing 20 has an elongated body 22
with a comically tapered section 24. Optional locator ring
26 is provided at the distal end of comically tapered
section 24. The front surface 27 is positioned in abutting
relation with the epicardium of a patient directly following
the piercing of the epicardium with the tip of fiber 18
during a TMR procedure. Locator ring 26 facilitates proper
orientation of the hand piece with respect to the heart
tissue. However, locator ring may be eliminated if it is
desired to improve visibility of the epicardium with some
trade-off of stability. Locator ring 26 can be formed
integrally with housing half-sections 20a and 20b or can be
removably fastened to tapered section 24. A ridged surface
28 is formed on an outer wall of housing half-sections 20a
and 20b to facilitate grasping of the device 10.
FIG. 4 illustrates hand piece 11 with housing
half-sections 20a and 20b and the internal components
separated. Housing half-sections 20a and 20b define a
central bore 30, a proximal recess 32, and a distal recess
34. The proximal recess 32 is configured to receive a
swivel connector 36 which is fastened to the optical fiber
casing 38. The swivel connector 36 has an annular flange 40
dimensioned to be received within an increased diameter
section 42 of proximal recess 32 to permit rotation of
housing 20 with respect to optical fiber casing 38.
As shown, the locator ring 26 has a cylindrical
body portion 44 having an annular flange 46 formed at its
proximal end. The cylindrical body portion 44 includes a
central bore 50 and is configured to be received within the
distal recess 34 defined by housing half-sections 20a and
20b. Central bore 50 of cylindrical body portion 44 is
aligned with a central opening 48 formed in the distal end
of the housing 20 and the central bore 30 of housing 20.
Locator ring 26 can either swivel, to allow independent
rotation of the hand piece relative thereto, or be fixed in
_g_
CA 02297295 2000-O1-19
WO 991W708 PC'fNS98/15139
place. The optical fiber 18 is slidably positioned within
central bores 30 and 50 such that it can be advanced through
opening 48 in housing 20. Pins or screws 49 can be used to
fasten the housing half-sections 20a and 20b together to
secure the locator ring 26 and the swivel connector 36 to
the housing 20. If locator ring 26 is eliminated, front
surface 31 of tapered portion 29 can act as the stop which
contacts the patient's outer epicardial surface to prevent
initial penetration of fiber 18 beyond distance Di. This
surface 31 can be buttressed slightly whereby it would form
a small diameter collar that is seated against the
epicardium during the channel formation procedure.
As seen in FIG. 3, the distal end of hand piece 11
corresponds to the front surface 27 of locator ring 26.
Allowing optical fiber 18 to protrude enables the surgeon to
pierce the epicardium with the tip of fiber 18 without
initially firing the laser, such that fiber 18 penetrates a
predetermined distance into the outer heart wall. This
initial penetration of the fiber is stopped at the
predetermined distance by means of the surface 27 contacting
the epicardial wall. The operator then depresses foot
actuator 16 to cause laser energy to be generated and form
the channel. By virtue of the initial penetration without
laser energy output, the transmyocardial channel that is
formed will be "capped" at the epicardium by the outer heart
tissue which is not ablated. This will become more apparent
below.
As an alternative to selecting a protruding
retraction position and utilizing the first surface 27 to
prevent excess initial penetration of fiber 18, the
advancing mechanism 12 (FIG. 1) can be designed in
conjunction with the control unit 17 to activate fiber 18 to
automatically advance longitudinally by the desired
predetermined initial distance without laser energy output.
(This initial distance will be designated hereafter as
distance Di). This~automatic initial advancement can be
-10-
CA 02297295 2000-O1-19
WO 99/04708 PCTNS98/15139
implemented either by activating an additional switch (not
shown) on unit 12 or 17, or it can advance automatically
whenever actuator 16 is initially depressed. In either of
these cases, the above-noted retracted position would
preferably not be adjustable but rather, would be preset to
approximately coincide with the distal surface 27. The
operator would then place the surface 27 directly on the
epicardium and then depress foot actuator 16 (or activate
the additional switch), whereupon the fiber 18 would first
automatically advance the distance Di without laser energy
being generated. Following the initial advance, laser
energy would automatically commence and the fiber
correspondingly advanced to create the TMR channel. In this
embodiment, selector switch 23 would be designed in
conjunction with advancing mechanism 12 to allow selection
of the initial penetration distance Di, not to select the
retracted position.
With either of the above approaches, i.e.,
retracted position protruding by distance Di or automatic
adva.~.=ement to distance Di without laser er_ergy, the
operar.or/surgeon preferably selects the initial penetration
distance Di. (Optionally, this distance could be preset and
not alterable by the operator). Typically,- a healthy heart
has a wall thickness of 10-l5mm. A diseased heart may be as
thick as 40mm (measured from the outer surface of the
epicardium to the inner surface of the endocardium). As
such, the initial penetration distance Di is typically
selected in the range of about 1 to about 2-Smm and
preferably from about 2 to about 5mm so that the fiber 18
penetrates slightly into the myocardium. This will ensure
that an adequate channel cap will be subsequently formed.
The diameter of the fiber (or fiber optic bundle) 18 is
typically in the range of about 0.5 to about 2.5mm, and
preferably about l.4mm. The TMR channel to be formed has
about the same size diameter as the fiber or fiber bundle
18.
-11-
CA 02297295 2000-O1-19
WO 99/Oa708
PCT/US98/15139
In either of the above methods, the heart tissue
may "tent" in response to the partially advanced fiber
pressing against the epicardium. If this occurs, upon
commencement of laser firing, the heart tissue will swiftly
move towards the handpiece (the "tent" will collapse). This
movement of the tissue will cause the outer layers of the
heart tissue to receive less laser energy, resulting in at
least a partial channel cap to aid in closing the channel.
Referring now to FIGS. 5-8, a method for producing
a TMR channel utilizing the laser ablation device 10 is
illustrated. As shown in FIG. 5, hand piece 11 is brought
in proximity to the epicardium 52 of a heart patient. Prior
to entry into the epicardium, the tip of optic fiber 18
protrudes slightly from the locator ring 26 by distance Di,
where Di is measured from the distal surface 18a of fiber 18
to the front surface 27 of locator ring 26. It is noted
that the surface 18a may be flat as shown; however, it could
alternatively be beveled to facilitate piercing of the
epicardium and preliminary advancement into the
2C epicardial/myocardial tissue. In any case, the distance Di
is selectable by means of select switch 23 (FIG. 1)
discussed earlier. with the tip of fiber 18 protruding in
this manner (retracted position), and without depressing the
foot actuator 16 to output laser energy, the fiber tip
surface 18a is brought into contact with epicardium 52 so as
to mechanically pierce or "tent" the epicardial outer
surface. The fiber tip initially advances through at least
a portion of the epicardium 52 and myocardium 50 either
without laser energy being generated or immediately after
lasing begins. As the tip of fiber 18 penetrates,
epicardial tissue (and as shown, myocardial tissue, if
desired) adjacent to the fiber tip is pushed aside. This
pushed aside tissue will not be ablated by the laser energy.
Tissue 53 will substantially return to its natural position
following channel formation and act as a cap to reduce
bleeding from the charnel as will become apparent below.
-12-
CA 02297295 2000-O1-19
WO 99/04708 PCT/US98/15139
In a preferred method, initial penetration of the
fiber occurs until the front surface 27 contacts the
epicardium 52 (FIG. 6). At this point, fiber 18 has
penetrated approximately the distance Di into the heart
tissue. Locator ring 26 enhances the surgeon's ability to
position and stabilize the laser device 10 with respect to
the heart, which can be beating during the procedure.
However, as explained above, some embodiments may not
utilize locator ring 26.
to As depicted in FIG. 7, the TMR channel is formed
by transmitting laser energy from the tip of fiber 1B to
ablate heart tissue while correspondingly advancing optical
fiber 18. The fiber tip is advanced through the myocardium
50 and endocardium 54 until it reaches its maximum extended
position corresponding to the distance D2 between fiber tip
surface 18a and the surface 27 of locator ring 26.
In methods disclosed herein, while forming the
channel below the channel cap, fiber 18 is preferably
advanced at a rate that. is coordinated with the power level
and the frequency of pulsing of the laser generator. For
example, optical fiber 18 can be advanced at a rate. of
between about 0.125mm/sec (0.005 in/sec) to about 12.7mm/sec
(0.5 in/sec) with a laser power level of about 10 mJ/mm2 to
about 60 mJ/mm2 and a pulsing frequency of about 5 Hz to
about 400 Hz. Preferably, the optical fiber is advanced at
a rate of about 0.75mm/sec to about 2.Omm/sec with a laser
power level of between about 30 mJ/mm2 to about 40 mJ/mm2
and a pulse frequency of about 20 to about 50 Hz. In a most
preferred embodiment, the rate of advancement of the optical
fiber is no greater than the rate of ablation of tissue in
order to minimize mechanical tearing by the fiber.
Alternatively, if some degree of mechanical tearing is
desired in addition to laser ablation, the advancing
mechanism can be set to advance the fiber at a rate greater
than the ablation rate. Studies have shown that a xenon
chloride excimer laser operating at a power level of about
-13-
CA 02297295 2000-O1-19
PC'TNS98/15139
35mJ/mm2 can ablate about 30-35 microns of animal heart
tissue per pulse.
As discussed above, the maximum extended position
corresponding to the distance D2 is selectable by the
surgeon by means of select switch 21. Once the maximum
extended position is reached, wherein fiber 18 typically
penetrates slightly into ventricle 56, output of laser
energy is automatically suspended. At this point, the
operator releases depression of foot actuator 16, causing
fiber 18 to retract to the retracted position, as depicted
in FIG. 8. The hand piece 11 is then drawn away from the
heart wall whereby transmyocardial channel 60 is completed.
The completed transmyocardial channel 60 is shown
in cross-section in FIG. 9A, while FIG. 9B shows the end
view, of the epicardium 52. The epicardium/myocardial tissue
53 that was pushed aside without being ablated during the
preliminary penetration of fiber 18, returns to its original
location coinciding with channel 60 upon the fiber's
withdrawal. This tissue 53 forms a flap that acts as a cap
for the channel 60 to reduce bleeding from the channel at
the epicardium 52. The interface 59 between the flap of
tissue 53 and the adjacent tissue is generally an annular
ring less than 360_ in extent. As shown, the cap 53 can
consist of both epicardial and myocardial tissue, but could
alternatively be just epicardial tissue.
Once channel 60 is completed, fiber 18 can be
moved to another location on the epicardium to begin forming
another channel, without the necessity of applying extended
pressure to the portion of the epicardium coinciding with
just-formed channel 60. The overall procedure wherein
dozens of channels 60 are typically formed can thus be
performed much faster as compared to other methods.
Three alternate methods of forming a TMR channel
having a channel cap will now be discussed with reference to
Figs. 10-19. In a first alternative method, the distal end
18a of the laser fiber 18 is initially flush with the distal
-14-
CA 02297295 2000-O1-19
WO 99/04708 PCTNS98I15139
end of laser handpiece 11 (FIG. 10). The fiber 18 is then
caused to puncture the epicardium 52 prior to or upon
commencement of laser firing (FIG. 11) by advancing the
fiber a distance Di prior to firing. The fiber is then
advanced at a specifically desired rate as the laser fires
to form the channel 60 (FIG. 12); and is withdrawn upon
completion of the channel 60 (FIG.13).
In a second alternative method, the laser fiber 18
can be placed against the epicardium 52 and initially
advanced at a rate faster than the tissue ablation rate of
the laser (FIG. 14). In this method, the fiber 18 will
mechanically pass through the heart tissue. After the fiber
18 travels through at least a portion of the epicardium 52,
the fiber advancement rate can be decreased to such a rate
that the laser energy ablates the tissue to form~the channel
60 (FIG. 15). The fiber 18 is then withdrawn from the
heart t;ssue (FIG. 16).
In a third alternative method shown by FIGS. 17
19, the fiber 18 pierces the heart tissue (FIG. 17) and is
advanced at a constant rate while the firing rate of the
laser energy is varied to allow mechanical passage of the
fiber 18 through at least a portion of the epicardium 52
(FIG. 18). More specifically, the amount of energy
delivered by the laser fiber 1B can be low, e.g., fewer
pulses per second, while passing through a portion of the
epicardium 52, and increased thereafter to complete the
channel 60. The fiber 18 is then withdrawn from the heart
tissue (FIG. 19).
It is also contemplated to produce multiple
channel caps in the epicardium sequentially in the same
channel by repetitively and consecutively advancing the
fiber and firing the laser. Multiple channel caps will
provide better assurance that the channel has been
successfully capped.
It will be understood that various modifications
can be made to the embodiments disclosed herein. For
-15-
CA 02297295 2000-O1-19
PCT/US98115139
example, other types of devices for delivering laser energy
could alternatively be used to produce TMR channels
utilizing the method of the present disclosure. For
instance, the method can be performed endoscopically,
wherein the fiber is entroduced through a small tube to
access the heart wall. Therefore, the above description
should not be construed as limiting, but merely as
exemplifications of preferred embodiments. Those skilled in
the art will envision other modifications within the scope
l0 and spirit of the claims appended hereto.
-16-