Note: Descriptions are shown in the official language in which they were submitted.
CA 02297444 2000-O1-26
A METHOD OF SEPARATING AN IMMISCIBLE LIQUID/LIQUID MIXTURE
AND APPARATUS THEREFOR
This application is divided from Canadian Patent
Application 2,110,026, filed November 25, 1993.
The present invention is directed to a method of
separating small amounts of a first liquid which are
immiscible but suspended in a second liquid, and to
separating apparatus therefor. More particularly, the
present invention is directed to a method of separating and
.15 removing a discontinuous liquid phase from a continuous
liquid phase, and to a separating element useful therefor.
Many industrial processes and apparatus, as
well as household devices, relate to the separation
of a liquid phase from another phase. In some
instances, particularly when water is the phase
present in minor amounts, chemical means may be used
to remove the water from the other components. Such
means for removing moisture, however, require the
replacement and/or regeneration of the reagents used
in the process. The reagents employed and the
products formed frequently introduce complications
relating to handling and disposal. Because of the
concomitant cost and, in some instances, .
inconvenience associated with such processes,
physical methods and apparatus have been preferred
to chemical means for removal of small amounts of a
liquid phase from other phases.
A method of coalescing an immiscible liquid
suspended in another phase and a coalescing device,
frequently termed a "coalesces", have found
- 1 -
CA 02297444 2000-O1-26
widespread use in removing liquid from both the
gaseous phase, such as in aerosols, and from
suspensions of one liquid in another liquid. Such
devices are particularly effective where the volume
of liquid removed is small in comparison.to the
volume of the phase from which it is removed.
Typically, the equipment necessary to remove a
liquid aerosol from a gas tends to be less
complicated than that used to separate two liquid
l0 phases in which a first liquid phase is immiscible
and suspended in a second liquid phase. This is
generally true because in air/liquid suspensions,
gravitational effects tend to be more significant
while surface energy, surface tension or interfacial
tension effects tend to be less significant than
with liquid/liquid suspensions.
The spectrum of applications where coalescers
have been used to remove minor amounts of a first
liquid phase, known as a "discontinuous phase" or
"suspended phase", from a second liquid phase in
which it is suspended, known as the "continuous
phase" or "suspending phase", covers a considerable
range of situations. For example, coalescers have
been used most often to remove or separate small
amounts of moisture from petroleum based fuels,
including gasoline, diesel and aviation fuels, such
as kerosene; remove moisture from cleaning fluids;
separate oil from coolants and parts cleaners;
remove oil contamination found in natural bodies of
water; separate immiscible solvent systems used in
extraction processes, etc.
Numerous mechanisms and models have been
proposed to describe coalescence of a droplet of the
discontinuous phase from the continuous phase and
the ease or difficulty of separation of the
immiscible phases. The factors which affect the
- 2 -
CA 02297444 2000-O1-26
coalescence process include the physical properties
of the phases, such as density, viscosity, surface
tension, and interfacial tension (IFT). In
addition, the properties of the system, such as drop
size, curvature of the interface, temperature,
concentration gradients and vibrations also affect
coalescence significantly. While any or all of
these factors may be significant in a particular
situation, properties such as density, drop size and
interfacial tension appear to be among the factors
which are of most significance and often over which
the least control can be exercised in difficult
separations of two immiscible liquids. Thus, all
other things being equal, where the densities of two
liquids differ only slightly, separation becomes
more difficult. This is also true of the
interfacial tensions of the liquids involved. In
those situations in which the droplets are greater
than 10~ (primary emulsions) coalescence and
separation is much easier to effect frequently with
the discontinuous phase settling by gravity after
coalescence to form a heterogeneous layer. When the
droplets are smaller than 10~, particularly less
than 1~ in diameter, secondary emulsions or
secondary hazes result from which the discontinuous
phase is much more difficult to coalesce. The
latter frequently occurs where the emulsion has been
formed by rigorous agitation or the inclusion of a
surface active agent. Where emulsification to form
the secondary haze occurs purely by mechanical
means, coalescence may be accomplished much more
readily by conventional coalescence methods and
apparatus. Where the secondary haze results from
surface active materials, which influence the
interfacial tensions of the liquids, separation
becomes more difficult.
- 3 -
CA 02297444 2000-O1-26
The type of coalescer employed depends on the
difficulty of separation or coalescence, as
influenced by the factors identified above. Thus,
in some situations, equipment may be very simple,
such as those employing baffles, and range to more
complex devices containing different types of
packing. The type of fluids being separated
frequently determines the packing used. Thus, both
the shape of the packing material and its
composition influence the efficiency of coalescence
and separation. For example, the coalescing
apparatus used to separate oil and water typically
contain tubes, plates, disks, spears, rods, fibers
or other internal structures designed to capture
oil. Conventionally, glass has been the most often
used packing material and while in some instances
membranes have been employed in coalescers, as well
as the packings listed above, fibers have been the
preferred form of packing. Currently, glass fibers
seem to have found the most widespread application
in coalescers.
In recent years, both household and industrial
requirements have led to the demand for purer
liquids, including drinking water, solvents, liquids
used in industrial processes, and fuels. To satisfy
the more stringent specifications required for such
materials, requirements have increased with regard
to the effectiveness, efficiency and capacity of
equipment used to purify these liquids.
Manufacturers of such equipment have also striven to
provide greater durability and longer interval
periods between maintenance, regeneration or
replacement of components. In the field of
liquid/liquid separation, coalescers have frequently
been expected to perform a filtration function to
remove particulate matter, in addition to their
- 4 -
CA 02297444 2000-O1-26
primary function of coalescing a discontinuous
phase.
A typical, conventional coalescing-separating
apparatus is illustrated in Figure 1. The
coalesces-separator unit 10 includes a housing 12
having a divided base. An inlet 14 is provided to
introduce contaminated liquid through the housing,
the liquid then passing through an inlet chamber 16
and thereafter through a coalesces inlet 18 into a
l0 coalesces cartridge 20. After passing in an inside-
out flow direction through an appropriate packing
which defines the walls 22 of the coalesces
cartridge, the fluid passes into the body of the
housing and thereafter through the walls 32 of the
separator cartridge 30 in an outside-in flow path.
The external surface of the walls of the separator
are provided with a material having a surface energy
such that because of the surface tensions of the
continuous and discontinuous phases, the liquid
forming the continuous phase can pass through the
walls of the separator and into the separator body
while the liquid which is immiscible therewith is
prevented from entering the separator body. In
effect, the liquid forming the discontinuous phase,
which is coalesced into larger droplets by the
coalesces, is repelled in the vicinity of the
separator wall 32. The continuous phase which
enters the separator cartridge 30, through the
separator wall 32, thereafter passes through the
separator outlet 28 into the outlet chamber 26 and
finally out the housing outlet 24. The coalesced
drops of liquid originally in the discontinuous
phase flow to the floor or base 36 of the housing
unit, situated above the inlet chamber 16 and outlet
chamber 26, and out the discontinuous phase outlet
or drain 34.
- 5 -
CA 02297444 2000-O1-26
In some industries, the demands for increased
capacity have resulted in an increased size of the
coalesces units. Figure 2 represents a plan view of
the interior of a conventional coalescing-separating
apparatus intended to provide large scale capacity
for separation of a discontinuous phase. As may be
noted, while the apparatus includes only two
separator elements, numerous coalesces units are
provided. In this arrangement, fluid enters the
inlet 14 of the housing 12 where it then flows, by
separate paths, into the inlets (not shown) of the
different coalesces units and afterwards through the
packing of each coalesces unit 20 into the housing.
The liquid then passes into the section of the
housing containing the separator elements 30 where
the fluid, largely depleted of the discontinuous
phase liquid, passes through the walls 32 of the
separator units, into the body of the separator
units, thereafter passing through the outlet of each
of the separator units and out of the housing outlet
24. While the capacity of the apparatus shown in
Figure 2 has been increased as compared to the type
illustrated in Figure 1~, such an arrangement results
in an uneven flow distribution. That is, a fluid
flow or velocity gradient exists between the
different regions within the housing. In the
arrangement shown in Figure 2, the gradient exists
as a side-to-side gradient in which the row of
coalesces units closest to the separators process
more fluid than do the remaining coalesces units.
At the same time, the separator units have an uneven
flow distribution about their circumferences because
of their proximity to the coalesces units.
As indicated above, secondary emulsions or
hazes present one of the most difficult separation
problems where physical methods are used exclusively
- 6 -
CA 02297444 2000-O1-26
to separate and remove the discontinuous or
dispersed phase. While coalesces-separator devices
have been used with varying degrees of success to
purify the continuous phase in such applications,
the method and apparatus are accompanied'by various
shortcomings. First, 100% coalescence and removal
of the discontinuous phase proves difficult simply
because of the very small droplet size of the
dispersed phase, which itself may be caused in part
by the presence of a surface active substance.
Secondly, in those situations in which a surface
active material is present, which is a common
situation, the change of surface tension
attributable to the surface active substances make
coalescence difficult, short of removing those
surface active substances prior to a coalescing
treatment. Third, after a period of use, the
surface active substances found in many of these
chemically induced emulsions are believed to coat
the active surfaces of the coalesces packing, which
currently is most often glass fibers, thus
"disarming" or rendering the coalesces ineffective.
For such reasons, coalesces-separator devices do not
provide the degree of purity sought from liquids
containing such surface active substances and/or
require frequent changing of coalesces elements.
This type of problem is being encountered much
more frequently in fuel related industries.
Petroleum based fuels tend to pick up moisture,
particularly upon storage. Filter-coalescer-
separator devices have conventionally been used to
remove entrained water from such fuels. In recent
years, however, additives, particularly surfactants,
have been used in increasing amounts in such fuels.
Accordingly, to achieve the same minimal
concentrations of moisture, treatments to remove
CA 02297444 2000-O1-26
moisture after blending, transporting and storage of
such fuels have required more frequent changing of
coalescing units. Although the inclusion of
phenolic or acrylic resins which primarily act as
binding agents for glass fiber packings has had a
collateral effect in reducing disarming somewhat but
disarming still occurs in high surfactant-containing
liquids.
The present invention relates to
coalesces-separator apparatus which overcomes many
of the shortcomings of conventional coalesces
devices. Because of the improved flow distribution
resulting from the present invention, the life of
the coalesces units employed is significantly
increased and effective separation of a
discontinuous phase, such as water typically found
in petroleum based fuels, is greatly increased. In
addition, because of the arrangement of the
components of the present invention, a more compact
unit may be prepared which achieves the same or an
improved level of performance as compared to larger
conventional units.
A liquid purification system in accordance with the
present invention includes at least one coalescing
assembly, each of which includes at least one coalescing
element or unit for coalescing the discontinuous or
suspended (rather than dissolved) phase of a mixture of
immiscible liquids into droplets, and at least one
separating assembly, each of which includes at least one
element or unit for separating the coalesced droplets from
the continuous phase. Usually, the coalescing assemblies
and/or elements) and the separating assemblies
_ g _
CA 02297444 2000-O1-26
and/or elements) are arranged in stacked or
superposed relationship. Typically, the coalescing
elements) and separating elements) are enclosed
within a housing having fluid, particularly liquid,
inlet and outlet passages. The housing includes an
outlet for the liquid which originally formed the
continuous phase and usually an outlet for the
liquid which originally formed the discontinuous
liquid phase.
An aspect of the present invention provides
a system for separation of two partially or
wholly immiscible liquids including at least one
coalescing element and at least one separating
element in which the coalescing elements) includes
a porous material having a surface energy (or
critical wetting surface tension) which is greater
than the surface tension of the continuous liquid
phase but less than the surface tension of the
discontinuous liquid phase. Preferably, the
material forming the phase separating portion of the
coalescer has a fibrous configuration.
Another aspect of the present invention relates
to a method of separating a discontinuous phase
liquid, such as water, from a continuous phase
liquid, particularly an organic liquid, such as a
fuel. The method involves introducing a mixture of
the discontinuous and continuous phase liquid to at
least one coalescing element that includes a packing
material having a critical wetting surface tension
intermediate the critical wetting surface tension of
the discontinuous and continuous phase liquid to
form droplets of the discontinuous phase.
Thereafter, the mixture of the continuous phase
liquid and the droplets of the discontinuous phase
liquid are conducted to at least one separating
element which permits passage of the continuous
_ g _
CA 02297444 2003-04-07
phase liquid but subsr:.<~ntially .resists or prevents passage
of the discontinuous phase liquid drop>lets, whereby the
continuous phase liqu:ic~ is separated from the droplets of
discontinuous phase liquid.
Thus, in one aspect, the present invention provides a
separating medium for separating a first liquid from a
second liquid in whicru the first= liquid is wl-iolly or partly
immiscible with, and forms a discc.~ntinuous phase with, the
second liquid, the separating medium comprising a
calendered, porous, fibrous st.ruci:~ure including polytetra-
fluoroethylene fibers waving d.ia~meters up to about 70 ~.z:m,
and a fluorocarbon binder.
In anoi~her aspect::, the pre.~ent invention provides .a
separating Element fozv separating a first liquid from a
second liquid in which the firs-: :i.iquid is wholly or partly
immiscible with, and .forms a discontinuous phase with, the
second liquid, the separating t.=._lement comprising the
separating medium as defined he rein. 'The separating
element further comprises a po.~ymeric or metal mesh support
sleeve and a metal support core, Each of the fibrous
structure, :support sleeve, and :.upport core have a
cylindrical configuration and cc;ncentric relationship, and
end caps sealingly attached to t:he~ fibrous st:ructure,
support sleeve, and support: core.
2_5 Figure 1 illustrates an el.evational, sectional view
with a partial cutaway of a r_onventiona_l. coalescer-
separator liquid separation system.
Figure 2 shows a plan view of the interior of a
conventional coalescer-separator liquid separation system
having a plurality of separating elemc_mt;; and a plurality
of coalescing elements .
CA 02297444 2003-04-07
Figure 3a ii.lustrates an embodiment of the present
invention in which coalescing element:: are superposed above
separating elements.
Figure 3b is a sec tional view of the embodiment of
Figure 3a taken <along ~.ine III-III.
Figure 4 illustrates another embodiment of the present
invention i:n whic-.;h se~:~arati.ng e:l.ements are superposed above
coalescing elements.
figure '. il7.ustr~:rtes an embodiment of the invention in
which a plu.ra.lity of c.,oalescind elements are arranged in
series.
Figure 6 ill.ustra.tes an embodiment of tpe invention in
which a plurality of coalescing elements and a plurality of
separating elements are arrange<~ i.n superposE~d anc~
alternating relationship.
As indicated above, the pr went: invention i s directed
to an. immiscible liquid! liquid c;oalesc irrg and separating
system which, in ~~ompari,son to c:onvent:ional liquid
coalescing-separating systems, ~>rovides longer useful life
of the coalesces elements, may ~~e fog°rned as a smaller unit
than a similar convent:i.onal system of comparable capacity
and performance, and, because of the arrangerrrent of the
elements, results in an improved flow distribution which. is
more effective in separating liguid c:anuponent.~~.
In describing the present inventic>n, terms such as
"coalesces", "coa:Lescing elerrrent", "c:oalescinc~
-10a-
CA 02297444 2000-O1-26
unit" and like terms, in both singular and plural,
have been used to describe the device or article
which coalesces the discontinuous or polydivided
phase of a mixture of immiscible liquids to form
droplets. Regardless of the term used, the
coalescing step employing such device occurs in the
same manner. While the term "coalesces" generically
describes such a device and the term "coalescing
element" describes one component unit or cartridge
of a system which may contain multiple coalescing
and separating units, the present invention may be
construed as containing as few as one coalesces unit
in a coalesces-separator system to a plurality of
such units. In addition, such coalescing units may
be fixed and not removable (without doing
significant damage to the system), or preferably,
contain easily removable and replaceable elements.
In a similar manner, terms such as "separator",
"separating element", "separator units", and like
terms have meanings similar to each other as do
those relating to coalescers, discussed above.
When a liquid is brought into contact with the
upstream surface of a porous medium and a small
pressure differential is applied, flow into and
through the porous medium may or may not occur. A
condition in which no flow occurs is that in which
the liquid does not wet the material of which the
porous structure is made.
A series of liquids can be prepared, each with
'a surface tension of about 3 dynes/cm higher
compared with the one preceding. A drop of each may
then be placed on a porous surface and observed to
determine whether it is absorbed quickly, or remains
on the surface. For example, applying this
technique to a 0.2 micrometer porous polytetra-
fluoroethylene (PTFE) filter sheet, instant wetting
- 11 -
CA 02297444 2000-O1-26
was observed for a liquid with a surface tension of
26 dynes/cm. However, the structure remains
unwetted when a liquid with a surface tension of 29
dynes/cm is applied.
Similar behavior is observed for porous media
made using other synthetic resins, with the
wet-unwet values dependent principally on the
surface characteristics of the material from which
the porous medium is made, and secondarily, on the
pore size characteristics of the porous medium. For
example, fibrous polyester, specifically
polybutylene terephthalate (hereinafter "PBT")
sheets, which have pore diameters less than about 20
micrometers, were wetted by a liquid with a surface
tension of 50 dynes/cm, but were not wetted by a
liquid with a surface tension of 54 dynes/cm.
In order to characterize this behavior of a
porous medium, the term "critical wetting surface
tension" (CWST) has been defined as described below.
The CWST of a porous medium may be determined by
individually applying to its surface, preferably
dropwise, a series of liquids with surface tensions
varying by 2 to 4 dynes/cm, and observing the
absorption or non-absorption of each liquid. The
CWST of a porous medium, in units of dynes/cm, is
defined as the mean value of the surface tension of
the liquid which is absorbed and that of a liquid of
neighboring surface tension which is not absorbed.
Thus, in the examples of the two preceding
paragraphs, the CWST's are, respectively 27.5 and 52
dynes/cm.
In measuring CWST, a series of standard liquids
for testing are prepared with surface tensions
varying in a sequential manner by about 2 to about 4
.dynes/cm. Ten drops of each of at least two of the
sequential surface tension standard liquids are
- 12 -
CA 02297444 2000-O1-26
independently placed on representative portions of
the porous medium and allowed to stand for 10
minutes. Observation is made after 10 minutes.
Wetting is defined as absorption into or obvious
wetting of the porous medium by at least nine of the
ten drops within 10 minutes. Non-wetting is defined
by non-absorption or non-wetting of at least nine of
the ten drops in 10 minutes. Testing is continued
using liquids of successively higher or lower
surface tension, until a pair has been identified,
one wetting and one non-wetting, which are the most
closely spaced in surface tension. The CWST is then
within that range and, for convenience, the average
of the two surface tensions is used as a single
number to specify the CWST.
Appropriate solutions with varying surface
tension can be prepared in a variety of ways,
however, those used in the development of the
product described herein were:
- 13 -
CA 02297444 2000-O1-26
TABLE 1
Surface Tension,
Solution or fluid (dynes/cm)
Sodium hydroxide in
water 94-110
Calcium chloride in
water 90-94
Sodium nitrate in water 75-87
Pure water 72.4
Acetic acid in water 38-69
Ethanol in water 22-35
n-Hexane 18.4
FC77~(3M Corp.) 15
FC84~ ( 3M Corp . ) 13
A first aspect of the present invention is
directed to a coalescing-separating system employing
both a coalescing assembly which includes at least
one coalescing element and a separating assembly
which includes at least one separating element in
which the coalescing assembly or element is arranged
with respect to the separating assembly or element
in a stacked or superposed relationship. When
cylindrical coalescing and separating elements are
employed, the cylindrical axes of the elements are
arranged substantially vertically. In its simplest
form, the present invention may include a single
coalescing element or coalesces and a single
separating element or separator. This arrangement
could be used for coalescers and separators formed
from any suitable media, which media are arranged in
any suitable configuration. In its simplest form,
the medium serving as the coalesces may be provided
- 14 -
CA 02297444 2000-O1-26
in sheet form and placed in proximate but spaced
relationship to the separator, which also may be in
sheet form. In such an embodiment, both the
coalescing element and the separating element,
independent of each other, may be formed either as
flat sheets or as pleated or corrugated sheets in
which the peaks and troughs of each sheet lie in
planes parallel to one another. The preferred
configuration of the coalesces- and separator is
cylindrical in which.the functional portion of the
coalesces and separator (i.e., that portion of the
coalesces or separator performs the coalescing or
separating function, respectively) is,
independently, formed as a cylinder around the axis
of the element. In either case, the functioning
portion of the element may be arranged as a
cylindrical sheet or mat, a cylindrical pleated
sheet or mat, or a helically or spirally wound sheet
or mat, the later pertaining particularly to
coalescers. In the case of separators, the
functioning portion of the element may also be a web
or, preferably, a screen.
The coalescers and separators or coalescing and
separating elements of the present invention may be
manufactured as a single unit with one or more
coalescing stages or portions and one or more
separating stages or portions. Most preferably, the
coalescing and separating elements are manufactured
and assembled as separate units. In practice this
permits removal and replacement of the separate
elements.
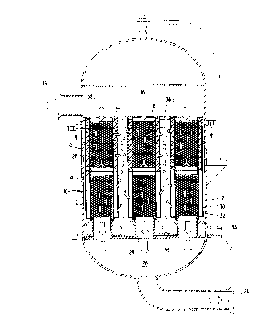
Figure 3a illustrates an embodiment of the
present invention in which a plurality of coalescing
elements 20 are individually superposed above a
plurality of separating elements 30. The coalescing
elements 20 and separating elements 30, illustrated
- 15 -
CA 02297444 2000-O1-26
in the embodiment of Figure 3a, are located within
housing 12. A liquid inlet is provided in a wall of
the housing for introducing liquid, in this
embodiment, above the coalescer elements. Liquid
inlets 18 are provided in the upper end of each
cylindrical coalescing element 20 for introduction
of contaminated liquid thereto. Each coalescing
element has a packing which defines the cylindrical
wall 22 of the coalescing element. The packing
contains a material which has a critical wetting
surface energy intermediate the surface tensions of
the liquids forming the continuous and discontinuous
phases.
In a like manner, each separating element
includes a perforated~wall 32 which is formed from,
or has an outer surface coating of, a material which
repels (or is not wetted by) a liquid of the
discontinuous phase, which may be termed the
"discontinuous phase~barrier material". Such a
material should not react with any liquid or other
substance present in the mixture of immiscible
liquids. When used as a coating on the wall of the
separator, such material should remain substantially
immobilized thereon. Typically, the critical
wetting surface energy of this material is selected
to permit passage of the liquid forming the
continuous phase through the small pores of the
material defining the wall of the separator element,
and when the separator is a cylindrical element, as
shown in Figure 3a, to thereby permit ingress of
that liquid to the separator but to repel or prevent
ingress to the liquid which forms the discontinuous
phase. For example, in systems in which water is
the discontinuous phase, materials are selected as,
or are coated on, the wall of the separator which
have a critical surface energy or CWST below the
- 16 -
CA 02297444 2000-O1-26
surface tension of water. For applications in which
water or a liquid having a similar surface tension
constitutes the discontinuous phase, materials
preferred for use as the discontinuous phase barrier
material for forming or coating the separating
element wall include silicones, such as a silicone
treated paper, and, preferably fluoropolymeric
materials of which fluorocarbons or perfluorocarbons
or perfluoro resins are particularly preferred.
Examples of preferred materials for use as the
packing or coating in the separator include
polytetrafluorolethylene (PTFE) or.other
polyfluorinated polymers such as fluorinated
ethylene propylene (FEP) resins.
A preferred embodiment includes a coating of
one of these materials on a stainless steel screen,
or a pleated paper pack. Other suitable materials
include those disclosed in the United States Patent
to Miller et al. (U. S. Patent 4,759,782),
Generally, the functional or discontinuous phase
barrier material portion, which is also the
continuous phase liquid-passing portion, of the
separator is selected to have pores smaller than a
substantial amount of the droplets of the liquid
which originally formed the discontinuous phase.
Typically the pore size of the functional part of
the separator wall is selected to be about 5~ to
about 140~C, preferably about 40~c to about 100.
Most preferably, and particularly when the
discontinuous phase is water, the pore size is about
80~.
Other media suitable for use as the functional
or discontinuous phase barrier material portion of
the separating element are porous, fibrous
fluorocarbon structures of the type described in
- 17 -
CA 02297444 2000-O1-26
United States Patent to Hurley et al. (U. S. Patent
No. 9,716,079). .Such materials are porous, fibrous'
structures having good structural integrity which include
fluorocarbon polymer fibers and a fluorocarbon binder.
Such media, while suitable for use in the present
invention, are intended primarily as support and drainage
layers in filtration cartridges.
Although sharing some similarities in
composition and preparation with the structures
described by Hurley et al., the medium most
preferred in the present invention is a calendared,
porous, fibrous fluorocarbon structure which
includes PTFE fibers in a fluorocarbon binder,
preferably a FEP binder. The fibers employed are
bleached and water washed PTFE fibers having
diameters ranging up to about 70 micrometers,
preferably from about 54 to about 70 micrometers.
Most preferred are PTFE fibers having a nominal
diameter of about 65 micrometers. This material is
prepared to have a sheet weight of about 15 to about
35 grams/ft2, preferably about 15 to about 25
grams/ft2. Most preferred is a medium having a~sheet
weight of about 21.5 grams/ft2.
As indicated above, although similarities exist
in both the preparation and composition between the
preferred porous, fibrous fluorocarbon media used as
the discontinuous phase barrier material of the
present invention and the media described in U.S.
Patent No. 4,716,074, major distinctions also exist
between these materials. Thus, the material which
is most preferred in the subject application is
calendared to a thickness of about 50 to about 90%,
preferably about 75%, of its original thickness.
Such calendaring raises both the DP and bubble
- 18 -
CA 02297444 2000-O1-26
points of the media and produces a more efficient
separating medium which achieves a substantially
uniform flow velocity perpendicular to and in
contact with all portions of the upstream surface.
In contrast, an uncalendared material demonstrates
both a high cross flow (movement or diffusion in an
edge-to-edge direction rather than surface-to-
surface direction) and a substantially imperceptible
resistance to fluid flow in an upstream-to-
downstream direction through the medium.
Essentially, fluids passing through such media take
the path of least resistance and may not contact all
portions of the medium. Thus, the process of
calendaring the medium provides the qualities
desirable for the medium of the present invention
while making such a medium substantially unsuitable
as a support and drainage layer. Likewise, a
material which demonstrates suitability as a support
and drainage layer is frequently not particularly
effective as a discontinuous phase barrier material.
This preferred medium, having an average
thickness before calendaring of about 0.015 to about
0.025 inch, preferably about 0.018 to about 0.022
inch and most preferably about 0.019 inch is
calendared to a thickness of about 0.004 to about
0.009 inch, preferably about 0.005 to about 0.007
inch and most preferably to about 0.006 inch. The
calendaring is performed at ambient temperature
under a pressure suitable to achieve a compression
and reduction in thickness to produce the DP and
Bubble Point sought. The calendared product has a
first Bubble Point (which reflects the size of the
largest pore), measured in ethyl alcohol of about
0.5 to about 4 inches (about 1.3 to about 10.2 cm)
of water, preferably about 2 to about 3.5 inches
(about 5.1 to about 8.9 cm) of water, preferably
- 19 -
CA 02297444 2000-O1-26
about 2.75 inches (about 7 cm) of water. The
calendared medium also has a Mean Pore Bubble Point
measured in ethyl alcohol of about 2 to about to
inches (about 5.1 to about 25.4 cm) of water,
preferably about 3.5 to about 6 inches (about 8.9 to
about 15.2 cm) of water. Most preferably the Mean
Pore Bubble Point is about 4.5 inches (11.4 cm) of
water. The calendared sheet of PTFE fibers bound
with FEP binder has a pressure drop across the
medium (L1P) as measured with a face velocity of air
at 28 ft/min., of about 0.5 to about 12 inches
(about 28 to about 1.17 Frazier number or about 1.3
to about 30.5 cm) of water, preferably about 1 to
about 5 inches (about 14 to about 2.8 Frazier number
or about 7.6 to about 12.7 cm) of water and most
preferably 1.4 inches (about 10 Frazier number or
about 3.5 cm) of water.
After passing into the separator 30 through
wall 32 in an outside-in direction, the liquid
forming the continuous phase passes out of separator
outlet 28 and into the outlet chamber 26.
Thereafter, the liquid which originally formed the
continuous phase passes out of the device through
outlet 24. The liquid which formed the
discontinuous phase in the original liquid mixture
collects at the floor or base 36 and is removed from
the apparatus through the discontinuous phase outlet
or drain 34.
In operation, a mixture of immiscible liquids
is introduced to the housing 12 through the
immiscible liquid inlet 14. After entering the
housing, the mixture flows in the direction of the
arrows shown in Figures 3a and 4. Namely, liquid
enters each coalescing element through the inlet
portion 18 in one of the end caps and, since the
other end cap 19 seals the unit completely, liquid
- 20 -
CA 02297444 2000-O1-26
flows through the porous packing which defines the
wall 22 of each coalescing element. Each coalescing
element is held in fixed position with respect to
another juxtaposed coalescing element and/or to the
housing wall. This may be achieved by specific
locating and/or fixing means (not shown) or,
alternatively, at least in part, by using liquid
barriers 38a, located between elements, or by liquid
barriers 38b, located between elements and the
interior wall. These barriers may be formed in
separate sections or as a single unit. These liquid
barriers primarily act as liquid sealing elements
and assure that the liquid flowing into the housing
under the force of gravity or an additional pressure
can only flow to the bottom of the housing by first
entering the inlet portion 18 of each of the
coalescing elements and flowing through the walls of
the coalescing elements. After passing through the
wall of the coalescing element in an inside-out
direction, the liquid flows into each separating
element through a wall portion 32 in an outside-in
direction. Due to the composition from which the
external wall of the separating element is formed or
on which a coating is placed, only the continuous
phase enters the separating element, leaving many of
the droplets of the discontinuous phase liquid
formed by the coalescing elements to fall to the
partition or bottom 36 located between and below the
separating elements (in the embodiment shown in
Figure 3a). This liquid is then removed from the
housing through the discontinuous phase outlet or
drain 34. The continuous phase liquid passes out of
each separating element through outlet 28 into the
outlet chamber 26 where it passes from the housing
through continuous phase outlet 24.
- 21 -
CA 02297444 2000-O1-26
Figures 3a and 3b illustrate an embodiment of
the present invention containing an assembly of
seven liquid coalescing elements superposed above an
assembly of seven liquid separators. However, while
this is a preferred embodiment and arrangement, the
present invention is not limited thereto and other
embodiments and variations are possible. The
particular number and arrangement of separating and
coalescing elements depends on the specific mixture
being separated. The arrangement shown in Figure 3a
is most suitable, and is preferred, for immiscible
liquid mixtures in which the discontinuous phase is
more dense than the continuous phase, as for
example, a mixture in which water is suspended in a
petroleum based fuel. In such a situation, the more
dense discontinuous phase would tend to move in the
direction of the separating elements 30 after
passing through the coalescing elements 20. Where
the discontinuous phase is less dense than the
continuous phase, for example, water suspended in
CC14, it is preferred to position separating elements
above coalescing elements. An embodiment such as
this is illustrated in Figure 4. While the
foregoing represents the preferred arrangements,
where the discontinuous phase is present at very low
inlet concentrations, for example, concentrations of
about up to about 0.02, the reverse orientation of
Figure 4 has been shown to be relatively effective
even when the discontinuous phase is more dense.
Also, rather than a single coalescing element being
arranged in superposed relationship with respect to
each separating element, a coalescing assembly
composed of a plurality of coalescing elements may
be superposed in series with respect to each
separating element.
- 22 -
CA 02297444 2000-O1-26
The series relationship could take a variety of forms. In
those embodiments in which a plurality of coalescing elements
are used for each separating element employed, and more than one
separating element may be used, the coalescing elements are
arranged, for example, within an assembly, in parallel
relationship with one another and collectively in series and
superposed relationship with the one or more separating elements
employed. In this arrangement, a tier of coalescing elements,
arranged in parallel with one another, would be placed above or
below one or more separating elements. In this embodiment,
although one coalesing element may be arranged coaxially with
respect to each separating element employed, such an arrangement
is not required. An example of this embodiment is illustrated
in Figure 5, showing two coalescing elements arranged in
parallel with one another and placed above two separating
elements. The reference numbers in Figure 5 correspond to those
used in Figure 3.
In another e.nbodiment of the present invention, the
coalescing (C) and separating (S) elements are arranged in
alternating series, and preferably, coaxial, head to tail
arrangement (i.e., C-S-C-S), as illustrated, for example, in
Figure 6. The reference numbers in Figure 6 correspond to those
used in Figure 3. Such an arrangement might be used with
mixtures which are difficult to separate. With this
arrangement, the liquid originally present in the discontinuous
phase, which not sufficiently coalesced to be rejected by the
walls of the separator, is passed on to the next coalesces in
the series, the droplets of liquid which were formed from the
discontinuous liquid growing in size after passing through each
successive stage.
. In another embodiment of the present invention, one which
is preferred, a coalescing assembly is formed from a plurality
of coalescing elements which are arranged in superposed, stacked
and coaxial arrangement in series with one another and are
collectively positioned in series with a separating
- 23 -
CA 02297444 2000-O1-26
element (e. g., C-C-C-S). Further details of such
arrangement are indicated below.
In addition, while Figure 3b indicates six
superposed coalescing-separating elements placed
radially around a central separating-coalescing
element, the number of radially placed separating
and coalescing elements in superposed relationship
may be increased or decreased as may the centrally
positioned separating and coalescing elements.
Although the radially arranged elements result in
the most compact liquid purification device with the
best flow distribution, other arrangements, such as
a linear or rectangular arrangement may be used for.
particular purposes.
In those instances in which the coalescing and
separating elements are manufactured as separate
units, the blind or closed end caps of the
coalescing and separating elements may be designed
such that they interlock with one another.
Alternatively, means may be provided to locate each
element within the housing so that they remain in
superposed positions. When a plurality of
coalescing elements are employed for each separating
element used, the coalescing elements may be
arranged in series with respect to one another (for
example, C-C-C-S), preferably in a stacked or
superposed arrangement. In such an arrangement, the
coalescing elements may be interconnected in a
number of ways. For example, as shown in Fig. 5 those
coalescing elements that first encounter the incoming
mixture of immiscible liquids may be joined to successive
downstream coalescing elements in a head-to-tail
arrangement by conduits which join an outlet portion
of each coalescing element (which differ from the
blind end caps of the coalescing elements of Figures
3a and 4 in that they have fluid outlets in the
- 24 -
CA 02297444 2000-O1-26
downstream end caps) to the next downstream element.
In such an arrangement, the outlet end of each
conduit would be connected to the inlet portion of
the coalescing element next in series and all of the
coalescing elements in the series would have both
inlet and outlet portions with the exception of the
last coalescing elements in each series which are
located in a stacked relationship with respect to
the separating elements and would have only a fluid
inlet. These last or end-of-series coalescing
elements would be essentially as shown in Figure 3a,
having an end cap with an inlet and a blind or
sealed end cap at the other end of the coalescing
element. In an alternative arrangement, rather than
using conduits between successive coalescing
elements, the fluid outlet portion of one coalescing
element may be constructed to sealingly engage the
inlet portion of the downstream coalescing element.
In still another embodiment using a plurality
of coalescing elements for each separating element,
the coalescing elements, either individually or as
assemblies of a plurality of coalescing elements,
could be placed above one another in series,
preferably stacked, but spaced apart from one
another. In this embodiment, all of the coalescing
elements would include inlet portions 18 and blind
end caps 19 at the opposite end of each element, such
as those shown in Figures 3a and 4. Rather than
liquid flowing continuously from one coalescing
element to the next downstream coalescing element in
the series through the center of each element and
out through the packing defining the walls of the
last coalescing element, as in the embodiment
discussed immediately above, each coalescing element
would be provided with a closed or sealed end cap
and liquid would flow only out through the walls of
- 25 -
CA 02297444 2000-O1-26
each coalescing element and into the inlet portion
of the next successive downstream coalescing
element. In such an instance, it would be
preferable to use multiple barriers, such as 38a and
38b, to separate each successive coalescing element
or tier of coalescing elements to direct liquid flow
passing through the walls of the coalescing element
in that tier or assembly to the inlet of the
coalescing element in the next downstream tier.
l0 Figure 3a illustrates a device in which the
liquid mixture inlet 14 is located in the wall of
the housing immediately above the coalescer elements
while the purified continuous phase liquid outlet 24
is located in the bottom of the housing
communicating with the outlet chamber 26. Although
these are preferred arrangements, the inlets and
outlets can be located elsewhere in the housing.
For example, in the embodiment shown in Figure 3a,
inlet 14 may be located in the top of the housing 12
while the liquid outlet 24 might be located in the
wall of the housing. In the latter instance, the
device would preferably have a flat bottom rather
than the spherical bottom illustrated in Figure 3a
and the outlet 24 would be located close to the base
of the device. In embodiments in which the
separating elements are positioned above the
coalescing elements as in Figure 4, the relative
positions of inlets and outlets may be reversed from
those described for the embodiment shown in Figure
3a. For example, the liquid mixture inlet 14 is
located in the lower part of the housing below the
coalescing elements while the continuous phase
liquid outlet 24 is located at the upper part of the
housing.
In embodiments of the present invention in
which the coalescing elements and/or separating
- 26 -
CA 02297444 2000-O1-26
elements are intended for easy removal from the
device for replacement or regeneration, the housing
is designed such that either the top or bottom of
the housing is removable. Since most frequently the
coalescing elements will be discarded and replaced,
the preferred system for the type of embodiment
illustrated in Figure 3a in which coalescing
elements are superposed above separating elements
includes a housing with a removable top or cover
portion 38. Most preferably, the top is a swing-up
cover, but alternatively, the top could include
threading or pins to engage like threading or a
bayonet base portion in the housing wall 42 or could
be a spring loaded, counterbalanced, hinged cover as
described by Miller et al., in U.S. Patent No.
4,419,234. In those systems in which the separating
elements are located above the coalescing elements,
the housing may be constructed to include a
removable bottom.
A pressurized feed may be used in some
instances. Accordingly, the wall of the housing may
also be provided with vents and pressure release
valves, as well as fittings for inlet and outlet
pressure gauges.
As indicated above, while in many instances
separation can be adequately obtained by using
coalescing elements and separating elements in equal
numbers, to provide as compact a unit as possible
with the smallest volume housing possible, and an
adequate separation and satisfactory capacity in
terms of flow rates, in many situations it is
desirable to increase the ratio of the number of
coalescing elements to the number of separating
elements employed. While substantially the same as
the ratio of coalescing elements to separating
elements in many situations, the more pertinent
- 27 -
CA 02297444 2000-O1-26
parameter is the ratio of effective surface area of
the coalescing elements to the effective surface
area of the separating elements. To some extent
this depends upon the size, shape and configuration
of the functionally effective portions of the
coalescing and separating elements. In most
situations this corresponds to the planar surface
area or cylindrical surface area (the height times
the circumference of the functionally effective
portion), also known as the "projected surface
area", of the cylindrical element. When a pleated
element is used either in the coalescing element or
the separating element, a more pertinent measurement
is the "effective surface area". This measurement
of surface area departs somewhat from the
measurement of the planar or cylindrical surface
area since it is the actual area of the material as
measured wrien the pleats or corrugations are removed
and the material is extended (or the height times
number of pleats times the depth of the pleat times
2). This measurement of surface area is greater
than the cylindrical surface area. This may be
taken into account in determining effective surface
area.
In those instances in which the size, shape and
configuration of both the separating elements and
coalescing elements are the same, it is merely
necessary to express the ratio of surface areas as a
ratio of the number of units. Alternatively, when
the configuration and diameter of both the
separating elements and coalescing elements are the
same, it may only be necessary to compare the height
of the coalescing element to that of the separating
element.
As suggested above, the ratio of the effective
surface area of the coalescer or coalescing element
- 28 -
CA 02297444 2000-O1-26
to that of the separator or separating element
varies with the separation to be effected. The
factors to be taken into account in determining the
appropriate ratio are the nature of the liquids
which form the suspended or discontinuous phase and
the suspending or continuous phase, the nature of
the packing and discontinuous phase liquid barrier,
and the volume and/or flow rate of the liquid
mixture. These factors take into account the
chemical and physical properties of both the liquids
and dissolved materials (such as surface active
substances) and the functional portion of the
coalescing and separating elements, as well as their
interaction with one another. For most purposes,
however, this ratio ranges from about 0.25 to 1-
to about 10 to 1. When water forms the
discontinuous phase and the liquid forming the
continuous phase has a high viscosity, preferably,
the ratio is about 5 to 1 to about 10 to 1.
Alternatively, when the liquid in the continuous
phase has a low viscosity preferably the ratio is
about 0.25 to 1 to about 4 to 1. As used in
discussing the present invention, "high viscosity"
means about 50 cp or greater and "low viscosity"
refers to less than about 5 cp.
In the stacked coalescer-separator arrangement
of the present invention, any packing may be
employed to form the coalescer wall through which
the immiscible mixture of liquids passes which does
not chemically react with or absorb any of the
components of the liquid mixtures. Typically this
would include materials such as glass, cork, and
nylon. However, other materials, such as those
listed in United States Patent to Pall et al. (U. S.
Patent No. 3,268,442)
- 29 -
CA 02297444 2000-O1-26
could be used in the stacked arrangement of the
present. invention .
In a particular aspect of the present
invention, which is not restricted to the superposed
arrangement of coalesces and separator, but which is
preferably used therewith to provide even further
benefits, a packing material is chosen for the
coalesces having specific surface energy properties.
In this preferred aspect of the present
invention, the packing is selected with a
' consideration of the mixture of liquids to be
separated. In particular, the surface energy or
CWST of the packing material is selected to be less
than the surface tension of the discontinuous phase
liquid and greater than the surface tension of the
continuous phase. Suitable for use in the present
invention as packing materials for the coalescing
element are those having a pore size in the range of
about 0.5~ to about 25~,, preferably, about 0.5~ to
about 3~. (especially for liquids having low IFTs)
and most preferably, about 3~. This is particularly
preferred as the pore size of the preferred packing
material discussed below. In general, the effective
pore size may be selected based on the relationship
a = 50/8.P. where ~. = effective pore size in microns
and B.P. - the open end bubble point of the material
in inches of water using an ethanol containing
liquid. (The constant is determined, in part, by
the thickness and nature of the material used and
the conditions of measurement and is known as the
"capture efficiency". For the material preferred as
the packing in the present invention the value is
50. For glass fiber packings this value would
typically be 150.) In many situations, and
particularly in those situations in which water is
present as the discontinuous phase, polyesters,
- 30 -
CA 02297444 2000-O1-26
including polycarbonates, are preferred as the
packing material. Among the preferred polyesters,
polyethylene terephthalate and polybutylene
terephthalate are preferred with the latter being
most preferred. Because of cost considerations and
the pressure drop across the packing (DP), these
materials are preferably used in fiber form,
although in some instances membranes may be used.
The fibers may be used as woven mats but non-woven
mats are generally preferred. It has been found
that materials with the above described critical
wetting surface tensions, and particularly
polyesters, are much less readily disarmed than
conventional materials and their use results in
extended lifetimes for the coalescing elements. In
addition, such materials are effective in separating
liquids having very low IFTs, typically at or below
dynes/cm and preferably below 10 dynes/cm.
As the preferred fibrous mats used as the
20 packing in the coalescing elements of the present
invention, mats containing uniform fiber diameters
as well as mats having stepped or graded fiber
diameters across the depth of the mat (i.e., from
one surface to the opposite parallel surface) may be
used. Preferred are non-woven mats containing at
least a partially graded fiber diameter structure
and most preferred are mats arranged in cylindrical
fiber structures having a graded fiber diameter
structure in at least a portion of the structure in
the radial direction. It is also preferred that
such a structure have a substantially constant voids
volume over at least a substantial portion of the
structure, also, as mentioned above, in the radial
direction. One of the preferred embodiments
includes constant fiber diameters in the downstream
portion with the upstream portion being profiled
- 31 -
CA 02297444 2000-O1-26
from the fiber diameter of the downstream portion up
to a larger diameter. The fibers employed to make
such mats are preferably substantially free of
fiber-to-fiber bonding but are secured to one
another by mechanical entanglement or intertwining.
The fibers employed to make the non-woven mats are
preferably synthetic, polymeric microfibers, most
preferably thermoplastic in nature. Examples of
such thermoplastic microfibers include polyolefins,
polyamides and polyesters. Such a packing material
and cylindrical structures provided with such
materials are available from Pall Corporation and
are described in U.S. Patent Nos. 4,594,202 and
4, 726, 901.
Typically, the voids volumes of such materials are
in the range of from about 60-95%, most preferably
from about 75 to about 85%. They also typically
have annular thicknesses from about 0.4 to about 1
inch (1.0 - 2.5 cm). The fiber diameter ranges from
about 1.5~, or less up to about 20u or more. When
the product is prepared to obtain a voids volume in
the range of about 75 to about 85%, the fiber
diameters are preferably selected to be below about
20~. The packing may also include a "final
coalescing layer" of fine fibers having diameters no
more than about 5~C and preferably about 3~ to about
5~. These fine coalescing fibers are present in a
downstream layer having a thickness of about 0.1 to
about 0.5 inches (about 2.5 to about 12.7 mm).
Each coalescing element may be provided with an
upstream or downstream support and/or drainage
material. Since most cylindrical coalesces elements
are employed in situations in which flow is in an
inside-out direction, provisions are generally taken
to protect the downstream surface of the packing
from damage and undue compression induced by
- 32 -
CA 02297444 2000-O1-26
elevated pressures or turbulent flow conditions.
Thus in order to retain structural integrity and
allow free flow of liquid, a plastic (e.g. PVC
coated glass) or stainless steel is disposed or
wound around the packing. Downstream of the
packing, a needled felt or air laid fiber batting,
preferably formed from polyester, may be placed.
This element, which has very large pores, i.e.,
significantly larger than those of the packing, is
provided to reduce turbulence and to orient or
"straighten" fluid f low. Optionally, a "sock" or
outer sleeve formed from Remay~ Orlon~or cotton may
be located downstream of the turbulence-reducing
layer to prevent fiber migration from the latter
layer. In addition, an upstream filter material may
be provided to capture particulate material before
it contacts the packing of the coalescing element.
Preferably, this is a depth filter having effective
pore sizes significantly larger than the packing
material of the coalesces so as not to inhibit flow
into the coalesces. In a preferred embodiment, such
as that discussed above, the packing used in the
coalescing element may be of a profile type having
graded pores which taper from the upstream surface
to the downstream surface. This type of structure
functions to trap dirt or particulate material in
the larger pores and to perform a coalescing
function in the downstream narrower pores. All of
these cylindrically configured layers are enclosed
within end caps.
The end cap, core and any support elements may
be manufactured from materials which are inert to
the liquid being treated. Typically, these will be
formed from fiberglass, a metal such as stainless
steel or, preferably, plastic.
- 33 -
CA 02297444 2000-O1-26
The separator may include elements similar to
those of the coalescing elements, such as end caps,
core and, to a lesser extent, support and drainage
layers. These also may be formed from the same
materials used to form like elements in the
coalescing elements. The separator may be formed as
or include a porous packing or coated element which
allows free flow of the continuous phase liquid but
repels the liquid which originally formed the
discontinuous phase. Preferably, this is a
stainless steel screen, for example a 100 x 100 wire
mesh screen, coated with PTFE. Most preferably, the
functional portion of the separating element is
formed from the medium described above, a calendared
web of PTFE fibers and FEP binder. The separating
element may be provided with a downstream metal or
plastic core. Any plastic which is inert or highly
resistant to the liquids being treated and any
additives or contaminants found in the liquids, and
which has satisfactory strength and rigidity may be
used to form the core. Exemplary are polyesters,
including polycarbonates such as Lexan~ polyamides
and Delrin°. As with the coalescing elements,
separating elements may be provided with an open
pore sleeve to assist fluid flow distribution along
the height dimension of the unit intermediate the
discontinuous phase barrier or repelling layer and
the core. Preferred is a pleated material known as
Epocel~ (available from Pall Corporation) which is
formed from cellulose and a phenolic binder. A
preferred embodiment of the present invention
employs, proceeding in an upstream-to-downstream
direction, a sleeve of a calendared medium formed
from PTFE fibers and a FEP binder (as described
above), a polymeric or metal mesh support sleeve and
a metal support core.
- 34 -
CA 02297444 2000-O1-26
In the present invention, the critical surface
energy or CWST of the functional part of the
separator packing or coating is lower than the
surface tension of the discontinuous phase. Thus, in
situations in which water is present, the present
invention is primarily effective to remove water in
a discontinuous phase from another liquid having a
surface tension lower than that of water.
Generally, where water is in the continuous phase
and the liquid to be removed constitutes the
discontinuous or suspended phase and has a surface
tension lower than that of water, a separator having
a packing or coating with a surface energy below the
surface tension of water would be ineffective since
it would prevent passage of the water but might
permit flow of the liquid in the discontinuous phase
through the walls of the separator without improving
the coalescence thereof. To employ a coating having
a surface energy higher than the surface tension of
water would allow both the continuous and
discontinuous phase liquids to pass through the
separator, also proving ineffective.
The following example indicates the manner in
which the present invention is used. The invention
should not however be construed as being in any way
limited thereto.
Example
A coalescer-separator system of the present
invention was tested for removal of water from
gasoline. An apparatus was constructed as described
above. A housing containing a single coalescing
element superposed in a coaxial arrangement over a
single separating element. The ratio of effective
surface area of the coalescing element to the
separating element was about 3 to 1. The packing
- 35 -
CA 02297444 2000-O1-26
which defined the. walls of the coalescing element
was formed from a profiled polybutylene
terephthalate fiber mat in which about the upstream
0.10 inch was formed from coarse fibers (about 40 to
60 ~.) and the downstream remaining portion of the
mat was formed from fine fibers (about 3 to about 5
~C) having an effective pore size of about 3 ~, and a
voids volume of about 75%. The coalesces element
was provided with end caps formed from stainless
steel and an 1.815" internal diameter stainless
steel core. A support cage formed from a fiberglass
mesh coated with polyvinyl chloride and secured with
a nylon hot melt bead which was located downstream
of the element and upstream of an air laid polyester
bat. The separating elements contained a
discontinuous phase liquid barrier of Teflon coated
on a 100 x 100 stainless steel mesh. The separator
included the same end caps and core as the
coalesces. A corrugated Epocel~ pack provided
downstream of the separator's Teflon coating.
The coalesces-separator housing was connected
to a 500 gallon gasoline storage tank by means of a
closed loop system provided with valves (globe and
conventional), flow controllers (Kates) and fluid
pumps to control the pressure and flowrate of liquid
in the system as well as to create a fuel and water
emulsion. The system was also provided with an Aqua
Glo~device (available from Gammon Technical Products
Company) to determine the concentration of water in
the system.
The gasoline employed in the tests contained a
commercial additive mixture which included, among
other things, a surfactant or engine detergent. The
additive composition was blended with a high test
gasoline in three times the typical concentration of
- 36 -
CA 02297444 2000-O1-26
a commercially available gasoline to create an
extreme disarming process stream.
After initiating the flow of gasoline in the
system and bleeding air from a vent in the housing,
the flowrate of fuel through the system was set at 5
gallons per minute. Water was then introduced to
the system and adjusted by means of a rotameter.
After reaching equilibrium (as determined by a
constant DP), and water began to collect, downstream
water concentrations were determined with the Aqua-
Glo. The upstream concentration of the water was
then readjusted at approximately 10 minute intervals
and the procedure was repeated. The concentration
of water was increased from 2-7% (vol/vol) to the
values indicated and with the results shown in Table
2, below. Upstream and downstream fuel samples were
removed for Clear and Bright analysis.
Table 2
Time Water
(minutes) Injection Aqua-Glo
% (vol/vol) (ppm water)
At equilibrium 2 5
15 3 5
25 4 8
35 5 8.5
45 6 12
55 7 18
60 7 16
65 7 19
- 37 -