Note: Descriptions are shown in the official language in which they were submitted.
CA 02299790 2005-09-01
-1 -
Title: METHOD AND APPARATUS FOR STIMULATING HEAVY
OIL PRODUCTION
FIELD OF THE INVENTION
This invention relates to the extraction of hydrocarbons such
as heavy oil and bitumen. In particular this invention relates to reducing the
viscosity of hydrocarbons such as heavy oil in situ to permit the heavy oil to
flow more readily and thus to improve the recovery thereof.
BACKGROUND OF THE INVENTION
Heavy oils refer to crude oils which have high specific gravity
and viscosity and are therefore difficuit to extract commercially because they
do not readily flow. Heavy oils are found, for example, in the tar sand
deposits in Alberta, Canada. Typically these heavy oils will have viscosities
greater than 1000 centiPoise or specific gravities greater than .934 at 60 F
(i.e. less than 20 API). There has long been sought a means to accelerate
the heavy oil production process by permitting the oil to flow more readily
thereby increasing the rate of return on capital and decreasing the financial
risk of such heavy oil production projects.
One thermal extraction technique, called fireflood, is generally
uneconomic due to very severe operating problems including corrosion,
scale precipitation and explosion hazards after breakthrough, not to mention
the difficulty in controlling the process and the production of plugging
deposits such as coke.
Another prior approach that has had some merit is to use
steam in a thermal stimulation for improving heavy oil extraction. Steam
raises the temperature of the oil and thereby reduces its viscosity and allows
it to flow more easily. Steam stimulation is subject to a number of problems,
including heat losses during injection, clay swelling problems, thief zones,
emulsions, capillary surface tension effects and lack of confinement for
CA 02299790 2005-09-01
-2-
shallower zones. Further, injecting steam creates water (condensate) in the
formation which is much less viscous than oil and which will therefore be
preferentially produced due to relative permeability effects. Preferential
production of water perversely makes the oil production or recovery more
difficult.
An additional problem, which has become more important
recently, is that most thermal recovery processes such as steam require
large amounts of methane gas to be burned to provide the energy to
vaporize the water above grade. This can lead to the emission of enormous
amounts of greenhouse gases such as carbon dioxide. For example a
100,000 bbl oil/day heavy oil facility requires 200,000 - 300,000 bbl water
/day to be converted into steam at 200 C. Thus, for a methane gas burner
system, to recover 100,000bbl oil/day requires producing more than 12
million pounds per day of carbon dioxide emissions. The two main
traditional approaches used in steam recovery systems have been "huff and
puff' (i.e., cyclic steaming) and steam floods. Recently, however, steam
assisted gravity drainage (SAGD) has become popular.
SAGD begins with the formation of a steam chamber in the
formation. The steam is injected into the chamber and transfers heat to the
surface of the chamber thereby mobilizing oil at the chamber surface. The
heated oil flows down the walls of the chamber underthe influence of gravity
and drains into the producing well, thereby increasing the size of the
chamber. The advantage of SAGD is that the countercurrent flow of steam
upwards into the reservoir and oil down and out of the reservoir is relatively
efficient, thus the heavy oil production rates are high enough to provide
favourable economics in some situations.
There are many possible SAGD geometries including single
well (injection and production from the same well) and dual or multiple well.
The wells may be either horizontal or vertical. Generally horizontal wells are
favoured by producers because they offer a longer exposure to the pay zone
and thereby offer increased production rates for highly viscous oils.
CA 02299790 2005-09-01
-3-
Single well SAGD offers the least capital cost, but heat losses
due to countercurrent flow of steam into and oil out of the wellbore are
severe. Quite simply, as the hot steam going into the well passes the cold
oil coming out of the well and the steam loses heat to the oil. For example,
at an injection pressure of 1000 psig and 285 C, the enthalpy of the steam
is 1192 btu/Ib and the enthalpy of the water is 542 btu/Ib. Due to
countercurrent heat exchange the produced fluids (water and oil) are at the
same temperature as the injected steam. For typical injection conditions,
the steam quality is 80% (i.e., 80% vapour and 20% liquid). Thus, the
maximum heat delivered to the formation is only the latent heat of
vaporization (i.e. about 50% of the total heat input). With additional heat
losses through the well casing, the net heat delivery to the formation is
quite
low and thus this process is inefficient.
There have also been in the past suggestions to use cold
solvent vapour to lower the viscosity of the heavy oil in situ. This was first
proposed by Nenniger' (1979). This idea has shown much promise for
production of heavy oil with minimal environmental impact, primarily because
such a process does not require heating large volumes of steam nor huge
amounts of fresh water suitable for steam generation. Energy requirements
for solvent extraction are expected to be less than 4% of those required for
steam extraction. Insitu recovery has minimal environmental impact
compared to surface mining techniques.
The physics of cold solvent stimulation are not fully
understood. The measured solvent diffusion rates are typically 100 - 1000
times higher than predicted by theory2,3. A key economic requirement is
Nenniger, E.H., Hydrocarbou Recovery, Canadian Patent 1,059,432
2 Duiui, S.G.; E.H. Nenniger, V.S.V. Rajan, A Study of Bitumen Recovery by
Gravity Drainage
Using Low Temperature Soluble Gas Iniectiou, The Cauadiau Jounial of Chemical
Eugiueeriug, Vol
67, December 1989.
3 Lim, et al, Three dimensional Scaled Physical Modelling of Solvent Vapour
Extraction of Cold Lake Bitumen, JCPT, April 1996, Page 37
CA 02299790 2005-09-01
-4-
efficient recovery of the solvent, so light gases such as ethane and propane
which can be recovered by pressure blowdown are generally preferred. A
recent study has reported the ratio of ethane solvent loss to bitumen
produced, was as low as seven percent (wt/wt). However, the calculated
production rates for solvent extraction are marginal for commercial
application and to date there has never been a successful commercial pilot.
In a bench test4 warm solvent (propane) was injected into a
sample of warmed heavy oil. This experiment showed that if the solvent
temperature was raised and the heavy oil temperature was also raised to the
same temperature (ie. Isothermal conditions) production rates could be
increased about 20 fold simply by increasing the temperature from 20 C to
90 C.
This observation led to the development of the Vapex process4
which proposes to combine solvent with steam or hot water heated above
grade to provide downhole heat. Because of the water/steam this process
suffers from all the problems mentioned above (countercurrent heat
exchange, formation damage problems with clays, emulsions, capillary
pressure, water treatment, water supply, reduced oil relative permeability
due to high water saturations and the like).
A key requirement for both steam assisted gravity drainage
and solvent assisted gravity drainage is the formation of a steam or solvent
chamber in the reservoir. The chamber allows efficient countercurrent flow
of solvent vapour (or steam) upwards. and flow of the heavy crude
downwards along the walls of the chamber. The predicted oil drainage rate
is proportional to the square root of the height of the chamber (reference 4).
Thus the oil production rates are predicted to be very small initially and
then
grow with time until the roof of the chamber encounters a boundary such as
an impermeable shale.
This has been confirmed by lab tests which have shown that
4 See Table 1 and Figure 7 of Butler et al, A New Process for Recovering Heavy
Oils using Hot
Water aiid Hydrocarbon Vapours, JCPT Jaii 1991, pg 100
CA 02299790 2005-09-01
-5-
the maximum oil production rates will not occur until a large solvent chamber
is formed. Unfortunately, in the field this means that peak oil production
rates do not occur until 3-4 years after the well is placed on production.
Thus, for solvent vapour extraction the peak oil production
rates are not typically achieved until perhaps three years after the capital
costs of the well and the production facilities are incurred. The delayed
production response decreases the rate of return and increases the risk to
the operator. For example thief zones, etc, may not be identified until
substantial costs have been incurred (i.e. until after three years of solvent
injection).
Thus, there is a need for the solvent chamber to be quickly
established. For example, the capital cost of drilling and completing a
horizontal well pair might be typically 1,800,000 dollars. The minimum
internal rate of return for a oil project is typically about 15%. Thus, the
opportunity cost of a one year delay in the peak production rate is 275K$.
If peak production is accelerated, so it occurs in the first year rather than
the
third, then the value added by early development of the solvent chamber
would be about 800K$ per well pair.
Thus, while the cold solvent vapour extraction process has
great advantages due to energy efficiency and minimal environmental
damage, it has never been successfully used. The primary reason is the
cold solvent vapour production rates are too low to be economic, particularly
with a 3 - 4 year delay in achieving peak production rates. Another way of
looking at this issue, is to apply a discount to value of the produced oil if
the
production is delayed. At 15% rate of return, the 3 year delay gives a
discount of 33%, so the value of the oil production is reduced by 1/3. In
other words, if the market price of oil is 20$/bbl, the effective price the
producer receives is only 14$/bbl, due to the three year delay. Obviously
this delayed startup has a huge negative impact on the commercial
feasibility of this environmentally friendly technology.
What is desired is a way of stimulating production of heavy oil
CA 02299790 2005-09-01
-6-
which is energy efficient and yet is effective. In this respect it should not
require the use of very high temperatures or high energy use rates as is the
case presently. Further, it would be preferable to avoid introduction of steam
or water into the formation which has negative effects on the production
rates.
SUMMARY OF THE INVENTION
What is desired therefore, is a means to accelerate the oil production
rate by encouraging the rapid extraction of heavy oil or bitumen. According
to the present invention it is possible to accelerate the extraction process
by
the injection of heated solvent vapor into the reservoir in the absence of a
water/steam phase under certain predetermined conditions. As the solvent
condenses on the cold bitumen surface it supplies heat to the bitumen
interface, by releasing the latent heat of condensation, and greatly
accelerates the extraction without the problems associated with a liquid
water phase. Furthermore, by using solvent condensation as a heat transfer
mechanism, it is possible to significantly increase the proportion of solvent
in the bitumen solvent blend, thereby reducing blend viscosity, improving
drainage rates (production) and also achieving enhanced insitu upgrading
of the oil. Further according to the present invention countercurrent heat
exchange losses can be avoided by injecting the heated solvent from an
injection well and removing the produced fluid from an adjacent well which
is communication with the injection well. Thus, the present invention
contemplates establishing such a connection between the production and
injections wells prior to injecting a surface heated solvent vapor.
The present invention also takes into consideration various
additional factors such as the kinetics of extraction, hydraulics and heat
transfer for hot gas delivery to the reservoir and recovery and recycle of
solvent from the produced fluid.
Accordingly, in the present invention, there is a provided a
CA 02299790 2005-09-01
-7-
method of enhanced heavy oil recovery, said method comprising the steps
of:
a) establishing a flow path between an injection well and a
production well through an oil bearing formation;
b) vapourizing a solvent to inject into said formation;
c) pressurizing said formation sufficiently to raise a condensation
temperature of said solvent above an original formation temperature;
d) delivering said vapourized solvent to said formation to permit said
solvent to condense within said formation, and to release the latent heat of
condensation to said formation at said condensation temperature; and
e) extracting heavy oil from said production well.
According to another aspect of the present invention, there is
provided a method of enhanced heavy oil recover, said method comprising:
a) selecting a solvent having a predetermined latent heat of
condensation;
b) selecting a predetermined amount of heat to deliver to a
formation;
c) vapourizing said selected solvent to permit said solvent to be
delivered to said formation in a vapour state;
d) delivering said solvent to said formation at a rate sufficient to
deliver the predetermined amount of heat;
e) condensing said solvent onto said formation to deliver said
predetermined amount of heat to said formation; and
f) recovering, from said formation, a solvent/heavy oil blend.
According to another aspect of the present invention, there is
provided a method of enhanced hydrocarbon recovery comprising the steps
of:
a) injecting a vapourized condensing solvent into a formation;
b) condensing said solvent in said formation to deliver a latent heat
of condensation to said formation to heat said hydrocarbon to reduce a
viscosity of said hydrocarbon;
CA 02299790 2005-09-01
- $ -
c) dissolving said solvent into said hydrocarbon to form a
solvent/hydrocarbon blend having a further reduced viscosity; and
d) recovering from said formation said reduced viscosity hydrocarbon
blend.
According to another aspect of the present invention, there is
provided a method of improved hydrocarbon recovering comprising:
a) heating said hydrocarbon to be recovered insitu in the presence
of a solvent to increase the solvent penetration rate into said hydrocarbon.
According to another aspect of the present invention, there is
provided a method of increasing the value of a recovered hydrocarbon
comprising the steps of:
a) injecting a condensing solvent vapour into a formation;
b) said solvent vapour at a pressure above its condensation
pressure (at original reservoir temperature);
c) condensing said solvent into said heated hydrocarbon thereby
providing a mobile hydrocarbon fraction; and
d) extracting said mobile hydrocarbon fraction from said formation;
whereby asphaltenes remain in said formation in a substantially
immobilized hydrocarbon fraction.
According to another aspect of the present invention, there is
provided a method of enhanced recovery of hydrocarbon from a
hydrocarbon bearing formation said method comprising the steps of:
a) establishing a flow path between an injection well and a
production well;
b) injecting a heated solvent into said formation under sufficient
pressure to raise a condensation temperature of said solvent above a
formation temperature;
c) heating said formation by condensing said solvent onto said
formation to help remove hydrocarbons from said formation and to create a
chamber;
d) increasing the size of the chamber by continuing to inject heated
CA 02299790 2005-09-01
-9-
solvent;
e) reducing the injection pressure as said chamber expands; and
f) reducing the energy delivered to said formation as said pressure
is reduced.
According to another aspect of the present invention, there is
provided a method of enhanced hydrocarbon recovery comprising the steps
of:
a) injecting a heated solvent into a formation under sufficient initial
injection pressure to raise a condensation temperature of said heated
solvent above a naturally occurring formation temperature; and
b) reducing, over time, said injection pressure from said initial
pressure.
BRIEF DESCRIPTION OF THE DRAWINGS
Reference will now be made, by way of example only, to
preferred embodiments of the invention as illustrated in the accompanying
drawings and in which:
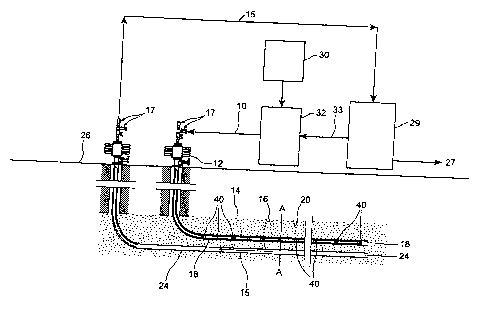
Figure 1 illustrates a process schematic of the present
invention showing formation of a solvent chamber;
Figure 2 illustrates the solvent chamber along section A-A of
Figure 1 in more detail;
Figure 3 is a graph which shows a relationship between
viscosity and temperature for Athabasca bitumen, and the predicted
relationship between diffusion rate and temperature based on the Stokes-
Einstein equation;
Figure 4 is a graph which illustrates a relationship between
temperature rise and volume of a theoretical reservoir heated at a constant
power rate of 1 megawatt;
Figure 5 is a graph which illustrates the vapour pressure of
propane solvent as a function of temperature;
Figure 6 is a graph which shows the latent heat of vaporization
CA 02299790 2005-09-01
-10-
for propane solvent as a function of temperature and the mass of propane
solvent vapour required to deliver one megawatt of heat (via latent heat of
condensation);
Figure 7 is a graph which shows the volumetric heat capacity
of vapour (via latent heat of condensation) as a function of temperature for
several solvents compared to steam;
Figure 8 is a graph which shows volume fraction of propane
solvent in produced fluid vs chamber temperature;
Figure 9 illustrates the bitumen- propane blend viscosity at 8C
as a function of propane solvent volume fraction and the favorable reduction
in viscosity at higher solvent ratios;
Figure 10 illustrates the propane solvent/bitumen blend
viscosity as a function of temperature; and
Figure 11 illustrates the extraction rate forthe heated propane
solvent vapour as a function of temperature and how the rate is limited by
mass transfer at temperatures below 40C and limited by heat transfer at
temperatures above 40C.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
Figure 1 shows a schematic of a process of stimulating heavy
oil or bitumen recovery according to the present invention. Generally, a hot
solvent 10 is injected down an injection well 12 into a reservoir 14. The hot
solvent 10 is most preferably a vapour, enters a solvent chamber 16 through
a perforated or slotted casing 18 or the like and flows out to condense on the
cold bitumen interface 20 to form a solvent/bitumen blend. The terms
"bitumen" and "heavy oil" are used interchangably in this specification and
for
the purposes of this invention means hydrocarbons which are recovered from
naturally occurring formations and which in their natural state are
generallytoo
viscous to readily flow into a production well. It will be appreciated that
the
present invention is most suitable for such formations as tar sands, but may
also be used in other formations.
CA 02299790 2005-09-01
-11-
The solvent bitumen blend 15 formed at the interface drains to
the bottom of the chamber 16 (shown at 22 in Figure 2), where it is removed
via a production well 24 and produced to surface 26. Valves 17 are located at
each well head. The bitumen is separated from the solvent at the surface 26
and the bitumen is sold 27. The separation at 29 of a solvent such as propane
from the bitumen, might involve a flash at a temperature above the critical
temperature of the solvent. The present invention comprehends that there
may be several stages of separation to maximize solvent recovery, which of
course will minimize solvent losses in the sold bitumen. It will be
appreciated
by those skilled in the art that some factors to consider in establishing
solvent
recovery are energy efficiency, reliability, and potential for fouling
problems
(i.e. deposition of asphaltenes). The recovered solvent 33 is then compressed,
and/or heated at 32 and then reinjected into the injection well 18. Additional
make up solvent is added as needed to replace the void volume created by
the extracted bitumen at 30. It may also be necessary to remove light gases
from solvent/bitumen blend which may have been co-produced from the
reservoir. These may also be used as fuel in re-heating the solvent.
The present invention comprehends a process in which a flow
path has already been established between an injection well and a production
well. This flow path could be established by any of a number of means
including downhole heaters or the like. The establishment of a flow
connection is desirable because this avoids countercurrent heat losses which
might otherwise occur. However, this step is not deemed essential if such
countercurrent heat losses can be mitigated through use of other strategies
such as insulated injection tubing or the like. It will be appreciated though
that
the most preferred form of the present invention is a flow through from an
injection well to a production or recovery well.
Figure 2 shows the solvent chamber 16 formed in this formation
in more detail. Also shown is a pressure containment layer, such as shale
barrier layer 21. The heated solvent vapour rises within the chamber 16 to
condense on the walls and roof 19 of the chamber 16. As the solvent
CA 02299790 2005-09-01
-12-
condenses it releases its latent heat of condensation thereby heating the
bitumen interface at the chamber surface. As the solvent dissolves and is
mixed into the bitumen the bitumen is upgraded by the precipitation out of
asphaltenes. At this stage the bitumen begins to flow as its viscosity has
been
lowered by two effects namely, the heating effect from the latent heat of
condensation and the dilution effect from being blended with the now liquid
solvent. The bitumen- solvent liquid blend 25 drains along the wall or down
off the ceiling into the sump 22. The liquid is then drained into the
production
well 24. As will be more fully understood from the description below, the
production of bitumen solvent blend is preferably restricted to avoid solvent
gas bypassing. This is accomplished via a steam trap type control as currently
practiced in SAGD technology.
Figure 3 shows the viscosity of a typical Athabasca bitumen as
a function of temperature by way of example. The Stokes-Einstein law states
that the diffusion coefficient for any solvent is inversely related to the
solute
viscosity. Using this relationship an estimate can be made of the improvement
in the diffusion coefficent as the temperature is increased and the bitumen
viscosity decreases. For example, at 40C the diffusion coefficient is
increased
by 100 fold above that at 8C (i.e. original reservoir temperature).
The thermal diffusivity in the Athabasca tar sands is typically
about 100 times larger than the molecular diffusivity at 8C. Thus, Figure 3
shows that the heat transfer process becomes the rate limiting process step
at temperatures above 40C, while the molecular diffusion will be the rate
limiting process step at temperatures below 40C.
Figure 4 shows the volume of reservoir heated per day with a
power delivery rate of 1 megawatt. This figure illustrates a simple heat
balance and does not reflect any heat transfer limitations. As a point of
reference 1 megawatt will heat 600m3/day of reservoir from 8C to 70C.
Assuming a recovery rate of about 80% recovery of the original bitumen in
place (assuming 35% porosity and 85% bitumen saturation) 1 megawatt will
provide 140 m3/day of bitumen production at 70C.
CA 02299790 2005-09-01
-13-
Figure 5 shows the vapour pressure of one preferred solvent,
propane, as a function of temperature. As can now be appreciated, the
present invention comprehends enhancing the delivery of heat to the heavy
oil insitu by increasing the pressure of the solvent vapour which 'in tum
increases the dew point temperature. As the vapour pressure is increased the
dew point temperature increases. Above the critical temperature a separate
liquid phase ceases to exist, so the vapour pressure concept no longer
applies. By way of example, assuming that the solvent used is propane, at
70C the vapour pressure of propane is about 375 psia. This means that if the
solvent chamber is pressurized to 375 psia, then liquid propane will condense
on any surface which is at a temperature below 70C. This condensation will
eventually heat the surface (via the latent heat of condensation), to a
temperature approaching 70C. Conversely if the target temperature was 40C,
then the pressure of propane in the chamber would have to be held only at
about 200 psia. Thus, according to the present invention by pre-heating a
solvent under predetermined pressure and injecting the same into a formation,
a predetermined amount of heat can be delivered to a formation by controlling
the injection rate of heated solvent vapour.
Figure 6 shows the latent heat of condensation for propane as
a function of temperature. As the temperature approaches the critical
temperature the latent heat of vaporization drops to zero. Figure 6 also shows
the metric tons of propane required per day to supply 1 megawatt via the
latent heat of condensation. At 70C about 350 metric tons of propane vapour
per day are required to supply one megawatt of heat. Thus, according to the
present invention heat can be delivered at a predetermined rate to the
hydrocarbon bearing formation by latent heat of condensation. As will now be
appreciated with appropriate pressure maintenance such a heat delivery
mechanism avoids many of the problems of the prior art.
Figure 7 compares the latent heat of condensation as a function
of temperature for several solvents and water. The latent heat is presented
on a volumetric basis (i.e. per m3 of saturated vapour at temperature and
CA 02299790 2005-09-01
-14-
pressure). The saturation pressure is the same thing as the vapour pressure
and can be obtained from Figure 5. On this basis, propane at 70C has a
latent heat content comparable to steam at 180C. Ethane has an even higher
heat content but is not as useful due to its low critical temperature. Figure
7
also shows that butane would be useful if one wanted to achieve a reservoir
temperature between 85 and 115C. While ethane, butane and propane are
all possible solvents, many other solvents could also be used without
departing from the present invention. Essentially for the purposes of this
invention, the term solvent means any material which mixes with oil in a
liquid
phase and which can be injected into a formation as a gas to deliver a latent
heat of condensation to the formation. Solvents which are substantially
miscible with the hydrocarbon or bitumen are preferred. By way of example,
light volatile hydrocarbons such as propane, propylene, butane, ethylene,
ethane and pentane are most preferred. While many solvents are available,
the most preferred ones will have a dew point temperature above a formation
temperature at reasonable operating pressures (i.e. belowformation orcasing
fracture pressures).
Retuming again to propane, Figure 8 shows the volume fraction
of propane in the bitumen propane blend as a function of temperature. This
graph was derived from Figures 4 and 6, which show bitumen production and
solvent injection rate at 1 megawatt of heat delivery. Figure 8 shows a great
advantage of the present invention, namely that solvent proportion in the
blend can be increased by operating at higher temperatures. This increased
solvent proportion at high temperatures is made possible because the solvent
circulation rate is determined by heat transfer requirements rather than
solubility in the bitumen under those conditions. In other words, to deliver
the
desired rate of heat transfer involves injecting enough solvent under pressure
to provide the predetermined heat. This higher injection rate leads to a
higher
solvent fraction in the produced blend, with a beneficially lowered blend
viscosity.
Figure 9 shows the blend viscosity at 8C as a function of
CA 02299790 2005-09-01
= -15-
propane volume fraction. It is clear that higher solvent proportions in the
blend
are very advantageous in terms of reducing viscosity. As the solvent
proportion increases the viscosity of the blend decreases quite rapidly. This
low blend viscosity provides rapid drainage of the bitumen from the chamber
interface and exposes fresh coid bitumen to fresh hot condensing solvent
vapour. Figure 9 also shows the approximate viscosity range expected for a
typical VAPEX solvent/oil ratio at bracket 100 and the preferred much lower
approximate viscosity range preferred for the present invention at higher
solvent oil ratios at range 102.
Figure 10 shows the blend viscosity as a function of
temperature. At 70C the blend viscosity is reduced by at least 10 fold over
blend viscosity at original reservoir temperature. This again increases
extraction relative to an unheated or ambient process.
Consider the rate of bitumen extraction with warm solvent
vapour according to the present invention. Making a determination of this rate
in advance is complicated because factors to simultaneously consider include
heat, mass and momentum transfer in a porous medium. Furthermore, the
measured mass transfer rates (diffusion coefficients) for cold solvent vapour
extraction are higher than predicted by theory. Therefore the calculation
which follows is an approximation only.
Consider temperatures above 40 C where the molecular
diffusivity is higher than the thermal diffusivity. Assuming the process is
limited by thermal diffusivity it is possible to model the process as a
solvent
SAGD with appropriate adjustments to the viscosity and permeability. Butler
(Canadian Patent 1,130,201, pg. 19) gives a formula which states thatthe rate
is proportional to (k/u). =(k*p/p). O'Rourke, J.C. (Canadian Joumal of
Petroleum Technology, Sept. 1999, pg, 50, Fig. 5.1) reports that the SAGD
extraction rate at 200 C is about 5cm/day.
A condensing propane flood will increase permeability k by 4-5,
because there is no relative permeability reduction due to high water
saturations from steam (see Table I on pg. 14 of Butler Canadian Patent
CA 02299790 2005-09-01
-16-
1,130,201). Op is reduced by'/~ due to the lower density difference between
condensed and vaporized propane relative to water and steam (i.e. 0.5 for
propane vs 1 for water). The blend viscosity p at 40 C is 0.3cP vs 10cP for
steam at 200 C. Therefore, the production rate using solvent vapours at 40 C
is predicted to increase by (4*0.5*10/0.3)" = 8 above the rate for SAGD at
200 C.
With the SAGD extraction rate of 5 cm/day at 200 C (where
cm/day equals the distance the steam chamber expands), one can predict a
hot pressurized propane solvent vapour extraction rate according to the
present invention of about 8 x 5= 40cm/day. Thus, the present invention, with
condensing propane in gravity drainage solvent extraction process can give
bitumen production rates about 8 times larger than a SAGD, with about 1/6 of
the energy requirement of a SAGD (due to the lower reservoir temperature
40 C vs 200 C) and 1/6 of the greenhouse gas emissions. Furthermore, the
produced oil will more valuable due to the insitu upgrading (i.e. loss of
undesirable asphaltenes).
Figure 11 shows the extraction rate as a function of
temperature for a heat transfer limited case for propane. The formation
extraction temperature is shown ranging from 10 degrees C to 80 degrees C
in ten degree increments. As noted earlier, the mass transfer rate via
molecular diffusion will be limiting at lower temperatures, so a different
mechanism occurs at temperatures below 40 C. Dunn et al. (Canadian
Journal of Chemical Engineering, Vol. 67, December 1989, pg. 979) present
an analogous equation for the case where mass transfer is limiting. In this
case the rate is proportional to (D/v)'2.=(D*p/N)'2.
Assuming that the extraction rate at 40 C is the same for the
heat transfer limited case above (i.e. 40cm/day) as the mass transfer rate
limited case (since the rates must converge to the same value at some
temperature). The variation in D (diffusion rate) is known from Figure 3. The
blend viscosity is known from Figure 10. Figure 11 also shows the predicted
extraction rates for temperatures less than 40 C where the mass transfer is
CA 02299790 2005-09-01
-17-
the rate limiting step. This low temperature part of the curve is very steep,
due
to the relationship between viscosity and diffusion coefficient. Thus a
relatively small increase in the solvent vapour chamber temperature can
increase the extraction rates significantly.
It will now be understood that as the chamber grows in size, the
requirements for solvent, (such as propane vapour) delivery will rapidly
increase due to the increased surface area if the temperature is to be
maintained. The ability to deliver hot vaporized propane to the injection well
may become rate limiting. To some extent, the solvent vapour delivery can
be improved by injecting at higher pressures and temperatures. However, this
will require very high bitumen-propane separation rates in the surface
facilities.
For example, consider a heat delivery of 1 megawatt at 70 C.
This requires 350 metric tons per day of propane vapour delivered to the
reservoir. At saturation pressure of 375 psia at 70 C, the propane vapour
requirement is about 8800m3/day. This gives a velocity of 5m/s in 7" casing
and a pressure drop of about 1 psi/100m. Over 700 meters of horizontal
injection well the total pressure drop is less than 3 psi, which corresponds
to
a hydrostatic head variation of about 3 meters of propane bitumen blend. (n.b.
the pressure drop along the horizontal section is less than 7 psi due to
leakoff
into the formation). If the injection and production wells are separated by 5
meters, the liquid interface can be kept between the injector and the
producer.
Considerthe case where SAGD production is 3000 bopd so the
predicted production according to the present invention will be 24000 bopd at
40 C. This yields a propane volume fraction of 0.67, so the propane injection
rate will be 48000 bbl/day of liquid solvent equivalent. This corresponds to a
volumetric flowrate of about 220,000 m3/day of vapour at 200psia and 40 C.
In 9" casing the velocity is 65m/s which gives a pressure gradient of 100
psi/100m. It is desirable to minimize the pressure gradient along the injector
and to this end flow control means 40 (see Figures 1 and 2) can be used. For
example, the pressure gradient can be mitigated by using larger casing or a
CA 02299790 2005-09-01
-18-
tubing string with orifices or the like to help distribute the solvent more
evenly.
The orifices can be metered to deliver a constant flow over different
pressures, or can be designed to yield a variable flow at different pressures.
Further, the flow control means can be varied along the length of the well to
yield a more constant injection pressure in spite of line losses. Of course,
at
such high volumes, an additional challenge will be to separate the solvent
from the bitumen at surface.
At some point increasing the injection/separation rates probably
won't be practical. When the supply of propane vapour to the reservoir
becomes rate limiting, the pressure in the solvent chamber will begin to drop.
This will lead to a reduction in the dew point or saturation temperature and a
reduction in the solvent penetration rate as the bitumen surface viscosity is
increased and the molecular diffusivity the solvent is reduced. Thus, it is
anticipated that the pressure in the solvent chamber will gradually decrease
with time and the process will eventually trend towards a process at the
original ambient temperature of the reservoir. Thus, the present invention
comprehends an extraction process which begins hot and pressurized and in
which overtime both heat and pressure are reduced as the production volume
increases. The supply bottleneck for solvent vapour could also be mitigated,
by using shorter horizontal wells, but this may not be economically desirable.
It can now be appreciated that a cold or ambient process may be used once
the solvent chamber has been made large enough by the hot process first to
give reasonable production rates.
Thus the proposed hot vapour extraction technique will be most
useful for providing high initial production rates by rapidly forming a
chamber
of size and quickly recovering the upfront capital costs. By growing a
chamber quickly, the hot vapour extraction technique described here will allow
the operator to have a large chamber much more quickly and thereby allow
subsequent energy efficient, cold extraction to proceed economically.
Forexample, one can now estimate the minimum chamber size
at 40 C and 200 psi for 1 megawatt of heat via condensing vapour. At
CA 02299790 2005-09-01
-19-
40cm/day x 750 m long x.35 porosity x.85 oil saturation x.8 recovery factor,
the production rate is 71 m3 of solvent per meter of chamber circumference.
Therefore for 270m3/day of bitumen production, the circumference of the
solvent chamber must be greater than 4m, or the solvent chamber diameter
should be larger than about 2 m. Since this is small relative to the distance
between the wells (5 m), high rates of bitumen extraction should be feasible
immediately after breakthrough between the wells.
The advantages of the present invention can now be
understood. The priorart, a cold (unheated) solvent vapour extraction process
the solvent- bitumen ratio is largely determined by the solubility of propane
in
the bitumen (it also depends somewhat on the mobility of the blend).
However, with a heated pressurized solvent vapour the solvent
injection rate is determined by the heat balance. In other words, the amount
of liquid solvent condensed within the reservoir depends on the volumetric
heating requirements required to heat the reservoir to the dewpoint of the
solvent. (i.e. the temperature difference between the solvent vapour at its
dewpoint temperature and the ambient reservoir temperature, the heat
capacity of the reservoir and the latent heat of vaporization of the
solvent.).
Thus the first advantage is that the solvent - bitumen ratio is uncoupled so
that
higher solvent proportions can be achieved in the blend.
A second advantage is that higher propane ratios provide a
higher degree of deasphalting and thereby enhance the value of the produced
oil (i.e. add up to 30% of incremental value to the oil). For a 100,000 bopd
facility each dollar of incremental value/bbl adds 36 million dollars per year
to
the cash flow, so a higher degree of insitu upgrading could add up to 100
million dollars of cashflow to a project annually.
A third advantage is that the solvent penetration rate into the
bitumen increases as the bitumen temperature is raised, because the diffusion
rate increases as the viscosity is decreased, and thermal diffusivity is 100x
faster than molecular diffusion at ambient reservoir temperature.
CA 02299790 2005-09-01
-20-
A fourth advantage of higher solvent ratios is that the bitumen
solvent blend will have significantly lower viscosities than a cold or ambient
process and therefore will drain faster and thereby speed up the extraction
process. This is important because the production rate is minimal for the
first
three years of a cold start Vapex due to the small size of the solvent
chamber.
At 15% rate of return, the three year delay in the cash flow reduces the value
of the oil production by 30%. For example if the oil is sold for 20$/bbl, the
3
year delay means that the producer is effectively paid only 14$/bbl. Thus, on
a 100,000 bopd facility, the fast start up will add $600,000/day of value to
the
production or 220million$ of value to the cash flow per year.
As will be appreciated with higher production rates fewer wells
are required to produce the same cash flow which is more efficient
economically.
A further advantage of the present invention is that the elevated
reservoir pressure can enormously simplify production of the fluids. For
example, at elevated reservoir pressure it may not be necessary to supply a
recovery pump on the production well side, because the reservoir pressure
may be sufficient to overcome the hydrostatic head. In this case the
production well would be choked back to maintain the pressure in the
horizontal portion of the production well above the bubble point, in a manner
analogous to the steam trap technique used for SAGD. This could save 3M$.
A further advantage of the present invention is that the energy
requirements are quite modest compared to SAGD. For example, if the entire
reservoir is heated to 40 C, instead of the 200 C for SAGD, then the
greenhouse gas emissions are reduced by about 80%. This is particularly
significant, since greenhouse gas emissions from heavy oil, bitumen and tar
sands account for 25% of the excess above Canada's obligation under the
Kyoto Accord.
As will be appreciated by those skilled in the art, off setting
these benefits are the requirement to recover and recycle higher volumes of
solvent per bbl of bitumen production. It is expected that in the end stages
of
CA 02299790 2005-09-01
-21 -
the extraction process, the solvent recovery may become a bottleneck, so
solvent pressure (i.e. dewpoint temperature) in the solvent chamber will be
reduced. However, this will help to offset higher heat losses to the
overburden
as the chamber spreads along the top of the oil bearing zone. Thus, the final
stages of extraction may occur at ambient reservoirtemperature as previously
described.
Thus we can see that the advantages of hot solvent gas
injection include accelerated cash flow (fast start up), increased cash flow
(upgrading) delayed capital expenditures, reduced solvent inventory and
lifting
costs, reduced energy costs (relative to steam) and reduced greenhouse gas
emissions (relative to steam). The hot solvent extraction process described
here has the potential to add about 1 million$/day of incremental value to a
100,000 bopd cold vapex project.
As will be appreciated, the example reference conditions
discussed in this patent have been injection of propane solvent vapour at
40 C and 200psia. This particular choice of solvent, temperature and
pressure was intended to teach by way of preferred example only. The
optimum choice of temperature and solvent for a particular reservoir will
depend on both cost factors (i.e., solvent separation rates) and bitumen
production rates.
While the foregoing description of the present invention
includes various altematives and variations, it will be apparent to those
skilled
in the art that various additional modifications are possible without
departing
from the broad spirit of the invention as noted in the appended claims. Some
of the variations are discussed above, such as the various pressures and
temperatures which are suitable for the different solvents which are suitable
according to the present invention. Others will be apparent to those skilled
in
the art. What is considered important in this invention is the selection of a
suitable solvent which can effectively deliver heat to the formation by a
latent
heat of condensation to decrease the viscosity of the hydrocarbons being
recovered.