Note: Descriptions are shown in the official language in which they were submitted.
CA 02300615 2007-05-29
50304-6
-1-
CONTINUOUS PRODUCTION OF ACTIVATED SILICA
BACKGROUND OF THE IlVVENTION
Field of the Invention
The present invention relates to a method and apparatus for the continuous
production of activated silica. More particularly, the present invention
relates to
the use of a tubular pipeline reactor comprised of an elastomeric material and
preferably the use of a shorter tubular pipeline reactor followed by a holding
tank,
for the continuous production of activated silica.
Descri~tion of the Related Art
The recent development of organic polymeric coagulants and flocculation
aids has greatly contributed to the efficiency of water treatment facilities.
There is
however increasing concern about the toxicity of these polymers which
generally
consist of polyamino- or polyamide chains.
"Activated Silica" is a family of highly dispersed polymeric silica or
insoluble metallic silicates which has long found use as an effective and
nontoxic
CA 02300615 2000-03-10
-2-
coagulation aid. The use of activated silica as a coagulant aid in water
treatment
has been recognized for many years, it being known for example that if
activated
silica is added along with alum to raw water, the resulting floc settles at a
faster
rate than alum alone, and a lower turbidity may be obtained. This is
particularly
important for a water treatment plant because floc settling at a faster rate
enables
settling basins to operate at higher flowrates without raising turbidity
levels and
possibly lowering them. Hence the capacity of a water treatment plant can be
increased or it can operate at higher peak flowrates during periods of high
water
demand and yet maintain the same turbidity of settled water.
Activated silica is also used in waste water treatrnent plants where its
addition to the primary clarifier can improve settling rates and lower
suspended
solids carry over to the Biological Reactors. Activated silica has also been
added
to digested sludge prior to dewatering process to improve de-waterability
characteristics and obtain a higher concentration of tbtal suspended solids.
Activated silica was first used commercially for the purification of potable
drinking water almost 60 years ago by J. R. Baylis (J. Am. Water Works Assn.
29:9 (1937) 1355-96), and an application is also described by. R.W. Pitman and
G.W. Wells (J. Am. Water Works Assn. 60:10 (1968) 1167-1172). Stumm,
Huper and Champlin (Enviro. Sci. and Technology 1:3 (1967) 221-7) have studied
the flocculation of alumina and silver bromide sols with activated silica,
while J.
D. Rushmere (U.S. Patent Nos. 4,927,498 5/22/90; 5,185,206 2/9/93) and
CA 02300615 2000-03-10
-3-
Rushmere & Moffett (U.S. Patent No. 5,482,693 1/9/1996) have described its
application in combination with cationic polymers as a retention/drainage aid
in
papermaking.
Activated silica (or "polysilicic microgels") is formed when dilute aqueous
solutions of alkali metal silicates or polysilicates are reacted with mineral
acids or
multivalent metal ions such as calcium, iron or aluminum. Activating agents
which produce the required polymerization of soluble alkali silicates include:
sulfuric acid, hydrochloric acid, chlorine gas or carbon dioxide (J.R. Baylis,
U.S.
Patent No. 2,217,466 10/8/1940), alum (C.L. Baker & C.H. Dedrick, U.S. Patent
No. 2,310,009 2/21943); ammonium sulfate (R.W. Pitman and G.W. Wells
JAWWA 60 #10 (1968) 1167-1172); sodium chromate, sodium aluminate and
metaphosphate (R.C. Merrill Ind Eng. Chem 40 (1948) 1355); sodium bicarbonate
and sodium bisulfate (H.R. Hay U.S. Patent No. 2,444,774 7/6/48; JAWWA 36
(1944) 626-636). Hasegawa et.al. (T. Hasegawa et:al. U.S. Patent No.
4,923,629, 5/8/1990; Water Science Technology (Kyoto) 23 (1991) 1713-1722)
have also taught a method of producing activated silica under acidic
conditions
after which the anionic species so produced into cationic form by addition of
the
salts of aluminum or iron. A description of the chemistry is to be found in J.
G.
Vail ("Soluble Silicates" Vol II New York: Reinhold, 1960), K.R. Lange and
R.W. Spenser (Envir. Sci. and Technology 2:3 (1968) 212-6).
CA 02300615 2000-03-10
-4-
Activated silica performance is best when manufactured immediately prior
to use, which usually means that on site preparation at the application point
such
as a potable water treatment facility or pulp and paper mill is required. This
dictates a number of important aspects of the manufacturing equipment which
should have the following characteristics: (1) relatively low cost, (2)
compact,
(3) low maintenance. Low cost is also important if activated silica is to be
chosen
relative to organic coagulants and flocculation aids. Capacity requirements
also
dictate that it is preferred to produce the activated silica microgels in a
reasonable
time period, i.e., not more than about 15 to 30 minutes, before the microgel
is
required for use, it being appreciated that the shorter the time, the greater
the
possible throughput of the equipment without the risk of solidification.
A step in the production of activated silica microgels is the aging of the
initial sols so as to permit growth of the silica micelles to the optimum
molecular
weight efficiency. Generally, efficiency increases with the molecular weight,
a
limit being reached when the whole sets into a solid mass, the "gel point."
However, as Pitman & Wells point out, control of the degree of activation is a
critical aspect of the production of such polysilicic microgels, and although
laboratory results looked encouraging, this difficulty has often precluded it
from
being used in a plant situation. The importance of control has also been
emphasized.
CA 02300615 2000-03-10
-5-
Further methods for the production of activated silica are discussed by J.G.
Vail in "Soluble Silicates" Vol II(Reinhold, N.Y., 1960) which reveals that
aqueous solutions of alkali silicates may be acidified by acidic salts and
gases such
as borax, sodium bisulfite; potassium dichromate, sodium bicarbonate, sodium
acid phosphate, carbon dioxide, sulfur dioxide and chlorine as well as
ammonium,
aluminum, ferric and ferrous salts, or alkaline salts of polyvalent metals
such as
aluminum and zinc. The gel time can range from minutes to days or months
depending on a number of factors including pH, silica concentration,
temperature
and the presence of neutral salts. For commercial applications, relatively
short gel
times, on the order of several minutes to an hour, are preferred. Laboratory
work
completed on various activated silica types has shown that the best
performance
was obtained at about 30-70 % of gel time. Complete gellation is to be avoided
since once gelled the solutions have little benefit either for water treatment
or as
drainage aid. By far the biggest problem in the prep`aration of activated
silica, and
one which has precluded its wide use in spite of its efficiency being well
known
for over 60 years, is plugging and scale formation of the reaction equipment
and
feed lines.
There are two main reasons for this plugging and scale formation: poor
control of the polymerization process which leads to premature or localized
gellation, the result of poor mixing or extended well times in "dead zones" in
the
equipment, and an inevitable by-product of the reaction, a small amount of the
CA 02300615 2000-03-10
-6-
dispersed silica or metal silicates which is precipitated as a silica or
aluminosilicate scale. The rate of reaction being temperature dependent, this
problem is exacerbated by the wide swings in water temperature common in water
treatment plants, where a variation of 25-30 C over the course of the year is
by
no means uncommon.
A method of producing activated silica which would perform continuously,
with the same degree of efficiency and without scale formation under the wide
range of conditions common in water treatment facilities has long been needed.
Until now it has been recommended that activation take place in two steps.
In the first, an unstable sol dispersion is prepared, and in the second, this
dispersion is stabilized by dilution, which greatly slows down the
polymerization
process (A.E. Griffm, "Preparation and Use of Activated Silica AWW46 (1954)
571-575).
Although a number of different methods of nianufacture of activated silica
have been disclosed in the literature, none to date has met the requirements
described above.
Thus, F. Smith (U.S. Patent Nos. 3,963,640; 6/15/76 and 4,147,657 (to
the PQ Corporation)) correctly describes how to manufacture activated silica
by
co-mixing the reagents under conditions of high shear, but fails to reveal
that the
by-product scale which rapidly forms in the mixing chamber is not readily
removed. The process disclosed by Smith involves preparation of a stable
CA 02300615 2000-03-10
-7-
dispersion of activated silica which is soluble in hydrochloric acid, which
reagent
is to be used for the removal of any scale which might form in the apparatus.
The
rapid formation of scale, and difficulty and hazard of using hydrochloric acid
for
its removal, quickly renders this process uneconomical. A similar story
applies to
the manufacture of this product using chlorine gas in the so called "Silactor"
Unit.
The advantage of this process (which was marketed under the trade name "WT
Silactor" by Wallace & Tiernan Inc., Belleville, N.J and described by A.E.
Griffin JAWWA 45 (1953) 1107), is that all the chlorine was available as NaOC1
for normal disinfecting functions. However these Silactor units also required
a
high degree of maintenance, and are no longer employed commercially.
A more recent development in the production of activated silica is that
described by Moffett and Rushmere (U.S. Patent Nos. 5,503,820 4/2/96;
5,648,055 7/15/97). Although these disclosures do represent an advance in the
art, they still have serious shortcomings. -
According to the processes disclosed by Moffett and Rushmere a low
concentration polysilicate microgel is prepared by (a) mixing a stream of a
solution of water soluble silicate with a stream of a strong acid having a pKa
less
than 6 into a mixing zone where the streams converge at an angle of not less
than
30 degrees, and at a rate sufficient to produce a mixture with a Reynolds
number
of at least 4000, having a silica concentration in the range from about 1.0 to
6.0
wt %, and a pH in the range from 2 to 10.5, (b) aging the silicate/acid
mixture for
CA 02300615 2000-03-10
-8-
a period of time sufficient to achieve a desire level of partial gelation (c)
diluting
the aged mixture to a silica concentration not greater than about 1 % whereby
the
gelation is stabilized. These authors note that undesirable silica deposition
does
occur during the production of activated silica according to their method, and
that
the build up of silica or aluminosilicates on internal surfaces of the
apparatus,
impedes the functioning of moving parts and restricts the fluid flow. The
process
disclosed requires several pumps, and a pH probe to control the reaction. A
reservoir containing heated NaOH with a timer and pump assembly to
periodically
flush the system to solubilize and remove deposits of silica is also
described,
which introduces a fourth process step. A particular problem related to this
particular disclosure is silica build up on the pH probe which is required as
their
technique relies on pH measurement to control the reaction, and this build up
prevents monitoring of this "critical control parameter" for microgel
production.
Although these authors demonstrate that high turbulence is able to reduce the
formation of siliceous deposits to some extent, it is tiot eliminated. The
solution
offered by Moffett and Rushmere is extremely complex and requires the
installation of hot caustic soda for periodic cleaning by circulation. This
requires
various timing mechanisms, or dual operating systems, the one operating while
the
other is cleansed of silica (or aluminosilicate). Since activated silica can
only
compete with organic flocculation aids if the cost, size and maintenance of
the
equipment is comparable to the simple mix of organic polymers with water, the
CA 02300615 2000-03-10
-9-
complexity, cost and physical size of the equipment described by Moffett and
Rushmere renders the process commercially unacceptable to the vast majority of
applications. This method also has the disadvantage of preferring a strong
acid,
such as sulfuric acid, which for safety reasons is increasingly unpopular in
many
work place environments.
SUMMARY OF THE INVENTION
The invention herein described is an improvement over the prior art, and
with particular reference to the most recent disclosures, reduces the number
of
steps from four to two, and utilizes as reagent a dilute solution of carbonic
acid.
The process of the present invention comprises dissolving carbon dioxide
in water to form carbonic acid, mixing the carbonic acid with sodium silicate
under turbulent hydraulic conditions, and allowing the carbonic acid and
sodium
silicate to react to produce activated silica. It is preferred that the
carbonic acid
and sodium silicate react in a tubular pipeline reactor which is fabricated
from
flexible elastomeric material. The swelling and contracting of the tubing,
which
can be achieved in many different ways, allows the scale to be released from
all
surfaces of the reactor. Thus, the process of the present invention can
successfully and econonzically be run continuously.
In another embodiment, there is provided an apparatus for continuously
producing activated silica from sodium silicate using carbon dioxide. The
CA 02300615 2000-03-10
-10-
apparatus comprises a mixing section where carbon dioxide is dissolved in
water
to form carbonic acid. The apparatus further comprises a contacting section
where said carbonic acid is mixed with sodium silicate solution under
turbulent
hydraulic conditions. The carbonic acid and sodium silicate then react to
produce
activated silica in a reaction section of the apparatus. The reaction section
preferably comprises a tubular pipeline reactor which is fabricated from
flexible,
elastomeric material. In a most preferred embodiment, the flexible,
elastomeric
material comprises a fluorocarbon polymer such as a tetrafluoroethylene
polymer
or a fluorinated ethylene-propylene polymer e.g., available under the
trademark
Teflon , or the material is at least coated with such a polymer material,
which
facilitates the removal of scale.
In another embodiment, there is provided a method and apparatus for the
production of activated silica, which uses a tubular pipeline reactor followed
by a
holding tank. The tubular reactor is elastomeric, and relatively short, so the
reaction is not completed in the tubular reactor. The reactive mixture is
passed to
the holding tank to permit further reaction/polymerization. In a most
preferred
embodiment, the contents of the holding tank are removed by a water eductor,
which stops the polymerization and dilutes the product at the same time.
In another embodiment of the present invention, there is provided a water
treatment facility which contains the apparatus of the present invention for
continuously producing activated silica from sodium silicate using carbon
dioxide
CA 02300615 2007-05-29
50304-6
- 11 -
in accordance with the present invention. Another
embodiment of the present invention is a pulp and paper mill
which comprises an apparatus for continuously producing
activated silica from sodium silicate using carbon dioxide
in accordance with the present invention. The activated
silica prepared by the apparatus can then be used
respectively in the treatment of water in the water
treatment facility or in the pulp and paper mill.
In another embodiment of the present invention,
there is provided an apparatus for continuously producing
activated silica from sodium silicate using carbon dioxide,
comprising: (a) a mixing section where carbon dioxide is
dissolved in water to form carbonic acid; (b) a contacting
section where said carbonic acid is mixed with a sodium
silicate solution under turbulent hydraulic conditions;
(c) a reaction section where the carbonic acid and sodium
silicate react to produce the activated silica, wherein said
reaction section comprises a tubular pipeline reactor which
is fabricated from a flexible, elastomeric material.
In another embodiment of the present invention,
there is provided an apparatus for continuously producing
activated silica from sodium silicate using carbon dioxide,
comprising: (a) a mixing section where carbon dioxide is
dissolved in water to form carbonic acid; (b) a contacting
section where said carbonic acid is mixed with a sodium
silicate solution under turbulent hydraulic conditions;
(c) a reaction section where the carbonic acid and sodium
silicate react to produce the activated silica, wherein the
contacting section comprises: a junction at which the sodium
silicate solution meets the carbonic acid solution; and an
additional water inlet upstream to the said junction, to
prevent formation and deposition of scale at said junction.
CA 02300615 2007-05-29
50304-6
- lla -
In another embodiment of the present invention,
there is provided an apparatus for continuously producing
activated silica from sodium silicate using carbon dioxide,
comprising: (a) a mixing section where carbon dioxide is
dissolved in water to form carbonic acid; (b) a contacting
section where said carbonic acid is mixed with a sodium
silicate solution under turbulent hydraulic conditions;
(c) a reaction section where the carbonic acid and sodium
silicate react to produce the activated silica, wherein the
sodium silicate concentration is equivalent to a range of
3-28% Si02 in the solution contacting the carbonic acid
solution.
In another embodiment of the present invention,
there is provided an apparatus for continuously producing
activated silica from sodium silicate using carbon dioxide,
comprising: (a) a mixing section where carbon dioxide is
dissolved in water to form carbonic acid; (b) a contacting
section where said carbonic acid is mixed with a sodium
silicate solution under turbulent hydraulic conditions;
(c) a reaction section where the carbonic acid and sodium
silicate react to produce the activated silica, wherein the
activated silica produced is in the range 0.5-5.0% Si02.
In another embodiment of the present invention,
there is provided an apparatus for continuously producing
activated silica from sodium silicate using carbon dioxide,
comprising: (a) a mixing section where carbon dioxide is
dissolved in water to form carbonic acid; (b) a contacting
section where said carbonic acid is mixed with a sodium
silicate solution under turbulent hydraulic conditions;
(c) a reaction section where the carbonic acid and sodium
silicate react to produce the activated silica, wherein the
CO2 gas is dissolved in water at a pressure in the range
50-200 psig.
CA 02300615 2007-05-29
50304-6
- llb -
In another embodiment of the present invention,
there is provided an apparatus for continuously producing
activated silica from sodium silicate using carbon dioxide,
comprising: (a) a mixing section where carbon dioxide is
dissolved in water to form carbonic acid; (b) a contacting
section where said carbonic acid is mixed with a sodium
silicate solution under turbulent hydraulic conditions;
(c) a reaction section where the carbonic acid and sodium
silicate react to produce the activated silica, wherein the
pH at the end of the apparatus is in the range 6-9.
In another embodiment of the present invention,
there is provided an apparatus for continuously producing
activated silica from sodium silicate using carbon dioxide,
comprising: (a) a mixing section where carbon dioxide is
dissolved in water to form carbonic acid; (b) a contacting
section where said carbonic acid is mixed with a sodium
silicate solution under turbulent hydraulic conditions;
(c) a reaction section where the carbonic acid and sodium
silicate react to produce the activated silica, wherein the
mixture of carbonic acid solution and sodium silicate flows
through the tubular reactor under turbulent conditions such
that the Reynolds number exceeds 3000.
In another embodiment of the present invention,
there is provided an apparatus for continuously producing
activated silica from sodium silicate using carbon dioxide,
comprising: (a) a mixing section where carbon dioxide is
dissolved in water to form carbonic acid; (b) a contacting
section where said carbonic acid is mixed with a sodium
silicate solution under turbulent hydraulic conditions;
(c) a reaction section where the carbonic acid and sodium
silicate react to produce the activated silica, wherein the
contact time between carbonic acid solution and sodium
silicate solution is in the range of from 2 to 20 minutes.
CA 02300615 2007-05-29
50304-6
- llc -
In another embodiment of the present invention,
there is provided a method for continuously producing
activated silica from sodium silicate using carbon dioxide,
which comprises: dissolving carbon dioxide in water to form
carbonic acid, mixing the carbonic acid with a sodium
silicate solution under turbulent hydraulic conditions, and
allowing the carbonic acid and sodium silicate to react to
provide activated silica, wherein the reaction is conducted
in a reactor fabricated from flexible, elastomeric material.
In another embodiment of the present invention,
there is provided an apparatus for continuously producing
activated silica from sodium silicate solution and carbon
dioxide gas comprising, a) a gas to liquid mass transfer
section in which carbon dioxide gas is contacted with water
flowing counter-currently and dissolves to form carbonic
acid solution; b) a mixing section where said carbonic acid
solution is mixed with the sodium silicate solution under
turbulent hydraulic conditions; c) a reaction section where
further turbulent mixing occurs in a short length of tubing
followed by a partial polymerization stage in a holding tank
where longer residence time is achieved so that further
polymerization and formation of activated silica can take
place; d) a dilution zone where an eductor powered by
potable or clean water draws the polymer of activated silica
from the holding tank and dilutes it at the same time; and
e) a polymer delivery line in which the partially
polymerized activated silica is transported to a line where
it is mixed with alum dispersed in water.
CA 02300615 2007-05-29
50304-6
-
- lid
BRIEF DESCRIPTION OF THE FIGURES OF THE DRAWINGS
FIG. 1 is a schematic diagram of an apparatus for producing activated
silica by reacting carbonic acid solution with sodium silicate.
FIG. 2 shows in detail contacting device for mixing carbonic acid and
sodium silicate employed in the apparatus of Figure 1.
FIG. 3 is a schematic diagram of an alternative apparatus for producing
activated silica including a Venturi type dissolution device.
FIG. 4 is a schematic diagram of an apparatus for in-line dilution of
sodium silicate before addition to the mixing device.
FIG. 5 is a schematic diagram of a further alternative apparatus for
producing activated silica including a Venturi type dissolution device and a
holding tank.
CA 02300615 2000-03-10
-12-
FIG. 6 is a schematic diagram of a modified alternative apparatus for
producing activated silica, where the activated silica is further diluted by
water
after formation.
FIG. 7 is a schematic diagram of a fnrther modified alternative apparatus
for producing activated silica including a pressurized reaction vessel.
FIG. 8 is a schematic diagram of a preferred apparatus employing a
holding tank with a water eductor for removing the contents of the holding
tank.
DETAILED DESCRIPTION OF THE INVENTION
In the process of the present invention, solutions of carbonic acid and
alkali silicates are admixed, preferably in a tubular reactor, under turbulent
hydraulic conditions, preferably where depressurization of the carbon dioxide
contributes to the turbulence in the tube. This allows suitable mixing to take
place
at Reynolds numbers lower than the 4000-6000 desciibed by Moffett and
Reynolds. The Reynolds number at the mixing point however is preferably higher
than 3000. Reynolds number is a dimensionless number used in engineering to
describe liquid flow conditions within a tube or pipe. Numbers below 2000
represent laminar flow and numbers of 2500 and above represent turbulent flow.
As a general rule, the larger the Reynolds number the better the mixing.
Reynolds number, (Re) for simple flow in a pipe or tube, is determined from
the
equation.
CA 02300615 2000-03-10
-13-
Re= Qxd
Dxu
Where:
Q = Flow in cubic feet per second
d=Density in pounds per cubic foot
D= Pipe diameter in feet
u=Viscosity in pounds per foot second
Although the use of carbon dioxide as an activating agent has been
previously described in the literature, no suitable method of commercially
utilizing
this reagent has been described. Carbon dioxide has a number of advantages
over
other activating materials. Carbon dioxide activated silica demonstrated good
activity already at 10-30 % of gel time. Most mineral acids such as sulphuric,
present hazards in handling and use because of their corrosive nature. Carbon
dioxide when dissolved in water forms a weak acid solution and this can be
used
in place of sulphuric acid to produce activated silica according to the
following
equation.
Na2O.(SiO2)3.2 + H20 + 2CO2 = 2NaHCO3 + 3.2SiO2.
Many of the salts described above, although weaker acids, have the
disadvantage
of being in solid form, which adds to the complexity of safety and handling.
To overcome these difficulties a continuous process has been designed in
which carbon dioxide is first dissolved in water under pressure, (preferably
50-
200 psig, more preferably 100 psig) and this solution is mixed with sodium
silicate
CA 02300615 2000-03-10
-14-
solution in a mixer that avoids the problem of deposition of silica. The
mixture
flows through a pipeline in which there is sufficient residence time so that
reaction
takes place. Perhaps the most innportant embodiment of the invention arises
from
the discovery that the deposition of scale can be eliminated by choosing
suitable
turbulent flow conditions and the use of flexible tubing. Choice of the
correct
polymeric material for the tube combined with swelling and contracting of this
tubing which takes place during the course of production releases the scale
from
all surfaces. Preferred materials for the tubing include fluorocarbon polymers
such as Teflon , or Teflon coated materials. The swelling and contracting can
also be triggered by periodically closing an outlet valve on the tubing,
increasing
the inlet pressure, preferably bits maximum value, followed by re-opening the
outlet valve, which results in any scale being flushed out. The process is
also
simplified both in that no separate dilution step is required, and the
requirement
for a pH is eliminated. It is, however, recognized that use of a flexible tube
for
the prevention of scale could also apply to other activating reagents
mentioned
above, carbon dioxide is to be preferred both for safety reason and because of
the
contribution to turbulence offered by the release of bubbles from solution. An
alternative or additional means for the prevention of scale formation is the
use of
an additional water inlet at a slightly upstream of the point where the
solution of
silicate and the carbonic acid meet.
CA 02300615 2000-03-10
-15-
In the apparatus of the invention a stream of pressurized water at a
controlled flowrate is passed into a device for dissolving CO2. Pressurized
carbon
dioxide gas from a storage tank is metered in a dissolving device where it
contacts
water and dissolves forming carbonic acid solution. The carbonic acid solution
flows into mixing device where it contacts and mixes with sodium silicate
solution
which preferably has a concentration of 3-28%, preferably 14%, Si02, at the
point
of contact. The mixture then flows through a long tubular reactor, or
alternately a
short tubular reactor with an in-line detention vessel, where the carbonic
acid
solution reacts with the sodium silicate solution so that after a certain
residence
time (between 2 and 20 minutes; preferably between 6 and 10 minutes) activated
silica is formed. The tubular reactor is preferably maintained under pressure
until
the activated silica has been formed in order to keep the CO2 dissolved. The
flowrate of COZ is controlled by a valve on the CO2 supply line that receives
a
signal from a pH probe and controller located at the end of the tubular
reactor.
This probe senses the final pH of the activated silica which preferably has a
concentration of 0.5-5.0% Si02 before discharging. This final pH is preferably
in
the range 6-9, and more preferably about 7. The flowrate through the tubular
reactor is preferably such that the Reynolds number exceeds 3000.
In a preferred embodiment, the process and apparatus involves a holding
tank. The tubular reactor is short, and only starts the reaction. The short
tubular
reactor is characterized by a short retention time and high velocity, e.g., 2-
10
CA 02300615 2007-05-29
50304-6
-16-
ft/sec, more preferably greater than 3 ft/sec, and most preferably from 4-6
ft/sec.
The reactive mixture is then passed from the tubular reactor to the holding
tank to
permit further reaction/polymerization. The tank is preferably open at the top
and
conical shaped at the bottom, allowing for easy cleaning. The contents/product
are removed from the tank by a water eductor, which stops the polymerization
and
dilutes the product at the same time.
Referring to the figures of the drawing, the apparatus in Fig. 1 produces
activated silica by reacting carbonic acid solution with sodium silicate in a
horizontal or vertical tubular serpentine reactor. Clean water passes through
pipe
1 into a pump 2 where the pressure is raised to a range 60-200 psig. From here
it
passes through pipe 3 into flowmeter 4. The flow is controlled through a flow
controller 5 that sends a signal to a flow control valve 6 placed at the end
of the
tubular reactor 7.
CO2 gas from a cryogenic storage container 21 is reduced in pressure by a
regulator 22, and passes by line 23 through a flow qontrol valve 24 into a
flowmeter 25 where the flow can be read. From here it passes through line.26
into a diffuser 27 where the CO2 is diffused and dissolved into the water. The
CO2 dissolves in the first few lengths of the tubular reactor. COZ addition is
controlled by a signal to the flow control valve 24 on the CO2 line 23, from a
pH
probe 28 and controller 29 at the end of the tubular reactor.
CA 02300615 2000-03-10
-17-
Sodium silicate solution is stored in a small storage tank 30, and is fed
through line 31 to a metering pump 32, where a controlled dosage is pumped by
line 33 into a mixing device 341ocated at the bend on the upleg 38 of the
tubular
reactor 7. It is mixed with the carbonic acid solution in a mixing device 34
that is
continuously diluted with water as shown in greater detail in Fig. 2. The
carbonic
acid solution reacts with the sodium silicate solution as it continues to mix
by
flowing turbulently through the remaining lengths of the tubular reactor. The
reacted mixture leaves the tubular reactor through a pressure control valve 6,
passes through pH probe 28, and exits through line 40 to be used, e.g. by
mixing
with raw rater and alum in a water treatment plant.
The contacting device 34 shown in Fig. 2 is for mixing carbonic acid with
sodium silicate. Sodium silicate solution is pumped by the metering pump
through
line 33. This line passes through a tee 35, through a connector and into
another
tee 36 where the sodium silicate mixes with the carbonic acid solution
entering
through the side arm 37 of tee 36.
Additional water from line 54 is measured by flowmeter 56 and flow,
controlled by valve 55, is added through the side arm 39 of tee 35 and passes
on
the outside of line 33, going through the connector and discharging slightly
ahead
of the sodium silicate in tee 36. This additional water flow prevents silica
deposition at the point where sodium silicate mixes with the carbonic acid
solution. The mixture flows into an up leg 38 of the tubular reactor 7. From
here
CA 02300615 2000-03-10
-18-
as shown in Fig. 1 the mixture flows through the rest of the tubular reactor
with
sufficient retention time for reaction between carbonic acid solution and
sodium
silicate to form activated silica. The mixture leaves the tubular reactor
through
valve 6 and passes through pH probe 28 into line 40 that leads to the addition
point with alum in the raw water line.
The apparatus shown in Fig. 3 is a variation on that shown in Fig. 1, as the
CO2 is dispersed and dissolved in a vertical venturi type dissolution device,
compared to a number of length of tubular reactor. Clean water passes through
pipe 101 into a pump 102 where the pressure is raised to a range 60-200 psig.
From here it passes through pipe 103 into flowmeter 104. The flow is
controlled
through a flow controller 105 that sends a signal to a flow control valve 106
placed at the end of the tubular reactor 107. CO Z gas from a cryogenic
storage
container 121 is reduced in pressure by a regulator 122, and passes by line
123
through a flow control valve 124 into a flowmeter 125 where the flow can be
read. From here it passes through line 126 into a vertically arranged venturi
dissolution device 127 where the CO2 dissolves in the water flowing through
it.
CO2 addition is controlled by a signal to the flow control valve 124 on the CO
2
line 123, from a pH probe 128 and controller 129 at the end of the pipeline.
Sodium silicate solution is stored in a small storage tank 130, and is fed
through line 131 to a metering pump 132, where a controlled dosage is pumped
by
line 133 into a mixing device 134 located at the bend on the upleg of pipe 138
of
CA 02300615 2007-05-29
50304-6 -
-19-
the tubular reactor 107. It is mixed with the carbonic acid solution in a
mixing
device 134 that is continuously diluted with water as shown in greater detail
in
Fig. 2 (item 34). The carbonic acid solution reacts with the sodium silicate
solution as it continues to mix by flowing turbulently through the rem~n g
lengths of the tubular reactor. The reacted mixture leaves the tubular reactor
through pressure control valve 106, passes through pH probe 128, and exits
through
line 140 to be used.
If a concentrated solution of sodium silicate is desired as a starting
material, the apparatus shown in Fig. 4 provides a method for in line dilution
of
sodium silicate with water before addition to a mixing device 234. Undiluted
sodium silicate is held in a storage tank 230 from which it exits through line
231
into a metering pump 232 discharging into a venturi mixer 253. Dilution water
from line 250 enters a flowmeter 251 and the flow is controlled by valve 252
before discharging into the inlet of the venturi mixef 253 and mixing with the
sodium silicate so that the homogenized mixture discharges through line 233
into
the contacting device 234 for mixing carbonic acid and diluted sodium silicate
(see
also 34 as shown in Fig. 2). The line passes through a tee 235, through a
connector and into another con.nector tee 236 where the sodium silicate mixes
with
the carbonic acid solution entering through the side arm 237 of tee 236. The
mixture flows into an upleg 238 of the tubular reactor. Additional water from
line
CA 02300615 2000-03-10
-20-
254 is measured by flowmeter 256, with flow controlled by valve 255 and is
added via line 239.
The apparatus shown in Fig. 5 is a variation on that shown in Fig. 3, as
the mixture of dissolved carbon dioxide and sodium silicate solution is
discharged
into a tank where the reaction is completed. Clean water passes through pipe
301
into a pump 302 where the pressure is raised to a range 60-200 psig. From here
it
passes through pipe 303 into flowmeter 304. The flow is controlled through a
flow controller 305 that sends a signal to a flow control valve 306 placed at
the
end of the tubular pipe from the venturi mixing device 327.
CO2 gas from a cryogenic storage container 321 is reduced in pressure by a
regulator 322, and passes by line 323 through a flow control valve 324 into a
flowmeter 325 where the flow can be read. From here it passes through line 326
into a vertically arranged venturi dissolution device 327 where the CO2 is
dissolved in the water. CO2 addition is controlled by a signal to the flow
control
valve 324 on the COZ line 323, from a pH probe 328 and controller 329 at the
end
of the tubular pipeline 338.
Sodium silicate solution is stored in a small storage tank 330, and is fed
through line 331 to a metering pump 332, where a controlled dosage is pumped
by
line 333 into a mixing device 334 located on the pipe 338. It is mixed with
the
carbonic acid solution in a mixing device 334 that is continuously diluted
with
water, e.g. as shown in greater detail in Fig. 2 or Fig. 4. The carbonic acid
CA 02300615 2000-03-10
-21-
solution reacts with the sodium silicate solution as it continues to mix by
flowing
turbulently through pipe 338 passing through flow control valve 306 and pH
sensor 328 before discharging by line 340 into holding tank 341. It remains in
the
holding tank long enough to complete the reaction and form activated silica.
The
product discharges from the tank through line 342 into pump 343 and is pumped
through line 344 to be used.
The apparatus shown in Fig. 6 is a variation on the apparatus shown in
Fig. 5, as the micro-polymer discharging from holding tank 441 through line
442
is diluted by a stream of water from line 446. The dilution water is delivered
through line 449 and is metered by rotameter 448 and flow is controlled by
valve
447 discharging by line 446 and diluting the micro-polymer in line 442. There
is
a non-return valve 445 installed in line 442 to prevent back-flow into tank
441.
The diluted micro-polymer is pumped by pump 443 through line 444 to be used.
The remaining portion of system in Fig. 6 is the sanfe as that illustrated in
Fig. 5.
The apparatus shown in Fig. 7 is a variation on the apparatus shown in
Fig. 6, as the tank 441 that operated at atmospheric pressure is replaced by
tank
561, which operates under pressurized conditions. The carbonic acid - sodium
silicate mixture flows through line 540 and enters the pressurized holding
tank
561. Here, after a certain detention time during which the micro-polymer is
formed, it flows through a non-return valve 563 through line 562 into a
venturi
mixer 564. Water from line 568 flows through a flowmeter 567 at a flowrate
CA 02300615 2000-03-10
-22-
controlled by valve 566 into line 565 that enters venturi mixer 564 where the
micro-polymer is diluted with water. The diluted mixture leaves through line
569
to be used. The remaining portion of the apparatus illustrated is the same as
shown in Figs. 5 and 6.
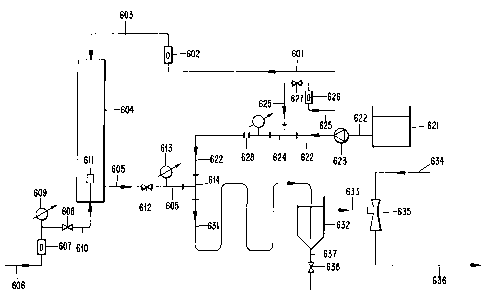
The system illustrated in Fig. 8 shows a holding tank with a water eductor
for removing the contents thereof. More preferably, potable or clean water is
added through line 601 and passes through a flow-meter 602 where its flow is
measured. The flow continues through line 603 into the top of a pressurized
column 604. The water flows down the column and exits at the outlet line 605
close to the bottom.
Carbon dioxide gas from a cylinder or tank is passed through line 606 into
a gas flow-meter 607 where the gas flow-rate is measured. The flow is
controlled
by a valve 608 on the outlet at a pressure slightly greater than the column
pressure
as indicated by a pressure gauge 609. -
The carbon dioxide flows into the bottom of-the column 604 by line 610
and into a diffuser 611 where fine gas bubbles are formed. The bubbles rise up
the column counter-current to the flow of water downwards and resulting in
almost complete dissolution of the carbon dioxide. The carbon dioxide solution
(carbonic acid) leaves the column through line 605 and the flow is controlled
by a
flow control valve 612, and indicated by the water flow-meter 602 on the inlet
line
603 to the top of the column.
CA 02300615 2000-03-10
-23-
As the carbonic acid solution flows through the water flow control valve
612 there is a reduction in pressure as indicated by the pressure gauge 613.
The
flow continues through line 605 into the side arm of a mixing tee 614. Sodium
silicate solution is stored in a tank 621 and exits through line 622 into a
metering
pump 623, that pumps a controlled flow of sodium silicate through a
continuation
of line 622 into a mixing tee 624.
Potable or clear water is added through line 625 through flow-meter 626
and the flow-rate is controlled by valve 627. The controlled flow of water
continues through line 625 into the side arm of a mixing tee 624 where it
mixes
with and dilutes the solution of sodium silicate. This mixture continues
through
an extension of line 622 into a mixing orifice 628 where further mixing takes
place. The diluted sodium silicate solution continues through line 622 into
the
mixing tee 614. In this mixing tee 614, the carbonic acid solution flowing
through
line 605 mixes with the sodium silicate solution.
There is further mixing in line 631 that is connected to the outlet of the
mixing tee 614. During this time in the mixing line 631, there is a chemical
reaction between the carbonic acid solution and the sodium silicate solution
and
silica sol (activated silica) starts to form. Line 631 discharges into the
bottom of
an holding tank 632 that is preferably designed with a conical bottom. As the
mixture flows into the bottom of the tank 632, there is a small release of gas
CA 02300615 2007-05-29
50304-6
-24-
bubbles that keeps the mixture completely mixed. In the tank 632, there is
sufficient residence time to allow further polymerization of the sol.
The mixture leaves tank 632 through line 633 that is connected to eductor
635. This eductor is powered by potable or clean water from a treated source
that
enters the eductor through line 634. The eductor draws the mixture from tank
632
through Iine 633 and discharges it with dilution from water into line 636.
This
dilution prevents further polymerization of the silica sol and prevents
scaling in
line 636. Line 636 discharges the mixture into a raw water supply line close
to
where alum is added.
The aging tank 632, is open to the atmosphere and is designed to be readily
accessible so that scale deposits may be easily cleaned out and discharged
through
the bottom of the tank by opening valve 638 on line 637.
The eductor 635 is designed so that it can be easily dismantled and cleaned
out in the event of scale deposition.
The present invention wiIl be further illustrated in greater detail by the
following example. It is understood that this example is given by way of .
illustration and is not meant to limit the disclosure or the claims that
follow. All
percentages in the examples, and elsewhere in the specification, are by weight
unless otherwise specified.
CA 02300615 2000-03-10
-25-
EXAMPLE
Conditions
In this example water pumped to a pressure of 90 psig and controlled to a
flowrate of 5liters/min was passed through the first downleg of a tubular
reactor.
COZ gas from a storage container was reduced in pressure to 100 psig by a
regulator and sent through a flowmeter before being added to the first downleg
of
the tubular reactor where the CO2 dissolves to form carbonic acid. In the next
upleg sodium silicate solution is pumped into the tubular reactor through a
mixing
device where the carbonic acid solution contacts the sodium silicate solution
and
starts to react. The reaction continues under pressurized turbulent mixing
conditions before leaving the reactor by a pressure reducing valve and flowing
through a pH probe. The COZ flow was adjusted to give a pH = 7Ø The
following results were obtained:
Results -
Gel time = 20 mins
Jar Tests
Alum addition mg/l 45 45 45 45
1%SiO2 mg/l 0 0.5 1.0 1.5
Settling rate mins 2 1.5 1.0 0.5
Turbidity NTU 0.33 0.33 0.31 0.32
CA 02300615 2000-03-10
-26-
This example shows that the settling rate increased by a factor of 4 without
increasing turbidity. This data was generated at a temperature of 24.5 C. It
is
well known that the effectiveness of activated silica is much greater at
temperatures below 15 C where the flocculating kinetics of alum are slower.
While the invention has been described with preferred embodiments, it is
to be understood that variations and modifications may be resorted to as will
be
apparent to those skilled in the art. Such variations and modifications are to
be
considered within the purview and the scope of the claims appended thereto.