Note: Descriptions are shown in the official language in which they were submitted.
CA 02301024 2000-02-15
WO 00/00592 PCT/KR99/00212
1
MONOCLONAL ANTIBODY 3-6-A SPECIFIC TO SURFACE OF
DENDRITIC CELLS AMONG THE PERIPHERAL BLOOD LEUKOCYTES.
TECHNICAL FIELD
The present invention relates to a monoclonal antibody 3-6-A that reacts only
with dendritic cells. More particularly, the monoclonal antibody 3-6-A reacts
with
extracellular region of DM a exclusively expressed on the surface of dendritic
cells
among the peripheral blood leukocytes. Therefore, the present invention
relates to
the usability of the monoclonal antibody 3-6-A in line with dendritic cell-
specific
surface marker.
BACKGROUND ART
When being exposed to external antigens as a consequence of pinocytosis or
pathogenic infection, dendritic cells (hereinafter referred to as DCs) ingest
the
antigens, degrade the proteins and present them on their surfaces in the form
of
peptides bound to MHC class II molecules. Even though the population of DCs
are
less than 1 % of the total PBL, DCs are much more potent in antigen
presentation to
T cells than monocytes and macrophages (Pierre et al., Nature 388:787 (1987)).
Once sensitized to external antigens, DCs home back to lymph nodes with
secreting a specific C-C chemokine. In the lymph nodes, sensitized DCs
activate
naive T cells (Adema et al., Nature 387:713 (1997); Ingulli E et al., J. Exp.
Med.,
CA 02301024 2000-02-15
WO 00/00592 PCT/KR99/00212
2
185:2133 (1997)) to induce cellular immunity against the antigens (Steinman R.
M.,
Annu. Rev. Immunolo., 9:271 (1991)). DCs are reported to play an important
role in
the positive and negative selection of T cells in the thymus (Carlos A.,
Immunol.
Today 18). Other experiments have shown that the chemokines secreted from DCs
S are involved in the homing of T cells to lymph nodes (Adema et al., Nature
387:713
(1997); Ingulli et al., Exp. Med., 185:2133 (I997)).
In addition, DCs have a capacity to induce IL-12 and activate cancer cell-
specific CTLs which are ei~ective to suppress cancer generation and cancer
cell
proliferation (Gabrilovich et al., Cell Immunol. 170:111-9 (1996): Vezzio et
al., Int.
Immunol. 8:19963-70 (1996); Young et al., J. Exp. Med. 183:7-11 (1996)). By
taking advantage of these functions of DCs, intensive research has recently
been
directed to the use of DCs in the development of immuno-therapy for cancer
(Gilboa
et al., Cancer Immunol. Immunother., 46:82-87 (1998); Nestle et al., Nat.
Med.,
4:328 (1998); Song et al., J. Exp. Med., 186:1247 (1997)).
1 S Besides, DCs are known to take an essential role in the pathogenesis of
AIDS
(Fauci, Science, 262:1011 (1993); Pantaleo et al., Nature, 362:3SS (1993);
Embretson et al., nature, 362:359 (1993); Haynes et al., Science, 271:324
(1996)).
Patients infected with HIV-1 virus usually undergo asymptomatic period from 3
to 1 S
years for which only a tracing amount of HIV-1 virus is found in the blood
stream of
patients. However, a large quantity of the virus and infected CD4+ T cells are
found
around DCs in lymph nodes (Pantaleo et al., Nature 362:3SS (1993)). It means
that
the HIV replication is active in the lymphoid organ throughout the period of
clinical
latency, even at times when minimal viral activity is demonstrated in blood.
For this
CA 02301024 2000-02-15
WO 00/00592 PCT/KR99/00212
3
phenomenon, they give an explanation as follows: DCs are exposed and infected
with
a large number of HIV-1 during the primary viremia and return to lymph nodes
in
which they stimulate naive T cells and then spread the virus to those
activated CD4+
T cells. DC-mediated HIV-1 spreading cause active infection of CD4+ T cell and
shortage of T cells in lymph nodes, resulting in the depletion of CD4+ T cells
in
blood stream to the level of AIDS (Blauvelt et al., Clin. Invest. 100:2043
(1997);
McCloskey et al., J. Immunol. 158:1014 {1997)). A report discloses that, when
purified, primary CD4+ T cells are not susceptible to HIV-1 in the absence of
antigen
presenting cells such as DCs or macrophages ,and DCs are much more efficient
in
transmission of HIV-1 to CD4+ T cells than macrophages (Joo et al., J. Kor.
Soc.
Microbiol., 30:77 (1995)). These research results are examples that concretely
show
that DCs are greatly responsible for the AIDS progression.
During the last several years, DCs have been highly focussed by
immunologists and related scientists for their special features and
characteristics as
mentioned above. However, DC studies have not so much progressed as generally
expected in other experiments because of the following limitations. First, DC
compose less than 1 % of the total PBL and do not increase in their population
in
vitro even in the presence of granulocyte macrophage-colony stimulating factor
(GM-
CSF) which has been reported to support the DC survival up to 6 weeks
(Marcowitz
and Engleman, J. Clin. Invest. 85:955, 1990). Second, human DC-specific
surface
markers have not yet been identified.
Limited cell population and lack of a DC-specific surface marker have made
it difficult to use pure and enough amounts of DCs for further experiments.
CA 02301024 2000-02-15
WO 00/00592 PCT/KR99/00212
4
Nonetheless, several laboratories persisted with their investigations, leading
to the
current acceptances about the special characteristics of DCs. Up to now, DCs
have
been purified from the PBL generally through the negative selection procedure
described elsewhere (Freudenthal et al., Proc. Natl. Acad. Sci 87:7698
(1990)). In
the negative selection method, DCs are isolated by removing other immunocytes
such as T cells, B cells, monocytes and macrophages from the PBL through the
following experimental procedures; Ficoll gradient, E-rosetting, adhesive
panning, Fc
panning, metrizamide gradient centrifugation, and antibody panning processes.
These procedures are so pains-taking and sophisticate that the procedures may
not be
10 applicable to general laboratories.
Recently, several investigators have tried to generate DCs by differentiating
bone marrow stem cells (CD34+, CD 11 c+) or monocytes in vitro in the presence
cytokines such as GM-CSF and IL-4 (Bender et al., J. Immunol. Methods 196:121-
135 (1996)). Most of the recent DC studies for immunotherapy exploit this
method
1 S to prepare DCs. However, this method also has its own disadvantages in
that it
requires a high cost and takes a long period of time for differentiation. In
addition,
even though the differentiated cells look like DC morphology, these cells are
still in
controversy for their biological functions whether they are authentic DCs and
are able
to take place real DC in vivo when required in the immunotherapy.
20 Therefore, in order to study DCs to a practical level, first of all, it is
very
essential to identify DC-specific surface markers and generate monoclonal
antibodies
against it. Those monoclonal antibodies would be very useful not only to study
DCs
but also applicable to positive selection of DCs from the PBL.
CA 02301024 2000-02-15
WO 00/00592 PC'T/KR99/00212
For that purpose, a few monoclonal antibodies have been suggested and used
for DC-detecting antibody. However, most of them are not suitable enough to be
used for detection and isolation of DCs from PBL owing to their own
limitations.
For instance, monoclonal antibody (mAb) against HB 15 molecule (CD83) reacts
only
S with activated DCs, but not with the naive DC at all. Our present
investigations have
shown that CD83 is not sufficiently expressed even in the activated DCs when
compared to other cell-specific surface markers. Moreover, the mAb against
CD83 is
not so much specific as it reacts with activated monocytes /macrophages as
well as
with activated DCs. Nevertheless, It has been widely used in identifying the
monocyte-derived DCs in vitro (Fearnley et al., Blood, 89:3708 (1997); Zhou et
al.,
J. Immunol., 154:3821 (1995)).
Besides, CD 11 c and CD 1 a have been reported as DC-specific surface
markers (Gao et al., Immunology 91:135 (1997); Lardon et al., Immunology
91:553
( 1997); Ruedl et al., Immunol. 266:1801 ( 1996)). However, they have a
problem in
their specificity to DCs. CDllc is expressed not only in DCs but also in
macrophages, granulocytes and NK cells, while CD 1 a is found on the
thymocytes
and Langerhans cells, as well. These results are partially confirmed in our
present
experiments.We found that CDllc was expressed substantially on the surface of
DCs, but was also expressed on the B cells and monocytes in a similar or
reduced
amount. Whereas, CDla was not expressed on the surface of naive DCs. These
results indicate that CD 1 a, CD 11 c and CD83 are not suitable for DC-
specific surface
markers.
CA 02301024 2000-02-15
WO 00/00592 PCT/KR99/00212
6
DISCLOSURE OF THE INVENTION
With this background in mind, the present inventors made a cDNA library of
DC and examine the library to seek for a DC specific surface marker. The
inventors
found that the HLA-DM a lb (hereinafter referred to as DM a ) genes are
expressed
only in DCs among the PBLs through a series of experiments, plaque-lifting and
southern blot differential hybridization, sequencing and quantitative RT-PCR
(Bae et
al., Mol. Cells, 5:569, 1995). A monoclonal antibody was generated against
extracellular region of DM a , and then called 3-6-A. Mab 3-6-A was tested for
its
specificity to DCs together with other suggested monoclonal antibodies.
Therefore, it is an object of the present invention to provide a novel
monoclonal antibody, which exclusively recognizes DCs among the PBL.
It is another object of the present invention to provide a hybridoma cell,
which produces the novel monoclonal antibody.
In order to produce a monoclonal antibody against the DM a protein, which
is specifically expressed in DCs among PBLs, there was made an attempt in
which
the DM a protein was expressed in E. coli and inoculated into BALB/c mice for
immunization. Any of the monoclonal antibodies thus obtained, however, were
revealed to be incapable of recognizing normal DM a which is expressed in DCs
or
Raji cells (Kim et al., Mol. Cells, 6:684 (1996)). DM a shows amino acid
sequence
homology over 75% in between human and mouse, suggesting that it would be
CA 02301024 2000-02-15
WO 00/00592 PCT/KR99/002I2
7
difficult to obtain any anti-human DM a monoclonal antibody in BALB/c mice
with
recombinant h-DM a protein.
The reason why the monoclonal antibodies generated by Kim et al (Mol,
Cells, 6:684 (1996)) could not recognize the normal DM a expressed in DCs, is
believed to be attributed to the following possibilities. The recombinant DM a
would
be lost with its B-cell epitopes by lacking in glycosylation or suructural
disruption
when expressed as a form of inclusion body.
In the present invention, recombinant DM a proteins were obtained from a
baculovirus system. After being proven to maintain the antigenicity of the
normal
DM a expressed in Raji cells or DCs, the recombinant DM a proteins were used
to
generate the monoclonal antibody 3-6-A. The monoclonal antibody 3-6-A of the
present invention was found to be an IgGl subclass having yl isotype K-chain
as
analyzed by IsoStrip TM kit (Boehringer Mannheim).
The monoclonal antibody 3-6-A shows a strong reactivity to the normal
DM a expressed in DCs and Raji cells, and the reactivity is very specific only
to
DCs among the primary immunocytes. In addition, the antibody 3-6-A was found
to
stain deeply not only the cytoplasm but also the cell surface of DCs. These
properties
indicate that only DCs among the PBL express DM a in a substantial amount on
their surface as well as in the cytoplasm. This result is quite different from
the
previous reports addressing that the DM proteins are endosomal compartments
and
are localized only in the cytoplasm (Karlsson et al., Science, 266:1569
(1994);
Sanderson et al., Science, 266:1566 (1994)). Present inventors found that the
CA 02301024 2000-02-15
WO 00/00592 PCT/KR99/002I2
8
discrepancy was attributed to the different cell types used for the
experiments.
Actually, Raji cells were stained with 3-6-A only when fixed and permeabilized
first,
but not stained without fixation, indicating that DM is localized only in the
cytoplasm in Raji cells, but not on the cell surface, as reported previously.
These
results strongly suggest that the DM expression pattern is likely to be cell
type
specific. Present inventors found first in the world that the DM is
exclusively
expressed on the surface of DCs among the PBL.
Recently, several reports have addressed that DM play an important role in
MHC class II-dependent antigen presentation as a sharperon protein (Moms et
al.,
Nature 368:551, 1994 ; Karlsson et al., Science 266:1569, 1994). Sanderson et
al
(Immunology 4:87, 1996) reported by co-immunoprecipitation experiments that DM
induces CLIP dissociation from the HLA-DR and facilitates peptide binding to
DR
molecules. But the present finding that substantial amounts of DM are
expressed on
the surface of DC suggests that the DM molecules have some other important
funtions in DC, at least in parts, as well as a chaperon function. First of
all, our
findings strongly suggest that the DM molecules can be used as DC-specific
surface
markers.
The monoclonal antibody 3-6-A of the present invention can be used not only
for the investigation of the DM functions when expressed on the surface of
DCs, but
also for the development of devices used for the positive selection of DCs
among the
PBLs. DCs play an essential role in immune responses. Particularly, DCs are
essential materials to study the immunotherapy against cancer. Accordingly, it
is
urgently demanded to develop a positive selection method for the purification
of
CA 02301024 2000-02-15
WO 00/00592 PCT/KR99100212
9
DCs. Once established by using the monoclonal antibody 3-6-A, developed in the
present invention, the positive selection device will be very helpful to study
DC-
mediated researches and the immunotherapy against cancer. The present
invention
was accomplished by preparing the monoclonal antibody 3-6-A on the basis of
the
research results of the inventors and assaying it for its specificity and
binding
capacity to DCs.
BRIEF DESCRIPTION OF THE DRAWINGS
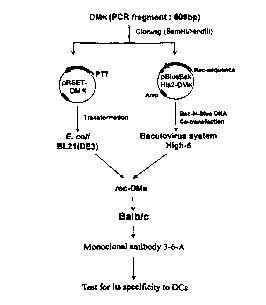
Fig. 1 is a flow diagram briefly showing the procedures used for generation
and characterization of the monoclonal antibody 3-6-A;
Fig. 2 is a SDS-PAGE photograph showing the expresion of recombinant
DM a a in a transformed E. coli;
Fig. 3 is a photograph showing positive plaques formed as a consequence of
the production of recombinant virions in the High-5 cells when transfected
with a
recombinant baculovirus DNA;
Fig. 4 is a Western blot photograph showing the normal expression of a
recombinant DM a in the baculovirus system;
Fig. 5 is a Western blot photograph showing the reactivity of mouse anti-
recombinant DM a antiserum to the authentic DM a expressed in Raji cells;
Fig. 6 is a Western blot photograph showing the reactivity of the monoclonal
antibody 3-6-A to the DM a expressed in E. coli and cells;
CA 02301024 2000-02-15
WO 00/00592 PCT/KR99/00212
Fig. 7A is a fluorescence microscopic photograph showing the the cytoplasm
and surface of DCs and Raji cells stained with the monoclonal antibody 3-6-A;
Fig. 7B is a FACS histogram of the Raji cells when stained with the
monoclonal antibody 3-6-A before or after fixation;
5 Fig. 8 is a fluorescence microscopic photographs showing the specificity of
the monoclonal antibody 3-6-A to DCs in comparison with those of other
commercially available DC-specific monoclonal antibodies when used forstaining
the
purified primary immune cells;
Fig. 9 is a dot immunoblot photograph comparing the reactivity and
10 specificity of the monoclonal antibody 3-6-A and commercially available, DC-
specific monoclonal antibodies to purified DCs; and
Fig. 10 is a FACS histogram showing the reactivity of the monoclonal
antibody 3-6-A to purified primary immune cells, respectively.
BEST MODES FOR CARRYING OUT THE INVENTION
To accomplish the objects of the present invention, first, a 609 by DNA
fragment coding for an extracellular region (corresponding to a base sequence
from
122-731 ) of a DM a gene is cloned into a baculovirus transfer vector,
pBlueBacHis2A, to give a recombinant plasmid, pBlueBacHis2A-DM a . This
recombinant plasmid, together with a baculovirus full-length DNA, Bac-N-Blue
(Invitrogen), is co-transfected into High-5 cells (Invitrogen), resulting in
the
production of a recombinant baculovirus Bac-N-Blue-DM a , which is then
infected
CA 02301024 2000-02-15
WO 00/00592 PCT/KR99/00212
11
into mass cultured High-5 cells. The infected High-5 cells are harvested,
whose
extracts are applied to the Ni+-NTA column (Qiagen) for purification of the
recombinant DM a protein. BALB/c mice are immunized with the pure recombinant
DM a protein and their splenocytes are excised and undergo hybridization with
mouse myeloma Sp2/0 cells, resulting in production of the hybridoma cell, KHB-
DM, which produce the monoclonal antibody 3-6-A. The monoclonal antibody 3-6-
A is now being obtained by culturing the hybridoma cell, KHB-DM.
Either from a buffy coat or leukopak, supplied from Korean Red Cross Blood
Bank, DCs and other immune cells (T cells, B cells and monocytes) were
purified
through the procedures as reported previously (Bae et al., Mol. Cells, 5, 569,
1995).
EXAMPLE 2: mRNA Extraction and DM a -cDNA Synthesis from the DCs
From the DCs obtained in Example 1, mRNA was extracted according to a
known technique (Bae et al., Mol. Cells, 5, 569, 1995). The mRNA was incubated
with 1 ~c g of an oligo-dT primer at 37 ° C for 2 min in a reverse
transcription
solution (RNase inhibitor 0.5 ,u 1, Sx first strand synthesis reaction buffer
4 ~c 1, 0.1
mM DTT 2 ,u 1, 10 mM dNTP 1 ,u 1), followed by the addition of 5 units of an
AMV-reverse transcriptase. This solution was incubate at 37 ° C for 40
min and
CA 02301024 2000-02-15
WO 00/00592 PCT/KR99/00212
12
then further incubated at 45 ° C for 30 min to synthesize cDNA. A DM a
gene was
amplified by PCR using the cDNA as template and primers shown below at Table
1.
TABLE 1 : Primers for DM a
BamH I-DM 5'-GAT AAG GAT CCG TCC AAG CTC CTA-3'122-137
Sense primer
HindIII-DM Anti-5'-GT TCA AAG CTT TCA CTC CAG CAG 732-712
ATC
sense primer TGA GGG-3'
The PCR was performed by repeating a thermal cycle consisting of 94 °
C/1 min, 40
C/30 sec and 72 ° C/45 sec, 5 times, and then, a thermal cycle
consisting of 94
° C/1 min and 72 ° C/l min, 25 times, so as to produce a 609 by
DNA fragment
encoding an extracellular region (base sequence 12-732) of the DM a gene.
EXAMPLE 3: Cloning and Expression of DM a in E. coli
The DNA fragment amplified by PCR in Example 2 was double digested with
BamH I and Hind III and inserted into the plasmid pRSET/A (Invitrogen) opened
with the same restriction enzymes, to give a recombinant plasmid pRSET-DM a .
This recombinant pRSET-DM a was transformed into E. coli BL21(DE3) which
was, then, spread on an LB medium containing ampicillin. The positive colonies
were inoculated in a M-9 ampicillin broths (1% bactotryptone, Na2HP04, KH2PO4,
CA 02301024 2000-02-15
WO 00/00592 PCT/KR99/00212
13
NaCI, NH4C1, 2M glucose, 0.5% diamine, 1M MgS04, 1M CaCl2) and cultured with
agitation. When the absorbance of the cultures reached 0.5, IPTG was added to
a
final concentration of 1 mM to induce protein expression at 37 ° C for
2 hours. The
expression of recombinant DM a in the transformed E. coli was identified by
SDS-
PAGE analysis in which a band was detected at 29 kDa (Fig. 2). In Fig. 2, Lane
1 is a
protein size marker, lane 1 the lysate of E. coli BL21 (DE3) transformed only
with
the pRSET/A plasmid, lane 2 the lysate of E. coli BL21 (DE3) transformed with
the
recombinant DM a plasmid and lane 3 a DM a protein sample purified from the
recombinant bacteria of lane 2 with the aid of an Ni+-NTA resin (Qiagen).
EXAMPLE 4: Construction of Recombinant Baculovirus Ex~~ressinst DM a
The DNA fragment amplified by PCR in Example 2 was digested with BamH
I and Hind III and inserted into the plasmid pBlueBacHis2A (Invitrogen) opened
with
the same restriction enzymes. The ligate was transformed into E. coli BL21
(DE3)
which was then cultured in the same manner as described in Example 2 to obtain
a
recombinant plasmid pBlueBacHis2A-DM a . Together with the baculovirus full-
length DNA Bac-N-Blue {Invitrogen) linearized by Bsu36I digestion, the
recombinant plasmid pBlueBacHis2A-DM a was transfected into High-5 cells
(Invitrogen) with the aid of Lifofectin (Gibco BRL). Five to 7 days culture in
a liquid
medium after transfection, the supernatant was harvested and reinfected at a
diluted
concentration into High-5 cells. Two days after inoculation, the cells were
covered
CA 02301024 2000-02-15
WO 00/00592 PCT/KR99/00212
14
with a 1 % low-melting agarose mixture (prepared by mixing 50 mg/ml of X-gal,
2x
TNM-FH medium with 2% low-melting agarose in an equal volume) and cultured for
3-5 days further. Blue plaques were selected as positive clones (panel A in
Fig. 3).
The selected clones were further purified by repeating the above culturing
procedures
three times. They were amplified in High-5 cells to obtain a large quantity of
recombinant baculovirus Bac-N-Blue-DM a .
EXAMPLE 5: Analysis of the Cloned DM a in the Recombinant Baculovirus
From the recombinant baculovirus Bac-N-Blue-DM a obtained in Example
4, the genomic DNA was extracted for PCR analysis. PCR was performed using the
primers BaclBac2 of Table 2 (included in the Bac-N-Blue transfection kit,
Invitrogen) with the genomic DNA serving as a template to identify the
recombinant
baculovirus is harboring the DM gene.
TABLE 2: BaclBac2 primers
Bac 1 primer 5'-TTT ACT GTT TTC GTA ACA GTT TTG -44~ -21
Bac 2 primer 5'-CAA CAA CGC ACA GAA TCT AGE-3' +794 +77
EXAMPLE 6: Expression and purification of DM a from the Recombinant
CA 02301024 2000-02-15
WO 00!00592 PC'f/KR99/00212
High-5 cells were infected with the recombinant baculovirus Bac-N-Blue-
DM a at 5 MOI and then cultured at 27 ° C for 5 days in a humid
incubator. The
recombinant DM a proteins were extracted from the infected cells and purified
by
using the Ni+-NTA resin column (Qiagen).
5
EXAMPLE 7: Immunization of BALB/c mouse with Recombinant DM a
The DM a protein obtained in Example 6 was mixed with an equal volume
of the Freund's adjuvant {Sigma) and inoculated into 5-6 week old BALB/c mice
at a
10 dose of 50 ,u g/mouse. Inoculated mice were then boosted secondarily and
tertiarily
with the same antigen at a dose of 25 ,u g/mouse in the 3 weeks interva. After
the
tertiary immunization, Each BALB/c mouse was bleed from the ocular vein to
analyze the antiserum.
15 EXAMPLE 8: Reactivit~~ of Antiserum to the Recombinant DM a
The High-5 cells infected with the recombinant baculovirus Bac-N-Blue-
DM a were homogenized and the proteins obtained from the disrupted cells were
separated by SDS-PAGE. The proteins separated on an SDS-PAGE were
transblotted to a nitrocellulose membrane using a transferring solution (48 mM
tris-
HCI, 39 mM glycin, 20% methanol, 1.3 mM SDS) in a semi-dry gel blotter (Bio-
Rad). This blotted nitrocellulose membrane was blocked with a blotto {5% (w/v)
CA 02301024 2000-02-15
WO 00/00592 PCT/KR99/00212
16
non-fat milk, 0.02% NaN3 in PBS) for 30 min, washed three times with PBS, and
placed for 30 min at room temperature in a 10 ml PBS solution containing 5 ~c
1 of
the antiserum obtained in Example 7. Washing 3 times with PBS, membrane was
soaked in the PBS solution containing goat anti-mouse-IgG-alkaline phosphatase
S (Sigma) as a second antibody, and then visualized by adding a NBTBCIP
solution
{66 ,u 1 of 50 mg/ml NBT (nitro blue tretrzolium) and 30 ,u 1 of 50 mg/ml BCIP
(5-
bromo-4-chloro-3-indolyl phosphate) in 10 ml of an AP buffer ( 100 mM NaCI, 5
mM MgCl2, 100 mM Tris-HCI, pH 9.5)} as a substrate for the enzymereaction.
When a band appeared as a consequence of the enzymatic reaction in a dark
place,
stop solution (20mM EDTA, 150mM NaCI in 10 mM Tris-Cl, pH 8.0) was added to
terminate the reaction.
This Western blotting analysis showed that the recombinant baculovirus Bac-
N-Blue-DM a normally expressed DM a in High-5 cells, as shown in Fig. 4. In
Fig.
4, lane M is a protein size marker, lane 1 an uninfected High-5 cell lysate,
lane 2 a
1 S wild baculovirus-infected High-5 cells lysate, lane 3 lysate of High-5
cell infected
with the recombinant baculovirus bac-N-Blue-DM a , lane 4 E. coli
BL21 (DE3)lysate as a negative control, and lane 5 lysate of the recombinant
E. coli
BL21(DE3) expressing the recombinant DM a .
EXAMPLE 9: Reactivit~r of Antiserum to Authentic DM a
CA 02301024 2000-02-15
WO 00/00592 PCT/KR99/00212
17
Western blot analysis was carried out in a similar manner to that of Example
8, using 1x106 untreated Raji cells and 1x106 Raji cells treated with Y-
interferon
(Sigma) at a concentration of 100 unit/ml for 3 days. The result is given in
Fig. 5. In
Fig. 5, lane 1 is a control Jurkat cell lysate, lane 2 a Raji cell lysate and
lane 3 the
5 lysate of the Raji cells treated with y-interferon.
This analysis shows that the antiserum obtained from the BALB/c mice
immunized with the recombinant DM a reacts well with the authentic DM a
expressed in Raji cells.
10 EXAMPLE 10. ProdLCtion of H;rbridoma cells Expr~~j"ngll~Ionoclonal antibody
The mice immunized with the recombinant DM a , obtained from the
expression in a baculovirus system in Example 7, were euthanized by backbone
dislocation and their spleens were excised. 1.4x10' spleenocytes were
suspended in
10 ml of a glucose-rich DMEM medium, mixed with 3x106 cells of the mouse
15 myeloma cell SP2/O (Sp2/O-Agl4 KTCC CRL1581) and washed with a glucose-
rich DMEM medium. To the cell pellet, 1 ml of a 50% PEG-4000 solution (Gibco
BRL) was added for 1 min to induce cell fusion. After a glucose-rich DMEM
medium was slowly added at a rate of 1 ml/min, the cells were well mixed and
centrifuged. The cell pellet was suspended in 5 ml of lx DMEM2~i, and
distributed
20 to the multiwell plate at an aliquot of 50 ~c 1 per well where mouse
macrophages
were cultured as a feeder cell (Antibody Lab., Mannual ed. H. D. Lane, p220,
1989)
and then cultured for one day. Then, an equal volume of 2x HAT medium was
added
CA 02301024 2000-02-15
WO 00/00592 PCT/KR99/00212
18
to each well. During a long culture period of time, fused cells were
colonized.
Hybridoma cells colonized in HAT medium were selected from 18 wells.
Reacting with DM a
Each of the 18 colonies obtained in Example 10 was cultured in a large
volume of a medium and the supernatant was analyzed by ELISA, dot immunoblot
hybridization and Western blot hybridization. 96-well plates were coated
overnight at
4 ° C with the DM cr proteins prepared in Examples 3 and 6 to a final
concentration
of 0.5 ,u g per well. Plates were washed twice with 100 ~c 1 of PBS containing
0.05 % Tween 20. A 1 % non-fat milk solution (dissolved in deionized water,
added
with 0.02% NaN3) was added to each wells at an amount of 90 ,u 1 per well and
allowed to stand at 37 ° C for 1 hour. Subsequently, reaction was
performed with the
antiserum of Example 7 for 37 ° C for 1 hour in the wells. After
washing, a
secondary antibody (goat-anti-mouse-IgG-AP 1/1000 diluted) was added and
incubated at 37 ° C for 2 hours. After being washed twice, each wells
were filled
with an alkaline phosphatase substrate solution (p-nitrophenyl phosphate 1 mg
in
10% diethanol amine buffer 1 ml (diethanol amine 97 ml, NaN3 0.2 g (0.02%),
MgCl2 6H20 100 mg (0.01%) in DDW 800 ml; total 1 liter)) 50 a 1 per well and
incubated at 37 ° C for 30 min.
CA 02301024 2000-02-15
WO 00/00592 PCT/KR99/00212
19
When the reaction was completed, absorbance was measured with an ELISA
reader (Molecular Devices) at 405 nm. Western blotting was earned out in the
same
manner as in Example 8, while dot immunoblot hybridization was performed
following the previous method (Kim et al., Mol. Cells, 6,684, 1996).
The results are summarized at Table 3, below. At Table 3, the reactivity of
the monoclonal antibody to the recombinant DM a protein expressed in E. coli
and
baculovirus system, was measured by ELISA and Western blot hybridization while
the reactivity to authentic DM a expressed in various cells were measured by
dot
immunoblot and Western blot hybridization. The experiment have shown that the
14
monoclonal antibodies all strongly react with the recombinant DM a expressed
in E.
coli, but none of them, except the monoclonal antibody 3-6-A, reacts with the
recombinant DM a expressed in High-5 cells. The monoclonal antibody 3-6-A was
found to have strong reactivity to DCs among the PBLs as measured by Western
blot
and dot immunoblot hybridization. Also, the monoclonal antibody 3-6-A was
highly
reactive to Raji cells in Western blot hybridization. A weak positive response
was
occasionally detected in B cell fractions in Western blot hybridization. This
weak
detection offers the possibility that activated B cells might express DM.
Based on
these results, the monoclonal antibody 3-6-A against DM a was selected out of
the
14 monoclonal antibodies. The hybridoma cells which express the monoclonal
antibody 3-6-A, were named KHB-DM and were deposited in the Korean Collection
for Type Cultures, Korean Research Institute of Bioscience and Biotechnology
(deposition No. KCTC-0485BP).
CA 02301024 2000-02-15
WO 00/00592 PCT/KR99/00212
EXAMPLE 12: Reactivity of Monoclonal Antibody 3-6-A to the Authentic DM a
Monoclonal antibody 3-6-A was tested for its reactivity to the recombinant
5 DM a and authentic DM a normally expressed in Raji cells and DCs in Western
blot hybridization. The result is given in Fig. 6. As shown in Fig. 6, the
monoclonal
antibody 3-6-A is highly reactive to the 34 lcDa DM a, expressed normally in
Raji
and DCs. In Fig. 6, lane 1 is a negative control of E.coli BL21 (DE3) lysate,
lane 2 a
lysate of E. coli expressing DM a , lane 3 a lysate of High-S cells expressing
DM a ,
10 lane 3 a Raji cell lysate, and lane 5 a DC lysate.
TABLE 3: Binding Capacity of Monoclonal Ab to DM a
Abs Anti
ens
Recombinant Cells
DM
E. coli Hi PBL T B MC DC Ra'i
h-5
DM a pAb ++++ +++~- + _ _
1 _ 1 _H -H. _ _ _ _ _ _
1~3-A +-~-+-+ - _ _ _ _ _ _
1-8-G ++++ _ _ _ _ _ _ _
1-10-A +++ + _ _ _ _ _ _
1-11-E +++-~- - - _ _ _ _ _
2-11-G ++++ - - _ _ _ _ _
2-12-A ++++ - _ _ - _ _ _
3-4-B -+-~+ - _ _ _ _ _ _
3-5-B ++++ _ _ _ _ _ _ _
3-b-A ++++ +++ + _ _ +++
4=1-C ++ - _ _ _ _ _ _
4-2-B +~-~-+ _ _ _ _ _ _ _
5-3-G +++ _ _ _ _ _ _ _
5-5-D ~-t-++ - _ _ _ _ _ _
CA 02301024 2000-02-15
WO 00/00592 PCT/KR99/00212
21
note: biding capacity was measured by ELISA, dot immunoblot and/or Western
blot
hybridization.
results similar to those of negative controls
S + : positive results, the number of this symbol shows the intensity of the
positive signal, and is increased one by one whenever .the absorbance at 405
nm
increases by 0.2 from the basic positive value which is the mean value of
negative
control plus SSD (standard deviation). ++-~+ is used for all reactions whose
ELISA
value is equal to or higher than 4 symbols.
In Western blot and dot immunoblot, the symbol '+' was used only when a
detectable signal is clearly repeated. The number of symbols is increased as
the
comparative intensity increases.
t : weak or non-repetitive signal.
EXAMPLE 13: Mass Production of Monoclonal A_ntbod,~6~-A
The hybridoma cell KHB-DM was seeded at a density of 2x105 cells per ml
of a high glucose DMEM2oo~ media. Four days later at a maximum growth, culture
supernatants were harvested as a monoclonal antibody 3-6-A source. At this
time,
the concentration of monoclonal antibody reached to approximately 40 ~u g per
ml of
the culture medium.
In order to produce a large amount of the monoclonal antibody, hybridoma
cells were cultured in mouse abdominal cavity. BALB/c mouse was injected first
CA 02301024 2000-02-15
WO 00/00592 PCT/KR99/00212
22
with 1 ml of prestane intraperitoneally. One week later, the mice were
inoculated
with 5x106 hybridoma cells per mouse. After 7-14 days, when the abdominal
cavities of the mice were swollen enough, ascitic fluid was taken by 5-10 ml
per
mouse with 18-gauge needle attached to a 10 ml syringe, and then spin at 1,500
rpm
for 10 min. The supernatant obtained was added with 0.02 % NaN3 and stored at -
70 ° C or less. The monoclonal antibody concentration was 5-9 mg per ml
in the
ascitic fluid.
The monoclonal antibody 3-6-A produced by the hybridoma cell KHB-DM in
Example 11 was examined for its isotype using the isotyping kit, IsoStripTM
(Boehringer mannheim). The monoclonal antibody 3-6-A was found IgGl isotype
having >G-light chain.
I?.S~
Microscopic slide glass were coated for 15 min with a 1 mg/ml poly-L-lysine
(Mw 400,000) solution and washed with deionized distilled water. Raji cells
(ATCC
CCL8b) and DCs, isolated as in Example 1, were diluted to 105 cells/ml,
attached to
the slide coated with poly-L-lysine and then washed with PBS. 100 ~c 1 of the
monoclonal antibody (culture supernatant) obtained in Example 13 were added to
the
CA 02301024 2000-02-15
WO 00/00592 PCT/KR99/00212
23
cell-attached slide, which was allowed to stand for 1 hour in a C02 incubator
and
then washed three times with PBS. After reaction with the second antibody
(anti-
mouse-IgG-FITC) at 37 ° C C02 incubator for 30 min, the slide was
washed twice
with PBS and then observed under a fluorescence microscope (Nikon E-600 Epi-
5 fluorescent microscope).
To stain the cytoplasm of DC with 3-6-A, the DCs-attached slides were fixed
by immersing them in an organic solvent (50% methanol and 50% acetone) prior
to
staining with the monoclonal antibody 3-6-A. After fixation, DCs were stained
with
the monoclonal antibody 3-6-A and the second antibody in the same manner as
above
10 and observed in the fluorescence microscope.
In Fig. 7A, panel a is a microscopic photograph of DC, magnified by 400
times at a microscope (Nikon TMS), panel b is a fluorescence microscopic
photograph of a DC stained with the PE-conjugated HLA-DR (Becton-Dikinson) as
a
control, panel c is that of a DC bound and stained with the antibody 3-6-A and
FITC-
15 conjugated second antibody,respectively, panel d is that of a DC which were
fixed
first and then stained in the same manner shown in panel c, panel a and f are
those of
unfixed and fixed Raji cells, respectively, which were stained in the same
manner.
Fig. 7B shows the FACS results of unfixed and fixed Raji cells stained with
the monoclonal antibody 3-6-A and FITC-conjugated second antibody. Half of a
Raji
20 cell fraction was fixed with Cytofix/cytoperm Cytostain~ Kits(Pharmingen)
by
following th evender's manual. Unfixed and fixed Raji cells were treated with
the
monoclonal antibody 3-6-A at 4 ° C for 30 min, washed twice with PBS,
and then
stained at 4 ° C for 30 min with the same FITC-conjugated second
antibody as
CA 02301024 2000-02-15
WO 00/00592 PCT/KR99/00212
24
mentioned above. Stained cells were washed twice with PBS and analyzed
immediately by FACStar (Becton-Dickinson). In Fig. 7B, panel a and b are the
FACS results of unfixed and fixed Raji cells, respectively.
As apparently shown in Fig. 7, DM a is expressed in a large quantity on the
surface of DC as well as in its cytoplasm (Fig. 7A panel c and d), which is
contrary to
the previous reports insisting that DM is only an endosomal compartment.
Whereas,
Raji cells were also deeply stained by the 3-6-A when fixed prior to staining
(Fig.7A
panel f and 7B panel b). Unfixed Raj i cells, however, were not stained at all
with the
same antibody (Fig.7A panel a and 7B panel a). The results from Raji cells
indicate
that the DM molecules are localized only iun the cytoplasm, but not on the
surface of
Raji cells, as mentioned in the previous reports (Karlsson et al., Science
266; Science
226:1599-1573, 1994; Sanderson et al., Science 266:1566-1569, 1994).
T cells, B cells, monocytes and DCs were isolated in the same manner as in
Example 1. Each cells were diluted to 105 cells per ml of PBS and treated at 4
° C for
30 min with 100 a 1 of the monoclonal antibody 3-6-A (culture supernatant)
obtained
in Example 13. After centrifugation at 750 g, the cell pellet was washed twice
with
PBS, suspended in 200 a 1 of PBS and stained at 4 ° C for 30 min with
10 ~c 1 (1
,u g) of the second antibody, anti-mouse-IgG-FITC (sigma). After
centrifugation, the
cell pellet was washed twice with PBS and attached to a coating slide. Stained
cells
CA 02301024 2000-02-15
WO 00/00592 PCT/KR99/00212
were analyzed with a fluorescence microscope as mentioned in Example 15 and
its
results are given in Fig. 8. In Fig. 8, column T stands for T cells, column B
for B
cells, column Mc for monocytes/macrophages, and column DC for DCs. Samples
were reacted with commercially available various monoclonal antibodies and the
5 monoclonal antibody 3-6-A and then stained with an FITC-conjugated second
antibody.
As shown in the bottom of Fig. 8, the monoclonal antibody 3-fi-A highly
reactive with DCs, but not with other immune cells at all. These data
demonstrate
that DM molecules are exclusively expressed on the surface of DCs among
primary
10 immune cells. In contrast, the antibodies against CD 11 c or CD83, widely
used for
DC analysis, are not so much specific to DCs among the PBL as shown in Fig 8.
They react with not only DCs, but also B cells and monocytes. Particularly, as
for
CD83, known to be expressed in the activated DC fraction (Zhou L. J., J.
Immunol.
149, 735, 1992), its monoclonal antibody (anti-CD83: Immunotech Co.) showed
15 reactivity, even if weak, to monocytes as well as to naive DCs in a similar
intensity.
Monoclonal antibody to Fascin, reported allegedly to be expressed only in the
cytoplasm of DCs, could not recognize the DCs when unfixed. From these data
apparently shown in Fig. 8, it was reconfirmed that the monoclonal antibody 3-
6-A
produced from the present inversion has higher specificity and more potent
affinity
20 to DCs than any other conventional monoclonal antibodies.
CA 02301024 2000-02-15
WO 00/00592 PCT/KR99/00212
26
The affinity of the monoclonal antibody 3-6-A to DCs was compared with
those of other conventional DC-specific monoclonal antibodies. DCs were
isolated in
the same manner as in Example 1 and subjected to dot immunoblot hybridization
{Kim et al., Mol. Cells, 6, 684, 1996) with the monoclonal activity 3-6-A,
together
with other commercially available monoclonal antibodies.
Each fraction of DCs containing 105 cells, isolated in the same manner as in
Example 1, were stained with 1 ~ g of each of an anti-CD 1 a monoclonal
antibody
(Becton-Dickinson), an anti-CD 11 c monoclonal antibody (Becton-Dickinson), an
anti-CD83 monoclonal antibody (Immunotech. Co.), an anti-Fascin monoclonal
antibody (granted from NIH AIDS Research and Reference Reagent Program), and
the monoclonal antibody 3-6-A in the same manner as in Example 15. Cells
treated
with the first antibodies were stained with an alkaline phosphatase-conjugated
with
goat-anti-mouse-IgG (Promega) in the same manner as in Example 15, followed by
being blotted to a nitrocellulose membrane with the aid of a dot blotter (Bio-
Rad).
The membrane was subjected to the chlomogenic reaction as shown in Example 8.
The dot immunoblot hybridization result is given in Fig. 9. In Fig. 9, the dot
blot signal of 1, 2, 3 and 4 result from the experiments of DC staining with
anti-
CD 1 a, anti-CD 11 c, anti-CD83 and the anti-Fascin monoclonal antibodies,
respectively as controls. Whereas, the dot blot signal S shows the DC fraction
stained with the monoclonal antibody 3-6-A. Consistent with the result shown
in
Example 16, the monoclonal antibody 3-6-A shows the most potent afllnity to
DCs
among the monoclonal antibodies tested (Blot 5 in Fig. 9). Next to the
monoclonal
antibody 3-6-A, the anti-CD 11 c antibody was the most potent in the affinity
to DCs
CA 02301024 2000-02-15
WO 00/00592 PCT/KR99/00212
27
(Blot 2 in Fig. 9), but was also reactive with monocytes and B-cell fraction
(Fig. 8).
The antibodies against CD83 or Fascin were found inefficient to react with
naive
DCs.
E~ AMP . . 18: FA nawsis of Primar5r I mLne ('.ell W h Monoclonal_ A'ntibodv
3-6-A
The primary immune cells, including T cells, B cells, monocytes and DCs,
isolated as in Example 1, were treated with the monoclonal antibody 3-6-A at 4
° C
for 30 min. Being washed twice with PBS, cells were treated with the second
antibody goat-anti-mouse-IgG-FITC (Sigma) at 4 ° C for 30 min. Stained
cells were
washed twice with PBS, and then immediately analyzed with the FACStar plus
(Becton-Dickinson).
The FACS analysis results are given in Fig. 10. In Fig. 10, panel A shows
FACS diagrams of the immune cells purled in the same manner as in Example 1,
while panel B shows FACS diagrams of the immune cells purified from the PBL
cultured for 1 week in an RPMI,~,o medium prior to isolation. The FACS
diagrams
for T cells are in column 1, for monocytes in column 2, for B cells in column
3 and
for DCs in column 4. As shown in columns 1 and 2, neither T cells nor
monocytes
/macrophages, whether isolated primarily or from the cultured PBL, reacted
with the
monoclonal antibody 3-6-A. As for B veils, when primarily isolated, they did
not
react with the monoclonal antibody 3-6-A at all. In contrast, when isolated
from the
PBL cultured for one week, tiny amounts of B cell fraction were stained with
the
CA 02301024 2000-02-15
WO 00/00592 PCf/KR99/00212
28
antibody 3-6-A (column 3 in Fig. 10). Whereas, more than 60% of the isolated
DC
fractions were deeply stained with the monoclonal antibody 3-6-A. Not a big
difference was found in the FACS results between the cells isolated primarily
and
after the culture for one week (column 4 in Fig. 10). These FACS data also
5 demonstrate that the monoclonal antibody 3-6-A reacts specifically with DCs
among
primary immunocytes.
EXAMPLE 19: Examination of the Antigenicitv between DM a Proteins Expressed
10
The same procedure as in Example 7 was repeated to obtain antisera (anti-
DM a -polyclonal antibodies) from the mice immunized with the recombinant DM a
proteins which were produced from E. coli in Example 3 and from the
baculovirus
system in Example 6. Using the polyclonal antibodies, the recombinant DM a and
15 authentic DM a expressed in Raji cells were subjected to Western blot
hybridization.
The antiserum obtained from the mice immunized with the recombinant
DM a expressed in E. coli, well recognized the recombinant DM a expressed in
E.
coli, but not the authentic DM a expressed in Raji cells. In contrast, the
antiserum
taken from the mice immunized with the DM a expressed in the baculovirus
system,
20 well responded to the recombinant DM a as well as the authentic DM a
expressed
in Raji cells. This result suggests that the recombinant DM a expressed in E.
coli is
likely to be different from the normal DM a proteins in antigenicity. It is
believed
CA 02301024 2000-02-15
WO 00/00592 PCT/KR99/00212
29
that this difference might cause the previous art to fail in producing the
monoclonal
antibody which is able to react with the normal DM a , when mice were
immunized
with the DM a expressed in E. coli (Kim et al., Mol. Cells, 6, 684, 1996).
INDUSTRIAL APPLICABILITY
As described hereinbefore, the monoclonal antibody 3-6-A of the present
invention shows a potent reactivity to the authentic DM a normally expressed
in
DCs and Raji cells. Particularly noteworthy is that the monoclonal antibody 3-
6-A is
very specific and strong binding capacity to DCs among the PBL. The monoclonal
antibody 3-6-A stains DC on their surface as well as in the cytoplasm. Being
very
helpful in studying the functions of DM molecules, therefore, the monoclonal
antibody 3-6-A of the present invention can be used as a DC-specific surface
marker,
which seems to be strong enough to allow positive selection of DCs from PBLs.
The
present invention will be very useful in the biomedical industry, contributing
to the
advance in the research on the immunotherapy against cancers or other chronic
diseases.