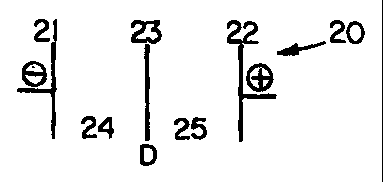Note: Descriptions are shown in the official language in which they were submitted.
CA 02301035 2000-02-15
WO 99/09234 PCT/US98/16942
Title: ELECTROSYNTHESIS OF HYDROXYLAMMONIUM SALTS AND
HYDROXYLAMINE USING A MEDIATOR, A CATALYTIC FILM,
METHODS OF MAKING THE CATALYTIC FILM, AND
ELECTROSYNTHESIS OF COMPOUNDS USING THE CATALYTIC
FILM
Technical Field
The present invention relates to methods for preparing
hydroxylammonium salts and hydroxylamine using a mediator, a catalytic
film, methods of making the catalytic film, and methods of using the
catalytic film. More particularly, the invention relates to a catalytic film
formed on an electrode from the interaction of a film forming compound
and nitrate ions.
Background of the Inventjon
Hydroxylammonium salts are compounds which have a variety of
applications. For instance, hydroxylammonium nitrate may be used as a
component of liquid propeilant and as a reducing agent in photographic
operations. In some of these applications, it is desirable that a
hydroxylammonium salt solution of high purity is available.
There exist several production methods to manufacture hydroxyl-
ammonium salts. In the case of hydroxylammonium nitrate for example,
some of these methods include: electrodialysis of hydroxylammonium
chloride and nitrate; reaction of hydroxylammonium sulfate and barium
nitrate; three-step cation exchange process employing hydroxylammonium
sulfate and nitric acid; and electrolytic reduction of nitric acid. Some of
1
SUBSTITUTE SHEET (RULE 26)
CA 02301035 2000-02-15
WO 99/09234 PCT/US98/16942
these methods, however, do not provide hydroxylammonium salt solutions
of high purity which some applications of the compound require. As a
result, procedures have been developed to purify the hydroxylammonium
salt solutions produced by existing methods. Nevertheless, there remains
a substantial demand for large quantities of high purity hydroxyl-
ammonium salt solutions. There also is a demand for an efficient process
of making hydroxylammonium salts.
Hydroxylamine is useful as an intermediary in chemical processes
especially in the pharmaceutical and agricultural industries. It is also
useful in stripper formulations. Stripper formulations may be used to
remove photoresists from or clean a substrate. For example,
hydroxylamine stripper solutions are used to remove polyamide coatings
from metal foil. Hydroxylamine stripper solutions are utilized in the
printed circuit board and semiconductor industries.
Frequently, solutions of hydroxylamine, especially solutions
prepared from hydroxylammonium salts, contain undesirable amounts of
impurities such as salts, ammonium ions, metals and organic materials.
Thus, there exists a need for hydroxylamine solutions having high purity.
There also is a demand for an efficient process of making hydroxylamine.
The production of hydroxylamine by the electroreduction of nitric
oxide in sulfuric acid is described by L.J.J. Janssen et al in Electrochimica
Acta, 1977, Vol. 22, pp. 27-30 and by M.L. Bathia et al in The Canadian
Journal of Chemical Engineering, Vol. 57, October 1979, pp. 631-7.
Janssen et al utilize a platinum cathode, and Bathia et al utilize a cathode
bed of tungsten carbide particles. The electroreduction of nitric oxide on
bulk platinum in perchloric acid and sulfuric acid solutions is described by
J.A. Colucci et al in Electrochimica Acta, Vol. 30, No. 4, pp. 521-528,
1985.
U.S. Patent 5,281,311 relates to a process in an electrolysis cell
involving (A) providing an electrolysis cell containing an anolyte
=compartment containing an anode, a catholyte compartment containing an
2
CA 02301035 2000-02-15
WO 99/09234 PCT/US98/16942
oxygen-consuming cathode and an anionic divider separating the anolyte
and catholyte compartments; (B) providing an aqueous solution containing
an acid and water to the anolyte compartment, and an aqueous solution
containing hydroxylamine salt, water and optionally, an acid to the
catholyte compartment; (C) charging an oxygen-containing gas to the
catholyte compartment; (D) passing a direct current through the
electrolysis cell for a period of time effective to reduce the acid content in
the catholyte compartment and/or to convert the salt to a hydroxylamine;
and (E) recovering a hydroxylamine or a hydroxylamine salt solution
containing a reduced amount of acid from the catholyte compartment.
U.S. Patent 5,447,610 relates to preparing hydroxylamine and
hydroxylammonium salts by electrolytically reducing a mixture containing
at least one nitrogen oxide and either a neutral electrolyte to form
hydroxylamine or an acidic electrolyte such as an organic or inorganic acid
to form a hydroxylammonium salt. The electrolytic reduction is conducted
in an electrolysis cell containing an anolyte compartment containing an
anode, a catholyte compartment containing a cathode, and a divider
separating the anolyte and catholyte compartments where the mixture of
at least one nitrogen oxide and the electrolyte is present in the catholyte
compartment, and an acid is present in the anolyte compartment.
Summary of the Invention
In one embodiment, the present invention relates to a catalytic film
made by applying an electric current to an electrochemical cell comprising
two electrodes and a solution comprising a film forming compound and a
nitrate ion source.
In another embodiment, the present invention relates to a method of
making a catalytic film comprising: applying an electric current to an
electrochemical cell comprising an anode, a cathode and a solution
comprising a film forming compound and a nitrate ion source thereby
forming the catalytic film.
3
*rB
CA 02301035 2000-02-15
WO 99/09234 PCT/US98/16942
In another embodiment, the present invention relates to a method of
using a catalytic film formed on a cathode made by applying an electric
current to a first electrochemical cell comprising an anode and the cathode
and a film forming solution comprising a film forming compound and a
nitrate ion source, comprising: providing a second electrochemical cell
comprising an anode, the cathode having the catalytic film, and a reactant
solution comprising reactants; applying an electric current to the second
electrochemical cell; and recovering a product from the second
electrochemical cell.
In another embodiment, the present invention relates to a method of
preparing a hydroxytammonium salt, involving the steps of: providing an
electrochemical cell containing an anode, a cathode, and a divider
positioned between the anode and the cathode, to define a catholyte
compartment between the cathode and the divider and an anolyte
compartment between the anode and the divider; charging the catholyte
compartment with a first solution comprising a nitrogen containing
compound and a mediator and the anolyte compartment with a second
solution comprising an ionic compound; passing a current through the
electrochemical cell to produce a hydroxylammonium salt in the catholyte
compartment; and recovering the hydroxylammonium salt from the
catholyte compartment.
In another embodiment, the present invention relates to a method of
making a hydroxylammonium salt by reducing a nitrogen containing
compound, where a mediator is used with the nitrogen containing
compound.
In another embodiment, the present invention relates to a method of
preparing hydroxylamine, involving the steps of: providing an
electrochemical cell containing an anode, a cathode, and a divider
positioned between the cathode and the anode, to define a catholyte
compartment between the cathode and the divider and an anolyte
compartment between the divider and the anode; charging the catholyte
4
CA 02301035 2000-02-15
WO 99/09234 PCT/US98/16942
compartment with a solution comprising a hydroxylammonium salt and a
mediator, and the anolyte compartment with a first electrolyte solution;
passing a current through the electrochemical cell to produce
hydroxylamine in the catholyte compartment; and recovering
hydroxylamine from the catholyte compartment.
In another embodiment, the present invention relates to a method of
making hydroxylamine from a hydroxylammonium salt in an
electrochemical cell, where a mediator is used with the hydroxylammonium
salt.
In another embodiment, the present invention relates to a method of
preparing a hydroxylammonium salt, involving the steps of: providing an
electrochemical cell containing an anode, a cathode, and a divider
positioned between the anode and the cathode, to define a catholyte
compartment between the cathode and the divider and an anolyte
compartment between the anode and the divider, wherein the cathode has
a film thereon formed from a mediator; charging the catholyte
compartment with a first solution comprising a nitrogen containing
compound and the anolyte compartment with a second solution comprising
an ionic compound; passing a current through the electrochemical cell to
produce a hydroxylammonium salt in the catholyte compartment; and
recovering the hydroxylammonium salt from the catholyte compartment.
In one embodiment, the present invention provides inexpensive and
uncomplicated electrochemical methods of efficiently preparing various
compounds of high purity including but not limited to hydroxylammonium
salts, hydroxylamine and adiponitrile.
Brief Description of the Drawinas
Figure 1 is a schematic cross-section of an electrochemical cell
useful in preparing a catalytic film according to the invention.
Figure 2 is a schematic cross-section of an electrochemical cell
useful in preparing a catalytic film according to the invention.
5
CA 02301035 2000-02-15
WO 99/09234 PCT/US98/16942
Figure 3 is a schematic cross-section of an electrochemical cell
useful in preparing a catalytic film according to the invention.
Figure 4 is a schematic cross-section of an electrochemical cell
useful in preparing hydroxylammonium salts and hydroxylamine according
to the invention.
Figure 5 is a schematic cross-section of an electrochemical cell
useful in preparing hydroxylammonium salts and hydroxylamine according
to the invention.
Figure 6 is a schematic cross-section of an electrochemical cell
useful in preparing hydroxylammonium salts and hydroxylamine according
to the invention.
Qescri tip on of_the ereferred Embodiments
In one embodiment, the invention relates to a catalytic film and
methods of forming the catalytic film. The catalytic film is formed on an
electrode, typically in an electrochemical cell containing at least a cathode,
an anode and a solution containing a film forming compound and nitrate
ions. In a preferred embodiment, the catalytic film is formed on a cathode
of an electrochemical cell.
Although not wishing to be bound by any theory, it is believed that
interactions between the film forming compound and nitrate ions form a
catalytic film on an electrode. The chemical identity of the catalytic film
formed on the electrode due to the presence of a film forming compound is
unknown. However, the catalytic film forms substantially uniformly and
smoothly over the electrode. The catalytic film typically is dark orange to
brown in color. The catalytic film is typically solid versus porous. The film
strongly adheres to the electrode. The catalytic film has an apparent
catalytic effect of promoting the conversion of at least one of a nitrogen
containing compound to a hydroxylammonium salt and acrylonitrile to
adiponitrile. Although not wishing to be bound by any theory, it is believed
that the catalytic film may increase the overpotential for hydrogen
6
CA 02301035 2000-02-15
WO 99/09234 PCT/US98/16942
evolution at the cathode thereby promoting the formation of a product,
such as hydroxylammonium salts, hydroxylamine or adiponitrile.
The thickness of the catalytic film formed on the electrode depends
upon various conditions such as the length of time that the film forming
compound and nitrate ions are permitted to interact, the strength of the
electric current, the relative concentrations of the film forming compound
and nitrate ions, and other process parameters. The catalytic film typically
has a thickness of at least about 0.1 nm, and typically from about 0.1 nm
to about 500 ,um. In another embodiment, the catalytic film has a
thickness of at least about 0.5 nm, and typically from about 0.5 nm to
about 100 um. In another embodiment, the catalytic film has a thickness
of at least about 1 nm, and typically from about 1 nm to about 10 ,um.
The catalytic film forms fairly rapidly during the first hour of applied
electric current, and may last (retain apparent catalytic effect) for at least
3 months. In this connection, once an electrode (such as a cathode) has
such a catalytic film formed thereon, it is not necessary to include a film
forming compound in the solutions charged to the electrochemical cell for
chemical processing. In other words, when an electrochemical cell
containing an electrode with such a catalytic film thereon is emptied, the
solution recharged to cell need only contain the reactants for producing a
desired compound.
Mediators or film forming compounds include organic mediators or
organic film forming compounds and inorganic mediators or inorganic film
forming compounds. Organic film forming compounds or organic
mediators include one or more aromatic compounds and heterocyclic
compounds capable of forming a catalytic film in the presence of nitrate
ions. As used herein, the terms film forming compound and mediator are
interchangeable (they refer to the same compounds); however, the term
film forming compound is generally used to indicate the formation of a film
without regard to the use of the film while the term mediator is generally
used to indicate the formation of a film and the simultaneous use of the
7
*rB
CA 02301035 2000-02-15
WO 99/09234 PCT/US98/16942
film to form a final product, such as a hydroxylammonium salt. Preferred
film forming compounds or mediators include amino-aromatic compounds
and quinone compounds. Specific examples of film forming compounds
include 1,4-phenylenediamine; 1,3-phenylenediamine;
tetracyanoquinodimethane; N,N,N',N'-tetramethyl-p-phenylenediamine;
aminophenois such as p-aminophenol, m-aminophenol and o-aminophenol;
aminothiophenols; tetrathiafulvalene; thianthrene; tri-N-p-tolyamine;
ferrocene; methylviologen dichloride hydrate; quinone compounds such as
hydroquinone, aminoanthraquinones, aminoanthraquinone-2-sulfonic acid
sodium salt, anthraquinone-1,5-disulfonic acid disodium salt, and
anthraquinone-2,6-disulfonic acid disodium salt; aniline compounds such as
acetanilide, 4-bromo-2,3,5,6-tetrafluoroaniline, 4,4'-oxydianiline, and 4'-
aminoacetanilide; 1,10-phenanthroline; phenazine; 1,8-
diaminonaphthalene; 1,4-diacetylbenzene; terephthaidicarboxaldehyde;
terephthalic acid; and 2,5-dichloro-1,4-phenylenediamine.
Inorganic mediators or inorganic film forming compounds include
metal mediators and non-organic mediators capable of being reversibly
reduced and oxidized. For instance, inorganic mediators include metals
(represented as Me) having an oxidized and reduced form, such as Mel"+X)+
and Me"+, respectively. Inorganic mediators include at least one of a
cesium compound, a chromium compound, a cobalt compound, a copper
compound, a manganese compound, a periodate compound, a silver
compound, a sodium compound, a tin compound, a titanium compound,
and a zinc compound. Specific examples of inorganic mediators include
Ag2+/Ag+, Ce4+/Ce3+, Co3+/CoZ+, Cra+/Cr2+, Cu2+/Cu+, Mn3+/Mn2+,
Sn2+/Sn4+, Ti3+/Ti4+, Zn2+/Zn , 104 /103 , and Na+/Na(Hg). Inorganic
mediators can be added to an electrochemical cell in metal form (adding
metal powder) or in salt form. Salts of the metals mentioned above are
known, such as acetate, bromide, carbonate, chloride, fluoride, iodide,
nitrate, oxalate, phosphate and sulfate salts (see also the various anions of
8
CA 02301035 2000-02-15
WO 99/09234 PCTlUS98/16942
the hydroxylammonium salts described below), and thus a long list is not
included here.
Determination of whether a prospective compound may be classified
as a film forming compound involves assessing whether a film formed by
the prospective compound in accordance with the invention promotes the
conversion of a reactant compound into a desired compound. In one
embodiment, the prospective compound may be classified as a film
forming compound if it forms a catalytic film and promotes the conversion
of a reactant to a product at a rate faster than the conversion under the
same conditions except that the catalytic film is not used. In another
embodiment, the prospective compound may be classified as a film
forming compound if it forms a catalytic film and promotes the conversion
of a nitrogen containing compound to a hydroxylammonium salt at a rate
faster than the conversion under the same conditions except that the
catalytic film is not used. In yet another embodiment, the prospective
compound may be classified as a film forming compound if it forms a
catalytic film and promotes the conversion of acrylonitrile to adiponitrile at
a rate faster than the conversion under the same conditions except that
the catalytic film is not used.
Nitrate ions may be obtained from one or more nitrate ion sources.
Sources of nitrate ions include nitric acid, alkali metal nitrates such as
sodium nitrate, potassium nitrate and rubidium nitrate, alkaline earth metal
nitrates such as magnesium nitrate, calcium nitrate and strontium nitrate,
transition metal nitrates such as copper nitrate, nickel nitrate, manganese
nitrate, silver nitrate, zinc nitrate, etc., ammonium nitrate, quaternary
ammonium nitrates such as tetramethylammonium nitrate, tetraethylam-
monium nitrate, tetrapropylammonium nitrate, tetrabutylammonium nitrate,
tetra-n-octylammonium nitrate, methyltriethylammonium nitrate,
diethyldimethylammonium nitrate, methyltripropylammonium nitrate,
methyltributylammonium nitrate, cetyltrimethylammonium nitrate,
trimethylhydroxyethylammonium nitrate, trimethylmethoxyethylammonium
9
CA 02301035 2000-02-15
WO 99/09234 PCT/US98/16942
nitrate, dimethyldihydroxyethylammonium nitrate, methyltrihydroxy-
ethylammonium nitrate, phenyltrimethylammonium nitrate, phenyltriethyl-
ammonium nitrate, benzyltrimethylammonium nitrate, and
benzyltriethylammonium nitrate, quaternary phosphonium nitrates such as
tetramethylphosphonium nitrate, tetraethylphosphonium nitrate,
tetrapropylphosphonium nitrate, tetrabutylphosphonium nitrate,
trimethylhydroxyethylphosphonium nitrate, dimethyidihydroxy-
ethylphosphonium nitrate, methyltrihydroxyethylphosphonium nitrate,
phenyltrimethylphosphoniurn nitrate, phenyltriethylphosphonium nitrate and
benzyltrimethylphosphonium nitrate, and tertiary sulfonium nitrates such as
trimethylsulfonium nitrate, triethylsulfonium nitrate, tripropylsulfonium
nitrate, and combinations thereof.
Once the catalytic film is formed on an electrode, typically the
cathode, the electrochemical cell may be emptied, and solutions containing
the reactants of a desired chemical reaction charged to the cell.
Alternatively, the catalytic film coated electrode may be removed from the
cell and transferred to another electrochemical cell where the desired
chemical reaction is carried out. Alternatively, the catalytic film coated
electrode may be used during and after its formation without solution
change by incorporating the reactants of a desired chemical reaction in the
cell with the mediator or film forming compound.
The electrochemical cells suitable for preparing the catalytic film can
assume a number of different configurations. In one embodiment, the
electrochemical cell contains at least one compartment including an anode
and a cathode (see Figure 1). In a preferred embodiment, the
electrochemical cell contains at least two compartments including an
anode, a cathode and a divider (see Figure 2). In another embodiment, the
electrochemical cell contains at least three compartments including an
anode, a cathode, a bipolar membrane and a divider (see Figure 3).
General speaking, the electrochemical cells may be composed of cell
materials which are compatible with the materials being charged into the
CA 02301035 2004-08-04
WO 99/09234 PCT/US98/16942
cells. The cell materials must be particularly able to tolerate an acidic
environment and sometimes a basic environment.
The cells may be adapted to operate at atmospheric pressure or at
elevated pressures. In one embodiment the cell is one capable of operating
at elevated pressures of at least about 1 psig up to about 10 psig or
higher. Since the anode and cathode do not directly enter into the
reaction, they also may be made from a variety of materials that do not
react with the solutions added to the cells or the catalytic films formed in
the cells.
Suitable cathodes may comprise carbon such as graphite, stainless
steel, glassy carbon, titanium, titanium oxide ceramic, niobium, tungsten
carbide, silver, lead, chromium, zinc, mercury, manganese dioxide or
platinum. For example, the cathode may comprise tungsten carbide,
platinum on carbon, silver on carbon, manganese dioxide on carbon, or a
platinized titanium. Graphite or carbon felt may be used with the cathode
to increase the active surface area of the cathode. Cathodes under the
trade designation Ebonex may also be used.
In some embodiments, a gas is introduced into an electrochemical
cell and the cathode is a gas diffusion cathode. The gas-diffusion cathode
may comprise a conventional cathode structure formed of a suitable
porous hydrophobic material such as polytetrafluoroethylene (PTFE), mixed
with carbon black and an optional catalyst. Commercially available gas
diffusion cathodes include an ELAT type gas diffusion cathode having an
integrated stainless steel mesh current collector with an alloy of PtCo on a
hydrophobic PTFE containing Vultan XC-72 carbon and an EFCG type gas
diffusion cathode having an integrated stainless steel mesh current
collector with an alloy of PtCo on a To~y carbon substrate.
Various materials can be used as anodes in the electrochemical cells.
For example, the anode may be made, of metals such as coated titanium
electrodes, tantalum, zirconium, hafnium or alloys of the same. Generally,
the anodes will have a non-passivable and catalytic film which may
=TrA&M8& 11
CA 02301035 2004-08-04
WO 99/09234 PCT/US98/16942
comprise metatiic noble metals such as platinum, iridium, rhodium,
ruthenium or alloys thereof, or a mixture of electroconductive oxides
containing at least one oxide or mixed oxides of a noble metal such as =
platinum, iridium, ruthenium, palladium or rhodium. In one embodiment,
the anode is a dimensionally stable anode such as an anode having a titanium
base with ruthenium and/or iridium oxides thereon.
Most of the electrochemical cells utilized in making and using the
catalytic film of the present invention contain at least one divider or
separator, such as ionic or nonionic selective membranes. The dividers
and/or bipolar membranes function as diffusion barriers and/or gas
separators.
In one embodiment, the dividers or separators which can be utilized
in the present invention can be selected from a wide variety of
microporous diffusion barriers, screens, filters, diaphragms, etc., which
contain pores of the desired size allow anions and/or cations of various
chemical compounds to migrate toward one of the electrodes. The
microporous dividers can be prepared from various materials including
plastics such as polyethylene, polypropylene and Teflon, ceramics, etc.
Microporous dividers such as nonionic dividers can be used, for example,
in addition to the dividers listed in the Figures. Specific examples of
commercially available microporous separators include: Celanese Ceigard
and Norton itex.
In one embodiment, the divider is an anion selective membrane.
Any anion selective membrane may be utilized including membranes used
in processes for the desalination of brackish water. Preferably, anion
selective membranes should be selective with respect to the particular
anions present in the cell (e.g., nitrate and halide ions). The preparation
and structure of anionic membranes are described in the chapter entitled
"Membrane Technology" in Encyclopedia of Chemical Technology, Kirk-
Othmer, Third Ed., Vol. 15, pp. 92-131, Wiley & Sons, New York, 1985.
12
iTodemak
CA 02301035 2004-08-04
WO 99/09234 PCTlUS98/16942
Among the anion selective membranes which may be utilized and
which are commercially available are the following: AMFL~ON, Series 310,
based on fluorinated polymer substituted with quaternary ammonium
groups produced by American Machine and Foundry Company; IONAC MA
3148, MA 3236 and MA 3475, based on polymer substituted with
quaternary ammonium derived from heterogenous polyvinyfchloride
produced by Ritter-Pfaulder Corp., Permutit Division; Tosfle3c IE-SF 34 or
IE-SA 48 made by Tosoh Corp. which is a membrane designed to be stable
in alkaline media; NEOSEPTA AMH, NEOSEPTA ACM, NEOSEPTA AFN or
NEOSEPTA ACLE-SP from Tokuyama Soda Co.; and Selemion AMV and
Selemio AAV from Asahi Glass.
In one embodiment, the divider is a cation selective membrane. The
cation selective membranes used in the cells and the process of the
invention may be any of those which have been used in the
electrochemical purification or recycling of chemical compounds.
Preferably, the cation-exchange membranes should contain a highly durable
material such as the membranes based on the fluorocarbon series, or from
less expensive materials of the polystyrene or polypropylene series.
Preferably, however, the cation selective membranes useful in the present
invention include fluorinated membranes containing cation selective groups
such as perfluorosulfonic acid and perfluorosulfonic and/perfluorocarboxylic
acid, perfluorocarbon polymer membranes such as sold by the E. 1. dupont
Nemours & Co. under the general trade designation "Nafion such as
DuPont's Cationic Nafion 423 and 902 membrane. Other suitable cation
selective membranes include styrenedivinyl benzene copolymer membranes
containing cation selective groups such as sulfonate groups, carboxylate
groups, etc. Raiporeationic R1010, (from Pall RAI), and NEOSEPTA
CMH and NEOSEPTA CM1 membranes from Tokuyama Soda are useful
particularly with the higher molecular compounds. The preparation and
*TtademartC 13
CA 02301035 2004-08-04
WO 99/09234 PCT/US98/16942
structure of cation selective membranes are described in the chapter
entitled "Membrane Technology" in Encyclopedia of Chemical Technology,
Kirk-Othmer, Third Ed., Vol. 15, pp. 92-131, Wiley & Sons, New York,
1985.
The bipolar membranes used in the electrochemical celis are
composite membranes containing three parts: a cation selective side or
region, an anion selective side or region, and an interface between the two
regions. When a direct current passes across a bipolar membrane, with
the cation selective side toward or facing the cathode, electrical
conduction is achieved by the transport of H+ and OH" ions which are
produced by the dissociation of water which occurs at the interface under
the influence of an electrical field. Bipolar membranes are described, for
example, in U.S. Patents 2,829,095, 4,024,043 (single film bipolar
membranes) and in 4,116,889 (cast bipolar membranes). The bipolar
membranes useful in the present invention include NEOSEPTA BIPOLAR 1
by Tokuyama Soda, WSI B1PO~ R, and Aqualytics Bipolar membranes.
In one embodiment, the electrochemical cells contain at least one
compartment. In a preferred embodiment, the electrochemical cells
contain at least two compartments; namely, a catholyte compartment and
an anolyte compartment. In another embodiment, the electrochemical cells
- contain at least three compartments; namely, a catholyte compartment, an
anolyte compartment and another compartment such as a buffer
compartment, a pass compartment, a base compartment, an acid
compartment, and the like. Buffer compartments typically are positioned
between two bipolar membranes or a bipolar membrane and an electrode.
A pass compartment is typically positioned between two cation selective
membranes or two anion selective membranes and serve to further purify
the final product. Bases and acids are typically formed in the base
compartment and acid compartment, respectively.
=~~ 14
CA 02301035 2000-02-15
WO 99/09234 PCT/US98/16942
The catholyte compartment (or the compartment of a one
compartment cell) of the electrochemical cells (generally next to the
cathode) contains a solution of a film forming compound and nitrate ions.
Aqueous solutions are preferred. In one embodiment, the concentration of
the film forming compound may be from about 1 mM to about 1 M. In
another embodiment, the film forming compound concentration is from
about 5 mM to about 500 mM. In yet another embodiment, the film
forming compound concentration is from about 10 mM to about 100 mM.
In one embodiment, the concentration of the nitrate ion source may be
from about 0.001 M to about 10 M. In another embodiment, the nitrate
ion source concentration is from about 0.01 M to about 1 M. In yet
another embodiment, the nitrate ion source concentration is from about
0.1 M to about 0.5 M.
The anolyte compartment as well as the remaining compartments, if
present, of the electrochemical cells (generally next to the anode) contain a
solution of an ionic compound (an electrolyte solution). An ionic
compound is any compound that fully or partially ionizes in solution. Ionic
compounds include acids, bases, and salts. Aqueous solutions are
preferred. The ionic compound in the anolyte compartment may be the
same or different from the ionic compound in any other compartment.
Any suitable ionic compound can be used in the anolyte and other
compartments, but in a preferred embodiment, the ionic compound in the
anolyte and other compartments is an acid or a nitrate ion source. The
concentration of the ionic compound in the anolyte and other compartment
is from about 0.1 M to about 10 M, and preferably from about 2 M to
about 6 M. The concentration of the ionic compound in the anOlyte
compartment may be the same, higher or lower than the concentration of
the ionic compound in the other compartments.
The electric current applied between the anode and cathode depends
upon how many, if any, dividers are positioned between the anode and
cathode and the concentrations of components. In one embodiment, a
CA 02301035 2000-02-15
WO 99/09234 PCT/US98/16942
current density is applied between the anode and cathode with an apparent
current density of about 0.01 ASI (amps per square inch) to about 10 ASI,
more often from about 1 ASI to 5 ASI at about 1 volt to about 10 volts
and about 2 volts to about 5 volts, respectively. The current is applied to
the electrochemical cell for a period of time effective to produce the
catalytic film on the cathode in the catholyte compartment (or the
compartment of a one compartment cell) at a desired thickness.
The electrochemical cell may be maintained at a temperature
suitable for the production of the catalytic film. The temperature is
typically from about -20 C to about 70 C. In another embodiment, the
temperature is from about 1 C to about 30 C. Formation of the catalytic
film may be monitored by visual observation.
Examples of electrochemical cells useful in the present invention are
discussed below and shown in Figures 1, 2 and 3.
Referring to Figure 1, the electrochemical cell 10 is made of a
cathode 11 and an anode 12. The electrochemical cell 10 contains one
compartment 13. In operation of the electrochemical cell illustrated in
Figure 1, a solution containing a film forming compound and a nitrate ion
source is charged to the compartment 13. An electrical potential is
established and maintained between the anode and the cathode to produce
a flow of current across the electrochemical cell whereupon a catalytic film
is produced on the cathode 11 in the compartment 13.
Referring to Figure 2, the electrochemical cell 20 is made of a
cathode 21, an anode 22, and a divider 23. The electrochemical cell 20
contains two compartments; namely, a catholyte compartment 24 and an
anolyte compartment 25. In operation of the electrochemical cel4
illustrated in Figure 2, a solution containing a film forming compound and a
nitrate ion source is charged to the catholyte compartment 24. An
electrolyte solution containing an ionic compound is charged to the anolyte
compartment 25. An electrical potential is established and maintained
between the anode and the cathode to produce a flow of current across
16
CA 02301035 2000-02-15
WO 99/09234 PCT/US98/16942
the electrochemical cell whereupon a catalytic film is produced on the
cathode 21 in the catholyte compartment 24.
Referring to Figure 3, the electrochemical cell 30 is made of a
cathode 31, an anode 32, and in sequence beginning at the cathode 31, a
bipolar membrane 33 and a divider 34. The bipolar membrane 33 has an
anion selective side (not shown) facing the anode and a cation selective
side (not shown) facing the cathode. The electrochemical cell 30 contains
three compartments; namely, a catholyte compartment 35, a middle
compartment 36, and an anolyte compartment 37. In operation of the
electrochemical cell illustrated in Figure 3, a solution containing a film
forming compound and a nitrate ion source is charged to the catholyte
compartment 35. A solution containing an ionic compound is charged to
the middle compartment 36 and the anolyte compartment 37. The ionic
compound of the middle compartment is the same or different than the
ionic compound in the anolyte compartment. An electrical potential is
established and maintained between the anode and the cathode to produce
a flow of current across the electrochemical cell whereupon a catalytic film
is produced on the cathode 31 in the catholyte compartment 35.
The following specific examples further illustrate the preparation of
the catalytic film according to the present invention. Unless otherwise
indicated in the examples and elsewhere in the specification and claims, all
parts and percentages are by weight, temperatures are in degrees
centigrade, and pressures are at or near atmospheric pressure.
Example 1
An electrochemical cell according to Figure 2 is provided containing
an anode made of ruthenium oxide coated titanium, a cathode rt-iade of
graphite, and a Nafion 423 cation selective membrane as the divider. A
solution containing 1 M nitric acid and 50 mM 1,4-phenylenediamine is
charged to the catholyte compartment. A solution of 4 M nitric acid is
charged to the anolyte compartment. Nitric acid is added to the catholyte
compartment to maintain the nitric acid concentration between 0.5 M and
17
CA 02301035 2000-02-15
WO 99/09234 PCT/US98/16942
1 M under application of an electrical current. While maintaining the
temperature between 5 C and 10 C, a current of 5 Amps (0.3 ASI) and a
cell voltage of about 3.5 volts is applied for 6 hours. The catholyte is
stirred under application of the current. A deep orange to brown colored
film uniformly forms over the cathode.
Example 2
An electrochemical cell according to Figure 3 is provided containing
an anode made of ruthenium oxide coated titanium, a stainless
steel cathode, a Tokuyama Bipolar 1 bipolar membrane, a Asahi glass AAV
anion selective membrane as the divider. A solution of 0.5 M nitric acid is
charged to the middle compartment, a solution of 0.3 M nitric acid is
charged to the anolyte compartment, and a solution of 1.7 M
hydroxylamine nitrate, 0.7 M nitric acid and 50 mM of 1,4-
phenylenediamine is charged to the catholyte compartment. While
maintaining the temperature between 5 C and 10 C, a current of 5 Amps
and a cell voltage of about 9.1 volts is applied for 2 hours. The catholyte
is stirred under application of the current. A deep orange to brown colored
film uniformly forms over the cathode.
Exam le3
An electrochemical cell according to Figure 2 is provided containing
an anode made of ruthenium oxide coated titanium, a cathode made of
graphite, and a Nafion 423 cation selective membrane as the divider.
Graphite felt is attached to the graphite cathode to enhance the active
cathode surface area. A solution containing 1 M nitric acid and 50 mM to
100 mM anthraquinone-2,6-disulfonic acid disodium salt is charged to the
catholyte compartment. A solution of 4 M nitric acid is charged to the
anolyte compartment. Nitric acid is added to the catholyte compartment
to maintain the nitric acid concentration between 0.5 M and 1 M under
application of an electrical current. While maintaining the temperature
between 5 C and 10 C, a current of 15 Amps (1 ASI) and a cell voltage of
about 3.5 volts is applied for 24 hours. The catholyte is stirred under
18
CA 02301035 2000-02-15
WO 99/09234 PCT/US98/16942
application of the current. A deep orange to brown colored film uniformly
forms over the cathode.
Exam Ig e 4
An electrochemical cell according to Figure 2 is provided containing
an anode made of ruthenium oxide coated titanium, a cathode made of
graphite, and a Nafion 423 cation selective membrane as the divider.
Graphite felt is attached to the graphite cathode to enhance the active
cathode surface area. A solution containing 1 M nitric acid and 50 mM to
400 mM 4,4'-oxydianiline is charged to the catholyte compartment. A
solution of 4 M nitric acid is charged to the anolyte compartment. Nitric
acid is added to the catholyte compartment to maintain the nitric acid
concentration between 0.5 M and 1 M under application of an electrical
current. While maintaining the temperature between 5 C and 10 C, a
current of 30 Amps (2 ASI) and a cell voltage of about 6 volts is applied
for 8 hours. The catholyte is stirred under application of the current. A
deep orange to brown colored film uniformly forms over the cathode.
Example 5
The general procedure of Example 1 is repeated except that a piece
of graphite felt is attached to the graphite cathode to enhance the cathode
surface area. A solution containing 0.5 M nitric acid and 50 mM 1,4-
phenylenediamine is charged to the catholyte compartment. Concentrated
nitric acid is added to the catholyte compartment to maintain the nitric acid
concentration between 0.5 M and 1 M under application of an electrical
current. While maintaining the temperature between 5 C and 10 C, a
current of 20 amps (5 ASI) and a cell voltage of about 5.5 volts is applied
for 16 hours. The catholyte is stirred under application of the current. A
deep orange to brown colored film uniformly forms over the cathode.
Exapl l~e 6
The general procedure of Example 1 is repeated except that a
solution containing 1.0 M nitric acid and 70 ppm p-aminophenol is charged
to the catholyte compartment. A solution of 4 M nitric acid is charged to
19
CA 02301035 2000-02-15
WO 99/09234 PCT/US98/16942
the anolyte compartment. Concentrated nitric acid is added to the
catholyte compartment to maintain the nitric acid concentration between
0.5 M and 1 M under application of an electrical current. While
maintaining the temperature between 5 C and 10 C, a current of 20 amps
(5 ASI) and a cell voltage of about 5 volts is applied for 30 hours. The
catholyte is stirred under application of the current. A deep orange to
brown colored film uniformly forms over the cathode.
Example 7
The general procedure of Example 1 is repeated except that a
solution of 1 M nitric acid and 100 ppm hydroquinone is charged to the
catholyte compartment. A solution of 4 M nitric acid is charged to the
anolyte compartment. Concentrated nitric acid is added to the catholyte
compartment to maintain the nitric acid concentration between 0.5 M and
1 M under application of an electrical current. While maintaining the
temperature between 5 C and 10 C, a current of 20 amps (5 ASI) and a
cell voltage of about 6.5 volts is applied for 1 hour. The catholyte is
stirred under application of the current. A deep orange to brown colored
film uniformly forms over the cathode.
Exam ip e 8
An electrochemical cell according to Figure 2 is provided containing
an anode made of ruthenium oxide coated titanium, a cathode made of
graphite, and a Nafion 423 cation selective membrane as the divider. A
solution containing 1 M sodium nitrate and 50 mM 1,4-phenylenediamine
is charged to the catholyte compartment. A solution of 4 M nitric acid is
charged to the anolyte compartment. Nitric acid is added to the catholyte
compartment to maintain the sodium nitrate concentration between 0.5 M
and 1 M under application of an electrical current. While maintaining the
temperature between 5 C and 10 C, a current of 5 Amps (0.3 ASI) and a
cell voltage of about 3.5 volts is applied for 6 hours. The catholyte is
stirred under application of the current. A deep orange to brown colored
film uniformly forms over the cathode.
CA 02301035 2000-02-15
WO 99/09234 PCT/US98/16942
Example 9
An electrochemical cell according to Figure 1 is provided containing
an anode made of ruthenium oxide coated titanium and a cathode made of
graphite. A solution containing 1 M tetrabutylammonium nitrate and 50
mM 1,4-phenylenediamine is charged to the compartment. Nitric acid is
added to the compartment to maintain the tetrabutylammonium nitrate
concentration between 0.5 M and 1 M under application of an electrical
current. While maintaining the temperature between 5 C and 10 C, a
current of 5 Amps (0.3 ASI) and a cell voltage of about 3.5 volts is applied
for 6 hours. The compartment is stirred under application of the current.
A deep orange to brown colored film uniformly forms over the cathode.
Using the cathode having a catalytic film thereon, the synthesis of
various compounds is facilitated. For example, the conversion of a
nitrogen containing compound to a hydroxylammonium salt, the conversion
of a hydroxylammonium salt to hydroxylamine, and the conversion of
acrylonitrile to adiponitrile are facilitated by the catalytic film of the
present
invention.
Generally speaking, an electrochemical cell containing an electrode
having a catalytic film thereon is used to facilitate the synthesis of various
compounds. In a preferred embodiment, the electrode is a cathode. The
synthesis of various compounds may be carried out in the electrochemical
cell in which the catalytic film is formed, or an electrode on which the
catalytic film is formed may be transferred to another electrochemical cell.
Any electrochemical cell suitable for the synthesis of a particular
compound may be equipped with an electrode having a catalytic film
thereon. For example, the electrochemical cells of Figures 2 and 3 are
suitable for making a hydroxylammonium salt and adiponitrile.
The electrochemical cells can be operated batchwise or in a
continuous operation. Circulation is effected by pumping and/or by gas
evolution. In one embodiment, the concentration of ionic compound in the
catholyte, anolyte and/or recovery compartments is maintained at a
21
CA 02301035 2000-02-15
WO 99/09234 PCT/US98/16942
substantially constant concentrations by the monitoring and employment
of feeds into the compartments, such as a water feed into the anolyte
compartment.
In one embodiment of the invention, the catalytic film is used to
electrochemically convert a nitrogen containing compound to a hydroxyl-
ammonium salt or convert a hydroxylammonium salt to hydroxylamine. In
particular, the nitrogen containing compound is reduced to a
hydroxylammonium salt in the presence of a film formed by the film
forming compound on a cathode. Referring to Figure 2, a solution
containing a nitrogen containing compound is charged to the catholyte
compartment 24. An electrolyte solution containing an ionic compound is
charged to the anolyte compartment 25. In a preferred embodiment, the
ionic compound is an acid. In this embodiment, the divider 23 is preferably
a cation selective membrane. In some embodiments, additional dividers
may be used in the cell, but they are not generally required. An electrical
potential is established and maintained between the anode and the cathode
to produce a flow of current across the electrochemical cell whereupon a
hydroxylammonium salt is produced in the catholyte compartment 24. A
hydroxylammonium salt is recovered from the catholyte compartment 24.
The hydroxylammonium salt (or hydroxylamine described below) may be
purified by further treatment using one or more of distillation, reverse
osmosis, electrodialysis and ion exchange techniques.
Ion exchange techniques, using cation exchange resins and anion
exchange resins, are known to those skilled in the art. Distillation
techniques are known by those skilled in the art. For example, the
hydroxylamonium salt solution obtained from the catholyte corripartment
can be further purified using vacuum distillation.
Reverse osmosis membranes are available from Fluid Systems,
Filmtech, Osmonics, Inc., Desalination Systems Inc., and others. Specific
examples include Fluid Systems TFCL-HP thin film composite membrane.
Reverse osmosis membrane technology is known by those skilled in the
22
CA 02301035 2000-02-15
WO 99/09234 PCT/US98/16942
art. For example, the hydroxylamine solution obtained from the catholyte
compartment containing hydroxylammonium salts is sent through a reverse
osmosis membrane (for instance, polyamide based membrane) under high
pressure (over 100 and often over 500 psi). Some compounds pass
through the membrane whereas the hydroxylammonium salts do not.
Reverse osmosis membranes generally permit water and small molecular
weight organics (such as hydroxylamine) to pass through while not
permitting ionic compounds to pass.
The hydroxylammonium salt solution obtained from the catholyte
compartment can be further purified using electrodialysis in an
electrodialytic cell. Electrodialytic techniques are known by those skilled in
the art.
These additional procedures are effective for removing impurities
that may be present in the solution obtained from the compartments. The
impurities include undesirable salts, ammonium ions, metals and organic
materials.
In embodiments where hydroxylammonium salt is produced in the
catholyte compartment, a current is applied between the anode and
cathode with an apparent current density of about 0.1 ASI (amps per
square inch) to about 10 ASI, more often from about 2 ASI to 4 ASI at
about 3 volts to about 4 volts. The current is applied to the
electrochemical cell for a period of time effective to produce the
hydroxylammonium salt in the catholyte compartment.
The concentration of nitrogen containing compound in the catholyte
compartment may be from about 0.01 M to about 10 M. Preferably the
nitrogen containing compound concentration is from about 0.5 M to about
1 M. The concentration of the ionic compound in the anolyte
compartment may be from about 0.01 M to about 5 M. Preferably the
acid concentration is from about 0.5 M to about 1 M.
Nitrogen containing compounds are compounds containing at least
one atom of nitrogen and which are capable of being converted to a
23
CA 02301035 2000-02-15
WO 99/09234 PCT/US98/16942
hydroxylammonium salt in accordance with the present invention.
Examples of nitrogen containing compounds include nitric acid, alkali metal
nitrates such as sodium nitrate and potassium nitrate, alkaline earth metal
nitrates such as magnesium nitrate and calcium nitrate, alkali nitrites such
as sodium nitrite and potassium nitrite, alkaline earth metal nitrites,
nitrides
such as calcium nitride and magnesium nitride, organo-nitro compounds
such as nitromethane, nitroethane, nitropropane, nitrobutane,
nitrobenzene, etc., and nitrogen containing gases.
A nitrogen containing gas as used herein includes any gas containing
an atom of nitrogen. Examples of nitrogen containing gas include nitrogen
oxide gas and nitrogen-hydrogen gas. Nitrogen oxide gas as used herein is
intended to mean a gas containing nitrogen and oxygen atoms. Examples
of nitrogen oxide gas include one or more of nitric oxide (NO), nitrogen
dioxide (NO2), nitrogen trioxide (NO3), dinitrogen trioxide (N203), dinitrogen
pentoxide N205. Nitrogen-hydrogen gas includes ammonia, hydrazine, and
derivatives thereof. Nitrogen containing gas may also be any gas
containing at least a nitrogen containing gas, for instance, a mixture of one
or more inert gases and nitrogen oxide gas. Inert gases include nitrogen
and the noble gases. The noble gases include helium, neon, argon,
krypton, xenon and radon.
In embodiments where a gas is introduced into an electrochemical
cell, such as a nitrogen containing gas in a process for making a
hydroxylammonium salt, the cathode is a gas diffusion cathode. In these
embodiments, the electrochemical cell contains a gas chamber next to the
gas diffusion cathode. A nitrogen containing gas is injected into the gas
chamber and then forced through the gas diffusion cathode into the
catholyte compartment. Such methods are described in U.S. Patent
5,447,610 and U.S. Patent Application Serial No. 08/734,858, both of
which are hereby incorporated by reference. In one embodiment, the
cathode may contain a material which exhibits electrocatalytic activity for
24
CA 02301035 2000-02-15
WO 99/09234 PCT/US98/16942
nitrogen oxide reduction to hydroxylamine or hydroxylammonium salts.
The hydroxylammonium salts which can be produced in the
electrochemical cells from nitrogen containing compounds in accordance
with the process of the present invention may be represented by the
formula
(NRZHOH)+,,X-''
wherein each R is independently hydrogen or a hydrocarbon group
containing from 1 to about 8 carbon atoms, preferably 1 to about 6 carbon
atoms, X is an anion of an acid, such as any of the acids described above,
and y is a number equal to the valence of X. Specific examples of anions
include Cl-, Br-, SO4 2, HS04 , N03 , PO4 3, H2P041, HP04 2, etc.
Specific examples of hydroxylammonium salts which can be
prepared in accordance with this invention include hydroxylammonium
sulfate, hydroxylammonium nitrate, hydroxylammonium chloride,
hydroxylammonium bromide, hydroxylammonium fluoride,
hydroxylammonium formate, hydroxylammonium acetate,
hydroxylammonium phosphate, hydroxylammonium methylsulfonate,
hydroxylammonium toluene sulfonate, methylhydroxylammonium nitrate,
ethylhydroxylammonium nitrate, propylhydroxylammonium nitrate,
isopropylhydroxylammonium nitrate, and diethyihydroxylammonium nitrate,
phenylhydroxylammonium nitrate, etc.
The concentration of hydroxylammonium salt formed in the
catholyte compartment may be from about 0.1 M to about 10 M.
Preferably the hydroxylammonium salt concentration in the catholyte
compartment is from about 0.5 M to about 2 M.
In one embodiment, the ionic compound is an acid and.a solution of
the acid is an acidic electrolyte. An acid lowers the pH of a neutral
CA 02301035 2000-02-15
WO 99/09234 PCT/US98/16942
solution. Acids include organic and inorganic acids. Preferably, the acid is
not reactive at the cathode.
Specific examples of inorganic acids represented by formula HYX
which may be utilized in the acidic electrolyte with the nitrogen containing
compound include at least one of nitric acid, halogen acids such as
hydrofluoric acid, hydrochloric acid, hydrobromic acid and hydriodic acid,
sulfuric acid, sulfurous acid, perchloric acid, boric acid and phosphorus
acids such as phosphorous acid and phosphoric acid. Nitric acid and
sulfuric acid are preferred inorganic acids. Nitric acid and any other acid
are preferred combinations of acids. Examples of organic acids
represented by the formula H,,X include carboxylic and polycarboxylic acids
such as formic acid, acetic acid, propionic acid, citric acid, oxalic acid,
etc.; organic phosphorus acids such as dimethyiphosphoric acid and
dimethylphosphinic acid; or sulfonic acids such as methanesulfonic acid,
ethanesulfonic acid, 1 -pentanesulfonic acid, 1 -hexanesulfonic acid, 1-
heptanesulfonic acid, benzenesulfonic acid, toluenesulfonic acid, etc.
Nitric acid and any other acid are preferred combinations of acids.
In one embodiment, the ionic compound is a base and a solution of
the base is a basic electrolyte. A base increases the pH of a neutral
solution. Bases include organic and inorganic bases.
Bases include alkali metal and alkaline earth metal hydroxides,
silicates, phosphates, borates, carbonates, and mixtures thereof. For
example, the basic compound includes alkali metal hydroxides, alkaline
earth metal hydroxides, alkali metal silicates and so on. Alkali metals
include lithium, sodium, potassium, rubidium and cesium. Alkaline earth
metals include beryllium, magnesium, calcium, strontium, and barium.
Specific bases include sodium tetraborate, sodium carbonate, sodium
bicarbonate, sodium hydroxide, sodium phosphate, sodium pyrophosphate
and other polyphosphates, sodium silicate, potassium carbonate,
potassium bicarbonate, potassium hydroxide, potassium phosphate,
potassium pyrophosphate and other polyphosphates, calcium carbonate,
26
CA 02301035 2000-02-15
WO 99/09234 PCT/US98/16942
calcium hydroxide, calcium phosphate, calcium pyrophosphate, calcium
silicate, magnesium carbonate, magnesium hydroxide, magnesium
phosphate, magnesium pyrophosphate, and magnesium silicate.
Examples of electrochemical cells useful in the present invention are
discussed below and shown in Figures 1, 2, 3, 4, 5 and 6.
As needed, various compounds such as one or more acids, water,
one or more ionic compounds, nitrogen containing compounds, stabilizers,
hydrogen suppressors and the like may be added or recovered from the
catholyte, anolyte and other compartments in order to maintain efficient
operation of the electrochemical cell. For example, nitrogen containing
compound must be continuously or intermittently added to the catholyte
compartment. From time to time, it may also be necessary to
intermittently or continuously remove acid from the anolyte compartment.
In one embodiment, the solutions charged to the compartments
where a hydroxylammonium salt (or adiponitrile as described below) is
produced may also optionally contain a hydrogen suppressor. Hydrogen
suppressors include thio compounds such as thiourea, and quaternary
ammonium salts such as quaternary alkyl ammonium chlorides, nitrates,
sulfates, bromides, phosphates, carbonates and bicarbonates. Specific
quaternary alkyl ammonium ions include quaternary methyl ammonium,
quaternary ethyl ammonium, quaternary propyl ammonium, quaternary
butyl ammonium, dimethyidiethyl ammonium, methyltriethyl ammonium,
and so on. In one embodiment, the amount of hydrogen suppressor in the
solution may range from about 0.00 1 % to about 10% by weight of the
solution. In another embodiment, the amount of hydrogen suppressor in
the solution may range from about 0.01 % to about 1 % by weight of the
solution.
In another embodiment, the solutions charged to the compartments
where a hydroxylammonium salt is produced may also optionally contain a
stabilizer. In some instances, a stabilizer inhibits the decomposition of
hydroxylammonium salt. Examples of stabilizers include quinoline
27
CA 02301035 2000-02-15
WO 99/09234 PCT/US98/16942
derivatives, thiocarboxylic acids, thiosulfates, hydroxy anthraquinone, etc.
Specific examples include 8-hydroxyquinoline, morin hydrate and quercetin.
The amount of stabilizer in the solution may range from about 5x10'4% to
about 1 % by weight based on the weight of electrolytes present.
In one particular embodiment where hydroxylammonium salts are
produced (starting with a solution containing a nitrogen containing
compound and a mediator or an electrode having a mediator formed film
thereon), the electrochemical cell contains an anode, a cathode, and a
divider (see Figure 2). In this embodiment, the divider is preferably a
cation selective membrane. In some embodiments, additional dividers may
be used in the cell, but they are not generally required.
In one particular embodiment where hydroxylamine is produced
(starting with a solution containing a hydroxylammonium salt and a
mediator or an electrode having a mediator formed film thereon), the
electrochemical cell contains an anode, a cathode, a bipolar membrane,
and a divider (see Figure 3). In this embodiment, the divider is preferably
an anion selective membrane. In some embodiments, additional dividers
may be used in the cell, but they are not generally required.
For instance, in another particular embodiment where hydroxylamine
is produced (starting with a solution containing a hydroxylammonium salt
and a mediator or an electrode having a catalytic film thereon), the
electrochemical cell contains an anode, a cathode, a bipolar membrane,
and two dividers (see Figure 4). In this embodiment, the two dividers
include an anion selective membrane next to the anode and a cation
selective membrane next to the bipolar membrane.
In one particular embodiment where hydroxylamine is produced
(starting with a solution containing a hydroxylammonium salt and a
mediator or an electrode having a mediator formed film thereon), the
electrochemical cell contains an anode, a cathode and a divider (see Figure
5). In this embodiment, the divider is preferably an anion selective
membrane and the cathode is preferably a gas diffusion cathode.
28
CA 02301035 2000-02-15
WO 99/09234 PCT/US98/16942
In a particular embodiment where both a hydroxylammonium salt
and hydroxylamine are produced in a single cell, the electrochemical cell
contains an anode, a cathode, a bipolar membrane and a divider (see Figure
6). In this embodiment, the divider is preferably an anion selective
membrane.
Accordingly, methods of making hydroxylammonium salts from a
nitrogen containing compound involve the use of one electrochemical cell,
while methods of making hydroxylamine from a nitrogen containing
compound via a hydroxylammonium salt involve the use of one or at least
two electrochemical cells. In embodiments where two electrochemical
cells are used to make hydroxylamine, a hydroxylammonium salt is made in
a first electrochemical cell (such as the cell in Figure 2) and hydroxylamine
is made in a second electrochemical cell (such as the cell in Figures 3, 4 or
5).
In embodiments where only hydroxylammonium salts are produced,
hydroxylammonium salts and hydroxylamine are produced, or cells the
same as or analogous to the electrochemical cells of Figures 2 and 3 are
used, the catholyte compartment contains a solution of a nitrogen
containing compound and a mediator and optionally an acid. In
embodiments where the cathode of the electrocherriical cell has a mediator
formed film thereon, the catholyte compartment contains a solution of a
nitrogen containing compound and optionally an acid (the mediator may be
omitted because of the film). The choice of acid is determined by the
particular hydroxylammonium salt desired to be produced. The acid may
contain the anion of the desired hydroxylammonium salt. The
concentration of nitrogen containing compound may be from about 0.01 M
to about 10 M. Preferably the nitrogen containing compound
concentration is from about 0.5 M to about 1 M. The concentration of the
mediator, when present, may be from about 1 mM to about 1 M.
Preferably the mediator concentration, when present, is from about 10 mM
to about 100 mM. The concentration of acid may be from about 0.01 M
29
CA 02301035 2000-02-15
WO 99/09234 PCT/US98/16942
to about 5 M. Preferably the acid concentration is from about 0.5 M to
about 1 M.
In embodiments where only hydroxylamine is intended to be
produced or cells the same as or analogous to the electrochemical cells of
Figures 3 or 4 are used, the catholyte compartment contains a solution of
an ionic compound (an electrolyte solution). Any ionic compound can be
used in the catholyte compartment, but in a preferred embodiment, the
ionic compound in the catholyte compartment is a base. In these
embodiments, the concentration of the ionic compound in the catholyte
compartment is from about 0.01 M to about 10 M, and preferably from
about 0.1 M to about 1 M. The concentration of the ionic compound in
the catholyte compartment may be the same, higher or lower than the
concentration of the ionic compound in the other compartments, where
present.
In embodiments where only hydroxylamine is intended to be
produced or cells the same as or analogous to the electrochemical cell of
Figure 5 is used, the catholyte compartment contains a solution of a
hydroxylammonium salt and a mediator. In other embodiments where only
hydroxylamine is intended to be produced or cells the same as or
analogous to the electrochemical cell of Figure 5 is used and the cathode
of the electrochemical cell has a mediator formed film thereon, the
catholyte compartment contains a solution of a hydroxylammonium salt.
The concentration of hydroxylammonium salt may be from about 0.1 M to
about 10 M. Preferably the hydroxylammonium salt concentration is from
about 0.5 M to about 2 M. The concentration of the mediator, when
present, may be from about 1 mM to about 1 M. Preferably the mediator
concentration, when present, is from about 10 mM to about 100 mM.
The recovery compartment of the electrochemical cell (generally a
middle compartment and/or next to a bipolar membrane) initially contains a
solution optionally containing an ionic compound. The ionic compound in
the recovery compartment may be the same or different from the ionic
CA 02301035 2000-02-15
WO 99/09234 PCT/US98/16942
compounds in the other compartments, where present. The concentration
of the ionic compound in the recovery compartment is from about 0.01 M
to about 10 M, and preferably from about 0.1 M to about 0.5 M. The
concentration of the ionic compound in the recovery compartment may be
the same, higher or lower than the concentration of the ionic compound in
the other compartments. In some embodiments (see Figure 3 for
instance), the recovery compartment is charged with a solution of a
hydroxylammonium salt and a mediator (when there is no mediator fromed
film on the cathode). The concentration of hydroxylammonium salt may
be from about 0.1 M to about 10 M. Preferably the hydroxylammonium
salt concentration is from about 0.5 M to about 2 M. The concentration of
the mediator, when present, may be from about 1 mM to about 1 M.
Preferably the mediator concentration, when present, is from about 10 mM
to about 100 mM. In the embodiment of Figure 4, the feed compartment
(generally a middle compartment) is charged with a solution of a
hydroxylammonium salt and a mediator (same concentrations as above),
when present, and the recovery compartment contains a solution with an
optional ionic compound.
The concentration of the hydroxylammonium salt produced in the
catholyte compartment is from about 0.1 M to about 10 M, and preferably
from about 0.5 M to about 2 M. A portion of the hydroxylammonium salt
produced in the catholyte compartment is then either recovered or
physically transferred to another electrochemical cell or a recovery
compartment of the same cell (see, for example, Figure 6). This may be
accomplished on an intermittent or continuous basis by methods known to
those skilled in the art. The concentration of hydroxylamine produced in
the recovery compartment is from about 0.1 M to about 16 M, and
preferably from about 2 M to about 5 M.
Referring to Figure 6, the electrochemical cell 60 is made of a
cathode 61, an anode 62, and in sequence beginning at the cathode 61, a
bipolar membrane 63 and a divider 64. In a preferred embodiment, the
31
CA 02301035 2000-02-15
WO 99/09234 PCT/US98/16942
divider 64 is an anion selective membrane. The bipolar membrane 63 has
an anion selective side (not shown) facing the anode and a cation selective
side (not shown) facing the cathode. The electrochemical cell 60 contains
three compartments; namely, a catholyte compartment 65, a recovery
compartment 66, and an anolyte compartment 67.
In operation of the electrochemical cell illustrated in Figure 6, a
solution containing a nitrogen containing compound and a mediator is
charged to the catholyte compartment 65. An electrolyte solution
containing an ionic compound is charged to the recovery compartment 66
and the anolyte compartment 67. The ionic compound is at a first
concentration in the recovery compartment and at a second concentration
in the anolyte compartment 67. An electrical potential is established and
maintained between the anode and the cathode to produce a flow of
current across the electrochemical cell whereupon a hydroxylammonium
salt is produced in the catholyte compartment 65. A portion of the
catholyte solution containing the hydroxylammonium salt is either collected
or physically removed from the catholyte compartment 65 as shown by
line 68 and transferred to the recovery compartment 66. As a result of
the electrical potential maintained between the anode and the cathode, the
salt (anion) of the hydroxylammonium salt is attracted towards the anode
62 thereby passing through the divider 64 into the anolyte compartment
67. Hydroxylamine is produced in the recovery compartment 66.
Hydroxylamine is then recovered from the recovery compartment 66. The
hydroxylamine and/or hydroxylammonium salt (before it is charged to the
recovery compartment) may be purified by further treatment using one or
more of distillation, reverse osmosis, electrodialysis and ion exchange
techniques.
In a preferred embodiment, a portion of the solution in the anolyte
compartment may be physically removed and transferred, as shown by line
69, to the catholyte compartment 65. In an even more preferred
embodiment, the acid solution obtained from the anolyte compartment is
32
CA 02301035 2000-02-15
WO 99/09234 PCT/US98/16942
concentrated before it is added to the catholyte compartment. As the salt
anion from the hydroxylammonium salt migrates through the divider 64
into the anolyte compartment 67, an acid corresponding to the salt is
produced in the anolyte compartment.
As needed, various compounds such as one or more acids, water,
one or more ionic compounds, nitrogen containing compounds, mediators,
stabilizers and the like may be added or recovered from the catholyte,
recovery and anolyte compartments in order to maintain efficient operation
of the electrochemical cell. For example, nitrogen containing compound
must be continuously or intermittently added to the catholyte
compartment. From time to time, it may also be necessary to
intermittently or continuously remove acid from the anolyte compartment.
Although the embodiment described in Figure 6 illustrates the
formation of a generic hydroxylammonium salt, the electrochemical cells
and the method described can be utilized to prepare many desired specific
hydroxylammonium salts by utilizing the different acids described above.
Thus, a hydroxylammonium chloride salt can be prepared utilizing
hydrochloric acid solutions, a hydroxylammonium sulfate salt can be
prepared utilizing sulfuric acid solutions, a hydroxylammonium nitrate salt
can be prepared utilizing nitric acid solutions, hydroxylammonium borate
salts can be prepared utilizing boric acid, and formate or acetate salts can
be prepared by utilizing formic acid or acetic acid.
Referring to Figure 2, the electrochemical cell 20 is made of a
cathode 21, an anode 22, and a divider 23. In a preferred embodiment,
the divider 23 is a cation selective membrane. The electrochemical cell 20
contains two compartments; namely, a catholyte compartment' 24 and an
anolyte compartment 25.
In operation of the electrochemical cell illustrated in Figure 2, a
solution containing a nitrogen containing compound and a mediator is
charged to the catholyte compartment 24. An electrolyte solution
containing an ionic compound is charged to the anolyte compartment 25.
33
CA 02301035 2000-02-15
WO 99/09234 PCT/US98/16942
In a preferred embodiment, the ionic compound is an acid. An electrical
potential is established and maintained between the anode and the cathode
to produce a flow of current across the electrochemical cell whereupon a
hydroxylammonium salt is produced in the catholyte compartment 24. A
hydroxylammonium salt is recovered from the catholyte compartment 24.
The hydroxylammonium salt may be purified by further treatment using
one or more of distillation, reverse osmosis, electrodialysis and ion
exchange techniques.
Referring to Figure 3, the electrochemical cell 30 is made of a
cathode 31, an anode 32, and in sequence beginning at the cathode 31, a
bipolar membrane 33 and a divider 34. In a preferred embodiment, the
divider 34 is an anion selective membrane. The bipolar membrane 33 has
an anion selective side (not shown) facing the anode and a cation selective
side (not shown) facing the cathode. The electrochemical cell 30 contains
three compartments; namely, a catholyte compartment 35, a recovery
compartment 36, and an anolyte compartment 37.
In operation of the electrochemical cell illustrated in Figure 3, a
solution containing a hydroxylammonium salt and a mediator is charged to
the recovery compartment 36. A solution containing an ionic compound is
charged to the catholyte compartment 35 and the anolyte compartment
37. The ionic compound of the catholyte compartment is the same or
different than the ionic compound in the anolyte compartment. In a
preferred embodiment, the ionic compound in the catholyte compartment
is a base while the ionic compound in the anolyte compartment is an acid.
An electrical potential is established and maintained between the anode
and the cathode to produce a flow of current across the electrochemical
cell whereupon hydroxylamine is produced in the recovery compartment
36. As a result of the electrical potential maintained between the anode
and the cathode, the salt (anion) of the hydroxylammonium salt is
attracted towards the anode 32 thereby passing through the divider 34
into the anolyte compartment 37. Hydroxylamine is then recovered from
34
CA 02301035 2000-02-15
WO 99/09234 PCT/US98/16942
the recovery compartment 36. The hydroxylamine may be purified by
further treatment using one or more distillation, reverse osmosis,
electrodialysis and ion exchange techniques.
Referring to Figure 4, the electrochemical cell 40 is made of a
cathode 41, an anode 42, and in sequence beginning at the cathode 41, a*
bipolar membrane 43, a first divider 44 and a second divider 45. In a
preferred embodiment, the first divider 44 is a cation selective membrane
and the second divider 45 is an anion selective membrane. The bipolar
membrane 43 has an anion selective side (not shown) facing the anode
and a cation selective side (not shown) facing the cathode. The
electrochemical cell 40 contains four compartments; namely, a catholyte
compartment 46, a recovery compartment 47, a feed compartment 48,
and an anolyte compartment 49.
In operation of the electrochemical cell illustrated in Figure 4, a
solution containing a hydroxylammonium salt and a mediator is charged to
the feed compartment 48. A solution containing an ionic compound is
charged to the catholyte compartment 35 and the anolyte compartment
37. A solution optionally containing an ionic compound is charged to the
recovery compartment 47. The ionic compound of the catholyte
compartment is the same or different than the ionic compound in the
anolyte compartment (and/or recovery compartment). In a preferred
embodiment, the ionic compound in the catholyte compartment is a base
while the ionic compound in the anolyte compartment is an acid. An
electrical potential is established and maintained between the anode and
the cathode to produce a flow of current across the electrochemical cell
whereupon hydroxylamine is produced in the recovery compartment 47.
As a result of the electrical potential maintained between the anode and
the cathode, the salt (anion) of the hydroxylammonium salt is attracted
towards the anode 42 thereby passing through the second divider 45 into
the anolyte compartment 49. Hydroxylamine is then recovered from the
recovery compartment 47. The hydroxylamine may be purified by
*rB
CA 02301035 2000-02-15
WO 99/09234 PCT/US98/16942
further treatment using one or more of distillation, reverse osmosis,
electrodialysis and ion exchange techniques.
Referring to Figure 5, the electrochemical cell 50 is made of a
cathode 51, an anode 52, and a divider 53. In a preferred embodiment,
the divider 53 is an anion selective membrane and the cathode is a gas
diffusion cathode. The electrochemical cell 50 contains two
compartments; namely, a catholyte compartment 54 and an anolyte
compartment 55.
In operation of the electrochemical cell illustrated in Figure 5, a
solution containing a hydroxylammonium salt and a mediator is charged to
the catholyte compartment 54. A solution containing an ionic compound
is charged to the anolyte compartment 55. In a preferred embodiment, the
ionic compound in the anolyte compartment is an acid. An electrical
potential is established and maintained between the anode and the cathode
to produce a flow of current across the electrochemical cell whereupon
hydroxylamine is produced in the catholyte compartment 54. As a result
of the electrical potential maintained between the anode and the cathode,
the salt (anion) of the hydroxylammonium salt is attracted towards the
anode 52 thereby passing through the divider 53 into the anolyte
compartment 55. Hydroxylamine is then recovered from the catholyte
compartment 54. The hydroxylamine may be purified by further treatment
using one or more distillation, reverse osmosis, electrodialysis and ion
exchange techniques.
In another embodiment of the invention, the catalytic film is used to
electrochemically convert acrylonitrile to adiponitrile. In particular,
acrylonitrile is converted to adiponitrile in the presence of a film formed by
the film forming compound on a cathode. Referring to Figure 2, a solution
containing acrylonitrile is charged to the catholyte compartment 24. An
electrolyte solution containing an ionic compound is charged to the anolyte
compartment 25. In a preferred embodiment, the ionic compound is an
acid. In this embodiment, the divider 23 is preferably a cation selective
36
CA 02301035 2000-02-15
WO 99/09234 PCT/US98/16942
membrane. In some embodiments, additional dividers may be used in the
cell, but they are not generally required. An electrical potential is
established and maintained between the anode and the cathode to produce
a flow of current across the electrochemical cell whereupon adiponitrile is
produced in the catholyte compartment 24. Adiponitrile is recovered from
the catholyte compartment 24. Adiponitrile may be purified by further
treatment using one or more of distillation, reverse osmosis, electrodialysis
and ion exchange techniques.
In embodiments where adiponitrile is produced in the catholyte
compartment, a current is applied between the anode and cathode with an
apparent current density of about 0.1 ASI (amps per square inch) to about
10 ASI, more often from about 2 ASI to 4 ASI at about 3 volts to about 4
volts. The current is applied to the electrochemical cell for a period of time
effective to produce the adiponitrile in the catholyte compartment.
The concentration of acrylonitrile in the catholyte compartment may
be from about 0.01 M to about 10 M. Preferably the acrylonitrile
concentration is from about 0.5 M to about 1 M. The concentration of the
ionic compound in the anolyte compartment may be from about 0.01 M to
about 5 M. Preferably the ionic compound concentration is from about 0.5
M to about 1 M. Ionic compounds are described above.
The concentration of adiponitrile formed in the catholyte
compartment may be from about 0.1 M to about 10 M. Preferably the
adiponitrile concentration formed in the catholyte compartment is from
about 0.5 M to about 2 M.
The following specific examples further illustrate the preparation of
the hydroxylammonium salts and hydroxylamine according to the present
invention. Unless otherwise indicated in the examples and elsewhere in
the specification and claims, all parts and percentages are by weight,
temperatures are in degrees centigrade, and pressures are at or near
atmospheric pressure.
Example 10
37
CA 02301035 2000-02-15
WO 99/09234 PCT/US98/16942
An electrochemical cell according to Figure 2 is provided containing
an anode made of ruthenium oxide coated titanium, the cathode made
according to Example 1, and a Nafion 423 cation selective membrane as
the divider. A solution containing 1 M nitric acid is charged to the
catholyte compartment. A solution of 4 M nitric acid is charged to the
anolyte compartment. Nitric acid is added to the catholyte compartment
to maintain the nitric acid concentration between 0.5 M and 1 M under
application of an electrical current. While maintaining the temperature
between 5 C and 10 C, a current of 5 Amps (0.3 ASI) and a cell voltage
of about 3.5 volts is applied. The catholyte is stirred under application of
the current. A solution of 1.3 M hydroxylammonium nitrate and 0.8 M
nitric acid with no detectable ammonium nitrate is obtained from the
catholyte compartment. An overall current efficiency of 60% for
formation of hydroxylammonium nitrate is achieved.
Example 1 1
An electrochemical cell according to Figure 2 is provided containing
an anode made of ruthenium oxide coated titanium, the cathode made
according to Example 3, and a Nafion 423 cation selective membrane as
the divider. A solution containing 1 M nitric acid is charged to the
catholyte compartment. A solution of 4 M nitric acid is charged to the
anolyte compartment. Nitric acid is added to the catholyte compartment
to maintain the nitric acid concentration between 0.5 M and 1 M under
application of an electrical current. While maintaining the temperature
between 5 C and 10 C, a current of 15 Amps (1 ASI) and a cell voltage of
about 3.5 volts is applied. The catholyte is stirred under application of the
current. A solution of 1.1 M hydroxylammonium nitrate and 0.9 M nitric
acid and 0.05 M ammonium nitrate is obtained from the catholyte
compartment. An overall current efficiency of 35% for formation of
hydroxylammonium nitrate is achieved.
Example 12
38
CA 02301035 2000-02-15
WO 99/09234 PCT/US98/16942
An electrochemical cell according to Figure 2 is provided containing
an anode made of ruthenium oxide coated titanium, the cathode made
according to Example 4, and a Nafion 423 cation selective membrane as
the divider. A solution containing 1 M nitric acid is charged to the
catholyte compartment. A solution of 4 M nitric acid is charged to the
anolyte compartment. Nitric acid is added to the catholyte compartment
to maintain the nitric acid concentration between 0.5 M and 1 M under
application of an electrical current. While maintaining the temperature
between 5 C and 10 C, a current of 30 Amps (2 ASI) and a cell voltage of
about 6 volts is applied. The catholyte is stirred under application of the
current. A solution of 1.9 M hydroxylammonium nitrate and 0.8 M nitric
acid with no detectable ammonium nitrate is obtained from the catholyte
compartment. An overall current efficiency of 60% for formation of
hydroxylammonium nitrate is achieved.
Exam l~e 13
An electrochemical cell according to Figure 2 is provided containing
an anode made of ruthenium oxide coated titanium, the cathode made
according to Example 4, and a Nafion 423 cation selective membrane as
the divider. A solution containing 1 M nitric acid is charged to the
catholyte compartment. A solution of 4 M nitric acid is charged to the
anolyte compartment. Nitric acid is added to the catholyte compartment
to maintain the nitric acid concentration between 0.5 M and 1 M under
application of an electrical current. While maintaining the temperature
between 5 C and 10 C, a current of 15 Amps (1 ASI) and a cell voltage of
about 4.5 volts is applied. The catholyte is stirred under application of the
current. A solution of 1.2 M hydroxylammonium nitrate and 1.2 M nitric
acid and 0.1 M ammonium nitrate is obtained from the catholyte
compartment. An overall current efficiency of 40% for formation of
hydroxylammonium nitrate is achieved.
Example 14
39
CA 02301035 2000-02-15
WO 99/09234 PCT/US98/16942
An electrochemical cell according to Figure 2 is provided containing
an anode made of ruthenium oxide coated titanium, a cathode made of
niobium, and a Nafion 423 cation selective membrane as the divider. A
solution containing 1 M nitric acid and 50 mM 1,4-phenylenediamine is
charged to the catholyte compartment. A solution of 4 M nitric acid is
charged to the anolyte compartment. Nitric acid is added to the catholyte
compartment to maintain the nitric acid concentration between 0.5 M and
1 M under application of an electrical current. While maintaining the
temperature between 5 C and 10 C, a current of 5 Amps (0.3 ASI) and a
cell voltage of about 3 volts is applied. The catholyte is stirred under
application of the current. A film forms on the cathode after about 1 hour
and a solution of 0.8M hydroxylammonium nitrate and 0.9 M nitric acid
and 0.03 M ammonium nitrate is obtained from the catholyte
compartment. An overall current efficiency of 45% for formation of
hydroxylammonium nitrate is achieved.
Example 15
An electrochemical cell according to Figure 2 is provided containing
an anode made of ruthenium oxide coated titanium, the cathode made
according to Example 5, and a Nafion 423 cation selective membrane as
the divider. A solution containing 0.5 M nitric acid is charged to the
catholyte compartment. Concentrated nitric acid is added to the catholyte
compartment to maintain the nitric acid concentration between 0.5 M and
1 M under application of an electrical current. While maintaining the
temperature between 5 C and 10 C, a current of 20 amps (5 ASI) and a
cell voltage of about 5.5 volts is applied. The catholyte is stirred under
application of the current. A solution of 1.67 M hydroxylammonium
nitrate and 0.50 M nitric acid with no detectable ammonium nitrate is
obtained from the catholyte compartment. An overall current efficiency of
85% for formation of hydroxylammonium nitrate is achieved.
Exam lR e 16
CA 02301035 2000-02-15
WO 99/09234 PCT/US98/16942
An electrochemical cell according to Figure 2 is provided containing
an anode made of ruthenium oxide coated titanium, the cathode made
according to Example 6, and a Nafion 423 cation selective membrane as
the divider. A solution containing 1.0 M nitric acid is charged to the
catholyte compartment. A solution of 4 M nitric acid is charged to the
anolyte compartment. Concentrated nitric acid is added to the catholyte
compartment to maintain the nitric acid concentration between 0.5 M and
1 M under application of an electrical current. While maintaining the
temperature between 5 C and 10 C, a current of 20 amps (5 ASI) and a
cell voltage of about 5 volts is applied. A solution of 1.26 M
hydroxylammonium nitrate and 0.7 M nitric acid with no detectable
ammonium nitrate is obtained from the catholyte compartment. An overall
current efficiency of 74% for formation of hydroxyfammonium nitrate is
achieved.
am iPe17
An electrochemical cell according to Figure 2 is provided containing
an anode made of ruthenium oxide coated titanium, the cathode made
according to Example 7, and a Nafion 423 cation selective membrane as
the divider. A solution of 1 M nitric acid is charged to the catholyte
compartment. A solution of 4 M nitric acid is charged to the anolyte
compartment. Concentrated nitric acid is added to the catholyte
compartment to maintain the nitric acid concentration between 0.5 M and
1 M under application of an electrical current. While maintaining the
temperature between 5 C and 10 C, a current of 20 amps (5 ASI) and a
cell voltage of about 6.5 volts is applied. A solution of 1.3 M
hydroxylammonium nitrate and 0.8 M nitric acid with no detectable
ammonium nitrate is obtained from the catholyte compartment. An overall
current efficiency of 60% for formation of hydroxylammonium nitrate is
achieved.
Example 18
41
CA 02301035 2000-02-15
WO 99/09234 PCT/US98/16942
An electrochemical cell according to Figure 2 is provided containing
an anode made of ruthenium oxide coated titanium, the cathode made
according to Example 1, and a Nafion 423 cation selective membrane as
the divider. A solution of 1 M hydrochloric acid and 1 M nitrobenzene is
charged to the catholyte compartment of a cell. A solution of 4 M nitric
acid is charged to the anolyte compartment. While maintaining the
temperature between 25 C and 30 C, a current of 10 amps (2.5 ASI) and
a cell voltage of about 5.5 volts is applied. The catholyte is stirred under
application of the current. A solution of 0.9 M phenylhydroxylammonium
chloride is obtained from the catholyte compartment. An overall current
efficiency of 55% for formation of phenylhydroxylammonium chloride is
achieved.
Exam lp e 19
An electrochemical cell according to Figure 2 is provided containing
an anode made of ruthenium oxide coated titanium, the cathode made
according to Example 1, and a Nafion 423 cation selective membrane as
the divider. The general procedure of Example 10 is repeated except that
thiourea is also added into the catholyte compartment. A solution of 1 M
nitric acid and 250 mM of thiourea is charged to the catholyte
compartment. A solution of 4 M nitric acid is charged to the anolyte
compartment. Nitric acid is added to the catholyte compartment to
maintain the nitric acid concentration between 0.5 M and 1 M under
application of an electrical current. While maintaining the temperature
between 5 C and 10 C, a current of 45 amps (3 ASI) and a cell voltage of
about 6.5 volts is applied. The catholyte is stirred under application of the
current. A solution of 1.77 M hydroxylammonium nitrate and 0.5 M nitric
acid with no detectable ammonium nitrate is obtained from the catholyte
compartment. An overall current efficiency of 90% for formation of
hydroxylammonium nitrate is achieved.
Exam lp e 20
42
CA 02301035 2000-02-15
WO 99/09234 PCT/US98/16942
An electrochemical cell according to Figure 2 is provided containing
an anode made of ruthenium oxide coated titanium, the cathode made
according to Example 1, and a Nafion 423 cation selective membrane as
the divider. The general procedure of Example 10 is repeated except that
tetrabutylammonium chloride is added into the catholyte compartment. A
solution of 1 M nitric acid and 0.1 M tetrabutylammonium chloride is
charged to the catholyte compartment. A solution of 4 M nitric acid is
charged to the anolyte compartment. Nitric acid is added to the catholyte
compartment to maintain the nitric acid concentration between 0.5 M and
1 M under application of an electrical current. While maintaining the
temperature between 5 C and 10 C, a current of 45 amps (3 ASI) and a
cell voltage of about 6.5 volts is applied. The catholyte is stirred under
application of the current. A solution of 1.65 M hydroxylammonium
nitrate and 0.7 M nitric acid with no detectable ammonium nitrate is
obtained from the catholyte compartment. An overall current efficiency of
85% for formation of hydroxylammonium nitrate is achieved.
Exam le21
An electrochemical cell according to Figure 2 is provided containing
an anode made of ruthenium oxide coated titanium, the cathode made
according to Example 1, and a Nafion 423 cation selective membrane as
the divider. The general procedure of Example 10 is repeated except that
a solution 1 M nitrobenzene is also charged is added to the catholyte
compartment of a cell. A solution of 4 M nitric acid is charged to the
anolyte compartment. While maintaining the temperature between 25 C
and 30 C, a current of 10 amps (2.5 ASI) and a cell voltage of about 5.5
volts is applied. The catholyte is stirred under application of the current.
A solution of 0.9 M phenylhydroxylammonium nitrate is obtained from the
catholyte compartment. An overall current efficiency of 55% for
formation of phenylhydroxylammonium nitrate is achieved.
Example 22
43
CA 02301035 2000-02-15
WO 99/09234 PCT/US98/16942
An electrochemical cell according to Figure 2 is provided containing
an anode made of ruthenium oxide coated titanium, the cathode made
according to Example 3, and a Nafion 423 cation selective membrane as
the divider. The general procedure of Example 10 is repeated except that
a solution of 1.5 M of acrylonitrile and 0.2 M of tetraethylammonium p-
toluenesulfonate is charged to the catholyte compartment. A solution of 4
M nitric acid is charged to the anolyte compartment. While maintaining
the temperature between 25 C and 30 C, a current of 12 amps (3 ASI)
and a cell voltage of about 4.50 volts is applied. A solution of 0.45 M
adiponitrile is obtained from the catholyte compartment. An overall current
efficiency of 95% for formation of adiponitrile is achieved.
Example 23
An electrochemical cell according to Figure 2 is provided containing
an anode made of ruthenium oxide coated titanium, a cathode made of
graphite, and a Nafion 423 cation selective membrane as the divider. A
solution containing 1 M nitric acid and 50 mM 1,4-phenylenediamine is
charged to the catholyte compartment. A solution of 4 M nitric acid is
charged to the anolyte compartment. Nitric acid is added to the catholyte
compartment to maintain the nitric acid concentration between 0.5 M and
1 M under application of an electrical current. While maintaining the
temperature between 5 C and 10 C, a current of 5 Amps (0.3 ASI) and a
cell voltage of about 3.5 volts is applied. The catholyte is stirred under
application of the current. A solution of 13 M hydroxylammonium nitrate
and 0.8 M nitric acid with no detectable ammonium nitrate is obtained
from the catholyte compartment. An overall current efficiency of 60% for
formation of hydroxylammonium nitrate is achieved.
Exam Ip e 24
An electrochemical cell according to Figure 2 is provided containing
an anode made of ruthenium oxide coated titanium, a cathode made of
graphite, and a Nafion 423 cation selective membrane as the divider.
Graphite felt is attached to the graphite cathode to enhance the active
44
CA 02301035 2000-02-15
WO 99/09234 PCT/US98/16942
cathode surface area. A solution containing 1 M nitric acid and 50 mM
1,4-phenylenediamine is charged to the catholyte compartment. A
solution of 4 M nitric acid is charged to the anolyte compartment. Nitric
acid is added to the catholyte compartment to maintain the nitric acid
concentration between 0.5 M and 1 M under application of an electrical
current. While maintaining the temperature between 5 C and 10 C, a
current of 45 Amps (3 ASI) and a cell voltage of about 6.5 volts is applied.
The catholyte is stirred under application of the current. A solution of 1.7
M hydroxylammonium nitrate and 0.6 M nitric acid with no detectable
ammonium nitrate is obtained from the catholyte compartment. An overall
current efficiency of 75% for formation of hydroxylammonium nitrate is
achieved.
Exam lp e 25
An electrochemical cell according to Figure 3 is provided containing
an anode made of ruthenium oxide coated titanium, a stainless
steel cathode, a Tokuyama Bipolar 1 bipolar membrane, a Asahi glass AAV
anion selective membrane as the divider. A solution of 0.5 M sodium
hydroxide is charged to the catholyte compartment, a solution of 0.3 M
nitric acid is charged to the anolyte compartment, and a solution of 1.7 M
hydroxylamine nitrate, 0.7 M nitric acid and 50 mM of 1,4-
phenylenediamine is charged to the recovery compartment. While
maintaining the temperature between 5 C and 10 C, a current of 5 Amps
and a cell voltage of about 9.1 volts is applied. A solution containing 1.6
M hydroxylamine and 50mM of 1,4-phenylenediamine is recovered from
the recovery compartment. Pure hydroxylamine is obtained after
purification by distillation.
Exam IR e 26
An electrochemical cell according to Figure 2 is provided containing
an anode made of ruthenium oxide coated titanium, a cathode made of
graphite, and a Nafion 423 cation selective membrane as the divider.
Graphite felt is attached to the graphite cathode to enhance the active
CA 02301035 2000-02-15
WO 99/09234 PCT/US98/16942
cathode surface area. A solution containing 1 M nitric acid and 50 mM to
100 mM anthraquinone-2,6-disulfonic acid disodium salt is charged to the
catholyte compartment. A solution of 4 M nitric acid is charged to the
anolyte compartment. Nitric acid is added to the catholyte compartment
to maintain the nitric acid concentration between 0.5 M and 1 M under
application of an electrical current. While maintaining the temperature
between 5 C and 10 C, a current of 15 Amps (1 ASI) and a cell voltage of
about 3.5 volts is applied. The catholyte is stirred under application of the
current. A solution of 1.1 M hydroxylammonium nitrate and 0.9 M nitric
acid and 0.05 M ammonium nitrate is obtained from the catholyte
compartment. An overall current efficiency of 35% for formation of
hydroxylammonium nitrate is achieved.
Exam lp e 27
An electrochemical cell according to Figure 2 is provided containing
an anode made of ruthenium oxide coated titanium, a cathode made of
graphite, and a Nafion 423 cation selective membrane as the divider.
Graphite felt is attached to the graphite cathode to enhance the active
cathode surface area. A solution containing 1 M nitric acid and 50 mM to
400 mM 4-4'-oxydianiline is charged to the catholyte compartment. A
solution of 4 M nitric acid is charged to the anolyte compartment. Nitric
acid is added to the catholyte compartment to maintain the nitric acid
concentration between 0.5 M and 1 M under application of an electrical
current. While maintaining the temperature between 5 C and 10 C, a
current of 30 Amps (2 ASI) and a cell voltage of about 6 volts is applied.
The catholyte is stirred under application of the current. A solution of 1.9
M hydroxylammonium nitrate and 0.8 M nitric acid with no detectable
ammonium nitrate is obtained from the catholyte compartment. An overall
current efficiency of 60% for formation of hydroxylammonium nitrate is
achieved.
Exam lp e 28
46
CA 02301035 2000-02-15
WO 99/09234 PCT/US98/16942
An electrochemical cell according to Figure 2 is provided containing
an anode made of ruthenium oxide coated titanium, a cathode made of
graphite, and a Nafion 423 cation selective membrane as the divider.
Carbon felt is attached to the graphite cathode to enhance the active
cathode surface area. A solution containing 1 M nitric acid and 50 mM to
0.1 M tin chloride is charged to the catholyte compartment. A solution of
4 M nitric acid is charged to the anolyte compartment. Nitric acid is added
to the catholyte compartment to maintain the nitric acid concentration
between 0.5 M and 1 M under application of an electrical current. While
maintaining the temperature between 5 C and 10 C, a current of 15 Amps
(1 ASI) and a cell voltage of about 4.5 volts is applied. The catholyte is
stirred under application of the current. A solution of 1.2 M
hydroxylammonium nitrate and 1.2 M nitric acid and 0.1 M ammonium
nitrate is obtained from the catholyte compartment. An overall current
efficiency of 40% for formation of hydroxylammonium nitrate is achieved.
Exam Ip e 29
An electrochemical cell according to Figure 2 is provided containing
an anode made of ruthenium oxide coated titanium, a cathode made of
niobium, and a Nafion 423 cation selective membrane as the divider. A
solution containing 1 M nitric acid and 50 mM 1,4-phenylenediamine is
charged to the catholyte compartment. A solution of 4 M nitric acid is
charged to the anolyte compartment. Nitric acid is added to the catholyte
compartment to maintain the nitric acid concentration between 0.5 M and
1 M under application of an electrical current. While maintaining the
temperature between 5 C and 10 C, a current of 5 Amps (0.3 ASI) and a
cell voltage of about 3 volts is applied. The catholyte is stirred'under
application of the current. A solution of 0.8M hydroxylammonium nitrate
and 0.9 M nitric acid and 0.03 M ammonium nitrate is obtained from the
catholyte compartment. An overall current efficiency of 45% for
formation of hydroxylammonium nitrate is achieved.
Exam fp e 30
47
*rB
CA 02301035 2000-02-15
WO 99/09234 PCT/US98/16942
The general procedure of Example 23 is repeated except that a piece
of graphite felt is attached to the graphite cathode to enhance the cathode
surface area. A solution containing 0.5 M nitric acid and 50 mM 1,4-
phenylenediamine is charged to the catholyte compartment. Concentrated
nitric acid is added to the catholyte compartment to maintain the nitric acid
concentration between 0.5 M and 1 M under application of an electrical
current. While maintaining the temperature between 5 C and 10 C, a
current of 20 amps (5 ASI) and a cell voltage of about 5.5 volts is applied.
The catholyte is stirred under application of the current. A solution of
1.67 M hydroxylammonium nitrate and 0.50 M nitric acid with no
detectable ammonium nitrate is obtained from the catholyte compartment.
An overall current efficiency of 85% for formation of hydroxylammonium
nitrate is achieved.
Exam l~ e 31
The general procedure of Example 30 is repeated except that a
solution containing 1.0 M nitric acid and 70 ppm p-aminophenol is charged
to the catholyte compartment. A solution of 4 M nitric acid is charged to
the anolyte compartment. Concentrated nitric acid is added to the
catholyte compartment to maintain the nitric acid concentration between
0.5 M and 1 M under application of an electrical current. While
maintaining the temperature between 5 C and 10 C, a current of 20 amps
(5 ASI) and a cell voltage of about 5 volts is applied. A solution of 1.26 M
hydroxylammonium nitrate and 0.7 M nitric acid with no detectable
ammonium nitrate is obtained from the catholyte compartment. An overall
current efficiency of 74% for formation of hydroxylammonium nitrate is
achieved.
Example 32
The general procedure of Example 30 is repeated except that a
solution of 1 M nitric acid and 100 ppm hydroquinone is charged to the
catholyte compartment. A solution of 4 M nitric acid is charged to the
anolyte compartment. Concentrated nitric acid is added to the catholyte
48
CA 02301035 2000-02-15
WO 99/09234 PCT/US98/16942
compartment to maintain the nitric acid concentration between 0.5 M and
1 M under application of an electrical current. While maintaining the
temperature between 5 C and 10 C, a current of 20 amps (5 ASI) and a
cell voltage of about 6.5 volts is applied. A solution of 1.3 M
hydroxylammonium nitrate and 0.8 M nitric acid with no detectable
ammonium nitrate is obtained from the catholyte compartment. An overall
current efficiency of 60% for formation of hydroxylammonium nitrate is
achieved.
Exam lo e 33
The general procedure of Example 30 is repeated except that
thiourea is also added into the catholyte compartment. A solution of 1 M
nitric acid, 50 mM 1,4-phenylenediamine and 250 mM of thiourea is
charged to the catholyte compartment. A solution of 4 M nitric acid is
charged to the anolyte compartment. Nitric acid is added to the catholyte
compartment to maintain the nitric acid concentration between 0.5 M and
1 M under application of an electrical current. While maintaining the
temperature between 5 C and 10 C, a current of 45 amps (3 ASI) and a
cell voltage of about 6.5 volts is applied. The catholyte is stirred under
application of the current. A solution of 1.77 M hydroxylammonium
nitrate and 0.5 M nitric acid with no detectable ammonium nitrate is
obtained from the catholyte compartment. An overall current efficiency of
90% for formation of hydroxylammonium nitrate is achieved.
Exa l~e34
The general procedure of Example 30 is repeated except that
tetrabutylammonium chloride is added into the catholyte compartment. A
solution of 1 M nitric acid, 50 mM 1,4-phenylenediamine and 0:1 M
tetrabutylammonium chloride is charged to the catholyte compartment. A
solution of 4 M nitric acid is charged to the anolyte compartment. Nitric
acid is added to the catholyte compartment to maintain the nitric acid
concentration between 0.5 M and 1 M under application of an electrical
current. While maintaining the temperature between 5 C and 10 C, a
49
CA 02301035 2000-02-15
WO 99/09234 PCT/US98/16942
current of 45 amps (3 ASI) and a cell voltage of about 6.5 volts is applied.
The catholyte is stirred under application of the current. A solution of
1.65 M hydroxylammonium nitrate and 0.7 M nitric acid with no
detectable ammonium nitrate is obtained from the catholyte compartment.
An overall current efficiency of 85% for formation of hydroxylammonium
nitrate is achieved.
The present invention provides efficient, inexpensive and
uncomplicated electrochemical methods of preparing hydroxylammonium
salts, hydroxylamine and adiponitrile of high purity. Since the use of
mercury containing and/or lead containing cathodes is not required, the
present invention does not raise toxicity concerns and is environmentally
friendly. Since in some embodiments the use of gas permeable cathodes is
not required, the present invention is relatively inexpensive and
uncomplicated to practice.
While the invention has been explained in relation to its preferred
embodiments, it is to be understood that various modifications thereof will
become apparent to those skilled in the art upon reading the specification.
Therefore, it is to be understood that the invention disclosed herein is
intended to cover such modifications as fall within the scope of the
appended claims.