Note: Descriptions are shown in the official language in which they were submitted.
CA 02301347 2005-02-07
TITLE: INTERNAL COMBUSTIO'.N ENGINE EXHAUST
TREATING APPARATUS AND METHOD
INVENTOR: DOMINIC E. ;SCAPPATURA
FIELD OF THE INVENTION
This invernion relates to the treatment of the exhaust gases from a diesel
internal combustion
engine to reduce the harmful emissions, particularly carbon deposits (soot),
noxious odors, carbon
dioxide, carbon monoxide, nitrogen oxides, sulfur oxides, from a diesel motor
emissions gases.
DESCRIPT101 OF DR~V1~1NGS
Preferred embodiments of the invention as described below with reference to
the accompanying
drawin~a in which:
Figure 1 is a schematic representation of a diesel combustion engine exhaust
treating apparatus according to the present invention;
Figure 1 is a schematic representation of a unit for the function of removing
soot
particles and odor from a diesel engine according to the present invention;
Figure 3, 4 and S are schematics of the internal features of a heat exchanger
according to the present invention;
Figure b is a section through a first chemical reactor a fluid separator and a
fluid
holding tank of an apparatus according to the present invention;
Figure 7 is a section through a second chemical reactor, a chernieal gases
separator
and fluid neutralizing holding tank with a an elatronic damper according to
the
present invention;
Figure 8 is a schematic view of an alternate embodiment of the present
invention;
Figure 9 is a schematic view of the internal features of a unit for the
function of
removing soot particles and odor from a diesel engine according to the present
invention;
CA 02301347 2005-02-07
-2-
Figure 14 is a schematic view of the internal features of an optional soot
bath for
the function of removing minute particles from the exhaust and final removal
of
vapor according to the present invemion; and
Figu re I I is a cross-section view of the internal features of an optional
hot water
cleansing system for the interior of the soot separator for removing atl
leftover
chemical and particulate as the chemical is. water soluble.
I~ACkGROL''1D OF THE INVENTION
The combustion of diesel fuel in internal combustion engines usually depicts
the reaction of air and
the hydrocarbon contained in the fuel. Air chiefly comprises of nitrogtn and
oxygen gases which
produces oxides of nitrogen, carbon and unburned hydrocarbon, the l8tter being
mostly matter
which contributes to smog. In general fuels generally contain sulfur, oxides
of sulfur in
combustion chambers and a large amount of unburned hydrocarbons which will be
considered as
particulate
The oxides of carbon are carbon monoxide and carbon dioxide. Carbon monoxide
is an umwanted
gas as it is very poisonous; and carbon dioxide is also unwanted as it is a
contributor to
"greenhouse gases" which is thought to be a primary eontn'butor to global
warning.
Conventional diesel exhausts have a device which is known as a catalytic
converter which reduces
the amount of nitrogen oxides, and unburned hydrocarbons and carbon monoxide
but tends to
increase the emission of carbon dioxide and particulate which it does not
reduce.
The object of this invention is to provide an apparatus a,~d method for
treating diesel internal
combustion enuines exhaust to significantly reduce the particulate carbon
dioxide, nitrous oxide
and sulfur oxides emitted to the atmosphere
It is also another object of this invention to provide a method and apparatus
to neutralise acid
contained in the exhaust gases.
It is also an object of this invention to provide an apparatus which removes
particulate (soot) and
particulate matter (smog) from the exhaust gases.
It is the object of this invention to provide an apparatus as part of a diesel
powered motor {i.e.,
heavy equipment, trucks. tractors, buses and any diesel powered equipment), as
its exhaust
system
DESCRtPTIO'~ OF PREFERRED EMBODIM~NT_~
CA 02301347 2005-02-07
-3-
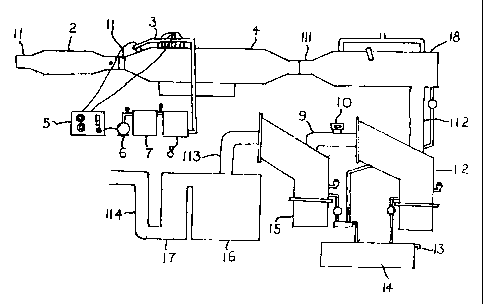
An apparatus according to the present irrvention for removing waste materials
from a diesel fueled
internal combustion engine exhaust as indicated in Figurs 1.
The apparatus in Figure I has a soot separator 4, which is connected through
an inlet 11 with a
diesel exhaust system and is connected after the catalytic: converter 2. The
soot separator ranoves
the soot by a chemical RP Super Filter Coat #421 injectad through injectors
for s speafed period
(seconds) over a set time (minutes). The soot collected then falls into the
reservoir for the soot
particles the exhaust with a major part of the soot particulate removed exits
the separator 4
through an inlet 111, and enters into the heat exchanger 18. The heat
exchanger 18 cools the
incoming exhaust gases, resulting in condensation of a portion of the gases
into a liquid
condensate. The condensate will principally be water which is typically formed
as a product in the
combustion of hydrocarbon fuels. As oxides of nitrogen; and sulfur are present
in the combustion
products these will combine with the condensed water vapor to form nitrogen
and sulfur oxide,
bases acids. including nitric and sulfuric acids.
The cooled gases and condensate exit the heat exchanger 18 through outlet 112
which fluidly
communicates into a fluid separator 12. The fluid separator 12 separates the
condensate from the
non-condensed gases. Condensate collects in the bottom of the fluid separator
12 from where it
drains into a holding tank 14. The condensate is ntutralized by coming into
contact with a
neutralizing agent in the first chemical reactor which ma;y form part ofthe
first fluid separator 12
or the holding tank 14. A drain T 3 Is provided for draining or overflowing
neutralized condensate
from the holdinsz tank 14
ion-condensed gases pass from the fluid separator 12 irtto a second chemical
reactor 15 through a
fluid canduii 9 ~°hich provides fluid communication between the
separator 12 and the second
chemical reactor 15
.4n air inlet 10 is provided into the fluid conduit 9 to adroit air into the
apparatus in the event that
a below ambient pressure should occur in the apparatus. It has been Found that
sudden
acceleration or deceleration ofthe vehicle to which the apparatus Figure 1 is
attached may give
rise to sudden pressure drops within the apparatus which results in a partial
vacuum causing air
inlet 10 to open.
The second chemical reactor I S receives non-condensed gases from the fluid
separator 12 which
will contain carbon monoxide, carbon dioxide and resulting from the combustion
of the diesel
hydrocarbon fuels and possibly also from the neutralisation reaction in the
first chemical reactor
from the neutralizing agent
Treated exhaust gases may be passed from the second reactor 1 S into a final
scrubber 16 and 17, if
one is provided through a fluid conduit 113 which provides fluid communication
there between.
The final scrubber 16 contains RP Super Filter Coat #421 which absorbs the
minute particles of
particulate (soot) in the treated exhaust gases, and the bath 17 removes any
vapors from the
reaction in the scrubber 16
CA 02301347 2005-02-07
-4-
Depending on the quality of the diesel exhaust efficiency is required, the
final scrubber 16 and
vapor remover 17 may not be necessary.
The treated and scrubbed exhaust gases pass out of the final scrubber 1b and
vapor remover 17
and are the vented into the atmosphere through an exhaust outlet 50 which may
be used to cool
the diesel engine when an air cooled engine is used.
The various components of the overall apparatus will now be described in more
detail.
Figure 2 illustrates the soot separator 4 which is connected to the diesel
engine by the inlet I I
from the catalytic converter 2. The soot collector 4 is designed is shown
a~tenu~lly by Figure Z
and internally by Figure 9. The soot separator 4 should reduce a major portion
of the soot
particulate from the exhaust by injecting a glue like chemical through
injectors 26 and 29 from a
chemical tank 8 through a carrier pipe 3. The chemical tank 3 is supplied by
air pressure from a
tank 7 which flows through 31 from 6 which is a small air compressor 23 and 24
respectively are
pressure gauges to control the air pressure. 25 is the filler cap through
which the chemical is
placed in the chemical tank #8. A panel 5 is set up for the control units 19
being a time delay
switch which control injectors 29. Fuses are contained in unit 20. The main
switch for the power
will be 21 An accelerator timer switch is used for injector 26 which controls
the sudden
acceleration of the diesel engine and picks up the soot particulate (soot) as
it enters the soot
separator 3? is the power line from the panel to 6 the compressor. 30 is the
lead wire from the
panel to the accelerator injector 26. At the end of the fed pipe 3 is a manual
release valve 28.
The paniculate reservoir 76 is at the base of the soot separator 4. Figure 9
shows a section
through the soot separator 4.
The components of the soot separator 4 exposed to exhaust gases are preferably
manufactured
from a heat and corrosion resistant material such as stainless steel. The soot
separator I2 has shell
casing containing an auger 8a of specified dimension for proper adaptation to
the diesel units. The
chemical feed pipe 3 feeds the chemical to the injectors. 29 which are
directed at 82 a stainless
steel mesh 1 ~'8 openings which are inserted into the auger blades 80. The
centre of the auger is
sealed onto a pipe 81 which runs the full length of the auger. The entrance of
the exhaust 11 has
at the immediate entry an accelerator injector 26 which is fed from the feed
pipe 3. A perforated
plate 78 may be set into the catch basin of which 77 is the bottom plate that
has larger holes to
accommodate the collected particulate which will be deposited into 76 the
reservoir. The exhaust
is returned back up into the auger by 79 deflectors attached to the bottom of
the auger blades and
are hooked at the base to deflect the air. The shell of tihe soot separator
should have small
perforations 83 so that any particulate from the injector 2b may be collected
and as the exhaust is
cleaned it exits through 11.
Figure 3 shows a cross section through the heat exchanger 18. The components
of the heat
exchanger exposed to exhaust gases are preferably manufactured from a heat and
corrosion
resistant material such as stainless steel. The heat exchanger 18 has a shell
or casing containing a
coolant chamber 35
CA 02301347 2005-02-07
The coolant chamber 35 fluidly intercommunicates with a coolarn inlet 34, end
a coolant outlet 42
so that the coolant fluid entering the coolant inlets 34 wi!! pass through the
coolant chamber 35 to
emerge from the coolant outlet 42.
The coolant chamber 35 extends around the exhaust tubes 41 which receive hot
exhaust gases
from the inlet 111 which is received by a cone shaped, receives 33 whie3t
contains 43 two cone
shaped baffles which deflect the gases evenly in the best exeiwnger 12 eMeriag
the txhaust tubes
41. Heat from the exhaust entering the inlet 33 is transnrotted through the
txbaust tubes 41 into
the coolant fluid contained within the coolant chamber 3b. The total cross-
soetionat area of the
exhaust tubes should be adequate to minimize flow restrictions through and
attendant back
pressure associated with the heat exchanger 18.
Spiral baffles 40 may be provided in the exhaust tubes 41 to promote swirling
of the exhaust
passing through the tubes and to conduct heat from the exhaust gases into the
coolant chamber 35
thereby promoting heat transfer and enhancing the efficiency of the heat
exchanger 18.
Figure 4 is also a cross-section of the heat exchanger 1.3 which shows the
exhaust outlet 112
containing the heat probe 37 on the exterior of the outlet I 12 with an
inferior sensor probe inside
of the outlet 112.
Figure 2 illustrates the connection for the purpose of the cooling of the heat
exchanger 18 to a
coolinu system as illustrated in Figure 5. Preferably the heat exchanger 18
should reduce the
exhaust temperature to a temperature of about 5° or 10°C
(approximately 40° to 50~'). In cold
weather this can be accomplished by connecting coolant inlet 47 and coolant
outlet 42 ofthe heat
exchanger 1 ~ to a radiator 44 which will be set up specifically for the
operation of this apparatus
.As the radiator 44 cannot cool coolant below ambient temperature, a further
source of cooling is
required in warm weather. This can be provided by the addition of an air
conditioning cooling
pump to the diesel engine. Reference 93 indicates an e~~aporator coil in a
cooling compressor for
cooling coolant flowing through a cooling conduit 47 providing fluid
communication between the
coolant into the heat exchanger and the radiator 44.
A coolam pump 46 is provided in the cooling conduit 47. The operator ofthe
coolant pump 4G is
controlled by a control system 37. Figure 4 which monitors the temperature by
a probe 38 which
monitors the temperature of the gaslcondensate exiting outlet 112 of the heat
exchanger 18.
Depending on the nature of the specific control system 3? and pump 93 selected
the control
system can either vary pump speed or simply turn the pumps on and off as
required to maintain the
temperature of the gaslcondensate exiting the outlet 112 within the desired
range. A temperature
sensor .38, Figure 4, may be mounted at a suitable location such as, for
example adjacent to outlet
I 12 to provide a temperature indicating signal to the control system 37.
Figure 6 shows a cross-section through of the fluid separator 12.
Condensatelgas emanating from
the outlet 112 of the heat exchanger 18 is directed into the bottom of the
fluid separator 12 from
CA 02301347 2005-02-07
-6-
the inlet 112 by a funnel shaped inlet guide 94. A block of a suitable
neutralizing aga~t such as
sodium carbonate (soda ash) 55 is located at the bottom of the fluid x~~rator
12 in the stream of
the condensate/gas emanating from the inlet guide 94. The soda ash block SS
reacts with the
acidic concentrate to neutralize acids contained in the condensate. The
neutralized condensate
flows through a fluid conduit into a one way drain valve 56 which is provided
to prevent the
return of the neutralized condensate from the holding tank 14 into the fluid
separator 12.
The condensate may be periodically drained from the balding tank 14 through a
drain 13 or left to
overflow. A valve is provided to open and close the drain 13.
Although the soda ash block 55 is shown in the fluid separator, ahernatively,
or additiona>>yy, soda
ash may be provided into the holding tank I4. The placement of the soda ash in
association with
the fluid separator 12 andlor the holding task I4 provides a fret chanacal
reactor for neutralizing
acids in the condensate.
Non-condensed gases pass upwardly in the direction of arrows 54 into a branch
95 of the fluid
separator towards an outlet 9. Baffles 51 and 52 are provided in the gas flow
path to trap
condensate carried in the gas. As the velocity ofthe exhaust diminishes and as
it changes direction
through the fluid separator, particulate matter tends to separate from the
exhaust in the fluid
separator and settle out with the condensate.
A further specially designed soda ash block 58 may be provided in the branch
96 as part of the
first chemical reactor to react with any condensate carried by the exhaust gas
flow into the branch
96 ,a stainless steel mesh screen 59 is provided across the branch 96 to
prevent the soda ash
block 59 from falling into the bottom of the separator 1:!. The soda ash block
55 will be activated
by 5 to 8oz of water poured through an intake funnel 54. All soda ash blocks
SS and 58 contain
time released pellets or time released layers of soda ash 1:o reconstitute the
chemical.
Flanges 57 are pro4~ided in the fluid separator 12 to allow disassembly for
replacement of the soda
ash blocks 55 and 58 respectively.
Treated gas exits the branch 95 of the separator through the fluid conduit 9
into a second chemical
reactor 15 such as shown in a detailed sectional view in lfignre 7. The
structure of the second
reactor is very similar outwardly to the first reactor. This reactor may be
made from non-
corrosive steel as is the first reactor 12 for protection purposes and the
interior of the unii IS may
be coated with a urethane or other suitable coating which is resistant to the
caustic materials such
as potassium hydroxide.
The exhaust gas is directed through the fluid conduit 9 down into the bottom
of the second
separator 15. The fluid conduit 9 has a funnel shaped bottom 94. A designed
block of potassium
hydroxide (containing time release pellets or time release layers of potassium
hydroxide to
reconstitute the chemical) 63 is located at the bottom of'.the reactor 16 in
the stream ofthe gas
emanating from the inlet guide (4 to 8oz of water must be added through 62 to
activate the
potassium hydroxide), The potassium hydroxide reacts with the carbon monoxide,
carbon dioxide
t t - I t t t
CA 02301347 2005-02-07
and nitrogen oxide which are absorbed into the activated hydroxide. As the gas
is forced down
through the second fluid conduit 9 onto the potassium hydroxide 63, it will
deposit whatever
vapor remaining and it is absorbed into the potassium hydroxide 63. As the
vapor is deposited
into the reactor 15 an automatic overflow 56 allows the extra hydroxide
condensate to leave and
be deposited into the holding tank 14 which contains a small amount of mild
acid 91 to neutralize
the caustic effect of the potassium hydroxide 63. A drain 13 is supplied to
drain the tank 14
occasionally.
The exhaust less a major portion of the carbon dioxide, warbon monoxide and
nitrogen oxide plus
other contaminants passed upwardly in the direction of arrows 64 into the
broach 95 of the reactor
towards an outlet and into a fluid conduit 6fi. Three to six batHes 61 are set
horizontally andlor
on an angle between the horizontal bales, across the second reactor 15 each
having holes with a
smaller size beginning at the lower baffles with the holes being enlarged as
you rise. Typical holes
would be 1 /8". 3i 15" and l l4". The baffles slow the exhaust speed and
pnwern an upward motion
of any of the potassium hydroxide contaminating fluid. The gases then enter a
branch where two
more solid baffles 60 are situated for further impeding the speed of the
exhaust and any vapors
present. A stainless steel wire mesh 69 supports a specialty designed
potassium hydroxide block
68 with the same properties of the one at the bottom of the ra~actor.
'The upper potassium hydroxide block 58 will pick up any further deposits of
carbon dioxide,
carbon monoxide, nitrogen oxide, and any other contaminants remaining and the
exhaust through
the exit pipe 6b into an electronic shutter 67 controlled fiom the ignition
lay to prevent
comaminated stases from entering the system while not in use, thence the
exhaust is released into
the atmosphere.
Two second chemical reactors are illustrated and described but it should be
appreciated that the
actual numbers may be increased by a particular application.
Figure 8 illustrates an altered embodiment similar to that described above but
in which the first
and second chemical reactors 12 and 15 respectively share a common holding
tank.
Neutralized condensate from the first reactor 12 drains through an automatic
drain 56 into the
holding tank 14. In the embodiment described above it was noted that
condensate from the
second chemical reactor 15 will typically be alkaline and therefore the
holding tank 14 associated
with the second reactor 15 contains a mild acid 91. In the embodiment
illustrated in Figure 'f the
second chemical reactor 15 drains through an automatic drain 56 and a first
drain conduit 73 into a
first chamber 74 in which it is neutralized prior to draining through a second
drain conduit 75 into
the holding tank 14
The first chamber 74 fluidly communicates with the heat exchanger 18 through a
pipe 72. The
heat exchanger 18 will typically contain acidic condensate 96 which may be
collected at 70 and 71
and drained through an automatic drain 56 and pipe 72 veto the first chamber
74. The acidic
concentrate 96 from the heat exchanger t 8 reacts with the alkaline oondensate
from the second
chemical reactor 15 to form a neutralized solution which drains into the
holding tank I4 through a
CA 02301347 2005-02-07
_g_
pipe 74 which provides fluid communication between the first chamber 74 and
the holding tank
14.
Figure 10 depicts a cross-section of 16 a chemical bath fir removal of minute
exhaust gases.
The inlet pipe 98 is perforated at it's lowermost end 87 b;y a plurality of
small holes 87 to radically
disperse the exhaust gases into a liquid 82 surrounding the pipe (RP Super
Filter Coat #421). This
chemical liquid would be used for the removal of minute ;particles of soot
remaining in the exhaust
The holes 87 extend at the end of the pipe 101, a distancE; corresponding to
approximately the
outside diameter of the inlet pipe 98 and the total area of the holes 87
should be approximately
twice the cross-sectioned area of the inside of the inlet pipe to minimize
flow restrictions. The
actual size and number of holes may vary somewhat in practice with the overall
object being to
provide enough holes to minimize flow restrictions with small enough holes to
provide good
dispersion of gas into the liquid 82 within the bath 16, without tending to
force the liquid 82 out of
the bath 16. Typically the holes may be on the order of 3/32 (approximately
2mm in diameter).
A series of inclined baffles 8, 99 and 100 extend across the bath 16, one
above the other in a zig
zag configuration except for the baffle plate 88 which is generally
horizontally disposed. The inlet
pipe 98 may extend down through the centre of the baffle: plates 84 through
100.
The level of the 8'' should be between the uppermost of the holes 87 and the
underside of baffle
plate 88.
The baffle plates 84 (two) 99 and 100 (two) are perforat<;d to allow the
upward passage of the
exhaust baffles through the bath 16. Typically the number of holes would be
greater and their
respective sizes smaller the closer the respective of the baffle 100, 84 and
99, into the bottom of
the first stage
The overall area of the holes in each baffle should be at least 1 3/4 times
across the sectional area
of the inside of the pipe 87 to minimize flow restriction. The typical hole
size may be 88 - 3/32"
(approximately 2mm), 100 - I/8" (approximately 2mm) each, 84 - 3/6"
(approximately 2mm)
each, and 99 - 3/8" (approximately 2mm).
The upper baffles 83 are not perforated and extend from the wall of 16 and
about half of the area
of 16 leaving a gap for the gases may flow. The gap should have an area of at
least 1 3/4 times the
cross-sectional area of the inside of the pipe 98 to minimize flow
restrictions. Chemicals are
inserted through 86 and when it is necessary to change the chemical (82 a tap
86 is provided).
The exhaust then enters a further drying area 17 when the exhaust enters 102
into a down pipe
containing a number of perforated baffles 89 that have 1/2" (approximately
2mm) holes in each of
the six baffles with enough area to minimize flow restrictions. The exhaust
direction 97 then exits
CA 02301347 2005-02-07
-9-
through 114 into the atmosphere or returned as a coolant for air cooled
engines as the exhaust air
will be clean and cool.
Figure 1 I is the interior section f Figure 9 which removes soot particulate
from the exhaust. This
is an optional unit designed for the purpose of cleaning the inlet of the soot
remover 4 by
connecting 106 to a hot or cold water outlet thence the water enters into the
centre pipe 81 which
will have small spray nozzles 1 OS in each section of the auger 80 by closing
the valve 104 in the
exit pipe 11 the water flow is restricted into the chamber. The particulate
will then be carried
down into the collector area 77 thence out into to a deposit area through a
tap 107 provided for
same.
A catalytic converter or scrubber can be incorporated into the connecting pipe
# III between the
condenser 4 and the soot separator 18 for the purpose of the removal of
hydrocarbons that may be
produced by the chemical Super Filter Coat used for the separation of the soot
particles in the
exhaust