Note: Descriptions are shown in the official language in which they were submitted.
CA 02302447 2000-02-29
WO 99/13099 PCT/US98/18216
ELECTROCHEMICAL SENSOR HAVING EQUALIZED ELECTRODE AREAS
Field of the Invention
The invention relates to electrochemical sensors, biomedical testing, and
blood analysis.
~ Background of the Invention -
Electrochemical assays for determining the concentration of enzymes or their
substrates
in complex liquid mixtures have been developed. For example. electrochemical
sensor
strips have been developed for the detection of blood glucose levels.
Electrochemical
sensor strips generally include an electrochemical cell in which there is a
working electrode
and a reference electrodes. The potential of the working electrode typically
is kept at a
constant value relative to that of the reference electrode.
Electrochemical sensor strips are also used in the chemical industry and food
industry, to
analyze complex mixtures. Electrochemical sensors are useful in biomedical
research,
where they can function as invasive probes, and for external testing (i.e.,
testing of blood
~ 5 obtained by a needle and syringe, or a lance).
Typical electrochemical sensors for blood analysis measure the amount of
analyte in a
blood sample by using a working electrode coated with a layer containing an
enzyme and a
redox mediator and a reference electrode. When the electrodes contact a liquid
sample
containing a species for which the enzyme is catalytically active, the redox
mediator
2o transfers electrons in the catalyzed reaction: When a voltage is applied
across the electrodes,
a response current results from the reduction or oxidation of the redox
mediator at the
electrodes. The response current is proportional to the concentration of the
substrate. Some
sensors include a dummy electrode coated with a layer containing,the redox
mediator but
lacking the enzyme. The response current at the dummy electrode represents a
background
25 response of the electrode in contact with the sample. A corrected response
is derived by
subtracting the response of the dummy electrode from the response of the
working electrode.
This dummy subtraction process substantially eliminates background
interferences, thereby
improving the signal-to-noise ratio in the electrode system.
Summary of the Invention
3o It has been discovered that equalizing the exposed areas of electrode
regions having no
electrochemical reaction components by which to generate a catalytic current
increases the
accuracy and precision of analyte concentration measurements. This result was
unexpected.
According to conventional electrochemical theory, the magnitude of current
from such areas
CA 02302447 2000-02-29
WO 99/13099 PCT/US98/18216
should be negligible relative to the current generated at the working areas of
the electrodes.
Based on this discovery, the invention features an improved electrode strip
for use in an
electrochemical sensor for measuring an analyte in a sample. The electrode
strip includes an
electrode support, which has a first support edge and a second support edge,
and an electrode
arrangement on the support. The electrode arrangement includes a working
electrode; a -
dummy electrode, and a reference electrode. The working electrode includes a
working
area, which contains assay reaction components, including an enzyme and a
redox mediator.
The working electrode also includes an extension, which is substantially free
of the enzyme
and redox mediator, and an outside edge. The dummy electrode includes a
working area,
l0 which contains assay reaction components, except the enzyme. The dummy
electrode also
includes an extension, which is substantially free of assay reaction
components, and an
outside edge. The reference electrode has a working area-facing side and an
extension-
facing side. The working area extension is located between the reference
electrode and the
first support edge. The dummy electrode extension is located between the
reference
electrode and the second support edge. The electrode strip includes a
dielectric coating,
which covers a portion of the support. The covered portion of the support
includes an area
located between the working electrode extension and the first support edge,
and an area
located between the dummy electrode extension and the second support edge, and
the
dielectric coating covers no portion of the outside edge of the working
electrode or the
outside edge of the dummy electrode.
Preferably, the dielectric coating surrounds the electrode arrangement in
which the
working electrode and the dummy electrode have equal areas. Preferably, each
electrode is a
printed electrode. Preferably, the enzyme is an enzyme that reacts with
glucose, for
example, glucose oxidase or glucose dehydrogenase. The redox mediator can be
any
electrochemically active compound that accepts or donates an electron to an
enzyme. Redox
mediators include ferrocene, ferrocene derivatives, ferricyanide, and osmium
complexes.
Unless otherwise defined, all technical and scientific terms used herein have
the same
meaning as commonly understood by one of ordinary skill in the art to which
this invention
belongs. In case of conflict, the present application, including definitions
will control. All
. publications, patent applications, patents, and other references mentioned
herein are
incorporated by reference.
Although methods and materials similar or equivalent to those described herein
can be
used in the practice or testing of the present invention, the preferred
methods and materials
CA 02302447 2000-02-29
WO 99/13099 PCT/US98/18216
3
are described below. The materials, methods, and examples are illustrative
only and not
intended to be limiting.
Other features and advantages of the invention will be apparent from the
detailed
description, and from the claims.
Brief Description of the Drawings
Fig. 1 is a top view of the electrode region of a prior art electrode sensor
strip.
Fig. 2 is a top view of a preferred embodiment of an electrode strip according
to the
present invention.
Fig. 3 is an exploded view of an electrode strip according to one embodiment
of the
l0 invention.
Fig. 4 is a perspective view of the assembled strip shown in Fig. 3.
Description of the Preferred Embodiments
In a multiple electrode sensor strip, equalization of electrode nonworking
areas, as well
as working areas, enhances the overall precision and accuracy of the
measurements made
using the sensor strip. The beneficial effect of area equalization is most
pronounced at
relatively low glucose concentrations. In the present invention, the exactness
and
reproducibility of electrode area equalization is improved by avoidance of
overlap between
the electrodes and a dielectric coating that defines an electrode exposure
area.
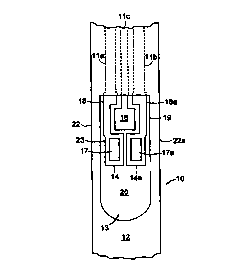
The invention is illustrated by comparison of Figs. 1 and 2, which depict a
prior art
2o electrode strip and an electrode strip of the invention, respectively.
Referring to Fig. 1, the
electrode strip 10 has three printed tracks of electrically conducting carbon
ink I la, l lb,
11 c. A dielectric coating 12 partially covers the electrode strip 10 and
defines an open area
13, which includes an electrode arrangement 19 and a sample loading area 20.
Each printed
track of electrically conducting carbon ink 11 a, 1 I b, 11 c terminates in an
electrode, in the
open area 13. One track I la terminates in a working electrode 14. A second
track 1 lb
terminates in a dummy electrode 14a. A third track terminates in a reference
electrode I6.
The working electrode 14 includes a working area 17, which contains components
of an
analyte assay reaction, including glucose oxidase and a ferrocene redox
mediator. The
working electrode also includes an extension 18, which is a non-working area,
i.e., it does
not contain any assay reaction components.
The dummy electrode includes 14a a working area 17a, which contains components
of
the assay assay reaction, except the enzyme, glucose oxidase. The dummy
electrode also
includes an extension 18a, which is a non-working area, i.e., it does not
contain any assay
CA 02302447 2000-02-29
WO 99/13099 PCT/US98/18216
4
reaction components. The geometry of the dummy electrode 14a forms a mirror
image of
the working electrode 14.
The reference electrode 16 is situated so that one side faces the working
areas 17, 17a,
~or~e side faces the working electrode extension 18, and one side faces the
dummy electrode
extension 18a.
In the prior art electrode depicted in Fig. l, dielectric coating 12 covers a
portion of the
working electrode 14 and dummy electrode 14a, thereby forming a pair of small
overlap
regions 21, 21 a.
Referring to Fig. 2, the electrode strip 10 has three printed tracks of
electrically
1 o conducting carbon ink 11 a, 11 b, 11 c. A dielectric coating 12 partially
covers the electrode
strip 10 and defines an open area 13, which includes an electrode arrangement
19 and a
sample loading area 20. Each printed track of electrically conducting carbon
ink 11 a, 11 b,
11 c terminates in an electrode, in the open area 13. One track 11 a
terminates in a working
electrode I 4. A second track I 1 b terminates in a dummy electrode 15. A
third track
terminates in a reference electrode 16.
The working electrode 14 includes a working area 17, which contains components
of an
analyte assay reaction, including glucose oxidase and a ferrocene redox
mediator. The
working electrode also includes an extension I 8, which is a non-working area,
i.e., it does
not contain any assay reaction components.
2o The dummy electrode includes 14a a working area 17a, which contains
components of
the assay assay reaction, except the enzyme, glucose oxidase. The dummy
electrode also
includes an extension 18a, which is a non-working area, i.e., it does not
contain any assay
reaction components. The geometry of the dummy electrode 14a forms a mirror
image of
the working electrode 14.
The reference electrode 16 is situated so that one side faces the working
areas 17, 17a,
one side faces the working electrode extension 18, and one side faces the
dummy electrode
extension 18a.
In the electrode depicted in Fig. 2, the dielectric coating 12 extends along
the electrode
support edges 22,22a. However, the width of the open area 13 is greater than
the width of
3o the electrode arrangement 19. This creates a gap between the outside edges
of the working
and electrodes, and the surrounding dielectric coating 12. Therefore, the
dielectric coating
12 covers no portion of the outside edge of the working electrode 14 or the
outside edge of
the dummy electrode 14a. The overlap regions 21, 21a, which are present in the
prior art
CA 02302447 2000-02-29
WO 99/13099 PCT/US98/18216
electrode strip 10 depicted in Fig. 1, are absent from the electrode strip 10
depicted in
Fig. 2.
A gap can be created between the outside edges of the electrodes and the
surrounding
dielectric coating by narrowing the electrodes, or by widening the open area,
or both. The
5 extent of electrode narrowing is limited, in part, by the overall resistance
of the electrode
system and printing tolerances.
As the width of the electrode arrangement 19 and the width of the open area 13
are made
closer, imperfect registration of electrode strip layers can cause the
dielectric coating 12
inadvertently to overlap an outside edge 23, 23a of the working electrode 14
or the dummy
t o electrode 14a. Preferably, the difference between electrode arrangement 19
width and open
area 13 width is great enough to accomodate layer registration tolerances in a
manufacturing
process without any overlap of dielectric coating 12 onto electrode edges.
Preferably, the dielectric coating is bonded securely to the electrode
support, mesh
layers, and to an electrode strip cover layer (e.g., polyester tape).
Preferably, the dielectric
layer is hydrophobic. This enhances its ability to confine an aqueous sample
to the electrode
area. Preferred materials for use as the dielectric coating are POLYPLAST? and
SERICARD? {Sericol Ltd., Broadstairs, Kent, UK), with SERICARD? being more
preferred.
The working electrode working area 17 is formed from an ink that includes an
enzyme, a
2o redox mediator, and a filler. The dummy electrode working area 17a is
formed from an ink
that includes the redox mediator and filler, but does not include the enzyme.
The respective
inks can be applied to the carbon tracks 11 a, 11 b by printing, to form
discrete working areas
17, 17a. When the analyte to be measured is blood glucose, the enzyme is
preferably
glucose oxidase, and the redox mediator is a ferrocene derivative.
Referring to Fig. 3, the various layers that make up the electrode strip are
layed down on
an electrode support 36. The electrode support is typically a plastic material
such as PVC,
polycarbonate, or polyester. Three printed tracks of electrically conducting
carbon ink 1 I a,
l lb, 1 Ic formed on the electrode support 36. Silver/silver chloride tracks
35a, 35b, 36c are
then overlayed onto the carbon ink tracks 11 a, i 1 b, 1 l c.
3o Referring to Fig. 3, two surfactant coated mesh layers 30, 31 overlay the
electrode
arrangement 19. The mesh layers protect the printed components from physical
damage.
They also facilitate wetting of the electrodes by the aqueous sample.
Preferably, the mesh
layers extend over the entire sample path, between and including, the sample
loading area 20
CA 02302447 2000-02-29
WO 99/13099 PCT/US98/18216
6
and the electrode arrangement 19. Finely woven nylon is suitable for the mesh
layers.
Alternatively, any woven or non-woven material can be used. For a detailed
discussion of
the mesh layers see Carter et al., U.S. Patent No. 5,628,890, which is herein
incorporated by
reference.
If the mesh material is hydrophobic (e.g., nylon or polyester), it 'is coated
with a -
surfactant. If a hydrophilic mesh is used, the surfactant coating can be
omitted.
Hydrophilicity of the mesh allows the sample to wick along the mesh layer to
the
electrodes. The wicking properties of the mesh can be controlled by changing
the type or
amount of surfactant on the mesh material. Various surfactants are suitable
for coating the
1o mesh material. A preferred surfactant is FC 170C FLUORAD' fluorochemical
surfactant
(3M, St. Paul, MN). FLUORAD' is a solution of a fluoroaliphatic oxyethylene
adduct,
lower polyethylene glycols, 1,4-dioxane, and water.
The preferred surfactant loading will vary depending on the type of mesh and
surfactant
used and the sample to be analyzed. It can be determined empirically by
observing flow of
~5 the sample through the mesh with different levels of surfactant. If two
mesh layers are used,
the second (upper) mesh layer preferably is hydrophilic, but not more
hydrophilic than the
first (lower) mesh layer. Accordingly, the first mesh layer can have a greater
load of
surfactant than the second mesh layer. With regard to the first mesh layer,
suitable
surfactant loading for most applications is about 15-20 ?g/mg of mesh (i.e.,
about 1.0
2o percent w/v). With regard to the second mesh layer, suitable surfactant
loading for most
applications is about 1-10 ?g/mg of mesh.
The mesh layers 30, 31 are held in place by a dielectric coating 12, which
impregnates
the periphery of the mesh layers 30, 31. The dielectric coating 12 can be
applied by screen
printing. The dielectric coating 12 covers no portion of the electrode
arrangement 19.
25 Preferably, the dielectric coating is hydrophobic, so that it efficiently
confines the sample.
Preferably, the hydrophobic dielectric coating is POLYPLAST' (Sericol Ltd.,
Broadstairs,
Kent, UK). More preferably, it is SERICARD' (Sericol).
The uppermost layer on the electrode strip is a cover layer 32. Preferably,
the cover
layer is substantially impermeable. A suitable material for formation of the
cover layer 32 is
3o a flexible polyester tape.
The cover layer 32 defines an upper boundary of the electrochemical cell
volume, and
thus, the cover layer 32 determines the maximum depth of the aqueous sample.
The cover
layer 32 fixes the upper boundary of the cell volume at a predetermined
height, which
CA 02302447 2000-02-29
WO 99/13099 PCT/US98/18216
7
depends on the thickness of the mesh layers 30, 31. The cell height, and thus
maximum
sample depth, is selected to ensure a suitably high solution resistance.
The cover layer 32 has an aperture 33 for sample access to the underlying mesh
layers
3.Or 31. The aperture 33 is located over the sample loading area 20, which is
adjacent to the
upstream ends of the working electrode 14 and dummy electrode 14a: The
aperture 33 cai~
be of any suitable size large enough to allow sufficient volume of sample to
pass through to
the mesh layers 30, 31. It should not be so large as to expose any portion of
the electrode
arrangement 19. The aperture 33 can be formed in the cover layer 32 by any
suitable
method, e.g., die punching.
to Cover layer 32 is peripherally affixed to the strip by means of a suitable
adhesive. The
cover layer 32 is not affixed in the area of the electrode arrangement 19, the
sample loading
area 20, or the area therebetween. Preferably, the cover layer 32 is affixed
by means of a hot
melt adhesive. The hot melt adhesive typically has a coating weight between 10
and 50
g/m2, preferably from 20 to 30 g/mz. Pressure sensitive adhesives or other
suitable
adhesives can also be used. When a heat sensitive dielectric coating 12 is
used, e.g.,
SERICARD', heat welding of the cover layer 32 should be carried out in a
manner that does
not damage the dielectric coating 12.
Optionally, the upper surface of the cover layer 32 can be coated with a layer
of silicone
or other hydrophobic coating. This helps to drive the applied sample onto the
hydrophlic
2o mesh layers 30, 31 at the sample loading area 20, thus facilitating the
application of small
volumes.
Referring to Fig. 4, an electrode strip 10 of the invention is connected, via
electrode
contacts 34, to a compatible meter (not shown), after a sample is placed in
aperture 33.
The following examples are intended to be illustrative and not limiting of the
invention.
Example 1 - Spiked Venous Blood Testing
Eleven batches of electrode strips essentially as shown in Fig. 2 were
constructed. In
addition, a batch of prior art control strips as shown in Fig. 1, were
constructed.
Samples of venous blood were collected in four studies and spiked with various
concentrations of glucose. Small volumes of each samples were applied to the
target area of
3o the sample and control strips and allowed to cover the working and
reference electrodes.
The responses of the strips to the glucose concentration in the blood were
measured after a
steady state response was achieved, using an appropriate meter.
The average calibration results for the eleven batches were calculated and are
listed in
CA 02302447 2000-02-29
WO 99/13099 PCTNS98/18216
8
Table 1. The data include the standard deviations of the results ("S.D.") and
the coefficients
of variation ("CV").
Table 1
Glucose Mean Pooled S.D. CV (%)
Level (mM) (mM) _ -- _ -
1 2.6 0.16 6.1
2 5.1 0.27 5.3
3 9.9 0.44 4.4
4 14.3 0.64 4.5
For comparison, the average precision results for the control batch are listed
in
Table 2. The standard deviations and coefficients of variation were
consistently higher in the
control strips.
Table 2
Glucose Pooled S.D. CV (%)
Level_ Mean (mM)
(mM)
1 2.7 0.28 10.2
2 5.1 0.34 6.7
3 10.1 0.52 5.1
4 15.1 0.71 4.7
Io Example 2 - Control Solution Testing
A standard precision test using 96 replicate measurements of an aqueous
solution
with a glucose concentration of 2.0 mM was carried out on eleven batches of
electrodes of this
invention. The results were compared to results from a corresponding test
carried out on control
(prior art) electrode batches. The results are shown in Table 3.
Table 3
_ Mean
Response SD % CV
(mM)
Test Batches 2.4 0.14 5.8
Control Batches 2.3 0.25 10.87
Other embodiments are within the following claims.