Note: Descriptions are shown in the official language in which they were submitted.
CA 02302590 2000-03-03
WO 99/11306 PCT/NZ98/00133
CODING OF SYRINGES TO MONITOR THEIR USE
INTRODUCTION
This invention relates to methods and apparatus for storage,
dispensing and use of administrable substances, particularly for
anaesthetics. Whilst the invention is primarily directed to
anaesthetics, the invention is not limited thereto and may be used in
other related areas.
BACKGROUND TO THE INVENTION
Hitherto, methods and apparatus for storage and use of
administrable substances such as anaesthetic drugs and the like,
have, in the main, relied upon the skill, alertness and self-imposed
systems of practitioners.
It has long been recognised that errors can and do occur,
sometimes with disastrous consequences, particularly in the area of
anaesthesia where on occasions, owing to tiredness, distraction,
adverse conditions (e.g. emergencies) or lack of attention to
procedures which have become routine, errors can be made which
can result in extremely serious consequences including patient
death.
A likelihood of errors is also exacerbated by an increasing
complexity of drug administration procedures, types of drugs and
their subsets, together with often potentially confusing markings,
packaging, concentrations and the like which all but the most alert
practitioner might otherwise mistake, especially in emergency or
other stressful circumstances.
Many aspects of anaesthesia have highly engineered safety
systems, for example, gas bottle pin index systems to prevent the
administration of a wrong gas from an anaesthetic machine.
Further, gas mixture control systems in place make the
CA 02302590 2000-03-03
WO 99/11306 PCT/NZ98/00133
2
administration of a hypoxic gas mixture virtually impossible. These
engineering advances operate in conjunction with procedural
approaches designed to enhance safety and are backed up by
monitors such as in line oxygen monitors and pulse oximeters. In
contrast, the administration of intravenous drugs has not changed
substantially for many decades, although the number, range and
complexity of drugs has undergone an exponential increase.
The flow-on effect is that in some countries practitioners, and
organisations such as the hospitals with whom they work often have
difficulty in obtaining at reasonable levels an appropriate degree of
negligence or malpractice cover, or the costs of dealing with an
accident can be astronomical. Further, there is a trend toward the
use of criminal law, for example manslaughter prosecutions, in cases
of drug administration error which is of concern to those involved in
anaesthesia and related activity.
OBJECTS OF THE INVENTION
It is an object of this invention to come some way in reducing,
the likelihood of errors in substance administration, and/or to at least
come some way in overcoming the abovementioned problems or at
least provide the public with a useful choice.
Other objects of this invention will become apparent from the
following description.
BROAD DESCRIPTION OF THE INVENTION
According to one aspect of this invention there is provided a
method of monitoring substance administration including the steps
of establishing first and second predetermined coded substance sites
for a predetermined coded substance carrier, placing said carrier in
an at least partially loaded condition prior to use in said first site and
after use in an at least partially discharged condition (relative to said
CA 02302590 2000-03-03
WO 99/11306 PCT/NZ98/00133
3
at least partially loaded condition) in said second site and
maintaining said carrier in said second site for a predetermined
period of time.
According to a further aspect of this invention there is provided
a method of monitoring substance administration including the steps
of forming a support device having a first predetermined coded
substance site for a predetermined coded loaded substance carrier,
forming a second predetermined coded site for such carrier, taking
said carrier from said first predetermined site for use and, after use,
positioning said carrier in the second site
According to a further aspect of this invention there is provided
apparatus for storage and use of at least one administrable
substance carrier including a support defining at least one coded site
in relation to which the predetermined coded carrier can be
positioned, said site coded and adapted to receive and a
predeterminedly coded, loaded carrier, said code provided to enable
user verification of said carrier relative to said at least one site.
According to a still further aspect of this invention there is
provided apparatus for storage and use of at least one administrable
substance carrier including a support defining at least one coded site
in relation to which the predetermined coded carrier can be
positioned, said site provided at least as a set of a first site and a
second site, said first site coded and adapted to receive a
predeterminedly coded, loaded carrier and a second site at least
partially commonly coded and also adapted to receive the carrier,
said code provided to enable user verification of said carrier relative
to said first and second sites.
According to a still further aspect of this invention there is
provided a package of at least one contained administrable
substance for administration in accordance with the method as
outline above, said package including a support as defined
CA 02302590 2000-03-03
WO 99/11306 PCT/NZ98/00133
4
hereinbefore, and wherein at least one of said first sites is charged
with a loaded, substantially corresponding coded carrier for said
administrable substance and means provided between said carrier
and said first coded site for verifying the correct site positioning of
said carrier on said site, a second coded site adapted for verification
of site position.
Other aspects of this invention will become apparent from the
following description. Modifications are envisaged and may be
incorporated without departing from the scope or spirit of the
invention.
DESCRIPTION OF THE INVENTION WITH REFERENCE TO THE
PREFERRED EMBODIMENTS
The preferred form of the invention will now be described with
reference to the accompanying drawings in which:
Figure 1 is a perspective view of an anaesthetic trolley showing
the apparatus of the invention mounted therewith.
Figure 2 is an alternative embodiment of the tray according to
the invention.
Figure 3 is an alternative embodiment of the tray according to
the invention.
While the preferred embodiment of the invention is
described with reference to anaesthesia processes and
anaesthetic products and the drawings, the invention is not
limited thereto. The invention is applicable in other areas of
practice where monitoring of use and a normally predetermined
sequence of use is desired.
CA 02302590 2000-03-03
WO 99/11306 PCT/NZ98/00133
Many anaesthesia practices are carried out according to
relatively standard and repeatable steps, although naturally
there often are variations. In other words, there is a sequence
through which the practitioner often passes during the course
of an operation. For example, the anaesthetist would normally
administer drugs or medications in types or classes, amounts
(usually volumes) and concentrations dependent on, amongst
other things, body mass, degree of anaesthesia required, age,
blood pressure, specific patient criteria etc., however, the
drugs used in the main generally tend to follow certain
predetermined sets of procedures.
There has always previously been a propensity for the
practitioner to rely on a combination of skill, experience,
memory, colleague verification and verification in relation to
notes and procedures to ensure correct drugs are used. The
present invention provides a means of reducing reliance on the
above procedures to reduce mistakes. In particular, the
invention provides a basis for reliance upon sequencing,
monitoring and verification, utilising such features as coding,
including colour codes, bar codes with comparison against
predetermined data and similar techniques and combinations
thereof to achieve risk reduction.
With reference to Figure 1 typically drug ampoules are
stored in the drawer D of an anaesthetist's trolley T. There is
usually no uniformity of presentation, either visually or spacially
and traditionally anaesthetists draw up contents of the
ampoules into syringes for administration of the drugs in many
steps, all of which are highly error prone.
The present invention provides both a means and
apparatus to minimise errors utilising in the preferred form of
the invention prefilled colour coded carriers in the form of
syringes S (see Figures 2 and 3). The syringes S will usually be
CA 02302590 2000-03-03
WO 99/11306 PCT/NZ98/00133
6
prefilled by a hospital pharmacy or pharmaceutical
manufacturer/supplier and be neatly colour coded by class of
drug and other details which may be necessary. Preferably the
colours indicate drug classes rather than individual drugs as a
drug error between classes is usually much more dangerous
than one within a class.
Whilst the preferred form of the invention as described
with reference to coding by colours, it is to be appreciated that
in alternative forms of the inventions, alternative coding can be
incorporated including any one or a combination of:
i. colour coding
ii. colour combinations
iii. pattern codes
iv. numeric codes
v. alpha codes
vi. bar codes
It is however to be appreciate that other forms and
combinations of coding may be adopted without departing from the
scope or sphere of the invention, as defined in the appended claims.
It will be appreciated that mass production of prefilled syringes
and the like is substantially less prone to error than traditional
techniques of staff filling to actual demand requirements. Colour
coding by class will also minimise the total colours used making the
classification system simpler. Whilst colour coding is preferred for
classes of drugs, in alternative arrangements it will also be
appreciated that a combination of drug class/individual drug may
also be provided, for example utilising a two-tier code system or
some other detectable identifier or combination of identifier.
Particularly with reference to Figure 2, in the preferred form
coloured syringe S labels S1 are used incorporating the name of the
drug in bold print of a size that they will wrap around the syringe S
CA 02302590 2000-03-03
WO 99/11306 PCT/NZ98/00133
7
barrel in a way that the colour code can be seen from any likely
syringe orientation. In other forms of the invention its is envisaged
the syringe body or plunger itself can be colour coded, such as at
manufacture.
In the preferred form syringe marking scales will be retained
and further, different densities or shades of colour on the label may
used to indicate the strength or concentration of the drug.
In alternative arrangements it is envisaged that syringes S or
other dispensing apparatus may be prefilled and supplied by drug
companies in a substantially complete state. By providing the drugs
in a "batch manufactured" manner it is envisaged that further risk
reduction will be achieved, the code can also hold this information if
required.
In the preferred form of the invention and with reference to
Figures 1, 2, and 3, syringes S are provided in conjunction with a
drug tray 1. It is envisaged that anaesthetic procedures will be
divided into preferably three classes according to
factors, such as complexity, for example "minor", "intermediate"
and "major". Sealed sterile plastic trays 1 will be prepackaged with
prefilled coded syringes S of the drug classes needed in the
"standard" anaesthetic procedure for each of the three classes,
resulting in three classes of drug tray 1.
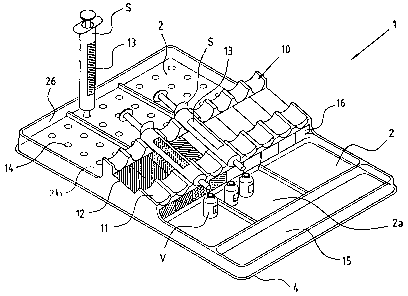
Referring predominantly to Figure 2 the tray 1 design preferably
incorporates separate sites or compartments 2 each, if required,
incorporating individually sealed rip-top covers 3 for each
compartment 2. Each compartment 2 is the same coded colour 2c
as the prefilled syringe S which that compartment 2 is intended to
house either by a suitable label or permanent marking 2c on the
compartments. The compartments 2 are preferably arranged in a
positionally sensitive manner allowing the syringes S to be used
CA 02302590 2000-03-03
WO 99/11306 PCT/NZ98/00133
8
from, for example, left to right across the tray 1 as the anaesthetic
procedure proceeds.
Each compartment 2 is preferably provided with two
subcompartments, a first subcompartment 2a or site, and a second
subcompartment 2b or site. The first subcompartment 2a is
preferably provided adjacent to a tray front 4 for preloaded, filled
syringes S and is designated the "ready" subcompartment 2a. The
other, preferably rearward second subcompartments 2b is provided
for used or empty syringes S (not shown) and is designated the
"used" subcompartment 2b.
In addition to compartments 2a prepacked with filled syringes
S, drug trays 1 in each class will also preferably provide also initially
empty compartments 2 (including both empty first and second
subcompartments 2a and 2b). These empty compartments 2 are
provided for use with drugs which are frequently but not always
used and are therefore considered not strictly "standard". The
additional compartments 2 can, for example, be supplied with
prefilled syringes S from a standard drug drawer D in the
anaesthetists trolley T before starting the anaesthetic procedure.
Coding systems and/or labelling will also be used in relation to
the additional components 2 by inserting, adhering or otherwise
positionally placing both on the syringe S and the additional
compartment 2 appropriate codes such as colour codes or other
identifier means.
Once a syringe S has been used, if further doses are required
these can be obtained by reloading the relevant "ready"
subcompartment 2a of the tray 1 with additional prefilled syringes S
from a source, perhaps a colour coded drug drawer D elsewhere on
the anaesthetists trolley, sympathetically or correspondingly set out
and possibly similarly or otherwise coded for ready verification.
Used syringes S will accumulate in the relevant "used"
CA 02302590 2000-03-03
WO 99/11306 PCT/NZ9S/00133
9
subcompartment 2b of the tray 1 as the anaesthetic proceeds and
be retained there until the completion of the whole procedure, thus
providing ready verification of the identity and amount of drug used
at any point in the procedure.
There will always be a certain number of drugs which are not
readily available in prefilled syringes S. In most instances, it is
envisaged that these drugs will be infrequently used, or are perhaps
drugs which are not stable in a plastic syringe S for long periods. A
section of the tray 1, for example a righthand section 5 thereof is
designed to accommodate drugs only available in ampoules.
In the preferred form of the invention, the coded compartments
2 in this section comprise three subcompartments, a forwardmost
compartment 2c for the placement of ampoule A from a colour
coded ampoule drawer (not shown) elsewhere in the drug trolley,
the middle subcompartment 2e for placement of the syringe S
conventionally filled from the ampoule A and colour coded; together
with a rearmost compartment 2c for an empty ampoule A (not
shown) after the syringe S has been filled.
It will be appreciated that in such a system, keeping track of
syringes S and ampoules A until completion of the procedure
maintains a visually striking monitor of drug administration and at
any time it is possible for practitioners to check at a glance what has
been administered and, equally important what has not been, to
reduce the potential for error to the individual anaesthetist and also
to enhance continuity where one anaesthetist hands over to another
during long anaesthetics.
Whilst the invention has been described with reference to a
series of "standard" combinations of anaesthetics, it is to be
appreciated that alternative arrangements can also provide for the
use of, for example, an emergency tray of a generally similar
specification to the standard anaesthetics tray 1 prepackaged with
CA 02302590 2000-03-03
WO 99/11306 PCT/NZ98/00133
prefilled colour codes syringes S of drugs used in an anaesthetic
emergency. An emergency drug tray of this type may have the
greatest potential to reduce drug error since it is during an
emergency that errors are most likely to occur. The emergency tray
1 may be stocked or restocked from an emergency "reserve" drug
drawer D in a similar way to the standard trays 1.
The invention envisaged that additional monitoring (including
preferably verification, and/or recordal) systems are incorporated
into the apparatus. It is envisaged in the preferred form of the
invention that each syringe S will incorporate some identification
means comparable against predetermined data, for example in a
prepared database, to positively identify the contained drug, for
example by class, individual drug, concentration and other relevant
data to the procedure. Preferably much of such information is
incorporated into a conveniently arranged code positioned on the
syringe S such as a bar code, however in alternative forms of the
invention, other identification means may be provided, for example
electronically stored and/or readable identification apparatus,
magnetic or digital devices, data information and the like.
In this arrangement, as each syringe S is taken from the ready
compartment 2a, it may be, for example, "swiped" under a
conveniently positioned reader as part of the drug administration
routine the detected code will be compared against the database
information and drug identified, whereupon a calm computer
generated voice will announce the name and dose of the drug just
swiped optionally coupled with a visual display. The response will
preferably occur at a time anticipated to be before the actual drug
administration. It is envisaged that this will considerably reduce the
risk of drug error by supplementing the anaesthetist's already
received information with further auditory/visual information to
hopefully allow correction of any errors before administration.
CA 02302590 2000-03-03
WO 99/11306 PCT/NZ98/00133
11
In the preferred form of the invention, information received by
the monitoring apparatus will be conveyed and stored as a record,
for example in a microprocessor based device including a database
of drug, drug use and patient information loaded thereon. It is
anticipated that the practitioner may, on receiving confirmation of
the identity of syringe S from the computerised announcement or
verification may physically confirm, for example by depressing a
"confirm" key, to confirm verification and/or administration, by
taking such action either prior to or subsequent to administration of
the identified drug. Measuring apparatus can also optionally be
provided connected either directly or indirectly with the syringe S to
monitor, measure and record amounts of such drug administration,
regardless of the syringe S volume as loaded.
In this way, it will be appreciated that both physical
confirmation and verification may be provided, and further, the
apparatus will provide a record of the actions of the practitioner. It
is envisaged that such a record may be valuable subsequently,
should complications arise, or other checking be considered
appropriate, and could also be integrated into or connected with
known recording apparatus recording general operations monitoring
equipment.
The monitoring method and apparatus may incorporate a series
"standard" or "specific" administrations previously worked out for
the anaesthetic procedure. In such circumstances, it is envisaged
that the monitoring apparatus will have such procedures entered into
the database and the monitoring apparatus will detect and then
compare the removal of syringes S from the "ready"
subcompartment 2a of the tray 1 against a predetermined "standard
administration order" and not only will provide auditory/visual
verification of the syringe S taken, but may also provide an auditory/
visual or other warning to the anaesthetist of any variation from the
predetermined routine of administration.
CA 02302590 2000-03-03
WO 99/11306 PCT/NZ98/00133
12
Whilst the invention has been described with reference to
syringes S and trays 1 with an associated bar code reader, it is
envisaged that in an alternative form of the invention the
compartments 2 are provided with suitable sensing or detection
means 6, for example positioned in the base 7 of each
subcompartment 2a/2b. Further, the syringes S are provided with
identification means thereon in the form of magnetic/digital devices
and others, which can be readily detected by the sensors 6 placed
within the base of the tray 1.
The monitoring apparatus is set up to distinguish individual
syringes S and drug classes and characteristics in the compartments
2 such that at any stage an accurate and reliable verification of
supply, use and countback of drugs/syringes used can be provided
and also be monitored against predetermined and anticipated usage
manually or via the database as a cross-checking procedure.
Whilst the invention has been described with reference to the
provision of sensors 6 placed within the base of the tray 1, in
alternative embodiments of the invention, it is envisaged that the
upper portion of the trolley T, or some other support apparatus
adapted to be used with the tray 1 of this invention may be provided
with suitable sensors; the tray 1 being provided of a means
substantially inert to interaction between the syringe code and the
sensor 6 so as enable simple formation of the trays, or provision of
the trays as a liner for separate support apparatus. In this way, it
will be appreciated that the cost of tray 1 can be kept to a minimum
and further, the sensors/monitoring apparatus will not interfere
unduly with necessary sterilisation and other hygiene steps
inevitably required.
In the preferred form of the invention, preferably the tray 1
apparatus is provided as a plastics or metal tray 1 able to be
sterilised and adapted for ready placement and holding of the
syringes S in the required layout for substantially standardised use
CA 02302590 2000-03-03
WO 99/11306 PCT/NZ98/00133
13
and providing the first "ready" and the second "used"
subcompartments 2a and 2b in a visually separate manner.
In the further embodiment of the invention as described
predominantly with reference to Figure 3, the drug tray 1 is vacuum
formed in a thin sheet plastics material, for example transparent or
translucent plastics sheet which is capable of being readily cleansed
by heat, irradiation and the like. The tray 1 is preferably arranged in
a generally "tapered" configuration so as to be "nestably stackable"
with similar trays 1, such that a "pack" of trays 1 can be supplied
for general use. Preferably the tray 1 is dimensioned for use with
the standard drugs trolley T, substantially as shown in Figure 1 and
further the outer peripheral dimensions of the tray 1 are such that
preferably a pair of trays 1 according to Figure 3 can be mounted
side-by-side on the standard drugs trolley T as is typically used in a
theatre or other hospital situation, although such use is not
essential.
In this form of the invention the sites or compartments 2 are
positioned on either side of an enlargement 10 upon which a
plurality of arcuate rests or syringe sites 11 are provided. The
syringe sites 11 are in this form inclined toward a front 4 of the tray
1 such that syringes S can be readily supported, and viewable by
the user. The syringe S after use is able to be positioned in the
second compartment 2b which has tapered apertures provided in the
second compartment 2b into which a boss B of the syringe S body
can optionally frictionally engage, to thus mount the syringe S neatly
in a secure and readily visible, verifiable substantially upright manner
after use.
The syringe sites 11 also include a predetermined array
(preferably three in respect to each compartment 2 "set") of arcuate
rests into which the syringe S can be mounted, inclined forwardly to
the user to provide good vision for the user and the syringe S and
CA 02302590 2000-03-03
WO 99/11306 PCT/NZ98/00133
14
coding (for example colour coding) at 12 on the sites 11, and on the
body of the syringe S.
It will be appreciated that correspondingly coded and possibly
prefilled syringes S or dedicated syringes S for particular drugs can
be readily positioned on the relevant sites 11 on the rests and on the
tray 1 in a verifiable positional relationship.
Preferably a supplementary area 15 is provided across the front
4 of the tray 1 for incidental items and the like as may be required
during the course of the anaesthesia operation.
It is envisaged that the enlargement 10 created by the raised
area defining the syringe sites 11 will readily enable the enclosed
mounting of the monitoring apparatus described hereinbefore, or at
least the sensor 6.
It is also envisaged that the drugs trolley T can be arranged on
it's upper portion thereof with an enlargement over which the tray 4
can fit. In this assembly coding 12 can be positioned either on the
trolley T prior to the application of a tray 1 thereover, where the
coding 12 can be "read" through transparent or translucent portions
of the tray 1, or alternatively, the coding 12 can be affixed on an
underside of the tray 1.
Preferably additional coding 12 may be provided substantially
corresponding on a front face 16 of the enlargement 10 to enable
additional simple code 12 verification relevant to the particular "row"
of the compartments, the syringe sites 11 and in the second
compartment 2b.
Where the invention incorporates the use of a "standard" drugs
tray 1 incorporating a series of "standard" combinations of
anaesthetics, it is to be appreciated that the drugs and drugs tray 1
may be stocked in a "package" form, where a recess provided
CA 02302590 2000-03-03
WO 99/11306 PCT/NZ98/00133
beneath the enlargement 10 is used for storage of the drugs,
syringes S and other items to be used in an anaesthesia operation,
optionally contained within a tear-off sheet plastics sheet and the
like releasably mounted across adjacent portions of an underside of
the tray 1, thus enclosing the items on the underside of the tray 1
which on removal therefrom can be used with the tray 1 in the
manner previously described.
The stackable nature of the tray in one alternative embodiment
enables a convenient "bulk" store of trays 1 to be held (for example
in packs of 10, 20 and the like) for convenient usage when required.
Tray 1 packages can incorporate sets of separate self-adhesive
labels or devices holding the codes and for mounting on the tray 1,
on syringes S and vials V or ampoules A for matching purposes.
The sets of codings may be arranged for either substantially
"standard" use codes or alternatively, for special or specific codes to
be provided in special use arrangements.
In one alternative form of the invention coded labels arranged
for the syringes S are provided in a substantially inverted L shaped
configuration, to enable positioning along the syringe body and
provision of a readily verifiable code together with a bar code (or
interactive indicator for a sensor/monitoring apparatus arrangement)
yet still leaving a visual "window" for use of syringe volume
graduations thereon.
In further alternative embodiments of the invention, it is
envisaged that the additional monitoring checking and notification
systems of the apparatus also provide the ability for users to enter
further information including, for example specific patient drug
allergies and furthermore, to hold on the database or library standard
codes and pharmaceutical details for drugs. This facility enables
enhancement of the monitoring and in particular, the warning facility
described in relation to the preferred embodiment, whereby should a
CA 02302590 2000-03-03
WO 99/11306 PCT/tYZ98/00133
16
user attempt to give a drug to which a patient is allergic or at
variance with predetermined protocols, a timely warning can be
given.
In a further embodiment of the invention, the apparatus can
verify and record not only drug identity and strength, but also
measure the amount of drug actually administered giving the user
additional information during the procedure, and also providing a
verifiable record subsequently. Furthermore, the code may
additionally provide a basis for drug batch identification and to
provide raw data and actuation for inventory information, control
and drug reordering.
In one embodiment of the invention the monitoring apparatus
may be integrated, preferably via a microprocessor to additionally
provide an integrated help facility for pharmaceutical information
such as dosages, drug properties and the like. One such use would
be for the database or library of information on commonly used
drugs to be accessible by the user who brings a coded syringe S or
other coded drug carrier into proximity with the reader or scanner of
the monitoring apparatus and, for example operates a specified key
or actuation device to access pharmaceutical information on the
drug and its properties during the course of the procedure.
Whilst the invention has been described with reference to a
tray 1 and to prefilled syringes S, the invention is not limited to such
arrangements and it is envisaged that other drug administration
apparatus can be provided and utilised in conjunction with the
methods and apparatus described.
Thus, by this invention there is provided a method and
apparatus for administration of substances which substantially
reduces the risk of errors and provides significant convenience and
security.