Note: Descriptions are shown in the official language in which they were submitted.
CA 02303132 2001-O1-31
I_NTRANASAL FORMULATION CONTAINING SCOPOLAMINE AND
METHOD OF TREATING MOTION SICKNESS
FIELD OF THE INVENTION
The present invention relates to pharmaceutical formulations containing
scopolamine.
More particularly, the present invention relates to nasal gel formulations for
intranasal
delivery of scopolamine, in particular, for preventing and/or treating motion
sickness.
BACKGROUND OF THE INVENTION
Scopolamine, and more particularly, its salt Scopolamine Hydrobromide, have
been
investigated for a variety of clinical indications. Examples of potential uses
for scopolamine
include the treatment of general nausea and/or vomiting, motion sickness,
peripheral vertigo,
post operative conditions and the use as an anesthetic.
Several delivery routes have been utilized for administering scopolamine.
These
include oral, transdermal, buccal and intranasal administration. Oral and
transdermal
administration, however, do not provide a rapid onset of a therapeutically
effective amount
of scopolamine as determined by bioavailability studies. Oral administration
of scopolamine
is further complicated by first-pass metabolism in the liver which can
significantly reduce
1
CA 02303132 2000-03-02
WO 99/12544 PCT/US98/18953
its bioavailability. Buccal administration of scopolamine has also been
investigated. It has
been reported, however, that the bioavailability from buccal administration
does not
significantly differ from oral administration.
Intranasal delivery of scopolamine has shown potential for the rapid onset of
a
therapeutically effective amount of the compound. For example, International
Application
No. PCT/US82/00941, published as WO 83/00286 on February 3, 1983 discloses
treatment
of sudden motion sickness with a nasal spray of scopolamine. While this
reference discusses
the rapid onset of scopolamine via the intranasal delivery route, there is no
consideration of
sustained delivery of scopolamine over a period of time or the storage
stability of such a
formulation.
While intranasal administration of various drugs such as scopolamine is known,
the
development of intranasal formulations to provide a therapeutically effective
amount of a
drug and the stability of the formulation over time is often unpredictable.
While many drugs
can be provided in intranasal formulations, the drug delivery offered by such
formulations
cannot be readily predicted and can dramatically differ between apparently
similar
formulations. Moreover, for an intranasal formulation of scopolamine to be
effective in the
prevention and treatment of acute conditions such as motion sickness, the
ability to provide
a therapeutically effective amount within 30 minutes, and desirably within 20
minutes, of
administration is necessary. Likewise, considerations such as providing a
therapeutically
effective amount of the drug as soon as possible, maintaining a
therapeutically effective level
over a sustained amount of time and stability of the formulation over time
must be balanced.
In considering the intranasal delivery of drugs, the pharmokinetics thereof
are often
considered. For example, ionization of a drug is believed to directly
influence membrane
penetration of the drug, and therefore, the absorption potential of the drug
into the blood
2
CA 02303132 2001-O1-31
stream. In particular, the ionization of a drug and therefore its absorption
potential, is largely
determined by the drug's dissociation constant, pKa, as well as the pH of the
solution in
which the drug is dissolved. As reported by Mayersohn in Modern Pharmaceutics,
Banker
& Rhodes, 1979, Ch. 2, Pg. 40, basic compounds are best absorbed from alkaline
solutions
where pH > pK,. Thus, it is generally believed that formulations for
delivering basic drugs,
in particular intranasal formulations, are best absorbed into the bloodstream
when the basic
drug is prepared in a formulation solution having a pH above the dissociation
constant of the
drug.
For example, scopolamine is known to be a basic drug. In order to provide
effective
membrane penetration and absorption through intranasal delivery, heretofor it
has been
understood that scopolamine hydrobromide should be formulated in a basic
solution having
a formulation pH greater than 7.6.
Intranasal formulations of scopolamine hydrobromide at pH levels below 7 have
been
investigated. For example, in "Absorption for Nasal Mucous Membrane: Systemic
Effect
of Hyoscine Following Intranasal Administration" by Tonndorf et al. in inn
Oto. Rhino.
La~vngol., vol. 62, 630, 1953, intranasal scopolamine spray formulations were
prepared at
a pH between 5.7-6Ø This reference discloses formulations which are
inherently inefficient
requiring 0.65 mg/ml and 0.4 mg/ml of scopolamine per dose, respectively. Such
high
dosages are wasteful of drug, add unnecessary cost to consumer and may cause
undesirable
side effects.
Accordingly, there is a need in the art for intranasal formulations that
provide a
therapeutically effective amount of scopolamine into the bloodstream within a
relatively
3
CA 02303132 2000-03-02
WO 99/12544 PCT/US98/18953
short time period (e.g., 30 minutes or less), that provide therapeutically
effective levels of
scopolamine for a sustained amount of time, that do not degrade over time and
are not
irntating to the nasal cavity.
~ilMA_RY OF THE INVENTION
It is an object of the present invention to provide an intranasal formulation
for
preventing and/or treating nausea and/or vomiting and other symptoms
associated with
motion sickness.
It is a further object of the invention to provide a scopolamine nasal
formulation
capable of prolonged shelf storage.
It is a further object of the invention to provide an intranasal formulation
capable of
achieving rapid plasma concentrations without achieving dangerous peak plasma
concentrations.
In the efficient attainment of these and other objects, the present invention
provides
an intranasal formulation including scopolamine in a pharmaceutically
acceptable carrier.
The formulation has a pH below about 4.0, desirably at or below about 3.5, and
a salt
concentration below about 200 mM, desirably at or below 100 mM, such as for
example at
or below 50 mM. Desirably, the formulation incorporates polyvinyl alcohol
therein.
Preferably the Garner is provided as an intranasal gel, with the polyvinyl
alcohol acting as
a gelling agent for the composition. The scopolamine is preferably provided as
a
pharmaceutically acceptable salt, such as for example, scopolamine
hydrobromide.
4
CA 02303132 2000-03-02
WO 99/12544 PCT/US98/18953
The present invention also relates to a method of preventing and/or treating
nausea
including administering intranasally to a mammal an effective amount of
scopolamine,
chemically modified equivalents and pharmaceutical salts thereof in a
pharmaceutically
acceptable Garner at a pH below about 4.0 and a salt concentration below about
200 mM;
with the carrier incorporating polyvinyl alcohol.
BR1FF nF~~RIpTION OF THE DRAWINGS
Figure 1 is a graph representing average plasma concentration over time of
intranasal
gel formulations incorporating different gelling agents.
Figure 2 is a graph representing product degradation as a function of the
percentage
formulation molarity over time for intranasal gel formulations.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
The present invention is an intranasal formulation for delivery of
scopolamine. As
used herein, "intranasal formulation" is intended to include a
pharmaceutically acceptable
carrier which incorporates the active agent, i.e., scopolamine. For purposes
of the present
invention, "pharmaceutical carrier" includes nasal sprays, nasal drops, gels,
ointments,
creams and the like. The present formulations may be administered using, for
example, a
nasal tampon or a nasal sponge containing the present formulation. Desirably,
however,
scopolamine is delivered in a gel formulation as set forth in more detail
below.
Polyvinyl alcohol (PVA) is known to enhance viscosity. It is well known that
PVA
should be used in a pH range of about 5 - 8. Thus, one skilled in the art
would not be
motivated to formulate PVA-containing pharmaceutical compositions at a pH
lower than 5
CA 02303132 2000-03-02
WO 99/12544 PCT/US98/18953
and would reject such a composition as a pharmaceutical earner for, e.g.,
scopolamine.
Surprisingly, it has been demonstrated in the present invention that gel
formulations of
scopolamine having PVA as the main gelling agent provide products with
excellent stability
which are superior to prior art spray formulations, as well as gel
formulations using methyl
cellulose. Thus, nasal gel preparations of scopolamine according to the
present invention are
prepared using PVA. The amount of PVA that can be used in the present
invention can vary
depending upon the specific formulation. In particular, the amount of PVA
contained in the
present invention is that amount which is sufficient to form a
pharmaceutically acceptable
gel. Desirably, PVA is present in the present formulations up to about 30%,
more desirably,
up to about 20%, such as for example up to about 10%.
Gel systems having as a main component a composition having similar properties
to
PVA and which provide results substantially as set forth in the Examples below
are also
contemplated by the present invention. For example, gelling agents including
the following
can be used as a substitute for or in addition to PVA: alginates, gums,
starches, polyacrylates,
dextrans, chitosans and mixtures thereof.
In the present invention, scopolamine is combined with the pharmaceutical
earner
at a pH of about 4. Desirably, scopolamine is combined with the pharmaceutical
carrier at
or below about pH 3.5.
In addition to maintaining the pH of the present formulation at or below 4,
the salt
concentration thereof must be maintained at or below about 200 mM. Desirably,
the salt
concentration is maintained at or below about 100 mM, such as for example at
or below
about 50 mM.
For purposes of the present invention, the term "scopolamine" as used herein
is
intended to include those pharmaceutically active scopolamine compositions set
forth in The
6
CA 02303132 2000-03-02
WO 99/12544 PCTNS98I18953
Merck Index (11th Edition on page 8363) including [7(S)-(1a,2~3,4~3,5a,7~3)]-a-
(Hydroxymethyl)benzeneacetic acid 9-methyl-3-oxa-9-azatricyclo-[3.3.02'4]non-7-
yl ester
and 6~i,7(3-epoxy-laH,SaH-tropan-3a-of (-)-tropate. Moreover, as used herein,
"scopolamine" also includes pharmaceutical salts and hydrated forms, as well
as all
chemically modified equivalents thereof. Scopolamine hydrobromide or
scopolammonium
bromide (C,~HZZBrN04~3HZ O), scopolamine hydrochloride (~, I~ C1N~,7 ),
methscopolamine
bromide and methscopolamine nitrate (C,gH24N20~) are examples of
pharmaceutical salts
which can be used in accordance with the present invention. As used herein,
"chemically
modified equivalents" is intended to include compositions which may have a
chemical
structure that differs from scopolamine but which functions in a similar
manner in the body,
such as for example prodrugs, analogs, biologically active fragments and the
like.
Such compositions have clinical utility to prevent and treat nausea and/or
vomiting
associated with, for example motion sickness. In addition, such compositions
can be used
as a sedative and as a pre-anesthetic. The present formulations, thus can be
used to treat
and/or prevent a variety of symptoms.
As set forth above, the present formulations can be used to both prevent and
treat
nausea and/or vomiting induced by motion sickness. Thus, the present
formulations can be
administered to a mammal prior to any symptoms associated with motion sickness
and can
prevent nausea and/or vomiting which are often symptoms thereof. Moreover,
once onset
of motion sickness symptoms has occurred in a patient, the present
formulations can be
administered to the patient and will provide alleviation or substantial
decrease of the nausea
and/or vomiting associated with motion sickness.
In the present invention, many other excipients, known from the pharmaceutical
literature, may be added to the formulations, such as preservatives,
surfactants, co-solvents,
7
CA 02303132 2000-03-02
WO 99/12544 PCT/US98/18953
adhesives, antioxidants, buffers, viscosity enhancing agents and agents to
adjust the pH or
the osmolarity.
The various forms of the intranasal formulations set forth above can
optionally
include a buffer to maintain the pH of the scopolamine formulation, a
pharmaceutically
acceptable thickening agent, humectant and surfactant. Desirably, the pH of
the buffer is
selected to maintain the stability of scopolamine. In particular, the pH of
the buffer is
selected to optimize the stability of the scopolamine in the present inventive
formulations.
As set forth previously, the pH of the buffer is desirably below about 4, more
desirably at or
below about 3.5. Buffers that are suitable for use in the present invention
include, for
example, hydrochloride, acetate, citrate, carbonate and phosphate buffers.
The viscosity of the compositions of the present invention can be maintained
at a
desired level using a pharmaceutically acceptable thickening agent. Thickening
agents that
can be used in accordance with the present invention include for example,
xanthan gum,
carbomer, polyvinyl alcohol, alginates, acacia, chitosans and mixtures
thereof. The
concentration of the thickening agent will depend upon the agent selected and
the viscosity
desired.
The compositions of the present invention also include a tolerance enhancer to
reduce
or prevent drying of the mucus membrane and to prevent irntation thereof.
Suitable
tolerance enhancers that can be used in the present invention include, for
example,
humectants, sorbitol, propylene glycol, mineral oil, vegetable oil and
glycerol; soothing
agents, membrane conditioners, sweeteners and mixtures thereof. The
concentration of the
tolerance enhancer(s) in the present compositions will also vary with the
agent selected.
In order to enhance absorption of the scopolamine through the nasal mucosa, a
8
CA 02303132 2001-O1-31
therapeutically acceptable surfactant may be added to the intranasal
formulation. Suitable
surfactants that can be used in accordance with the present invention include,
for example,
polyoxyethylene derivatives of fatty acid partial esters of sorbitol
anhydrides, such as for
example, Tween 80*Polyoxyl 40 Stearate,~Polyoxy ethylene 50
Stearat~*fusidates, bile salts
and Octoxynol* Suitable surfactants include non-ionic, anionic and cationic
surfactants.
These surfactants can be present in the intranasal formulation in a
concentration ranging from
about 0.001 % to about 20% by weight.
In the present invention other optional ingredients may also be incorporated
into the
nasal delivery system provided they do not interfere with the action of the
scopolamine or
significantly decrease the absorption of scopolamine across the nasal mucosa.
Such
ingredients can include, for example, pharmaceutically acceptable excipients
and
preservatives. The excipients that can be used in accordance with the present
invention
include, for example, bio-adhesives and/or swelling/thickening agents.
In the present invention, any other suitable absorption enhancers as known in
the art
may also be used.
Preservatives can also be added to the present compositions. Suitable
preservatives
that can be used with the present compositions include, for example, benzyl
alcohol,
parabens, thimerosal, chlorobutanol and benzalkonium, with benzalkonium
chloride being
preferred. Typically, the preservative will be present in the present
compositions in a
concentration of up to about 2% by weight. The exact concentration of the
preservative,
however, will vary depending upon the intended use and can be easily
ascertained by one
skilled in the art.
Another embodiment of the present invention is an intranasal formulation for
*Trade-mark
CA 02303132 2000-03-02
WO 99!12544 PCT/US98/18953
preventing or treating motion sickness. This formulation includes scopolamine
hydrobromide in a PVA gel solution at a pH at or below about 3.5 and a salt
concentration
below about 100 mM. In this embodiment, the PVA gel solution can include
mixtures of
other gelling agents or bio-adhesives, such as for example, alginates, gums,
starches,
polyacrylates, dextrans, chitosans and mixtures thereof. Moreover, the PVA
gelling agent
can be replaced with other similar gelling/bio-adhesives provided that such
agents produce
the surprising results as set forth in the examples of the present invention.
Another embodiment of the present invention is a method of preventing and/or
treating nausea and/or vomiting. This method includes administering
intranasally to a
mammal an effective amount of scopolamine, chemically modified equivalents and
pharmaceutical salts thereof in a pharmaceutically acceptable Garner at a pH
at or below
about 4.0 with a salt concentration at or below about 200 mM. The
pharmaceutically
acceptable carrier is desirably PVA, however, other gelling/bio-adhesives
which provide
results similar to those set forth in the present experimental examples are
also contemplated.
Examples of such gelling/bio-adhesives include, alginates, gums, starches,
polyacrylates,
dextrans, chitosans and mixtures thereof. Moreover, the pharmaceutically
acceptable carrier
can include PVA and mixtures of other appropriate gelling/bio-adhesives
provided the
formulation produces superior pharmacokinetic profiles along the lines set
forth in the
Examples.
The pharmaceutically acceptable carrier of the present invention is
specifically
designed for intranasal administration. Such formulations are safe and
effective for
intranasal delivery to mammals, including humans. Formulations of such
carriers are well
known in the art and specific examples thereof are provided below.
In the present embodiment of the invention, the salt concentration is
desirably at or,
CA 02303132 2000-03-02
WO 99/12544 PCT/US98/18953
below 100 mM, such as for example, 50 mM. As set forth above, the pH of the
present
formulation is desirably at or below about 3.5.
The following examples are set forth to illustrate the formulations of the
present
invention, as well as the surprising results achieved therewith. These
examples are provided
for purposes of illustration only and are not intended to be limiting in any
sense.
11
CA 02303132 2000-03-02
WO 99/12544 PCT/US98/18953
EXAMPLE 1
Inventive and Com~rative Formulations
Example 1 sets forth a comparative intranasal scopolamine hydrobromide nasal
gel formulation containing methyl cellulose with a buffer concentration of 25
mM
(Formulation 1 ) and three inventive intranasal scopolamine hydrobromide nasal
gel
formulations according to the present invention containing PVA at 20, 50 and
100 mM
buffer concentrations (Formulations 2, 3 and 4, respectively).
Nasal gel Formulation 1 containing a methyl cellulose gelling agent at a pH of
about 3.5 and having a scopolamine concentration of about 0.2 mg/0.1 gm at a
buffer
concentration of 0.025M (25 mM) was prepared as follows:
Formulation 1 - Methyl Cellulose (25 mM)
Composition Quantity -
100mL(Gm)
Scopolamine Hydrobromide, 0.20
USP
Citric Acid Anhydrous, USP 0.37
Sodium Citrate Dihydrate, 0.17
USP
Sodium Metabisulfite, NF 0.1
Glycerine (96%), USP 5.0
Methyl cellulose (4000 cps), 2.0
USP
Benzalkonium Chloride {50%), 0.04
NF
Purified Water, USP 100 Q.S.
Nasal gel Formulation 2 containing a PVA gelling agent at a pH of about 3.5
and
having a scopolamine concentration of about 0.2 mg/0.1 gm at a buffer
concentration of
0.02M (20 mM) was prepared as follows:
12
CA 02303132 2000-03-02
WO 99/12544 PCT/US98/18953
Formulation 2 - PVA (20 mM)
Composition Quantity -
100mL(Gm)
Scopolamine Hydrobromide, USP 0.20
Citric Acid Anhydrous, USP 0.32
Sodium Citrate Dihydrate, USP 0.098
Sodium Metabisulfite, NF 0.1
Glycerine (96%), USP 5.0
Polyvinyl Alcohol, USP 10.0
Benzalkonium Chloride (50%), 0.04
NF
Purified Water, USP 100 Q.S.
Formulation 3 - PVA (50 mM)
Composition Quantity -
100mL(Gm)
Scopolamine Hydrobromide, USP 0.20
Citric Acid Anhydrous, USP 0.73
Sodium Citrate Dihydrate, USP 0.34
Sodium Metabisulfite, NF 0.1
Glycerine (96%), USP 5.0
Polyvinyl Alcohol, USP 10.0
Benzalkonium Chloride (SO%), 0.04
NF
Purified Water, USP 100 Q.S.
13
*rB
CA 02303132 2000-03-02
WO 99/12544 PCT/US98/18953
Formulation 4 - PVA (100 mM)
Composition Quantity -
100mL(Gm)
Scopolamine Hydrobromide, USP 0.20
Citric Acid Anhydrous, USP 1.42
Sodium Citrate Dehydrate, USP 0.76
Sodium Metabisulfite, NF 0.1
Glycerine (96%), USP 5.0
Polyvinyl Alcohol, USP 10.0
Benzalkonium Chloride (50%), 0.04
NF
Purified Water, USP 100 Q.S.
EXAMPLE 2
~parison of Methxl Cellulose vs. PVA Formulations:
Scopolamine Absorption
Example 2 is a comparisonof scopolamineabsorptioninto the blood stream from
Formulation 1 (methyl cellulose at 25 mM buffer concentration)vs. Formulation2
(PVA
at 20 mM buffer concentration). Nasal gel formulations were prepared based on
Formulations 1-2 set forth above. These Formulations were adjusted to a pH
value of
about 3.5 with citric acid solution or sodium citrate solution as needed.
The nasal gel Formulations l and 2 were administered intranasally to 10
healthy
humans. The plasma concentration of scopolamine free base was measured in
these
individuals over time for a period of 240 minutes by LC/MS/MS. The average
results
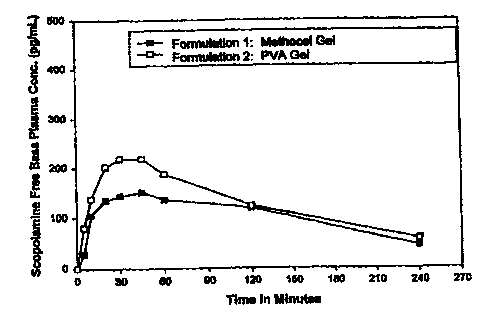
of these measurements are shown in Table 1, and depicted in Figure 1.
14
CA 02303132 2000-03-02
WO 99/12544 PCT/US98/18953
Compiarison of Methyl Cellulose vs PVA Formul~tions~
Scouolamine Absorption
Time (minutes)Formulation 1" Formulation
2"
0 0~0 0~0
5 28.4 t 45.1 80.7 ~ 58.3
10 106 t 157 138.6 ~ 86.7
20 136 t 144 203 f 124
30 144 ~ 100 2191 112
45 152 ~ 119 220 ~ 111
60 137 t 106 188 t 127
120 119 ~ 99.0 123 t 81.8
240 44.1 t 37.5 58.3 ~ 39.7
Cmax' (pg/ml) 204 ~ 161 248 ~ 123
T,"e~' (minutes)45.5 t 31.2 35.5 ~ 12.8
"Average Plasma Concentration of Scopolamine tree f3ase (pg~m~).
' C,".~ and Tm,x values represent an average of the respective Cm" and Tm,~
values obtained
for each patient.
As can be seen from these results, absorption of scopolamine into the blood
was
within acceptable limits as recognized by those skilled in the art for both
Formulations
1 and 2.
A comparison of the plasma concentrations of scopolamine free base for
Formulation 1 (methyl cellulose) with Formulation2 (PVA) demonstratesthe
unexpected
and surprising results achieved through the present invention. As is clearly
evident from
Figure 1 which is a graphic representation of the data set forth in Table l,
the average
plasma concentration over time of Formulation 1 (methyl cellulose) was
considerably
below that of Formulation 2 (PVA). Thus, the intranasal formulation according
to the
CA 02303132 2000-03-02
WO 99/12544 PCTIUS98/18453
present invention (Formulation 2) achieves significantly higher scopolamine
absorption
into the blood compared to intranasal Formulation 1 (methyl cellulose).
Additionally,
rapid onset of the drug is achieved with the present inventive formulation
(Formulation
2), as is evidenced by the graph which shows scopolamine free base
concentrations of
Formulation 2 which according to the present invention more than doubled that
of the
Formulation 1 in 5 minutes.
EXAMPLE 3
Comparison of Stabilitir of PVA and
Meth,rtl Cgllulose Intranasal Formulations
This Example provides stability data comparing Formulation 1 (methyl
cellulose)
with Formulation 2 (PVA) at various temperatures and relative humidities.
For purposes of the present examples, formulations having unacceptable
stability
exhibited more than 1 % degradation of product resulting in the formation of
tropic acid
over 6 months of storage at 40°C and 75% humidity in accordance with
generally
accepted FDA guidelines for minimum stability.
Nasal gel Formulations 1 and 2 having a scopolamine concentration of 0.2
mg/0.1 gm at a buffer concentration of 0.025M (25mM) and 0.02 (20 mM),
respectively
were prepared in accordance with the formulations of Example 1.
A. Stability at 40°CI75% RH
Formulations 1 and 2 were adjusted to a pH value of about 3.5 with citric acid
solution or sodium citrate solution as needed. The respective formulations
were stored
in a standard drug container in both upright and inverted positions at a
temperature of 40 °
C and 75 % relative humidity, over time for a period of 6 months. Various
measurements
were taken to represent stability of each formulation, including Scopolamine
HBr content
16
CA 02303132 2000-03-02
WO 99/12544 PCT/US98/18953
as a percentage, degradation of the product represented by the percentage of
tropic acid
appearing in the formulation and viscosity. The results are set forth in Table
2
(Formulation 1 - methyl cellulose) and Table 3 (Formulation 2 - PVA) below.
Table 2
S Methyl Cellulose (25 mM)
Container~ Scopolamine Degradation Viscosit3r
M hs Position HBr (% LCl Product TroRiclets)
Acid. %LCj
initial -- 3.48 102.6 not detectable4393
1 Upright 3.30 101.0 not quantifiable1452
1 Inverted 3.31 101.7 not quantifiable1341
2 Upright 3.13 102.3 not quantifiable--
2 Inverted 3.13 104.1 not quantifiable--
3 Upright 3.11 106.6 0.23 --
3 Inverted 3.09 104.2 0.23 --
6 Upright 3.03 101.4 0.45 206
6 Inverted 3.08 102.3 0.45 259
17
*rB
CA 02303132 2000-03-02
WO 99/12544 PCT/US98/18953
PVA (20 mM~
TIME Container~ Scopolamine Degradation Viscosity
ctsl
~Monthsl Position HBr (,% LC) Product (,Tro~fc
Acid. %LCl
initial -- 3.64 104.0 not detectable1711
1 Upright 3.52 100.9 not quantifiable1714
1 Inverted 3.50 100.8 not quantifiable1761
2 Upright 3.49 100.8 not quantifiable--
2 Inverted 3.46 101.0 not quantifiable--
3 Upright 3.31 102.8 0.21 --
3 Inverted 3.29 103.5 0.22 --
4 Upright 3.09 102.0 0.27 --
4 Inverted 3.11 103.0 0.27 --
5 Upright 3.08 102.7 0.33 --
5 Inverted 3.06 102.9 0.34 -- i
6 Upright 3.03 105.4 0.50 1564
6 Inverted 3.03 102.6 0.40 1618
As is evident from the data depicted in Table 2, Formulation 1 (methyl
cellulose)
provides stability with respect to scopolamine HBr content, maintaining 101.4%
and
102.3 % for upright and inverted containers, respectively, over 6 months
storage.
Moreover, the degradation of this gel formulation over time is within
acceptable limits,
demonstrated by 0.45 percent of tropic acid in the formulation after 6 months.
The
viscosity of Formulation 1 (methyl cellulose), however, rapidly decreased from
an initial
level of 4393 cts to 1452 cts and 1341 cts for upright and inverted
containers,
respectively, after only 1 month of storage, and further decreasing to 206 cts
and 209 cts
for upright and inverted containers, respectively, after 6 months storage,
demonstrating
an unacceptable change in viscosity for stability.
18
CA 02303132 2000-03-02
WO 99/12544 PCTNS98118953
As is evident from the data depicted in Table 3, Formulation 2 (PVA) remains
both chemically and physically stable over the entire 6 month period, as
evidenced by the
Scopolamine HBr content, degradation product and viscosity of the formulation
remaining within acceptable ranges, even after 6 months of storage time.
B. Stability at 30°C/60% RH
The stability of Formulations 1 and 2 was investigated at a temperature of 30
°C
at 60% relative humidity over the course of 6 months substantially as set
forth above.
The data from this investigation is set forth below in Table 4 (Formulation 1 -
methyl
cellulose) and Table 5 (Formulation 2 -PVA):
Table 4
Meth~~l Cellulose 25 m1!!I)
TIME S;ontainer~ Scopolamine. DegradationViscosi
(Montli,~i io HBr (% LCl Product T~ (ctrl
Acid. ,./oLCI
initial -- 3.48 102.6 not detectable4393
1 Upright 3.40 102.3 not detectable-
1 Inverted 3.42 102.7 not detectable-
2 Upright 3.28 104.6 not detectable-
2 Inverted 3.26 104.0 not detectable-
3 Upright 3.26 106.9 not quantifiable2082
3 Inverted 3.24 107.5 not quantifiable2233
6 Upright 3.15 103.4 not quantifiable1083
6 Inverted 3.16 103.2 not quantifiable1305
19
CA 02303132 2000-03-02
WO 99/12544 PCT/US98/18953
Table 5
PVA (20 mMl
TIME Containerp~ copolamine Degradation Viscosity
(cts)
Mph i i HBr (% LC) Product (Tropic
Acid, %LC~
initial -- 3.64 104.0 not detectable17I 1
1 Upright 3.60 101.5 not detectable--
1 Inverted 3.59 100.5 not detectable--
2 Upright 3.63 100.7 not detectable--
2 Inverted 3.66 101.7 not detectable--
3 Upright 3.51 104.7 not quantifiable1665
3 Inverted 3.52 104.3 not quantifiable1637
6 Upright 3.34 105.4 not quantifiable1633
6 Inverted 3.40 104.2 not quantifiable1595
As is evident from the data depicted in Table 4, Formulation 1 (methyl
cellulose)
stored at 30 ° C and 60% relative humidity provides stability with
respect to scopolamine
HBr content, maintaining 103.4% and 103.2 % for upright and inverted
containers,
respectively, over 6 months storage. Moreover, the degradation of this gel
formulation
over time is within acceptable limits, demonstrated by no quantifiable percent
of tropic
acid in the formulation after 6 months. The viscosity of Formulation 1,
however, again
rapidly decreased from an initial level of 4393 cts to 2082 cts and 2233 cts
for upright
and inverted containers, respectively, after 3 months of storage, and further
decreased to
1083 cts and 1305 cts for upright and inverted containers, respectively, after
6 months
storage, demonstrating an unacceptable change in viscosity for stability.
As is evident from the data depicted in Table 5, Formulation 2 (PVA) remains
both chemically and physically stable over the entire 6 month period, as
evidenced by the
Scopolamine HBr content, degradation product and viscosity of the formulation
remaining within acceptable ranges, even after 6 months of storage time.
CA 02303132 2000-03-02
WO 99/12544 PCT/US98/18953
C. Stability at 25°C/60% RH
The stability of Formulations 1 and 2 was investigated at a temperature of 25
°C
at 60% relative humidity over the course of 6 months substantially as set
forth above.
The data from this investigation is set forth below in Table 6 (Formulation 1 -
methyl
cellulose) and Table 7 (Formulation 2 -PVA):
Table 6
Met~rl Cellulose (25 mM)
T_ IME Container~ ScopslamineDegradation Viscosi
onths) Position HBr (% LCl Product (Tropic, (cts)i
A cid. %LC~
initial -- 3.48 102.6 not detectable4393
3 Upright 3.26 107.0 not detectable--
3 Inverted 3.32 102.4 not detectable--
6 Upright 3.19 103.2 not quantifiable1755
6 Inverted 3.19 103.2 not quantifiable2129
Table 7
PVA (20 mMl
TIME Container ~ Sconolamin~Degradation Viscosi
nths Position HBr~% LCl Product ~[, ~ctsl
ro~ic
Acid. %LCl
initial -- 3.64 104.0 not detectable1711
3 Upright 3.55 104.4 not detectable--
3 Inverted 3.56 103.8 not detectable--
6 Upright 3.46 104.0 not quantifiable1640
6 Inverted 3.47 104.6 not quantifiable1640
As is evident from the data depicted in Table 6, Formulation 1 {methyl
cellulose)
21
CA 02303132 2000-03-02
WO 99/12544 PCT/US98/18953
provides stability with respect to scopolamine HBr content, maintaining 103.2
% for both
the upright and inverted containers over 6 months storage. Moreover, the
degradation
of this gel formulation over time is within acceptable limits, demonstrated by
no
quantifiable percent of tropic acid in the formulation after 6 months. The
viscosity of
Formulation I (methyl cellulose), however, again decreased from an initial
level of 4393
cts to 1755 cts and 2129 cts for upright and inverted containers,
respectively, after 6
months storage, demonstrating an unacceptable change in viscosity for
stability.
As is evident from the data depicted in Table 7, Formulation 2 (PVA) remains
both chemically and physically stable over the entire 6 month period, as
evidenced by the
Scopolamine HBr content, degradation product and viscosity of the formulation
remaining within acceptable ranges, even after 6 months of storage time.
D. Stability at 15 ° C/40% RH
The stability of Formulations 1 and 2 was investigated at a temperature of I 5
°C
at 40% relative humidity over the course of 6 months substantially as set
forth above.
1 S The data from this investigation is set forth below in Table 8
(Formulation 1 - methyl
cellulose) and Table 9 (Formulation 2 -PVA):
ethyl Cellulose (25 mMli
n i r g~ Scouolamine]gradation Viscosi
(cts)
,(Months)Position HBr ~% LCl Product (Tropic
Acid. %LCj
initial -- 3.48 102.6 not detectable4393
3 Upright 3.35 106.5 not detectable--
3 Inverted 3.33 105.9 not detectable--
6 Upright 3.30 105.6 not detectable3418
6 Inverted 3.33 103.7 not detectable3220
22
CA 02303132 2000-03-02
WO 99/12544 PCT/US98/18953
PVA (~Q mMl
TIME container~H Scopolamine D~radation Viscosi
on_l~hs)Position HBr l% LCl Prnduct i(Tronic(cts)
Acid. %LCl
initial -- 3.64 104.0 not detectable1711
3 Upright 3.62 103.2 not detectable--
3 Inverted 3.62 103.4 not detectable--
6 Upright 3.56 104.2 not detectable1709
6 Inverted 3.55 104.1 not detectable1839
As is evident from the data depicted in Table 8, even with Formulation 1
(methyl
cellulose) stored at 15 ° C and 40% relative humidity, stability with
respect the viscosity
of the formulation decreased from an initial level of 4393 cts to 3418 cts and
3220 cts for
upright and inverted containers, respectively, after 6 months storage. Thus,
even under
cold storage conditions, the stability of Formulation 1 (methyl cellulose) was
inadequate,
as demonstrated by an unacceptable change in viscosity
As is evident from the data depicted in Table 9, Formulation 2 (PVA) remains
both chemically and physically stable over the entire 6 month period, as
evidenced by the
Scopolamine HBr content, degradation product and viscosity of the formulation
remaining within acceptable ranges, even after 6 months of storage time.
Thus, these data support the surprising conclusion that Formulation 2
containing
PVA as a gelling agent is consistentlymore stable comparedto Formulation 1
containing
methyl cellulose as a gelling agent over the entire 6 month investigational
period at a
variety of temperatures and relative humidities.
23
CA 02303132 2000-03-02
WO 99/12544 PCTIUS98/18953
EXAMPLE 4
Fffect of Molaritv on Stability of PVA Formulations
~9 mM, 50 mM and 100 mM)
Example 4 is a study of the effect of different molarities on PVA stability
over
time. In particular, Formulations 2, 3 and 4 were prepared substantially as
set forth in
Example 1 with molarities of 20 mM, 50 mM and 100 mM, respectively.
Formulations 2, 3 and 4 were adjusted to a pH value of about 3.5 with citric
acid
solution or sodium citrate solution as needed. The respective formulations
were stored
in a standard drug container in both upright and inverted positions at various
temperatures and relative humidity, over time for a period of 6 months.
Various
measurements were taken to represent stability of each formulation, including
Scopolamine HBr content as a percentage, degradation of the product
represented by the
percentage of tropic acid appearing in the formulation and viscosity. The
results are set
forth in Tables 10 - 21 below.
24
CA 02303132 2000-03-02
WO 99/12544 PCT/US98/18953
Table 10
formulation 2 at 40°C/75% Relative Humidity l%RHl
TIME Containerp~I- Scopolamine Degradation Viscosi
M~, onths)Po ' ' HBr (% LC? Product y , fctsl
n ronic
acid, %LC~
initial -- 3.64 104.0 not detectable1711
1 Upright 3.52 100.9 not quantifiable1714
I Inverted 3.50 100.8 not quantifiable1761
2 Upright 3.49 100.8 not quantifiable--
2 Inverted 3.46 101.0 not quantifiable--
3 Upright 3.31 102.8 0.21 --
3 Inverted 3.29 103.5 0.22 --
4 Upright 3.09 102.0 0.27 --
4 Inverted 3.11 103.0 0.27 --
5 Upright 3.08 102.7 0.33 --
5 Inverted 3.06 102.9 0.34 --
6 Upright 3.03 105.4 0.50 1564
6 Inverted 3.03 102.6 0.40 1618
CA 02303132 2000-03-02
WO 99/12544 PCT/US98/18953
~Qrmalation 3 at 40°C/75% RH
DIME Container~H Scopolamine Degradation Viscosity
(cts)
(Months)Position HBr l% LCl Product Tropic
A cid. %LCl
initial -- 3.50 103.1 not detectable1705
1 Upright 3.50 104.5 not quantifiable1659
1 Inverted 3.40 102.2 not quantifiable1696
2 Upright 3.40 102.5 0.22 --
2 Inverted 3.40 103.0 0.21 --
3 Upright 3.40 104.2 0.32 --
3 Inverted 3.40 104.5 0.31 --
4 Upright 3.34 104.0 0.43 --
4 Inverted 3.32 103.0 0.41 --
5 Upright 3.33 103.6 0.56 --
5 Inverted 3.32 103.4 0.60 --
6 Upright 3.32 104.5 0.68 1579
6 Inverted 3.30 103.0 0.66 1709
26
CA 02303132 2000-03-02
WO 99/12544 PCT/US98/18953
Formulation 4 at 40°C/75% RH
Containerp~ Scopolamine Dggradation Viscosity
M pt Position HBr l% LCD Product (Tropicfetal
Acid. %LC)
initial -- 3.52 106.5 not detectable1924
1 Upright 3.58 102.9 not quantifiable1751
1 Inverted 3.57 103.6 not quantifiable1755
2 Upright 3.49 102.5 0.35 --
2 Inverted 3.48 102.7 0.33 --
3 Upright 3.44 103.5 0.50 --
3 Inverted 3.44 103.5 0.48 --
6 Upright 3.39 101.5 1.00 1747
6 Inverted 3.39 101.4 0.99 1701
Formulation 2 at 30°C/60%
Container~ ScopolamineDegradation Viscos~~cts)
(Months)Position HBr (% LC) Product,(Trooic
A cid. %LCl
initial -- 3.64 104.0 not detectable1711
1 Upright 3.60 101.5 not detectable--
1 Inverted 3.59 100.5 not detectable--
2 Upright 3.63 100.7 not detectable--
2 Inverted 3.66 101.7 not detectable--
3 Upright 3.51 104.7 not quantifiable1665
3 Inverted 3.52 104.3 not quantifiable1637
6 Upright 3.34 105.4 ~ not quantifiable1633
6 Inverted 3.40 104.2 not quantifiable1595
27
CA 02303132 2000-03-02
WO 99/12544 PCT/US98/18953
Table 14
Formulation 3 at 30°C/60% RH
~'IME Container phi ScopolamineDegradation Viscosity~cts)
(MonthslP i i n HBr i[% Product (Tropic
LC) Acid. %LCl
initial -- 3.50 103.1 not detectable1705
1 Upright 3.60 104.4 not detectable1693
1 Inverted 3.60 104.4 not detectable1884
2 Upright 3.50 104.0 not quantifiable--
2 Inverted 3.50 103.1 not quantifiable--
3 Upright 3.50 105.3 not quantifiable--
3 Inverted 3.50 104.5 not quantifiable--
6 Upright 3.46 104.7 0.68 1610
6 Inverted 3.45 104.3 0.66 1618
Table 15
Formu~~tion 4 at 30 ° C/60% RH
TIME Containerp~ Scopolamine Degr lotion Viscosity
o thsl it'on HBr (% LC) Product lTrgyic(cts)
Acid. %LC)
initial -- 3.52 106.5 not detectable1924
1 Upright 3.57 104.6 not quantifiable--
1 Inverted 3.59 103.8 not quantifiable--
2 Upright 3.52 103.0 not quantifiable--
2 Inverted 3.51 103.7 not quantifiable--
3 Upright 3.48 104.5 not quantifiable1810
3 Inverted 3.50 104.8 not quantifiable1776
6 Upright 3.46 103.3 0.35 1770
6 Inverted 3.46 103.7 0.35 1709
28
CA 02303132 2000-03-02
WO 99/12544 PCT/US98/18953
Table 16
Formulation 2 at 25°C/60%RH
TIME Container~H ScopolamineDegradation Viscosity
~Monthsl os't'on HBr (% LCl Product Tropic~(ctsl
Acid. %LC)
initial -- 3.64 104.0 not detectable1711
3 Upright 3.55 104.4 not detectable--
3 Inverted 3.56 103.8 not detectable--
6 Upright 3.46 104.0 not quantifiable1640
6 Inverted 3.47 104.6 not quantifiable1640
Ta a 1
Formulation 3 at 25°C/60%RH
TIME Container~ Scopolamine Degradation Viscosity_(ctsl
(Months)Position HBr l% LC) Product Tropic
Acid, %LCl
initial -- 3.50 103.1 not detectable1705
1 S 3 Upright 3.50 104.8 not quantifiable--
3 Inverted 3.50 104.3 not quantifiable--
6 Upright 3.50 104.8 not quantifiable1633
6 Inverted 3.42 104.4 not quantifiable1938
29
CA 02303132 2000-03-02
WO 99/12544 PCT/US98/18953
Formulation 4 at 25°C/60%RH
TIME Container ~H ScopolamineDeEradation Viscosity_(cts)
on Po i ' HBr ~% LCD Product ~Trooic
n Acid. %LC~
initial -- 3.52 106.5 not detectable1924
3 Upright 3.51 ~ 104.3 not quantifiable--
3 Inverted 3.50 105.0 not quantifiable--
6 Upright 3.47 103.7 0.24 1770
6 Inverted 3.48 103.7 0.24 1701
Table 19
Formulation 2 at 15°C/40%RH
Containerp~-I Ss.QpolamineDegradation Viscosit~y,jcty
M~ oaths)Position HBr l% LCl Produc ro~ic
Acid. %LC~
initial -- 3.64 104.0 not detectable1711
1 S 3 Upright 3.62 103.2 not detectable--
3 Inverted 3.62 103.4 not detectable--
6 Upright 3.56 104.2 not detectable1709
6 Inverted 3.55 104.1 not detectable1839
CA 02303132 2000-03-02
WO 99/12544 PCT/US98/18953
Table 2U
Formulation 3 at 15°C/40%RH
TIME Container~H Scopolamine Degradation Vi~cositv
Months) Position HBr (% LCl Product (Tropicfctsl
Acid. %LC)
initial -- 3.50 103.1 not detectable1705
3 Upright 3.50 103.4 not detectable--
3 Inverted 3.50 104.7 not detectable--
6 Upright 3.51 104.1 not quantif 1701
able
6 Inverted 3.49 104.8 not quantifiable1686
fable 21
Formulation 4 at 15°C/40%RH
TIME Container~I ScopolamineDegradation Visco~ixy
M~, onths)Position HBr f% LCl Product,jTroyicfetal
Acid. %LCl
initial -- 3.52 106.5 not detectable1924
1 S 3 Upright 3.51 105.5 not detectable--
3 Inverted 3.52 105.6 not detectable--
6 Upright 3.49 104.5 not quantifiable1846
6 Inverted 3.49 104.0 not quantifiable1801
Tables 10-21 above demonstrate that Formulations 2 - 4 according to the
present
invention remain both chemically and physically stable when stored at varying
conditions
of temperature and humidity as measured by scopolamine HBr content,
degradation
product and viscosity. Each of these parameters remained within acceptable
limits as
recognized by one skilled in the art even after 6 months of storage. The data
indicate,
however, that at PVA concentrations at about 100 mM (Formulation 4) the
degradation
product as represented by the % tropic acid is at 1.00% and 0.99% after 6
months of
storage (Table 12). Thus, the 100 mM formulation (Formulation 4) approached
31
CA 02303132 2000-03-02
WO 99/12544 PCT/US98/18953
unacceptable levels representing chemical instability thereof at elevated
temperatures for
prolonged storage periods. Thus, the data suggest that nasal formulations at
PVA
concentrations above 100 mM are not likely to be useful due to chemical
instability.
AMPLE 4
Stabili r of PVA Formulations qt Different Buffer Concentrations
Example 4 is a direct comparison of the effect of the buffer concentration on
the
PVA Formulations (Formulations 2, 3 and 4) at 40°C/75%RH. This data is
set forth in
Table 22 and graphed in Figure 2, which demonstrates product degradation as a
function
of the percentage of tropic acid in the formulation over time.
Table 22
Effect of Buffer C~centration on Stabilihr of PVA Formulations
Buffer Tropic
Acid
(%
LC)
(ND=not
detectable;
NQ=not
quantifiable)
Molarity
Formulation(mM) 1 2 3 4 5 6
# nitialmonth month month month month month
2 20 ND NQ NQ 0.21 0.27 0.33 0.50
3 50 ND NQ 0.22 0.32 0.43 0.56 0.68
4 100 ND 0.20 0.35 0.50 -- -- 1.00
As is evident from Table 22 and Figure 2, Formulations 2, 3 and 4 prepared
according to the present invention with PVA remain physically and chemically
stable for
up to 6 months. In fact, the formulations at 20 mM and 50 mM, as represented
in Tables
10 and 11, respectively, provide excellent stability results even at the 6
month storage
time, while Formulation 4 (100 mM) as represented by Table 12, approaches
unstable
limits of 1.00 % tropic acid, representing chemical degradation at the 6 month
storage
date. Moreover, it can be recognized from these data that formulations
incorporating
PVA prepared at a pH of about 3.5 and at concentrations above 100 mM lose
chemical
stability during storage.
32
CA 02303132 2000-03-02
WO 99/12544 PCT/US98/18953
e5
Com an rison of Stabili~r of
Methsrl Cellulose vs. PVA Formulations
as a Function of Viscosity
Example 5 is a direct comparison of Formulation 1 (methyl cellulose) and
Formulation 2 (PVA) at 40°C/75%RH highlighting the viscosity data as
set forth in
Table 23 below.
Ta
Met 1 Cellulose vs. PVA Formulations
as a Function of Viscosity
Viscosity
(cts)
FormulationInitial1 month 6 month
#
1 4393 1452 206
2 1711 1714 1564
As is evident from a review of the results of Table 23, the nasal gel
formulation
prepared according to the present invention with polyvinyl alcohol as a
gelling agent in
a formulation at about pH 3.5 and concentration of 20 mM (Formulation 2)
maintains a
substantially constant viscosity over time, thus evidencing that such
formulations remain
chemically and physically stable for periods of 6 months. The nasal gel
formulation
prepared with methyl cellulose as a gelling agent in a formulation at about pH
3.~ and
concentration of 25 mM (Formulation 1 ) demonstrates a significant decrease in
viscosity
after only one month of storage, with a remarkable decrease after 6 months of
storage,
thus evidencing that such a formulation is chemically and physically unstable.
The invention being thus described, it will be obvious that the same may be
varied
in many ways. Such variations are not to be regarded as a departure from the
spirit and
scope of the invention and all such modifications are intended to be included
within the
scope of the following claims.
33