Note: Descriptions are shown in the official language in which they were submitted.
CA 02303460 2008-04-28
-1-
SYNERGISTIC EFFECTS OF OP/BMP MORPHOGENS
AND GDNF/NGF NEUROTROPHIC FACTORS
Field of the Invention
The present invention relates generally to methods and preparations for the
treatment of
mammalian subjects afflicted with, or at imminent risk of, damage or injury to
tissues, particularly
neural tissues, which express receptors for OPBMP morphogens and GDNF/NGF
neurotrophic
1
factors.
Background of the Invention
During development of the mammalian nervous system, differentiating neurons
from the
central and peripheral nervous systems send out axons that must grow and make
contact with
specific target cells. In some cases, neurons stay confined entirely within
the central nervous
system. In other cases, however, growing axons must cover enormous distances,
extending from
the CNS into the periphery of the body. In mammals, this stage of neurogenesis
is completed
during the embryonic phase of life and, it is believed, neuronal cells do not
multiply once they
have fully differentiated.
Dendritic growth occurs in two phases: initial extension followed by
elongation and
ramification. Purves el al., Nature 336:123-128 (1988). Some molecules,
including
neurotransmitters and hormones, have been shown to regulate expansion of an
existing dendritic
arbor. Much less is known, however, about the factors that influence early
events, and cause a
neuron to initially form dendrites. In certain classes of neurons, initial
dendritic sprouting occurs
as part of an intrinsic developmental program which is relatively independent
of trophic
interactions. Dotti el al., J. Neurosci. 8:1454-1468 (1988). In other classes
of neurons, however,
the regulation of the initial stages of dendritic growth appears to be quite
different. For example,
rat sympathetic neurons fail to form dendrites and extend only axons when they
are cultured in the
absence of non-neuronal cells. In contrast, co-culture with Schwann cells or
astrocytes causes
these neurons to form dendritic processes and to eventually generate a
dendritic arbor which is
comparable in size to that observed in situ. Tropea et al., rlia 1:380-392
(1988). Thus, it would
appear that specific trophic interactions are required to allow sympathetic
neurons to form
dendrites.
CA 02303460 2000-03-08
WO 99/12560 PCTIUS98/18772
-2-
The foregoing observations have been taken to support a theory that the in
situ environment
specifies formation of a dendritic arbor. The environment in the vicinity of
neural cells or
developing neural processes is thought to include electromagnetic,
electrochemical and/or
biochemical fields or gradients which positively and negatively influence the
extent and specificity
of dendritic outgrowth as well as the formation of synapses between dendrites
and nerve cell
bodies and axons. This theory, however, suffers from a paucity of identified
mediators which
have the capacity to cause neurons to sprout dendrites.
A host of neuropathies, some of which affect only a subpopulation or a system
of neurons in
the peripheral or central nervous systems have been identified. The
neuropathies, which may
affect the neurons themselves or the associated glial cells, may result from
cellular metabolic
dysfunction, infection, exposure to toxic agents, autoimmunity dysfunction,
malnutrition or
ischemia. In some cases the cellular dysfunction is thought to induce cell
death directly by
apoptosis. Oppenheim, Ann Rev. Neurosci. 14:37-43 (1991). In other cases, the
neuropathy may
induce tissue necrosis by stimulating the body's immune system, resulting in a
local inflammatory
response and cell lysis at the initial site of neural injury.
The ability of neurons within the peripheral nervous system to regenerate a
damaged neural
pathway is limited. Specifically, new axons and dendrites extend randomly, and
are often
misdirected, making contact with inappropriate targets that can cause abnormal
function. In
addition, where severed nerve processes result in a gap of longer than a few
millimeters, e.g.,
greater than 10 millimeters (mm), appropriate nerve regeneration does not
occur, either because
the processes fail to grow the necessary distance, or because of misdirected
axonal growth.
Efforts to repair peripheral nerve damage by surgical means has met with mixed
results,
particularly where damage extends over a significant distance. In some cases,
the suturing steps
used to obtain proper alignment of severed nerve ends stimulates the
formulation of scar tissue
which is thought to inhibit axon regeneration. Even where scar tissue
formation has been
reduced, as with the use of nerve guidance channels or other tubular
prostheses, successful
regeneration generally is still limited to nerve damage of less than 10
millimeters in distance.
It is now well established that various trophic factors play a critical role
in regulating the
survival and differentiation of developing neurons. Snider et al., Ann.
Neurol. 26:489-506
(1989). Most of the characterized actions of nerve trophic actors relate to
developmental events
and suggest that the temporal and local regulation of expression of these
proteins plays a role
during maturation of the nervous system. Nerve trophic factors are also
important in the function
CA 02303460 2000-03-08
WO 99/12560 PCT/US98/18772
-3-
of the adult nervous system for the maintenance of structural integrity and
regulation of plasticity.
Such processes are altered in neurodegenerative diseases and neurodegenerative
events following
acute injury to the nervous system. This has prompted speculation that nerve
trophic factors are
involved in the structural alterations which occur in response to injury and
disease.
Several well-characterized trophic factors have been shown to enhance the
survival and
differentiation of dopaminergic neurons in tissue culture and/or following
transplantation to the
anterior chamber of the eye. These trophic factors include fibroblast growth
factor (FGF),
epidermal growth factor (EGF), platelet-derived growth factor (PDGF),
transforming growth
factor-a (TGF-a), and glial cell-derived neurotrophic factor (GDNF), as well
as several Nerve
Growth Factor (NGF) related neurotrophins.
Nerve trophic factors are found among several protein superfamilies of
polypeptide growth
factors based on their amino acid sequence homology and/or their three-
dimensional structure.
MacDonald el al., Cell 73:421-424 (1993). One family of neurotrophic factors
is the
neurotrophin family. This family currently consists of Nerve Growth Factor
(NGF), Brain
Derived Neurotrophic Factor (BDNF), Neurotrophin-3 (NT-3), Neurotrophin-4/5
(NT-4/5), and
Neurotrophin-6 (NT-6). These neurotrophic factors affect specific neuronal
populations in the
central nervous system. The loss of such specific neurotrophic factors may be
responsible for
age-related declines in cell survival and/or function. While the cellular
source remains unclear,
there is evidence to suggest that neurons and glial cells are both capable of
secreting neurotrophic
factors.
The osteogenic protein/bone morphogenetic protein (OP/BMP) proteins form a
family, or
subfamily, within the larger TGF-(3 superfamily of proteins. That is, these
proteins form a distinct
subgroup, referred to herein as the "OP/BMP family of morphogens" or "OP/BMP
morphogens,"
within the loose evolutionary grouping of sequence-related proteins known as
the TGF-(3
superfamily. Members of this protein family comprise secreted polypeptides
that share common
structural features, and that are similarly processed from a pro-protein to
yield a carboxy-terminal
mature protein. OPBMP morphogens have been identified in developing and adult
rat brain and
spinal cord tissue, as determined both by northern blot hybridization of
morphogen-specific
probes to mRNA extracts from developing and adult nerve tissue and by
immunolocalization
studies. For example, northern blot analysis of developing rat tissue has
identified significant OP-
I mRNA transcript expression in the CNS. The mRNA of another OP/BMP family
member,
CA 02303460 2000-03-08
WO 99/12560 PCT/US98/18772
-4-
GDF-1, appears to be expressed primarily in developing and adult nerve tissue,
specifically in the
brain, including the cerebellum and brain stem, spinal cord and peripheral
nerves. BMP4 (also
referred to as BMP2B) and Vgr-1 transcripts also have been reported to be
expressed in nerve
tissue.
The morphogen OP-I was found to be localized predominantly to the
extracellular matrix of
the grey matter (neuronal cell bodies), distinctly present in all areas except
the cell bodies
themselves. In white matter, formed mainly of myelinated nerve fibers,
staining appears to be
confined to astrocytes (glial cells). A similar staining pattern also was seen
in newborn rat
(10 day old) brain sections.
In addition, OP-I has been specifically localized in the substantia nigra,
which is composed
primarily of striatal basal ganglia, a system of accessory motor neurons whose
function is
associated with the cerebral cortex and corticospinal system. Dysfunctions in
this subpopulation
or system of neurons are associated with a number of neuropathies, including
Huntington's chorea
and Parkinson's disease.
Summary of the Invention
The present invention is based, in part, upon the discovery that OPBMP
morphogens in
combination with GDNF/NGF neurotrophic factors show a synergistic effect in
promoting the
survival or growth, or inhibiting the death or degeneration, of mammalian
cells, particularly neural
cells, which express OP/BMP-activated serine/threonine kinase receptors and
GDNF/NGF-
activated tyrosine kinase receptors. Based on this discovery, the present
invention provides new
methods for in vivo and in vitro treatment of such cells, including in vivo
treatments for mammals
afflicted with, or at imminent risk of, damage or injury to such cells, as
well as new
pharmaceutical preparations for such in vivo and in vitro treatments.
Thus, in one aspect, the present invention provides methods for promoting the
survival or
growth of mammalian cells, particularly neural cells, by contacting the cells
with an effective
concentration of a preparation comprising a GDNF/NGF neurotrophic factor and
an OP/BMP
morphogen. Similarly, the present invention provides methods for inhibiting
the death or
degeneration of mammalian cells, particularly neural cells, by contacting the
cells with an effective
concentration of a preparation comprising a GDNF/NGF neurotrophic factor and
an OP/BMP
morphogen. Such methods may be employed in vitro (e.g., for improved cell
cultures) or in vivo
(e.g., for treating conditions affecting such cells in a mammalian subject).
CA 02303460 2000-03-08
WO 99/12560 PCT/US98/18772
-5-
In another aspect, the present invention specifically provides methods for
treating a
mammalian subject afflicted with, or at imminent risk of, damage or injury to
cells, particularly
neural cells, comprising contacting the neural cells with an effective
concentration of a
preparation comprising a GDNF/NGF neurotrophic factor and an OPBMP morphogen.
These
methods can be applied to either neurons or neuroglial cells, and to either
central or peripheral
nervous system cells.
The methods of the present invention are particularly suited to the treatment
of cells which
have been damaged or injured, or are at imminent risk of damage or injury, due
to mechanical
traumas such as blunt force traumatic brain injury, blunt force traumatic
spinal cord injury,
concussion, intracranial pressure due to cerebral edema or subdural haematoma,
broken or
crushed vertebra, and torn or severed nerves; chemical traumas such as those
arising from
exposure to neurotoxins or the side effects of chemotherapies; ischemic
injuries such as those
arising from stroke, cardiac arrest or failure; and neuropathic or
neurodegenerative damage or
injury such as those arising from neuropathic diseases including Parkinson's
disease, Huntington's
disease, Amyotrophic Lateral Sclerosis, Alzheimer's disease, epilepsy,
progressive muscular
atrophy, Charcot-Marie-Tooth disease, palsy, dementia, Shy-Drager disease,
Wernicke-Korsakoff
syndrome, and Hallervorden-Spatz disease.
In preferred embodiments, the OPBMP morphogens of the present invention
comprise
polypeptides having amino acid sequences with at least 70% homology, more
preferably 80%
homology, with the C-terminal seven-cysteine domain of human OP-1. In
particularly preferred
embodiments, the OPBMP morphogens comprise polypeptides having at least 60%
amino acid
identity, more preferably at least 70% identity, with the C-terminal seven-
cysteine domain of
human OP-l. In most preferred embodiments, the OPBMP morphogen comprises at
least the C-
terminal six- or seven-cysteine domain of a mammalian, preferably human, OP-l,
OP-2, OP-3,
BMP2, BMP3, BMP4, BMP5, BMP6, or BMP9 protein. Preferably, the OPBMP
morphogens
of the present invention are capable of inducing osteogenesis in the Reddi-
Sampath ectopic bone
assay.
In preferred embodiments, the GDNF/NGF neurotrophic factors of the present
invention
comprise at least the mature, functional form of a mammalian, preferably
human, GDNF, NGF,
BDNF, NT-3, NT-4, NT-5 or NT-6 protein.
CA 02303460 2008-04-28
-6-
In preferred embodiments the effective concentration of the preparation
comprises
between 0.1 ng/ml and 10 .tg/ml of an OPBMP morphogen and between 0.1 ng/ml
and 10 p.g/ml
of a GDNF/NGF neurotrophic factor, more preferably between 1 ng/ml and 100
ng/ml of either
an OPBMP morphogen or a GDNF/NGF neurotrophic factor and, most preferably,
between 1
ng/ml and 100 ng/ml of both an OPBMP morphogen and a GDNF/NGF neurotrophic
factor.
In another aspect, the present invention provides methods for promoting the
survival or
growth, or inhibiting the death or degeneration, of mammalian cells, including
non-neural cells,
which express an OPBMP-activated serine/threonine kinase receptor and a
GDNF/NGF-
activated tyrosine kinase receptor. Similarly, the present invention provides
methods for treating
a mammalian subject afflicted with damage or injury to cells, or at imminent
risk of damage or
injury to cells, including non-neural cells, which express an OPBMP-activated
serine/threonine
kinase receptor and a GDNF/NGF-activated tyrosine kinase receptor. These
methods also
comprise contacting such cells with an effective concentration of a
preparation comprising a
GDNF/NGF neurotrophic factor and an OPBMP morphogen, as described above.
In another aspect, the present invention provides for pharmaceutical
preparations for
promoting the survival or growth of mammalian cells, or inhibiting the death
or degeneration of
mammalian cells, particularly neural cells, comprising a GDNF/NGF neurotrophic
factor in
combination with an OPBMP morphogen.
The preferred methods, materials, and examples that will now be described are
illustrative
only and are not intended to be limiting. Other features and advantages of the
invention will be
apparent from the following detailed description, and from the claims.
Brief Description of the brawings
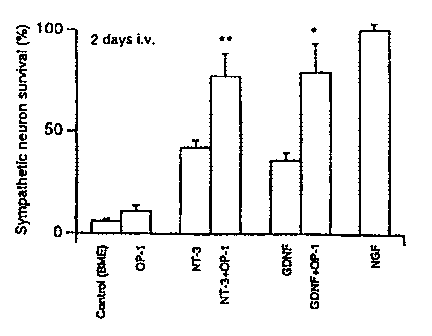
FIG. 1 is a bar graph showing the survival of dissociated sympathetic neurons
grown
in collagen gels after 2 (Fig. 1A) or 6 day (Fig. 1B) treatment with NT-3 (2
ng/ml) or GDNF
(50 ng/ml), in the presence or absence of OP-1.
Detailed Description of the Invention
The present invention provides new methods of treatment for mammalian subjects
afflicted
with, or at imminent risk of, damage or injury to tissues, particularly neural
tissues, comprising the
administration of a combination of an OPBMP morphogen and a GDNF/NGF
neurotrophic
factor. Surprisingly, it has been demonstrated that administration of these
agents in combination
CA 02303460 2000-03-08
WO 99/12560 PCTIUS98/18772
-7-
has a synergistic effect in promoting the survival and/or growth of neural
tissues, and in inhibiting
death or degeneration of neural tissues. In addition, the present invention
provides new
pharmaceutical preparations comprising an OPBMP morphogen in combination with
a
GDNF/NGF neurotrophic factor.
Without being bound to any particular theory of the invention, it is believed
that the
OPBMP morphogens and GDNF/NGF neurotrophic factors exert a synergistic effect
in
promoting the survival and/or growth of neural tissues, and in inhibiting
death or degeneration of
neural tissues, by acting through separate receptor-based signaling pathways.
In particular, it is
believed that the OPBMP proteins act through serine/threonine kinase receptors
and that the
GDNF/NGF neurotrophic factors of the invention act through tyrosine kinase
receptors such that,
when an OPBMP morphogen and a GDNF/NGF neurotrophic factor are administered in
combination, both the serine/threonine kinase and the tyrosine kinase signal
transduction
pathways are activated and a synergistic effect is produced.
Thus, the OPBMP morphogens have been shown to act through serine/threonine
kinase
receptors, and these receptors have been shown to be expressed in both
peripheral and central
nervous system tissues. For example, it has been shown by in situ
hybridization that several
classes of peripheral neurons express BMP type II serine/threonine kinase
receptors known to
bind members of the OPBMP morphogen family (Liu et al., Mol. Cell. Biol.
15:3479-3486
(1995); Rosenzweig et al., Proc. Natl. Acad. Sci. (USA) 92:7632-7636 (1995)),
and that both
OPBMP type I and type II serine/threonine receptors are expressed in the CNS.
Soderstrom et
al., Cell Tiss. Res. 286:269-279(1996a); Nosrat et al., Cell Tiss. Res.
286:191-207 (1996).
Similarly, the GDNF/NGF neurotrophic factors have been shown to act through
specific
tyrosine kinase receptors which are expressed in neural tissues. Thus, for
example, the signaling
pathway of GDNF has been shown to involve the activation of the tyrosine
kinase receptor Ret
(Trupp et al., Nature 381:785-789 (1996)), which is expressed in neural
tissues including
sympathetic, nodose and ciliary ganglia. Similarly, NT-3 acts via the TrkC
receptor and, to some
extent, via the TrkA receptor. See, e.g., Ebendal, J. Neurosci. Res. 32:461-
470 (1992). These
receptors are expressed in the brain and spinal cord as well as peripheral
neurons. See, e.g.,
Vazquez and Ebendal, Neuro Report 2:593-596 (1991); Pei and Ebendal, Exp.
Neurol. 132:105-
115 (1995); Soderstrom et al., Dev. Brain Res. 85:96-108 (1995); Hallbook et
al., Int. J. Dev.
Biol. 39: 855-868 (1995); Williams and Ebendal, Neuro Report 6:2277-2282
(1995); Williams et
al., Eur. J. Neurosci. 7:116-128 (1995).
CA 02303460 2000-03-08
WO 99/12560 PCT/US98/18772
-8-
Thus, in one aspect, the present invention provides methods of promoting the
survival and/or
growth of neural tissues, or of inhibiting death or degeneration of neural
tissues, by administering
to a mammal an OPBMP morphogen and a GDNF/NGF neurotrophic factor in
combination,
thereby activating both the OP/BMP-activated serine/threonine kinase pathway
and the
GDNF/NT-activated tyrosine kinase pathway. In another aspect, the present
invention provides
for new pharmaceutical preparations for use in promoting the survival and/or
growth of neural
tissues, or of inhibiting death or degeneration of neural tissues, and
comprising an OP/BMP
morphogen and a GDNF/NGF neurotrophic factor in combination.
In another aspect, the present invention provides methods and pharmaceutical
preparations
for the treatment of non-neural tissues. In particular, there are a variety of
non-neural tissues
which express OPBMP-activated serine/threonine kinase receptors and GDNF-
activated tyrosine
kinase receptors, including renal tissue and many thyroid papillary carcinomas
(see, e.g.,
Schuchardt et al., Nature 367: 380-383 (1994); Pachnis et al., Development
119:1005-1017
(1993)). Therefore, the present invention also provides methods of promoting
the survival and/or
growth of such non-neural tissues, or of inhibiting death or degeneration of
such non-neural
tissues, by administering to a mammal an OP/BMP morphogen and a GDNF/NGF
neurotrophic
factor in combination, thereby activating both the OPBMP-activated
serine/threonine kinase
pathway and the GDNF/NT-activated tyrosine kinase pathway. Similarly, the
present invention
provides for new pharmaceutical preparations for use in promoting the survival
and/or growth of
such non-neural tissues, or of inhibiting death or degeneration of such non-
neural tissues, and
comprising an OP/BMP morphogen and a GDNF/NGF neurotrophic factor in
combination.
A. OP/BMP Morphogens
The OP/BMP morphogens of the present invention are naturally occurring
proteins, or
functional variants of naturally occurring proteins, in the osteogenic
protein/bone morphogenetic
protein (OP/BMP) family within the TGF-P superfamily of proteins. That is,
these proteins form
a distinct subgroup, referred to herein as the "OP/BMP morphogens," within the
loose
evolutionary grouping of sequence-related proteins known as the TGF-(3
superfamily. Members
of this protein family comprise secreted polypeptides that share common
structural features, and
that are similarly processed from a pro-protein to yield a carboxy-terminal
mature protein. Within
the mature protein, all members share a conserved pattern of six or seven
cysteine residues
defining a 97-106 amino acid domain, and the active form of these proteins is
either a disulfide-
CA 02303460 2000-03-08
WO 99/12560 PCT/US98/18772
-9-
bonded homodimer of a single family member, or a heterodimer of two different
members. See,
e.g., Massague, Annu. Rev. Cell Biol. 6:597 (1990); Sampath et al., J. Biol.
Chem. 265:13198
(1990). For example, in its mature, native form, natural-sourced human OP-1 is
a glycosylated
dimer typically having an apparent molecular weight of about 30-36 kDa as
determined by
SDS-PAGE. When reduced, the 30 kDa protein gives rise to two glycosylated
peptide subunits
having apparent molecular weights of about 16 kDa and 18 kDa. The
unglycosylated protein has
an apparent molecular weight of about 27 kDa. When reduced, the 27 kDa protein
gives rise to
two unglycosylated polypeptide chains, having molecular weights of about 14
kDa to 16 kDa.
Typically, the naturally occurring OP/BMP proteins are translated as a
precursor, having
an N-terminal signal peptide sequence, a "pro" domain, and a "mature" protein
domain. The
signal peptide is typically less than 30 residues, and is cleaved rapidly upon
translation at a
cleavage site that can be predicted using the method of Von Heijne, Nucleic
Acids Research
14:4683-4691 (1986). The "pro" domain is variable both in sequence and in
length, ranging from
approximately 200 to over 400 residues. The pro domain is cleaved to yield the
"mature"
C-terminal domain of approximately 115-180 residues, which includes the
conserved six- or
seven-cysteine C-terminal domain of 97-106 residues. As used herein, the "pro
form" of an
OPBMP family member includes a protein comprising a folded pair of
polypeptides, each
comprising a pro domain in either covalent or noncovalent association with the
mature domains of
the OPBMP polypeptide. Typically, the pro form of the protein is more soluble
than the mature
form under physiological conditions. The pro form appears to be the primary
form secreted from
cultured mammalian cells. The "mature form" of the protein includes a mature C-
terminal domain
which is not associated, either covalently or noncovalently, with the pro
domain. Any preparation
of OP- I is considered to contain mature form when the amount of pro domain in
the preparation
is no more than 5% of the amount of "mature" C-terminal domain.
OPBMP family members useful herein include any of the known naturally-
occurring
native proteins including allelic, phylogenetic counterpart and other variants
thereof, whether
naturally-sourced or biosynthetically produced (e.g., including "muteins" or
"mutant proteins"), as
well as new, active members of the OPBMP family of proteins.
Particularly useful sequences include those comprising the C-terminal seven
cysteine
domains of mammalian, preferably human, human OP-1, OP-2, OP-3, BMP2, BMP3,
BMP4,
BMP5, BMP6, BMP8 and BMP9. Other proteins useful in the practice of the
invention include
CA 02303460 2008-04-28
-10-
active forms of GDF-5, GDF-6, GDF-7, DPP, Vg I, Vgr-1, 60A, GDF-1, GDF-3, GDF-
5,
GDF-6, GDF-7, BMP 10, BMP 11, BMP 13, BMP 15, UNIVIN, NODAL, SCREW, ADMP or
NURAL and amino acid sequence variants thereof. In one currently preferred
embodiment, the
OPBMP morphogens of the invention are selected from any one of. OP-1, OP-2, OP-
3, BMP2,
BMP3, BMP4, BMP5, BMP6, and BMP9.
Publications disclosing these sequences, as well as their chemical and
physical properties,
include: OP-I and OP-2: U.S. Pat. No. 5,011,691, U.S. Pat. No. 5,266,683, and
Ozkaynak et al.,
EMBO J. 9:2085-2093 (1990); OP-3: W094/10203; BMP2, BMP3, and BMP4: U.S. Pat.
No. 5,013,649, W091/18098, W088/00205, and Wozney et al., Science 242:1528-
1534 (1988);
BMP5 and BMP6: W090/11366 and Celeste et al., Proc. Natl. Acad. Sci. (USA)
87:9843-9847
(1991); Vgr-1: Lyons et al., Proc. Natl. Acad. Sci. (USA) 86: 4554-4558
(1989); DPP: Padgett
et al., Nature 325:81-84 (1987); Vgl: Weeks, Cell 51:861-867 (1987); BMP9:
W095/33830;
BMP10: W094/26893; BMP-11: W094126892; BMP12: W095/16035; BMP-13: W095/16035;
GDF-1: W092/003 82 and Lee et al., Proc. Natl. Acad. Sci. (USA) 88:4250-4254
(1991);
GDF-8: W094/21681; GDF-9: W094/15966; GDF- 10: W095/10539; GDF- 11:
W096/01845;
BMP-15: W096/36710; MP121: W096/01316; GDF-5 (CDMP-1, MP52): W094/15949,
W096/14335, W093/16099 and Storm et al., Nature 368:639-643 (1994); GDF-6
(CDMP-2,
BMP13): W095/01801, W096/14335 and W095/10635; GDF-7 (CDMP-3, BMPI2):
W095/10802 and W095/10635; BMP-3b: Takao et al., Biochem. Biophhys. Res. Comm.
219:656-662 (1996); GDF-3: W094/15965; 60A: Basler et a1., Cell 73:687-702
(1993) and
GenBank Accession No. L12032. In another embodiment, useful proteins include
biologically
active biosynthetic constructs, including novel biosynthetic proteins and
chimeric proteins
designed using sequences from two or more known OP/BMP family proteins. See
also the
biosynthetic constructs disclosed in U.S. Pat. No. 5,011,691
(e.g., COP-1, COP-3, COP-4, COP-51 COP-7, and COP-16).
In other preferred embodiments, the OPBMP morphogens useful herein include
proteins
which comprise an amino acid sequence sharing at least 70% amino acid sequence
"homology"
and, preferably, 75% or 80% homology with the C-terminal seven cysteine domain
present in the
active forms of human OP-1 (i.e., residues 330-431, as shown in SEQ ID NO: 2
of U.S. Pat. No.
5,266,683). In other preferred embodiments, the OP/BMP morphogens useful
herein include
proteins which comprise an amino acid sequence sharing at least 60% amino acid
sequence
identity and, preferably, 65% or 70% identity with the C-terminal seven
cysteine domain present
CA 02303460 2000-03-08
WO 99/12560 PCT/US98/18772
-11-
in the active forms of human OP-1. Thus, a candidate amino acid sequence can
be aligned with
the amino acid sequence of the C-terminal seven cysteine domain of human OP-1
using the
method of Needleman et al., J. Mol. Biol. 48:443-453 (1970), implemented
conveniently by
computer programs such as the Align program (DNAstar, Inc.). As will be
understood by those
skilled in the art, homologous or functionally equivalent sequences include
functionally equivalent
arrangements of the cysteine residues within the conserved cysteine skeleton,
including amino acid
insertions or deletions which alter the linear arrangement of these cysteines,
but do not materially
impair their relationship in the folded structure of the dimeric protein,
including their ability to
form such intra- or inter-chain disulfide bonds as may be necessary for
biological activity.
Therefore, internal gaps and amino acid insertions in the candidate sequence
are ignored for
purposes of calculating the level of amino acid sequence homology or identity
between the
candidate and reference sequences.
"Amino acid sequence homology" is understood herein to include both amino acid
sequence identity and similarity. Thus, as used herein, a percentage
"homology" between two
amino acid sequences indicates the percentage of amino acid residues which are
identical or
similar between the sequences. "Similar" residues are "conservative
substitutions" which fulfill
the criteria defined for an "accepted point mutation" in Dayhoff et a[, Atlas
of Protein Sequence
and Structure Vol. 5 (Suppl. 3), pp. 354-352 (1978), Natl. Biomed. Res.
Found., Washington,
D.C. Thus, "conservative amino acid substitutions" are residues that are
physically or functionally
similar to the corresponding reference residues, having similar size, shape,
electric charge, and/or
chemical properties such as the ability to form covalent or hydrogen bonds, or
the like. Examples
of conservative substitutions include the substitution of one amino acid for
another with similar
characteristics, e.g., substitutions within the following groups: (a) valine,
glycine; (b) glycine,
alanine; (c) valine, isoleucine, leucine; (d) aspartic acid, glutamic acid;
(e) asparagine, glutamine;
(0 serine, threonine; (g) lysine, arginine, methionine; and (h) phenylalanine,
tyrosine. The term
"conservative substitution" or "conservative variation" also includes the use
of a substituted
amino acid in place of an unsubstituted parent amino acid in a given
polypeptide chain, provided
that the resulting substituted polypeptide chain has biological activity
useful in the present
invention.
The OPBMP morphogens of the invention are characterized by biological
activities which
may be readily ascertained by those of ordinary skill in the art.
Specifically, an OPBMP
morphogen of the present invention is capable of inducing osteogenesis in the
Reddi-Sampath
CA 02303460 2008-04-28
-12-
ectopic bone assay (Sampath and Reddi, Proc. Natl. Acad. Sci. (USA) 78:7599-
7603 (1981)) or a
substantially equivalent assay.
The Reddi-Sampath ectopic bone assay is well known in the art as an assay of
osteogenic
activity. The assay, which can be easily performed, is described and discussed
in, for example,
Sampath and Reddi, Proc. Natl. Acad. Sci. (USA) 78:7599-7603 (1981); and
Wozney, "Bone
Morphogenetic Proteins," Progress in Growth Factor Research 1:267-280 (1989).
Many
equivalent assays, using other animals and tissue sites, may be employed or
developed by those of
skill in the art to evaluate the biological activity of the OP/BMP morphogens
of the present
invention. See, for example, the bioassays described in U.S. Pat. No.
5,226,683.
The OP/BMP morphogens contemplated herein can be expressed from intact or
truncated
genomic or cDNA or from synthetic DNAs in prokaryotic or eukaryotic host
cells. The dimeric
proteins can be isolated from the culture media and/or refolded and dimerized
in vitro to form
biologically active preparations. Heterodimers can be formed in vitro by
combining separate,
distinct polypeptide chains. Alternatively, heterodimers can be formed in a
single cell by co-
expressing nucleic acids encoding separate, distinct polypeptide chains. See,
for example,
W093/09229, or U.S. Pat. No. 5,411,941, for several exemplary recombinant
heterodimer
protein production protocols. Currently preferred host cells include, without
limitation,
prokaryotes including E. coli, or eukaryotes including yeast such as
Saccharomvices, insect cells,
or mammalian cells, such as CHO, COS or BSC cells. One of ordinary skill in
the art will
appreciate that other host cells can be used to advantage. Detailed
descriptions of the proteins
useful in the practice of this invention, including how to make, use and test
them for osteogenic
activity, are disclosed in numerous publications, including U.S. Pat. Nos.
5,266,683 and
5,011,691.
B. GDNF/NGF Neurotrophic Factors
The GDNF/NGF neurotrophic factors useful in the methods and preparations of
the present
invention include polypeptides, as well as functional variants of
polypeptides, comprising at least
the active portion of a mature mammalian protein selected from the group
consisting of GDNF,
BDNF, NGF, NT-3, NT-4, NT-5 and NT-6. Each of these preferred GDNF/NGF
neurotrophic
factors is discussed separately below.
1. GDNF. Glial cell-derived neurotrophic factor (GDNF) is a neurotrophic
factor that
belongs to the transforming growth factor-(3 (TGF-0) superfamily, but not to
the OP/BMP family
CA 02303460 2000-03-08
WO 99/12560 PCT/US98/18772
-13-
within the TGF-(i superfamily. GDNF displays potent survival and
differentiation-promoting
effects for dopaminergic neurons both in vitro. Lin et al., Science 260:1130-
1132 (1993)) and in
vivo in animal models (Hudson et al., Brain Res. Bull. 36:425-432 (1995);
Hoffer et al., Neurosci.
Lett. 182:107-111 (1994). GDNF has also been shown to have neurotrophic
effects for
cholinergic motor neurons of the brain stem and spinal cord. Oppenheim et al.,
Nature 373:344-
346 (1995); Yan et al., Nature 373:341-344 (1995). Descriptions of the active
portion of a
mature mammalian GDNF protein can be found in, for example, Lin et al.,
Science 260:1130-
1132 (1993); Lin et al., J. Neurochem. 63: 758-768 (1994); and PCT Publication
W097/11965.
In brief, GDNF is synthesized as a precursor and secreted as a mature protein
comprising 134
amino acids, including the seven cysteine domain which characterizes the TGF-
(3 superfamily of
proteins. A 133 amino acid variant of GDNF in which the initial Met residue
has been omitted
([Met']GDNF) has substantially equivalent biological activity. See, e.g.,
W097/11965. As used
herein, the term "GDNF" includes a polypeptide comprising at least the 134
amino acid mature
form, as well as a functional variant of the polypeptide, such as a
conservative amino acid
substitution variant or the 133 amino acid variant.
2. NGF. Nerve growth factor (NGF) is the best characterized member of the
"neurotrophin" protein family, other members of which include brain-derived
neurotrophic factor
(BDNF), neurotrophin-3 (NT-3), neurotrophin-4 (NT-4), neurotrophin-5 (NT-5),
and
neurotrophin-6 (NT-6), discussed below. NGF has been shown to play a role in
the regulation of
the initial stages of dendritic growth, and to aid in promoting the survival,
growth and/or repair of
cholinergic neurons, and maintaining the differentiated phenotype of
cholinergic neurons. For
example, NGF can cause a subpopulation of nodose neurons to form dendrites in
culture (De
Koninck et al., J. Neurosci. 13:577-585 (1993)) and can enhance the growth of
sympathetic
dendrites when injected in situ (Snider, J. Neurosci. 8:2628-2634 (1988)). NGF
alone, however,
does not support dendritic growth in cultures of sympathetic neurons.
Bruckenstein and Higgins,
Dev. Biol. 128:324-336 (1988). In addition, NGF has been shown to be effective
in preventing or
even reversing the atrophy of cholinergic neurons induced in a standard
fimbria/fornix axotomy
model of Alzheimer's Disease. See, e.g., Batchelor et al., J. Comp. Neurol.
284:187-204 (1989);
Hefti et al. , J. Neurobiol. 25:1418-1435 (1994); Olson et al., Neurochem. J.
25:1-3 (1994). As
used herein, the term "NGF" includes a polypeptide comprising at least the
mature form, as well
as a functional variant of the polypeptide, such as a conservative amino acid
substitution variant.
CA 02303460 2008-04-28
-14-
3. BDNF. Brain-derived neurotrophic factor (BDNF), another member of the
neurotrophin
family of proteins, has been shown to enhance dopamine uptake by fetal nigral
DA neurons in cell
culture (Beck et al., Neurosci. 52:855-866 (1993); Knusel et al., Proc. Natl.
Acad. Sci. (USA)
88:961-969 (1991)) and to partially protect DA neurons from the toxic effects
of the neurotoxins
N-methyl-4-phenylpyridinium ion and 6-hydroxydopamine (6-ODHA). Hyman el al.,
Nature
350:230-232 (1991). BDNF has also been shown to have a strong supportive
effect on the
survival of cultured nigral DA neurons. Beck et al., Neurosci. 52:855-866
(1993); Hyman et al.,
Nature 350:230-232 (1991); Knusel et al., Proc. Natl. Acad. Sci. (USA) 88:961-
965 (1991).
These findings suggested that BDNF might have a survival-promoting effect on
grafted DA
neurons in vivo. BDNF treatment enhances the behavioral effect of grafted
nigral DA neurons to
the DA depleted striatum of unilaterally 6-ODHA-lesioned rats as manifested by
a relative
reduction in amphetamine-induced turning at two weeks post-grafting. Sauer el
al., Brain
Research 626:37-44 (1993). However, this study failed to established any clear
cut difference
between treated and control animals in the extent of neurite outgrowth from
the grafted DA
neurons. Thus, although infusion with BDNF produced several behavioral and
morphological
effects in rats grafted with fetal nigral tissue, it was unable to increase
the survival rates of the
transplanted dopamine cells. As used herein, the term "BDNF" includes a
polypeptide comprising
at least the mature form, as well as a functional variant of the polypeptide,
such as a conservative
amino acid substitution variant.
4. NT-3. As used herein, "NT-3" means a human protein, or mammalian homologues
thereof, having a protein sequence essentially as published at Jones et al.,
Proc. Natl. Acad. Sci.
(USA) 87:8060-8064 (1990); Maisonpierre et al., Genomics 10:558-568 (1991);
Kaisho et al.,
FEBS Lett. 266:187-191 (1990); WO 91/03569; and available through
GenBanlrAccession No.
M37763. The isolation and/or characterization of human NT-3 is described in,
for example, Jones
et al., Proc. Natl. Acad. Sci. (USA) 87:8060-806 (1990); and Maisonpierre et
al., Science
247:1446-1451 (1990). The mouse NT-3 sequence is disclosed in Hohne et al.,
Nature 344:339-
T7
341 (1990); WO 91/03569, and GenBank Accession No. X53257. The rat NT-3 gene
sequence
is disclosed in Ernfors et al., Proc. Natl. Acad. Sci. (USA) 87:5454-5458
(1990); WO 91/03569;
rM
and GenBank Accession No. M34643. Comparison of the human NT-3 precursor to
the rat and
mouse NT-3 precursors reveals that the mature forms are identical. The gene
for human NT-3
encodes a putative 257 amino acid precursor with an 18 amino acid signal
peptide which is
processed to a 119 amino acid mature peptide. NT-3 RNA has been detected in
human CNS
CA 02303460 2000-03-08
WO 99/12560 PCTIUS98/18772
-15-
tissues including the cerebellum, nucleus basalis, basal ganglia, hippocampus
and visual cortex.
Maisonpierre et at, Genomics 10:558-568 (1991). Human NT-3 shares 56% amino
acid identity
with human NGF and human BDNF, and shares the conserved 6 cysteine with other
members of
the neurotrophin family, with the regions of greatest homology between the
three proteins being
clustered around these cysteine residues. The term "NT-3," as used herein,
includes a polypeptide
comprising at least the mature 119 amino acid form as disclosed in Jones et
at, Proc. Natl. Acad.
Sci. (LJSA) 87:8060-806 (1990), as well as a functional variant of the
polypeptide, such as a
conservative amino acid substitution variant.
5. NT-4. As used herein, "NT-4" means a human protein, or mammalian homologues
thereof, having a protein sequence essentially as published at Ip et at, Proc.
Natl. Acad. Sci.
(USA) 89:3060-3064 (1992); U.S. Pat. No. 5,364,769; and available through
GenBank Accession
No. M86528. The isolation and/or characterization of NT-4 is described in, for
example, Ip et
al., Proc. Natl. Acad. Sci. (USA) 89:3060-3064 (1992); Hallbook et at, Neuron
6:845-858
(1991); and Ibanez et at, Development 117:1345-1353 (1993). The complete NT-4
gene
sequence encodes a 236 amino acid, 27 kD precursor protein comprising a
putative signal peptide
sequence and pro- region of approximately 60 amino acids, which is believed to
be processed to a
130 amino acid mature human NT-4 polypeptide. The mature form of NT-4 shares
46.5%,
55.4% and 52.2% sequence identity with NGF, BDNF, and NT-3, respectively. NT-4
contains
the conserved 6 cysteines of the neurotrophin family, but contains a seven
amino acid insertion
located between the second and third cysteines. NT-4 has been demonstrated to
support the
survival of neurons of the trigeminal ganglion in the mouse (Ibanez et al.,
Development
117:1345-1353 (1993)) and dorsal root ganglia in the chicken (Ip et at, Proc.
Natl. Acad. Sci.
(USA) 89:3060-3064 (1992)). In the rat, NT-4 mRNA has been found in spinal
cord and several
brain regions including cerebellum and cortex. The term "NT-4," as used
herein, includes a
polypeptide comprising at least the mature 130 amino acid form as disclosed in
Ip et at, Proc.
Natl. Acad. Sci. (USA) 89:3060-3064 (1992), as well as a functional variant of
the polypeptide,
such as a conservative amino acid substitution variant.
6. NT-5. As used herein, the term "NT-5" means a human protein, or mammalian
homologue thereof, having a protein sequence essentially as described in
Berkemeier et at,
Neuron 7:857-866 (1991). The isolation and/or characterization of NT-5 is
described in, for
example, Berkemeier et at, Neuron 7:857-866 (1991) and Berkemeier et at, Som.
Cell Mol.
Genet. 18:233-245 (1992). The human gene for NT-5 encodes a putative 210 amino
acid, 22.4
CA 02303460 2000-03-08
WO 99/12560 PCT/US98/18772
-16-
kD precursor protein. The assigned initiation codon is followed by a putative
signal sequence
cleavage site at Ser - 24. The putative pre-pro sequence of NT-5 is
approximately 50 amino acids
shorter than those of the other neurotrophins. The overall homology of the NT-
5 precursor with
Xenopus NT-4, human NT-3, human BDNF and human NGF is 52%, 45%, 47% and 41%,
respectively. The mature NT-5 is 123 amino acids long and shares the 6
cysteines conserved in
the neurotrophin family. NT-5 shares 50% homology with NGF, 56% homology with
BDNF,
55% homology with NT-3 and 66% homology with Xenopus NT-4. The rat NT-5 gene
encodes
a 209 amino acid protein that is 91% identical to its human counterpart.
Berkemeier el al.,
Neuron 7:857-866 (1991). NT- 5 protein has been identified in adult rat brain,
and acts as a
survival factor for dorsal root ganglion sensory cells and promotes survival
and neurite outgrowth
from sympathetic ganglion neurons. Berkemeier et al., Neuron 7:857-866 (1991).
The term
"NT-5," as used herein, includes a polypeptide comprising the mature 123 amino
acid form, as
well as a functional variant of the polypeptide, such as a conservative amino
acid substitution
variant.
7. NT-6. As used herein, "NT-6" means a human protein, or mammalian homologue
thereof, having a protein sequence essentially as described in Gotz et al.,
Nature 327:266-269;
WO 95/26363 (1994); and available through GenBank Accession Nos. L36325 and
L36942. The
isolation and characterization of NT-6 is described in, for example, Gotz et
al., Nature 327:266-
269 (1994). The NT-6 precursor contains 286 amino acids, having a hydrophobic
domain at the
N terminus (residues 1-19) characteristic of a signal peptide, and a putative
pro- region at
residues 20-142. The putative mature protein begins at residue 143. NT-6
shares the conserved
6 cysteines found in all neurotrophins, but contains a 22 residue insert
between the second and
third conserved cysteine containing domain. The term "NT-6," as used herein,
includes a
polypeptide comprising the mature 119 amino acid form disclosed in Gotz et
al., Nature 327:266-
269 (1994), as well as a functional variant of the polypeptide, such as a
conservative amino acid
substitution variant.
C. Subjects for Treatment
Subjects for treatment according to the methods of the present invention
include, without
limitation, (1) those having neural tissues which have been damaged, or are at
imminent risk of
damage, due to mechanical traumas such as arise from blunt force traumas to
the head, cerebral
edema, subdural haematoma, broken or crushed vertebra, or torn or severed
nerves (including
those arising from surgical procedures); (2) those which have suffered, or are
at imminent risk of
CA 02303460 2000-03-08
WO 99/12560 PCT/US98/18772
-17-
suffering, chemical traumas to nervous tissue as may arise, for example, from
exposure to
neurotoxins (e.g., lead, ethanol, ammonia, formaldehyde, mercury) or the
administration of
chemotherapeutic agents with neurotoxic side effects (e.g., cisplatin); (3)
those which have
suffered, or are at imminent risk of, an ischemic injury to neural tissues
(e.g., cerebral stroke); and
(4) those afflicted with, or at imminent risk of, a neuropathy or
neurodegenerative disease.
Particularly contemplated is the treatment of human subjects diagnosed with,
or at imminent risk
of, a neuropathy or neurodegenerative disease selected from the group
consisting of amyotrophic
lateral sclerosis (ALS), progressive muscular atrophy, hereditary motor and
sensory neuropathy
(Charcot-Marie-Tooth disease), Alzheimer's Disease, epilepsy, Huntington's
Disease, Parkinson's
Disease, palsy, dementia, Shy-Drager disease, Wernicke-Korsakoff syndrome, and
Hallervorden-
Spatz disease.
Diseases such as ALS, progressive muscular atrophy, and hereditary motor and
sensory
neuropathy (Charcot-Marie-Tooth disease) all result, at least in part, from
degeneration of motor
neurons which are located in the ventral horn of the spinal cord. Thus, in one
embodiment, the
present invention provides for methods and preparations which employ an OPBMP
morphogen
in combination with a GDNF/NGF neurotrophic factor for the treatment of these
and other
conditions involving loss of or damage to motor neurons.
The hippocampus, a well-defined structure that is part of the cerebral cortex
of the brain, is
important in the formation of long term memory. Degeneration of pyramidal CAI
neurons, which
are located in the CAI region of the hippocampus, is one characteristic of
Alzheimer's disease.
These same neurons are selectively vulnerable to ischemic and anoxic damage
which occurs in
conditions such as stroke and head trauma. In addition, the CA I pyramidal
hippocampal neurons
as well as pyramidal neurons located in the CA3 region of the hippocampus are
selectively injured
in epilepsy. Thus, in one embodiment, the present invention provides for
methods and
preparations which employ an OPBMP morphogen in combination with a GDNF/NGF
neurotrophic factor for the treatment of these and other conditions involving
loss of or damage to
hippocampal neurons.
The majority of neurons in the striatum utilize GABA (4-aminobutyric acid) as
their
neurotransmitter, and may be referred to as "GABAnergic" neurons. The striatum
is also the
major target of the progressive neurodegeneration that occurs in Huntington's
disease, in which
striatal GABA-utilizing neurons (e.g., spiny cell neurons and enkephalin
neurons) atrophy and/or
CA 02303460 2000-03-08
WO 99/12560 PCT/US98/18772
-18-
die. Thus, in one embodiment, the present invention provides for methods and
preparations
which employ an OP/BMP morphogen in combination with a GDNF/NGF neurotrophic
factor for
the treatment of this and other conditions involving loss of or damage to
striatal or GABAnergic
neurons.
Dopamine-producing ("dopaminergic" or "DA") neurons are located primarily in
the
substantia nigra and, to a lesser extent, in the adjacent striatum (comprising
the caudate nucleus,
putamen, pallidum and locus coerulus). The neurons of the striatum express
receptors for
dopamine and are responsible for control of motor activity. Thus, degeneration
of DA neurons
results in a decrease of dopamine levels and loss of motor activity. In
particular, the progressive
degeneration of dopaminergic neurons in the substantia nigra can lead to the
slowing of initiation
and execution of voluntary muscle movement (bradykinesis), the muscular
rigidity, and tremor
which are characteristic of Parkinson's Disease. Thus, in one embodiment, the
present invention
provides for methods and preparations which employ an OPBMP morphogen in
combination
with a GDNF/NGF neurotrophic factor for the treatment of this and other
conditions involving
loss of or damage to dopaminergic neurons, including those of the substantia
nigra.
Other neuropathic conditions in which populations of neural cells are lost or
damaged
include palsy, dementia, Shy-Drager disease, Wernicke-Korsakoff syndrome, and
Hallervorden-
Spatz disease. Thus, in other embodiments, the present invention provides for
methods and
preparations which employ an OP/BMP morphogen in combination with a GDNF/NGF
neurotrophic factor for the treatment of these and other conditions involving
loss of or damage to
neural tissues.
D. Pharmaceutical Preparations
The pharmaceutical preparations of the present invention include at least one
OPBMP
morphogen in combination with at least one GDNF/NGF neurotrophic factor.
Preferably, such
preparations include an amount of each agent which is effective when
administered by alone.
Because of the synergistic effect of the agents in combination, however, the
pharmaceutical
preparations may contain an amount of each agent which, when administered in
the absence of the
other, would not be effective alone. The determinations of appropriate ratios
of the agents is
within the ability and discretion of one of ordinary skill in the art for the
treatment of a given
condition, for a given combination of OPBMP morphogen and GDNF/NGF
neurotrophic factor,
and given route of administration.
CA 02303460 2000-03-08
WO 99/12560 PCT/US98/18772
-19-
As a general matter, the pharmaceutical preparations of the present invention,
including at
least one OPBMP morphogen and at least one GDNF/NGF neurotrophic factor can be
tested
using in vitro assays, animal models, and clinical studies. Effective
concentrations of the
preparations are those sufficient to (1) cause enhanced neurite outgrowth in
an in vitro assay of
neuronal growth; (2) cause motor skill improvements in a standard animal model
of Parkinson's
disease; or (3) cause a clinically significant improvement in neurological
function when
administered to a mammalian subject (e.g., a human patient).
E. Methods and Formulations for Administration
The pharmaceutical preparations of the present invention, including at least
one OP/BMP
morphogen and at least one GDNF/NGF neurotrophic factor, can be administered
to an individual
by any suitable means, preferably directly (e.g., locally, as by injection to
a tissue locus) or
systemically (e.g., parenterally or orally). Where the preparation is to be
administered
parenterally, such as by intravenous, subcutaneous, intramuscular,
intraorbital, ophthalmic,
intraventricular, intracranial, intracapsular, intraspinal, intracisternal,
intraperitoneal, or buccal
administration, the preparation preferably comprises part of an aqueous
solution. The solution is
chosen to be physiologically acceptable such that, in addition to delivery of
the desired OPBMP
morphogen and GDNF/NGF neurotrophic factor to the patient, the solution does
not otherwise
adversely affect the patient's electrolyte and volume balance.
Useful solutions for parenteral administration can be prepared by a variety of
methods well
known in the pharmaceutical arts. See, e.g., Remington's Pharmaceutical
Sciences, Gennaro, A.,
ed., Mack Pub., (1990). Formulations can include, for example, polyalkylene
glycols such as
polyethylene glycol, oils of vegetable origin, hydrogenated naphthalenes, and
the like.
Formulations for direct administration, in particular, can include glycerol
and other preparations
of high viscosity. Biocompatible, preferably bioresorbable, polymers,
including, for example,
hyaluronic acid, collagen, polybutyrate, tricalcium phosphate, lactide and
lactide/glycolide
copolymers, can be useful excipients to control the release of the preparation
in vivo. Other
potentially useful parenteral delivery systems for these preparations include
ethylene-vinyl acetate
copolymer particles, osmotic pumps, implantable infusion systems, and
liposomes.
As will be appreciated by those skilled in the art, the amounts and/or
concentrations of the
agents present in a pharmaceutical preparation will vary depending upon a
number of factors,
including the dosage of the drug to be administered, the chemical
characteristics (e.g.,
CA 02303460 2008-04-28
-20-
hydrophobicity) of the agents employed, and the route of administration. The
preferred dosage
may also depend on such variables as the type and extent of the damage or
injury to be treated,
the overall health status of the subject, the relative biological efficacy of
the agents employed, the
presence of excipients or diluents, and the like.
As a general matter, the pharmaceutical preparations of the present invention
are
administered in amounts sufficient to achieve effective concentrations of the
OP/BMP morphogen
and the GDNF neurotrophic factor at a site of neural tissue damage or injury,
or at a site of
imminent risk of such injury. Preferably, the preparations contain an amount
of an OPBMP
morphogen such that, when administered by the chosen route of administration,
results in a
concentration of the OP/BMP morphogen of about 0.1 ng/ml to 10 gg/ml, more
preferably about
I ng/ml to 100 nglml. Similarly, the preparations preferably contain an amount
of a GDNF/NGF
neurotrophic factor such that, when administered by the chosen route of
administration, results in
a concentration of the GDNF/NGF neurotrophic factor of about 0.1 ng/ml to 10
g/ml, more
preferably about 1 ng/ml to 100 ng/ml. Because of the synergistic interactions
of the OPBMP
morphogens and GDNF/NGF neurotrophic factors, optimal concentrations and
ratios will vary
depending upon the particular agents employed.
Where injury to neurons of a neural pathway is induced deliberately as part
of, for example, a
surgical procedure, the OPBMP morphogen and GDNF/NGF neurotrophic factor
preferably are
provided just prior to, or concomitant with induction of the trauma.
Preferably, the morphogen
and neurotrophic factor are administered prophylactically in a surgical
setting.
Where the OPBMP morphogen and neurotrophic factor are to be provided to a site
to
stimulate axon regeneration, the pharmaceutical preparation preferably is
provided to the site in
association with a biocompatible, preferably bioresorbable carrier suitable
for maintaining the
proteins at the site in vivo, and through which a neural process can
regenerate. A currently
preferred carrier also comprises sufficient structure to assist direction of
axonal growth.
Currently preferred carriers include structural molecules such as collagen,
hyaluronic acid or
laminin, and/or synthetic polymers or copolymers of, for example, polylactic
acid, polyglycolic
acid or polybutyric acid. Currently most preferred are carriers comprising
tissue extracellular
matrix. These can be obtained commercially. In addition, a brain tissue-
derived extracellular
matrix can be prepared as described in PCT Publication W092/15323,
and/or by other means known in the art.
CA 02303460 2000-03-08
WO 99/12560 PCT/US98/18772
-21-
The currently preferred means for repairing breaks in neural pathways,
particularly pathways
of the peripheral nervous system, include providing the OP/BMP morphogen and
GDNF/NGF
neurotrophic factor to the site as part of a device that includes a
biocompatible membrane or
casing of a dimension sufficient to span the break and having openings adapted
to receive severed
nerve ends. The OP/BMP morphogen and GDNF/NGF neurotrophic factor are disposed
within
the casing, preferably dispersed throughout a suitable carrier, and are
accessible to the severed
nerve ends. Alternatively, the OP/BMP morphogen and GDNF/NGF neurotrophic
factor can be
adsorbed onto the interior surface of the casing, or otherwise associated
therewith. The casing
acts as a nerve guidance channel, aiding in directing axonal growth. Suitable
channel or casing
materials include silicone or bioresorbable materials such as collagen,
hyaluronic acid, laminin,
polylactic acid, polyglycolic acid, polybutyric acid and the like.
Examples
OPBMP Morphogen and GDNF/NGF Neurotrophic Factor Synergy
The OP/BMP morphogen and GDNF/NGF neurotrophic factor compositions described
herein enhance process formation in neural cells. A variety of peripheral
ganglia derived from
embryonic chickens were used as a model for the induction of nerve fiber
outgrowth by OP- I
(Creative Biomolecules, Hopkinton, MA) in combination with GDNF (PeproTech,
Rocky Hill,
New Jersey), NT-3 (Austral Biologicals, San Ramon, CA), activin A (Austral
Biologicals, San
Ramon, CA), or mouse (3-NGF.
Peripheral ganglia were obtained from embryonic day 9 (E9) chickens according
to the
method of Ebendal et al. (Ebendal, in IBRO Handbook series: Methods in the
Neurosciences
Vol. 12, pp. 81-93 (1989), John Wiley, Chichester). Sympathetic ganglia were
obtained from the
lumbar region. Ciliary ganglia were obtained from the orbit. Remak ganglia
were obtained from
the dorsal mesorectum. Dorsal root ganglia were obtained from the lumbo-sacral
region. Nodose
ganglia were obtained from the vagus nerve cranial to the heart. For some
experiments,
trigeminal ganglia were obtained and the maxillomandibular and ophthalmic
lobes were separated
before explantation. The ganglia were removed intact and maintained at 37 C
with 5% CO2 in a
collagen matrix or were dissociated into single neurons and spread into a thin
collagen gel
(Ebendal (1989), supra). In both instances, the gels were supplemented with
equal volumes of
Eagle's Basal Medium with 1% fetal calf serum. Control cultures consisted of
Eagle's Basal
Medium with 1% fetal calf serum, in some instances supplemented with the
buffer (25 mM
CA 02303460 2008-04-28
-22-
arginine, 150 mM NaCl, pH 9.0, 0.1 % Tween 80) at concentrations to match the
experimental
culture dilutions. Cultures were treated with control medium, OP-1, NT-3, GDNF
or NGF alone,
or in various combinations and at a series of concentrations for 2, 4 and 6
days.
The 6 day cultures were fed with fresh medium containing the growth factors at
day 4 post
incubation. Whole ganglia were examined under dark-field illumination whereas
dissociated
neurons were examined using phase-contrast optics. Surviving cells were
counted in a strip
across the plate to calculate the survival relative to the survival of cells
treated with NGF at 5
ng/ml for 2 days.
Previous studies have shown that NT-3 evokes fiber outgrowth in explanted
ciliary ganglia
and also elicits a weak response in sympathetic ganglia of the chicken embryo.
Ernfors el al.,
Proc. Natl. Acad. Sci. (USA) 87:5454-5458 (1990). OP-1 greatly enhanced fiber
outgrowth
induced by NT-3 treatment in E9 sympathetic ganglia. The effects were apparent
after 2 days in
culture and persisted after 4 days of culture. Optimal concentrations of NT-3
and OP-I were 10
ng/ml and 50 ng/ml, respectively. Treatment of ganglia cultures with OP-1 in
combination with
GDNF gave similar results. OP-I did not stimulate fiber outgrowth of any of
the whole explanted
E9 ganglia at doses of 0.1 ng/ml to 1000 ng/ml. Further, OP-1 also failed to
stimulate younger
ganglia (e.g., from embryonic day 4.5). The buffer (pH 9) failed to exert any
potentiating effect
on the NT-3 responses in sympathetic, ciliary or nodose ganglia.
Treatment of ciliary ganglia with 10 ng/ml NT-3 and 50 ng/ml OP- I resulted in
robust fiber
halos consisting of thick fascicles of neurites which radiated from the cell.
Treatment of ciliary
ganglia with 50 ng/ml GDNF and 50 ng/ml OP- I gave a fiber halo of ciliary
nerve fascicles after 2
days of culture. Ciliary ganglia extended fewer or less robust nerve fibers in
response to NT-3 (2
ng/ml and 10 ng/ml) and GDNF (50 ng/ml) but did not extend neurites in
response to 50 ng/ml
OP-I alone.
Thus treatment of neurons with a combination of OP-I and NT-3, or OP-1 and
GDNF,
suggested a synergistic effect of OP-1 on NT-3 or GDNF induced neurite
outgrowth. A
statistical analysis also shows differences in survival to be significant
(Figure 1) as compared with
the control (BME, or Basal Medium, Eagle's). Both OP-1 and NT-3 are required
from the
beginning of the treatment period to elicit the enhanced nerve outgrowth
response in the ciliary
ganglia.
CA 02303460 2000-03-08
WO 99/12560 PCT/US98/18772
-23-
Sensory neurons of the nodose ganglia were also treated with OP-1 (50 ng/ml)
alone and in
combination with NT-3 (2 ng/ml and 10 ng/ml) or OP-1 (50 ng/ml) in combination
with GDNF
(50 ng/ml). Treatment of sensory neurons with OP-1 alone did not elicit fiber
outgrowth.
However, OP- I enhanced NT-3 -induced neurite outgrowth after 2 and 4 days of
treatment.
Activin A (20 ng/ml) did not mimic the effects of OP-1 when tested alone or in
combination
with NT-3 (10 ng/ml), GDNF (50 ng/ml) or NGF (5 ng/ml) in the treatment of
sympathetic
ganglia.
OP- I is therefore capable of promoting NT-3 and GDNF induced nerve process
formation in
several classes of peripheral neurons.
Treatments for Alzheimer's Disease
Alzheimer's disease is a neurodegenerative disease in which there is
significant neuronal loss
of the large pyramidal cells of the parietal and frontal association areas,
hippocampus and
amagdyla. Cholinergic neurons of the basal forebrain and noradrenergic neurons
of the locus
coerulus are also severely affected.
In accordance with the present invention, an OP/BMP morphogen, preferably
human OP-1,
is administered to an Alzheimer's patient in combination with a GDNF/NGF
neurotrophic factor,
preferably GDNF or NT-3, in order to promote the growth and survival of cells
in this region, as
well as to inhibit the progressive loss of additional neuronal cells.
An aqueous, pharmaceutical preparation comprising an OPBMP morphogen and
GDNF/NGF neurotrophic factor is administered to the patient parenterally.
Preferably, the
preparation is administered intracerebrally by, for example,
intracerebroventricular or intrathecal
injection or infusion. In one embodiment, the cranium of the subject is
immobilized in a
stereotaxic device, a small hole is made in the skull, and the preparation is
injected or infused
directly into the affected regions of the brain. Dosages may be calculated to
achieve
concentrations of the OPBMP morphogen and GDNF/NGF neurotrophic factor of
about 0.1-10
p.g/ml, preferably about 1-100 ng/ml at the site of injection. Alternatively,
dosages may be
calculated to deliver about 10-100 pg/kg of the morphogen and neurotrophic
factor, preferably 1-
25 g/kg. Dosages are repeated daily or less frequently for a period of
several weeks to months
or, if needed, on a regular basis throughout the life of the subject.
Alternatively, a sustained
release device is implanted within the brain of the subject to cause a
continuous release of the
preparation over an extended period.
CA 02303460 2000-03-08
WO 99/12560 PCT/US98/18772
-24-
Treatments for Severed Nerve Fibers
Due to the limited ability of mammalian nerve cells grow or regenerate,
severed nerve fibers
frequently lead to permanent loss of the sensory or motor function associated
with the nerve.
Nerve fibers may be torn or severed in accidents (e.g., vehicular accidents)
or as an unavoidable
side effect of surgery.
In accordance with the present invention, an OPBMP morphogen, preferably human
OP-1,
is administered in combination with a GDNF/NGF neurotrophic factor, preferably
GDNF or NT-
3, to a subject having severed nerve fibers in order to promote reparative
growth and survival of
the damaged cells.
An aqueous, pharmaceutical preparation comprising an OPBMP morphogen and
GDNF/NGF neurotrophic factor is administered to the patient parenterally. In
the case of
damaged peripheral nerve fibers (e.g., in the sciatic nerve), the preparation
is preferably
administered in conjunction with the use of nerve guide channels which enclose
the ends of the
severed fibers and guide their growth together. In the case of severed fibers
in the spinal cord, the
preparation is preferably injected or infused into the CSF in the region of
the injury. Dosages may
be calculated to achieve concentrations of the OPBMP morphogen and GDNF/NGF
neurotrophic factor of about 0.1-10 gg/ml, preferably about 1-100 ng/ml at the
site of injection or
infusion. Alternatively, dosages may be calculated to deliver about 10-100
g/kg of the
morphogen and neurotrophic factor, preferably 1-25 pg/kg. In the case of a
spinal injury, dosages
are repeated daily or less frequently for a period of several weeks to months.
Alternatively, in the
case of severed peripheral nerves enclosed in nerve guidance channels, a
sustained release
formulation is employed in which the preparation is admixed with a matrix
material within the
nerve guidance channel.
Treatments for Stroke
Ischemic events, or strokes, in the brain may cause neural lesions which
result in permanent
loss of cognitive, sensory, or motor function. Immediately after the onset of
a stroke, inhibition
of the death or degeneration of cells in the ischemic area, as well as the
promotion of growth and
survival of these cells, is important to minimizing permanent damage.
In accordance with the present invention, an OPBMP morphogen, preferably human
OP-1,
is administered in combination with a GDNF/NGF neurotrophic factor, preferably
GDNF or NT-
3, to a subject suffering from a stroke.
CA 02303460 2000-03-08
WO 99/12560 PCT/US98/18772
-25-
An aqueous, pharmaceutical preparation comprising an OPBMP morphogen and
GDNF/NGF neurotrophic factor is administered to the patient parenterally.
Preferably, the
preparation is administered intracerebrally by, for example,
intracerebroventricular or intrathecal
injection or infusion. Dosages may be calculated to achieve concentrations of
the OPBMP
morphogen and GDNF/NGF neurotrophic factor of about 0.1-10 p.g/ml, preferably
about 1-100
ng/ml at the site of injection. Alternatively, dosages may be calculated to
deliver about 10-100
g/kg of the morphogen and neurotrophic factor, preferably 1-25 .tg/kg. Dosages
may be
continuous or frequent for a period of several days to weeks. First dosages
are preferably
administered within the first few hours on the onset of the stroke.