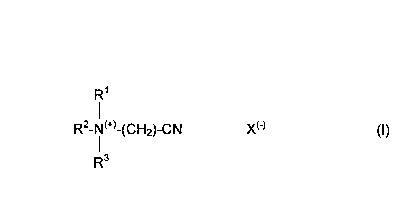Note: Descriptions are shown in the official language in which they were submitted.
CA 02303638 2000-03-31
Single- or Multi-phase Detergent Tablets Containing Special Bleach
Activators
Field of the Invention
This invention relates to detergent tablets which contain so-called
nitriloquats as bleach activators. More particularly, the invention relates to
laundry detergent tablets, cleaning tablets, more particularly dishwasher
tablets, bleach tablets and water softener tablets containing these bleach
activators.
Background of the Invention
Detergent compositions in the form of tablets have long been known
and are widely described in the prior art although, hitherto, tablets have not
been especially prominent on the market. The reason for this is that
tablets, despite a number of advantages, also have disadvantages which
have an adverse effect both on their production and use and on their
acceptance by consumers. The main advantages of tablets, such as
elimination of the need to measure out the quantity of product required by
the consumer, the higher density and hence the reduced packaging and
storage costs and an aesthetic aspect which should not be underestimated,
are offset by such disadvantages as the dichotomy between acceptable
hardness and sufficiently rapid disintegration and dissolution of the tablets
and numerous technological difficulties in production and packaging.
In particular, the dichotomy between a sufficiently hard tablet and a
sufficiently fast disintegration time is a central problem. Since sufficiently
stable, i.e. dimensionally stable and fracture-resistant, tablets can only be
produced by applying relatively high tabletting pressures, the tablet
ingredients are heavily compacted which delays disintegration of the tablet
in the aqueous wash liquor and hence leads to excessively slow release of
the active substances in the washing process. The delayed disintegration
of the tablets has the further disadvantage that conventional detergent
tablets cannot be flushed into the washing process from the dispensing
CA 02303638 2000-03-31
2
compartment of domestic washing machines because the tablets do not
disintegrate sufficiently quickly into secondary particles which are small
enough to be flushed into the drum of the washing machine from the
dispensing compartment.
Many proposals have been put forward in the prior art with a view to
overcoming the dichotomy between hardness, i.e. transportation and
handling stability, and easy disintegration of the tablets. One proposed
solution which is known in particular from the pharmaceutical field and
which has been extended to detergent tablets is to incorporate certain
disintegration aids which facilitate the access of water or which have a
swelling or effervescing or other disintegrating effect on contact with water.
Other proposed solutions from the patent literature include the tabletting of
premixes with certain particle sizes, the separation of individual ingredients
from certain other ingredients and the coating of individual ingredients or
the tablet as a whole with binders.
Bleaching compositions which contain bleach activators of the
nitriloquat type are described in the prior art. Thus, European patent
application EP 303 520 (Kao Corp.) discloses bleaching compositions
which contain a peroxide and a per acid precursor containing at least one
N+-CH2-CN or one N+(CH2CN)2 group. Detergent tablets are not
mentioned in this document.
European patent application EP 458 396 (Unilever) also describes
bleaching compositions which contain a peroxy bleaching agent and a per
acid precursor containing at least one N+-CH2-CN or N+(CH2CN)2 group.
This document also makes no reference either to particle sizes or to
detergent tablets.
Cationic nitrites with the general formula R'R"R"'N+CR'R2-CN and
their use as a bleach activator are described in European patent
application EP 464 880 (Unilever). This document also makes no refer-
ence whatever to detergent tablets.
CA 02303638 2000-03-31
3
Detergent tablets in which individual ingredients are separated from
others are described, for example, in EP-A-0 481 793 (Unilever). The
detergent tablets disclosed in this document contain sodium percarbonate
which is separated from all other components that could affect its stability
(for example bleach activators). Bleach activators of the nitriloquat type are
not mentioned in this document nor does it provide any information on the
pH values of the solutions of individual layers or the tablet as a whole.
None of the cited prior art documents which are concerned with
detergent tablets describes detergent tablets containing bleach activators
based on "nitriloquats". Nowhere is their any indication of a well-defined
particle size range for tablets or regions of tablets containing bleach
activators based on nitriloquats. None of the documents cited above is
concerned with improving the solubility of detergent tablets by the selective
use of these special bleach activators in regions with a defined pH value.
Accordingly, the problem addressed by the present invention was to
provide detergent tablets which would contain so-called nitriloquats as
bleach activators and which would combine high hardness with excellent
disintegration properties. The detergent tablets provided by the invention
would also be able to be dosed from the dispensing compartment without
the consumer experiencing any disadvantages through residues in the
dispensing compartment and too little detergent in the wash liquor.
Besides these tablet-specific properties, the cleaning performance of the
tablets according to the invention would also be exemplary. In particular,
the storage stability of the tablets and the maintenance of their bleaching
performance, even after prolonger storage, would be guaranteed.
Summary of the Invention
It has now been found that the stability of bleach activators based on
"nitriloquats" in detergent tablets can be increased by using these bleach
activators in tablets or regions of tablets which, after dissolution in water,
have a pH value below 10.
CA 02303638 2000-03-31
4
The present invention relates to single- or multi-phase detergent
tablets of compacted particulate detergent containing bleaching agents,
bleach activators) and optionally other detergent ingredients, characterized
in that the the tablets or at least one phase thereof contains) as bleach
activator a cationic nitrite corresponding to formula (I):
R'
R2- i ~+~-(CH2)-CN X~-~ (I)
R3
in which R' represents -H, -CH3, a C2_24 alkyl or alkenyl group, a substituted
C2_24 alkyl or alkenyl group with at least one substituent from the group
consisting of -CI, -Br, -OH, -NH2, -CN, an alkyl or alkenyl aryl group
containing a C~_24 alkyl group or a substituted alkyl or alkenyl aryl group
containing a C~_24 alkyl group and at least one other substituent at the
aromatic ring, R2 ad R3 independently of one another are selected from
-CH2-CN, -CH3, -CH2-CH3, -CH2-CH2-CH3, -CH(CH3)-CH3, -CHZ-OH, -CH2-
CH2-OH, -CH(OH)-CH3, -CH2-CH2-CH2-OH, -CHZ-CH(OH)-CH3, -CH(OH)-
CH2-CH3, -(CH2CH2-O)~H where n = 1, 2, 3, 4, 5 or 6 and X is an anion,
the pH value of a 1 % by weight aqueous solution of the tablet or the phase
containing the nitriloquat being below 10.
Detergent tablets according to the invention may be single- or multi-
phase tablets. According to the invention, the individual phases of the
tablet may have various three-dimensional forms. The most simple form is
a two-layer or multilayer tablet where each layer of the tablet represents a
phase. However, it is also possible in accordance with the invention to
produce multiphase tablets where individual phases are in the form of
incorporations in (an)other phase(s). Besides so-called ring/core tablets,
jacket tablets, for example, or combinations of the tablet forms mentioned
above are possible. Examples of multiphase tablets can be found in the
CA 02303638 2000-03-31
drawings of EP-A-0 055 100 (Jeyes) which describes lavatory cleaning
blocks. At present, the most widespread form of multiphase tablets are
two-layer or multilayer tablets. According to the present invention,
therefore, the phases of the tablet are preferably in the form of layers.
5 According to the invention, two-, three- and four-layer tablets are
preferred.
Detailed Description of the Invention
According to the invention, the pH value of a 1 % by weight aqueous
solution can be determined by preparing a solution of the tablet or the
phase containing the cationic nitrite of formula (I) in demineralized water.
To this end, a corresponding quantity of the tablet or the phase is dissolved
or suspended in water and the pH value is determined in known manner
using indicator papers or glass rods. Since certain methods for
determining pH are temperature-dependent, the values mentioned
throughout the present specification apply to a temperature of the solution
or suspension of 20°C.
In preferred single-phase or multi-phase detergent tablets, the pH
value of a 1 % by weight aqueous solution of the tablet or the phase
containing the nitriloquat is below 9, preferably below 8, more preferably
below 7 and most preferably below 6.
Within the pH range mentioned, single- or multi-phase detergent
tablets where the pH of a 1 % by weight aqueous solution of the tablet or
the phase containing the nitriloquat is below 6.5, preferably below 6, more
preferably below 5.5 and most preferably below 5 are preferred.
The tablets according to the invention may contain the cationic
nitrites corresponding to general formula (I) in varying quantities, the
quantity being determined by the application envisaged for the tablets.
Thus, laundry detergent tablets and dishwasher tablets normally contain
less bleach activator than, for example, bleach tablets which consist largely
of bleaching agent and bleach activator. According to the invention,
preferred detergent tablets are characterized in that they contain the
CA 02303638 2000-03-31
6
cationic nitrite corresponding to formula (I) in quantities of 0.1 to 20% by
weight, preferably in quantities of 0.25 to 15% by weight and more
preferably in quantities of 0.5 to 10% by weight, based on the weight of the
tablet.
In one particularly preferred embodiment, the detergent tablets
according to the invention contain the cationic nitrite corresponding to
formula (I) in relatively coarse form. In preferred detergent tablets, at
least
90% by weight of the particles of the cationic nitrite corresponding to
formula (I) are larger than 0.2 mm in size.
In another preferred embodiment of the invention, not only are more
than 90% by weight of the particles of the cationic nitrite larger than 0.2 mm
in size, a large percentage of even coarser particles is also present. Pre-
ferred detergent tablets are characterized in that at least 40% by weight,
preferably at least 50% by weight and more preferably at least 60% by
weight of the particles of the cationic nitrite corresponding to formula (I)
are
larger than 0.4 mm in size.
The percentage of particles larger than 200 Nm in size should
preferably be more than 90% by weight, based on all the particles of the
cationic nitrite. In order to have an advantageous homogeneous particle
size distribution, the bleach activators used should above all be free from
overly fine particles or dust, i.e. in one particularly preferred embodiment
should not contain any particles smaller than 0.2 mm in diameter. In
particularly preferred detergent tablets, the cationic nitrites are
substantially
free from particles below 0.2 mm in size. "Substantially free" in the context
of the present invention means contents below 2% by weight, preferably
below 1 % by weight and more preferably below 0.5% by weight, based on
the particles as a whole.
In particularly preferred detergent tablets, the cationic nitrite
corresponding to formula (I) has a mean particle size above 400 Nm,
preferably above 500 Nm, more preferably above 600 Nm and most
CA 02303638 2000-03-31
7
preferably above 700 Nm.
General formula (I) encompasses a number of cationic nitrites which
may be used in accordance with the present invention. In one particularly
advantageous embodiment, the detergent tablets according to the
invention contain cationic nitrites in which R' stands for methyl, ethyl,
propyl, isopropyl or an n-butyl, n-hexyl, n-octyl, n-decyl, n-dodecyl, n-tetra-
decyl, n-hexadecyl or n-octadecyl group. R2 and R3 are preferably selected
from methyl, ethyl, propyl, isopropyl and hydroxyethyl; one or both of these
substituents may with advantage even be a cyanomethylene group. In the
following Table, preferred cationic nitrites corresponding to formula (I) are
characterized by their substituents R', R2 and R3:
R _ R
_H -CHs _CH3
_H _CH2_CHs _CHs
-H _CH2_CH2_CHs _CHs
-H -CH(CH3)-CH3 -CH3
-H -C H2-O H -C H 3
-H -CH2-CH2-OH -CH3
-H -CH(OH)-CH3 -CH3
-H -CH2-CH2-CH2-OH -CH3
-H -CH2-CH(OH)-CH3 -CH3
-H -CH(OH)-CH2-CH3 -CH3
-H -(CHZCH2-O)~H -CHs
-H -(CH2CH2-O)2H -CHs
-H -(CHzCH2-O)sH -CH3
-H -(CHZCHZ-O)aH -CHs
-H -(CH2CH2-O)sH -CHs
-H -(CH2CH2-O)sH -CHs
-CHs -CHs -CHs
CA 02303638 2000-03-31
-CH3 -CHZ-CH3 -CH3
-CH3 -CH2-CH2-CH3 -CH3
-CH3 -CH(CH3)-CH3 -CH3
-CH3 -CH2-OH -CH3
-CH3 -CH2-CH2-OH -CH3
-CH3 -CH(OH)-CH3 -CH3
-CH3 -CHZ-CH2-CH2-OH -CH3
-CH3 -CH2-CH(OH)-CH3 -CH3
-CH3 -CH(OH)-CH2-CH3 -CH3
-CH3 -(CH2CH2-O)~H -CH3
-CHs -(CHZCH2-O)zH -CHs
-CHs -(CH2CH2-O)sH -CHs
-CHs -(CH2CH2-O)aH -CHa
-CHs -(CH2CH2-O)sH -CHs
-CHs -(CH2CHz-O)sH -CHs
-CH2-CH3 -CH2-CH3 -CH3
-CH2-CH3 -CH2-CH2-CH3 -CH3
-CHZ-CH3 -CH(CH3)-CH3 -CH3
-CH2-CH3 -CH2-OH -CH3
-CH2-CH3 -CH2-CH2-OH -CH3
-CH2-CH3 -CH(OH)-CH3 -CH3
-CH2-CH3 -CH2-CH2-CH2-OH -CH3
-CH2-CH3 -CH2-CH(OH)-CH3 -CH3
-CH2-CH3 -CH(OH)-CH2-CH3 -CH3
-CH2-CH3 -CH2-CH3 -CH2-CH3
-CH2-CH3 -CH2-CH2-CH3 -CHZ-CH3
-CH2-CH3 -CH(CH3)-CH3 -CH2-CH3
-CH2-CH3 -CH2-OH -CH2-CH3
-CH2-CH3 -CH2-CH2-OH -CH2-CH3
-CH2-CH3 -CH(OH)-CH3 -CH2-CH3
CA 02303638 2000-03-31
-CH2-CH3 -CH2-CH2-CH2-OH -CH2-CH3
-CHZ-CH3 -CH2-CH(OH)-CH3 -CHZ-CH3 '
-CH2-CH3 -CH(OH)-CH2-CH3 -CH2-CH3
-CH2-CH2-CH3 -CH2-CH2-CH3 -CH3
-CHZ-CH2-CH3 -CH(CH3)-CH3 -CH3
-CH2-CH2-CH3 -CH2-OH -CH3
-CH2-CH2-CH3 -CH2-CH2-OH -CH3
-CH2-CH2-CH3 -CH(OH)-CH3 -CH3
-CH2-CH2-CH3 -CH2-CH2-CH2-OH -CH3
-CH2-CH2-CH3 -CH2-CH(OH)-CH3 -CH3
-CH2-CH2-CH3 -CH(OH)-CH2-CH3 -CH3
-CH2-CH2-CH3 -CH2-CH2-CH3 -CH2-CH3
-CH2-CH2-CH3 -CH(CH3)-CH3 -CH2-CH3
-CHZ-CHZ-CHs -CH2-OH -CHZ-CH3
-CH2-CH2-CH3 -CH2-CH2-OH -CH2-CH3
-CH2-CH2-CH3 -CH(OH)-CH3 -CH2-CH3
-CH2-CH2-CH3 -CHZ-CHZ-CHZ-OH -CH2-CH3
-CH2-CH2-CH3 -CH2-CH(OH)-CH3 -CH2-CH3
-CH2-CH2-CH3 -CH(OH)-CH2-CH3 -CH2-CH3
-CH(CH3)-CH3 -CH(CH3)-CH3 -CH3
-CH(CH3)-CH3 -CH2-OH -CH3
-CH(CH3)-CH3 -CH2-CH2-OH -CH3
-CH(CH3)-CH3 -CH(OH)-CH3 -CH3
-CH(CH3)-CH3 -CH2-CH2-CHz-OH -CH3
-CH(CH3)-CH3 -CH2-CH(OH)-CH3 -CH3
-CH(CH3)-CH3 -CH(OH)-CH2-CH3 -CH3
-CH(CH3)-CH3 -CH3 -CH2-CH3
-CH(CH3)-CH3 -CH2-CH3 -CH2-CH3
-CH(CH3)-CH3 -CH2-CH2-CH3 -CH2-CH3
-CH(CH3)-CH3 -CH(CH3)-CH3 -CH2-CH3
CA 02303638 2000-03-31
-CH(CH3)-CH3 -CH2-OH -CH2-CH3
-CH(CH3)-CH3 -CHZ-CHZ-OH -CH2-CH3
-CH(CH3)-CH3 -CH(OH)-CH3 -CH2-CH3
-CH(CH3)-CH3 -CH2-CH2-CH2-OH -CH2-CH3
-CH(CH3)-CH3 -CH2-CH(OH)-CH3 -CH2-CH3
-CH(CH3)-CH3 -CH(OH)-CHZ-CH3 -CH2-CH3
In the interests of easier synthesis, preferred compounds are those
in which the substituents R' to R3 are identical, for example (CH3)3N~+~CH2-
CN X-, (CH3CH2)3N~+~CH2-CN X-, (CH3CH2CH2)3N~+~CH2-CN X-,
5 (CH3CH(CH3))3N~+~CH2-CN X- or (HO-CH2-CH2)3N~+~CHz-CN X-. According
to the invention, single- or multi-phase detergent tablets which contain a
cationic nitrite corresponding to formula (la):
R4
R5- i ~+~-(CH2)-CN X~-~ (la)
Rs
where R4, R5 and Rs independently of one another are selected from -CH3,
-CH2-CH3, -CH2-CH2-CH3, -CH(CH3)-CH3, in addition to which R4 may even
be -H, and X is an anion; preferably R5 = R6 = -CH3 and, more preferably,
R4 = R5 = Rs = _CH3~
as the cationic nitrite of formula (I) are particularly preferred.
According to the invention, detergent tablets containing
(CH3)3N~+~CH2-CN X, where X is an anion selected from the group
consisting of chloride, bromide, iodide, hydrogen sulfate, methosulfate, p-
toluene sulfonate (tosylate) or xylene sulfonate, as the cationic nitrite are
particularly preferred.
The detergent tablets according to the invention contain an
"activated bleaching" system, i.e. both bleaching agent and bleach
CA 02303638 2000-03-31
11
activator, cationic nitrites being used in accordance with the invention as
the bleach activator in order to obtain advantageous tablet properties. In
addition to the cationic nitrites, the detergent tablets according to the
invention may contain other bleach activators which are also described
hereinafter. However, the detergent tablets according to the invention
preferably contain the cationic nitrites corresponding to general formula (I)
as sole or princpal bleach activator, i.e. at least 50% by weight of the total
bleach activators present in the detergent tablets according to the invention
is made up of cationic nitrites corresponding to formula (I). Bleach
activators are added to bleach-containing detergents in order to obtain an
improved bleaching effect at washing temperatures of 60°C or lower.
According to the invention, compounds which form aliphatic peroxocar-
boxylic acids preferably containing 1 to 10 carbon atoms and more
preferably 2 to 4 carbon atoms and/or optionally substituted perbenzoic
acid under perhydrolysis conditions may be used as bleach activators in
addition to the cationic nitrite. Suitable additional bleach activators are
substances which contain O- and/or N-acyl groups with the number of
carbon atoms indicated and/or optionally substituted benzoyl groups.
Preferred additional bleach activators are polyacylated alkylenediamines,
more especially tetraacetyl ethylenediamine (TAED), acylated triazine
derivatives, more particularly 1,5-diacetyl-2,4-dioxohexahydro-1,3,5-triazine
(DADHT), acylated glycol urils, more particularly tetraacetyl glycol uril
(TAGU), N-acylimides, more particularly N-nonanoyl succinimide (NOSI),
acylated phenol sulfonates, more particularly n-nonanoyl- or isononanoyl-
oxybenzenesulfonate (n- or iso-NOBS), carboxylic anhydrides, more
especially phthalic anhydride, acylated polyhydric alcohols, more especially
triacetin, ethylene glycol diacetate and 2,5-diacetoxy-2,5-dihydrofuran.
In addition to or instead of the conventional bleach activators, so-
called bleach catalysts may also be incorporated in the tablets in addition
to the cationic nitrites of formula (I) in accordance with the present
CA 02303638 2000-03-31
12
invention. Bleach catalysts are bleach-boosting transition metal salts or
transition metal complexes such as, for example, Mn-, Fe-, Co-, Ru- or Mo-
salen complexes or carbonyl complexes. Mn-, Fe-, Co-, Ru-, Mo-, Ti-, V-
and Cu-complexes with N-containing tripod ligands and Co-, Fe-, Cu- and
Ru-ammine complexes may also be used as bleach catalysts. Bleach-
boosting active-substance combinations obtainable by thoroughly mixing a
water-soluble salt of a divalent transition metal selected from cobalt, iron,
copper and ruthenium and mixtures thereof, a water-soluble ammonium
salt and optionally a peroxygen-based oxidizing agent and an inert carrier
material may also be used as bleach catalysts in accordance with the
present invention. If bleach catalysts are used for the purposes of the
present invention, they are also governed by the particle size restrictions
mentioned in the foregoing.
A particularly preferred additional bleach activator, i.e. a bleach
activator used in addition to the cationic nitrite corresponding to formula
(I),
is N,N,N',N'-tetraacetyl ethylenediamine which is widely used in laundry/
dishwasher detergents. Accordingly, preferred detergent tablets are
characterized in that they contain a cationic nitrite corresponding to formula
(I) and tetraacetyl ethylenediamine (TAED) as bleach activators.
In "nitriloquat"/TAED combinations such as these, the ratio by weight
of "nitriloquat" to TAED is preferably in the range from 1:2 to 10:1. In one
particularly preferred embodiment, one third to two thirds of the total
quantity of bleach activator in combinations such as these consists of
cationic nitrite corresponding to formula (I).
The detergent tablets according to the invention contain the bleach
activators) in quantities of 0.5 to 30% by weight, preferably in quantities of
1 to 20% by weight and more preferably in quantities of 2 to 15% by
weight, based on the detergent tablet as a whole, reference being made
here to the quantity data mentioned in the foregoing for the cationic nitrite.
The total quantities of bleach activators may vary according to the
CA 02303638 2000-03-31
13
application envisaged for the tablets. Thus, in typical heavy-duty detergent
tablets, bleach activator contents of 0.5 to 10% by weight, preferably 2 to
8% by weight and more preferably 4 to 7% by weight are normal whereas
bleach tablets can have higher contents, for example from 5 to 30% by
weight, preferably from 7.5 to 25% by weight and more preferably from 10
to 20% by weight. The expert is not restricted in his freedom of formulation
and is able in this way to produce laundry detergent tablets, dishwasher
tablets or bleach tablets with a stronger or weaker bleaching effect by
varying the contents of bleach activator and bleaching agent.
The function of the bleach activators) in the detergent tablets
according to the invention is to activate the bleaching agents) at relatively
low washing temperatures and thus to provide for a high bleaching
performance, even at low temperatures. Sodium perborate tetrahydrate
and sodium perborate monohydrate are particularly important as bleaching
agents. Other useful bleaching agents are, for example, sodium
percarbonate, peroxypyrophosphates, citrate perhydrates and HZOZ-
yielding peracidic salts or peracids, such as perbenzoates,
peroxophthalates, diperazelaic acid, phthaloiminoperacid or diperdodecane
dioic acid. Where bleaching agents are used, it is again possible to leave
out surfactants and/or builders so that pure bleach tablets can be
produced. If such bleach tablets are to be added to laundry, a combination
of sodium percarbonate with sodium sesquicarbonate is preferably used
irrespective of what other ingredients the tablets contain. If detergent or
bleach tablets for dishwashing machines are being produced, bleaching
agents from the group of organic bleaches may also be used. Typical
organic bleaching agents are diacyl peroxides, such as dibenzoyl peroxide
for example. Other typical organic bleaching agents are the peroxy acids,
of which alkyl peroxy acids and aryl peroxy acids are particularly mentioned
as examples. Preferred representatives are (a) peroxybenzoic acid and
ring-substituted derivatives thereof, such as alkyl peroxybenzoic acids, but
CA 02303638 2000-03-31
14
also peroxy-a-naphthoic acid and magnesium monoperphthalate, (b)
aliphatic or substituted aliphatic peroxy acids, such as peroxylauric acid,
peroxystearic acid, s-phthalimidoperoxycaproic acid [phthaloiminoperoxy-
hexanoic acid (PAP)], o-carboxybenzamidoperoxycaproic acid, N-
nonenylamidoperadipic acid and N-nonenylamidopersuccinates. and (c)
aliphatic and araliphatic peroxydicarboxylic acids, such as 1,12-
diperoxycarboxylic acid, 1,9-diperoxyazelaic acid, diperoxysebacic acid,
diperoxybrassylic acid, diperoxyphthalic acids, 2-decyldiperoxybutane-1,4-
dioic acid, N,N-terephthaloyl-di(6-aminopercaproic acid).
Other suitable bleaching agents in dishwasher tablets are chlorine-
and bromine-releasing substances. Suitable chlorine- or bromine-releasing
materials are, for example, heterocyclic N-bromamides and N-chloramides,
for example trichloroisocyanuric acid, tribromoisocyanuric acid, dibromo-
isocyanuric acid and/or dichloroisocyanuric acid (DICA) and/or salts thereof
with cations, such as potassium and sodium. Hydantoin compounds, such
as 1,3-dichloro-5,5-dimethyl hydantoin, are also suitable.
In laundry detergent tablets and also dishwasher tablets, sodium
percarbonate is the preferred bleaching agent. "Sodium percarbonate" is a
non-specific term used for sodium carbonate peroxohydrates which, strictly
speaking, are not "percarbonates" (i.e. salts of percarbonic acid), but
hydrogen peroxide adducts with sodium carbonate. The commercial
material has the mean composition 2 Na2C03 ~ 3 H202 and, accordingly, is
not a peroxycarbonate. Sodium percarbonate forms a white water-soluble
powder with a density of 2.14 gcm3 which readily decomposes into sodium
carbonate and bleaching or oxidizing oxygen.
Sodium carbonate peroxohydrate was obtained for the first time in
1899 by precipitation with ethanol from a solution of sodium carbonate in
hydrogen peroxide, but was mistakenly regarded as peroxycarbonate. It
was only in 1909 that the compound was recognised as a hydrogen
CA 02303638 2000-03-31
peroxide addition compound. Nevertheless, the historical name "sodium
percarbonate" has been adopted in practice.
On an industrial scale, sodium percarbonate is mainly produced by
precipitation from aqueous solution (so-called wet process). In this pro-
s cess, aqueous solutions of sodium carbonate and hydrogen peroxide are
combined and the sodium percarbonate is precipitated by salting-out
agents (mainly sodium chloride), crystallization aids (for example polyphos-
phates, polyacrylates) and stabilizers (for example Mg2+ ions). The
precipitated salt which still contains 5 to 12% by weight of mother liquor is
10 then removed by centrifuging and dried at 90°C in fluidized bed
dryers.
The bulk density of the end product can vary between 800 and 1200 g/I
according to the production process. In general, the percarbonate is
stabilized by an additional coating. Coating processes and materials are
widely described in the patent literature. Basically, any commercially
15 available percarbonate types as marketed, for example, by Solvay Interox,
Degussa, Kemira and Akzo may be used in accordance with the present
invention.
The detergent tablets according to the invention may be single-
phase tablets, i.e. may have been tabletted from a premix to form a
homogeneous tablet. However, it is also possible and preferred in
accordance with the invention to provide multiphase tablets where active
substances can be divided up into spatially demarcated regions where this
appears appropriate. According to the invention, preferred detergent
tablets are two- or multi-phase detergent tablets where one phase contains
the cationic nitrite of formula (I) in quantities of 2.5% by weight,
preferably
5% by weight and more preferably 7.5% by weight, based on the weight of
the phase, while the other phases) is/are preferably free from the cationic
nitrite corresponding to formula (I) and, in particular, is/are free from all
bleach activators.
By dividing up the total mass of a tablet into different phases, an
CA 02303638 2000-03-31
16
increased content of certain ingredients, more particularly bleach
activators, can be achieved in an individual phase providing the content of
this ingredient in the other phases) is reduced accordingly without the total
content of this active substance in the tablet varying. As can be seen from
the above-mentioned quantities of bleach activators) in the tablet as a
whole and from the above-mentioned quantities of bleach activators) in an
individual phase, it is preferred in accordance with the invention to
concentrate the bleach activators) in one phase of the tablet so that the
corresponding phase has high bleach activator contents.
Multiphase tablets have the advantage over single-phase tablets
that the pH criterion according to the invention can be satisfied more easily
and universally in a phase than in the tablet as a whole. Due to the low pH
value which the phase is supposed to have after dissolution in water, the
use of raw materials with a relatively highly alkaline pH in unlimited
quantities is not possible and, according to the invention, should be
avoided. Acidifying agents may have to be added to ensure that the
cationic nitrites of formula (I) are present in the weakly alkaline to mildly
acidic medium required in accordance with the invention. However, if
indispensable alkaline raw materials are to be incorporated in the
detergent, large quantities of acidifying agent would have to be used for a
single-phase tablet. Through a phase split, the advantageous stabilization
of the cationic nitrite and the use of alkaline raw materials are both
possible
by the presence of the alkaline raw materials, such as carbonates,
phosphates, silicates, etc. in the phases) which is/are free from the
cationic nitrite corresponding to formula (I).
According to the invention, the release of the activated bleaching
system can also be delayed or accelerated so that further advantages in
regard to cleaning performance are obtained. Delayed release can be
obtained, for example, by coating particles or the entire phase while
accelerated release can be obtained, for example, by adding disintegration
CA 02303638 2000-03-31
17
aids to a phase. By applying such measures, it is possible, for example, to
release the cationic nitrite corresponding to formula (I) at the beginning of
the wash cycle, the wash liquor having a weakly alkaline or neutral to mildly
acidic pH value if only the nitrite-containing phase dissolves. After
dissolution of the other phase(s), the pH value may be drastically increased
according to the composition of the phase(s). Conversely, delayed release
of the cationic nitrite corresponding to formula (I) can also be achieved. If
in this case the accelerated dissolution of the other phases) in relation to
the nitrite-containing phase is intended to provide a high pH value, this can
be compensated by acidifying agent in the nitrite-containing phase if the
nitrite is to be released in the neutral to mildly acidic medium.
The division of the tablet into two or more phases may also be used
to separate incompatible ingredients. According to the invention, two-
phase or multiphase detergent tablets in which one phase contains the
cationic nitrite corresponding to formula (I) while another phase contains all
the bleaching agents) present in the tablets are preferred.
Where other ingredients are used besides bleaching agents and the
cationic nitrite corresponding to formula (I), certain ingredients are
preferably separated from one another. In the case of dishwasher tablets
and laundry detergent tablets, two- or multi-phase detergent tablets which
contain the cationic nitrite (I), bleaching agent and enzymes - the enzymes
not being present in the same phase as the bleaching agent and the
cationic nitrite - are preferred.
In addition, in the case of dishwasher tablets, two- or multi-phase
detergent tablets which contain the cationic nitrite (I), bleaching agent and
silver protector - the silver protector not being present in the same phase
as the bleaching agent and the cationic nitrite - are preferred.
The ingredients mentioned are described in detail hereinafter.
Besides the ingredients mentioned above (bleach activator and bleaching
agent), the detergent tablets according to the invention may contain other
CA 02303638 2000-03-31
18
ingredients in quantities determined by the particular application envisaged
for the tablets. Thus, substances from the groups of surfactants, builders
and polymers are particularly suitable for use in the detergent tablets
according to the invention. The expert will again have no difficulty in
selecting the individual components and the quantities in which to use
them. Thus, a heavy-duty detergent tablet will contain relatively large
quantities of surfactants) whereas a bleach tablet may well contain no
surfactant at all. The quantity of builders) used also varies according to
the particular application envisaged.
The detergent tablets according to the invention may contain any of
the builders normally used in detergents, i.e. in particular zeolites,
silicates,
carbonates, organic co-builders and - providing there are no ecological
objections to their use - also phosphates.
Suitable crystalline layered sodium silicates correspond to the
general formula NaMSixOZX+~y H20, where M is sodium or hydrogen, x is a
number of 1.9 to 4 and y is a number of 0 to 20, preferred values for x
being 2, 3 or 4. Crystalline layered silicates such as these are described,
for example, in European patent application EP-A-0 164 514. Preferred
crystalline layered silicates corresponding to the above formula are those in
which M is sodium and x assumes the value 2 or 3. Both (i- and 8-sodium
disilicates Na2Si205y HZO are particularly preferred, (i-sodium disilicate
being obtainable, for example, by the process described in International
patent application WO-A- 91/08171.
Other useful builders are amorphous sodium silicates with a
modulus (Na20:Si02 ratio) of 1:2 to 1:3.3, preferably 1:2 to 1:2.8 and more
preferably 1:2 to 1:2.6 which dissolve with delay and exhibit multiple wash
cycle properties. The delay in dissolution in relation to conventional
amorphous sodium silicates can have been obtained in various ways, for
example by surface treatment, compounding, compacting or by overdrying.
In the context of the invention, the term "amorphous" is also understood to
CA 02303638 2000-03-31
19
encompass "X-ray amorphous". In other words, the silicates do not
produce any of the sharp X-ray reflexes typical of crystalline substances in
X-ray diffraction experiments, but at best one or more maxima of the
scattered X-radiation which have a width of several degrees of the
diffraction angle. However, particularly good builder properties may even
be achieved where the silicate particles produce crooked or even sharp
diffraction maxima in electron diffraction experiments. This may be
interpreted to mean that the products have microcrystalline regions
between 10 and a few hundred nm in size, values of up to at most 50 nm
and, more particularly, up to at most 20 nm being preferred. So-called X-
ray amorphous silicates such as these, which also dissolve with delay in
relation to conventional waterglasses, are described for example in
German patent application DE-A-44 00 024. Compacted amorphous
silicates, compounded amorphous silicates and overdried X-ray-amorphous
silicates are particularly preferred.
The finely crystalline, synthetic zeolite containing bound water used
in accordance with the invention is preferably zeolite A and/or zeolite P.
Zeolite MAP~ (Crosfield) is a particularly preferred P-type zeolite.
However, zeolite X and mixtures of A, X and/or P are also suitable.
According to the invention, it is also preferred to use, for example, a co-
crystallizate of zeolite X and zeolite A (ca. 80% by weight zeolite X) which
is marketed by CONDEA Augusta S.p.A. under the name of VEGOBOND
AX~ and which may be described by the following formula:
nNa20 ~ (1-n)K20 ~ AI203 ~ (2 - 2.5)Si02 ~ (3.5 - 5.5) H20.
The zeolite may be used both as a builder in a granular compound and as
a kind of "powder" to be applied to the entire mixture to be tabletted, both
routes normally being used to incorporate the zeolite in the premix.
Suitable zeolites have a mean particle size of less than 10 ~,m (volume
CA 02303638 2000-03-31
distribution, as measured by the Coulter Counter Method) and contain
preferably 18 to 22% by weight and more preferably 20 to 22% by weight of
bound water.
The generally known phosphates may of course also be used as
5 builders providing their use should not be avoided on ecological grounds.
Among the large number of commercially available phosphates, alkali
metal phosphates have the greatest importance in the detergent industry,
pentasodium triphosphate and pentapotassium triphosphate (sodium and
potassium tripolyphosphate) being particularly preferred.
10 "Alkali metal phosphates" is the collective term for the alkali metal
(more particularly sodium and potassium) salts of the various phosphoric
acids, including metaphosphoric acids (HP03)~ and orthophosphoric acid
(H3P04) and representatives of higher molecular weight. The phosphates
combine several advantages: they act as alkalinity sources, prevent lime
15 deposits on machine parts and lime incrustations in fabrics and, in
addition,
contribute towards the cleaning effect.
Sodium dihydrogen phosphate (NaH2P04) exists as the dehydrate
(density 1.91 gcm-3, melting point 60°) and as the monohydrate (density
2.04 gcm 3). Both salts are white readily water-soluble powders which, on
20 heating, lose the water of crystallization and, at 200°, are
converted into the
weakly acidic diphosphate (disodium hydrogen diphosphate, Na2H2P20~)
and, at higher temperatures, into sodium trimetaphosphate (Na3P309) and
Maddrell's salt (see below). NaH2P04 shows an acidic reaction. It is
formed by adjusting phosphoric acid with sodium hydroxide to a pH value
of 4.5 and spraying the resulting "mash". Potassium dihydrogen phosphate
(primary or monobasic potassium phosphate, potassium biphosphate,
KDP), KH2P04, is a white salt with a density of 2.33 gcm-3, has a melting
point of 253° [decomposition with formation of potassium polyphosphate
(KP03)X] and is readily soluble in water.
Disodium hydrogen phosphate (secondary sodium phosphate),
CA 02303638 2000-03-31
21
Na2HP04, is a colorless, readily water-soluble crystalline salt. It exists in
water-free form and with 2 moles (density 2.066 gcm-3, water loss at
95°), 7
moles (density 1.68 gcm-3, melting point 48° with loss of 5 H20) and 12
moles of water (density 1.52 gcm-3, melting point 35° with loss of 5
H20),
becomes water-free at 100° and, on fairly intensive heating, is
converted
into the diphosphate Na4P20~. Disodium hydrogen phosphate is prepared
by neutralization of phosphoric acid with soda solution using phenol-
phthalein as indicator. Dipotassium hydrogen phosphate (secondary or
dibasic potassium phosphate), K2HP04, is an amorphous white salt which
is readily soluble in water.
Trisodium phosphate, tertiary sodium phosphate, Na3P04, consists
of colorless crystals which have a density of 1.62 gcm-3 and a melting point
of 73-76° (decomposition) as the dodecahydrate, a melting point of
100° as
the decahydrate (corresponding to 19-20% P205) and a density of 2.536
gcm-3 in water-free form (corresponding to 39-40% P205). Trisodium
phosphate is readily soluble in water through an alkaline reaction and is
prepared by concentrating a solution of exactly 1 mole of disodium
phosphate and 1 mole of NaOH by evaporation. Tripotassium phosphate
(tertiary or tribasic potassium phosphate), K3P04, is a white deliquescent
granular powder with a density of 2.56 gcm-', has a melting of 1340°
and is
readily soluble in water through an alkaline reaction. It is formed, for
example, when Thomas slag is heated with coal and potassium sulfate.
Despite their higher price, the more readily soluble and therefore highly
effective potassium phosphates are often preferred to corresponding
sodium compounds in the detergent industry.
Tetrasodium diphosphate (sodium pyrophosphate), Na4P20~, exists
in water-free form (density 2.534 gcm-3, melting point 988°, a figure
of 880°
has also been mentioned) and as the decahydrate (density 1.815 - 1.836
gcm-3, melting point 94° with loss of water). Both substances are
colorless
crystals which dissolve in water through an alkaline reaction. Na4P20~ is
CA 02303638 2000-03-31
22
formed when disodium phosphate is heated to >200° or by reacting
phosphoric acid with soda in a stoichiometric ratio and spray-drying the
solution. The decahydrate complexes heavy metal salts and hardness
salts and, hence, reduces the hardness of water. Potassium diphosphate
(potassium pyrophosphate), K4P20~, exists in the form of the trihydrate and
is a colorless hygroscopic powder with a density of 2.33 gcm-3 which is
soluble in water, the pH value of a 1 % solution at 25° being 10.4.
Relatively high molecular weight sodium and potassium phosphates
are formed by condensation of NaHZP04 or KH2P04. They may be divided
into cyclic types, namely the sodium and potassium metaphosphates, and
chain types, the sodium and potassium polyphosphates. The chain types
in particular are known by various different names: fused or calcined
phosphates, Graham's salt, Kurrol's salt and Maddrell's salt. All higher
sodium and potassium phosphates are known collectively as condensed
phosphates.
The industrially important pentasodium triphosphate, Na5P30~o
(sodium tripolyphosphate), is a non-hygroscopic white water-soluble salt
which crystallizes without water or with 6 H20 and which has the general
formula Na0-[P(O)(ONa)-O)"-Na where n = 3. Around 17 g of the salt free
from water of crystallization dissolve in 100 g of water at room temperature,
around 20 g at 60° and around 32 g at 100°. After heating of the
solution
for 2 hours to 100°, around 8% orthophosphate and 15% diphosphate are
formed by hydrolysis. In the preparation of pentasodium triphosphate,
phosphoric acid is reacted with soda solution or sodium hydroxide in a
stoichiometric ratio and the solution is spray-dried. Similarly to Graham's
salt and sodium diphosphate, pentasodium triphosphate dissolves many
insoluble metal compounds (including lime soaps, etc.). Pentapotassium
triphosphate, K5P30~o (potassium tripolyphosphate), is marketed for
example in the form of a 50% by weight solution (> 23% P205, 25% K20).
The potassium polyphosphates are widely used in the detergent industry.
CA 02303638 2000-03-31
23
Sodium potassium tripolyphosphates, which may also be used in
accordance with the invention, also exist. They are formed for example
when sodium trimetaphosphate is hydrolyzed with KOH:
(NaP03)3 + 2 KOH -~ Na3K2P30~o + H20
According to the invention, they may be used in exactly the same
way as sodium tripolyphosphate, potassium tripolyphosphate or mixtures
thereof. Mixtures of sodium tripolyphosphate and sodium potassium
tripolyphosphate or mixtures of potassium tripolyphosphate and sodium
potassium tripolyphosphate or mixtures of sodium tripolyphosphate and
potassium tripolyphosphate and sodium potassium tripolyphosphate may
also be used in accordance with the invention.
Organic cobuilders suitable for use in the detergent tablets
according to the invention are, in particular, polycarboxylates/polycarboxylic
acids, polymeric polycarboxylates, aspartic acid, polyacetals, dextrins,
other organic cobuilders (see below) and phosphonates. These classes of
substances are described in the following.
Useful organic builders are, for example, the polycarboxylic acids
usable, for example, in the form of their sodium salts, polycarboxylic acids
in this context being understood to be carboxylic acids which bear more
than one acid function. Examples of such carboxylic acids are citric acid,
adipic acid, succinic acid, glutaric acid, malic acid, tartaric acid, malefic
acid,
fumaric acid, sugar acids, aminocarboxylic acids, nitrilotriacetic acid (NTA),
providing their use is not ecologically unsafe, and mixtures thereof.
Preferred salts are the salts of the polycarboxylic acids, such as citric
acid,
adipic acid, succinic acid, glutaric acid, tartaric acid, sugar acids and
mixtures thereof.
The acids per se may also be used. Besides their builder effect, the
acids also typically have the property of an acidifying component and,
CA 02303638 2000-03-31
24
hence, also serve to establish a relatively low and mild pH value in
detergents. Citric acid, succinic acid, glutaric acid, adipic acid, gluconic
acid and mixtures thereof are particularly mentioned in this regard.
Other suitable builders are polymeric polycarboxylates such as, for
example, the alkali metal salts of polyacrylic or polymethacrylic acid, for
example those with a relative molecular weight of 500 to 70,000 g/mole.
The molecular weights mentioned in this specification for polymeric
polycarboxylates are weight-average molecular weights MW of the particular
acid form which, basically, were determined by gel permeation
chromatography (GPC) using a UV detector. The measurement was
carried out against an external polyacrylic acid standard which provides
realistic molecular weight values by virtue of its structural similarity to
the
polymers investigated. These values differ distinctly from the molecular
weights measured against polystyrene sulfonic acids as standard. The
molecular weights measured against polystyrene sulfonic acids are
generally higher than the molecular weights mentioned in this specification.
Particularly suitable polymers are polyacrylates which preferably
have a molecular weight of 2,000 to 20,000 g/mole. By virtue of their
superior solubility, preferred representatives of this group are the short-
chain polyacrylates which have molecular weights of 2,000 to 10,000
glmole and, more particularly, 3,000 to 5,000 g/mole.
Also suitable are copolymeric polycarboxylates, particularly those of
acrylic acid with methacrylic acid and those of acrylic acid or methacrylic
acid with malefic acid. Acrylic acidlmaleic acid copolymers containing 50 to
90% by weight of acrylic acid and 50 to 10% by weight of malefic acid have
proved to be particularly suitable. Their relative molecular weights, based
on the free acids, are generally in the range from 2,000 to 70,000 g/mole,
preferably in the range from 20,000 to 50,000 g/mole and more preferably
in the range from 30,000 to 40,000 g/mole.
The (co)polymeric polycarboxylates may be used either in powder
CA 02303638 2000-03-31
form or in the form of an aqueous solution. The content of (co)polymeric
polycarboxylates in the detergent is preferably from 0.5 to 20% by weight
and more preferably from 3 to 10% by weight.
In order to improve solubility in water, the polymers may also contain
5 allyl sulfonic acids, such as allyloxybenzene sulfonic acid and methallyl
sulfonic acid, as monomer.
Other particularly preferred polymers are biodegradable polymers of
more than two different monomer units, for example those which contain
salts of acrylic acid and malefic acid and vinyl alcohol or vinyl alcohol
10 derivatives as monomers or those which contain salts of acrylic acid and 2-
alkylallyl sulfonic acid and sugar derivatives as monomers.
Other preferred copolymers are those which are described in
German patent applications DE-A-43 03 320 and DE-A-44 17 734 and
which preferably contain acrolein and acrylic acid/acrylic acid salts or
15 acrolein and vinyl acetate as monomers.
Other preferred builders are polymeric aminodicarboxylic acids, salts
or precursors thereof. Particular preference is attributed to polyaspartic
acids or salts and derivatives thereof which, according to German patent
application DE-A-195 40 086, are also said to have a bleach-stabilizing
20 effect in addition to their co-builder properties.
Other suitable builders are polyacetals which may be obtained by
reaction of dialdehydes with polyol carboxylic acids containing 5 to 7
carbon atoms and at least three hydroxyl groups. Preferred polyacetals
are obtained from dialdehydes, such as glyoxal, glutaraldehyde, terephthal-
25 aldehyde and mixtures thereof and from polyol carboxylic acids, such as
gluconic acid and/or glucoheptonic acid.
Other suitable organic builders are dextrins, for example oligomers
or polymers of carbohydrates which may be obtained by partial hydrolysis
of starches. The hydrolysis may be carried out by standard methods, for
example acid- or enzyme-catalyzed methods. The end products are
CA 02303638 2000-03-31
26
preferably hydrolysis products with average molecular weights of 400 to
500,000 g/mol. A polysaccharide with a dextrose equivalent (DE) of 0.5 to
40 and, more particularly, 2 to 30 is preferred, the DE being an accepted
measure of the reducing effect of a polysaccharide by comparison with
dextrose which has a DE of 100. Both maltodextrins with a DE of 3 to 20
and dry glucose sirups with a DE of 20 to 37 and also so-called yellow
dextrins and white dextrins with relatively high molecular weights of 2,000
to 30,000 g/mole may be used.
The oxidized derivatives of such dextrins are their reaction products
with oxidizing agents which are capable of oxidizing at least one alcohol
function of the saccharide ring to the carboxylic acid function. Dextrins thus
oxidized and processes for their production are known, for example, from
European patent applications EP-A-0 232 202, EP-A-0 427 349, EP-A-0
472 042 and EP-A-0 542 496 and from International patent applications
WO 92/18542, WO 93/08251, WO 93/16110, WO 94/28030, WO 95/07303,
WO 95/12619 and WO 95/20608. An oxidized oligosaccharide
corresponding to German patent application DE-A-196 00 018 is also
suitable. A product oxidized at C6 of the saccharide ring can be particularly
advantageous.
Other suitable co-builders are oxydisuccinates and other derivatives
of disuccinates, preferably ethylenediamine disuccinate. Ethylenediamine-
N,N'-disuccinate (EDDS) is preferably used in the form of its sodium or
magnesium salts. Glycerol disuccinates and glycerol trisuccinates are also
preferred in this connection. The quantities used in zeolite-containing
and/or silicate-containing formulations are from 3 to 15% by weight.
Other useful organic co-builders are, for example, acetylated
hydroxycarboxylic acids and salts thereof which may optionally be present
in lactone form and which contain at least 4 carbon atoms, at least one
hydroxy group and at most two acid groups. Co-builders such as these are
described, for example, in International patent application WO-A-95/20029.
CA 02303638 2000-03-31
27
Another class of substances with co-builder properties are the
phosphonates, more particularly hydroxyalkane and aminoalkane phos-
phonates. Among the hydroxyalkane phosphonates, 1-hydroxyethane-1,1-
diphosphonate (HEDP) is particularly importance as a co-builder. It is
preferably used in the form of the sodium salt, the disodium salt showing a
neutral reaction and the tetrasodium salt an alkaline reaction (pH 9).
Preferred aminoalkane phosphonates are ethylenediamine tetramethylene
phosphonate (EDTMP), diethylenetriamine pentamethylenephosphonate
(DTPMP) and higher homologs thereof. They are preferably used in the
form of the neutrally reacting sodium salts, for example as the hexasodium
salt of EDTMP or as the hepta- and octasodium salts of DTPMP. Of the
phosphonates, HEDP is preferably used as a builder. In addition, the
aminoalkane phosphonates have a pronounced heavy metal binding
capacity. Accordingly, it can be of advantage, particularly where the
detergents also contain bleach, to use aminoalkane phosphonates, more
particularly DTPMP, or mixtures of the phosphonates mentioned.
In addition, any compounds capable of forming complexes with
alkaline earth metal ions may be used as co-builders.
The quantity of builder used is normally between 10 and 70% by
weight, preferably between 15 and 60% by weight and more preferably
between 20 and 50% by weight. The quantity of builder used is again
dependent upon the particular application envisaged, so that bleach tablets
can contain larger quantities of builders (for example between 20 and 70%
by weight, preferably between 25 and 65% by weight and more preferably
between 30 and 55% by weight) than, for example, laundry detergent
tablets (normally 10 to 50% by weight, preferably 12.5 to 45% by weight
and more preferably 17.5 to 37.5% by weight).
Preferred detergent tablets additionally contain one or more
surfactant(s). Anionic, nonionic, cationic and/or amphoteric surfactants or
mixtures thereof may be used in the detergent tablets according to the
CA 02303638 2000-03-31
28
invention. Mixtures of anionic and nonionic surfactants are preferred from
the performance point of view. The total surfactant content of the tablets is
from 5 to 60% by weight, based on the weight of the tablet, surfactant
contents above 15% by weight being preferred in the case of laundry
detergent tablets and dishwasher tablets normally containing less than 3%
by weight of surfactant.
The anionic surfactants used are, for example, those of the sulfonate
and sulfate type. Preferred surfactants of the sulfonate type are C9_~3 alkyl
benzenesulfonates, olefin sulfonates, i.e. mixtures of alkene and hydroxy-
alkane sulfonates, and the disulfonates obtained, for example, from C~2_~8
monoolefins with an internal or terminal double bond by sulfonation with
gaseous sulfur trioxide and subsequent alkaline or acidic hydrolysis of the
sulfonation products. Other suitable surfactants of the sulfonate type are
the alkane sulfonates obtained from C~2_~8 alkanes by sulfochlorination or
sulfoxidation and subsequent hydrolysis or neutralization. The esters of a-
sulfofatty acids (ester sulfonates), for example the a-sulfonated methyl
esters of hydrogenated coconut oil, palm kernel oil or tallow fatty acids.
Other suitable anionic surfactants are sulfonated fatty acid glycerol
esters, i.e. the monoesters, diesters and triesters and mixtures thereof
which are obtained where production is carried out by esterification by a
monoglycerol with 1 to 3 moles of fatty acid or in the transesterification of
triglycerides with 0.3 to 2 moles of glycerol. Preferred sulfonated fatty acid
glycerol esters are the sulfonation products of satturated C6_22 fatty acids,
for example caproic acid, caprylic acid, capric acid, myristic acid, lauric
acid, palmitic acid, stearic acid or behenic acid.
Preferred alk(en)yl sulfates are the alkali metal salts and, in
particular, the sodium salts of the sulfuric acid semiesters of C~2_~$ fatty
alcohols, for example coconut alcohol, tallow alcohol, lauryl, myristyl, cetyl
or stearyl alcohol, or C~o_2o oxoalcohols and the corresponding semiesters
of secondary alcohols with the same chain length. Other preferred
CA 02303638 2000-03-31
29
alk(en)yl sulfates are those with the chain length mentioned which contain
a synthetic, linear alkyl chain based on a petrochemical and which are
similar in their degradation behavior to the corresponding compounds
based on oleochemical raw materials. C~2_~6 alkyl sulfates and C~2_~5 alkyl
sulfates and also C~4_~5 alkyl sulfates alkyl sulfates are particularly
preferred
from the washing performance point of view. Other suitable anionic
surfactants are 2,3-alkyl sulfates which may be produced, for example, in
accordance with US 3,234,258 or US 5,075,041 and which are
commercially obtainable as products of the Shell Oil Company under the
name of DAN~.
The sulfuric acid monoesters of linear or branched C~_2~ alcohols
ethoxylated with 1 to 6 moles of ethylene oxide, such as 2-methyl-branched
C9_> > alcohols containing on average 3.5 moles of ethylene oxide (EO) or
C~2_~a fatty alcohols containing 1 to 4 EO, are also suitable. In view of
their
high foaming capacity, they are normally used in only relatively small
quantities, for example in quantities of 1 to 5% by weight, in dishwashing
detergents.
Other suitable anionic surfactants are the salts of alkyl sulfosuccinic
acid which are also known as sulfosuccinates or as sulfosuccinic acid
esters and which represent monoesters and/or diesters of sulfosuccinic
acid with alcohols, preferably fatty alcohols and, more particularly,
ethoxylated fatty alcohols. Preferred sulfosuccinates contain C8_~$ fatty
alcohol molecules or mixtures thereof. Particularly preferred
sulfosuccinates contain a fatty alcohol molecule derived from ethoxylated
fatty alcohols which, considered in isolation, represent nonionic surfactants
(for a description, see below). Of these sulfosuccinates, those of which the
fatty alcohol molecules are derived from narrow-range ethoxylated fatty
alcohols are particularly preferred. Alk(en)yl succinic acid preferably
containing 8 to 18 carbon atoms in the alk(en)yl chain or salts thereof may
also be used.
CA 02303638 2000-03-31
Other suitable anionic surfactants are, in particular, soaps. Suitable
soaps are, in particular, saturated fatty acid soaps, such as the salts of
lauric acid, myristic acid, palmitic acid, stearic acid, hydrogenated erucic
acid and behenic acid, and soap mixtures derived in particular from natural
5 fatty acids, for example coconut, palm kernel or tallow acids.
The anionic surfactants, including the soaps, may be present in the
form of their sodium, potassium or ammonium salts and as soluble salts of
organic bases, such as mono-, di- or triethanolamine. The anionic
surfactants are preferably present in the form of their sodium or potassium
10 salts and, more preferably, in the form of their sodium salts.
Preferred nonionic surfactants are alkoxylated, advantageously
ethoxylated, more especially primary alcohols preferably containing 8 to 18
carbon atoms and, on average, 1 to 12 moles of ethylene oxide (EO) per
mole of alcohol, in which the alcohol radical may be linear or, preferably,
15 methyl-branched in the 2-position or may contain linear and methyl-
branched radicals in the form of the mixtures typically present in oxoalcohol
radicals. However, alcohol ethoxylates containing linear radicals of
alcohols of native origin with 12 to 18 carbon atoms, for example coconut
oil, palm oil, tallow or oleyl alcohol, and on average 2 to 8 EO per mole of
20 alcohol are particularly preferred. Preferred ethoxylated alcohols include,
for example, C~2-~a alcohols containing 3 EO or 4 EO, C9_» alcohol
containing 7 EO, C~3-~5 alcohols containing 3 EO, 5 EO, 7 EO or 8 EO,
C~2_~$ alcohols containing 3 EO, 5 EO or 7 EO and mixtures thereof, such
as mixtures of C~2_~4 alcohol containing 3 EO and C~2_~$ alcohol containing
25 5 EO. The degrees of ethoxylation mentioned represent statistical mean
values which, for a special product, can be a whole number or a broken
number. Preferred alcohol ethoxylates have a narrow homolog distribution
(narrow range ethoxylates, NRE). In addition to these nonionic surfactants,
fatty alcohols containing more than 12 EO may also be used, examples
30 including tallow fatty alcohol containing 14 EO, 25 EO, 30 EO or 40 EO.
CA 02303638 2000-03-31
31
Suitable other nonionic surfactants are alkyl glycosides with the
general formula RO(G)X where R is a primary, linear or methyl-branched,
more particularly 2-methyl-branched, aliphatic radical containing 8 to 22
and preferably 12 to 18 carbon atoms and G stands for a glycose unit
containing 5 or 6 carbon atoms, preferably glucose. The degree of
oligomerization x, which indicates the distribution of monoglycosides and
oligoglycosides, is a number of 1 to 10 and preferably 1.2 to 1.4.
Another class of preferred nonionic surfactants which may be used
either as sole nonionic surfactant or in combination with other nonionic
surfactants are alkoxylated, preferably ethoxylated or ethoxylated and
propoxylated, fatty acid alkyl esters preferably containing 1 to 4 carbon
atoms in the alkyl chain, more especially the fatty acid methyl esters which
are described, for example, in Japanese patent application JP 581217598
or which are preferably produced by the process described in International
patent application WO-A-90/13533.
Nonionic surfactants of the amine oxide type, for example N-
coconutalkyl-N,N-dimethylamine oxide and N-tallowalkyl-N,N-dihydroxy-
ethylamine oxide, and the fatty acid alkanolamide type are also suitable.
The quantity in which these nonionic surfactants are used is preferably no
more than the quantity in which the ethoxylated fatty alcohols are used
and, more preferably, no more than half that quantity.
Other suitable surfactants are polyhydroxyfatty acid amides
corresponding to formula (II):
R~
R-CO-N-[Z] ( I I )
in which RCO is an aliphatic acyl group containing 6 to 22 carbon atoms,
R' is hydrogen, an alkyl or hydroxyalkyl group containing 1 to 4 carbon
atoms and [Z] is a linear or branched polyhydroxyalkyl group containing 3
to 10 carbon atoms and 3 to 10 hydroxyl groups. The polyhydroxyfatty acid
CA 02303638 2000-03-31
32
amides are known substances which may normally be obtained by
reductive amination of a reducing sugar with ammonia, an alkylamine or an
alkanolamine and subsequent acylation with a fatty acid, a fatty acid alkyl
ester or a fatty acid chloride.
The group of polyhydroxyfatty acid amides also includes compounds
corresponding to formula (III):
R'-O-R2
R-CO-N-[Z] (III)
in which R is a linear or branched alkyl or alkenyl group containing 7 to 12
carbon atoms, R' is a linear, branched or cyclic alkyl group or an aryl group
containing 2 to 8 carbon atoms and R2 is a linear, branched or cyclic alkyl
group or an aryl group or an oxyalkyl group containing 1 to 8 carbon atoms,
C~~ alkyl or phenyl groups being preferred, and [Z] is a linear polyhydroxy-
alkyl group, of which the alkyl chain is substituted by at least two hydroxyl
groups, or alkoxylated, preferably ethoxylated or propoxylated, derivatives
of that group.
[Z] is preferably obtained by reductive amination of a reduced sugar,
for example glucose, fructose, maltose, lactose, galactose, mannose or
xylose. The N-alkoxy- or N-aryloxy-substituted compounds may then be
converted into the required polyhydroxyfatty acid amides by reaction with
fatty acid methyl esters in the presence of an alkoxide as catalyst, for
example in accordance with the teaching of International patent application
WO-A-95107331.
According to the invention, preferred detergent tablets contain
anionic and/or nonionic surfactants) and have total surfactant contents
above 2.5% by weight, preferably above 5% by weight and more preferably
above 10% by weight, based on the weight of the tablet. Performance-
related advantages can arise out of certain quantity ratios in which the
individual classes of surfactants are used.
CA 02303638 2000-03-31
33
For example, particularly preferred detergent tablets are charac-
terized in that the ratio of anionic surfactants) to nonionic surfactants) is
from 10:1 to 1:10, preferably from 7.5:1 to 1:5 and more preferably from 5:1
to 1:2.
It can be of advantage from the performance point of view if certain
classes of surfactants are missing from certain phases of the detergent
tablets or from the entire tablet, i.e. from every phase. In another important
embodiment of the present invention, therefore, at least one phase of the
tablets is free from nonionic surfactants.
Conversely, a positive effect can also be obtained through the
presence of certain surfactants in individual phases or in the tablet as a
whole, i.e. in every phase. Introducing the alkyl polyglycosides described
above has proved to be of particular advantage, so that detergent tablets in
which at least one phase of the tablet contains alkyl polyglycosides are
preferred.
As with the nonionic surfactants, the omission of anionic surfactants
from individual phases or from all phases can result in detergent tablets
which are more suitable for certain applications. Accordingly, detergent
tablets where at least one phase of the tablet is free from anionic
surfactants are also possible in accordance with the present invention.
In order to facilitate the disintegration of heavily compacted tablets,
disintegration aids, so-called tablet disintegrators, may be incorporated in
them to shorten their disintegration times. According to Rompp (9th
Edition, Vol. 6, page 4440) and Voigt "Lehrbuch der pharmazeutischen
Technologie" (6th Edition, 1987, pages 182-184), tablet disintegrators or
disintegration accelerators are auxiliaries which provide for the rapid
disintegration of tablets in water or gastric juices and the release of the
pharmaceuticals in an absorbable form.
These substances, which are also known as "disintegrators" by
virtue of their effect, are capable of undergoing an increase in volume on
CA 02303638 2000-03-31
34
contact with water so that, on the one hand, their own volume is increased
(swelling) and, on the other hand, a pressure can be generated through the
release of gases which causes the tablet to disintegrate into relatively small
particles. Well-known disintegrators are, for example, carbonate/citric acid
systems, although other organic acids may also be used. Swelling dis-
integration aids are, for example, synthetic polymers, such as polyvinyl
pyrrolidone (PVP), or natural polymers and modified natural substances,
such as cellulose and starch and derivatives thereof, alginates or casein
derivatives.
Preferred detergent tablets contain 0.5 to 10% by weight, preferably
3 to 7% by weight and more preferably 4 to 6% by weight of one or more
disintegration aids, based on the weight of the tablet.
According to the invention, preferred disintegrators are cellulose-
based disintegrators, so that preferred detergent tablets contain a
cellulose-based disintegrator in quantities of 0.5 to 10% by weight,
preferably 3 to 7% by weight and more preferably 4 to 6% by weight. Pure
cellulose has the formal empirical composition (C6H~pO5)n and, formally, is
a ~3-1,4-polyacetal of cellobiose which, in turn, is made up of two molecules
of glucose. Suitable celluloses consist of ca. 500 to 5000 glucose units
and, accordingly, have average molecular weights of 50,000 to 500,000.
According to the invention, cellulose derivatives obtainable from cellulose
by polymer-analog reactions may also be used as cellulose-based
disintegrators. These chemically modified celluloses include, for example,
products of esterification or etherification reactions in which hydroxy
hydrogen atoms have been substituted. However, celluloses in which the
hydroxy groups have been replaced by functional groups that are not
attached by an oxygen atom may also be used as cellulose derivatives.
The group of cellulose derivatives includes, for example, alkali metal
celluloses, carboxymethyl cellulose (CMC), cellulose esters and ethers and
aminocelluloses. The cellulose derivatives mentioned are preferably not
CA 02303638 2000-03-31
used on their own, but rather in the form of a mixture with cellulose as
cellulose-based disintegrators. The content of cellulose derivatives in
mixtures such as these is preferably below 50% by weight and more
preferably below 20% by weight, based on the cellulose-based
5 disintegrator. In one particularly preferred embodiment, pure cellulose free
from cellulose derivatives is used as the cellulose-based disintegrator.
The cellulose used as disintegration aid is preferably not used in
fine-particle form, but is converted into a coarser form, for example by
granulation or compacting, before it is added to and mixed with the
10 premixes to be tabletted. Detergent tablets which contain granular or
optionally co-granulated disintegrators are described in German patent
applications DE 197 09 991 (Stefan Herzog) and DE 197 10 254 (Henkel)
and in International patent application WO-A-98/40463 (Henkel). Further
particulars of the production of granulated, compacted or co-granulated
15 cellulose disintegrators can also be found in these patent applications.
The
particle sizes of such disintegration aids is mostly above 200 Nm, at least
90% by weight of the particles being between 300 and 1600 Nm in size
and, more particularly, between 400 and 1200 Nm in size. According to the
invention, the above-described relatively coarse-particle cellulose-based
20 disintegrators described in detail in the cited patent applications are
preferably used as disintegration aids and are commercially obtainable, for
example under the name of Arbocel~ TF-30-HG from Rettenmaier.
Microcrystalline cellulose may be used as another cellulose-based
disintegration aid or as part of such a component. This microcrystalline
25 cellulose is obtained by partial hydrolysis of the celluloses under
conditions
which only attack and completely dissolve the amorphous regions (ca. 30%
of the total cellulose mass) of the celluloses, but leave the crystalline
regions (ca. 70%) undamaged. Subsequent de-aggregation of the
microfine celluloses formed by hydrolysis provides the microcrystalline
30 celluloses which have primary particle sizes of ca. 5 Nm and which can be
CA 02303638 2000-03-31
36
compacted, for example, to granules with a mean particle size of 200 Nm.
According to the present invention, single- or multi-phase detergent
tablets additionally containing a disintegration aid, preferably a cellulose-
based disintegration aid, preferably in granulated, cogranulated or
compacted form, in quantities of 0.5 to 10% by weight, preferably 3 to 7%
by weight and more preferably 4 to 6% by weight, based on the weight of
the tablet, are preferred.
Besides the ingredients mentioned (bleach activator, bleaching
agent, builder, surfactant and disintegration aid), the detergent tablets
according to the invention may also contain one or more substances from
the group of builders, enzymes, pH regulators, perfumes, perfume carriers,
fluorescers, dyes, foam inhibitors, silicone oils, redeposition inhibitors,
optical brighteners, discoloration inhibitors, dye transfer inhibitors and
corrosion inhibitors. These substances are described in the following.
Suitable enzymes are, in particular, those from the classes of
hydrolases, such as proteases, esterases, lipases or lipolytic enzymes,
amylases, cellulases or other glycosyl hydrolases and mixtures thereof. All
these hydrolases contribute to the removal of stains, such as protein-
containing, fat-containing or starch-containing stains, and discoloration in
the washing process. Cellulases and other glycosyl hydrolases can
contribute towards color retention and towards increasing fabric softness by
removing pilling and microfibrils. Oxidoreductases may also be used for
bleaching and for inhibiting dye transfer. Enzymes obtained from bacterial
strains or fungi, such as Bacillus subtilis, Bacillus licheniformis,
Streptomyces griseus, Coprinus cinereus and Humicola insolens and from
genetically modified variants are particularly suitable. Proteases of the
subtilisin type are preferably used, proteases obtained from Bacillus lentus
being particularly preferred. Of particular interest in this regard are enzyme
mixtures, for example of protease and amylase or protease and lipase or
lipolytic enzymes or protease and cellulase or of cellulase and lipase or
CA 02303638 2000-03-31
37
lipolytic enzymes or of protease, amylase and lipase or lipolytic enzymes or
protease, lipase or lipolytic enzymes and cellulase, but especially protease-
and/or lipase-containing mixtures or mixtures with lipolytic enzymes.
Examples of such lipolytic enzymes are the known cutinases. Peroxidases
or oxidases have also been successfully used in some cases. Suitable
amylases include in particular a-amylases, isoamylases, pullanases and
pectinases. Preferred cellulases are cellobiohydrolases, endoglucanases
and ~i-glucosidases, which are also known as cellobiases, and mixtures
thereof. Since the various cellulase types differ in their CMCase and
avicelase activities, the desired activities can be established by mixing the
cellulases in the appropriate ratios.
The enzymes may be adsorbed to supports and/or encapsulated in
shell-forming substances to protect them against premature decomposition.
The percentage content of the enzymes, enzyme mixtures or enzyme
granules may be, for example, from about 0.1 to 5% by weight and is
preferably from 0.5 to about 4.5% by weight.
The choice of the particular enzymes is also dependent on the
application envisaged for the detergent tablets according to the invention.
Particularly suitable enzymes for dishwasher tablets are those from the
classes of hydrolases, such as proteases, esterases, lipases or lipolytic
enzymes, amylases, glycosyl hydrolases and mixtures thereof. All these
hydrolases contribute to the removal of stains, such as protein-containing,
fat-containing or starch-containing stains. Oxidoreductases may also be
used for bleaching. Enzymes obtained from bacterial strains or fungi, such
as Bacillus subtilis, Bacillus licheniformis, Streptomyces griseus, Coprinus
cinereus and Humicola insolens and from genetically modified variants are
particularly suitable. Proteases of the subtilisin type are preferably used,
proteases obtained from Bacillus lentus being particularly preferred. Of
particular interest in this regard are enzyme mixtures, for example of
protease and amylase or protease and lipase or lipolytic enzymes or of
CA 02303638 2000-03-31
38
protease, amylase and lipase or lipolytic enzymes or protease, lipase or
lipolytic enzymes, but especially protease- and/or lipase-containing
mixtures or mixtures with lipolytic enzymes. Examples of such lipolytic
enzymes are the known cutinases. Peroxidases or oxidases have also
proved suitable in some cases. Suitable amylases include in particular a-
amylases, isoamylases, pullanases and pectinases.
In the case of dishwasher tablets also, the enzymes may be
adsorbed to supports and/or encapsulated in shell-forming substances to
protect them against premature decomposition. The percentage content of
the enzymes, enzyme mixtures or enzyme granules may again be, for
example, from about 0.1 to 5% by weight and is preferably from 0.5 to
about 4.5% by weight.
Dishwasher tablets according to the invention may contain corrosion
inhibitors to protect the tableware or the machine itself, silver protectors
being particularly important for dishwashing machines. Known corrosion
inhibitors may be used. Above all, silver protectors selected from the group
of triazoles, benzotriazoles, bisbenzotriazoles, aminotriazoles,
alkylaminotriazoles and the transition metal salts or complexes may
generally be used. Benzotriazole and/or alkylaminotriazole is/are
particularly preferred. In addition, dishwashing formulations often contain
corrosion inhibitors containing active chlorine which are capable of
distinctly reducing the corrosion of silver surfaces. Chlorine-free
dishwashing detergents contain in particular oxygen- and nitrogen-
containing organic redox-active compounds, such as dihydric and trihydric
phenols, for example hydroquinone, pyrocatechol, hydroxyhydroquinone,
gallic acid, phloroglucinol, pyrogallol and derivatives of these compounds.
Salt-like and complex-like inorganic compounds, such as salts of the
metals Mn, Ti, Zr, Hf, V, Co and Ce are also frequently used. Of these, the
transition metal salts selected from the group of manganese and/or cobalt
salts and/or complexes are preferred, cobalt(ammine) complexes,
CA 02303638 2000-03-31
39
cobalt(acetate) complexes, cobalt(carbonyl) complexes, chlorides of cobalt
or manganese and manganese sulfate being particularly preferred. Zinc
compounds may also be used to prevent corrosion of tableware.
In addition, the detergent tablets according to the invention may also
contain components with a positive effect on the removability of oil and fats
from textiles by washing (so-called soil repellents). This effect becomes
particularly clear when a textile which has already been repeatedly washed
with a detergent according to the invention containing this oil- and fat-
dissolving component is soiled. Preferred oil- and fat-dissolving compo-
nents include, for example, nonionic cellulose ethers, such as methyl
cellulose and methyl hydroxypropyl cellulose containing 15 to 30% by
weight of methoxyl groups and 1 to 15% by weight of hydroxypropoxyl
groups, based on the nonionic cellulose ether, and the polymers of phthalic
acid and/or terephthalic acid known from the prior art or derivatives thereof,
more particularly polymers of ethylene terephthalates and/or polyethylene
glycol terephthalates or anionically and/or nonionically modified derivatives
thereof. Of these, the sulfonated derivatives of phthalic acid and
terephthalic acid polymers are particularly preferred.
The tablets may contain derivatives of diaminostilbenedisulfonic acid
or alkali metal salts thereof as optical brighteners. Suitable optical
brighteners are, for example, salts of 4,4'-bis-(2-anilino-4-morpholino-1,3,5-
triazinyl-6-amino)-stilbene-2,2'-disulfonic acid or compounds of similar
composition which contain a diethanolamino group, a methylamino group,
an anilino group or a 2-methoxyethylamino group instead of the morpholino
group. Brighteners of the substituted diphenyl styryl type, for example
alkali metal salts of 4,4'-bis-(2-sulfostyryl)-Biphenyl, 4,4'-bis-(4-chloro-3-
sulfostyryl)-Biphenyl or 4-(4-chlorostyryl)-4'-(2-sulfostyryl)-Biphenyl, may
also be present. Mixtures of the brighteners mentioned above may also be
used.
Dyes and perfumes are added to the detergent tablets according to
CA 02303638 2000-03-31
the invention to improve the aesthetic impression created by the products
and to provide the consumer not only with the required washing
performance but also with a visually and sensorially "typical and
unmistakable" product. Suitable perfume oils or perfumes include
5 individual perfume compounds, for example synthetic products of the ester,
ether, aldehyde, ketone, alcohol and hydrocarbon type. Perfume com-
pounds of the ester type are, for example, benzyl acetate, phenoxyethyl
isobutyrate, p-tert.butyl cyclohexyl acetate, linalyl acetate, dimethyl benzyl
carbinyl acetate, phenyl ethyl acetate, linalyl benzoate, benzyl formate,
10 ethyl methyl phenyl glycinate, allyl cyclohexyl propionate, styrallyl
propionate and benzyl salicylate. The ethers include, for example, benzyl
ethyl ether; the aldehydes include, for example, the linear alkanals
containing 8 to 18 carbon atoms, citral, citronellal, citronellyloxy-
acetaldehyde, cyclamen aldehyde, hydroxycitronellal, lilial and bourgeonal;
15 the ketones include, for example, the ionones, a-isomethyl ionone and
methyl cedryl ketone; the alcohols include anethol, citronellol, eugenol,
geraniol, linalool, phenyl ethyl alcohol and terpineol and the hydrocarbons
include, above all, the terpenes, such as limonene and pinene. However,
mixtures of various perfumes which together produce an attractive perfume
20- note are preferably used. Perfume oils such as these may also contain
natural perfume mixtures obtainable from vegetable sources, for example
pine, citrus, jasmine, patchouli, rose or ylang-ylang oil. Also suitable are
clary oil, camomile oil, clove oil, melissa oil, mint oil, cinnamon leaf oil,
lime
blossom oil, juniper berry oil, vetiver oil, olibanum oil, galbanum oil and
25 labdanum oil and orange blossom oil, neroli oil, orange peel oil and
sandalwood oil.
The detergent tablets according to the invention normally contain
less than 0.01 % by weight of dyes whereas perfumes/fragrances can make
up as much as 2% by weight of the formulation as a whole.
30 The perfumes may be directly incorporated in the detergents
CA 02303638 2000-03-31
41
according to the invention, although it can also be of advantage to apply
the perfumes to supports which strengthen the adherence of the perfume
to the washing and which provide the textiles with a long-lasting fragrance
through a slower release of the perfume. Suitable support materials are,
for example, cyclodextrins, the cyclodextrin/perfume complexes optionally
being coated with other auxiliaries.
In order to improve their aesthetic impression, the detergents
according to the invention may be colored with suitable dyes. Preferred
dyes, which are not difficult for the expert to choose, have high stability in
storage, are not affected by the other ingredients of the detergents or by
light and do not have any pronounced substantivity for textile fibers so as
not to color them.
Detergent tablets are produced by applying pressure to a mixture to
be tabletted which is accommodated in the cavity of a press. In the most
simple form of tabletting, the mixture to be tabletted is tabletted directly,
i.e.
without preliminary granulation. The advantages of this so-called direct
tabletting lie in its simple and inexpensive application because no other
process steps and hence no other items of equipment are required.
However, these advantages are often offset by disadvantages. Thus, a
powder mixture which is to be directly tabletted must show adequate
plasticity and good flow properties and should not have any tendency to
separate during storage, transportation and filling of the die. With many
mixtures, these three requirements are very difficult to satisfy with the
result
that direct tabletting is often not applied, particularly in the production of
detergent tablets. Accordingly, the normal method of producing detergent
tablets starts out from powder-form components ("primary particles") which
are agglomerated or granulated by suitable methods to form secondary
particles with a larger particle diameter. These granules or mixtures of
different granules are then mixed with individual powder-form additives and
tabletted.
CA 02303638 2000-03-31
42
The present invention also relates to a process for the production of
single- or multi-phase detergent tablets by tabletting one or more
particulate premixes in known manner, characterized in that the premix for
the entire tablet or for at least one of the phases contains a cationic
nitrite
corresponding to formula (I):
R'
R2-N~+~-(CH2)-CN X~-~ (I)
R3
in which R' represents -H, -CH3, a C2_24 alkyl or alkenyl group, a substituted
C2_24 alkyl or alkenyl group with at least one substituent from the group
consisting of -CI, -Br, -OH, -NHZ, -CN, an alkyl or alkenyl aryl group
containing a C~_24 alkyl group or a substituted alkyl or alkenyl aryl group
containing a C~_24 alkyl group and at least one other substituent at the
aromatic ring, RZ ad R3 independently of one another are selected from
-CH2-CN, -CH3, -CH2-CH3, -CHZ-CH2-CH3, -CH(CH3)-CH3, -CHZ-OH, -CH2-
CH2-OH, -CH(OH)-CH3, -CH2-CHZ-CH2-OH, -CHZ-CH(OH)-CH3, -CH(OH)-
CH2-CH3, -(CH2CH2-O)nH where n = 1, 2, 3, 4, 5 or 6 and X is an anion,
the pH value of a 1 % by weight aqueous solution of the particular premix
being below 10.
In the production of single-phase detergent tablets, the entire premix
has to satisfy the pH criterion mentioned. In the production of multiphase
tablets by pressing various premixes onto or into one another, only the
premix containing the cationic nitrite of formula (I) need have a pH below 7
in the form of a 1 % by weight aqueous solution.
Preferred embodiments of the process according to the invention
should be regarded similarly to the detergent tablets according to the
invention. Thus, according to the invention, processes in which the pH
value of a 1 % by weight aqueous solution of the premix for the entire tablet
CA 02303638 2000-03-31
43
or for the phase containing the cationic nitrite (I) is below 9, preferably
below 8, more preferably below 7 and most preferably below 6, are
preferred.
In the process according to the invention also, the pH is preferably
within a relatively narrow range so that processes where the pH of a 1 % by
weight aqueous solution of the premix for the entire tablet or for the phase
containing the cationic nitrite (I) is below 6.5, preferably below 6, more
preferably below 5.5 and most preferably below 5 are preferred.
So far as other preferred embodiments of the process according to
the invention are concerned, reference is made to the foregoing
observations on the detergent tablets according to the invention. Thus,
cationic nitrite contents in the premix of 0.25 to 15% by weight and, more
particularly, 0.5 to 10% by weight, based on the premix, are preferred.
The foregoing observations on the particle sizes of the cationic nitrite
again apply. According to the invention, preferred processes are also
characterized in that at least 90% by weight of the particles of the cationic
nitrite corresponding to formula (I) have a particle size above 0.2 mm, at
least 40% by weight, preferably at least 50% by weight and more preferably
at least 60% by weight of the particles of the cationic nitrite corresponding
to formula (I) having a particle size above 0.4 mm. Particularly preferred
variants of the process are characterized in that the cationic nitrite
corresponding to formula (I) has a mean particle size above 400 Nm,
preferably above 500 pm, more preferably above 600 Nm and, in one
particularly preferred embodiment, above 700 Nm.
The foregoing observations also apply in regard to the cationic
nitrites preferably used. In particularly preferred processes, the premix
contains (CH3)3N~+~CH2-CN X, where X is an anion selected from the group
consisting of chloride, bromide, iodide, hydrogen sulfate, methosulfate, p-
toluene sulfonate (tosylate) or xylene sulfonate, as the cationic nitrite.
According to the invention. preferred detergent tablets are produced
CA 02303638 2000-03-31
44
by tabletting a particulate premix of surfactant-containing granules of at
least one type and at least one powder-form component subsequently
added. The surfactant-containing granules may be produced by
conventional industrial granulation processes, such as compacting,
extrusion, mixer granulation, pelleting or fluidized bed granulation. It is of
advantage so far as the subsequent detergent tablets are concerned if the
premix to be tabletted has a bulk density approaching that of typical
compact detergents. In one particularly preferred embodiment, the premix
to be tabletted has a bulk density of at least 500 g/I, preferably of at least
600 g/I and more preferably of at least 700 g/I.
In preferred variants of the process, the surfactant-containing
granules also satisfy certain particle size criteria. Thus, preferred
processes according to the invention are characterized in that the
surfactant-containing granules have particle sizes of 100 to 2000 Nm,
preferably 200 to 1800 Nm, more preferably 400 to 1600 Nm and most
preferably 600 to 1400 Nm.
Besides the active substances (anionic and/or nonionic and/or
cationic and/or amphoteric surfactants), the surfactant granules preferably
contain carrier materials which, in one particularly preferred embodiment,
emanate from the group of builders. Accordingly, particularly
advantageous processes are characterized in that the surfactant-containing
granules contain anionic and/or nonionic surfactants and builders and have
total surfactant contents of at least 10% by weight, preferably at least 20%
by weight and more preferably at least 25% by weight.
Before the particulate premix is compressed to form detergent
tablets, it may be "powdered" with fine-particle surface treatment materials.
This can be of advantage to the quality and physical properties of both the
premix (storage, tabletting) and the final detergent tablets. Fine-particle
powdering materials have been known for some time in the art, zeolites,
silicates and other inorganic salts generally being used. However, the
CA 02303638 2000-03-31
premix is preferably "powdered" with fine-particle zeolite, zeolites of the
faujasite type being preferred. In the context of the present invention, the
expression "zeolite of the faujasite type" encompasses all three zeolites
which form the faujasite subgroup of zeolite structural group 4 (cf. Donald
5 W. Breck: "Zeolite Molecular Sieves" John Wiley & Sons, New
York/London/Sydney/Toronto, 1974, page 92). Besides zeolite X, there-
fore, zeolite Y and faujasite and mixtures of these compounds may also be
used, pure zeolite X being preferred.
Mixtures or co-crystallizates of faujasite zeolites with other zeolites
10 which need not necessarily belong to zeolite structure group 4 may also be
used as powdering materials, in which case at least 50% by weight of the
powdering material advantageously consists of a faujasite zeolite.
According to the invention, preferred detergent tablets consist of a
particulate premix containing granular components and powder-form
15 substances subsequently added, the - or one of the - powder-form
components subsequently incorporated being a faujasite zeolite with
particle sizes below 100 Nm, preferably below 10 Nm and more preferably
below 5 Nm and making up at least 0.2% by weight, preferably at least
0.5% by weight and more preferably more than 1 % by weight of the premix
20 to be tabletted.
Besides the components mentioned (surfactant, builder and
disintegration aid), the premixes to be tabletted may additionally contain
one or more substances from the group of bleaching agents, bleach
activators, enzymes, pH regulators, perfumes, perfume carriers,
25 fluorescers, dyes, foam inhibitors, silicone oils, redeposition inhibitors,
optical brighteners, discoloration inhibitors, dye transfer inhibitors and
corrosion inhibitors. These substances are described in the foregoing.
The tablets according to the invention are produced by first dry-
mixing the ingredients - which may be completely or partly pregranulated -
30 and then shaping/forming, morre particularly tabletting, the resulting
CA 02303638 2000-03-31
46
mixture using conventional processes. To produce the tablets according to
the invention, the premix is compacted between two punches in a die to
form a solid compactate. This process, which is referred to in short
hereinafter as tabletting, comprises four phases, namely metering,
compacting (elastic deformation), plastic deformation and ejection.
The premix is first introduced into the die, the filling level and hence
the weight and shape of the tablet formed being determined by the position
of the lower punch and the shape of the die. Uniform dosing, even at high
tablet throughputs, is preferably achieved by volumetric dosing of the
premix. As the tabletting process continues, the top punch comes into
contact with the premix and continues descending towards the bottom
punch. During this compaction phase, the particles of the premix are
pressed closer together, the void volume in the filling between the punches
continuously diminishing. The plastic deformation phase in which the
particles coalesce and form the tablet begins from a certain position of the
top punch (and hence from a certain pressure on the premix). Depending
on the physical properties of the premix, its constituent particles are also
partly crushed, the premix sintering at even higher pressures. As the
tabletting rate increases, i.e. at high throughputs, the elastic deformation
phase becomes increasingly shorter so that the tablets formed can have
more or less large voids. In the final step of the tabletting process, the
tablet is forced from the die by the bottom punch and carried away by
following conveyors. At this stage, only the weight of the tablet is
definitively established because the tablets can still change shape and size
as a result of physical processes (re-elongation, crystallographic efFects,
cooling, etc.).
The tabletting process is carried out in commercially available tablet
presses which, in principle, may be equipped with single or double
punches. In the latter case, not only is the top punch used to build up
pressure, the bottom punch also moves towards the top punch during the
CA 02303638 2000-03-31
47
tabletting process while the top punch presses downwards. For small
production volumes, it is preferred to use eccentric tablet presses in which
the punches) is/are fixed to an eccentric disc which, in turn, is mounted on
a shaft rotating at a certain speed. The movement of these punches is
comparable with the operation of a conventional four-stroke engine.
Tabletting can be carried out with a top punch and a bottom punch,
although several punches can also be fixed to a single eccentric disc, in
which case the number of die bores is correspondingly increased. The
throughputs of eccentric presses vary according to type from a few hundred
to at most 3,000 tablets per hour.
For larger throughputs, rotary tablet presses are generally used. In
rotary tablet presses, a relatively large number of dies is arranged in a
circle on a so-called die table. The number of dies varies - according to
model - between 6 and 55, although even larger dies are commercially
available. Top and bottom punches are associated with each die on the
die table, the tabletting pressures again being actively built up not only by
the top punch or bottom punch, but also by both punches. The die table
and the punches move about a common vertical axis, the punches being
brought into the filling, compaction, plastic deformation and ejection
positions by means of curved guide rails. At those places where the
punches have to be raised or lowered to a particularly significant extent
(filling, compaction, ejection), these curved guide rails are supported by
additional push-down members, pull-down rails and ejection paths. The die
is filled from a rigidly arranged feed unit, the so-called filling shoe, which
is
connected to a storage container for the premix. The pressure applied to
the premix can be individually adjusted through the tools for the top and
bottom punches, pressure being built up by the rolling of the punch shank
heads past adjustable pressure rollers.
To increase throughput, rotary presses can also be equipped with
two filling shoes so that only half a circle has to be negotiated to produce a
CA 02303638 2000-03-31
48
tablet. To produce two-layer or multiple-layer tablets, several filling shoes
are arranged one behind the other without the lightly compacted first layer
being ejected before further filling. Given suitable process control, shell
and bull's-eye tablets - which have a structure resembling an onion skin -
can also be produced in this way. In the case of bull's-eye tablets, the
upper surface of the core or rather the core layers is not covered and thus
remains visible. Rotary tablet presses can also be equipped with single or
multiple punches so that, for example, an outer circle with 50 bores and an
inner circle with 35 bores can be simultaneously used for tabletting.
Modern rotary tablet presses have throughputs of more than one million
tablets per hour.
Where rotary presses are used for tabletting, it has proved to be of
advantage to carry out the tabletting process with minimal variations in the
weight of the tablets. Variations in tablet hardness can also be reduced in
this way. Minimal variations in weight can be achieved as follows:
- using plastic inserts with minimal thickness tolerances
- low rotor speed
- large filling shoe
- adapting the rotational speed of the filling shoe blade to the rotor speed
- filling shoe with constant powder height
- decoupling the filling shoe from the powder supply
Any of the nonstick coatings known in the art may be used to reduce
caking on the punch. Plastic coatings, plastic inserts or plastic punches are
particularly advantageous. Rotating punches have also proved to be of
advantage; if possible, the upper and lower punches should be designed
for rotation. If rotating punches are used, there will generally be no need
for a plastic insert. In that case, the surfaces of the punch should be
electropolished.
It has also been found that long tabletting times are advantageous.
These can be achieved by using pressure rails, several pressure rollers or
CA 02303638 2000-03-31
49
low rotor speeds. Since variations in tablet hardness are caused by
variations in the pressures applied, systems which limit the tabletting
pressure should be used. Elastic punches, pneumatic compensators or
spring elements in the force path may be used. The pressure roller can
also be spring-mounted.
Tabletting machines suitable for the purposes of the invention can
be obtained, for example, from the following companies: Apparatebau
Holzwarth GbR, Asperg; Wilhelm Fette GmbH, Schwarzenbek; Hofer
GmbH, Weil; Horn & Noack Pharmatechnik GmbH, Worms; IMA
Verpackungssysteme GmbH Viersen; KILIAN, Cologne; KOMAGE, Kell am
See, KORSCH Pressen GmbH, Berlin; and Romaco GmbH, Worms. Other
suppliers are, for example Dr. Herbert Pete, Vienna (AU); Mapag
Maschinenbau AG, Bern (Switzerland); BWI Manesty, Liverpool (GB); I.
Holand Ltd., Nottingham (GB); and Courtoy N.V., Halle (BE/LU) and
Medicopharm, Kamnik (SI). One example of a particularly suitable
tabletting machine is the model HPF 630 hydraulic double-pressure press
manufactured by LAEIS, D. Tabletting tools are obtainable, for example,
from Adams Tablettierwerkzeuge Dresden; Wilhelm Fett GmbH,
Schwarzenbek; Klaus Hammer, Solingen; Herber & Sohne GmbH,
Hamburg; Hofer GmbH, Weil; Horn & Noack, Pharmatechnik GmbH,
Worms; Ritter Pharmatechnik GmbH, Hamburg; Romaco GmbH, Worms
and Notter Werkzeugbau, Tamm. Other suppliers are, for example, Senss
AG, Reinach (CH) and Medicopharm, Kamnik (SI).
The tablets can be made in certain shapes and certain sizes.
Suitable shapes are virtually any easy-to-handle shapes, for example
slabs, bars, cubes, squares and corresponding shapes with flat sides and,
in particular, cylindrical forms of circular or oval cross-section. This last
embodiment encompasses shapes from tablets to compact cylinders with a
height-to-diameter ratio of more than 1.
The portioned pressings may be formed as separate individual
CA 02303638 2000-03-31
elements which correspond to a predetermined dose of the detergent.
However, it is also possible to form pressings which combine several such
units in a single pressing, smaller portioned units being easy to break off in
particular through the provision of predetermined weak spots. For the use
5 of laundry detergents in machines of the standard European type with
horizontally arranged mechanics, it can be of advantage to produce the
portioned pressings as cylindrical or square tablets, preferably with a
diameter-to-height ratio of about 0.5:2 to 2:0.5. Commercially available
hydraulic presses, eccentric presses and rotary presses are particularly
10 suitable for the production of pressings such as these.
The three-dimensional form of another embodiment of the tablets
according to the invention is adapted in its dimensions to the dispensing
compartment of commercially available domestic washing machines, so
that the tablets can be introduced directly, i.e. without a dosing aid, into
the
15 dispensing compartment where they dissolve on contact with water.
However, it is of course readily possible - and preferred in accordance with
the present invention - to use the detergent tablets in conjunction with a
dosing aid.
Another preferred tablet which can be produced has a plate-like or
20 slab-like structure with alternately thick long segments and thin short
segments, so that individual segments can be broken off from this "bar" at
the predetermined weak spots, which the short thin segments represent,
and introduced into the machine. This "bar" principle can also be
embodied in other geometric forms, for example vertical triangles which are
25 only joined to one another at one of their longitudinal sides.
In another possible embodiment, however, the various components
are not compressed to form a single tablet, instead the tablets obtained
comprise several layers, i.e. at least two layers. These various layers may
have different dissolving rates. This can provide the tablets with favorable
30 performance properties. If, for example, the tablets contain components
CA 02303638 2000-03-31
51
which adversely affect one another, one component may be integrated in
the more quickly dissolving layer while the other component may be
incorporated in a more slowly dissolving layer so that the first component
can already have reacted off by the time the second component dissolves.
The various layers of the tablets can be arranged in the form of a stack, in
which case the inner layers) dissolve at the edges of the tablet before the
outer layers have completely dissolved. Alternatively, however, the inner
layers) may also be completely surrounded by the layers lying further to
the outside which prevents constituents of the inner layers) from dissolving
prematurely.
In another preferred embodiment of the invention, a tablet consists
of at least three layers, i.e. two outer layers and at least one inner layer,
a
peroxy bleaching agent being present in at least one of the inner layers
whereas, in the case of the stack-like tablet, the two cover layers and, in
the case of the envelope-like tablet, the outermost layers are free from
peroxy bleaching agent. In another possible embodiment, peroxy
bleaching agent and any bleach activators or bleach catalysts present
and/or enzymes may be spatially separated from one another in one and
the same tablet. Multilayer tablets such as these have the advantage that
they can be used not only via a dispensing compartment or via a dosing
unit which is added to the wash liquor, instead it is also possible in cases
such as these to introduce the tablet into the machine in direct contact with
the fabrics without any danger of spotting by bleaching agent or the like.
Similar effects can also be obtained by coating individual
constituents of the detergent composition to be compressed or the tablet as
a whole. To this end, the tablets to be coated may be sprayed, for
example, with aqueous solutions or emulsions or a coating may be
obtained by the process known as melt coating.
After pressing, the detergent tablets have high stability. The fracture
resistance of cylindrical tablets can be determined via the diametral fracture
CA 02303638 2000-03-31
52
stress. This in turn can be determined in accordance with the following
equation:
2P
a=
~Dt
where a represents the diametral fracture stress (DFS) in Pa, P is the force
in N which leads to the pressure applied to the tablet that results in
fracture
thereof, D is the diameter of the tablet in meters and t is its height.
The invention may be varied in any number of ways as would be
apparent to a person skilled in the art and all obvious equivalents and the
like are meant to fall within the scope of this description and claims. The
description is meant to serve as a guide to interpret the claims and not to
limit them unnecessarily.