Note: Descriptions are shown in the official language in which they were submitted.
CA 02305183 2000-04-03
WO 99/17763 PCT/US98/20394
POSTSURGICAL TREATMENT WITH DICHLOROACETATE
FIELD OF INVENTION
The present invention relates to the field of cardiovascular disease and more
particularly, the treatment and p"revention of poor cardiac function following
surgery,
including, but not limited to, open heart surgery.
BACKGROUND
Poor cardiac function remains a significant problem in the post-operative
period, such
as the period following open heart surgery. Drugs used to treat this type of
cardiac
dysfunction have either forced the heart to work harder (e.g. inotropes), or
decreased the
work load faced by the heart (e.g. vasodilators, alpha, beta and calcium
channel blockers).
Unfortunately, both classes of drugs have deleterious side-effects.
For example, one of the most common inotropes used to improve cardiac function
is
digitalis. However, the dosage of digitalis is critical; intoxication can be
fatal. While the
overall level of toxicity is not clear, it has been estimated that
approximately 25% of
hospitalized patients taking digitalis show some signs of toxicity. See Beller
et al., "Digitalis
intoxication: A prospective clinical study with serum level correlations." N.
Engl. J. Med.
284:989 (1971).
Calcium channel blockers also have side-effects. Some, for example, are
reported to
aggravate myocardial ischemia. This may be due to excessive hypotension or
decreased
coronary perfusion. See Goodman and Gilman's The Pharmacological Basis of
Therapeutics
(Pergamon Press, Eighth Edition 1990) (pgs. 774-779). While the drug verapamil
is less
likely to have this problem, the use of the drug is limited. Indeed, it is
specifically
contraindicated where there are SA or AV nodal conduction disturbances.
What is needed is a safe and effective pharmacological approach to the
treatment and
prevention of cardiac failure. Such a treatment should permit broad use
without significant
side-effects.
SUMMARY OF THE INVENTION
The present invention relates to the field of cardiovascular disease and more
particularly, the treatment and prevention of poor cardiac function following
an ischemic
incident, a heart attack, or surgery. With regard to surgery, the procedure
can be used before,
-1-
CA 02305183 2000-04-03
WO 99/17763 PCT/US98/20394
during and following surgery, and the surgery can be general surgery (e.g.,
transplantation,
such as liver transplantation) or cardiac surgery, such cardiac surgery
including, but not
limited to, open heart surgery. The present invention relates to new methods
of treating poor
cardiac performance, such as that resulting from ischemia in a surgical
setting. In some
embodiments, a patient with a myocardial infarction (e.g., due to occlusion of
a coronary
artery) is treated by the methods of the present invention.
Both treatment and prevention are contemplated. In one embodiment, the present
invention contemplates a method comprising the steps of a) providing: i) a
subject having
symptoms of poor cardiac performance and ii) means for delivering a solution
of
dichloroacetate; and b) delivering said solution to said subject with said
delivering means
under conditions such that said subject has a blood (e.g. serum or plasma)
concentration of
dichloroacetate in the therapeutic range (such as a concentration of
approximately 0.5 mM or
greater). In another embodiment, the present invention contemplates a method
comprising the
steps of a) providing: i) a subject at risk of poor cardiac performance and
ii) means for
delivering a solution of dichloroacetate; and b) delivering said solution to
said subject with
said delivering means under conditions such that said subject has a blood
(e.g. serum or
plasma) concentration of dichloroacetate of greater than approximately 200 M,
more
preferably greater than 500 M, and still more preferably greater than 1 mM,
for a period of
time longer than I hours, and more preferably longer than 6 hours, and most
preferably 24
hours or longer. In one embodiment, said delivering of step (b) is performed
where the
conditions comprise a first administration, comprising a bolus, and a second
administration,
wherein said second administration comprises continuous administration.
It is not intended that the invention be limited to subjects with any one type
of
symptom of poor cardiac function. Also, the age, sex, or degree of disease
state is not
intended to be in any way limiting to the present invention, although the
invention can be
used with particular success on children and infants, including but not
limited to neonates.
The invention is also not limited by the cause of poor cardiac function,
although the
invention can be used with particular success with patients whose cardiac
function is poor
following surgery, such as open heart surgery. Of course, it is not intended
that the present
invention be limited to particular surgical procedures. Open heart surgery
using
cardiopulmonary bypass pump and aortic cross clamp is contemplated as one
example of
surgery putting patients at risk for poor cardiac function. This includes
simple lesions such as
atrial septal defect or ventricular septal defect, and complex lesions such as
transposition-
-2-
CA 02305183 2000-04-03
WO 99/17763 PCT/US98/20394
arterial switch, Tetralogy of Fallot, atrioventricular septal defect, repair
of total veins, Fontan
operation, etc. In some embodiments, the methods and compositions of the
present invention
find use in the treatment of myocardial infarction (e.g., during or following
thrombolysis).
For example, dichloroacetate solution can be supplied during reperfusion.
While it is not intended that the present invention be limited by the
particular delivery
means. One means is an intravenous means, such as that achieved by
introduction through an
intravenous drip. Other means include (but are not limited to) delivery with a
catheter. A
preferred means involves direct injection into the aorta.
The particular dosage is also not intended to be limiting. A variety of
temporal
protocols is contemplated. Delivery in a bolus as well as continuous delivery
is
contemplated. In a preferred embodiment, dichloroacetate (such as sodium
dichloroacetate) is
given in a bolus of at least 100 mg/kg of an approximately 100mg/mi solution
(1.0cc/kg
bolus) and, immediately thereafter, dichloroacetate is given as an infusion at
approximately
12.5 mg/kg/hr for greater than 10 hours, and more preferably, 24 hours or
more.
Higher dosages are permitted. Dichloroacetate does not have significant side-
effects,
although some patients experience mild drowsiness.
DEFINITIONS
The following definitions are to be used to further explain the invention and
should in
no way be used to limit the scope of the invention.
"Subject" as used herein refers to a vertebrate. Preferably, the vertebrate is
a human.
"Catheter" as used herein refers to a device for insertion into canals,
vessels,
passageway or body cavities.
"Cardiac disease" as used herein refers to a state in which the heart of a
subject is no
longer able to function within normal parameters.
"Internally" as used herein refers to the state of being inside the body.
"Temporal protocol" or "dosage regiment" as used herein refers to the time
sequence
for administration of drug, i.e. the amount of drug given over time.
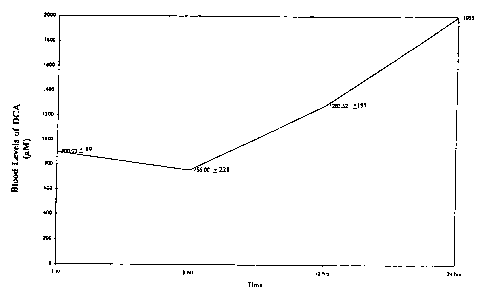
BRIEF DESCRIPTION OF THE FIGURES
Figure 1 shows the results of the unique dosage regiment of the present
invention,
whereby patient blood levels of dichloroacetate are maintained at high (and
therefore
therapeutic) levels over a 24 hour period.
-3-
CA 02305183 2000-04-03
WO 99/17763 PCT/US98/20394 -
DESCRIPTION OF THE INVENTION
The present invention relates to the field of cardiovascular disease and more
particularly, the treatment and prevention of poor cardiac function following
surgery,
including, but not limited to, open heart surgery. One proposed embodiment of
the invention
contemplates the use of a solution of dichloroacetate (typically sodium
dichloroacetate) to
reduce or eliminate the morbidity and mortality associated with open-heart
surgical
techniques, including but not limited to problems associated with the weaning
of patients
from the heart/lung machine after open heart surgery. The present method of
treatment is a
substantial improvement over existing techniques because it presents a
prevention and
treatment approach without significant side-effects.
A. HEART FUNCTION
Repetitive contraction of cardiac muscle requires an efficient and ready
source of ATP
production to sustain mechanical activity. There are two main mechanisms to
produce this
ATP in cardiac muscle: 1) glycolysis utilizing glucose as a substrate; and 2)
oxidative
metabolism utilizing lactate, glucose or fatty acids as substrates.
Glycolysis is an anaerobic process and produces 2ATP per mole of glucose
converted
to pyruvate. Fatty acid, lactate and glucose oxidation are aerobic processes,
that is, requiring
oxygen, and produce 129 moles of ATP, 18 moles of ATP and 36 moles of ATP per
mole of
substrate metabolized, respectively. Bing and colleagues identified that the
adult human heart
primarily utilizes glucose, lactate and fatty acids as the major sources of
energy. See R.J.
Bing et al., "Metabolic studies on the human heart in vivo. Studies on
carbohydrate
metabolism of the human heart," Am. J. Med. 15:284 (1953). There is, however,
a marked
difference in energy substrate utilization between neonatal and adult hearts,
with adult hearts
preferring fatty acid substrates and newborn hearts more resilient on glucose
and lactate as
energy substrates.
The type of energy substrate used by the heart can have a profound impact on
the
ability of the heart to withstand an episode of hypoxia or ischemia. See G.D.
Lopaschuk et
al., "Etomoxir, a carnitine palmitoyltransferase I inhibitor, protects hearts
from fatty acid-
induced ischemic injury independent of changes in long chain acylarnitine,"
Circ. Res.
63:1036 (1988). As a result, changes in energy substrate preference during
maturation of the
heart should influence the outcome of hypoxia or ischemia.
-4-
-------------
CA 02305183 2000-04-03
WO 99/17763 PCT/US98/20394
Both hypoxia and ischemia in the immature heart are relevant clinicai
problems, since
hypoxia can occur in the form of birth asphyxia, or with cyanotic congenital
heart disease,
and ischemia in the setting of surgery to correct congenital heart defects.
Differences in
myocardial energy substrate utilization may also affect the ability of the
newborn heart to
withstand ischemia.
Rapid return of myocardial oxidative metabolism is critical for post-operative
recovery
of ventricular function. The type of carbon substrate oxidized by the heart
during reperfusion
is also important for recovery. While it is not intended that the present
invention be limited
to any particular mechanism by which the methods and compositions achieve a
therapeutic
result, it is believed that increasing glucose oxidation at the expense of
fatty acid oxidation
will enhance the recovery of previously ischemic myocardium. The beneficial
effect of
glucose may well result from: 1) an increase in the ratio of ATP produced per
oxygen
consumed; 2) an increased availability of glycolytically-produced ATP from
membrane ion
pumps; 3) more rapid return of oxidative metabolism in the immediate
reperfusion period; or
4) a decrease in proton production due to an improved coupling between
glycolysis and
glucose oxidation.
Importantly, glucose is not the primary energy substrate of the heart during
perfusion.
Under non-ischemic conditions, as noted previously, fatty acids are the
primary energy
substrate in the adult heart, with glucose oxidation providing only 30 to 40
percent of
myocardial ATP production. In experimental studies, it has been demonstrated
that glucose
oxidation provides an even smaller portion of ATP production in hearts
reperfused following
a period of global ischemia. See G.D. Lopaschuk et al., "Glucose and palmitate
oxidation in
isolated working rat hearts reperfused after a period of transient global
ischemia," Circ. Res.
66:546 (1990). One of the primary factors resulting in low glucose oxidation
rates post-
ischemia is the circulating level of fatty acids; serum fatty acids are potent
inhibitors of
myocardial glucose oxidation.
In patients suffering a myocardial infarction or undergoing heart surgery,
serum fatty
acids can be markedly elevated. See G.D. Lopaschuk et al., "Plasma fatty acid
levels in
infants and adults following myocardial ischemia," Am. Heart J. 128:61 (1994).
These high
levels of fatty acids have been shown to potentiate ischemic injury in several
experimental
models including pig, dog, rabbit and rat hearts. See e.g. M. Saddik and G.D.
Lopaschuk
"Myocardial triglyceride turnover and contribution to energy substrate
utilization in isolated
working rat hearts," J. Biol. Chem. 266:8162 (1991). During and following
cardiopulmonary
-5-
CA 02305183 2000-04-03
WO 99/17763 PCT/US98/20394
bypass, elevations in fatty acid levels could potentially put the patients at
increased risk for
prolonged myocardial stunning, manifested by impaired cardiac function, or
prolonged
inotrope.
B. REVERSING FATTY ACID INHIBITION
In both aerobic and reperfused ischemic rat hearts, high levels of fatty acids
markedly
inhibit glucose oxidation rates. This is believed to be the result of marked
inhibition by fatty
acids of the pyruvate dehydrogenase complex (PDC), a key enzyme complex
regulating
carbohydrate oxidation.
It is further believed that overcoming fatty acid inhibition of PDC will
dramatically
increase glucose oxidation and improve functional recovery of ischemic hearts.
One of the
pharmacologic agents that is particularly effective in reversing fatty acid
inhibition of PDC is
dichloroacetate. Dichloroacetate (DCA) directly stimulates PDC, resulting in a
marked
stimulation of glucose oxidation. See J.J. McVeigh and G.D. Lopaschuk
"Dichloroactetate
stimulation of glucose oxidation improves recovery of ischemic rat hearts,"
Am. J. Physiol.
259:H 1070 (1990). Because infants are noted to have the highest fatty acid
levels during and
after cardiac surgery, and the lowest rates oxidation for ATP production, it
is logical that they
may benefit the most from an agent which alters substrate metabolism thus
improving not
only oxidation but functional recovery. Experimental studies have demonstrated
that
administration of DCA results in a dramatic stimulation of glucose oxidation
during
reperfusion of previously ischemic hearts. Again, while an understanding of a
precise
mechanism is not necessary to the practice of the invention, it is believed
that, by selectively
stimulating glucose oxidation, secondary to a stimulation of PDC, DCA
significantly improves
the coupling of glycolysis and glucose oxidation during reperfusion of
ischemic hearts. This
has the effect of decreasing proton production (H+) due to ATP hydrolysis
originating from
glycolysis uncoupled from glucose oxidation. By doing so, DCA results in a
dramatic
improvement in cardiac efficiency during reperfusion, since less ATP is
utilized to deal with
intracellular ionic in the post-ischemic period.
In adult studies, the present inventors have demonstrated that DCA
administration
significantly stimulates PDC in heart muscle, strongly suggesting that glucose
oxidation is
increased. See Thannikkuto et al., "Dichloroacetate (DCA) stimulates pyruvate
dehydrogenase complex (PDC) activity in hearts of patients undergoing coronary
artery
bypass grafting (CABG)" Can. J. Cardiol. 10(suppl. C):130C (1994). In a pilot
project in
-6-
I
CA 02305183 2006-01-27
. . I
which DCA was administered to pediatric patients. the present inventors
observed a
significant drop in the requirements for inotrooes in a immediate post
operative period. See
R.L. Collins-Nakai et al., "Dichloroacetic acid !, (DCA) after open heart
surgery in infants and
children," Cad. J. Cardiol. 11(suppl. E):106E (1995).
Unfortunately, due to the very short half-life of dichloroacetate (i.e.
approximately 40
minutes), the appropriate dosage regiment for optimum therapeutic effect has
not been
obtained. The present invention provides methods and compositions that
optimize the
therapeutic effect of dichloroacetate when used to provide myocardial
protection and
treatment, during and after cardiac surgery, andi in particular, surgery in
the pediatric patient.
The present invention contemplates that the appropriate regiment for optimum
therapeutic
effect involves, in part, a longer temporal protocol, i.e. administration for
periods longer than
-1 hour, and more preferably, longer than 10 hours, and still more preferably
24 hours or
more. This is in contrast to single bolus administrations of dichloroacetate
which have been
found to provide blood levels of the drug in the therapeutic range for less
than one hour.
Dichloroacetate is commerically available (typcially as a salt). Preparation
of the
compound and detection of patient levels can be performed using a variety of
techniques,
such as those discussed in U.S. Patent No. 5,587,397 to Fox.
EXPERIMENTAL
The following example serves to illustrate certain preferred embodiments and
aspects
of the present invention and is not to be construed as limiting the scope
thereof.
EXAMPLE
This example describes the use of dichloroacetate administered in a bolus
followed by
infusion for 24 hrs in pediatric patients after cardiopulmonary bypass.
Patient Selection: All patients from new-born to six years of age who require
open
heart surgery are candidates for administration of dichloroaeetate. Neonates
are included as
they are likely to benefit most from the DCA because of developmental changes
in
myocardial metabolism. There are no patient contraindications to DCA, but it
should be
noted that the use of corticosteroids or nicotinic: acid in a patient within
24 hrs prior to
surgery may change free fatty acid levels. Patients with requirements for
insulin or a
diagnosis of diabetes can be included, as myocardial function is enhanced in
such patients as
-7-
~I
CA 02305183 2000-04-03
WO 99/17763 PCT/US98/20394
well. Although insulin requirements may change slightly because of the DCA, in
the
immediate post-operative period insulin requirements may change dramatically
anyway and
close observation would be required in such patients.
Other Drugs: All procedures and drugs normally given for infants and children
undergoing cardiopulmonary bypass are given as routinely administered.
Introduction of
inotropes in the post-operative period is most easily documented if introduced
in a stepwise
manner. For purposes of the experiment, both calcium and sodium bicarbonate
will be
considered to be inotropes, as they both may significantly change the levels
of glucose
oxidation in the myocardium. Other inotropes to be considered include:
epinephrine,
dobutamine, dopamine, norepinephrine, phentolamine, phenylephrine and
amrinone. The use
of other drugs such as vasodilators, diuretics and analgesics or others, are
continued as
required. Routine post-operative care and management of complications is also
contemplated.
Administration of Dichloroacetate: DCA, in a bolus of 100 mg/kg of 10 mg/ml
solution (1.0cc/kg bolus) is injected into the proximal aorta immediately
prior to
discontinuing the aortic cross-clamp. Immediately thereafter, an infusion of
DCA at 12.5
mg/kg/hr is initiated and run for 24 hours or longer. Based on the
pharmacokinetics of DCA,
this dosage regiment is designed to continuous maintain plasma levels of DCA
in the
therapeutic range of [0.2-1 mM] - in contrast to transient therapeutic levels.
Measuring Blood Levels: Blood samples are collected from the indwelling lines
of
patients in citrate-containing tubes. These samples are centrifuged to
separate the plasma and
frozen. The frozen samples are later analyzed for DCA concentration by using a
high
performance liquid chromatography technique (HPLC). See generally Thannikkuto
et al.,
"Dichloroacetate (DCA) stimulates pyruvate dehydrogenase complex (PDC)
activity in hearts
of patients undergoing coronary artery bypass grafting (CABG)" Can. J.
Cardiol. 10(suppl. .
C):130C (1994). Briefly, the analysis was performed on a IonoSpher A (250 X
4.6mm L X
ID) column accompanied by a guard column AX. Both of the columns were
purchased from
Chrompack Canada. The mobile phase used was 10-3 M pyromellitate buffer (pH
3.8 - 4.0) at
a flow rate of 3.0 mmL/min. Detection was at 320nm UV. The sample size
injected was
201iL. The results are shown in Figure 1. The unique dosage regiment of the
present
invention clearly results in continuously maintained patient blood levels of
dichloroacetate in
the therapeutic range. The patients are also observed to require fewer drugs
(e.g. inotropes)
following surgery (data not shown).
-8-
CA 02305183 2000-04-03
WO 99/17763 PCT/US98/20394
From the above it is clear that the present invention provides a method of
treating
poor cardiac performance that is both effective and safe. The method results
in the need for
fewer cardiac performance-enhancing drugs in the first hour after cardiac
surgery, and less
time on the ventilator and in the intensive care unit. Any further
improvements and
modifications which become apparent to persons of ordinary skill in the art
only after reading
this disclosure, the drawings and the following claims are deemed within the
spirit and scope
of the present invention.
-9-