Note: Descriptions are shown in the official language in which they were submitted.
' CA 02305979 2000-04-12
WO 99/20724 PCT/US98/21677
1
Liquid Hard-Surface Cleaning Compositions
Technical Field
The present invention relates to liquid compositions for cleaning hard-
surfaces.
~o Background of the invention
Liquid compositions for cleaning hard-surfaces have been disclosed in the art.
Much of the focus for such compositions has been on providing outstanding
cleaning on a variety of surfaces and soils. However, such compositions are
~5 not fully satisfactory from a consumer viewpoint, especially regarding the
soil
release properties imparted to the hard-surfaces treated therewith. Indeed,
consumers are looking for liquid cleaning compositions, whereby next-time
(subsequent) cleaning is more facilitated.
The object of the present invention is to formulate a liquid cleaning
composition
2o for removal of various soils from hard-surfaces, that will facilitate the
next-time
cleaning operation.
It has now been found that the next-time cleaning performance is improved
when a hard-surface has first been treated with a liquid composition
comprising
25 particular antiresoiling ingredients, namely a polyalkoxylene glycol
diester as
defined herein, as a first antiresoiling ingredient, together with a
vinylpyrrolidone
homopolymer or copolymer, as a second antiresoiling ingredient. Indeed, the
compositions of the present invention allow improved next-time cleaning
performance, as compared to the same compositions comprising only one of
3o said antiresoiling ingredients as defined herein, at the same total level
of
antiresoiling ingredients, or another antiresoiling polymeric ingredient like
for
example poly (trimethyl aminoethyl) methacrylate at the same total level of
antiresoiling ingredients.
35 More particularly, it has surprisingly been found that the use of such a
polyalkoxylene glycol diester, as defined herein, together with such a
vinylpyrrolidone homopolymer or copolymer, such as a quaternized or
CA 02305979 2000-04-12
WO 99120724 PCT/US98121677
2
unquaternized vinylpyrrolidone/dialkylaminoalkyl acrylate or methacrylate
copolymer, results in a synergistic effect on next-time cleaning performance.
Advantageously, the compositions herein may be used to clean hard-surfaces
made of a variety of materials like glazed and non-glazed ceramic tiles,
vinyl,
no-wax vinyl, linoleum, melamine, glass, plastics, plastified wood, both in
neat
and diluted conditions, e.g., up to a dilution level of 1:400
(composition:water).
A further advantage of the present invention is that the next-time cleaning
performance is obtained with the compositions according to the present
invention on various types of stainslsoils including typical greasy stains
like
kitchen grease and other tough stains such as burntlsticky food residues
typically found in kitchens, while delivering good gloss to said surfaces.
i 5 Another advantage associated to the compositions according to the present
invention comprising the polyalkoxylene glycol diester and vinylpyrrolidone
homopolymer or copolymer, is that they have the ability to provide good shine
to the surface they have cleaned. Indeed, less formation of watermarks and/or
even limescale deposits are observed on a surface having been cleaned with
2o the compositions of the present invention and later comes in contact with
water,
for example, during a rinse operation. Advantageously, the shine benefit
delivered to the surface even persists after several cycles of rinsing, thus
providing long lasting protection against formation of watermarks andlor even
limescale deposits on the surface, and hence long lasting shiny surfaces.
2s Another advantage of the liquid compositions of the present invention is
that not
only next-time cleaning performance is improved, but that also good first time
cleaning performance is delivered.Yet a further advantage of the compositions
of the present invention is that faster drying is obtained on the surfaces
that
have been cleaned therewith, this both when used diluted or when used neat.
3o In other words, the housewife will have the advantage to shorten the total
time
of the cleaning operation of hard-surfaces and diminish the inconvenience of
having wet floors in her home.
Background art
....._. _....__...... .._..__T.._.___ __...._... _ ...
" CA 02305979 2000-04-12
WO 99/20724 PCT/US98/2167 7
3
WO 94/26858 discloses a liquid hard-surface composition (pH 2-8) with
nonionic surfactants (1-30%) and anionic polymers having an average
molecular weight of less than 1,000,000, said polymers being free of
quaternary
nitrogen groups. Said compositions bring initial cleaning benefit in addition
to
the anti-soiling benefit. Indeed, WO 94126858 discloses that acrylic,
methacryiic
and malefic anhydride derivatives such as copolymers of styrene with malefic
produce a streak-free finish after drying. No liquid compositions comprising a
combination of a polyalkoxylene glycol diester together with a
vinylpyrrolidone
~o homopolymer or copolymer are disclosed.
EP-A-635 567 discloses liquid compositions for cleaning solid surfaces
comprising a cleaning agent capable of being deposited on the surface during
cleaning and of forming a dried layer adhered to the surface, said layer
having
a cohesive strength such that at least outermost surface portion of the layer
is
~5 removable by further washing: Polyvinylpyrrolidone is disclosed. However,
no
polyalkoxylene glycol diesters are disclosed.
2o Summary of the invention
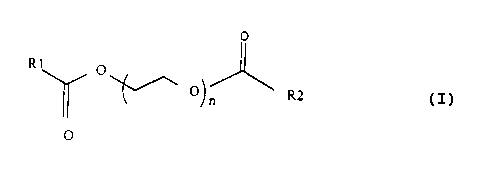
The present invention encompasses a liquid hard-surface cleaning composition
comprising a pofyalkoxylene glycol diester according to the formula:
O
R1
O ~ Ol R2
,n
O
so wherein the substituents R1 and R2 each independently are substituted or
unsubstituted, saturated or unsaturated, linear or branched hydrocarbon chains
having from 1 to 36 carbon atoms and wherein n is an integer from 10 to 400,
and a vinylpyrrolidone homopolymer or copolymer.
CA 02305979 2000-04-12
WO 99/20724 PCT/US98I21677
4
The present invention also encompasses a process of cleaning hard-surfaces
wherein a liquid composition as defined herein above, is contacted with said
surfaces.
Detailed Description of the invention
~o
The liguid compositions:
As a first essential ingredient, the compositions according to the present
invention comprise a polyalkoxylene glycol diester or a mixture thereof, as
~ 5 defined herein after.
Typically, the compositions of the present invention comprise from 0.001 % to
20% by weight of the total composition of said polyalkoxylene glycol diester
or a
mixture thereof, preferably from 0.01 % to 10%, more preferably from 0.1 % to
20 5% and most preferably from 0.2% to 2%.
Suitable polyalkoxylene glycol diesters for use herein have the following
formula
0
RI
R2
I
In this formula the substituents R1 and R2 each independently are substituted
or unsubstituted, saturated or unsaturated, linear or branched hydrocarbon
ao chains having from 1 to 36 carbon atoms, and n is an integer of from 10 to
400.
Preferably R1 and R2 each independently are substituted or unsubstituted,
linear or branched alkyl groups or alkenyl groups having from 1 to 36 carbon
CA 02305979 2000-04-12
WO 99/20724 PCT/US98/216'?
atoms, preferably from 1 to 30, more preferably from 1 to 24, even more
preferably from 1 to 22 and most preferably from 1 to 18, or aryl groups
having
up to 36 carbon atoms, preferably from 6 to 36, more preferably from 6 to 30.
Preferably n is an integer from 20 to 400, more preferably from 40 to 300,
even
5 more preferably from 40 to 200 and most preferably from 40 to 150.
The preferred polyalkoxylene glycol diesters for use according to the present
invention have a molecular weight of at least 200, more preferably from 400 to
10,000 and most preferably from 800 to 6,000.
~o
Suitable polyalkoxylene glycol diesters for use herein include O'O-distearyl
polyethylene glycol diester (MW 6000) or O'O-dioleyl polyethylene glycol
diester
(MW 560).
~ s Such polyalkoxylene glycol diesters may be commercially available from
Akzo
Nobel under the name KESSCO PEG 600UDS~, or from Lonza under the
name Pegosperse~ or from Huls under the name Marlosol FS~.
As a second essential ingredient, the compositions according to the present
zo invention comprise a vinylpyrrolidone homopolymer or copolymer or a mixture
thereof.
Typically, the compositions of the present invention comprise from 0.001 % to
20% by weight of the total composition of a vinylpyrrolidone homopolymer or
2s copolymer or a mixture thereof, preferably from 0.01 % to 10%, more
preferably
from 0.1 % to 5% and most preferably from 0.2% to 2%.
Suitable vinylpyrrolidone homopolymers for use herein is an homopolymer of N-
vinyfpyrrolidone having the following repeating monomer:
H
I
C-CH,
I '
N
H_, C~ ~C=O
I I
H~ C- CH,
n
CA 02305979 2000-04-12
WO 99120724 PCT/US98/21677
6
wherein n (degree of polymerisation) is an integer of from 10 to 1,000,000
preferably from 20 to 100,000, and more preferably from 20 to 10,000.
Accordingly, suitable vinylpyrrolidone homopolymers ("PVP") for use herein
have an average molecular weight of from 1,000 to 100,000,000 , preferably
from 2,000 to 10,000,000 , more preferably from 5,000 to 1,000,000 , and most
preferably from 50,000 to 500,000.
Suitable vinylpyrrolidone homopolymers are commercially available from ISP
Corporation, New York, NY and Montreal, Canada under the product names
PVP K-15~ (viscosity molecular weight of 10,000), PVP K-30~ (average
molecular weight of 40,000), PVP K-60~ (average molecular weight of
160,000), and PVP K-900 (average molecular weight of 360,000). Other
~ 5 suitable vinylpyrroiidone homopolymers which are commercially available
from
BASF Cooperation include Sokaian HP 165~ and Sokalan HP 12~;
vinylpyrrolidone homopolymers known to persons skilled in the detergent field
(see for example EP-A-262,897 and EP-A-256,696).
2o Suitable copolymers of vinylpyrrolidone for use herein include copolymers
of N-
vinylpyrrolidone and alkylenically unsaturated monomers or mixtures thereof.
The alkylenically unsaturated monomers of the copolymers herein include
unsaturated dicarboxylic acids such as malefic acid, chloromaleic acid,
fumaric
25 acid, itaconic acid, citraconic acid, phenylmaleic acid, aconitic acid,
acrylic acid,
N-vinylimidazole and vinyl acetate. Any of the anhydrides of the unsaturated
acids may be employed, for example acrylate, methacrylate. Aromatic
monomers like styrene, sulphonated styrene, alpha-methyl styrene, vinyl
toluene, t-butyl styrene and similar well known monomers may be used.
The molecular weight of the copolymer of vinylpyrrolidone is not especially
critical so long as the copolymer is water-soluble, has some surface activity
and
is adsorbed to the hard-surface from the liquid composition or solution (i.e.
under dilute usage conditions) comprising it in such a manner as to increase
the
hydrophilicity of the surface. However, the preferred copolymers of N-
vinylpyrrolidone and alkylenically unsaturated monomers or mixtures thereof,
~
CA 02305979 2000-04-12
W O 99/20724 PCTN S981216 7 7
7
have a molecular weight of between 1,000 and 1,000,000 , preferably between
10,000 and 500,000 and more preferably between 10,000 and 200,000.
For example particularly suitable N-vinylimidazole N-vinylpyrrolidone polymers
for use herein have an average molecular weight range from 5,000-1,000,000,
preferably from 5,000 to 500,000, and more preferably from 10,000 to 200,000.
The average molecular weight range was determined by light scattering as
described in Barth H.G. and Mays J.W. Chemical Analysis Vol 113,"Modern
Methods of Polymer Characterization".
~o
Such copolymers of N-vinylpyrrolidone and alkylenically unsaturated monomers
like PVPlvinyl acetate copolymers are commercially available under the trade
name Luviskol0 series from BASF.
~5 Particular preferred copolymers of vinylpyrrolidone for use in the
compositions
of the present invention are quaternized or unquaternized
vinylpyrrolidone/dialkylaminoalkyl acrylate or methacrylate copolymers.
The vinylpyrrolidoneldialkylaminoalkyl acrylate or methacrylate copolymers
20 (quaternised or unquatemised) suitable for use in the compositions of the
present invention are according to the following formula:
N O R,
CH-CH, CH~-C
m
(c=o ~,
T
O-R,-N (R3)~R4.X
2s in which n is between 20 and 99 and preferably between 40 and 90 mol% and
m is between 1 and 80 and preferably between 5 and 40 mol%: R1 represents
H or CH3; y denotes 0 or 1; R2 is -CH2-CHOH-CH2- or CXH2x, in which x=2 to
18; R3 represents a lower alkyl group of from 1 to 4 carbon atoms, preferably
methyl or ethyl, or
CA 02305979 2000-04-12
WO 99/20724 PCT/US98/2167''
8
CH~
R4 denotes a lower alkyl group of from 1 to 4 carbon atoms, preferably methyl
or ethyl; X- is chosen from the group consisting of CI, Br, I, 1/2S04, HS04
and
CH3S03. The polymers can be prepared by the process described in French
Pat. Nos. 2,077,143 and 2,393,573.
The preferred quaternized or unquaternized vinylpyrrolidoneldialkylaminoalkyl
acrylate or methacryiate copolymers for use herein have a molecular weight of
between 1,000 and 1,000,000, preferably between 10,000 and 500,000 and
more preferably between 10,000 and 100,000.
Such vinylpyrrolidone/dialkylaminoalkyl acrylate or methacrylate copolymers
are
commercially available under the name copolymer 8450, Gafquat 734~, or
~ 5 Gafquat 755~ from ISP Corporation, New York, NY and Montreal, Canada or
from BASF under the tradename Luviquat~.
Most preferred herein is quaternized copolymers of vinyl pyrrolidone and
dimethyl aminoethymethacrylate (polyquaternium-11 ) available from BASF.
The present invention is based on the finding that the liquid compositions of
the
present invention provide improved next-time cleaning performance when a
hard-surface has been first treated therewith. Although not wishing to be
bound
by theory, it is speculated that the first antiresoiling ingredient, i.e.,
polyalkoxylene glycol diester, and the second antiresoiling ingredient, i.e.,
vinylpyrrolidone homopolymer or copolymer, have in common the property of
adsorbing to a hard-surface being first cleaned therewith, in such a manner
that
a hygroscopic layer is left behind. The resulting hygroscopic layer can
attract
and retain ambient atmospheric water vapor to more effectively reduce
3o adhesion of soils once treated and/or facilitate removal of soils
subsequently
deposited thereon, i.e. less work (e.g. less scrubbing andlor wiping andlor
less
chemical action) is required to remove the soils in the next-time cleaning
operation, as compared to a similar soiled hard-surface which has been first
CA 02305979 2000-04-12
WO 99/20724 PCT/US98/2167'
9
cleaned with the same compositions without the first or second antiresoiling
ingredients according to the present invention.
More particularly, it has surprisingly been found that there is a synergistic
effect
on next-time cleaning performance associated with the use of such a
polyalkoxylene glycol diester and a vinylpyrrolidone homopolymer or copolymer,
as defined herein. Indeed, the next-time cleaning performance delivered by
combining a polyalkoxyiene glycol diester and a vinylpyrrolidone homopolymer
or copolymer, as defined herein, in a liquid composition, is superior than the
~o next-time cleaning performance delivered by for example the same
composition, but comprising only one of those ingredients at the same total
level of antiresoiling ingredients.
in a preferred embodiment of the compositions of the present invention the
~ 5 polyalkoxylene glycol diester as defined herein, and the vinylpyrrolidone
homopolymer or copolymer, as defined herein, are present at a weight ratio of
the polyalkoxylene glycol diester to the vinylpyrrolidone homopolymer or
copolymer of from 1:100 to 100:1, preferably from 1:10 to 10:1 and more
preferably from 1:2 to 2:1.
Also an advantage of the present invention is that effective next-time
cleaning
performance can be obtained at low total level of antiresoiling ingredients.
In a
preferred embodiment the compositions herein comprise from 0.1 % to 10% by
weight of the total composition of the pofyalkoxylene glycol diester, and the
vinylpyrrolidone homopolymer or copolymer, preferably from 0.2% to 5%, more
preferably from 0.3% to 2% and most preferably from 0.3% to 1.5%.
Surprisingly, effective next-time cleaning performance is delivered not only
when a composition of the present invention is contacted to the hard-surface
to
clean in its neat form, but also in its diluted form, e.g. up to a dilution
level
3o water: composition (400:1 ).
An advantage of the compositions of the present invention is that the first
time
cleaning performance is also increased, as compared for example to the same
compositions without said vinylpyrrolidone homopolymer or copolymer.
CA 02305979 2000-04-12
WO 99/20724 PCT/US98/2167
By "cleaning performance", it is meant herein cleaning on various types of
soils
including greasy soils, like kitchen grease or burntlsticky food residues
typically
found in a kitchen (e.g., burnt milk) and the like.
The first time dilute cleaning performance may be evaluated by the following
test method: Tiles of enamel, vinyl or ceramic are prepared by applying to
them
a representative greaselparticulate artificial soil, followed by aging. The
test
compositions and the reference composition are diluted (e.g.,
composition:water 1:50 or 1:100), applied to a sponge, and used to clean the
1o tiles with a Sheen scrub tester. The number of strokes required to clean to
100% clean is recorded. A minimum of 6 replicates can be taken with each
result being generated in duplicate against the reference on each soiled tile.
The next-time dilute cleaning performance may be evaluated by the following
test method: Following the procedure detailed for first time cleaning the
tiles
used for this previous test are taken and resoiled directly without first
being
further washed or rinsed. The cleaning procedure is then repeated using the
Sheen scrub tester, taking care that the test compositions are used to clean
the
same part of the tile as was previously cleaned by them. The number of
2o strokes required to clean to 100% clean is recorded. A minimum of 6
replicates
can be taken with each result being generated in duplicate against the
reference on each soiled tile. This resoiling and cleaning procedure can be
repeated up to 5 times.
2s The test method for evaluating neat cleaning performance is identical to
above
except that the test compositions and reference are used undiluted and that
after cleaning a rinsing cycle is performed with clean water. This rinsing
cycle
may be repeated up to 5 times prior to the resoiling step for next time
cleaning
evaluation.
Another advantage of the compositions of the present invention is that these
compositions when applied on the surface to be cleaned either in their neat or
diluted form dry faster than the same compositions comprising only the first
or
second antiresoiling ingredients as described herein at the same total level
of
antiresoiling ingredients. Thus, the present invention also encompasses the
use
of a polyalkoxylene glycol diester and vinylpyrrolidone homopolymer or
copolymer, in a liquid composition, for faster evaporation of said composition
CA 02305979 2000-04-12
WO 99/20724 PCT/US98/21b7
11
when used to clean a hard-surface in its diluted or neat form, and/or faster
evaporation of water subsequently coming into contact with said surface for
rinsing off the surface after cleaning.
Although not wishing to be bound by theory, it has been observed that hard
surfaces often have low affinity with water. This means that, when water gets
in
contact with hard-surfaces, its spreading, which is controlled by the
interfacial
energy (i.e., solid/liquid surface tension), is very limited. Indeed, it has
been
observed that the most stable configuration for water is grouping in spherical
1o droplets rather than forming a thin film uniformly spread over the surface.
Thus
requiring more time to completely evaporate from said surface. It has now been
found that when the polyalkoxylene glycol diester and vinylpyrrolidone
homopolymer or copolymer are added together into a liquid hard-surface
cleaning composition according to the present invention a hydrophilic layer is
15 left on a hard-surface cleaned with said composition, said hydrophilic
layer
leaves the water coming in contact with the surface that has been first
cleaned
with said composition (e.g., water which is used to rinse off the surfaces
having
been so treated) uniformly spread over the surface ("sheeting effect") instead
of
forming droplets. This way, the evaporation of the composition itself and
2o subsequent water coming into contact with the surface for example in the
rinsing step is accelerated.
Not to be bound by theory, it is believed that the antiresoiling ingredients
described herein also have the ability to form a film on the surface of the
user
25 skin, thereby providing improved skin mildness. In another embodiment, the
present invention thus also encompasses the use of a polyalkoxylene glycol
diester and/or polyvinyl pyrrolidone homopoiyrner or copolymer, in a liquid
composition, for improved skin mildness.
3o An additional advantage related to the use of the polyalkoxylene glycol
diester
and/or polyvinyl pyrrolidone homopolymer or copolymer herein is that, as they
adhere on hard surface making them more hydrophilic, the surfaces themselves
become smoother (this can be perceived by touching said surfaces) and this
contributes to convey perception of surface perfectly cleaned.
The compositions according to the present invention particularly suitable for
the
cleaning of a hard-surface are liquid compositions. The liquid compositions of
CA 02305979 2000-04-12
WO 99/20724 PCT/US98I216~'
12
the present invention are preferably but not necessarily formulated as aqueous
compositions. Aqueous compositions typically comprise from 50% to 99% by
weight of the total composition of water, preferably from 60% to 95%, and more
preferably from 80% to 95%.
The liquid compositions herein may be formulated in the full pH range of 0 to
14, preferably 1 to 13. Typically, the compositions herein are formulated in a
neutral to highly alkaline pH range from 7 to 12, preferably from 9 to 11 and
more preferably from 9.5 to 11. The pH of the compositions herein can be
1o adjusted by any of the means well-known to those skiJied in the art such as
acidifying agents like organic or inorganic acids, or alkalinising agents like
NaOH, KOH, K2C03, Na2C03 and the like. Preferred organic acids for use
herein have a pka of less than 6. Suitable organic acids are selected from the
group consisting of citric acid, tactic acid, glycolic acid, succinic acid,
glutaric
acid and adipic acid and mixtures thereof. A mixture of said acids may be
commercially available from BASF under the trade name Sokalan~ DCS.
Optional ingredients:
2o The liquid compositions according to the present invention may comprise a
variety of optional ingredients depending on the technical benefit aimed for
and
the surface treated.
Suitable optional ingredients for use herein include surfactants, builders,
chelants, polymers, solvents, buffers, bactericides, hydrotropes, colorants,
stabilisers, radical scavengers, bleaches, bleach activators, suds controlling
agents like fatty acids, enzymes, soil suspenders, dye transfer agents,
brighteners, anti dusting agents, dispersants, dye transfer inhibitors,
pigments,
dyes and/or perfumes.
Surfactants
The liquid compositions of the present invention preferably comprise a
surfactant, or mixtures thereof. Said surfactant may be present in the
compositions according to the present invention in amounts of from 0.1 % to
50% by weight of the total composition, preferably of from 0.1 % to 20% and
more preferably of from 1 % to 10%.
CA 02305979 2000-04-12
WO 99/20724 PCT/US98/2167~,
13
Surfactants are desired herein as they further contribute to the cleaning
performance and/or gloss benefit of the compositions of the present invention.
Surfactants for use herein include nonionic surfactants, anionic surfactants,
cationic surfactants, amphoteric surfactants, zwitterionic surfactants, and
mixtures thereof.
Particularly preferred surfactants are the nonionic surfactants. Suitable
nonionic
surfactants for use herein include a class of compounds which may be broadly
defined as compounds produced by the condensation of alkylene oxide groups
(hydrophilic in nature) with an organic hydrophobic compound, which may be
branched or linear aliphatic (e.g. Guerbet or secondary alcohols) or alkyl
aromatic in nature. The length of the hydrophilic or polyoxyalkylene radical
which is condensed with any particular hydrophobic group can be readily
adjusted to yield a water-soluble compound having the desired degree of
balance between hydrophilic and hydrophobic elements. For example, a well-
known class of nonionic synthetic detergents is made available on the market
under the trade name "Pluronic". These compounds are formed by condensing
ethylene oxide with an hydrophobic base formed by the condensation of
propylene oxide with propylene glycol. The hydrophobic portion of the molecule
2o which, of course, exhibits water-insolubility has a molecular weight of
from
about 1500 to 1800. The addition of polyoxyethylene radicals to this
hydrophobic portion tends to increase the water-solubility of the molecule as
a
whole and the liquid character of the products is retained up to the point
where
poiyoxyethylene content is about 50% of the total weight of the condensation
product.
Other suitable nonionic synthetic detergents include
(i) The polyethylene oxide condensates of alkyl phenols, e.g., the
condensation products of alkyl phenols having an alkyl group containing
3o from about 6 to 12 carbon atoms in either a straight chain or branched
chain configuration, with ethylene oxide, the said ethylene oxide being
present in amounts equal to 10 to 25 moles of ethylene oxide per mole of
alkyl phenol. The alkyl substituent in such compounds may be derived
from polymerized propylene, diisobutylene, octane, and nonane;
(ii) Those derived from the condensation of ethylene oxide with the product
resulting from the reaction of propylene oxide and ethylene diamine
CA 02305979 2000-04-12
WO 99/20724 PCT/US98121677
14
products which may be varied in composition depending upon the balance
between the hydrophobic and hydrophilic elements which is desired.
Examples are compounds containing from about 40% to about 80%
polyoxyethylene by weight and having a molecular weight of from about
5000 to about 11000 resulting from the reaction of ethylene oxide groups
with a hydrophobic base constituted of the reaction product of ethylene
diamine and excess propylene oxide, said base having a molecular weight
of the order of 2500 to 3000;
(iii) The condensation product of aliphatic alcohols having from 8 to 18
carbon
atoms, in either straight chain or branched chain configuration, with
ethylene oxide, e.g., a coconut alcohol ethylene oxide condensate having
from 10 to 30- moles of ethylene oxide per mole of coconut alcohol, the
coconut alcohol fraction having from 10 to 14 carbon atoms;
(iv) Trialkyl amine oxides and trialkyl phosphine oxides wherein one alkyl
group ranges from 10 to 18 carbon atoms and two alkyl groups range from
1 to 3 carbon atoms; the alkyl groups can contain hydroxy substituents;
specific examples are dodecyl di(2-hydroxyethyl)amine oxide and
tetradecyl dimethyl phosphine oxide.
2o Also useful as a nonionic surfactant are the alkylpolysaccharides disclosed
in
U.S. Patent 4,565,647, t_lenado, issued January 21, 1986, having a
hydrophobic group containing from about 6 to about 30 carbon atoms,
preferably from about 10 to about 16 carbon atoms and polysaccharide, e.g., a
polyglycoside, hydrophilic group containing from about 1.3 to about 10,
preferably from about 1.3 to about 3, most preferably from about 1.3 to about
2.7 saccharide units. Any reducing saccharide containing 5 or 6 carbon atoms
can be used, e.g., glucose, galactose, and galactosyl moieties can be
substituted for the glucosyl moieties. (Optionally the hydrophobic group is
attached at the 2-, 3-, 4-, etc. positions thus giving a glucose or galactose
as
opposed to a glucoside or galactoside.) The intersaccharide bonds can be,
e.g., between the one position of the additional saccharide units and the 2-,
3-,
4-, and/or 6- positions of the preceding saccharide units.
Optionally, and less desirably, there can be a polyalkyleneoxide chain joining
the hydrophobic moiety and the polysaccharide moiety. The preferred
alkyleneoxide is ethylene oxide. Typical hydrophobic groups include alkyl
groups, either saturated or unsaturated, branched or unbranched containing
CA 02305979 2000-04-12
WO 99/20724 PCT/US98/21677
from about 8 to about 18, preferably from about 10 to about 16, carbon atoms.
Preferably, the alkyl group can contain up to about 3 hydroxy groups and/or
the
polyalkyleneoxide chain can contain up to about 10, preferably less than 5,
alkyleneoxide moieties. Suitable alkyl polysaccharides are octyl, nonyldecyi,
5 undecyldodecyl, tridecyl, tetradecyl, pentadecyl, hexadecyl, heptadecyl, and
octadecyl, di-, tri-, tetra-, penta-, and hexaglucosides, galactosides,
lactosides,
glucoses, fructosides, fructoses and/or galactoses. Suitable mixtures include
coconut alkyl, di-, tri-, tetra-, and pentaglucosides and tallow alkyl tetra-,
yenta-,
and hexaglucosides.
The preferred alkylpolyglycosides have the formula:
R2~(CnH2n~)t(9lucosyl)x
1 s wherein R2 is selected from the group consisting of alkyl, alkylphenyl,
hydroxyalkyl, hydroxyalkylphenyl, and mixtures thereof in which the alkyl
groups
contain from about 10 to about 18, preferably from about 12 to about 14,
carbon atoms; n is 2 or 3, preferably 2; t is from 0 to about 10, preferably
0; and
x is from about 1.3 to about 10, preferably from about 1.3 to about 3, most
2o preferably from about 1.3 to about 2.7. The glycosyl is preferably derived
from
glucose. To prepare these compounds, the alcohol or alkylpolyethoxy alcohol
is formed first and then reacted with glucose, or a source of glucose, to form
the
glucoside (attachment at the 1-position). The additional glycosyl units can
then
be attached between their 1-position and the preceding glycosyl units 2-, 3-,
4-
25 andlor fi- position, preferably predominantely the 2- position.
Although not preferred, the condensation products of ethylene oxide with a
hydrophobic base formed by the condensation of propylene oxide with
propylene glycol are also suitable for use herein. The hydrophobic portion of
3o these compounds will preferably have a molecular weight of from about 1500
to
about 1800 and will exhibit water insolubility. The addition of
polyoxyethylene
moieties to this hydrophobic portion tends to increase the water solubility of
the
molecule as a whole, and the liquid character of the product is retained up to
the point where the polyoxyethylene content is about 50% of the total weight
of
35 the condensation product, which corresponds to condensation with up to
about
40 moles of ethylene oxide. Examples of compounds of this type include
CA 02305979 2000-04-12
WO 99/20724 PCT/US98/2167
16
certain of the commercially available PluronicTM surfactants, marketed by
BASF.
Also not preferred, although suitable for use as nonionic surfactants herein
are
s the condensation products of ethylene oxide with the product resulting from
the
reaction of propylene oxide and ethylenediamine. The hydrophobic moiety of
these products consists of the reaction product of ethylenediamine and excess
propylene oxide, and generally has a molecular weight of from about 2,500 to
about 3,000. This hydrophobic moiety is condensed with ethylene oxide to the
1o extent that the condensation product contains from about 40% to about 80%
by
weight of polyoxyethylene and has a molecular weight of from about 5,000 to
about 11,000. Examples of this type of nonionic surfactant include certain of
the commercially available TetronicTM compounds, marketed by BASF.
15 Other suitable nonionic surfactants for use herein include polyhydroxy
fatty acid
amides of the structural formula
2o O R1
(I) R2-C-N-Z
wherein : R1 is H, C1-C4 hydrocarbyl, 2-hydroxy ethyl, 2-hydroxypropyl, or a
2s mixture thereof, preferably C1-C4 alkyl, more preferably C1 or C2 alkyl,
most
preferably C1 alkyl (i.e., methyl); and R2 is a C5-C31 hydrocarbyl, preferably
straight chain C7-C1g alkyl or alkenyl, more preferably straight chain Cg-C17
alkyl or alkenyl, most preferably straight chain C11-C17 alkyl or alkenyl, or
mixtures thereof; and Z is a polyhydroxyhydrocarbyl having a linear
hydrocarbyl
3o chain with at least 3 hydroxyls directly connected to the chain, or an
alkoxylated
derivative (preferably ethoxylated or propoxylated) thereof. Z preferably will
be
derived from a reducing sugar in a reductive amination reaction; more
preferably Z is a glycityl. Suitable reducing sugards include glucose,
fructose,
maltose, lactose, galactose, mannose, and xylose. As raw materials, high
35 dextrose com syrup can be utilised as well as the individual sugars listed
above. These corn syrups may yield a mix of sugar components for Z. It
should be understood that it is by no means intended to exclude other suitable
CA 02305979 2000-04-12
WO 99/20724 PCT/US98/2167','
17
raw materials. Z preferably will be selected from the group consisting of -CH2-
' ~ . (CHOH)n-CH20H, -CH(CH20H)-(CHOH)n-1-CH20H, -CH2-
(CHOH)2(CHOR')(CHOH)-CH20H, where n is an integer from 3 to 5, inclusive,
and R' is H or a cyclic or aliphatic monosaccharide, and alkoxylated
derivatives
thereof. Most preferred are glycityls wherein n is 4, particularly -CH2-
(CHOH)4
CH20H.
In Formula (I}, R1 can be, for example, N-methyl, N-ethyl, N-propyl, N-
isopropyl, N-butyl, N-2-hydroxy ethyl, or N-2-hydroxy propyl.
1o R2-CO-N< can be, for example, cocamide, stearamide, oleamide, lauramide,
myristamide, capricamide, palmitamide, tallowamide, etc.
Z can be 1-deoxyglucityl, 2-deoxyfnlctityl, 1-deoxymaltityl, 1-deoxylactityl,
1-
deoxygalactityl, 1-deoxymannityl, 1-deoxymaltotriotityl, etc.
In one embodiment herein suitable nonionic surfactants for use herein are
polyethylene oxide condensates of alkyl phenols, condensation products of
primary and secondary aliphatic alcohois with from about 1 to about 25 moles
of ethyelene oxide, alkylpolysaccharides, and mixtures thereof. Most preferred
are Cg-C14 alkyl phenol ethoxylates having from 3 to 15 ethoxy groups and Cg-
2o C1g alcohol ethoxylates (preferably C10 avg.) having from 2 to 10 ethoxy
groups, and mixtures thereof.
Particularly preferred surfactants include also the anionic surfactants.
Suitable
anionic surfactants for use herein include alkali metal (e.g., sodium or
potassium) fatty acids, or soaps thereof, containing from about 8 to about 24,
2s preferably from about 10 to about 20 carbon atoms.
The fatty acids including those used in making the soaps can be obtained from
natural sources such as, for instance, plant or animal-derived glycerides
(e.g.,
palm oil, coconut oil, babassu oil, soybean oil, castor oil, tallow, whale
oil, fish
30 oil, tallow, grease, lard and mixtures thereof). The fatty acids can also
be
synthetically prepared (e.g., by oxidation of petroleum stocks or by the
Fischer-
Tropsch process). Alkali metal soaps can be made by direct saponification of
fats and oils or by the neutralization of the free fatty acids which are
prepared in
a separate manufacturing process. Particularly useful are the sodium and
s5 potassium salts of the mixtures of fatty acids derived from coconut oil and
tallow, i.e., sodium and potassium tallow and coconut soaps.
CA 02305979 2000-04-12
WO 99/20724 PCT/US98/21677
18
The term "tallow" is used herein in connection with fatty acid mixtures which
typically have an approximate carbon chain length distribution of 2.5% C14,
29% C16, 23% C18, 2% palmitoleic, 41.5% oleic and 3% linoleic (the first three
fatty acids listed are saturated). Other mixtures with similar distribution,
such as
the fatty acids derived from various animal tallows and lard, are also
included
within the term tallow. The tallow can also be hardened (i.e., hydrogenated)
to
convert part or all of the unsaturated fatty acid moieties to saturated fatty
acid
moieties. When the term "coconut" is used herein it refers to fatty acid
mixtures
which typically have an approximate carbon chain length distribution of about
8% C8, 7% C10, 48% C12, 17% C14, 9% C16, 2% C18, 7% oleic, and 2%
finoleic (the first six fatty acids listed being saturated). Other sources
having
similar carbon chain length distribution such as palm kernel oil and babassu
oil
are included with the term coconut oil.
Other suitable anionic surfactants for use herein include water-soluble salts,
particularly the alkali metal salts, of organic sulfuric reaction products
having in
the molecular structure an alkyl radical containing from about 8 to about 22
carbon atoms and a radical selected from the group consisting of sulfonic acid
and sulfuric acid ester radicals. Important examples of these synthetic
2o detergents are the sodium, ammonium or potassium alkyl sulfates, especially
those obtained by sulfating the higher aicohols produced by reducing the
glycerides of tallow or coconut oil; sodium or potassium alkyl benzene
sulfonates, in which the alkyl group contains from about 9 to about 15 carbon
atoms, especially those of the types described in U.S. Pat. Nos. 2,220,099 and
2,477,383, incorporated herein by reference; sodium alkyl glyceryl ether
sulfonates, especially those ethers of the higher alcohols derived from tallow
and coconut oil; sodium coconut oil fatty acid monoglyceride sulfates and
sulfonates; sodium or potassium salts of sulfuric acid esters of the reaction
product of one mole of a higher fatty alcohol (e.g., tallow or coconut oil
3o alcohols) and about three moles of ethylene oxide; sodium or potassium
salts of
alkyl phenol ethylene oxide ether sulfates with about four units of ethylene
oxide per molecule and in which the alkyl radicals contain about 9 carbon
atoms; the reaction product of fatty acids esterified with isothionic acid and
neutralized with sodium hydroxide where, for example, the fatty acids are
derived from coconut oil; sodium or potassium salts of fatty acid amide of a
methyl taurine in which the fatty acids, for example, are derived from coconut
oil; and others known in the art, a number being specifically set forth in
U.S.
CA 02305979 2000-04-12
WO 99/20724 PCT/US98/2167?
19
Pat. Nos: 2,486,921, 2,486,922 and 2,396,278, incorporated herein by
reference.
Suitable zwitterionic detergents for use herein comprise the betaine and
betaine-like detergents wherein the molecule contains both basic and acidic
groups which form an inner salt giving the molecule both cationic and anionic
hydrophilic groups over a broad range of pH values. Some common examples
of these detergents are described in U.S. Pat. Nos. 2,082,275, 2,702,279 and
2,255,082, incorporated herein by reference. Preferred zwitterionic detergent
1o compounds have the formula:
R2
I
R1 -N+-CH2-R4-Y-
I
R3 X
2o wherein R1 is an alkyl radical containing from 8 to 22 carbon atoms, R2 and
R3
contain from 1 to 3 carbon atoms, R4 is an alkylene chain containing from 1 to
3 carbon atoms, X is selected from the group consisting of hydrogen and a
hydroxyl radical, Y is selected from the group consisting of carboxyl and
sulfonyl radicals and wherein the sum of R1, R2 and R3 radicals is from 14 to
24 carbon atoms.
Amphoteric and ampholytic detergents which can be either cationic or anionic
depending upon the pH of the system are represented by detergents such as
dodecylbeta-alanine, N=alkyltaurines such as the one prepared by reacting
dodecyiamine with sodium isethionate according to the teaching of U.S. Pat.
3o No. 2,658,072, N-higher alkylaspartic acids such as those produced
according
to the teaching of U.S. Pat. No. 2,438,091, and the products sold under the
trade name "Miranol", and described in U.S. Pat. No. 2,528,378, said patents
being incorporated herein by reference. Additional synthetic detergents and
listings of their commercial sources can be found in McCutcheon's Detergents
and Emulsifiers, North American Ed. 1980, incorporated herein by reference.
CA 02305979 2000-04-12
WO 99/20724 PCT/US98121677
Suitable amphoteric surfactants include the amine oxides corresponding to the
formula:
RR'R"NCO
5 wherein R is a primary alkyl group containing 6-24 carbons, preferably 10-18
carbons, and wherein R' and R" are, each, independently, an alkyl group
containing 1 to 6 carbon atoms. The arrow in the formula is a conventional
representation of a semi-polar bond. The preferred amine oxides are those in
which the primary alkyl group has a straight chain in at least most of the
~o molecules, generally at least 70%, preferably at least 90% of the
molecules,
and the amine oxides which are especially preferred are those in which R
contains 10-18 carbons and R' and R" are both methyl. Exemplary of the
preferred amine oxides are the N-hexyldimethylamine oxide, N-
octyldimethylamine oxide, N-decyldimethylamine oxide, N-dodecyl
~ 5 dimethylamine oxide, N-tetradecyldimethylamine oxide, N-hexadecyl
dimethylamine oxide, N-octadecyldimethylamine oxide, N-eicosyldimethylamine
oxide, N-docosyldimethylamine oxide, N-tetracosyl dimethylamine oxide, the
corresponding amine oxides in which one or both of the methyl groups are
replaced with ethyl or 2-hydroxyethyl groups and mixtures thereof. A most
2o preferred amine oxide for use herein is N-decyldimethylamine oxide.
Other suitable amphoteric surfactants for the purpose of the invention are the
phosphine or sulfoxide surfactants of formula:
R R' R" ADO
wherein A is phosphorus or sulfur atom, R is a primary alkyl group containing
6-
24 carbons, preferably 10-18 carbons, and wherein R' and R" are, each,
independently selected from methyl, ethyl and 2-hydroxyethyl. The arrow in the
3o formula is a conventional representation of a semi-polar bond.
Cationic surfactants suitable for use in compositions of the present invention
are those having a long-chain hydrocarbyl group. Examples of such cationic
surfactants include the ammonium surfactants such as 'alkyldimethylammonium
halogenides, and those surfactants having the formula:
[R2(OR3)y~[R4(OR3)y~2R5N+X_
CA 02305979 2000-04-12
WO 99/20724 PCT/US98/216 %~
21
wherein R2 is an alkyl or alkyl benzyl group having from 8 to 18 carbon atoms
in the alkyl chain, each R3 is selected from the group consisting of -CH2CH2-,
-CH2CH(CH3r, -CH2CH(CH20H)-, -CH2CH2CH2-, and mixtures thereof;
s each R4 is selected from the group consisting of C1-C4 alkyl, C1-C4
hydroxyalkyl, benzyl ring structures formed by joining the two R4 groups, -
CH2CHOH-CHOHCOR6CHOHCH20H wherein R6 is any hexose or hexose
polymer having a molecular weight less than about 1000, and hydrogen when y
is not 0; R5 is the same as R4 or is an alkyl chain wherein the total number
of
1o carbon atoms of R2 plus R5 is not more than about 18; each y is from 0 to
about 10 and the sum of the y values is from 0 to about 15; and X is any
compatible anion.
Other cationic surfactants useful herein are also described in U.S. Patent
1 s 4,228,044, Cambre, issued October 14, 1980, incorporated herein by
reference.
Perfumes
Suitable perfumes for use herein include materials which provide an olfactory
2o aesthetic benefit andlor cover any "chemical" odour that the product may
have.
The main function of a small fraction of the highly volatile, low boiling
(having
low boiling points), perfume components in these perfumes is to improve the
fragrance odor of the product itself, rather than impacting on the subsequent
odor of the surface being cleaned. However, some of the less volatile, high
25 boiling perfume ingredients provide a fresh and clean impression to the
surfaces, and it is desirable that these ingredients be deposited and present
on
the dry surface. Perfume ingredients can be readily solubilized in the
compositions, for instance by the nonionic detergent surfactants.The perfume
ingredients and compositions suitable to be used herein are the conventional
so ones known in the art. Selection of any perfume component, or amount of
perfume, is based solely on aesthetic considerations.
Suitable perfume compounds and compositions can be found in the art
including U.S. Pat. Nos. : 4,145,184, Brain and Cummins, issued March 20,
s5 1979; 4,209,417, Whyte, issued June 24, 1980; 4,515,705, Moeddel, issued
May 7, 1985; and 4,152,272, Young, issued May 1, 1979, all of said patents
being incorporated herein by reference.ln general, the degree of substantivity
of
CA 02305979 2000-04-12
WO 99120724 PCT/US98/21677
22
a perfume is roughly proportional to the percentages of substantive perfume
material used. Relatively substantive perfumes contain at least about 1 %,
preferably at least about 10%, substantive perfume materials.Substantive
perfume materials are those odorous compounds that deposit on surfaces via
the cleaning process and are~detectable by people with normal olfactory
acuity.
Such materials typically have vapour pressures lower than that of the average
perfume material. Also, they typically have molecular weights of about 200 and
above, and are detectable at levels below those of the average perfume
material.Perfume ingredients useful herein, along with their odor character,
and
their physical and chemical properties, such as boiling point and molecular
weight, are given in "Perfume and Flavor Chemicals (Aroma Chemicals),"
Steffen Arctander, published by the author, 1969, incorporated herein by
reference.
Examples of the highly volatile, Low boiling, perfume ingredients are :
anethole,
~ 5 benzaldehyde, benzyl acetate, benzyl alcohol, benzyl formate, iso-bomyl
acetate, camphene, ciscitral (neral), citronellal, citronellol, citronellyl
acetate,
para-cymene, decanal, dihydrolinalool, dihydromyrcenol, dimethyl phenyl
carbinol, eucaliptol, geranial, geraniol, geranyl acetate, geranyl nitrite,
cis-3-
hexenyl acetate, hydroxycitronellal, d-limonene, linalool, linalool oxide,
linalyl
2o acetate, finalyl propionate, methyl anthranilate, alpha-methyl ionone,
methyl
nonyl acetaldehyde, methyl phenyl carbinyl acetate, laevo-menthyl acetate,
menthone, iso-menthone, mycrene, myrcenyl acetate, myrcenol, nerol, neryl
acetate, nonyl acetate, phenyl ethyl alcohol, alpha-pinene, beta-pinene,
gamma-terpinene, alpha-terpineol, beta-terpineol, terpinyl acetate, and
2s vertenex (para-tertiary-butyl cyclohexyl acetate). Some natural oils also
contain
large percentages of highly volatile perfume ingredients. For example,
Lavandin
contains as major components : Linalool; linalyl acetate; geraniol; and
citronellol.
Lemon oil and orange terpenes both contain about 95% of d-limonene.
Examples of moderately volatile perfume ingredients are : amyl cinnamic
3o aldehyde, iso-amyl salicylate, beta-caryophyllene, cedrene, cinnamic
alcohol,
coumarin, dimethyl benzyl carbinyl acetate, ethyl vanillin, eugenol, iso-
eugenol,
flor acetate, heliotropine, 3-cis-hexenyl saiicyfate, hexyl salicylate, lilial
(para-
tertiarybutyl-alpha-methyl hydrocinnamic aldehyde), gamma-methyl ionone,
nerolidol, patchouli alcohol, phenyl hexanol, beta-selinene, trichioromethyl
35 phenyl carbinyl acetate, triethyl citrate, vanillin, and veratraldehyde.
Cedarwood terpenes are composed mainly of alpha-cedrene, beta-cedrene,
and other C15H24 sesquiterpenes.
~.._.._ -.__.__...._.___..-
CA 02305979 2000-04-12
WO 99/20724 PCT/US98121677
23
Examples of the less volatile, high boiling, perfume ingredients are
benzophenone, benzyl salicylate, ethylene brassyiate, galaxolide (1,3,4,6,7,8-
hexahydro-4,6,6,7,8,8-hexamethyl-cyclopenta-gama-2-benzopyran), hexyf
cinnamic aldehyde, lyral (4-(4-hydroxy-4-methyl pentyl)-3-cyclohexene-10-
s carboxaldehyde), methyl cedrylone, methyl dihydro jasmonate, methyl-beta-
naphthyl ketone, musk indanone, musk ketone, musk tibetene, and phenylethyl
phenyl acetate.
Selection of any particular perfume ingredient is primarily dictated by
aesthetic
~o considerations.
The compositions herein may comprise a perfume ingredient, or mixtures
thereof, in amounts up to 5.0% by weight of the total composition, preferably
in
amounts of 0.1 % to 1.5%.
~ s Chelatinq a4ents
Another class of optional compounds for use herein include chelating agents or
mixtures thereof. Chelating agents can be incorporated in the compositions
herein in amounts ranging from 0.0% to 10.0% by weight of the total
2o composition, preferably 0.1 % to 5.0%.
Suitable phosphonate chelating agents for use herein may include alkali metal
ethane 1-hydroxy diphosphonates (HEDP), alkylene poly (alkylene
phosphonate), as well as amino phosphonate compounds, including amino
25 aminotri(methylene phosphoric acid) (ATMP), nitrilo trimethylene
phosphonates
(NTP), ethylene diamine tetra methylene phosphonates, and diethylene
triamine yenta methylene phosphonates (DTPMP). The phosphonate
compounds may be present either in their acid form or as salts of different
cations on some or all of their acid functionalities. Preferred phosphonate
3o chelating agents to be used herein are diethylene triamine yenta methylene
phosphonate (DTPMP) and ethane 1-hydroxy diphosphonate (HEDP). Such
phosphonate chelating agents are commercially available from Monsanto under
the trade name DEQUEST~~
35 Polyfunctionally-substituted aromatic chelating agents may also be useful
in the
compositions herein. See U.S. patent 3,812,044, issued May 21, 1974, to
CA 02305979 2000-04-12
WO 99/20724 PCT/US9812167
24
Connor et al. Preferred compounds of this type in acid form are
dihydroxydisulfobenzenes such as 1,2-dihydroxy -3,5-disulfobenzene.
A preferred biodegradable chelating agent for use herein is ethylene diamine
N,N'- disuccinic acid, or alkali metal, or alkaline earth, ammonium or
substitutes
ammonium salts thereof or mixtures thereof. Ethylenediamine N,N'- disuccinic
acids, especially the (S,S) isomer have been extensively described in US
patent 4, 704, 233, November 3, 1987, to Hartman and Perkins.
Ethylenediamine N,N'- disuccinic acids is, for instance, commercially
available
under the tradename ssEDDS~ from Paimer Research Laboratories.
Suitable amino carboxyiates for use herein include ethylene diamine tetra
acetates, diethylene triamine pentaacetates, diethylene triamine pentaacetate
(DTPA),N- hydroxyethylethylenediamine triacetates, nitrilotri-acetates,
i5 ethyienediamine tetrapropionates, triethylenetetraaminehexa-acetates,
ethanol-
diglycines, propylene diamine tetracetic acid (PDTA) and methyl glycine di-
acetic acid (MGDA), both in their acid form, or in their alkali metal,
ammonium,
and substituted ammonium salt forms. Particularly suitable amino carboxylates
to be used herein are diethylene triamine yenta acetic acid, propylene diamine
2o tetracetic acid (PDTA) which is, for instance, commercially available from
BASF
under the trade name Trilon FS~ and methyl glycine di-acetic acid (MGDA).
Further carboxylate chelating agents for use herein include salicylic acid,
aspartic acid, glutamic acid, glycine, malonic acid or mixtures thereof.
BUIIderS:
The liquid compositions of the present invention may also comprises a builder
or a mixture thereof, as an optional ingredient. Suitable builders for use
herein
so include polycarboxylates and polyphosphates, and salts thereof. Typically,
the
compositions of the present invention comprise up to 20.0 % by weight of the
total composition of a builder or mixtures thereof, preferably from 0.1 % to
10.0% , and more preferably from 0.5% to 5.0%.
ss Suitable and preferred polycarboxylates for use herein are organic
polycarboxylates where the highest LogKa, measured at 25°C/0.1 M ionic
strength is between 3 and 8, wherein the sum of the LogKCa + LogKMg,
CA 02305979 2000-04-12
WO 99/20724 PCTNS98/2167?
measured at 25°C10.1 M ionic strength is higher than 4, and wherein
LogKCa =
LogKMg ~ 2 units, measured at 25°CI0.1 M ionic strength.
Such suitable and preferred polycarboxylates include citrate and complexes of
5 the formula:
CH(A){COOX)-CH(COOX)-O-CH(COOX)-CH(COOX)(B)
wherein A is H or OH; B is H or -O-CH(COOX~CH2(COOX); and X is H or a
~o salt-forming cation. For example, if in the above general formula A and B
are
both H, then the compound is oxydissuccinic acid and its water-soluble salts.
If
A is OH and B is H, then the compound is tartrate monosuccinic acid (TMS)
and its water-soluble salts. If A is H and B is -O-CH(COOX)-CH2(COOX), then
the compound is tartrate disuccinic acid (TDS) and its water-soluble salts.
~ 5 Mixtures of these builders are especially preferred for use herein.
Particularly
TMS to TDS, these builders are disclosed in U.S. Patent 4,663,071, issued to
Bush et al., on May 5, 1987.
Still other ether polycarboxylates suitable for use herein include copolymers
of
2o malefic anhydride with ethylene or vinyl methyl ether, 1, 3, 5-trihydroxy
benzene-2, 4, 6-trisulfonic acid.
Other useful polycarboxylate builders include the ether
hydroxypolycarboxylates represented by the structure
HO-[C(R)(COOM}-C(R)(COOM}-OJn-H
wherein M is hydrogen or a cation wherein the resultant salt is water-soluble,
preferably an alkali metal, ammonium or substituted ammonium cation, n is
3o from about 2 to about 15 (preferably n is from about 2 to about 10, more
preferably n averages from about 2 to about 4) and each R is the same or
different and selected from hydrogen, C1~ alkyl or C1~. substituted alkyl
(preferably R is hydrogen).
Suitable ether polycarboxylates also include cyclic compounds, particularly
alicyclic compounds, such as those described in U.S. Patents 3,923,679;
CA 02305979 2000-04-12
WO 99/20724 PCT/US98/216 7 7
26
3,835,163; 4,158,635; 4,120,874 and 4,102,903, all of which are incorporated
herein by reference.
Preferred amongst those cyclic compounds are dipicolinic acid and chelidanic
acid.
Also suitable polycarboxylates for use herein are mellitic acid, succinic
acid,
polymaleic acid, benzene 1,3,5-tricarboxylic acid, benezene pentacarboxylic
acid, and carboxymethyloxysuccinic acid, and soluble salts thereof.
~o
Still suitable carboxylate builders herein include the carboxylated
carbohydrates
disclosed in U.S. Patent 3,723,322, Diehl, issued March 28, 1973, incorporated
herein by reference.
~5 Other suitable carboxylates for use herein, but which are less preferred
because they do not meet the above criteria are alkali metal, ammonium and
substituted ammonium salts of polyacetic acids. Examples of polyacetic acid
builder salts are sodium, potassium, lithium, ammonium and substituted
ammonium salts of ethylenediamine, tetraacetic acid and nitrilotriacetic acid.
25
Other suitable, but less preferred polycarboxylates are those also known as
alkyliminoacetic builders such as methyl imino diacetic acid, alanine diacetic
acid, methyl glycine diacetic acid, hydroxy propylene imino diacetic acid and
other alkyl imino acetic acid builders.
Also suitable in the compositions of the present invention are the 3,3-
dicarboxy-
4-oxa-1,6-hexanediotes and the related compounds disclosed in U.S. Patent
4,566,984, Bush, issued January 28, 1986, incorporated herein by reference.
Useful succinic acid builders include the C5-C20 alkyl succinic acids and
salts
3o thereof. A particularly preferred compound of this type is
dodecenylsuccinic
acid. Alkyl succinic acids typically are of the general formula R-
CH(COOH)CH2(COOH) i.e., derivatives of succinic acid, wherein R is
hydrocarbon, e.g., C10-C20 alkyl or alkenyl, preferably C12-C16 or wherein R
may be substituted with hydroxyl, sulfo, sulfoxy or sulfone substituents, all
as
35 described in the above-mentioned patents.
CA 02305979 2000-04-12
WO 99/20724 PCT/US98/21677
27
The succinate builders are preferably used in the form of their water-soluble
salts, including the sodium, potassium, ammonium and alkanolammonium salts.
Specific examples of succinate builders include : laurylsuccinate,
myristylsuccinate, paimitylsuccinate, 2-dodecenylsuccinate (preferred), 2-
pentadecenylsuccinate, and the like. Laurylsuccinates are the preferred
builders of this group, and are described in European Patent Application
86200690.5/0 200 263, published November 5, 1986.
Examples of useful builders also include sodium and potassium
carboxymethyloxymalonate, carboxymethyloxysuccinate, cis-cyclo-
hexanehexacarboxylate, cis-cyclopentane-tetracarboxylate, water-soluble
polyacrylates and the copolymers of malefic anhydride with vinyl methyl ether
or
ethylene.
Other suitable polycarboxylates are the polyacetal carboxylates disclosed in
U.S. Patent 4,144,226, Crutchfield et al., issued March 13, 1979, incorporated
herein by reference. These polyacetal carboxylates can be prepared by
bringing together, under polymerization conditions, an ester of glyoxylic acid
2o and a polyerization initiator. The resulting polyacetal carboxylate ester
is then
attached to chemically stable end groups to stabilize the polyacetal
carboxylate
against rapid depolymerization in alkaline solution, converted to the
corresponding salt, and added to a surfactant.
Polycarboxylate builders are also disclosed in U.S. Patent 3,308,067, Diehl,
issued March 7, 1967, incorporated herein by reference. Such materials
include the water-soluble salts of homo- and copolymers of aliphatic
carboxylic
acids such as malefic acid, itaconic acid, mesaconic acid, fumaric acid,
aconitic
acid, citraconic acid and methylenemalonic acid.
Suitable polyphosphonates for use herein are the alkali metal, ammonium and
alkanolammonium salts of polyphosphates (exemplified by the
tripolyphosphates, pyrophosphates, and glassy polymeric meta-phosphates),
phosphonates. The most preferred builder for use herein is citrate.
Divalent ions:
CA 02305979 2000-04-12
WO 99/20724 PCT/US9812167 7
28
The compositions according to the present invention may further comprise a
divalent ion, or mixtures thereof. All divalent ions known to those skilled in
the
art may be used herein. Preferred divalent ions to be used herein are calcium,
zinc, cadmium, nickel, copper, cobalt, zirconium, chromium and/or magnesium
and more preferred are calcium, zinc andlor magnesium. Said divalent ions
may be added in the form of salts for example as chloride, acetate, sulphate,
formate and/or nitrate or as a complex metal salt. For example, calcium may be
added in the form of calcium chloride, magnesium as magnesium acetate or
io magnesium sulphate and zinc as zinc chloride. Typically such ions may be
present at a level up to 3 %, preferably from 0.001 % to 1 % by weight of the
total composition.
Other antiresoiling ingredients:
The compositions of the present invention particularly suitable for the
cleaning
of a hard-surface may comprise another antiresoiling ingredient on top of the
polyalkoxylene glycol diester and polyvinyl homopolymer or copolymer as
described herein before. Suitable additional antiresoiling ingredients for use
2o herein include those selected from the group consisting of polyalkoxylene
glycol, mono- and dicapped polyalkoxylene glycol and a mixture thereof, as
defined herein after. The compositions of the present invention may comprise
up to 20% by weight of the total composition of such another antiresoiling
ingredient or a mixture thereof, preferably from 0.01 % to 10%, more
preferably
from 0.1 % to 5% and most preferably from 0.2% to 2%.
Suitable pofyalkoxylene glycols for use herein are according to the following
formula H-O-(CH2-CHR20)n-H.
3o Suitable monocapped polyalkoxylene glycols for use herein are according to
the following formula R1-O-(CH2-CHR20)n-H.
Suitable dicapped polyalkoxylene glycols for use herein are according to the
formula R1-O-(CH2-CHR20)n-Rg.
In these formulas the substituents R1 and R3 each independently are
substituted or unsubstituted, saturated or unsaturated, linear or branched
CA 02305979 2000-04-12
WO 99/20724 PCT/US98/21677
29
hydrocarbon chains having from 1 to 30 carbon atoms, or amino bearing linear
or branched, substituted or unsubstituted hydrocarbon chains having from 1 to
30 carbon atoms, R2 is hydrogen or a linear or branched hydrocarbon chain
having from 1 to 30 carbon atoms, and n is an integer greater than 0.
Preferably R1 and R3 each independently are substituted or unsubstituted,
linear or branched alkyl groups, alkenyl groups or aryl groups having from 1
to
30 carbon atoms, preferably from 1 to 16, more preferably from 1 to 8 and most
preferably from 1 to 4, or amino bearing linear or branched, substituted or
~o unsubstituted alkyl groups, alkenyl groups or aryl groups having from 1 to
30
carbon atoms, more preferably from 1 to 16, even more preferably from 1 to 8
and most preferably from 1 to 4. Preferably R2 is hydrogen, or a linear or
branched alkyl group, alkenyl group or aryl group having from 1 to 30 carbon
atoms, more preferably from 1 to 16, even more preferably from 1 to 8, and
i ~ most preferably R2 is methyl, or hydrogen. Preferably n is an integer
greater
than 1, more preferably from 5 to 1000, more preferably from 10 to 100, even
more preferably from 20 to 60 and most preferably from 30 to 50.
The preferred polyalkoxyiene glycols, mono and dicapped polyalkoxylene
2o glycols to be used herein have a molecular weight of at least 200, more
preferably from 400 to 5000 and most preferably from 800 to 3000.
Suitable monocapped polyalkoxylene glycols for use herein include 2-
aminopropyl polyethylene glycol (MW 2000), methyl polyethylene glycol (MW
25 1800) and the like. Such monocapped polyalkoxylene glycols may be
commercially available from Hoescht under the polyglycol series or Hunstman
under the tradename XTJ~. Suitable poiyalkoxylene glycols to be used herein
are polyethylene glycols like polyethylene glycol (MW 2000).
3o Suitable dicapped polyalkoxylene glycols for use herein include O,O'-bis(2-
aminopropyl)polyethylene glycol (MW 2000), O.O'-bis(2-
aminopropyl)polyethyiene glycol (MW 400), O,O'-dimethyi polyethylene glycol
(MW 2000), dimethyl polyethylene glycol (MW 2000), or mixtures thereof. A
preferred dicapped polyalkoxylene glycol for use herein is dimethyl
35 polyethylene glycol (MW 2000). For instance dimethyl polyethylene glycol
may
be commercially available from Hoescht as the polyglycol series, e.g. PEG
DME-2000, or from Huntsman under the name Jeffamine0 and XTJ~.
CA 02305979 2000-04-12
WO 99/20724 PCT/US98/21677
These polyalkoxylene giycols, mono- or dicapped polyalkoxylene glycois
contribute to the benefit of the liquid hard-surface compositions of the
present
invention, i.e. they help further improving the next-time cleaning performance
of
5 the composition herein. Dicapped polyalkoxylene glycols are highly preferred
herein.
Suds controlfina agents:
io The compositions according to the present invention may further comprise a
suds controlling agent such as 2-alkyl alkanol, or mixtures thereof, as a
preferred optional ingredient. Particularly suitable to be used in the present
invention are the 2-alkyl alkanols having an alkyl chain comprising from 6 to
16
carbon atoms, preferably from 8 to 12 and a terminal hydroxy group, said alkyl
~5 chain being substituted in the a position by an alkyl chain comprising from
1 to
10 carbon atoms, preferably from 2 to 8 and more preferably 3 to 6. Such
suitable compounds are commercially available, for instance, in the Isofol~
series such as Isofol~ 12 (2-butyl octanol) or Isofol~ 16 (2-hexyl decanol).
2o Other suds controlling agents may include alkali metal (e.g., sodium or
potassium) fatty acids, or soaps thereof, containing from about 8 to about 24,
preferably from about 10 to about 20 carbon atoms.
The fatty acids including those used in making the soaps can be obtained from
25 natural sources such as, for instance, plant or animal-derived glycerides
(e.g.,
palm oil, coconut oil, babassu oil, soybean oil, castor oil, tallow, whale
oil, fish
oil, tallow, grease, lard and mixtures thereof). The fatty acids can also be
synthetically prepared (e.g., by oxidation of petroleum stocks or by the
Fischer-
Tropsch process).Alkali metal soaps can be made by direct saponification of
3o fats and oils or by the neutralization of the free fatty acids which are
prepared in
a separate manufacturing process. Particularly useful are the sodium and
potassium salts of the mixtures of fatty acids derived from coconut oil and
tallow, i.e., sodium and potassium tallow and coconut soaps.The term "tallow"
is
used herein in connection with fatty acid mixtures which typically have an
approximate carbon chain length distribution of 2.5% C14, 29% C16, 23% C18,
2% palmitoleic, 41.5% oleic and 3% linoleic (the first three fatty acids
listed are
saturated). Other mixtures with similar distribution, such as the fatty acids
CA 02305979 2000-04-12
WO 99/20724
PCT/US98/2167~
31
derived from various animal tallows and lard, are also included within the
term
tallow. The tallow can also be hardened (i.e., hydrogenated) to convert part
or
all of the unsaturated fatty acid moieties to saturated fatty acid moieties.
When
the term "coconut" is used herein it refers to fatty acid mixtures which
typically
have an approximate carbon chain length distribution of about 8% C8, 7% C10,
48% C12, 17% C14, 9% C16, 2% C18, 7% oleic, and 2% linoleic (the first six
fatty acids listed being saturated). Other sources having similar carbon chain
length distribution such as palm kernel oil and babassu oil are included with
the
term coconut oil.
Other suitable suds controlling agents are exemplified by silicones, and
silica-
silicone mixtures. Silicones can be generally represented by alkylated
polysiloxane materials while silica is normally used in finely divided forms
exemplified by silica aerogels and xerogels and hydrophobic silicas of various
1 s types. These materials can be incorporated as particulates in which the
suds
controlling agent is advantageously releasably incorporated in a water-soluble
or water-dispersible, substantially non-surface-active detergent impermeable
carrier. Alternatively the suds controlling agent can be dissolved or
dispersed in
a liquid carrier and applied by spraying on to one or more of the other
2o components.
A preferred silicone suds controlling agent is disclosed in Bartollota et al.
U.S.
Patent 3 933 672. Other particularly useful suds controlling agents are the
self-
emulsifying silicone suds controlling agents, described in German Patent
25 Application DTOS 2 646 126 published April 28, 1977. An example of such a
compound is DC-544, commercially available from Dow Corning, which is a
siloxane-glycol copolymer.
Especially preferred silicone suds controlling agents are described in
3o Copending European Patent application N°92201649.8. Said
compositions can
comprise a silicone/silica mixture in combination with fumed nonporous silica
such as AerosilR.
Especially preferred suds controlling agent are the suds controlling agent
35 system comprising a mixture of silicone oils and the 2-alkyl-alcanols.
CA 02305979 2000-04-12
WO 99/20724 PCT/US98I216T
32
Typically, the compositions herein may comprise up to 4% by weight of the
total
composition of a suds controlling agent, or mixtures thereof, preferably from
0.1 % to 1.5% and most preferably from 0.1 % to 0.8%.
Solvents
The compositions of the present invention may further comprise a solvent or a
mixtures thereof. Solvents for use herein include all those known to the those
skilled in the art of hard-surfaces cleaner compositions. Suitable solvents
for
~o use herein include ethers and diethers having from 4 to 14 carbon atoms,
preferably from 6 to 12 carbon atoms, and more preferably from 8 to 10 carbon
atoms, glycols or alkoxylated glycols, alkoxylated aromatic alcohols, aromatic
alcohols, aliphatic branched alcohols, alkoxyiated aliphatic branched
alcohols,
alkoxylated linear C1-C5 alcohols, linear C1-C5 alcohols, C8-C14 alkyl and
~s cycloalkyl hydrocarbons and halohydrocarbons, C6-C16 glycol ethers and
mixtures thereof.
Suitable glycols to be used herein are according to the formula HO-CR1 R2-OH
wherein R1 and R2 are independently H or a C2-C10 saturated or unsaturated
2o aliphatic hydrocarbon chain and/or cyclic. Suitable glycols to be used
herein
are dodecaneglycol and/or propanediol.
Suitable alkoxylated glycols to be used herein ale according to the formula R-
(A)n-R1-OH wherein R is H, OH, a linear saturated or unsaturated alkyl of from
2s 1 to 20 carbon atoms, preferably from 2 to 15 and more preferably from 2 to
10,
wherein R1 is H or a linear saturated or unsaturated alkyl of from 1 to 20
carbon
atoms, preferably from 2 to 15 and more preferably from 2 to 10, and A is an
alkoxy group preferably ethoxy, methoxy, andlor propoxy and n is from 1 to 5,
preferably 1 to 2. Suitable alkoxylated glycols to be used herein are methoxy
30 octadecanol and/or ethoxyethoxyethanol.
Suitable alkoxylated aromatic alcohols to be used herein are according to the
formula R (A)n-OH wherein R is an alkyl substituted or non-alkyl substituted
aryl
group of from 1 to 20 carbon atoms, preferably from 2 to 15 and more
35 preferably from 2 to 10, wherein A is an alkoxy group preferably butoxy,
propoxy and/or ethoxy, and n is an integer of from 1 to 5, preferably 1 to 2.
CA 02305979 2000-04-12
WO 99/20724 PCT/US98/21677
33
Suitable alkoxylated aromatic aicohols are benzoxyethanoi and/or
benzoxypropanol.
Suitable aromatic alcohols to be used herein are according to the formula R-OH
s wherein R is an alkyl substituted or non-alkyl substituted aryl group of
from 1 to
20 carbon atoms, preferably from 1 to 15 and more preferably from 1 to 10. For
example a suitable aromatic alcohol to be used herein is benzyi alcohol.
Suitable aliphatic branched alcohols to be used herein are according to the
~o formula R-OH wherein R is a branched saturated or unsaturated alkyl group
of
from 1 to 20 carbon atoms, preferably from 2 to 15 and more preferably from 5
to 12. Particularly suitable aliphatic branched alcohols to be used herein
include
2-ethylbutanol and/or 2-methylbutanol.
~s Suitable alkoxylated aliphatic branched afcohols to be used herein are
according to the formula R (A)n-OH wherein R is a branched saturated or
unsaturated alkyl group of from 1 to 20 carbon atoms, preferably from 2 to 15
and more preferably from 5 to 12, wherein A is an alkoxy group preferably
butoxy, propoxy and/or ethoxy, and n is an integer of from 1 to 5, preferably
1
2o to 2. Suitable alkoxylated aliphatic branched alcohols include 1-
methylpropoxyethanol and/or 2-methylbutoxyethanol.
Suitable alkoxylated linear C1-C5 aicohols to be used herein are according to
the formula R (A)n-OH wherein R is a linear saturated or unsaturated alkyl
2s group of from 1 to 5 carbon atoms, preferably from 2 to 4, wherein A is an
alkoxy group preferably butoxy, propoxy and/or ethoxy, and n is an integer of
from 1 to 5, preferably 1 to 2. Suitable alkoxylated aliphatic linear C1-C5
alcohols are butoxy propoxy propanol (n-BPP), butoxyethanol, butoxypropanol,
ethoxyethanol or mixtures thereof. Butoxy propoxy propanol is commercially
3o available under the trade name n-BPPO from Dow chemical.
Suitable linear C1-C5 alcohols to be used herein are according to the formula
R-OH wherein R is a linear saturated or unsaturated alkyl group of from 1 to 5
carbon atoms, preferably from 2 to 4. Suitable linear C1-C5 alcohols are
3s methanol, ethanol, propanol or mixtures thereof.
CA 02305979 2000-04-12
WO 99120724 PCT/US98/21677
34
Other suitable solvents include butyl diglycol ether (BDGE), butyltriglycol
ether,
ter amilic alcohol and the like. Particularly preferred solvents to be used
herein
are butoxy propoxy propanol, butyl digiycol ether, benzyl alcohol,
butoxypropanol, ethanol, methanol, isopropanol and mixtures thereof.
Typically, the compositions of the present invention comprise up to 20% by
weight of the total composition of a solvent or mixtures thereof, preferably
from
0.5% to 10% by weight and more preferably from 1 % to 8%.
1o Bleaching components
The liquid compositions herein may also comprise a bleaching component. Any
bleach known to those skilled in the art may be suitable to be; used herein
including any peroxygen bleach as well as a chlorine releasing component.
Suitable peroxygen bleaches for use herein include hydrogen peroxide or
sources thereof. As used herein a source of hydrogen peroxide refers to any
compound which produces active oxygen when said compound is in contact
with water. Suitable water-soluble sources of hydrogen peroxide for use herein
2o include percarbonates, preformed percarboxyiic acids, persilicates,
persulphates, perborates, organic and inorganic peroxides andlor
hydroperoxides.
Suitable chlorine releasing component for use herein is an alkali metal
hypochforite. Advantageously, the composition of the invention are stable in
presence of this bleaching component. Although alkali metal hypochlorites are
preferred, other hypochlorite compounds may also be used herein and can be
selected from calcium and magnesium hypochlorite. A preferred alkali metal
hypochlorite for use herein is sodium hypochlorite.
Bleach activators
The compositions of the present invention that comprise a peroxygen bleach
may further comprise a bleach activator or mixtures thereof. By "bleach
activator", it is meant herein a compound which reacts with peroxygen bleach
CA 02305979 2000-04-12
WO 99/20724 PCT/US98/2167'
like hydrogen peroxide to form a peracid. The peracid thus formed constitutes
the activated bleach. Suitable bleach activators to be used herein include
those belonging to the class of esters, amides, imides, or anhydrides.
Examples of suitable compounds of this type are disclosed in British Patent GB
5 1 586 769 and GB 2 143 231 and a method for their formation into a grilled
form is described in European Published Patent Application EP-A-62 523.
Suitable examples of such compounds to be used herein are tetracetyl ethylene
diamine (TAED), sodium 3,5,5 trimethyl hexanoyloxybenzene sulphonate,
diperoxy dodecanoic acid as described for instance in US 4 818 425 and
io nonylamide of peroxyadipic acid as described for instance in US 4 259 201
and
n-nonanoyloxybenzenesulphonate (NOBS). Also suitable are N-acyl
caprolactams selected from the group consisting of substituted or
unsubstituted
benzoyl caprolactam, octanoyl caprolactam, nonanoyl caprolactam, hexanoyl
caprofactam, decanoyl caprolactam, undecenoyl caprolactam, formyl
~ 5 caprolactam, acetyl caprolactam, propanoyl caprolactam, butanoyl
caprolactam
pentanoyl caprolactam or mixtures thereof. A particular family of bleach
activators of interest was disclosed in EP 624 154, and particularly preferred
in
that family is acetyl triethyl citrate (ATC). Acetyl methyl citrate has the
advantage that it is environmental-friendly as it eventually degrades into
citric
2o acid and alcohol. Furthermore, acetyl triethyl citrate has a good
hydrolytical
stability in the product upon storage and it is an efficient bleach activator.
Finally, it provides good building capacity to the composition.
Packaging form of the compositions
The compositions herein may be packaged in a variety of suitable detergent
packaging known to those skilled in the art. The liquid compositions are
preferably packaged in conventional detergent plastic bottles.
3o In one embodiment the compositions herein may be packaged in manually
operated spray dispensing containers, which are usually made of synthetic
organic polymeric plastic materials. Accordingly, the present invention also
encompasses liquid cleaning compositions of the invention packaged in a spray
dispenser, preferably in a trigger spray dispenser or pump spray dispenser.
Indeed, said spray-type dispensers allow to uniformly apply to a relatively
large
area of a surface to be cleaned the liquid cleaning compositions suitable for
use
CA 02305979 2000-04-12
WO 99/20724 PCT/US98/216
36
according to the present invention. Such spray-type dispensers are
particularly
suitable to clean vertical surfaces.
Suitable spray-type dispensers to be used according to the present invention
include manually operated foam trigger-type dispensers sold for example by
Specialty Packaging Products, Inc. or Continental Sprayers, Inc. These types
of dispensers are disclosed, for instance, in US-4,701,311 to Dunnining et al.
and US-4,646,973 and US-4,538,745 both to Focarracci. Particularly preferred
to be used herein are spray-type dispensers such as T 8500~ commercially
~o available from Continental Spray International or T 8100~ commercially
available from Canyon, Northern Ireland. In such a dispenser the liquid
composition is divided in fine liquid droplets resulting in a spray that is
directed
onto the surface to be treated. Indeed, in such a spray-type dispenser the
composition contained in the body of said dispenser is directed through the
~ 5 spray-type dispenser head via energy communicated to a pumping mechanism
by the user as said user activates said pumping mechanism. More particularly,
in said spray-type dispenser head the composition is forced against an
obstacle, e.g. a grid or a cone or the like, thereby providing shocks to help
atomise the liquid composition, i.e. to help the formation of liquid droplets.
The process of cleaning a hard-surface:
The present invention also encompasses a process of cleaning hard-surfaces
wherein a liquid composition comprising a polyalkoxylene glycol diester and a
vinylpyrrolidone homopolymer or copolymer as described herein before, is
contacted with said surfaces.
By "hard-surfaces", it is meant herein any kind of surfaces typically found in
houses like kitchens, bathrooms, or in car interiors or exteriors, e.g.,
floors,
walls, tiles, windows, sinks, showers, shower piastified curtains, wash
basins,
3o WCs, dishes, fixtures and fittings and the like made of different materials
like
ceramic, vinyl, no-wax vinyl, linoleum, melamine, glass, any plastics,
plastified
wood, metal or any painted or varnished or sealed surface and the like. Hard-
surfaces also include household appliances including, but not limited to,
refrigerators, freezers, washing machines, automatic dryers, ovens, microwave
ovens, dishwashers and so on.
CA 02305979 2000-04-12
WO 99/20724 PCT/US98121677
37
The liquid compositions of the present invention may be contacted to the
surface to be cleaned in its neat form or in its diluted form.
By "diluted form" it is meant herein that said liquid composition is diluted
by the
user typically with water. The composition is diluted prior use to a typical
dilution
level of 10 to 400 times its weight of water, preferably from 10 to 200 and
more
preferably from 10 to 100. Usual recommended dilution level is a 1.2% dilution
of the composition in water.
In the preferred process of cleaning hard-surfaces according to the present
invention where said composition is used in diluted form, there is no need to
rinse the surface after application of the composition in order to obtain
excellent
first and next-time cleaning performance and also excellent end result surface
appearance.
The present invention will be further illustrated by the following examples.
2o Examples
The following compositions were made by mixing the listed ingredients in the
listed proportions. All proportions are % by weight of the total composition.
Excellent first and next-time cleaning performance and good gloss were
delivered to the hard-surfaces cleaned with these compositions both under neat
2s and diluted conditions, e.g. at a dilution level of 50:1 to 200:1
(water:composition).
CA 02305979 2000-04-12
WO 99/20724 PCTNS98/21677
38
Compositions (weight%):
A B C D E F
Nonionic surfactants
C 9-11 E05 2.4 1.9 2.5 - - 2.5
C12,14 E05 3.6 2.9 2.5 2.5 - 2.5
C7-9 E06 - - - - 3.2
Dobanol~ 23-3 - - - - 1.3 -
A021 1.0 0.8 4.0 - 1.9 2.0
Anionic surfactants
NaPS - - - - - -
NaLAS - - - 4.0 0.9 0.8
NaCS 1.5 2.6 - 2.3 1.2 1.5
C8-AS _ _ _ - 0.8 -
Isalchem~ AS 0.6 0.6 - - - -
Buffer
Na2C03 0.6 0.13 0.6 1.0 1.0 0.1
Citrate 0.5 0.56 0.5 - - 0.6
Caustic 0.3 0.33 0.3 - - 0.3
Suds control
Fatty Acid 0.6 0.3 0.5 0.4 0.4 0.5
Isofol12~ 0.3 0.3 - 0.3 0.3 0.3
Polymers
Kessco 6000DS~ 0.4 - 0.3 - 0.5 0.35
Marlosol FS~ - 0.4 - 0.5 - -
PVP K60~ - - - 0.5 - -
PVP K90~ 0.3 0.4 0.6 - 0.5 0.3
Minors and water ----- ----- up to 100% ----
~H 9.5 7.4 9.5 10.5 10.75 7.5
CA 02305979 2000-04-12
WO 99/20724 PCTNS98/21677
39
G H I J K L M
Nonionic surfactants
C 9-11 E05 - - 2.5 2.4 - 2.5 0.030
C12,14 E05 - - 2.5 3.6 - 2.5 0.030
C7-9 E06 3.2 8 - - 3.2 - -
Dobanol~ 23-3 1.3 3.2 - - 1.3 - -
A021 1.9 4.8 2.0 1.0 1.9 2.0 0.024
Anionic surfactants
NaPS - 3.0 - - - - -
NaLAS 0.9 - 0.8 - 0.9 0.8 0.009
NaCS 1.2 3.0 1.5 1.5 1.2 1.5 0.018
Cg-AS 0.8 2.0 - - 0.8 - -
Isalchem~ AS - - - 0.6 - - -
Buffer
Na2C03 1.0 2.0 0.2 0.6 1.0 0.2 0.002
Citrate - - 0.75 0.5 - 0.75 0.009
Caustic - - 0.5 0.3 - 0.5 0.006
Suds control
Fatty Acid 0.4 0.8 0.4 0.6 0.4 0.4 0.005
Isofol12~ 0.3 - 0.3 0.3 0.3 0.3 0.004
Pol~rmers
Kessco 6000DS~ 0.5 0.75 0.5 0.5 0.5 0.4 0.006
PVP K90~ - 0.5 0.5 - - 0.3 0.006
PVP K60~ 0.5 - - 0.5 0.5 - -
Minors and water --- -- up to 100% --- --- ___
pH 10.7 10.75 9.5 9.5 10.75 9.5 8.5
CA 02305979 2000-04-12
PCT/US98/21677
WO 99/20724
PVP K60~ and PVP K900 are vinylpyrrolidone homopolymers (average
molecular weight of 160,000), commercially available from ISP Corporation,
New York, NY and Montreal, Canada.
Kessco 6000DS~ is O,O'- distearyl polyethylene glycol diester commercially
5 available from Akzo Nobel.
Marlosol FS~ is O,O'-dioleyl polyethylene glycol diester commercially
available
from Huls.
Isofol 12~ is 2-butyl octanol
Dobanol~ 23-3 is a C12-C13 EO 3 nonionic surfactant commercially available
from SHELL.
C8-AS is octyl sulphate available from Albright and Wilson, under the
tradename Empimin~ LV 33.
A021 is a C12-14 E021 alcohol ethoxylate.
Isalchem0 AS is a branched alcohol alkyl sulphate commercially available from
Enichem.