Note: Descriptions are shown in the official language in which they were submitted.
CA 02306155 2000-04-11
WO 99/24037 PCTNS9$/23549
-1-
USE OF QUINAZOLINE DERIUATIUES AS TYROSINE KINASE INHIBITORS FOR TREATING
COLONIC POLYPS
This invention relates to the use of certain quinazoline compounds in the
5 treatment and inhibition of colonic polyps.
Colonic Polyps occur in both a familial pattern (Familial Adenomatous Polyps
(FAP) and sporadically. FAP afflicts approximately 25,000 patients in the US:
while it
is estimated that sporadic adenomatous polyps (SAP) occur in approximately 2
million
10 people per year in the US alone. All these patients are at risk for
developing
adenocarcinoma of the colon. In the case of FAP, that risk is virtually 100%
and these
patients usually undergo a colectomy at an early age. Patients with sporadic
polyps are
treated with polypectomy and require periodic colonoscopic exar~fination
because of
their inherent risk of developing recurrent polyps. In fact, parents and
siblings of these
15 patients are also at increased risk for developing colorectal cancer.
The genetic basis for FAP has been linked to the presence of mutations in the
APC gene. Similar APC mutations have been found in patients with sporadic
polyps.
Biochemically, the APC mutation occurs in conjunction with the increased
expression
of cyclooxygenase enzymes, particularly COX-2. These enzymes are essential for
the
20 production of prostenoids, (prostaglandin's; (PG's)) that mediate a number
of
functions in the bowel including motility, vascular tone, angiogenesis and
mucosal
protection. PG's are also purported to discourage apoptosis and this is
proposed as an
explanation for polyp formation.
The therapy of FAP and SAP has focused on inhibiting COX enzymes.
25 Considerable evidence exists for the efficacy of COX inhibitors in reducing
polyp
formation. These COX inhibitors are predominantly NSAID's such as clinoril,
sulindac, piroxicam and etodoloc, all of which appear to be equivalent in
their action. A
major problem with NSAID therapy has been the development of serious side
effects
including peptic ulceration, and cholestatic hepatitis and renal papillary
necrosis. Long
30 term therapy with NSAIDs for the treatment of polyps is therefore
considered to be
impractical.
It has recently been proposed that the activation and overexpression of COX-2
in adenomatous polyps is due to activation of the epidermal growth factor
receptor
(EGFR). EGFR stimulation by one of it's ligands - amphiregulin (AR), induces
the
35 nuclear targeting of COX-2, release of PG's and subsequent mitogenesis, in
polarized
CA 02306155 2000-04-11
WO 99/24037 PCT/US98/Z3549
-2-
colonic epithelial cells. COX-2 inhibitors have been shown to prevent this
series of
events.
DESCRIPTION OF THE INVENTION
5 This invention provides a method of treating or inhibiting colonic polyps in
a
mammal in need thereof which comprises administering to said mammal a compound
of
formula 1:
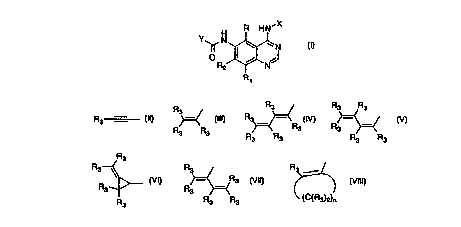
H R HN'X
Y II N ~ ~N
O R2 / N J
R~
1
10 wherein:
X is phenyl optionally substituted with one or more substituents selected from
the
group consisting of halogen, alkyl of 1-6 carbon atoms, alkoxy of 1-6 carbon
atoms, hydroxy, trifluoromethyl, cyano, nitro, carboxy, carboalkoxy of 2-7
carbon atoms, carboalkyl of 2-7 carbon atoms, amino, and alkanoylamino of 1-
15 6 carbon atoms;
R and R1 are each, independently, hydrogen, halogen, alkyl of 1-6 carbon
atoms,
alkoxy of 1-6 carbon atoms, hydroxy, or trifluoromethyl;
R2 is hydrogen, alkyl of 1-6 carbon atoms, alkoxy of 1-6 carbon atoms,
hydroxy,
trifluoromethyl;
20 Y is a radical selected from the group consisting of
R Rs Rs Rs
, , _ ,Rs , R3
R3 - ~ R3 ,
R3 R3 R R R~R
3 3 3 3
R3
R3 ~ R3 R R3 ~
R , R 3 , and
3 3 \
R R3 R3 ~C~R3)2)n
3
CA 02306155 2000-04-11
WO 99IZ4037 PCT/US98/23549
-3-
R3 is independently hydrogen, alkyl of 1-6 carbon atoms, carboxy, carboalkoxy
of 1-6
carbon atoms, phenyl, or carboalkyl of 2-7 carbon atoms;
n = 2-4;
or a pharmaceutically acceptable salt thereof, with the proviso that each R3
of Y may be
5 the same or different.
The pharmaceutically acceptable salts are those derived from such organic and
inorganic acids as: acetic, lactic, citric, tartaric, succinic, malefic,
malonic, gluconic,
hydrochloric, hydrobromic, phosphoric, nitric, sulfuric, methanesulfonic, and
similarly
10 known acceptable acids.
The alkyl portion of the alkyl, alkoxy, carboalkoxy, carboalkyl, and
alkanoylamino substituents include both straight chain as well as branched
carbon
chains, e.g. methyl, ethyl, n-propyl, i-propyl, n-butyl, i-butyl, s-butyl, t-
butyl, n-
15 pentyl or n-hexyl. Carboxy is defined as a -C02H radical. Carboalkoxy of 2-
7 carbon
atoms is defined as a -C02R" radical, where R" is an alkyl radical of 1-6
carbon
atoms. Carboalkyl is defined as a -COR" radical, where R" is an alkyl radical
of 1-6
carbon atoms. When used herein the term halogen refers to chlorine, bromine,
iodine
or fluorine. When X is substituted, it is preferred that it is mono- , di- ,
or tri-
20 substituted, with monosubstituted being most preferred. When a compound of
this
invention contains an assymetric center, this invention covers the individual
R and S
entantiomers as well as the racemate with respect to such compound.
Of the compounds of this invention, preferred members include those in which
25 R, R~, and R2 are hydrogen; and those in which R, R~, and R2 are hydrogen
and X is
phenyl either unsubstituted or monosubstituted with halogen or alkyl of 1-6
carbon
atoms. One group of compounds within the invention are those wherein X is
monosubstituted in the 3-position, preferably by a halogen, more preferably by
bromine. A further group of compounds within the invention are those wherein Y
is
30 -CH=CH-C02H, -CH=CH-C02Et, -CH=C(Me)2, -CH=CH-Et, -CH=CH-CH3,
-CH=CH-CH=CH-CH3~ -CH=CH2, -CH=CH-Ph, -CH~CH-CH3 or 2-cyclo-
pentene. R3 is preferably hydrogen, methyl, ethyl, phenyl, C02H or C02Et.
CA 02306155 2000-04-11
WO 99124037 PCT/US98/23549
-4-
A process for the preparation of a compound of Formula 1 as defined above
which
comprises:
a) reducing a 6-amino-4-anilinoquinazoline of Formula 6 wherein R, R 1, R2 and
5 X are as defined above
R HN~x
H2N \ ~ N
J
R2
R~
6
with:
10
i) an acid chloride of Formula 7, wherein Y is as defined above
//O
Y~C I
15 ii) a mixed anhydride of Formula 8, wherein Y is as defined above
O
r
~OCORa
8
or iii) a cyclic anhydride of Formula 11 wherein each R5 is independently
20 hydrogen, phenyl, or alkyl of 1-6 carbon atoms
O
R5
O
R5
~~ O
b) reacting a compound of Formula 17 wherein R, R1, R2 and Y are as defined
25 above and Hal is any suitable halogen
CA 02306155 2000-04-11
WO 99/24037 PCT/US98/23549
-5-
R Hal
Y II N ~ ~N
o ~ ~ NJ
Rp
R1
17
with an aniline of Formula 18 wherein X is as defined above
X-NH2
18
or c) reacting a compound of Formula 24 wherein R, R 1, R2 and Y are as
defined
above
R
YuN ~ C N
R2 ~ N~N(CH3)2
R~
10
with an aniline of Formula 25
H2N-X
15
wherein X is as defined above.
The preparation of the compounds of this invention encompassed by Formula 9
is described below in Flowsheet A where R, R1, R2, R3, X, and n are as defined
above
20 and R4 is alkyl of 1-6 carbon atoms (preferably isobutyl). Y' is a radical
selected from
the group consisting of:
CA 02306155 2000-04-11
WO 99/24037 PCT/US98/23549
-6-
R R3 R3 Rs,
s~ R, _
R ~ ~ ~~--(~ s
3 ' '
R 3 , R3
Rs R s Rs R~a R3 R's
R~s
Rs~ ~ R3 Ra _--
R~3 , and
R3 ~ Rs
C R'
fig R g ~ ~ 3)2)n
wherein each R'3 is independently alkyl of 1-6 carbon atoms, carboxy,
carboalkoxy of
1-6 carbon atoms, phenyl, or carboalkyl of 2-7 carbon atoms. According to the
5 sequence of reaction outlined in flowsheet A, a 5-vitro-anthranilonitrile of
Formula 2 is
heated at about 100°C with or without solvent containing an excess of
dimethylformamide dimethyl acetal to furnish an amidine of Formula 3 . Heating
a
solution of amidine 3 and the aniline 4 in acetic acid for 1 to S hours gives
the 6-nitro-
4-anilinoquinazolines of Formula 5. Reduction of the vitro group of 5 using a
10 reducing agent such as iron in an acetic acid-alcohol mixture at elevated
temperature
gives the 6-amino-4-anilinoquinazolines of Formula 6 . Acylation of 6 with
either an
acid chloride of Formula 7 or a mixed anhydride of Formula 8 (which is
prepared from
the corresponding carboxylic acid) in an inert solvent such as tetrahydrofuran
(THF) in
the presence of an organic base such as pyridine or triethylamine gives the
compounds
15 of this invention represented by Formula 9. In those cases where 7 or 8
have an
asymmetric carbon atom, they can be used as the racemate or as the individual
R or S
entantiomers in which case the compounds of this invention will be in the
racemic or R
and S optically active forms, respectively. The 5-vitro-anthranilonitriles of
Formula 2
needed to prepare the compounds of this invention are either already known to
the art or
20 can be prepared by procedures known in the art as detailed in the following
references:
Baudet, RecLTrav.Chim.Pays-Bas, 43, 710 ( 1924); Hartmans, Recl.Trav.Chim.Pays-
Bas, 65, 468, 469 (1946) ; Taylor et al., J.Amer.Chem.Soc., 82, 6058,6063
(1960);
Taylor et al., J.Amer.Chem.Soc., 82, 3152,3154 (1960); Deshpande; Seshadri,
Indian
J.Chem., 11 , 538 (1973); Katritzky, Alan R.; Laurenzo, Kathieen S.,
J.Org.Chem.,
25 51 (1986); Niclas, Hans-Joachim; Bohle, Matthias; Rick, Jens-Detlev;
Zeuner,Frank;
Zoelch, Lothar, Z.Chem., 25(4), 137-138 (1985).
CA 02306155 2000-04-11
WO 99/24037 PCT/US9$/23549
_ '7 _
FLOWSHEET A
R R X-NH2
02N I ~ CN (CH3)2NCH(OCH3)2 02N \ CN
I / ~ CHg O
R2 / NH2 DMF RZ N N(CH3)2
R~ Rt
2 3
R HN'X R HN'X
O 2N ~ ~ N Fe H2N \ ~ N
I / J CH3C02H, C2H50H~ I /
R2 ~ ~ N R2 N
R~ Ri
5 6
O ~~O
// ,X
°r OCOR4 H R HN
7 8 ~~N \ ~N
THF, pyridine, or (C2Hs)3N O I /
R2 N
R~
9
The preparation of the compounds of this invention encompassed by Formula
12 is described below in Flowsheet B wherein R, R1, R2, X, and n are described
above. Each RS is independently hydrogen, phenyl, or alkyl of 1-6 carbon
atoms.
5 According to the reaction outlined in Flowsheet B, the 6-amino-4-
anilinoquinazolines of
Formula 10 (prepared as in Flowsheet A) are acylated with a cyclic anhydride
of
Formula 11 in an inert solvent such as tetrahydrofuran in the presence of a
basic
catalyst such as pyridine or triethylamine.
CA 02306155 2000-04-11
WO 99/24037 PCT/US98/23549
-g_
FLOWSHEET B
O
R5
O
R HN'X R5 R5 R5 ~ R HN'X
H2N O
~N 11 H02C~ ~ ~N
- , p
~ NJ THF, pyridine, or (C2H5)3N R2 N
R~ R~
10 12
Representative compounds of this invention were evaluated in several standard
pharmacological test procedures that showed that the compounds of this
invention
possess significant activity as inhibitors of protein tyrosine kinases, and
are
S antiproliferative agents. Based on the activity shown in the standard
pharmacological
test procedures, the compounds of this invention are therefore useful as
antineoplastic
agents. The test procedures used and results obtained are shown below.
The preparation of the compounds of this invention encompassed by Formula
10 19 is described below in Flowsheet C wherein Y', R4, and X are described
above.
According to the reactions outlined in Flowsheet C, 4-choro-6-
nitroquinazoline, 13,
(Morley, JS. and Simpson,J. Chem.. Soc., 360 ( 1948)) is reduced to 6-amino-4-
chloroquinazoline, 14, using a reducing agent such as sodium hydrosulfite in a
two
phase system consisting of tetrahydrofuran and water in the presence of a
small amount
15 of phase transfer catalyst. Acylation of 14 with either an acid chloride of
Formula 15 or
a mixed anhydride of Formula 16 (which is prepared from the corresponding
carboxylic acid) in an inert solvent such as tetrahydrofuran (THF) in the
presence of an
organic base such as pyridine or N-methyl morpholine gives the compounds of
Formula 17. In those cases where 15 or lb have an asymmetric carbon atom, they
can
20 be used as the racemate or as the individual R or S entantiomers in which
case the
compounds of this invention will be in the racemic or R and S optically active
forms,
respectively. Heating a compound of Formula 17 with an aniline of Formula 18,
in a
inert solvent such as isopropanol, gives the compounds of this invention
represented by
Formula 19.
25
CA 02306155 2000-04-11
WO 99/24037 PCT/US98/23549
-9-
FLOWSHEET C
I
I
02N ~ ~ N Na2S204, (C8H»)sNCH3+ CI' H2N I ~ ~ N
NJ THF, H20 / NJ
14
13
Y'---~ or Y ~OCOR CI
CI
~ X-NH2
15 16 Y~~ ~ ~N 18
'O I / N J (CH3jpCHOH
THF, pyridine, or
N-methyl morpholine
17
H H N~
Y~~N ~ ~ N
o ~ / NJ
19
The preparation of the compounds of this invention encompassed by Formula
5 26 is described below in Flowsheet D wherein Y', Rq., and X are described
above.
According to the reactions outlined in Flowsheet D, the nitro group of 20
(prepared as
in Flowsheet A) is reduced to the corresponding amino compound 21 using a
palladium
catalyst and a source of hydrogen which can be hydrogen itself or cyclohexene.
Acylation of 21 with either an acid chloride of Formula 22 or a mixed
anhydride of
10 Formula 23 (which is prepared from the corresponding carboxylic acid) in an
inert
solvent such as tetrahydrofuran (THF) in the presence of an organic base such
as
pyridine or N-methyl morpholine gives the compounds of Formula 24. In those
cases
where 22 or 23 have an asymmetric carbon atom, they can be used as the
racemate or
as the individual R or S entantiomers in which case the compounds of this
invention
CA 02306155 2000-04-11
WO 99124037 PCT/US98/23549
- 10-
will be in the racemic or R and S optically active forms, respectively.
Heating a
compound of Formula 24 with an aniline of Formula 25, in a inert solvent such
as
acetic acid gives the compounds of this invention represented by Formula 26.
FLOWSHEET D
02N I ~ C N cyclohexene H2N ~ C N
/
/ N~N{CH~)2 CH30H, Pd/C N N(CH3)2
O //O
// Y'
Y ~Ci or ~OCOR4 'Y ~ ~ CN
22 ~ O
--~ N N(CH3)2
THF, pyridine, or (C2H5)3N
H2N_X Y N H N'X
25 ~ ~ ~ N
acetic acid O I /
N
5
The ability of the compounds of this invention to treat or inhibit colonic
polyps
10 was demonstrated in an i~ v_ ivo standard pharmacological test procedure as
described
below. The compound of Example 9 was evaluated in this procedure, which
emulates
familial adenomatous polyps (FAP) in humans, as a representative compound of
this
invention. The Min mouse used in this test procedure, currently the best
available
model for FAP, is a strain which has lost both copies of the APC gene. These
animals
15 develop multiple intestinal polyps (Adenomas) that ultimately progress to
form
adenocarcinomas. The polyps that develop in Min mice express EGFR and have
activated COX-2. NSAID's such as sulindac and etodoloc can reduce (but not
eradicate) intestinal polyp formulation in these animals indicating that COX-2
and the
CA 02306155 2000-04-11
WO 99/24037 PCT/US98/23549
-11-
ultimate production of PG's is likely responsible for these effects. The
following
briefly describes the procedure used and the results obtained in this standard
pharmacological test procedure.
5 The compound of Example 9 was blended with a standard marine chow and
animals were given ad libitum access to the food. Based on estimated food
consumption, the compound of Example 9 was added at a concentration
commensurate
with animals ingesting 20 mg/kg/day. At day 30, 4 treated + 4 control (chow
alone)
animals were sacrificed and assessed for polyp number. All control animals had
greater
10 than 30 polyps in their bowel, while the treated animals had none.
Identical results
were observed at 60 days - when 15 animals/group were assessed. The control
animals
had greater than 30 (larger) polyps while the treated animals had none.
These data demonstrate that the compounds of this invention effectively
inhibit
15 polyp formation in animals having mutations in their APC genes. Based on
the results
obtained in this standard pharmacological test procedure, the compounds of
this
invention are useful in treating or inhibiting the formation of colonic
polyps.
The compounds of this invention may formulated neat or may be combined with
20 one or more pharmaceutically acceptable carriers for administration. For
example,
solvents, diluents and the like, and may be administered orally in such forms
as tablets,
capsules, dispersible powders, granules, or suspensions containing, for
example, from
about 0.05 to 5% of suspending agent, syrups containing, for example, from
about 10
to 50% of sugar, and elixirs containing, for example, from about 20 to 50%
ethanol,
25 and the like, or parenterally in the form of sterile injectable solution or
suspension
containing from about 0.05 to 5% suspending agent in an isotonic medium. Such
pharmaceutical preparations may. contain, for example, from about 0.05 up to
about
90% of the active ingredient in combination with the carrier, more usually
between
about 5 % and 60% by weight.
30 The effective dosage of active ingredient employed may vary depending on
the
particular compound employed, the mode of administration and the severity of
the
condition being treated. However, in general, satisfactory results are
obtained when
the compounds of the invention are administered at a daily dosage of from
about 0.5 to
about 1000 mg/kg of animal body weight, optionally given in divided doses two
to four
35 times a day, or in sustained release form. For most large mammals the total
daily
dosage is from about 1 to 1000 mg, preferably from about 2 to 500 mg. Dosage
forms
CA 02306155 2000-04-11
WO 99/24037 PCT/US98/23549
-12-
suitable for internal use comprise from about 0.5 to 1000 mg of the active
compound in
intimate admixture with a solid or liquid pharmaceutically acceptable carrier.
This
dosage regimen may be adjusted to provide the optimal therapeutic response.
For
example, several divided doses may be administered daily or the dose may be
5 proportionally reduced as indicated by the exigencies of the therapeutic
situation.
These active compounds may be administered orally as well as by intravenous,
intramuscular, or subcutaneous routes. Solid carriers include starch, lactose,
dicalcium
phosphate, microcrystalline cellulose, sucrose and kaolin, while liquid
carriers include
sterile water, polyethylene glycols, non-ionic surfactants and edible oils
such as corn,
10 peanut and sesame oils, as are appropriate to the nature of the active
ingredient and the
particular form of administration desired. Adjuvants customarily employed in
the
preparation of pharmaceutical compositions may be advantageously included,
such as
flavoring agents, coloring agents, preserving agents, and antioxidants, for
example,
vitamin E, ascorbic acid, BHT and BHA.
15 The preferred pharmaceutical compositions from the standpoint of ease of
preparation and administration are solid compositions, particularly tablets
and hard-
filled or liquid-filled capsules. Oral administration of the compounds is
preferred.
In some cases it may be desirable to administer the compounds directly to the
airways in the form of an aerosol.
20 These active compounds may also be administered parenterally or
intraperitoneally. Solutions or suspensions of these active compounds as a
free base or
pharmacologically acceptable salt can be prepared in water suitably mixed with
a
surfactant such as hydroxy-propylcellulose. Dispersions can also be prepared
in
glycerol, liquid polyethylene glycols and mixtures thereof in oils. Under
ordinary
25 conditions of storage and use, these preparation contain a preservative to
prevent the
growth of microorganisms.
The pharmaceutical forms suitable for injectable use include sterile aqueous
solutions or dispersions and sterile powders for the extemporaneous
preparation of
sterile injectable solutions or dispersions. In all cases, the form must be
sterile and
30 must be fluid to the extent that easy syringability exists. It must be
stable under the
conditions of manufacture and storage and must be preserved against the
contaminating
action of microorganisms such as bacteria and fungi. The Garner can be a
solvent or
dispersion medium containing, for example, water, ethanol, polyol (e.g.,
glycerol,
propylene glycol and liquid polyethylene glycol), suitable mixtures thereof,
and
35 vegetable oils.
CA 02306155 2000-04-11
WO 99/24037 PCT/US98/23549
-13-
The preparation of representative examples of the compounds of this invention
is described below.
Example 1
5 N' (2-Cyano-4-nitrophenyll-N N-dimethylformamidine
A 40.8 g portion of 5-vitro-anthranilonitrile and 40 ml of N, N-
dimethylformamide dimethyl acetal were heated on a steam bath for 2 hours. The
solvents were removed at reduced pressure and the residue was taken up in
methyiene
10 chloride. After passing this solution through Magnesol the solvent was
removed.
After washing with ether 50.8 g of N'-(2-cyano-4-nitrophenyl)-N,N-dimethyl-
formamidine was obtained.
Example 2
15 N-(3-Bromophenvl)-6-vitro-4-quinazolinamine
A solution of 23.74 ml of 3-bromo aniline and 40.5 g N'-(2-cyano-4-
nitrophenyl)-N,N-dimethylformamidine in 100 ml of glacial acetic acid was
stirred and
heated in an oil bath at 148°C for 1.5 hours. On cooling, filtration of
the resulting solid
20 gives a quantitative yield of N-(3-bromophenyl)-6-vitro-4-quinazolinamine:
mp = 267-
270°C; mass spectrum (m/e): 345.
Example 3
N-(3-Bromonhenyrll-4 6-auiyzolig famine
25
A mixture of 34.5 g of N-(3-bromophenyl)-6-vitro-4-quinazolinamine and 16.8
g of iron powder in 150 ml of ethanol and 150 ml of glacial acetic acid was
heated in an
oil bath at 120°C for 2 hours. After filtration of the solid, solid
sodium carbonate was
added to the filtrate giving a solid. This was filtered, and the solid was
extracted with
30 methanol. The extracts were treated with charcoal and evaporated to a
solid. After
washing the solid with ether 27.5 g of N-{3-bromophenyl)-4,6-quinazolindiamine
was
obtained: mass spectrum (m/e): 315.
CA 02306155 2000-04-11
WO 99/24037 PCT/US98123549
- 14-
Example 4
4 ff4 fl3 Brom~henv_1,L)aminol-6-q,~inazolinvlLaminol-4-oxo-(Z)-~-butenoic
acid
A 15 ml portion of pyridine was added to 1.6 g of N-(3-bromophenyl)-4,6-
5 quinazolindiamine and 0.6 g of malefic anhydride. After stirnng overnight,
the solvents
were removed on the rotary evaporator. The solid was taken up in about 400 ml
of hot
ethanol and the insoluble material filtered to give 0.33 g of 4-[[4-[(3-
Bromophenyl)amino]-6-quinazolinyl]amino]-4-oxo-(Z)-2-butenoic acid: mass
spectrum
(m/e): M+H 413, 415.
10
Example 5
4 f~4 f(3 Bromo~hen~laminol6-qu_inazoli ~laminol4-oxo-(E)-2-butenoic acid
ethyl
se
15
A solution of N-(3-bromophenyl)-4,6-quinazolindiamine in 15 ml of pyridine
was cooled in an ice bath and a solution of 1.22 g of ethyl fumaryl chloride
in 10 ml of
methylene chloride was added dropwise. After stirring for 1.5 hours, the
reaction was
allowed to come to room temperature. The solvents were removed at reduced
pressure
20 and the residue was treated with water. The red solid was filtered and
extracted into
hot acetone. After filtration of the insoluble material, the filtrate was
concentrated to
give 0.45 g of 4-[[4-[(3-Bromophenyl)amino]-6-quinazolinyl]amino]-4-oxo-(E)-2-
butenoic acid, ethyl ester: mp = 259-263°C, mass spectrum (m/e): M+H
441, 443.
25 Example 6
N f4 fl3-Brom~y~yaminol-6-a~inazoli~rll-3-methyl-2-butenamide
A solution of 1.58 g of N-(3-bromophenyl)-4,6-quinazolindiamine in 15 ml of
pyridine was cooled in an ice bath and a solution of 0.67 ml of 3,3-
dimethylacryloyl
30 chloride in 7 ml of ether was added dropwise. After stirnng and cooling for
2 hours,
the solvents were removed at reduced pressure. The residue was treated with
water
and the resulting solid was recrystallized from methyl cellusolve to give 0.97
g of N-[4-
[(3-bromophenyl)amino]-6-quinazolinyl]-3-methyl-2-butenamide: mp = 300-
301°C,
mass spectrum (m/e): 396, 398.
35
CA 02306155 2000-04-11
WO 99/24037 PCT/US98/23549
- 15-
Example 7
N f4 fl3 Bromo~yl)aminol-6-qu~~olinvll-(E)-2-butenamide
A solution of 1.6 g of N-(3-bromophenyl)-4,6-quinazolindiamine in 15 ml of
5 pyridine was cooled in an ice bath and a solution of 0.57 ml of trans-
crotonoyl chloride
in 6 ml of ether was added dropwise. After stirring and cooling for 2 hours,
the
solvents were removed at reduced pressure. The residue was treated with water
and
the resulting solid recrystallized from n-butanol to give 0.69 g of N-[4-[(3
bromophenyl)amino]-6-quinazolinyl]-(E)-2-butenamide: mp - 153-160°C,
mass
10 spectrum (m/e): M+H 383, 385.
Example 8
N f4 f(3 Bromonhenyl)aminol-6-q~inazolinvll-2-met~yl-2-pronenamide
15
A solution of 1.6 g of N-(3-bromophenyl)-4,6-quinazolindiamine in 15 ml of
pyridine was cooled in an ice bath and a solution of 0.59 ml of methacryoyl
chloride in
6 ml of ether was added dropwise. After stirring and cooling for 2 hours, the
solvents
were removed at reduced pressure. The residue was treated with water and the
20 resulting solid was taken up in n-butanol (warming). Addition of ether to
the cooled
solution gives 0.44 g of N-[4-[(3-bromophenyl)amino]-6-quinazolinyl]-2-methyl-
2-
propenamide: mp = 40-245°C, mass spectrum (m/e): M+H 383, 385.
Example 9
25 N f4 f(3-Bromouheny~)aminol-6-quinazolinvll-2-butynamide
A solution of 0.50 g of 2-butynoic acid in 10 ml of tetrahydrofuran was cooled
in an ice bath. A 0.79 ml portion of isobutyl chlorofolmate followed by a 0.66
ml
portion of N-methyl morpholine were added. After about 1 minute a solution of
1.6 g
30 of N-(3-bromophenyl)-4,6-quinazolindiamine in 10 ml of pyridine was added.
The
reaction was allowed to come to room temperature and stir overnight. The
solvents
were removed at reduced pressure and the solid was recrystallized from n-
butanol to
give 1.07 g of N-[4-[(3-bromophenyl)amino]-6-quinazolinyl]-2-butynamide: mass
spectrum (m/e): 381, 383.
35
CA 02306155 2000-04-11
WO 99/24037 PCT/US98/23549
- 16-
Example 10
~[j~, l3-Bromophenvllaminol~quinazolinvllaminol-4-oxo-(E)-2-butenoic acid
A 2.5 ml portion of 10 N aqueous sodium hydroxide was added to 2.3 g of 4-[[4-
[(3-
5 bromophenyl)amino)-6-quinazolinyl]amino]-4-oxo-(E)-2-butenoic acid ethyl
ester
(Example 5) in 25 ml of ethanol. After stirring for an hour, 2.1 ml of
concentrated
hydrochloric acid was added, and the reaction was stirred an additional 2
hours. The
resulting solid was recrystallized from n-butanol to give 0.97 g of 4-[[4-[(3
Bromophenyl)amino]-6-quinazolinyl]amino]-4-oxo-(E)-2-butenoic acid: mass
spectrum
10 (m/e): M+H 413.
Example 11
N f4 fl3 Bromo~he~,~)aminol-6-quinazoliny~,l-2 4-hexadienamide
15
A solution of 0.67g of 2,4-hexadienoic acid in 10 ml of tetrahydrofuran was
cooled in an ice bath. A 0.79 ml portion of isobutyl chloroformate followed by
a 0.66
ml portion of N-methyl morpholine were added. After about 1 minute a solution
of 1.6
g of N-(3-bromophenyl)-4,6-quinazolindiamine in 10 ml of pyridine was added.
The
20 reaction was allowed to come to room temperature and stir overnight. The
solvents
were removed at reduced pressure and the solid was recrystallized to give 1.0
g of N-
[4-[(3-bromophenyl)amino]-6-quinazolinyl]-2,4-hexadienamide: mp = 258-
260°C.
25 Example 12
N-[4-t( -Bromophenyl)aminol-6-auinazolin3r11-2-c3rclo~nteneamide
A solution of 0.43 g of 2-cyclopedtenoic acid in Sml of tetrahydrofuran was
cooled in an ice bath. A 0.49 ml portion of isobutyl chloroformate followed by
a 0.41
30 ml portion of N-methyl morpholine were added. After about 1 minute a
solution of 1.0
g of N-(3-bromophenyl)-4,6-quinazolindiamine in 10 ml of pyridine was added.
The
reaction was allowed to come to room temperature and stir overnight. Another
0.5
equivalents of mixed anhydride was added. The mixture was stirred for 5 hours.
The
solvents were removed at reduced pressure and the solid was purified by
35
CA 02306155 2000-04-11
WO 99/24037 PCT/US98/Z3549
-17-
chromatography on silica gel to give 0.30 g of N-[4-[(3-bromophenyl)amino]-6-
quinazolinyl)-2-cyclopenteneamide: mass spectrum (m/e): 409 (M+H, EI).
Example 13
5 ~1-f4-fl3-Brom~heny~)aminol-6-quinazolinyil-2- r~openamide
A solution of 2.0 g of N-(3-bromophenyl)-4,6-quinazolindiamine in 10 ml of
pyridine was cooled in an ice bath and a solution of 0.61 ml of acryoyl
chloride in 30
ml of ether was added dropwise at 0°C. After stirring at room
temperature for 3.5
10 hours, the solvents were removed at reduced pressure. The residue was
purified by
chromatography to give 0.2 g of N-[4-[(3-bromophenyl)amino)-6-quinazolinyl)-2-
propenamide: mass spectrum (m/e): M+H 369.
15 Example 14
~T-[g-fl3-Bromophen~lamino]-6~- uinazoliny],1~3-phenyl-2-t~rODVnamidel
A solution of 0.93 g of 3-phenyl-2-propynoic acid in lOml of tetrahydrofuran
was cooled in an ice bath. A 0.82 ml portion of isobutyl chloroformate
followed by a
20 0.69 ml portion of N-methyl morpholine were added. After about 1 minute a
solution
of 1.0 g of N-(3-bromophenyl)-4,6-quinazolindiamine in 7 ml of pyridine was
added.
The reaction at 0°C for 1 hr.. The solvents were removed at reduced
pressure and the
solid was purified by chromatography on silica gel to give 0.01 g of N-[4-[(3
bromophenyl)amino]-6-quinazolinyl]-(3-phenyl-2-propynamide): mass spectrum
(m/e):
25 443.2, 445.2 (M+H, electrospray).
Example I5
6-amino-4.-chloroquinazoline
30 A mixture consisting of 3.25 g of 4-chloro-6-nitroquinazoline, 10.8 g of
sodium hydrosulfite, and 0.3 g of the phase transfer catalyst (CgH 17)3NCH3+
Cl- in
97 mi of tetrahydrofuran and 32 ml of water was stirred rapidly for 2 hours.
The
mixture was diluted with ether and the organic layer was separated. The
organic
solution was washed with brine and then dried over magnesium sulfate. The
solution
35 was passed through a small column of silica gel. The solvent was removed at
30°C at
reduced pressure giving 6-amino-4-chloroquinazoline which is used in the next
step
without additional purification.
CA 02306155 2000-04-11
WO 99/24037 PCT/US98/23549
- 18-
Example 16
j4-chloro-6-auinazo]iny]]-2-butvnamide
5 A solution of 1.64 g of 2-butynoic acid in 46 ml of tetrahydrofuran was
cooled
in an ice bath. A 2.34 ml portion of isobutyl chloroformate followed by a 4.13
ml
portion of N-methyl morpholine were added. After about 10 minutes, this was
poured
into a solution of 6-amino-4-chloroquinazoline in 46 ml tetrahydrofuran. This
mixture
was stirred at room temperature for 2 hours. The mixture was poured into a
mixture of
10 brine and saturated sodium bicarbonate and extracted with ether. The ether
solution was
dried over magnesium sulfate and filtered. The solvent was removed giving j4-
chloro-
6-quinazolinyl]-2-butynamide as colored oil that was used in the next step
without
additional purification.
IS Example 17
I~ [4 fl3-Bromophen~~aminol-~uinazolin~rll-2-but~tnamide
A solution consisting of 1.76 g of j4-chloro-6-quinazolinyl]-2-butynamide and
1.23 g of 3-bromo aniline was refluxed under an inert atmosphere in 23 ml of
20 isopropanol for 40 minutes. The mixture was cooled to room temperature and
200 ml of
ether was added giving 0.4 g of N-[4-[(3-bromophenyl)amino]-6-quinazolinyl]-2-
butynamide as the hydrochloride salt. Neutralizing with sodium bicarbonate
solution,
extracting with ethyl acetate, removal of the solvent, and recyrstallization
from 1-
butanol gives N-[4-[(3-bromophenyl)amino]-6-quinazolinyl]-2-butynamide as the
free
25 base.
Example 18
1~'- g Amino-2-c3ranQ,phenylZN N-dimethvlformamidine
A solution of 6.0 g (27.5 mmol) of N'-(2-cyano-4-nitrophenyl)-N,N
30 dimethylformamidine, 33.9 g (41.8 ml, 412.4 mmol) of cyclohexene, and 0.6 g
of
IO% Pd/C in 360 ml of methanol was refluxed for 4 hrs. The hot mixture was
filtered
through Celite. Solvent was removed and the residue was recrystallized from
chloroform-carbon tetrachloride giving 4.9 g (95%) of the title compound as a
light
gray crystalline solid. mass spectrum (m/e):188.9 (M+H, electrospray).
CA 02306155 2000-04-11
WO 99/24037 PCT/US98/23549
- 19-
Example 19
N-[3-Cyapo--4-[[Sdime ~rlamino)methylenelaminol phenvll-2-bu namide
To a solution of 2.01 g (23.9 mmol) of 2-butynoic acid and 2.9 ml (22.3
mmol) isobutyl chloroformate in 30 ml tetrahydrofuran was stirred at
0°C under
5 nitrogen as 2.42 g (2.63 ml, 22.3 mmol) of N-methyl morpholine was added
over 3
min, After stirring for 15 min., a solution of N'-(4-amino-2-cyanophenyl)-N,N-
dimethylfonmamidine and 1.6 g ( 1.75 ml, 15.9 mmol) of N-methyl morpholine in
25
ml tetrahydrofuran was added over 4 min. The mixture was stirred 30 min. at
0°C and
30 min. at room temperature. The mixture was diluted with 70 ml of ethyl
acetate and
10 poured into a mixture of brine and saturated sodium bicarbonate. The
organic layer was
dried (MgS04) and filtered through a pad of silica gel. The solvent was
removed and
the residue was stirred with 50 ml of ether. The suspended solid was collected
to give
3.61 g (89%) of an off white solid. mass spectrum (m/e): 255.0 (M+H,
electrospray).
15
Example 20
ty-~4-i v s-t~romo~nen3rl ~arrnnol-o-qulnazounm i-~-omvnamlae
A solution of 3.0 g ( 11.8 mmol) of N-[3-cyano-4-[[(dimethylamino)
methylene]amino] phenyl]-2-butynamide and 2.23 g ( 12.98 mmol) of 3-bromo
aniline
20 in 18 ml of acetic acid was refluxed gently with stirnng under nitrogen for
1 hr 15 min..
The mixture was cooled in an ice bath and a solid mass formed. The solid was
collected
by filtration and washed with ether-acetonitrile 1:1 to give a yellow solid
which was
recrystailized from ethanol giving 2.51 g of N-[4-[(3-bromophenyl)amino]-6-
quinazolinyl]-2-butynamide : mass spectrum (m/e): 381, 383.